Wu discusses a study by Graessl et al. that describes a Rho GTPase signaling network that combines positive and negative feedback to regulate subcellular contraction patterns.
Abstract
The nature of signal transduction networks in the regulation of cell contractility is not entirely clear. In this study, Graessl et al. (2017. J. Cell Biol. https://doi.org/10.1083/jcb.201706052) visualized and characterized pulses and waves of Rho activation in adherent cells and proposed excitable Rho signaling networks underlying cell contractility.
Contraction is one of the most basic mechanobiological processes involved with cellular shape changes. It is not a passive relaxation process after cell protrusion or stretching. Contractile materials made of partially overlapping arrays of actin and myosin filaments are the earliest identified active force-generating devices. Increasing evidence suggests that contractions are frequently pulsatile. Although there has been considerable interest in understanding how the coordination of contraction pulses in multicellular systems is linked with morphogenesis events by looking at the phase relationship of the contraction pulses in neighboring cells, much less is known about origin of the contractile pulses within single cells and whether or how different subcellular regions coordinate local contractions.
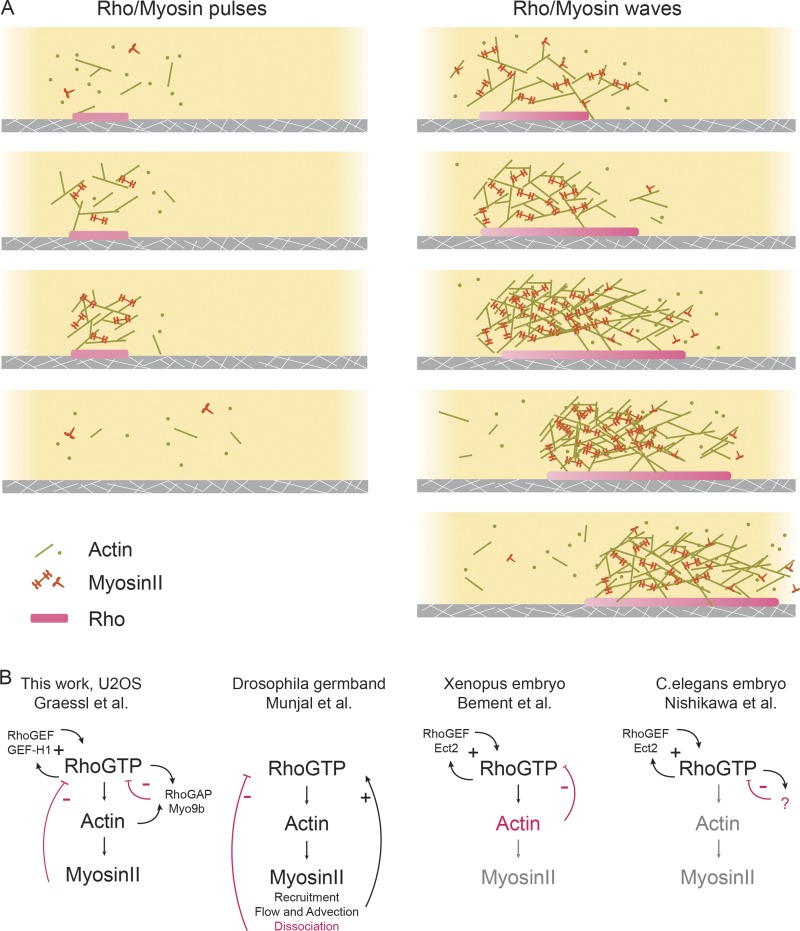
In a visually compelling and timely study, Graessl et al. observed and characterized the activation of Rho, a critical regulator of cell contractility, in single cells. By imaging the basal surface of the adherent cells, they reveal a plethora of dynamic activity for Rho. Despite the overall irregularity and heterogeneity both within a single cell and between cells, a number of ordered patterns are consistently found (Fig. 1 A). First, Rho activation in the basal state is very local and transient, with characteristic lifetimes of 20–25 s and decay lengths of ∼2 µm. Graessl et al. (2017) paid particular attention to cells with local pulses that recur at the same location with variable intervals of 2–7 min between peaks. Intriguingly, in cells with artificially elevated levels of Rho activation (by either nocodazole treatment, which presumably releases microtubule-associated Rho guanine nucleotide exchange factor [GEF] or overexpresses the Rho GEF GEF-H1), waves of Rho activation can be readily observed with a propagating speed of 0.3–0.4 µm/s.
Figure 1.
Excitable Rho signaling networks underlying contractile patterns. (A) Schematic illustration of Rho and actin dynamics in contractile pulses and waves. (B) Various models of the activator–inhibitor network proposed in different experimental systems. Texts and arrows in black or magenta indicate components involved in positive or negative feedbacks, respectively. Text in gray indicates components not essential for the feedback loops.
The visualization of these patterns was made possible by an activity sensor using the GTPase binding domain of the Rho effector Rhotekin that binds selectively to the active Rho (including RhoA, RhoB, and RhoC). The compatibility with total internal reflection fluorescence (TIRF) imaging is an additional advantage of the single-cell experimental system compared with developmental systems. In fact, although the detection benefited from the sensor, utilization of the Rho sensor is not absolutely essential under this experimental condition because Rho recruitment to the plasma membrane could also be visualized with TIRF and display similar dynamic patterns as the sensor. Graessl et al. (2017) primarily characterized Rho patterns in U2OS cells (osteosarcoma cells), but the findings are likely applicable to other adherent cells, including embryonic stem cells. Similar observations of Rho pulses were recently reported in U2OS cells by a different group (Baird et al., 2017), and Rho waves were also reported in primary human endothelial cells (Reinhard et al., 2016) as well as Xenopus laevis/starfish oocytes and embryos (Bement et al., 2015).
These pulses and propagating waves are hallmarks of excitable dynamics and are modeled after the firing and propagation of action potentials in neurons. Subsequently, they were extended to chemical systems made up of a minimal activator–inhibitor network whereby the activator can activate the inhibitor, which, delayed in its effect, serves to suppress the activation. Interesting spatial patterns can also emerge if both the activator and the inhibitor spread in space; when the activator spreads faster than the inhibitor, traveling waves of the activation can appear. Conversely, if the inhibitor spreads faster than the activator, local maxima would form and be locked in space.
To establish the molecular identity of the activator and inhibitor, Graessl et al. (2017) systematically characterized the phase-shifted recruitment of Rho regulators and downstream effectors. Among them, the Rho GEF GEF-H1 (ARHGEF2), preceded Rho activation by ∼2 s, whereas the Rho effector FHOD1 (∼6 s), actin (∼11 s), RhoGAP Myo9b (∼1 s), and myosin II (∼40 s) showed delayed accumulation. They next examined how the expression level of these regulators modulated the network dynamics. Based on a series of quantitative measurements, they propose that the positive feedback between GEF-H1 and Rho acts as the primary activator, whereas actomyosin and the associated RhoGAP Myo9b act as the delayed inhibitor (Fig. 1 B).
Specifically, if GEF-H1 is part of the positive feedback loop that amplifies Rho activation, GEF-H1 needs to interact with active Rho in addition to binding inactive Rho. Based on previous findings which have suggested that the Pleckstrin homology (PH) domain of GEF-H1 could interact with active Rho without blocking the interaction between inactive Rho and GEF-H1’s Dbl homology domain, Graessl et al. (2017) hypothesized that the PH domain is essential for the positive feedback. To test this, they employed a PH domain mutant that could not bind to active Rho and assumed that the mutant did not interfere with GEF activity. This mutant indeed blocked the fluctuating Rho activity, similar to the GEF-deficient mutant; therefore, GEF-H1 is concluded to be part of the amplification loop for Rho pulses.
Graessl et al. (2017) also identified two inhibitors that act at different stages. Myo9b was named as the fast inhibitor because of its minimal delay in recruitment (1 s later than Rho), and the observation that cells overexpressing Myo9b but not the GAP-deficient Myo9b mutant show pulses of decreased widths (from a mean of ∼45 s to ∼30 s) and increased frequencies (from 2–7-min intervals to a frequency distribution with peaks at 2–7 min and 30–50 s). Graessl et al. (2017) concluded that myosin II was the slow inhibitor because of the longer delay in the recruitment of myosin IIa (40 s later than Rho), the anticorrelation with GEF-H1, and the inhibitory effects of blebbistatin (myosin II inhibitor) and Y27632 (ROCK inhibitor) on Rho pulses (i.e., waves and pulses disappeared).
Because of the fundamental importance of cell contractility in cell and tissue morphogenesis, contraction pulses have been extensively investigated in various experimental systems. There are a few interesting differences between the proposed mechanisms in this work and recent studies on similar topics that are perhaps worth noting.
For example, Graessl et al. (2017) show in their study that the stimulating effect of GEF-H1 on Rho waves is shared with another Lbc family GEF, leukemia-associated Rho GEF (LARG), but not with RhoGEF Ect2. This differs from other work, which has reported that Ect2 is essential for the excitable dynamics of RhoA in some systems (Bement et al., 2015; Nishikawa et al., 2017). Graessl et al. (2017) found that constitutively active Ect2 could still stimulate Rho activity dynamics but only in ∼20% of the U2OS cells, compared with the stronger stimulating effect of the constitutively active mutant of GEF-H1 (∼50% with propagating waves). Rho waves stimulated by constitutively active Ect2 are limited to the cell periphery and display a mutually exclusive pattern compared with active Ect2, in contrast with GEF-H1–induced Rho activity waves, which were predominantly localized to central cell regions and share the same phase as active GEF-H1. The reasons for the differential effects remain unclear, but it may be related to different Rho isoforms (Reinhard et al., 2016) and the context-dependent role of GEF-induced positive feedback as well as the contributions of membrane geometry.
The model proposed that myosin II and Myo9b act as part of the core negative feedback network driving the Rho pulsing dynamics. The recruitment of Myo9b was only minimally delayed compared with activation of Rho (∼1 s delay for WT Myo9b and ∼3 s delay for a GAP mutant). As such, the profile of Myo9b in fact overlaps almost completely with that of Rho, raising the question of whether Myo9b is the true inhibitor driving the pulsatile Rho activation. It is important to note that not all negative regulators are the inhibitor driving the pulsing dynamics, and some negative regulators could be involved in regulating the amplitude or duration of the activation and do not constitute the minimal activator–inhibitor network sustaining the oscillations (Xiong et al., 2016). Myo9b certainly limits the accumulation of active Rho, but the lifetime of the inhibitor in the activation–inhibitor network defines the refractory period of Rho activation, and this does not seem to be the case for Myo9b. Intriguingly, overexpressing Myo9b does have an effect on the pulsing period of Rho, but it appeared to do so by converting the excitable dynamics to more regular sinusoidal oscillations. An additional frequency peak (30–50 s) was created without affecting the original peak at 2–7 min. Considering the heterogeneity of the excitable dynamics and the sensitivity of the signaling networks to the mechanical property of the cortex, more acute perturbations will be necessary to fully interpret this result.
Whether myosin II acts as the delayed inhibitor in contractile pulses in general is a puzzling question. Although many contractile patterns in multicellular systems are clearly dependent on Rho activity, surprisingly, Rho activation has not always been directly visualized. This might be a result of the sensitivity of developmental systems to overexpression of the sensor, which could sequester active Rho if not properly controlled. Given the lack of direct visualization of Rho activation and the fact that myosin was often used as a proxy, it is not surprising that earlier research shares a myosin-centric view and argues that cortical instability requires negative feedback from myosin II (Fig. 1 B; Munjal et al., 2015). There are compelling experimental and theoretical arguments suggesting that collective myosin II motor activity could be intrinsically unstable and capable of sustaining oscillations (Jülicher and Prost, 1997). The lack of regular pulsing or wavelike propagation in multicellular systems was previously used to imply that a reaction–diffusion signaling network upstream of cell pulsing was unlikely (Solon et al., 2009); perhaps this needs to be revisited in light of the demonstration of Rho waves in many single cellular systems.
If Rho activity cycles are closely coupled with myosin II–based contractile cycles, does that mean that they belong to the same feedback loop, or are they two separate but interlinked networks? In a different excitable system responsible for chemotaxis, there is compelling evidence indicating that membrane-localized excitable signaling networks (composed of PI(3)K, Ras, and Rac GTPases) are coupled with a separate actin oscillator to drive motility (Huang et al., 2013). When it comes to contractile pulses, results from different experimental systems barely agree with each other on this fundamental question (Fig. 1 B). In Xenopus and starfish embryos, Bement et al. (2015) showed that the myosin II inhibitor blebbistatin had no significant effect on Rho wave period. Under the same conditions, cortical contraction was suppressed, suggesting that the signaling networks in the absence of myosin II–dependent contractility are sufficient to display excitable dynamics (Fig. 1 B). Two recent studies in Caenorhabditis elegans embryos suggest a similar conclusion (Robin et al., 2016; Nishikawa et al., 2017). Based on both experimental results and theoretical considerations, Nishikawa et al. (2017) and Robin et al. (2016) proposed that a myosin-independent RhoA pacemaker controls contractile pulses. Both groups observed that RhoA pulses persisted in the absence of the myosin II pulses in a mutant background, indicating that the Rho oscillator can be uncoupled from myosin II. In addition, Nishikawa et al. (2017) reported that a simple theoretical model based on myosin II–dependent contractile instability could not quantitatively recapitulate the experimentally observed spacing between myosin II foci. Different from the model of Bement et al. (2015), where actin is the delayed inhibitor, Nishikawa et al. (2017) argue that actin could not be the inhibitor in C. elegans based on how changes of RhoA relate to the changes of actin (slope of the nullcline). Hence, there appears to be an actin- and myosin-independent inhibitor that sets the rhythm for Rho pulses, whose nature remains to be identified (Fig. 1 B).
Graessl et al. (2017) suggest that there might be at least two types of Rho waves; one is myosin II–dependent, and the other is not. If so, what could be the parameters that determine how the membrane-localized signaling networks engage force-generating machinery and fine-tune its responses? It is clear that contraction is not always pulsatile, but given the acute sensitivity of contractile pulses to the mechanical properties of the cell cortex and the external environment, including substrate rigidity Graessl et al. (2017), cell adhesion (He et al., 2010), and tissue elasticity, it seems that the excitable dynamics as characterized by Graessl et al. (2017) offer a great opportunity to dissect the molecular circuits underlying cellular mechanosensing.
Acknowledgments
The author acknowledges the Singapore Ministry of Education (2015-T2-1-122) and Singapore Ministry of Health (NMRC/OFIRG/0038/2017) for support.
The author declares no competing financial interests.
References
- Baird M.A., Billington N., Wang A., Adelstein R.S., Sellers J.R., Fischer R.S., and Waterman C.M.. 2017. Local pulsatile contractions are an intrinsic property of the myosin 2A motor in the cortical cytoskeleton of adherent cells. Mol. Biol. Cell. 28:240–251. 10.1091/mbc.E16-05-0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bement W.M., Leda M., Moe A.M., Kita A.M., Larson M.E., Golding A.E., Pfeuti C., Su K.-C., Miller A.L., Goryachev A.B., and von Dassow G.. 2015. Activator-inhibitor coupling between Rho signalling and actin assembly makes the cell cortex an excitable medium. Nat. Cell Biol. 17:1471–1483. 10.1038/ncb3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graessl M., Koch J., Calderon A., Kamps D., Banerjee S., Mazel T., Schulze N., Jungkurth J.K., Patwardhan R., Solouk D., et al. . 2017. An excitable Rho GTPase signaling network generates dynamic subcellular contraction patterns. J. Cell Biol. 10.1083/jcb.201706052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Wang X., Tang H.L., and Montell D.J.. 2010. Tissue elongation requires oscillating contractions of a basal actomyosin network. Nat. Cell Biol. 12:1133–1142. 10.1038/ncb2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.-H., Tang M., Shi C., Iglesias P.A., and Devreotes P.N.. 2013. An excitable signal integrator couples to an idling cytoskeletal oscillator to drive cell migration. Nat. Cell Biol. 15:1307–1316. 10.1038/ncb2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jülicher F., and Prost J.. 1997. Spontaneous Oscillations of Collective Molecular Motors. Phys. Rev. Lett. 78:4510–4513. 10.1103/PhysRevLett.78.4510 [DOI] [Google Scholar]
- Munjal A., Philippe J.-M., Munro E., and Lecuit T.. 2015. A self-organized biomechanical network drives shape changes during tissue morphogenesis. Nature. 524:351–355. 10.1038/nature14603 [DOI] [PubMed] [Google Scholar]
- Nishikawa M., Naganathan S.R., Jülicher F., and Grill S.W.. 2017. Controlling contractile instabilities in the actomyosin cortex. eLife. 6:058101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard N.R., van Helden S.F., Anthony E.C., Yin T., Wu Y.I., Goedhart J., Gadella T.W.J., and Hordijk P.L.. 2016. Spatiotemporal analysis of RhoA/B/C activation in primary human endothelial cells. Sci. Rep. 6:25502 10.1038/srep25502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin F.B., Michaux J.B., McFadden W.M., and Munro E.M.. 2016. Excitable RhoA dynamics drive pulsed contractions in the early C. elegans embryo. 10.1101/076356 [DOI] [PMC free article] [PubMed]
- Solon J., Kaya-Copur A., Colombelli J., and Brunner D.. 2009. Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell. 137:1331–1342. 10.1016/j.cell.2009.03.050 [DOI] [PubMed] [Google Scholar]
- Xiong D., Xiao S., Guo S., Lin Q., Nakatsu F., and Wu M.. 2016. Frequency and amplitude control of cortical oscillations by phosphoinositide waves. Nat. Chem. Biol. 12:159–166. 10.1038/nchembio.2000 [DOI] [PubMed] [Google Scholar]