Abstract
Background
Studies assessing outcomes in occult breast cancer have often included women treated before the routine use of MRI. We undertook to examine outcomes in patients presenting with axillary adenopathy and no primary detectable on MRI or other imaging modalities.
Methods
All patients with axillary nodal metastases consistent with breast carcinoma, and no detectable breast primary on physical exam, mammography, or MRI treated between 1/1/1996 and 6/30/2011 were identified from an institutional database. Data was collected on local, regional, and distant recurrences.
Results
Thirty-eight patients were identified. Modified radical mastectomy (MRM) was performed in 13, while 25 underwent axillary dissection (ALND) and whole-breast radiotherapy (WBRT). Most women had pathological N1 disease (median number of positive nodes 2 [MRM cohort] and 3 [ALND+WBRT cohort], p=0.38). All patients received chemotherapy, with 30 of the 38 patients (79%) treated receiving an anthracycline and taxane. Regional nodal radiation was used in 60% of those with ALND+WBRT and in all of the 46% of MRM patients who received chest wall radiotherapy. At a median follow up of 7 years, there were no nodal recurrences. Two patients treated with ALND+WBRT developed in-breast recurrences; none in the MRM group developed local recurrence. The proportion that developed distant disease was similar between the MRM cohort (1 of 13) and the ALND+WBRT cohort (2 of 25).
Conclusion
Breast cancer presenting as axillary adenopathy but no detectable primary is rare. Breast conservation with WBRT is a viable option for patients diagnosed with occult breast cancer and a negative preoperative MRI.
Keywords: occult breast cancer, MRI, outcomes, whole-breast radiation therapy
INTRODUCTION
Occult breast cancer denotes the clinical scenario of axillary nodal metastases consistent with a breast carcinoma arising in the absence of any identifiable primary breast tumor (pT0N+) despite full clinical and radiological assessment. It is an uncommon presentation, with contemporary reports suggesting that less than 0.3% of breast cancer cases are occult.1,2 Historically, the surgical management of these patients mandated a modified radical mastectomy (MRM).3
A preoperative MRI has become standard of care for breast cancer patients with axillary metastases in whom clinical examination, mammography, and ultrasound have failed to identify the primary tumor.4 The use of MRI identifies a suspect lesion in 72% of cases with normal mammography and ultrasound, and the majority of these (>85%) prove to be the primary breast carcinoma upon biopsy.5 As a result of the enhanced MRI sensitivity, any putative primary lesion requires pathologic confirmation through either a second-look ultrasound and biopsy, MRI guided biopsy, or, failing these, excisional biopsy prior to deciding on management.
Given its low incidence, evidence assessing outcomes following treatment for occult breast cancer has often been accumulated over long study periods with inherent heterogeneity in both the diagnosis and treatment of occult breast cancer. Axillary lymph node dissection (ALND) followed by whole-breast radiotherapy (WBRT) has emerged as a viable treatment option.6–8 A recent meta-analysis identified studies comprising only 241 patients of whom less than half had a preoperative breast MRI9, and concluded that equivocal rates of local control and overall survival are achievable with breast ALND and WBRT.10 Notwithstanding this, population studies suggest that almost half of patients with occult breast are still treated with MRM.11,12
Currently, the diagnosis of occult breast cancer should be limited to patients who present with an axillary metastasis without any evidence of clinical, mammographic, sonographic, or MRI evidence of breast disease. Accordingly, the aim of this study was to compare disease-free survival (DFS) between patients with true occult breast cancer treated with MRM or ALND with WBRT.
MATERIALS AND METHODS
Patients who were treated for an occult breast cancer (pT0N+) between 01/01/1996 and 06/30/2011 were identified from a prospective institutional database after receipt of Memorial Sloan Kettering Cancer Center (MSKCC) institutional review board approval. Occult breast cancer was defined as adenocarcinoma histologically consistent with a breast origin that presented with axillary metastases in the absence of primary breast tumor on physical examination, imaging, or preoperative biopsy.9 Patients eligible for inclusion were those who presented with biopsy proven axillary lymph node metastases and were evaluated with a breast MRI prior to definitive axillary surgery. All MRI studies prior to July 2009 were performed on a 1.5T MRI scanner after which both 1.5T and 3T MRI scanners were used within the department. All patients underwent ALND and either mastectomy or primary breast radiotherapy. Throughout the entire study period, MRM or ALND with WBRT were offered as treatment options in the scenario of occult breast cancer.
Patients who were not evaluated with a breast MRI prior to ALND and patients with a history of prior ipsilateral breast cancer were excluded. Those with a history of contralateral breast cancer were excluded, as axillary metastases in most of these cases represented probable contralateral axillary metastases. Patients in whom the biopsy histology definitively concluded that the axillary metastasis was of non-breast origin were also excluded. Patients in whom the presenting mass was felt to be arising in the axillary tail of the breast and not a true nodal metastasis were excluded.
Patient characteristics at presentation, including clinical stage and systemic staging investigations performed were recorded. The method of initial axillary node biopsy type (excisional versus needle biopsy), and results including the use of additional immunohistochemical markers of CK7 and CK20 in addition to estrogen receptor (ER), progesterone receptor (PR), and HER2 was evaluated. Breast MRI results, including the use of diagnostic excisional biopsy, were noted. Treatment details incorporating surgery, the use of chemotherapy, adjuvant radiotherapy, and endocrine therapy were available. Patient outcomes, including date of last follow-up and the occurrence of ipsilateral or contralateral breast events, regional or distant metastases as well as overall survival were collected.
Categorical variables were summarized with number and percentage, whereas continuous variables were summarized using median and range. To test for differences between groups, Fisher’s exact test and the Kruskal-Wallis test were used for categorical and continuous variables, respectively. The STEEP system was proposed in 2007 to standardize the definitions used as breast cancer clinical trial end points.13 For assessment of DFS in this study, we used the end point of invasive DFS and DCIS end point as outlined in the STEEP system. Accordingly, the occurrence of ipsilateral breast tumor recurrence or contralateral breast cancer (in situ and invasive), regional or distant recurrence, or death attributable to any cause was considered a primary end point. This time was calculated from the date of ALND to the date of recurrence or death. Patients alive without recurrence were censored on their date of last visit. This time was calculated from the date of ALND to the date of recurrence or death. Patients alive without recurrence were censored on their date of last visit. The Kaplan-Meier method estimated time to recurrence. A p-value < 0.05 was considered statistically significant. All statistical analyses were conducted with R software version 3.2.5 (R Core Development Team, Vienna, Austria).
RESULTS
During the study period, 38 patients who met the eligibility criteria were identified. In the same period, 18994 patients underwent surgical treatment for invasive breast cancer at our center. Twenty-five patients (66%) were treated ALND and WBRT, while the remaining 13 (34%) underwent an MRM (Table 1).
Table 1.
Patient demographics, nodal stage at presentation and initial evaluation
| ALND + WBRT n=25 |
MRM n=13 |
P Value | ||
|---|---|---|---|---|
| Demographics | Age, years, median | 59 | 53 | 0.878 |
|
| ||||
| Clinical Presentation | N1 | 22 (88%) | 10 (77%) | 0.392 |
|
| ||||
| N2/3 | 3 (12%) | 3 (23%) | ||
|
| ||||
| Systemic Staging | CT and Bone Scan | 9 (36%) | 3 (23%) | 0.773 |
|
| ||||
| Bone scan alone | 1 (4%) | 0 | ||
|
| ||||
| CT alone | 1 (4%) | 0 | ||
|
| ||||
| PET CT | 14 (56%) | 10 (77%) | ||
|
| ||||
| Biopsy Type | Excisional | 13 (52%) | 5 (38%) | 0.506 |
|
| ||||
| Needle Biopsy | 12 (48%) | 8 (62%) | ||
|
| ||||
| Additional biopsy following MRI | 3 (12%) | 2 (15%) | 1.000 | |
|
| ||||
| Biopsy Pathology | CK-7 Pos (n=29 tested) | 18 90%) | 9 (100%) | 1.000 |
|
| ||||
| CK-20 Pos (n=26 tested) | 0 | 0 | – | |
|
| ||||
| Grade 3 | 23 (92%) | 13 (100%) | 0.538 | |
ALND, axillary lymph node dissection; WBRT, whole-breast radiation therapy; MRM, modified radical mastectomy; CT, computed tomography; PET, positron emission tomography
Patients with mobile adenopathy, clinical stage N1 comprised 88% and 77% of those in the ALND+WBRT and MRM groups, respectively. Almost half of the patients were diagnosed by means of an excisional biopsy of the palpable lymph node (n = 18, 47%). In more recent years, needle biopsy (either core needle biopsy or fine needle aspiration) was more frequently utilized, with 77% of patients presenting between 2009 and 2011 diagnosed by needle biopsy. Not all biopsy samples were subject to immunohistochemical staining for CK7 and CK20, but in those in whom they were used, CK7 was positive in the majority of cases and CK20 was not positive in any.
Systemic staging was performed in most patients, with positron emission tomography-computed tomography (PET-CT) (n = 24, 63%) or CT and bone scan (n = 12, 32%) comprising the most common staging modalities. Some patients had staging following excisional biopsy; in the 10 patients staged with a PET-CT prior to axillary surgery, the median axillary standard uptake value maximum was 13.7 (range 3.6–24).
All patients were evaluated with an MRI preoperatively. Five patients underwent an MRI-guided biopsy prior to definitive surgery, the results of which were benign. An additional 11 patients (44%) who elected breast conservation underwent a breast imaging-guided excision of a radiological abnormality in the ipsilateral breast, none of which yielded ductal carcinoma in situ (DCIS) or invasive breast cancer (Table 2). In two patients treated with mastectomy, the final pathology identified a focus of DCIS with microinvasion. The imaging in these patients was not reviewed for the purposes of this study, but one patient had a preoperative MRI guided biopsy that returned a benign diagnosis.
Table 2.
Surgical Management and Final Pathology
| ALND + WBRT n=25 |
MRM n=13 |
P Value | ||
|---|---|---|---|---|
| Surgical Management | ALND | 25 | 13 | – |
|
| ||||
| Mastectomy | 0 | 13 | – | |
|
| ||||
| Excisional biopsy of imaging abnormality at time of ALND | 11 (44%) | 0 | – | |
|
| ||||
| Pathology | Number of nodes removed (median) | 20 | 23 | 0.47 |
|
| ||||
| Nodes positive: 0–3† | 15 (60%) | 7 (54%) | 0.23 | |
| 4–10 | 9 (36%) | 3 (23%) | ||
| > 10 | 1 (4%) | 3 (23%) | ||
|
| ||||
| Largest size nodal met (median) | 28mm | 20mm | 0.06 | |
|
| ||||
| Extranodal extension | 10 (40%) (Missing: n=9) |
4 (31%) (Missing n=5) |
0.67 | |
|
| ||||
| Subtype | ER+/HER2− | 6 (24%) | 3 (23%) | 0.89 |
|
| ||||
| ER+/HER2+ | 5 (20%) | 2 (15%) | ||
|
| ||||
| ER−/HER2+ | 3 (12%) | 3 (23%) | ||
|
| ||||
| Triple negative | 7 (28%) | 4 (31%) | ||
|
| ||||
| ER−/HER2 unknown | 4 (16%) | 1 (8%) | ||
One patient diagnosed by core needle biopsy had complete pathological response after neoadjuvant chemotherapy with no positive nodes identified on pathological analysis of ALND.
ALND, axillary lymph node dissection; WBRT, whole-breast radiation therapy; MRM, modified radical mastectomy; ER, estrogen receptor
Among the 29 patients who underwent surgery as the first treatment, the median number of positive axillary nodes was 2 for the entire group and 3 for the 17 patients diagnosed by needle biopsy (range 1–28). The final burden of axillary disease was similar between those treated with ALND with WBRT or MRM, with 60% and 54%, respectively, having 3 or fewer positive nodes. In 22 of 38 cases (58%), the nodal metastases were ER negative, with the triple-negative subtype the most commonly represented.
Neoadjuvant chemotherapy (NAC) was given in 9 patients, of whom 6 had undergone nodal excisional biopsy for diagnosis (Table 3). One of these 6 had residual adenopathy found in the ALND specimen after NAC, and 2 of the 3 who had undergone needle biopsy for diagnosis had residual nodal disease. Selection of patients for NAC was at the discretion of the treating surgeon as opposed to on the basis of a department guideline. All patients received chemotherapy as part of their treatment; in 30 of the 38 patients (79%), this regimen included an anthracycline and a taxane. For patients who elected breast conservation, all received adjuvant WBRT. The regional nodes were included in the field of radiation in 60% of these cases. In the MRM group, 46% of patients were treated with postmastectomy radiotherapy, and in all of these cases, the treatment encompassed regional nodal radiation. Supraclavicular nodes were treated in all 21 cases that received regional nodal irradiation with treatment additionally to the axilla in 9 patients and both axilla and internal mammary nodes in 3 patients (Table 3).
Table 3.
Adjuvant Treatment and Outcomes
| ALND + WBRT n=25 |
MRM n=13 |
||
|---|---|---|---|
| Chemotherapy | Neoadjuvant | 5 (20%) | 4 (31%) |
|
| |||
| Post needle biopsy | 1 (4%) | 2 (15%) | |
|
| |||
| Post excisional biopsy | 4 (16%) | 2 (15%) | |
|
| |||
| Adjuvant | 20 (80%) | 9 (69%) | |
|
| |||
| Radiotherapy | Whole breast | 25 (100%) | – |
|
| |||
| Chest wall | – | 6 (46%) | |
|
| |||
| Regional nodes Supraclavicular nodes alone Supraclavicular nodes + axilla Supraclavicular, internal mammary nodes + axilla |
15 (60%) 6 8 1 |
6 (46%) 3 1 2 |
|
|
| |||
| No radiation therapy | 0 | 7 (54%) | |
|
| |||
| Adjuvant Endocrine Therapy | 14 (56%) | 6 (46%) | |
ALND, axillary lymph node dissection; WBRT, whole-breast radiation therapy; MRM, modified radical mastectomy
The median duration of follow-up among survivors was 7 years (range 1.5–18 years). No local or regional recurrence events were noted in the MRM group. In the WBRT group, 2/25 (8%) developed an ipsilateral breast cancer (Table 4). One patient had ER positive disease and the ipsilateral breast cancer was diagnosed 4.2 years after her initial surgery while still receiving adjuvant tamoxifen. The other ipsilateral breast tumor was in a patient with initial ER negative disease, 4.6 years after surgery. Overall, there appeared to be no significant difference in DFS between the 2 groups as illustrated by the Kaplan Meier curve in Fig. 1. The sample size was too small for formal statistical comparison of DFS between the two groups, but as illustrated by the Kaplan Meier curve (Fig.1), the confidence intervals for DFS at 10 years are wide and overlap, which suggests that there is not a significant difference in DFS between the two groups at 10 years.
Table 4.
Outcomes
| ALND + WBRT n=25 |
MRM n=13 |
||
|---|---|---|---|
| Outcome | Alive and disease free | 19 | 10 |
|
| |||
| In-breast recurrence | 2 | 0 | |
|
| |||
| Regional node recurrence | 0 | 0 | |
|
| |||
| Contralateral primary | 1 | 1 | |
|
| |||
| Death from other cause | 1 | 1 | |
|
| |||
| Distant recurrence | 2 | 1 | |
ALND, axillary lymph node dissection; WBRT, whole-breast radiation therapy; MRM, modified radical mastectomy
Fig 1.
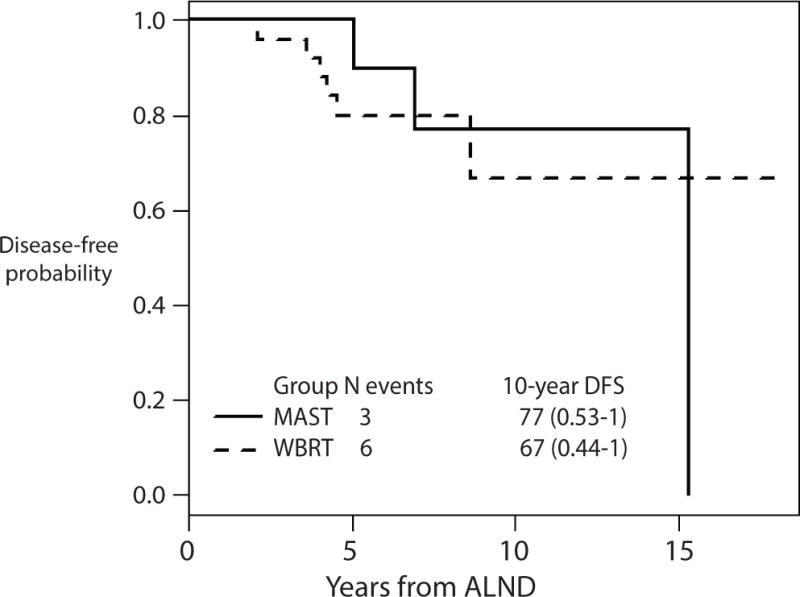
Disease-free survival
For calculation of disease-free survival, the occurrence of ipsilateral breast tumor recurrence or contralateral breast cancer (in situ and invasive), regional or distant recurrence, or death attributable to any cause was considered an event.
DISCUSSION
Occult breast cancer is a clinical entity not commonly encountered as evidenced by the dearth of studies identified in the aforementioned recent meta-analysis.9 In the current study, occult breast cancer represented less than 0.5% of breast cancers surgically treated at our center during the study period. The application of breast MRI as a routine component of the evaluation of patients in whom mammography and ultrasound have been unable to locate a breast primary will undoubtedly further diminish the frequency with which women with truly imaging occult breast cancers are identified. Historically, the rate of identifying a focus of invasive breast cancer upon histological analysis of mastectomy specimens in patients with clinical- and imaging-occult breast cancer patients ranged from 35%3,14 to 82%.15 The variation is explainable as the studies examined practice over long periods of time with inconsistency in the threshold for pre-mastectomy biopsy of indeterminate areas on mammography.
The experience with MRI in the setting of occult breast cancer has been gleaned over a shorter time period, and it is accepted that in 70–80% of cases with normal physical examination and mammography, MRI will identify a lesion suspect of a primary breast cancer.5 The false-negative rate of MRI in this setting is not as well reported, with few contemporary studies reporting on patients with a negative MRI who undergo a mastectomy. Orel et al identified 2 false-negative MRIs in 22 patients (9%) between 1993 and 1997.16 In our current study, of the 13 patients treated with mastectomy in which MRI had not identified a primary, two (15%) patients had an area of DCIS with microinvasion on final pathology.
Because MRI identifies the primary in a majority of patients presenting with mammographically occult breast cancer, the small proportion of women in whom no primary is detectable by any method, including MRI, must have a low tumor burden. In conventional breast-conserving therapy, it is assumed that subclinical disease remains even after lumpectomy with clear margins.17 Radiation is used to eradicate this undetectable disease; several randomized trials of lumpectomy with WBRT vs. MRM have shown equivalent outcomes.18,19 With the adoption of breast MRI that is more sensitive than conventional imaging, the same rationale has been used to conserve the breast in women presenting with nodal metastases and no identifiable primary. None of the comparative studies have shown a significant increase in rates of locoregional recurrence in patients treated with WBRT as compared to MRM.7,14,20,21 On meta-analysis, the rates of locoregional control, distant metastasis, and mortality are similar between patients who undergo MRM and those who have ALND and WBRT.10 The addition of WBRT when the breast is preserved is an important component of the treatment, as its omission results in a significantly higher rate of locoregional recurrence due to failure to either excise or irradiate the breast primary and perhaps a worse long-term survival.14,22,23
To date, studies on occult breast cancer have not limited their cohort to patients with true occult primaries by current standards as defined by a negative breast MRI. Our study is the largest series to date comparing outcomes for patients with truly occult breast cancer (not detectable by any clinical or radiological investigation including MRI) treated with either mastectomy or WBRT. Our findings echo those of previous reports that show breast conservation for patients with true occult breast cancer is a safe option with low rates of local and regional recurrence. In our series, 2 of 25 patients (8%) in whom the breast was preserved and treated with WBRT experienced an in breast recurrence, without distant metastases. Previous reports of in-breast recurrences in contemporarily treated cohorts range from 0% (of 24 patients)10 to 6% (average of 5 years from diagnosis).22
Buoyed by the success of neoadjuvant chemotherapy in obtaining high rates of axillary complete response in women who present with node-positive breast cancer, there is interest in axillary conservation, particularly in patients with triple-negative and HER2 positive breast cancer.24,25 In the recent report by Rueth et al of patients with occult breast cancer, a complete axillary response was seen in 60% of patients who had an ALND after neoadjuvant chemotherapy.10 The high rate of both ER negative cancers (58%) and triple-negative breast cancer (33%) in this cohort would seem to support the neoadjuvant chemotherapy approach for many of these patients. Our subtype findings are similar to those in previously published occult breast cancer cohorts with rates of ER negativity ranging from 32% to 63% and triple-negative disease reported in 18% to 33% of cases.10,20,22,26
It is conceivable that in carefully selected patients who present with occult primary breast cancer and demonstrate an excellent clinical and radiological axillary response to neoadjuvant systemic therapy, a sentinel lymph node biopsy for axillary staging may have a role. Three prospective studies have shown the accuracy of SLNB in women with axillary nodal metastases who receive neoadjuvant chemotherapy, become clinically node negative, and undergo dual-agent mapping with at least 3 nodes retrieved.27–29 Techniques to ensure removal of nodes with biopsy-confirmed metastasis including pathological assessment for tumor regression30 and the placement of a biopsy marker clip31 may improve the accuracy of nodal staging if certain patients with occult breast cancer are selected for sentinel lymph node biopsy following neoadjuvant chemotherapy.
As a retrospective study of only 38 patients, the limitations of this study must be acknowledged. While the cohort was selected to ensure standardization of preoperative evaluation with MRI, the patients were accrued over a 15-year study period. The study period coincided with many important treatment advances in breast cancer management. This is exemplified by the expanding role of neoadjuvant chemotherapy, the use of which was not a standard guideline for these patients during the study period. A high proportion of patients were also diagnosed by means of an excisional lymph node biopsy, which may still occasionally be required in the setting of a suspected lymphoma, but needle biopsy should be used as the current standard of care for patients presenting with clinical lymphadenopathy.32 Most importantly, the small numbers preclude the ability to identify patients with occult breast cancer who are at highest risk of local or regional recurrence and determine if there exists a group in whom primary breast radiotherapy may not represent sufficient local control.
With the universal recognition of the role for breast MRI in evaluation of patients who present with axillary lymph node metastases and normal physical examination, mammography, and ultrasound, the true definition of occult breast cancer must now include a negative MRI. In this cohort, whose members all received contemporary chemotherapy, the outcomes for patients treated with breast conservation through WBRT following ALND were comparable to patients treated with modified radical mastectomy with low rates of local recurrence, verifying breast conservation as a real option in those with true occult breast cancer. Recent studies suggest further progress may be achieved for women with occult breast cancer in whom neoadjuvant chemotherapy may result in avoidance of ALND.
Synopsis.
In patients presenting with axillary adenopathy and no detectable breast primary on MRI or other imaging modalities, we find breast conservation with WBRT to be a viable option with low rates of local recurrence in this cohort of patients with occult breast cancer.
Acknowledgments
The preparation of this study was supported by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center. No financial or material support was provided for this research.
Footnotes
Disclosures: The authors have no conflicts of interest or commercial interests to disclose.
References
- 1.Fayanju OM, Jeffe DB, Margenthaler JA. Occult primary breast cancer at a comprehensive cancer center. J Surg Res. 2013 Dec;185(2):684–9. doi: 10.1016/j.jss.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Couder F, Schmitt C, Treilleux I, Tredan O, Faure C, Carrabin N, et al. Axillary lymph node metastases with an occult breast: About 16 cases from a cohort of 7770 patients. Gynécologie Obstétrique Fertil. 2015 Sep;43(9):588–92. doi: 10.1016/j.gyobfe.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Ashikari R, Rosen PP, Urban JA, Senoo T. Breast cancer presenting as an axillary mass. Ann Surg. 1976 Apr;183(4):415–7. doi: 10.1097/00000658-197604000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchanan CL, Morris EA, Dorn PL, Borgen PI, Van Zee KJ. Utility of breast magnetic resonance imaging in patients with occult primary breast cancer. Ann Surg Oncol. 2005 Dec;12(12):1045–53. doi: 10.1245/ASO.2005.03.520. [DOI] [PubMed] [Google Scholar]
- 5.de Bresser J, de Vos B, van der Ent F, Hulsewé K. Breast MRI in clinically and mammographically occult breast cancer presenting with an axillary metastasis: a systematic review. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2010 Feb;36(2):114–9. doi: 10.1016/j.ejso.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Campana F, Fourquet A, Ashby MA, Sastre X, Jullien D, Schlienger P, et al. Presentation of axillary lymphadenopathy without detectable breast primary (T0 N1b breast cancer): experience at Institut Curie. Radiother Oncol J Eur Soc Ther Radiol Oncol. 1989 Aug;15(4):321–5. doi: 10.1016/0167-8140(89)90077-7. [DOI] [PubMed] [Google Scholar]
- 7.Vlastos G, Jean ME, Mirza AN, Mirza NQ, Kuerer HM, Ames FC, et al. Feasibility of breast preservation in the treatment of occult primary carcinoma presenting with axillary metastases. Ann Surg Oncol. 2001 Jun;8(5):425–31. doi: 10.1007/s10434-001-0425-6. [DOI] [PubMed] [Google Scholar]
- 8.Vilcoq JR, Calle R, Ferme F, Veith F. Conservative treatment of axillary adenopathy due to probable subclinical breast cancer. Arch Surg Chic Ill 1960. 1982 Sep;117(9):1136–8. doi: 10.1001/archsurg.1982.01380330004002. [DOI] [PubMed] [Google Scholar]
- 9.Macedo FIB, Eid JJ, Flynn J, Jacobs MJ, Mittal VK. Optimal Surgical Management for Occult Breast Carcinoma: A Meta-analysis. Ann Surg Oncol. 2016 Jun;23(6):1838–44. doi: 10.1245/s10434-016-5104-8. [DOI] [PubMed] [Google Scholar]
- 10.Rueth NM, Black DM, Limmer AR, Gabriel E, Huo L, Fornage BD, et al. Breast conservation in the setting of contemporary multimodality treatment provides excellent outcomes for patients with occult primary breast cancer. Ann Surg Oncol. 2015 Jan;22(1):90–5. doi: 10.1245/s10434-014-3991-0. [DOI] [PubMed] [Google Scholar]
- 11.Walker GV, Smith GL, Perkins GH, Oh JL, Woodward W, Yu T-K, et al. Population-based analysis of occult primary breast cancer with axillary lymph node metastasis. Cancer. 2010 Sep 1;116(17):4000–6. doi: 10.1002/cncr.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fayanju OM, Stoll CRT, Fowler S, Colditz GA, Jeffe DB, Margenthaler JA. Geographic and temporal trends in the management of occult primary breast cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2013 Oct;20(10):3308–16. doi: 10.1245/s10434-013-3157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hudis CA, Barlow WE, Costantino JP, Gray RJ, Pritchard KI, Chapman J-AW, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol Off J Am Soc Clin Oncol. 2007 May 20;25(15):2127–32. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 14.He M, Tang L-C, Yu K-D, Cao A-Y, Shen Z-Z, Shao Z-M, et al. Treatment outcomes and unfavorable prognostic factors in patients with occult breast cancer. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2012 Nov;38(11):1022–8. doi: 10.1016/j.ejso.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 15.Merson M, Andreola S, Galimberti V, Bufalino R, Marchini S, Veronesi U. Breast carcinoma presenting as axillary metastases without evidence of a primary tumor. Cancer. 1992 Jul 15;70(2):504–8. doi: 10.1002/1097-0142(19920715)70:2<504::aid-cncr2820700221>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 16.Orel SG, Weinstein SP, Schnall MD, Reynolds CA, Schuchter LM, Fraker DL, et al. Breast MR imaging in patients with axillary node metastases and unknown primary malignancy. Radiology. 1999 Aug;212(2):543–9. doi: 10.1148/radiology.212.2.r99au40543. [DOI] [PubMed] [Google Scholar]
- 17.Holland R, Veling SH, Mravunac M, Hendriks JH. Histologic multifocality of Tis, T1-2 breast carcinomas. Implications for clinical trials of breast-conserving surgery. Cancer. 1985 Sep 1;56(5):979–90. doi: 10.1002/1097-0142(19850901)56:5<979::aid-cncr2820560502>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 18.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002 Oct 17;347(16):1233–41. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 19.Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002 Oct 17;347(16):1227–32. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 20.Woo SM, Son BH, Lee JW, Kim HJ, Yu JH, Ko BS, et al. Survival outcomes of different treatment methods for the ipsilateral breast of occult breast cancer patients with axillary lymph node metastasis: a single center experience. J Breast Cancer. 2013 Dec;16(4):410–6. doi: 10.4048/jbc.2013.16.4.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foroudi F, Tiver KW. Occult breast carcinoma presenting as axillary metastases. Int J Radiat Oncol Biol Phys. 2000 Apr 1;47(1):143–7. doi: 10.1016/s0360-3016(99)00542-8. [DOI] [PubMed] [Google Scholar]
- 22.Barton SR, Smith IE, Kirby AM, Ashley S, Walsh G, Parton M. The role of ipsilateral breast radiotherapy in management of occult primary breast cancer presenting as axillary lymphadenopathy. Eur J Cancer Oxf Engl 1990. 2011 Sep;47(14):2099–106. doi: 10.1016/j.ejca.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Masinghe SP, Faluyi OO, Kerr GR, Kunkler IH. Breast radiotherapy for occult breast cancer with axillary nodal metastases–does it reduce the local recurrence rate and increase overall survival? Clin Oncol R Coll Radiol G B. 2011 Mar;23(2):95–100. doi: 10.1016/j.clon.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Mamtani A, Barrio AV, King TA, Van Zee KJ, Plitas G, Pilewskie M, et al. How Often Does Neoadjuvant Chemotherapy Avoid Axillary Dissection in Patients With Histologically Confirmed Nodal Metastases? Results of a Prospective Study. Ann Surg Oncol. 2016 May 9; doi: 10.1245/s10434-016-5246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014 Jul 12;384(9938):164–72. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 26.Sohn G, Son BH, Lee SJ, Kang EY, Jung SH, Cho SH, Baek S, et al. Treatment and survival of patients with occult breast cancer with axillary lymph node metastasis: a nationwide retrospective study. J Surg Oncol. 2014 Sep;110(3):270–4. doi: 10.1002/jso.23644. [DOI] [PubMed] [Google Scholar]
- 27.Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013 Oct 9;310(14):1455–61. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013 Jun;14(7):609–18. doi: 10.1016/S1470-2045(13)70166-9. [DOI] [PubMed] [Google Scholar]
- 29.Boileau J-F, Poirier B, Basik M, Holloway CMB, Gaboury L, Sideris L, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol Off J Am Soc Clin Oncol. 2015 Jan 20;33(3):258–64. doi: 10.1200/JCO.2014.55.7827. [DOI] [PubMed] [Google Scholar]
- 30.Barrio AV, Mamtani A, Edelweiss M, Eaton A, Stempel M, Murray MP, et al. How Often Is Treatment Effect Identified in Axillary Nodes with a Pathologic Complete Response After Neoadjuvant Chemotherapy? Ann Surg Oncol. 2016 Oct;23(11):3475–80. doi: 10.1245/s10434-016-5463-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients With Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection. J Clin Oncol. 2016 Apr 1;34(10):1072–8. doi: 10.1200/JCO.2015.64.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris-Stiff G, Cheang P, Key S, Verghese A, Havard TJ. Does the surgeon still have a role to play in the diagnosis and management of lymphomas? World J Surg Oncol. 2008;6:13. doi: 10.1186/1477-7819-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


