SUMMARY
Th17 cells are potent mediators in autoimmune diseases and RORγt is required for their development. Recent studies have shown that RORγt+ Treg cells in the gut regulate intestinal inflammation by inhibiting effector T cell function. In the current study, we report that RORγt+ Treg cells were also found in lymph nodes following immunization. Not only distinct from intestinal RORγt+ Treg in their transcriptomes, peripheral RORγt+ Treg cells were derived from Foxp3+ thymic Treg cells, in an antigen-specific manner. Development of these RORγt+ Treg cells, coined as T regulatory 17 (Tr17) cells, depended on IL-6/Stat3 signaling. Tr17 cells showed suppressive activity against antigen-specific effector T cells in vitro. In addition, Tr17 cells efficiently inhibited myelin-specific Th17 cell-mediated CNS auto-inflammation in a passive EAE model. Collectively, our study demonstrates Tr17 cells as effector Treg cells that potentially restrict autoimmunity.
In brief
Kim et al. find that RORγt+Foxp3+ T regulatory 17 (Tr17) cells are induced in lymph nodes after immunization. Tr17 cells are generated from thymic Treg cells in an antigen-specific manner through Stat3 signaling. Their data suggest that Tr17 cells represent antigen-specific effector Treg cells that can regulate Th17 cell-dependent autoimmunity.
INTRODUCTION
IL-17-producing T helper cells (Th17 cells) have been associated with the progression of autoimmune diseases (Dong, 2008). An orphan nuclear hormone receptor RORγt is required for the development of Th17 cells; RORγt-deficient T cells are impaired in Th17 differentiation (Ivanov et al., 2006). Accordingly, in experimental autoimmune encephalomyelitis (EAE) model, the severity of autoimmunity in the central nervous system (CNS) is significantly decreased in RORγt-deficient mice compared to control mice, along with the decreased Th17 cells in the CNS, indicating that RORγt-dependent Th17 generation is critical for the development of CNS autoimmunity (Ivanov et al., 2006).
Foxp3+ regulatory T cells (Treg cells) are essential for preventing autoimmunity against self-antigens and preventing tissue destruction resulted from excessive immune responses. Recent studies have shown that Treg cells differentiate into distinct subsets to inhibit distinct T helper cell subsets (Campbell and Koch, 2011; Sakaguchi et al., 2013). For example, T-bet+CXCR3+ Treg cells were required for the inhibition of Th1 cell-mediated inflammation, while Irf4 expression in Foxp3+ Treg cells was necessary to prevent Th2 cell-mediated spontaneous immunopathology (Koch et al., 2009; Zheng et al., 2009). In addition, CXCR5+ follicular regulatory T cells (Tfr cells), whose development was dependent on Bcl6, were critical for regulating germinal center reactions mediated by CXCR5+Bcl6+ follicular helper T cells (Tfh cells) (Chung et al., 2011; Linterman et al., 2011). Moreover, Stat3 or IL-10R deficiency selectively in Treg cells led to dysregulation of Th17 cell responses and the subsequent development of inflammation in Th17 cell-rich mucosal tissues such as lung, skin, and intestine, suggesting that IL-10-mediated Stat3 activation in Treg cells is critical for Th17 regulation (Chaudhry et al., 2009; Chaudhry et al., 2011).
RORγt+Foxp3+CD4+ T cells or IL-17-producing Foxp3+ Treg cells have been demonstrated both in mouse and human (Du et al., 2014). However, whether RORγt+Foxp3+CD4+ T cells represent a subset of Treg cells, a precursor of Th17 cells, or an intermediate differentiation stage with a bipolar potential to develop into either Treg cells or Th17 cells has been a matter of debate. Human studies showed that CD4+CD25hiCD45RA−HLA-DR− Treg cells or CD4+CD25hiCCR6+ Treg cells produce IL-17, but their suppressive activity against effector cells is maintained unless the stimulation is too strong (Beriou et al., 2009; Voo et al., 2009). A mouse study also found that RORγt+Foxp3int cells that highly express membrane-bound TGFβ can regulate autoimmune diabetes (Tartar et al., 2010). In contrast, several mouse studies identified RORγt+Foxp3+ cells as one of the intermediate stages during Th17 cell development both in vitro and in vivo although their function has not been addressed (Ichiyama et al., 2008; Yang et al., 2008; Zhou et al., 2008). In addition, others found that RORγt expression in Foxp3+ cells represents an unstable subpopulation of Treg cells that can convert to IL-17-producing cells or pathogenic Treg cells to promote the development of autoimmune diseases or cancer (Blatner et al., 2012; Komatsu et al., 2014). Two recent reports demonstrated the enrichment of RORγt-expressing Foxp3+ Treg cells in the mouse colon (Ohnmacht et al., 2015; Sefik et al., 2015). These gut RORγt+ Treg cells were originated from naïve CD4+ T cell precursors, dependent on intestinal microbiota. Although gut RORγt+ Treg cells were required to inhibit intestinal inflammation mediated by Th1/Th17 cells (Sefik et al., 2015) or Th2 cells (Ohnmacht et al., 2015), whether RORγt+ Treg cells are also present outside the gut and regulate peripheral T helper cell immune responses is unknown.
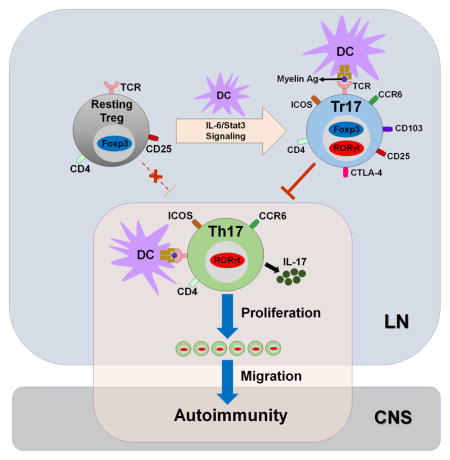
In this study, we identified RORγt-expressing Foxp3+ Treg cells that were induced in lymphoid tissues after immunization. These RORγt+ Treg cells selectively co-expressed chemokine receptor CCR6 and represented activated Treg cells with high proliferative potential. We found that RORγt+CCR6+ Treg cells shared similar molecular regulation with Th17 cells for their development and most of them were derived from thymic Treg cells. Moreover, RORγt+CCR6+ Treg cells potently inhibited Th17 cell-mediated CNS auto-inflammation in a Th17 transfer passive EAE model. Thus, immunization-induced LN RORγt+ Treg cells, which we name as T regulatory 17 cells (Tr17 cells), represent a type of Ag-specific effector Treg cells with inhibitory function against autoreactive effector T cells.
RESULTS
RORγt+CCR6+Foxp3+ T cells are a distinct Treg cell subset
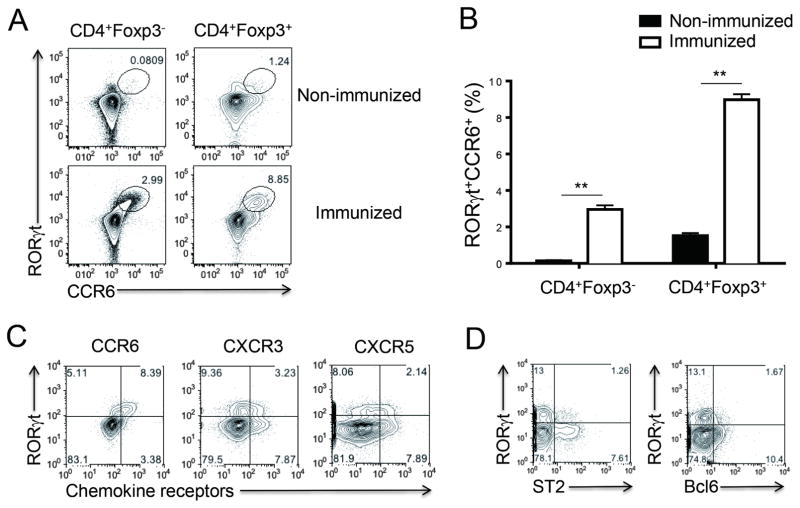
Although RORγt+Foxp3+ Treg cells have been shown in the gut constitutively, less than 2 % of Treg cells in the peripheral lymph nodes were RORγt+ in unimmunized C57BL/6 mice; upon subcutaneous (s.c.) immunization with myelin oligodendrocyte glycoprotein (MOG) peptide emulsified in complete Freund’s adjuvant (CFA), about 10 % of CD4+Foxp3+ Treg cells expressed RORγt in draining lymph nodes (dLNs) (Figures 1A and 1B). The RORγt expression was highly correlated with cell surface expression of CCR6, a chemokine receptor preferentially expressed by Th17 cells (Yamazaki et al., 2008), in both Treg cells and non-Treg cells (Figure 1A). In addition, most of the RORγt+ Treg cells were negative for CXCR3 and CXCR5, markers for T-bet+ and Bcl6+ Treg cells, respectively (Figure 1C). Interestingly, RORγt+ Treg cells were mutually exclusive from Bcl6+ Treg cells as well as ST2+ Treg cells, a subpopulation of Treg cells recently identified in mucosal tissues (Figure 1D) (Arpaia et al., 2015; Molofsky et al., 2015; Schiering et al., 2014).
Figure 1. Induction of RORγt+CCR6+ Treg cells after immunization.
C57BL/6 mice were s.c. immunized with MOG/CFA. Seven days later, dLNs were isolated for analysis. (A–B) RORγt and CCR6 expressions in CD4+Foxp3+ cells or CD4+Foxp3− cells were analyzed by flow cytometry. Non-immunized mice were included as a control. (C) Expression of CCR6, CXCR3 or CXCR5 was compared with RORγt expression in CD4+Foxp3+ cells. (D) Expression of ST2 or Bcl6 was compared with RORγt expression in CD4+Foxp3+ cells. Data are representatives of at least two independent experiments. **, p<0.005.
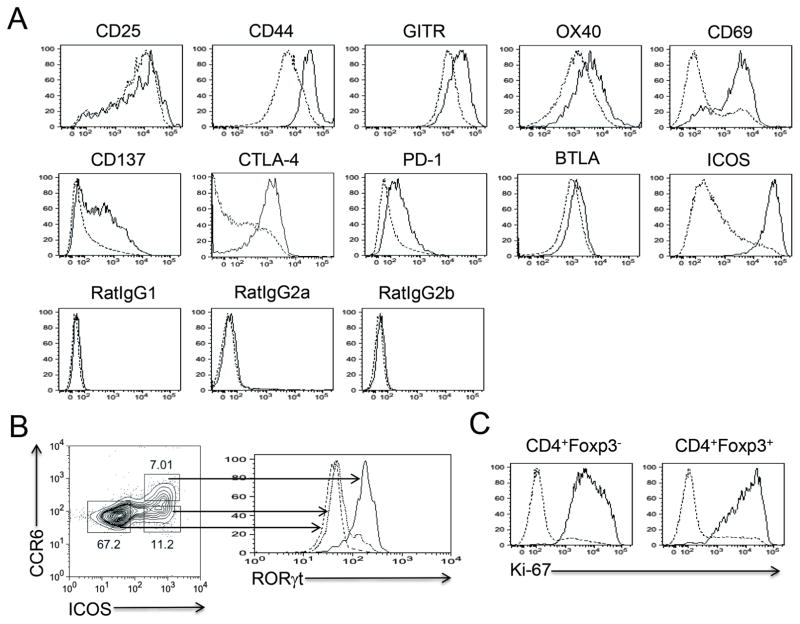
To characterize RORγt+ Treg cells, we analyzed the expression of Treg cell-associated cell surface molecules. Compared to RORγt−CCR6− Treg cells, RORγt+CCR6+ Treg cells highly expressed cell activation markers including CD25, CD44, GITR, OX40 and CD69 (Figure 2A). In addition, the expression levels of CD137, CTLA-4, PD-1, and BTLA, which have been attributed to the functional aspects of Treg cells, were also higher in RORγt+CCR6+ Treg cells than in RORγt−CCR6− Treg cells. Of note, most of the RORγt+CCR6+ Treg cells were selectively ICOShi, resulting in exclusive expression of RORγt in ICOShiCCR6+ Treg cells compared to ICOShiCCR6− Treg cells or ICOSloCCR6− Treg cells (Figures 2A and 2B). The cell surface marker expression pattern of RORγt+CCR6+ non-Treg cells was very similar to that of RORγt+CCR6+ Treg cells except the low expression of CD137 (Figure S1). Consistent with their activated phenotype, most of the RORγt+CCR6+ Treg cells as well as RORγt+CCR6+ non-Treg cells were actively proliferating cells with the high levels of Ki-67 expression (Figure 2C). Collectively, these results suggest that RORγt+CCR6+ Treg cells induced following immunization are activated cells.
Figure 2. Treg cell-specific cell surface marker expression in RORγt+CCR6+ Treg cells.
(A) Seven days after MOG/CFA s.c. immunization, dLNs were isolated from C57BL/6 mice. Cell surface marker expression in RORγt+CCR6+CD4+Foxp3+ cells (Solid) was compared with that in RORγt−CCR6−CD4+Foxp3+ cells (Dashed). (B) Seven days after MOG/CFA immunization, RORγt expression in each CD4+Foxp3+ subsets in dLNs was analyzed after gating based on ICOS and CCR6 expressions. (C) Ki-67 expression in RORγt+CCR6+ (Solid) or RORγt−CCR6− (Dashed) cells was analyzed. Data are representatives of at least two independent experiments. See also Figure S1.
RORγt+CCR6+ Treg cells are derived from Foxp3+ thymic Treg cells
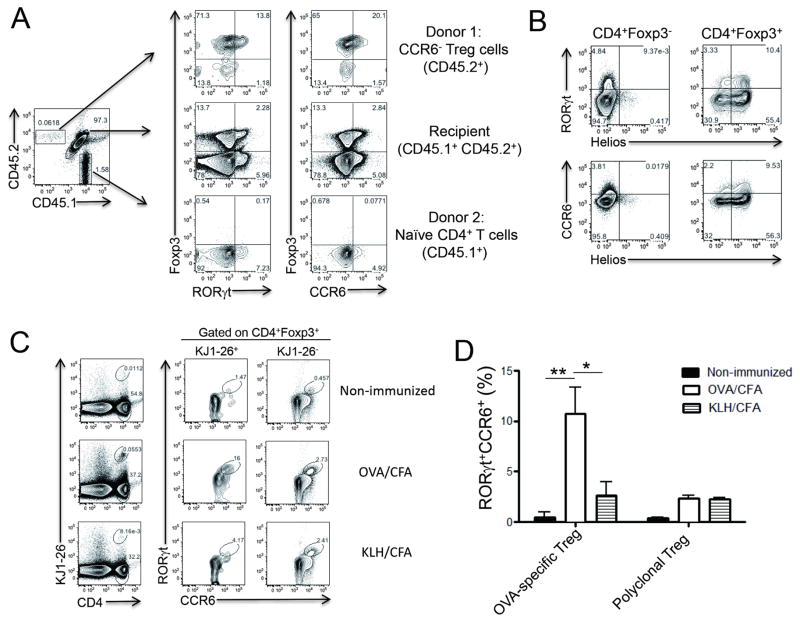
Next, we examined the origin and antigen specificity of RORγt+ Treg cells. In order to determine whether RORγt+ Treg cells are preferentially derived from thymic Foxp3+ Treg (tTreg) cells or naïve CD4+ T cell precursors, we co-transferred CD4+CCR6−YFP+ Treg cells isolated from Foxp3YFP-Cre reporter mice (CD45.2+) with naïve CD4+ T cells isolated from congenic mice (CD45.1+) into CD45.1+CD45.2+ recipient mice. Seven days after immunization with MOG/CFA, about 10% of host Treg cells were RORγt+CCR6+. Of interest, the majority of the transferred CCR6− tTreg cells maintained their Foxp3 expression and 15–20 % of them were RORγt+CCR6+ (Figure 3A). In contrast, few, if any, Foxp3+ cells were induced from naïve CD4+ T cells. These results suggest that RORγt+ Treg cells are preferentially derived from tTreg cells after immunization. In support of this notion, unlike their intestinal counterparts (Ohnmacht et al., 2015; Sefik et al., 2015), more than 75% of the RORγt+CCR6+ Treg cells induced by MOG/CFA immunization expressed Helios, one of the markers preferentially expressed by tTreg cells (Figure 3B) (Thornton et al., 2010). To directly confirm their thymic origin, we sorted Treg cells from thymocytes of Foxp3GFP reporter mice and co-transferred them with naïve CD4+ T cells into TCRβ−/− mice. Seven days after MOG/CFA immunization, we confirmed that more than 95% of the RORγt+CCR6+ Treg cells were originated from thymic Treg cells (Figure S2).
Figure 3. Origin of RORγt+CCR6+ Treg cells.
(A) CD4+YFP+CCR6− Treg cells isolated from Foxp3YFP-Cre reporter mice (CD45.2+) were mixed with CD4+CD25−CD44loCD62L+ naïve T cells isolated from B6.SJL mice (CD45.1+) in the 1:9 ratio and then were adoptively transferred to CD45.1+CD45.2+ recipient mice. One day after the transfer, recipient mice were subcutaneously immunized with MOG/CFA. Seven days later, CD4+ T cells in dLNs were analyzed for Foxp3, RORγt and CCR6 expressions after gating populations based on CD45.1 and CD45.2 expression. (B) Helios expression in CD4+Foxp3+ or CD4+Foxp3− cells in dLNs was compared with RORγt or CCR6 expression. (C–D) OVA-specific CD4+KJ1-26+GFP+CCR6− Treg cells isolated from DO11.10xFoxp3GFP reporter mice were adoptively transferred to BALB/c mice. One day after the transfer, mice were s.c. immunized with OVA/CFA or KLH/CFA or left untreated. Seven days after immunization, KJ1-26+ or KJ1-26− CD4+Foxp3+ Treg cells in dLNs were analyzed for RORγt and CCR6 expressions. Data are representatives of two independent experiments. *, p<0.05. **, p<0.005. See also Figure S2.
To assess whether RORγt+ Treg cell induction is antigen-specific, OVA-specific CCR6− Treg cells isolated from DO11.10xFoxp3GFP mice were adoptively transferred to naïve BALB/c recipient mice followed by s.c. immunization with either OVA/CFA or keyhole limpet hemocyanin (KLH)/CFA. Without immunization, very few CD4+KJ1-26+ cells were recovered and less than 0.5 % of KJ1-26− polyclonal Treg cells were RORγt+CCR6+ (Figures 3C and 3D). Interestingly, however, the numbers of OVA-specific KJ1-26+ Treg cells were remarkably increased by OVA antigen immunization, while 10–16% of the OVA-specific Treg cells became RORγt+CCR6+. In contrast, we could not observe any increase in the frequencies of OVA-specific CD4+KJ1-26+ Treg cells after KLH immunization, and most of the donor Treg cells remained RORγt−CCR6−. Similar induction efficiency of RORγt+CCR6+ cells among KJ1-26− Treg cells between OVA and KLH immunization rules out the possibility of intrinsic difference in immunization protocol (Figures 3C and 3D). Altogether, these results indicate that unlike intestinal RORγt+ Treg cells, those in the peripheral lymphoid tissues mainly originated from tTreg cells in an antigen-specific manner after immunization.
Immunization-induced peripheral RORγt+Foxp3+ cells are distinct from conventional Treg or colonic RORγt+ Treg cells
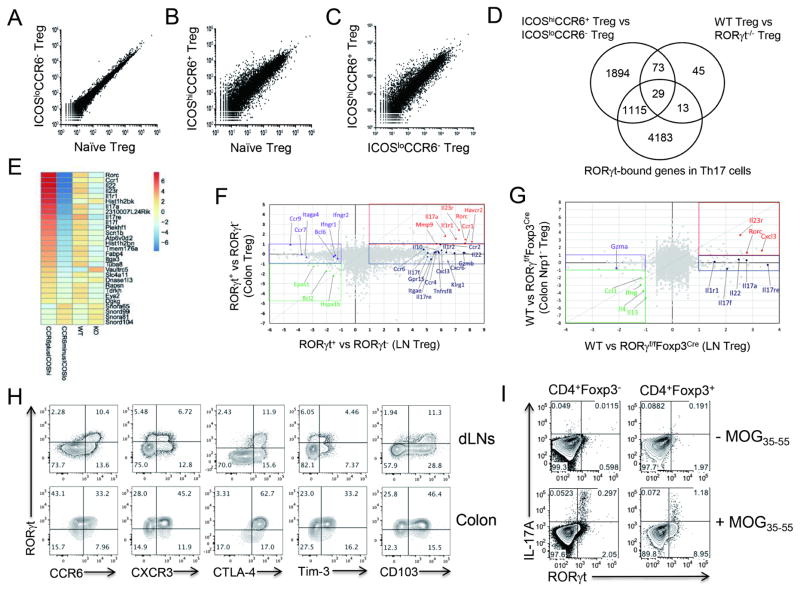
To further characterize RORγt+ Treg cells in their transcriptome, we isolated RORγt+CCR6+ and RORγt−CCR6− Treg cells from dLNs of MOG/CFA-immunized Foxp3YFP-Cre reporter mice by flow cytometry, based on their ICOS and CCR6 expression patterns (Figures S3A and S3B). We confirmed that CD4+YFP+ICOShiCCR6+ cells and CD4+YFP+ICOSloCCR6− cells represent RORγt+ Treg cells and RORγt− Treg cells, respectively, at the mRNA level by RT-PCR (Figure S3C). Then, we compared the gene expression profiles by performing RNA-sequencing (RNA-seq) analysis. Based on transcriptomic profiling by RNA-seq analysis, ICOShiCCR6+ Treg cells were found to be distinct from ICOSloCCR6− Treg cells, which were relatively similar to CCR6−CXCR3−CXCR5− naive Treg cells isolated from unimmunized mice (Figures 4A–4C). Total 3111 genes were differentially regulated in ICOShiCCR6+ Treg cells compared to ICOSloCCR6− Treg cells (Figure 4D).
Figure 4. Transcriptional profiling of RORγt+CCR6+ Treg cells.
(A–C) Normalized read counts of the indicated cells are plotted. (D) Common differentially regulated genes between “ICOShiCCR6+ Treg vs ICOSloCCR6− Treg” and “WT Treg (WT) vs RORγt−/− Treg (KO)” were compared and shown in Venn diagram. RORγt-bound gene loci identified from ChIP-sequencing data of in vitro generated Th17 cells were further compared. (E) Heat map shows 29 common differentially regulated genes selected from (D). (F) Relatively up-regulated or down-regulated genes in LN ICOShiCCR6+ Treg cells (RORγt+ Treg) over ICOSloCCR6− Treg cells (RORγt− Treg) were compared with those in colonic RORγt+ Treg cells over colonic RORγt−Treg cells. (G) Comparison of RORγt-dependent genes (WT vs RORγf/fFoxp3Cre mice) between Colon Nrp1− Treg cells and LN Treg cells isolated from s.c. immunized mice. Values in F–G indicate log2(fold change). (H) Expressions of indicated molecules in CD4+Foxp3+ cell in dLNs or colon were analyzed by flow cytometry seven days after MOG/CFA s.c. immunization. (I) Antigen-specific expressions of IL-17A and RORγt in CD4+Foxp3+ cells or CD4+Foxp3− cells were analyzed 24 hours after MOG peptide restimulation. Brefeldin A and monensin were added for last 6 hrs of culture. Data are representatives of at least two independent experiments. See also Figure S3.
To sort out RORγt-dependent genes among them, we generated RORγ-floxed mice and then crossed them to Foxp3YFP-Cre mice to specifically delete RORγt in Treg cells. We then performed RNA-seq analysis for CD4+YFP+ cells isolated either from RORγf/fFoxp3YFP-Cre mice (RORγt−/− Treg cells) or from RORγ+/+Foxp3YFP-Cre mice (WT Treg cells) 7 days after MOG/CFA immunization. When we compared differentially regulated genes between ICOShiCCR6+ Treg and ICOSloCCR6− Treg cells with those between WT Treg and RORγt−/− Treg cells, 102 common genes were selected (Figure 4D). Then, we further identified 29 RORγt-bound loci among them based on ChIP-sequencing analysis of in vitro generated Th17 cells (Figure 4E) (Ciofani et al., 2012). Intriguingly, a number of Th17 cell-related genes, such as Rorc, Il22, Il23r, Il1r1, Il17a, and Il17f, were highly expressed by ICOShiCCR6+ Treg cells, suggesting that ICOShiCCR6+RORγt+ Treg cells share some of the features of Th17 cells (Figure 4E). We confirmed the high expression of the selected genes (Il17a, Il17f, Il22, Il1r1, Il23r, and Il10) by ICOShiCCR6+ Treg cells at the mRNA level, while the expression of irrelevant Th1-specific genes (Tbx21 and Ifng) was not increased (Figure S3C).
To see whether peripheral RORγt+ Treg cells are similar to colonic RORγt+ Treg cells, we compared the transcriptome of these two RORγt+ Treg populations (Sefik et al., 2015). A fold change/fold change comparison between colonic RORγt+ Treg cells and LN RORγt+ Treg cells, after calculating the fold change based on their respective RORγt− populations, revealed that several Th17 cell-related genes (Rorc, Il23r, Il1r1 and Il17a) were highly expressed by both colonic RORγt+ Treg and peripheral RORγt+ Treg cells (Figure 4F). Genes selectively upregulated in RORγt+ Treg cells in peripheral LNs compared to colonic ones include those encoding Th17 cell-associated effector cytokines and IL-17 receptor family member (Il22, Il17f and Il17re), Treg cell-associated effector cytokines (Gzmb and Il10), G protein-coupled receptors (Gpr15), integrin (Itgae), chemokine receptors (Ccr2, Cxcr6, Ccr4, Ccr6), costimulatory molecule (Tnfrsf8) and Helios (Ikzf2). In contrast, gut homing integrin and receptor (Itaga4 and Ccr9), IFN-γ receptors (Ifngr1 and Ifngr2) and transcription factor Bcl6 were selectively down-regulated in LN Treg cells (Figure 4F). We further compared RORγt-dependent, differentially regulated genes in LN Treg cells to those in colonic Treg cells. Accordingly, several Th17 cell-associated effector cytokines (Il17a, Il17f and Il22) and cell surface receptors (Il1r1 and Il17re) were selectively elevated in LN Treg cells in an RORγt-dependent manner, while Il23r and chemokine Cxcl3 were commonly up-regulated by both populations. In contrast, expression of Th1 (Ifng) and Th2 (Il4 and Il13) effector cytokines were significantly inhibited by RORγt in both colon and LN Treg cells (Figure 4G). We confirmed the relatively high expression of CCR6, CD103, Helios and IL-17A in peripheral RORγt+ Treg cells compared to colonic RORγt+ Treg cells at the protein level, while granzyme B expression level was not consistent with the transcriptome analysis result (Figures 4H, S3D and S3E). In contrast, Tim-3 (encoded by Havcr2) expression level was higher in colonic RORγt+ Treg cells than in peripheral RORγt+ Treg cells at the protein level, despite the common upregulation of the molecule by both populations in transcriptome analysis (Figures 4F and 4H). Of note, about 10–25 % of peripheral RORγt+ Treg cells expressed IL-17A, whereas colonic RORγt+ Treg cells did not produce IL-17A (Figures 4I and S3E). Based on this finding, we named peripheral RORγt+ Treg cells as T regulatory 17 (Tr17) cells. Altogether, these results indicate that immunization-induced peripheral RORγt+ Tr17 cells are distinct from conventional Treg cells as well as gut RORγt+ Treg cells.
Factors involved in the generation of RORγt+CCR6+ Tr17cells
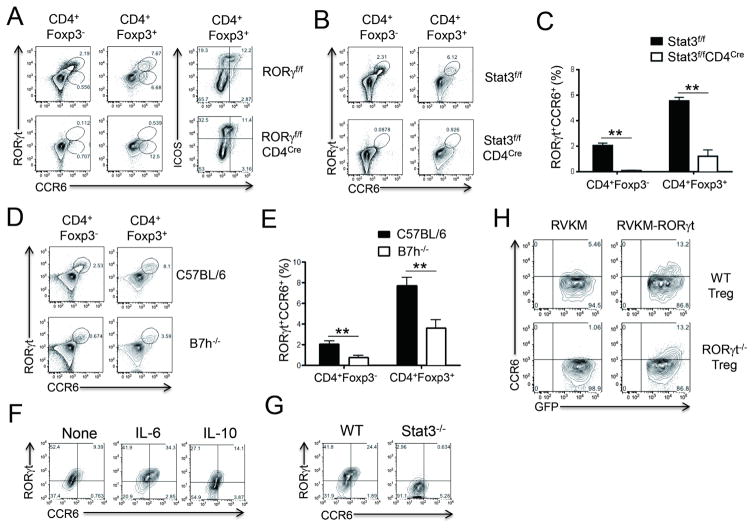
Increased expression of Th17 cell-associated genes in Tr17 cells prompted us to address whether these cells share the developmental cues with Th17 cells. To test whether RORγt is required for the generation of Tr17 cells following immunization, RORγf/fCD4Cre mice were immunized with MOG/CFA. As expected, the induction of RORγt+CCR6+ Tr17 cells was completely abolished in RORγf/fCD4Cre mice (Figure 5A). Intriguingly, however, we could still detect some CCR6intICOShi Treg cells in RORγf/fCD4Cre mice, suggesting the RORγt-independent CCR6 expression in Treg cells despite the suboptimal level of expression (Figure 5A). A previous report has shown that Stat3 in Treg cells is essential for the regulation of Th17 cells, at least in part, by inducing the expression of CCR6 (Chaudhry et al., 2009). Thus, we determined whether Stat3 is required for Tr17 cell generation using Stat3f/fCD4Cre mice. After immunization with MOG/CFA, the induction of RORγt+CCR6+ Tr17 cells was completely abrogated in the absence of Stat3 in T cells, demonstrating that Stat3 is indispensable for the generation of Tr17 cells (Figures 5B and 5C). We and others previously found that ICOS-ICOSL (B7h) signaling is critical for Th17 cell development in vivo (Bauquet et al., 2009; Park et al., 2005). Increased expression of ICOS in RORγt+CCR6+ Treg cells prompted us to define whether ICOS-ICOSL signaling is also involved in the generation of Tr17 cells. When we immunized B7h-deficient mice with MOG/CFA, we found a significant reduction in RORγt+CCR6+ Tr17 cell generation compared to control mice (Figures 5D and 5E). Taken together, these results suggest that Tr17 cells utilize similar molecular machinery for their development as Th17 cells.
Figure 5. Requirements for the induction of RORγt+CCR6+ Treg cells.
(A–E) Seven days after MOG/CFA s.c. immunization in indicated mice, dLNs were isolated for the analysis of RORγt+CCR6+ cell induction in CD4+Foxp3+ cells or CD4+Foxp3− cells. (F) CD4+CCR6−GFP+ Treg cells isolated from Foxp3GFP mice were stimulated with anti-CD3 and anti-CD28 Abs for 4 days in the presence of IL-6 (20 ng/ml) or IL-10 (100 ng/ml). RORγt and CCR6 expressions in gated CD4+Foxp3+ cells were analyzed by flow cytometry. (G) CD4+CD25hiCCR6− Treg cells isolated from Stat3f/fCD4Cre mice or littermate control mice were stimulated with anti-CD3 and anti-CD28 Abs in the presence of IL-6 (20 ng/ml). RORγt and CCR6 expressions in gated CD4+Foxp3+ cells were analyzed. (H) CD4+CD25hiCCR6− Treg cells isolated from RORγf/fCD4Cre mice (RORγt−/− Treg) or littermate control mice (WT Treg) were transduced with retroviral vector expressing RORγt (RVKM- RORγt) or empty vector (RVKM). CCR6 and GFP expressions were analyzed after gating on GFP+ cells. Data are representatives of at least two independent experiments. **, p<0.005.
RORγt induced by IL-6-Stat3 in Treg cells regulates CCR6 expression
It has been demonstrated that IL-10-IL-10R signaling in Treg cells is mandatory to induce Stat3 activation and subsequent regulation of Th17 cells by Treg cells (Chaudhry et al., 2011). Thus, we hypothesized that IL-10-mediated activation of Stat3 induces RORγt and CCR6 expression in Treg cells. To address this hypothesis, CCR6− Treg cells isolated from Foxp3 reporter mice were TCR-stimulated in the presence of IL-6 or IL-10. TCR activation alone induced few, if any, RORγt+CCR6+Foxp3+ cells. Interestingly, exogenous IL-6 induced a considerable proportion of RORγt+CCR6+ population among Foxp3+ cells while the effect of exogenous IL-10 was minimal (Figure 5F). As expected, Stat3 expression in Treg cells was essential for IL-6-mediated induction of RORγt+CCR6+Foxp3+ cells (Figure 5G).
Next, we addressed whether RORγt transduction is sufficient to induce CCR6 expression in Treg cells. To this end, we isolated CCR6− Treg cells from RORγf/f CD4Cre mice or littermate control mice and then transduced them with retroviral vector expressing RORγt-GFP or empty vector expressing GFP alone. Although WT Treg cells expressed a marginal level of CCR6 even without RORγt transduction, RORγt−/− Treg cells were totally deficient in CCR6 expression without RORγt transduction. In addition, CCR6 expression was dramatically increased in GFP+ cells of WT Treg cells by RORγt transduction in a GFP intensity-dependent manner. Of note, RORγt transduction was adequate to restore CCR6 expression in RORγt−/− Treg cells at a comparable level to that in WT Treg cells (Figure 5H). These results suggest that RORγt expression in Treg cells is dependent on IL-6-mediated Stat3 signaling and, in turn, RORγt expression is essential and sufficient for CCR6 expression in Treg cells.
RORγt+CCR6+ Tr17 cells have inhibitory function against Ag-specific Th17 and Th1 cells in vitro
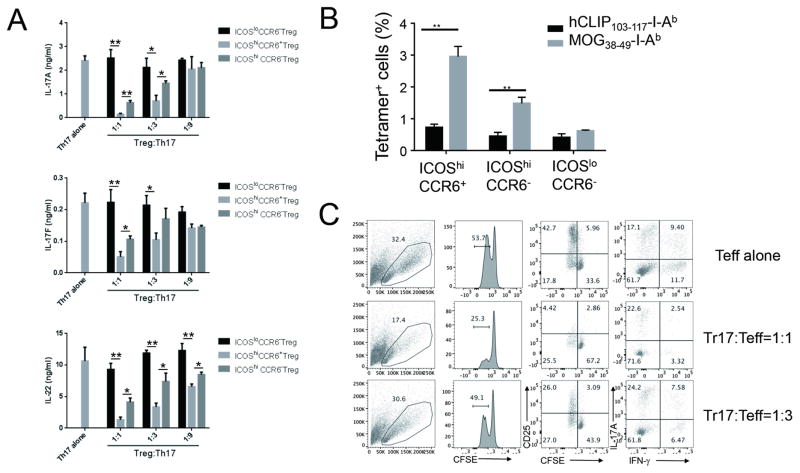
To address whether Tr17 cells can regulate Ag-specific Th17 cell response, CD4+ICOShiCCR6+GFP− Th17 cells were isolated from dLNs of MOG/CFA-immunized Foxp3GFP reporter mice. Three days after MOG peptide stimulation, a copious amount of IL-17A, IL-17F, and IL-22 was detected in the culture supernatant. Of note, ICOShiCCR6+RORγt+ Tr17 cells significantly suppressed cytokine production by Th17 cells in a Treg cell to Th17 cell ratio-dependent fashion. In contrast, ICOSloCCR6− resting Treg cells failed to do so, even at the highest Treg to Th17 ratio (1:1) (Figure 6A). Of interest, ICOShiCCR6+ Tr17 cells were almost three times more potent at suppressing Ag-specific Th17 cells than ICOShiCCR6− activated Treg cells (Figure 6A). We also observed that ICOShiCCR6+ Treg cells were superior at suppressing IFN-γ production as well as IL-17A production by total ICOShi effector CD4+ T cells compared to ICOShiCCR6− Treg cells and ICOSloCCR6− Treg cells (Figure S4A). These results indicate that Tr17 cells are Ag-specific Treg cells with potent regulatory activity against Ag-specific Th17 cells and Th1 cells in vitro.
Figure 6. ICOShiCCR6+RORγt+ Treg cells are Ag-specific regulatory T cells with potent suppressive activity in vitro.
(A) ICOShiCCR6+GFP−CD4+ Th17 cells were isolated seven days after MOG/CFA s.c. immunization of CD45.2+Foxp3GFP reporter mice. In parallel, ICOShiCCR6+GFP+CD4+ Treg cells, ICOSloCCR6−GFP+CD4+ Treg cells, and ICOShiCCR6−GFP+CD4+ Treg cells were isolated from MOG/CFA immunized CD45.1+CD45.2+ Foxp3GFP reporter mice. Th17 cells were stimulated with MOG peptide (50 μg/ml) in the presence of irradiated T cell-depleted splenocytes (TdS, CD45.1+). Three fold serially diluted number of each Treg cells were added to the culture to test the suppressive activity. Three days later, IL-17A, IL-17F or IL-22 production in the culture supernatant was analyzed by ELISA. (B) Foxp3GFP reporter mice were s.c. immunized with MOG/CFA. Seven days later, MOG-specific cells in indicated populations of dLNs were identified by tetramer staining. (C) ICOShiGFP−CD4+CD45.1+ T effector cells (Teff) were labeled with CFSE (2.0 μM) and then were stimulated with splenic CD11c+ DCs in the presence or absence of ICOShiCCR6+GFP+CD4+CD45.2+ Tr17 cells in the indicated Tr17 to Teff ratio. Three days later, FSC value, CFSE dilution, CD25 expression and cytokine production of CD45.1+ Teff cells were analyzed by flow cytometry after PMA/Ionomycin restimulation. Data are representatives of at least two independent experiments. *, p<0.05. **, p<0.005. See also Figure S4.
RORγt+CCR6+ Tr17 cells inhibit proliferation and activation of effector cells
Because RORγt+ Tr17 cells exerted more potent suppressive function than resting Treg cells, we hypothesized that Ag-specific Treg cells were enriched within the RORγt+ subpopulation. To test this hypothesis, we benefit from MOG38–49-I-Ab tetramer, which enabled us to detect MOG-reactive CD4+ T cells by flow cytometry. Of interest, we observed that ICOShiCCR6+ Tr17 cells contained more MOG-specific cells than ICOShiCCR6− or ICOSloCCR6− Treg cells, suggesting that enrichment of Ag-specific Treg cells contributed to the enhanced suppression meditated by Tr17 cells (Figures 6B and S4B).
We then determined the mode of action by which Tr17 cells inhibited effector T cells. After labeling CD4+GFP(Foxp3)−ICOShi effector T cells with CFSE dye to monitor cell proliferation, we restimulated the cells with MOG peptide. Three days after restimulation, about 50% of effector cells diluted CFSE and highly expressed cell activation marker CD25, suggesting the proliferation and activation of the MOG-specific effector T cells. When we added titrated numbers of ICOShiCCR6+ Tr17 cells to the culture, effector cell proliferation and activation were significantly reduced in a Treg to Teff ratio dependent manner (Figure 6C). Of note was that the frequency of Th1 cells was considerably reduced by Tr17 cells, while that of Th17 cells was maintained (Figure 6C). These results suggest that the suppressive mode of action exerted by Tr17 cells might be different depending on the type of effector T cells. We observed a similar pattern of suppression at the single cell level in the presence of polyclonal restimulation (Figure S4C).
Since our transcriptome analysis revealed that Tr17 cells up-regulated Il10 transcript and it was confirmed by RT-PCR (Figures 4F and S3C), we tested whether IL-10 is required for the suppression mediated by Tr17 cells. As shown in Figure S4D, Tr17 cell-mediated suppression of IL-17A and IFN-γ production by effector T cells was significantly impaired by the anti-IL-10R blocking antibody, implying that Tr17 cell-produced IL-10 is, at least in part, involved in their suppressive function.
RORγt+CCR6+ Tr17 cells can dampen Th17 cell-driven autoimmunity
Having shown that RORγt+CCR6+ Tr17 cells are activated cells with potent suppressive activity against Th17 cells in vitro, we set out to assess suppressive function of Tr17 cells under inflammatory conditions in vivo. To assess whether RORγt+Foxp3+ Tr17 cells were required for the regulation of Ag-specific Th17 cells in vivo, we compared EAE progression between RORγf/fFoxp3Cre mice and littermate control mice. Despite the potent suppressive activity of Tr17 cells in vitro, EAE progression was not exacerbated in Tr17 cell-deficient RORγf/fFoxp3Cre mice compared to control mice (data not shown). We also found that frequency and total number of Treg cells in CNS were not significantly different between RORγf/fFoxp3Cre mice and control mice, suggesting that RORγt expression in Treg cells was not essential for CNS homing of Treg cells (data not shown).
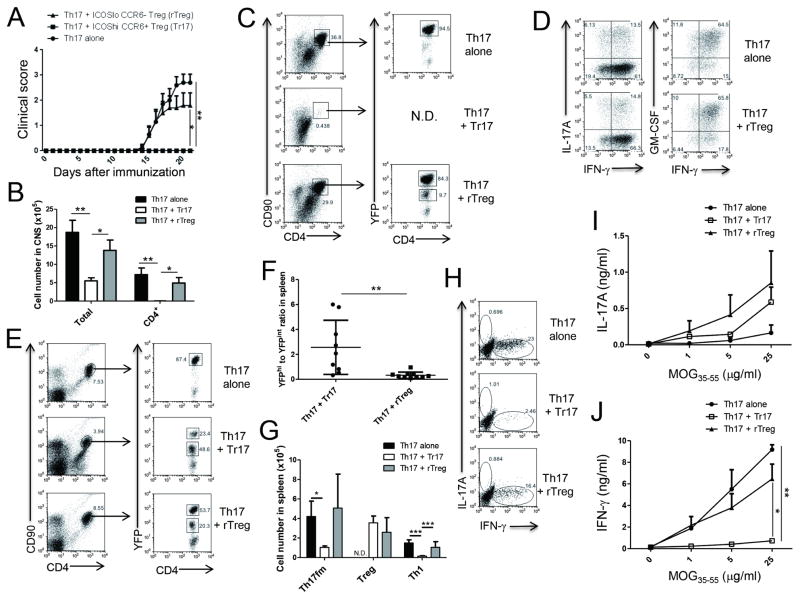
According to a recent study, it is possible that depletion of RORγt+ Treg cells is needed to examine their function rather than deletion of RORγt expression in Treg cells (Levine et al., 2017). However, availability of an experimental system for selective depletion of RORγt+ Treg cells in vivo is limited at the moment. To directly examine whether Tr17 cells can regulate Ag-specific Th17 cells in vivo, we isolated CD4+YFPhi Th17 fate-mapped (Th17fm) cells from dLNs of MOG/CFA-immunized IL-17FCrexRosa26YFP fate-mapping mice and confirmed that about 50% of the CD4+YFPhi cells produced IL-17A after MOG restimulation (Figure S5A). We then adoptively transferred Th17fm cells alone or together with either ICOShiCCR6+YFP+ Tr17 cells or ICOSloCCR6−YFP+ resting Treg cells (rTreg) into RAG1−/− mice at 4:1 Treg to Th17fm ratio (Figure S5B). After immunization with MOG peptide emulsified in incomplete Freund’s adjuvant (IFA) followed by PTX injections, the recipient mice transferred with Th17fm cells alone developed sequential clinical signs of regular EAE. Notably, the disease progression by Th17fm cells was completely abrogated by the co-transfer of Tr17 cells (Figure 7A). Interestingly, however, about 60 % of rTreg cells co-transferred recipient mice developed the disease with similar onset and severity of the disease as Th17fm cells alone transferred mice, while remainder of the mice did not develop disease (Figure 7A). Of note, CD4+ T cells were barely detectable in mice co-transferred with Tr17 while a significant infiltration of CD4+ T cells was found in those with Th17fm alone or with rTreg co-transfer (Figures 7B and 7C). Since Th17fm cells permanently express YFP and its intensity is much higher than that in Treg cells in this experimental system, we could differentiate YFPhi Th17fm cells from YFPint Treg cells after induction of EAE. Analysis of YFP expression in total CD4+ T cells in CNS confirmed that a majority of the CNS infiltrated CD4+ T cells were Th17fm cells in both Th17 alone transferred mice and diseased rTreg co-transferred mice (Figure 7C). We found that most of the YFPhi Th17fm cells had been converted to IFN-γ+GM-CSF+ cells, while some cells still produced IL-17A (Figure 7D). When we analyzed YFP expression in total CD4+ T cells in spleen, the ratio of YFPint Treg cells to YFPhi Th17fm cells in Tr17 cells co-transferred mice was 2.56 ± 0.72, which was about eight times higher than that in rTreg cell co-transferred mice (0.32 ± 0.08) (Figures 7E and 7F). We also confirmed that absolute number of YFPhi Th17fm cells were significantly reduced by the co-transfer of Tr17 cells (Figure 7G). These results suggest that Tr17 cells efficiently regulated expansion of Ag-specific Th17 cells in the periphery. Intracellular cytokine staining after MOG peptide restimulation revealed that most of the MOG-specific Th17fm cells in spleen already converted to IFN-γ-producing Th1 cells after EAE induction under the lymphopenic condition. The absolute number and frequency of IFN-γ-producing exTh17 cells were significantly suppressed by Tr17 cells while rTreg cells marginally suppressed IFN-γ production at the single cell level (Figures 7G and 7H). After three days of culture with MOG peptide, splenocytes isolated from Th17fm alone transferred mice produced a significant amount of IFN-γ with little production of IL-17A. Interestingly, IFN-γ production by splenocytes was significantly reduced by co-transfer of Tr17 cells, while IL-17A production was marginally increased compared to Th17fm alone (Figures 7I and 7J). In contrast, co-transfer of rTreg cells did not significantly suppress IFNγ production by splenocytes. Taken together, these results indicate that thymic Treg-derived RORγt+ Tr17 cells can suppress Th17 cell-mediated CNS inflammation by repressing peripheral expansion and CNS migration of Th17 cells.
Figure 7. ICOShiCCR6+RORγt+ Treg cells can regulate Th17 cell-mediated CNS autoimmunity.
CD4+YFPhi Th17 cells isolated from MOG/CFA immunized IL-17FCrexRosa26YFP fate mapping mice were adoptively transferred into RAG1−/− mice. CD4+YFP+ICOShiCCR6+ cells (Tr17) or CD4+YFP+ICOSloCCR6− cells (rTreg) isolated from MOG/CFA immunized Foxp3YFP-Cre mice were co-transferred with Th17 cells in some recipient mice. All the recipient mice were immunized with MOG/IFA one day after adoptive transfer. Pertussis toxin was injected on the day of immunization and two days later. (A) EAE score was monitored daily. Th17 alone (n=10), Th17 + Tr17 (n=9) and Th17 + rTreg (n=10). (B) The numbers of total cells and CD4+ T cells in CNS were analyzed. (C) Representative dot plot of YFP expression in gated CD4+CD90+ T cells in CNS is shown. (D) Cytokine production in CNS CD4+YFPhi T cells was analyzed after PMA/Ionomycin stimulation. (E) YFP expression in splenic CD4+ T cells is shown. (F) YFPint Treg cell to YFPhi Th17 fate-mapped cell ratio in spleen was analyzed. (G) Absolute numbers of CD4+YFPhi Th17 fate-mapped cells (Th17fm), CD4+YFPint Treg cells (Treg) and MOG-specific CD4+IFN-γ+ Th1 cells in spleen are shown. (H) MOG-specific cytokine production by splenic CD4+ T cells was analyzed after MOG peptide (50 μg/ml) restimulation. (I–J) IL-17 (I) and IFN-γ (J) production by splenocytes were analyzed by ELISA three days after restimulation with titrated doses of MOG peptide. *, p<0.05. **, p<0.005. N.D. Not detected. Data are combined results (A and F) or representatives (B–E and G–J) of two independent experiments. See also Figure S5.
DISCUSSION
Recent studies have identified intestinal RORγt+ Treg cells and found that these microbiota-induced Treg cells were required for the regulation of specific T helper cells in the gut (Ohnmacht et al., 2015; Sefik et al., 2015). The immunization-induced RORγt+ Tr17 cells in the current study share some features with the intestinal RORγt+ Treg cells, including: 1) CD44hi, ICOShi, CTLA4hi, CD103hi activated phenotype, 2) CCR6 expression, 3) Stat3-dependent development, 4) distinct from ST2+ Treg cells and 5) elevated expression of genes encoding Il1r1, Il23r, Havrc2 and Il10. However, the peripheral RORγt+ Tr17 cells are distinct from intestinal RORγt+ Treg cells. First, we found that peripheral RORγt+ Tr17 cells are mostly Helios+Nrp-1+ tTreg cells, whereas intestinal RORγt+ Treg were totally Helios−Nrp-1−, indicating a different origin of peripherally induced Treg cells (pTreg). Second, unlike intestinal RORγt+ Treg cells, about 10% of peripheral RORγt+ Tr17 cells produced IL-17A upon restimulation. Third, Stat3 was essential for the development of peripheral RORγt+ Tr17 cells, whereas Stat3 was not absolutely required for RORγt expression in intestinal Treg cells. Finally, gene expression profiling data obtained from two independent transcriptome analysis revealed that these two Treg cell populations have some similarities but are distinct from each other.
The identity of RORγt+Foxp3+ cells has been debatable. We hypothesized that there are at least three different possibilities on the fate of immunization-induced peripheral RORγt+Foxp3+ cells: 1) transient precursor cells during Th17 cell development from naïve CD4+ T cells, 2) unstable Foxp3+ Treg cells that can convert to IL-17-producing pathogenic cells under inflammatory conditions, 3) activated Treg cell subpopulation generated under the Th17-prone microenvironment. To address whether a considerable proportion of Th17 cells undergo RORγt+Foxp3+ intermediate stage as demonstrated before (Ichiyama et al., 2008; Yang et al., 2008; Zhou et al., 2008), we generated Foxp3CrexRosa26YFP mice to track Th17 cells that expressed Foxp3 at any stage throughout their developmental process. Unexpectedly, however, we found few, if any, YFP+IL-17A+ or YFP+IFN-γ+ cells in CNS of Foxp3CrexRosa26YFP mice after EAE induction (data not shown). This result indicates that the contribution of RORγt+Foxp3+ intermediate precursor to the total Th17 cell pool is minor at least in our EAE model. In addition, low expression level of IL-17A compared to that of Th17 cells and no evidence for pathogenic potential in our cell adoptive transfer EAE experiment indicate that peripheral RORγt+Foxp3+ cells are not a distinct IL-17-producing pathogenic cell population. Finally, high expression of Treg cell-associated cell surface molecules, stable expression of Foxp3, increased proliferative potential, Stat3-dependent development and strong suppressive activity against effector cells suggest that peripheral RORγt+Foxp3+ cells are activated Treg cells with potent suppressive activity induced under Th17-prone microenvironment in a Stat3-dependent manner as previously suggested (Yang et al., 2015).
Although we have observed that RORγt+ Tr17 cells had strong suppressive activity against MOG-specific Th17 cells in vitro and in vivo, we did not see any difference in onset and severity of the EAE between RORγt+ Treg cell-deficient RORγf/fFoxp3Cre mice and WT mice. It is plausible that RORγt and CCR6 expression would rather mark the Ag-specific activated Treg cells generated under the Th17 cell-promoting microenvironment than are directly involved in Treg cell function. Considering that total ICOShi Treg cell frequency were not changed after immunization even in the absence of RORγt (Figure 4A), ICOShiCCR6− Treg cells might have compensated for the absence of ICOShiRORγt+CCR6+ Treg cells in RORγf/fFoxp3Cre mice. A recent study demonstrated that depletion of T-bet+ Treg cells rather than deletion of Tbx21 gene in Treg cells resulted in Th1-dependent immunopathology (Levine et al., 2017). In this regard, selective depletion of RORγt+CCR6+ Treg cells after their development is required to address the functional relevance of the population in EAE as previously suggested (Kim et al., 2007). Another possibility is that the numbers of Tr17 cells generated in EAE were not sufficient to suppress autoimmunity during excessive inflammation reaction.
The role of CCR6 in EAE has been controversial (Elhofy et al., 2009; Kleinewietfeld et al., 2005; Liston et al., 2009; Moriguchi et al., 2013; Reboldi et al., 2009; Villares et al., 2009; Yamazaki et al., 2008). We and others have demonstrated that CCR6 expression was required for Th17 cells to enter into CNS to initiate CNS inflammation by showing that EAE development is attenuated in CCR6-deficient mice (Liston et al., 2009; Reboldi et al., 2009; Yamazaki et al., 2008). In contrast, others showed that CCR6 deficiency exacerbated EAE progression by regulating different immune cells such as Treg cells or dendritic cells (Elhofy et al., 2009; Villares et al., 2009). In the current study, we found that most of the adoptively transferred RORγt+CCR6+ Tr17 cells reside in the periphery without migrating to CNS, while they suppress MOG-specific Th17-mediated CNS inflammation in a Th17 cell transfer EAE model (Figures 7C and 7E). In addition, Treg cell-specific deletion of RORγt in RORγf/fFoxp3Cre mice did not critically affect the CNS homing of Treg cells after EAE induction (data not shown). These results indicate that RORγt-dependent CCR6 expression in Treg cells is neither sufficient nor necessary for CNS homing of Treg cells. As previously suggested by others, it is conceivable that CCR6 expression is not required for Treg cells to migrate to CNS once CNS inflammation is initiated by Th17 cells (Reboldi et al., 2009). Also, we do not rule out RORγt-independent CCR6 expression in Treg cells since we detected CCR6int Treg cells even in the absence of RORγt. The role of CCR6 in EAE progression warrants more detailed analysis with the cell type-specific deletion of CCR6 in different immune cells.
The role of Treg cells in human multiple sclerosis has been unclear. Although the frequency of CD4+CD25hi Treg cells in peripheral blood was not significantly different between MS patients and healthy subjects, a decrease in Foxp3 expression and a defect in Treg function have been found in MS patients (Kleinewietfeld and Hafler, 2014; Venken et al., 2008; Viglietta et al., 2004). In contrast, a recent study did not support that Treg function is defective in MS patients (Michel et al., 2008). Since most of these studies focused on conventional Treg cells, it is important to dissect Treg cell subsets based on additional cellular markers. In this regard, it has been reported that CD103+ Treg cells are increased in relapsing-remitting MS patients especially after IFN-β treatment (Venken et al., 2008). In addition, CD39+ Treg cells that are selectively required for Th17 cell regulation has been identified in MS patients (Borsellino et al., 2007). Whether a human counterpart for RORγt+ Tr17 cells can be found in MS patients and if there is any relationship between the frequency of RORγt+CCR6+ Treg cells and the progression of MS will be important questions to be elucidated in the future.
Overall, we found that RORγt+CCR6+Foxp3+ Tr17 cells induced by subcutaneous immunization represent activated Ag-specific effector regulatory T cells. The RORγt+ Tr17 can regulate Th17-mediated CNS autoimmunity by inhibiting the peripheral expansion and CNS migration of myelin-specific Th17 cells. This study will provide a guide to the development of an effective Treg cell immunotherapy against multiple sclerosis and other autoimmune diseases.
EXPERIMENTAL PROCEDURES
Mice
Mice were bred and maintained in the specific pathogen-free (SPF) animal facility at UT MD Anderson Cancer Center in accordance with institutional guidelines. We generated RORγf/f mice, in which exon 2 and 3 of Rorc gene are flanked by LoxP sites. The resultant RORγf/f mice were bred to FLPeR mice to remove Neomycin resistance cassette and then back-crossed to C57BL/6 mice for six generations. Foxp3YFP-Cre knock-in mice and Foxp3GFP reporter mice were kindly provided by Dr. Alexander Rudensky (Memorial Sloan-Kettering Cancer Center). Foxp3GFP-Cre BAC transgenic mice were kindly provided by Drs. Jeffrey Bluestone (UCSF) and Shao-Cong Sun (UT MD Anderson Cancer Center). Stat3f/fCD4Cre and B7h−/− mice were previously described (Nurieva et al., 2003; Yang et al., 2007). C57BL/6 and BALB/c mice were purchased from Jackson Laboratories. Foxp3GFP reporter mice on BALB/c background and DO11.10 mice were obtained from Jackson Laboratories and were crossed in our animal facility. Female and male mice at 8–12 weeks of age were used for experiments.
Antigen-specific induction of RORγt+CCR6+ Treg cells in vivo
CD4+KJ1-26+CCR6−GFP+ Treg cells isolated from DO11.10xFoxp3GFP/Y male mice were adoptively transferred into BALB/c recipient mice. One day after the transfer, recipient mice were untreated or immunized with either ovalbumin (OVA) (Grade V, Sigma) or keyhole limpet hemocyanin (KLH) (Sigma), both of which were emulsified in IFA supplemented with M. tuberculosis extract. Seven days after immunization, CD4+KJ1-26+ T cells in draining inguinal lymph nodes were analyzed for RORγt, CCR6 and Foxp3 expression.
Induction of EAE
For active EAE induction, mice were s.c. immunized with MOG35–55/CFA at the tail base at day 0. Pertussis toxin (List Biological Laboratories) was i.p. injected at day 0 and day 2. For Th17 transfer passive EAE, CD4+YFPhi Th17 cells isolated from MOG35–55/CFA-immunized IL-17FCrexRosa26YFP fate-mapping mice were adoptively transferred into RAG1−/− mice (2.0 × 104 Th17 cells/mouse). To test the suppressive activity of Treg cells, CD4+ ICOShiCCR6+YFPint Tr17 cells or CD4+ICOSloCCR6−YFPint resting Treg cells isolated from MOG35–55/CFA-immunized Foxp3YFP-Cre mice were adoptively transferred into RAG1−/− mice together with Th17 cells (8.0 × 104 Treg cells/mouse). The recipient mice were immunized with MOG35–55/IFA followed by two times PTX injection on the day of immunization and two days later. EAE disease progression was monitored daily and the disease score was assigned on a scale as follows: 0, no disease; 1, loss of tail tonicity; 2, wobbly gait; 3, hindlimb paralysis; 4, hindlimb and forelimb paralysis; 5, moribund.
Statistical analysis
Statistical analysis was performed by two-tailed t-test. A P-value less than 0.05 was considered statistically significant. *, p<0.05. **, p<0.005. All error bars were drawn based on standard errors.
Supplementary Material
Highlights.
RORγt+Foxp3+CD4+ Tr17 cells are found in lymph nodes after immunization.
Tr17 cells up-regulate Treg cell-associated effector molecules and CCR6.
Tr17 cells are originated from resting Treg cells via Stat3 signaling.
Tr17 cells potentially modulate Th17 cell-driven CNS autoimmunity.
Acknowledgments
The following reagents were obtained through the NIH Tetramer Core Facility: hCLIP103–117-I-Ab and MOG38–49-I-Ab. This research was supported in part by grants from National Institutes of Health to C.D. (R01AR050772) and Cancer Prevention and Research Institute of Texas (RP130078) and Department of Defense (W81XWH-16-1-0100) to S.H.C. This research was also supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (0430-20160039). B.-S.K. received postdoctoral fellowships from Arthritis Foundation of USA. C.D. is a Bayer Chair Professor at Tsinghua University.
Footnotes
ACCESSION NUMBERS
The accession number for RNA-Seq data reported in this paper is GEO:GSE103319.
AUTHOR CONTRIBUTIONS
C.D., S.H.C., Y.C. and B.-S.K. designed the research and analyzed the data. B.-S.K. performed most of the experiments, and K.I., X.C., K.T., Y.-h.L. and S.H.C. participated in specific experiments. X.Y. and Y.-B. Z. generated RORγf/f mice. R.N. provided B7h−/− mice. B.-S.K., H.L., N.A.M., L.Z., and W.J. analyzed RNA-sequencing data. B.-S.K, S.H.C., and C.D. prepared the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, Treuting PM, Rudensky AY. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell. 2015;162:1078–1089. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beriou G, Costantino CM, Ashley CW, Yang L, Kuchroo VK, Baecher-Allan C, Hafler DA. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113:4240–4249. doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatner NR, Mulcahy MF, Dennis KL, Scholtens D, Bentrem DJ, Phillips JD, Ham S, Sandall BP, Khan MW, Mahvi DM, et al. Expression of RORgammat marks a pathogenic regulatory T cell subset in human colon cancer. Sci Transl Med. 2012;4:164ra159. doi: 10.1126/scitranslmed.3004566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, Centonze D, Bernardi G, Dell’Acqua ML, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Bruning JC, Muller W, et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34:566–578. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, Wang YH, Lim H, Reynolds JM, Zhou XH, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofani M, Madar A, Galan C, Sellars M, Mace K, Pauli F, Agarwal A, Huang W, Parkurst CN, Muratet M, et al. A validated regulatory network for Th17 cell specification. Cell. 2012;151:289–303. doi: 10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- Du R, Zhao H, Yan F, Li H. IL-17+Foxp3+ T cells: an intermediate differentiation stage between Th17 cells and regulatory T cells. J Leukoc Biol. 2014;96:39–48. doi: 10.1189/jlb.1RU0114-010RR. [DOI] [PubMed] [Google Scholar]
- Elhofy A, Depaolo RW, Lira SA, Lukacs NW, Karpus WJ. Mice deficient for CCR6 fail to control chronic experimental autoimmune encephalomyelitis. J Neuroimmunol. 2009;213:91–99. doi: 10.1016/j.jneuroim.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama K, Yoshida H, Wakabayashi Y, Chinen T, Saeki K, Nakaya M, Takaesu G, Hori S, Yoshimura A, Kobayashi T. Foxp3 inhibits RORgammat-mediated IL-17A mRNA transcription through direct interaction with RORgammat. J Biol Chem. 2008;283:17003–17008. doi: 10.1074/jbc.M801286200. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- Kleinewietfeld M, Hafler DA. Regulatory T cells in autoimmune neuroinflammation. Immunol Rev. 2014;259:231–244. doi: 10.1111/imr.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinewietfeld M, Puentes F, Borsellino G, Battistini L, Rotzschke O, Falk K. CCR6 expression defines regulatory effector/memory-like cells within the CD25(+)CD4+ T-cell subset. Blood. 2005;105:2877–2886. doi: 10.1182/blood-2004-07-2505. [DOI] [PubMed] [Google Scholar]
- Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, Tanaka S, Bluestone JA, Takayanagi H. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med. 2014;20:62–68. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- Levine AG, Medoza A, Hemmers S, Moltedo B, Niec RE, Schizas M, Hoyos BE, Putintseva EV, Chaudhry A, Dikiy S, et al. Stability and function of regulatory T cells expressing the transcription factor T-bet. Nature. 2017;546:421–425. doi: 10.1038/nature22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston A, Kohler RE, Townley S, Haylock-Jacobs S, Comerford I, Caon AC, Webster J, Harrison JM, Swann J, Clark-Lewis I, et al. Inhibition of CCR6 function reduces the severity of experimental autoimmune encephalomyelitis via effects on the priming phase of the immune response. J Immunol. 2009;182:3121–3130. doi: 10.4049/jimmunol.0713169. [DOI] [PubMed] [Google Scholar]
- Michel L, Berthelot L, Pettre S, Wiertlewski S, Lefrere F, Braudeau C, Brouard S, Soulillou JP, Laplaud DA. Patients with relapsing-remitting multiple sclerosis have normal Treg function when cells expressing IL-7 receptor alpha-chain are excluded from the analysis. J Clin Invest. 2008;118:3411–3419. doi: 10.1172/JCI35365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AB, Van Gool F, Liang HE, Van Dyken SJ, Nussbaum JC, Lee J, Bluestone JA, Locksley RM. Interleukin-33 and Interferon-gamma Counter-Regulate Group 2 Innate Lymphoid Cell Activation during Immune Perturbation. Immunity. 2015;43:161–174. doi: 10.1016/j.immuni.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi K, Miyamoto K, Tanaka N, Yoshie O, Kusunoki S. The importance of CCR4 and CCR6 in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2013;257:53–58. doi: 10.1016/j.jneuroim.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Mai XM, Forbush K, Bevan MJ, Dong C. B7h is required for T cell activation, differentiation, and effector function. Proc Natl Acad Sci U S A. 2003;100:14163–14168. doi: 10.1073/pnas.2335041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnmacht C, Park JH, Cording S, Wing JB, Atarashi K, Obata Y, Gaboriau-Routhiau V, Marques R, Dulauroy S, Fedoseeva M, et al. MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science. 2015;349:989–993. doi: 10.1126/science.aac4263. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, Sallusto F. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013;13:461–467. doi: 10.1038/nri3464. [DOI] [PubMed] [Google Scholar]
- Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–568. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM, Burzyn D, Ortiz-Lopez A, Lobera M, Yang J, et al. MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science. 2015;349:993–997. doi: 10.1126/science.aaa9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartar DM, VanMorlan AM, Wan X, Guloglu FB, Jain R, Haymaker CL, Ellis JS, Hoeman CM, Cascio JA, Dhakal M, et al. FoxP3+RORgammat+ T helper intermediates display suppressive function against autoimmune diabetes. J Immunol. 2010;184:3377–3385. doi: 10.4049/jimmunol.0903324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken K, Hellings N, Thewissen M, Somers V, Hensen K, Rummens JL, Medaer R, Hupperts R, Stinissen P. Compromised CD4+ CD25(high) regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology. 2008;123:79–89. doi: 10.1111/j.1365-2567.2007.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villares R, Cadenas V, Lozano M, Almonacid L, Zaballos A, Martinez AC, Varona R. CCR6 regulates EAE pathogenesis by controlling regulatory CD4+ T-cell recruitment to target tissues. Eur J Immunol. 2009;39:1671–1681. doi: 10.1002/eji.200839123. [DOI] [PubMed] [Google Scholar]
- Voo KS, Wang YH, Santori FR, Boggiano C, Wang YH, Arima K, Bover L, Hanabuchi S, Khalili J, Marinova E, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Yang XO, Chung Y, Fukunaga A, Nurieva R, Pappu B, Martin-Orozco N, Kang HS, Ma L, Panopoulos AD, et al. CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol. 2008;181:8391–8401. doi: 10.4049/jimmunol.181.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang BH, Hagemann S, Mamareli P, Lauer U, Hoffmann U, Beckstette M, Fohse L, Prinz I, Pezoldt J, Suerbaum S, et al. Foxp3 T cells expressing RORgammat represent a stable regulatory T-cell effector lineage with enhanced suppressive capacity during intestinal inflammation. Mucosal Immunol. 2015 doi: 10.1038/mi.2015.74. [DOI] [PubMed] [Google Scholar]
- Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, Corcoran L, Treuting P, Klein U, Rudensky AY. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.