Abstract
Background
The aim of this study was to investigate the efficacy and safety of chemotherapy (CT) combined with stereotactic radiotherapy (SRT) in the treatment of nasopharyngeal carcinoma (NPC).
Material/Methods
A total of 329 NPC patients without any previous treatment were included in this study between January 2009 and November 2013. These patients were divided into three groups: CT group (n=114), SRT group (n=109), and CT + SRT group (n=106). Contrast-enhanced nasopharyngeal computed tomography (CT)/magnetic resonance (MR) scan was performed on the third month after treatment. Short-term efficacy was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST). Toxicity was graded according to the Acute Radiation Morbidity Scoring Criteria (RTOG) and the World Health Organization (WHO) toxicity grading scale. Overall survival (OS), progression free survival (PFS), and incidence rate of acute toxicity (grade ≥3) were calculated after a 24 month follow-up.
Results
Total response rate of all patients was 85.41%. Compared with the CT group and the SRT group, the CT + SRT group showed a substantially improved efficacy in NPC treatment. The incidence rate of the acute toxicity in the CT + SRT group was slightly higher than in the CT group and the SRT group, but the difference was not statistically significant. No treatment-related deaths were observed. The CT + SRT group had the highest two-year OS and PFS, followed by the CT group and the SRT group.
Conclusions
It was shown that NPC patients treated with CT + SRT had better short- and long-term efficacy than those treated with CT or SRT alone.
MeSH Keywords: Antineoplastic Agents; Nasopharyngeal Neoplasms; Prognosis; Radiosurgery; Toxicity Tests, Acute
Background
Nasopharyngeal cancer (NPC) is a type of squamous cell carcinoma; its pathological changes occur in the nasopharyngeal epithelium layer [1]. NPC is aggressive in its behavior, including early lymphatic spread, and it has a high incidence of hematological transmission [2]. It accounts for 0.6% of all cancers in the world and is known for its distinct variant morbidity in different ethnic groups and geographical regions [3]. In particular, NPC has a high prevalence in the southern region of China [4]. Epstein-Barr virus infection is considered to be a major pathogenic factor associated with NPC not only in endemic areas but also in non-endemic areas [5]. Among the traditional treatments for NPC, radiotherapy is a primary treatment. However, radiotherapy has many side effects such as swallowing difficulties, neuroendocrine dysfunction, and even hearing problems [6]. In addition, a very recent study found that after radiotherapy, NPC patients who have high pretreatment serum LDH showed significantly worse treatment outcomes [7]. Thus, although the development of NPC treatment has benefited from advanced techniques in radiotherapy, chemo-radiotherapy has been proposed to be more beneficial than radiotherapy alone for NPC patients [8]. Finding a safer and more effective therapeutic regimen for NPC patients and exploring treatment outcome of such therapies is urgently needed.
Stereotactic radiotherapy (SRT) is a high dose therapeutic method used for tumors with normal tissues reserved; it offers better mechanical precision by its stereotactic performance and it is applied in some malignancies such as head, neck, and epipharyngeal carcinomas [9]. SRT uses a three-dimensional (3D) framework especially designed for positioning during treatment; using targeted high-dose regions is helpful for preserving surrounding normal structures [10]. It has been reported that chemotherapy plus radiotherapy contributes to improvement in overall survival (OS) in NPC [11]. In addition, in the past ten years, there have been improvements in combined chemo-radiation for standard treatment of primary advanced NPC [12]. In particular, SRT combined with chemotherapy was shown to be a successful treatment in a case of choriocarcinoma [13]. SRT combined with chemotherapy is one of the new treatments considered for NPC patients and it is predicted to have a better survival rate. With the aim of improving quality of life for NPC patients and offering better therapeutic regimens for clinical NPC treatment, this study explored the short- and long-term efficacy of stereotactic SRT combined with chemotherapy in the treatment of NPC in a Chinese population.
Material and Methods
Study participants
Between January 2009 and November 2013, a total of 329 NPC patients from Zhejiang Cancer Hospital were enrolled in this study. The inclusion criteria were as follows: 1) patients who were diagnosed in the Ophthalmology and Otolaryngology Departments of Zhejiang Cancer Hospital and confirmed by imaging data and pathological examinations of specimens as having NPC; 2) patients who had primary nasopharyngeal carcinoma without receiving any medicine, chemo-radiotherapy, or immune-biological treatments; and 3) patients who had complete clinical data; patients who had no tumors in other areas or major heart, liver, or kidney disease.
Clinical data for study inclusion included: 1) pathological type was non-keratinizing or undifferentiated carcinoma (the World Health Organization (WHO) type II or III); 2) clinical stage was stage III to IV (according to the fifth edition of the American Joint Committee on Cancer (AJCC) Staging System); 3) Karnofsky performance scale (KPS) score ≥80; 4) hepatorenal function was normal: 5) alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels ranged between 0 and 2 times; 6) creatinine clearance rate ≤60 mL/minute; 7) routine blood examination appeared normal: and 8) leukocyte level was 4,000/uL and platelet level was 100,000/uL.
Patients were randomly divided into three groups: CT group (n=114), SRT group (n=109), and CT + SRT group (n=106). This study was approved by the ethical committee of Zhejiang Cancer Hospital. All patients enrolled in this study provided written informed consent.
Treatment regimens
Conventional chemotherapy was performed for patients in the CT group using Varian 2300C/D linear accelerator (Varian Medical Systems, Palo Alto, CA, USA) with 6MV-X. Dose for facio-cervical field and tangential field of middle and lower neck was GT36Gy/18 times, 2Gy per time, five times a week. Attention was payed to avoid overlaps between the two fields on cervical lumps. Field and dose was adjusted according to the condition of the patient. Nasopharyngeal irradiation dose was GT69Gy to 78.98Gy (median, 70Gy). When the neutrophil level was ≥1,000/μL (≤1,499/μL), platelet level ≥50,000/μL (≤74,999/μL), or creatinine clearance rate > 40 mL/minute (≤59 mL/minute), cisplatin dose was reduced to 60 mg/m2; when the neutrophil level was ≤1,000/μL, platelet level ≤50,000/μL, or creatinine clearance rate ≤40 mL/minute, it was considered time to cease chemotherapy. In the case of grade 2 mucositis, the 5-fluorouracil (5-FU) dose was decreased by 200 mg/m2; in the case of mucositis above grade 3, the 5-FU dose was decreased by 400 mg/m2.
SRT was performed for patients in the SRT group: patients first received radical radiation therapy and rested for one to three months (1.5 months on average). Nasopharyngeal examination and CT scanning were arranged for all patients. SRT was arranged for patients who showed reversal of disease. A 3D treatment planning system ARTP (Shanghai TOPSLANE Technolage Co., Ltd., Shanghai, China) and 6 MV linear accelerator (Primus, Siemens, Erlangen, Germany) were used for 3D conformal radiation therapy. Patients were positioned using fixation face mask or head, shoulder, or head mask. CT scanning was performed on the positioned part with a thickness of 5–7 mm. Digitalized images were transferred to ARTP. Tumor range and organ at risks (OAR), like eyeball, brainstem, and pituitary gland were contoured by the physician. The target area covered nasopharyngeal tumor, parapharyngeal space, and cervical lymph node, which were double checked by a senior radiation physician. Four to nine noncoplanar fields were used so that the planning target volume (PTV) was covered by 95% isodose curve. Dose tolerance of OARs was considered. Radiotherapy regimens were evaluated and optimized according to the dose-volume histogram (DVH). Fractionated radiotherapy dose was DT3.0–3.5Gy, three times per week, 10 to 14 times altogether. Cervical lymph nodes of both sides were contoured in the target treatment area. Three to five noncoplanar fields were used. Fractionated radiotherapy dose was DT3.0–3.2Gy, three times per week, eight to ten times altogether. Chemotherapy combined with SRT was performed for the patients in the CT + SRT group: adaptive radiotherapy was performed using stereotactic concurrent blocking method with linear accelerator 6MV-X. Continuous CT scans were performed on the face and cervix and the obtained imaging data were transferred to the SRT treatment planning system, where clinical target volume (CTV) and PTV were contoured. PTV was externally expanded for 5–10 mm in margin size, compared with CTV. Isocenter location and incident beam direction was determined by the axial, coronal, and sagittal sections of PTV. Five or six coplanar or noncoplanar beam fields were selected. Shaped as the reflection of the tumor when irradiated by the beam, a lead block was made. PTV was covered by 100% isodose line, and prescription dose for the target area was determined. Treatment regimens were examined through DVH. Treatment regimen was platinum (cis-platinum complexes (DDP)/nedaplatin) + 5-FU). On the first day, 60–100 mg/m2 of DDP was used and during the following four days (day 2 to day 5), 500–1,000 mg/m2 of 5-FU was used. In the CT + SRT group, patients received SRT and CT. CT regimen was 50–80 mg/m2 of DDP every three weeks or 20–40 mg/m2 of DDP every week [14].
Efficacy and toxic reaction evaluation
At the third month after treatment, contrast-enhanced CT/MR scan of the head and cervix and thorough examination were performed to evaluate short-term efficacy. Efficacy was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST): 1 was complete response (CR): target lesions disappeared for more than four weeks; 2 was partial response (PR): target lesions shrank by more than 50% for at least four weeks; 3 was stable disease (SD): lump shrank by less than 50% or grew by less than 25%; and 4 was progressive disease (PD): target lesions grew by more than 25% or new target lesions appeared. Patients were told to return to the hospital for examination once every three months during the following two years, once every six months during the following five years and once every year after five years.
Side effects in the three groups were compared. Patients were graded according to the acute radiation morbidity scoring criteria (RTOG). 1 was grade 0: no change over baseline.; 2 was grade 1: mild dysphagia or odynophagia, does not require anodyne but may require soft diet; 3 was grade 2: mucositis, may produce inflammatory serum secretions, moderate dysphagia or odynophagia, may require narcotic analgesics, may require puree or liquid diet; 4 was grade 3: integrated fibrous mucositis, severe dysphagia or odynophagia with dehydration, requiring narcotic analgesics and requiring nasogastric feeding tube, intravenous fluids or hyperalimentation, and 5 was grade 4: mucosal ulceration, hemorrhage, and necrosis. Side effects of chemotherapy were evaluated according to the WHO toxicity grading scale. Patients were graded 0–IV according to their hematological toxicity, gastrointestinal reaction, renal damage, and hair loss [15]. The symptoms of patients whose toxic reaction was ≥3 were recorded.
Follow-up
Information like efficacy, survival, and tumor control of NPC patients were collected through medical records, radiotherapy records, radiotherapy plans, outpatient records, and phone calls. The follow-up lasted for 24 months. Tumor control, OS, progression free survival (PFS), incidence rate of acute toxicity (grade ≥3) and efficacy were calculated.
Statistical analysis
All statistical analyses were performed using SPSS 20.0 software (SPSS, Chicago, IL, USA). Data are presented as means ± standard deviations and the χ2 test was used for the comparison. Enumeration data was expressed by percentage, and OS, locoregional control, and metastasis-free survival were analyzed by using Kaplan-Meier method. The difference in OS among the three groups was performed using the log-rank test. The χ2 test was used for comparing incidence rates of toxicity among the three groups.
Results
Baseline characteristics of NPC patients in the CT, SRT, and CT + SRT groups
From January 2009 to January 2014, a total of 329 NPC patients were diagnosed in the Ophthalmology and Otolaryngology Departments of Zhejiang Cancer Hospital, which included 252 males and 77 females with a mean age of 45.13±8.14 years (range from 19 to 72 years). Among them, 289 had squamous cell carcinoma and 40 had non-squamous cell carcinoma. All KPS scores were higher than 80. Clinical stages were as follows: 228 patients were in stage III, 101 patients were in stage IV, 170 patients were in stage T1–T2, and 159 patients were in stage T3–T4. Clinical factors among the three groups, including sex, age, pathological type, staging system, and clinical stage, were comparable (p>0.05) (Table 1).
Table 1.
Baseline characteristics of NPC patients among the CT, SRT and CT + SRT group.
| Characteristic | CT (n=114) | SRT (n=109) | CT + SRT (n=106) | P |
|---|---|---|---|---|
| Sex | ||||
| Male | 90 (78.95) | 76 (69.72) | 86 (81.13) | 0.109 |
| Female | 24 (21.05) | 33 (30.28) | 20 (18.87) | |
| Age (years) | ||||
| >40 | 85 (74.56) | 74 (67.89) | 76 (71.70) | 0.543 |
| ≤40 | 29 (25.44) | 35 (32.11) | 30 (28.30) | |
| Type of pathology | ||||
| Undifferentiated | 102 (89.47) | 96 (88.07) | 84 (79.25) | 0.066 |
| Differentiated | 12 (10.53) | 13 (11.93) | 22 (20.75) | |
| Tumor stage | ||||
| T1–T2 | 59 (51.75) | 52 (47.71) | 59 (55.66) | 0.506 |
| T3–T4 | 55 (48.25) | 57 (52.29) | 47 (44.34) | |
| LNM stage | ||||
| N0–N1 | 26 (22.81) | 26 (23.85) | 24 (22.64) | 0.974 |
| N2–N3 | 88 (77.19) | 83 (76.15) | 82 (77.36) | |
| Clinical stage | ||||
| III | 81 (71.05) | 80 (73.39) | 67 (63.21) | 0.238 |
| IV | 33 (28.95) | 29 (26.61) | 39 (36.79) | |
NPC – nasopharyngeal carcinoma; CT – chemotherapy; SRT – stereotactic radiotherapy. LNM – lymph node metastasis.
Comparison of the efficacy of NPC patients among the CT, SRT, and CT + SRT groups
Contrast-enhanced CT/MR scan combined with fiber optic bronchoscope for all 329 patients was performed within three months after radiotherapy. The results indicated that CR was achieved in 110 patients, PR was achieved in 171 patients, SD was achieved in 48 patients; the total response rate (RR; RR=CR + PR/total cases) was 85.41%. In total, 88 patients achieved CR and PR in the CT group (RR=77.19%), 92 patients achieved CR and PR in the SRT group (RR=84.40%) and 101 patients achieved CR and PR in the CT + SRT group (RR=95.28%). Compared with the CT and SRT group, efficacy in the CT + SRT group was improved remarkably (p=0.004 <0.05) (Table 2).
Table 2.
Efficacy in NPC patients among the CT, SRT and CT + SRT group.
| Group | CR | PR | SD | χ2 | P |
|---|---|---|---|---|---|
| C group | 34 (29.82) | 54 (47.37) | 26 (22.81) | 15.53 | 0.004 |
| SR group | 33 (30.28) | 59 (54.13) | 17 (15.60) | ||
| C+SR group | 43 (40.57) | 58 (54.72) | 5 (4.72) |
NPC – nasopharyngeal carcinoma; CT – chemotherapy; SRT – stereotactic radiotherapy; CR – complete response; PR – partial response; SD – disease stable.
Comparison of acute toxicity of NPC patients among the CT, SRT, and CT + SRT groups
A total of 329 patients completed chemotherapy and radiotherapy as scheduled. All patients in the CT and SRT groups completed chemotherapy or radiotherapy. In the CT + SRT group, a total of 75.47% (80/106) of patients completed seven cycles of chemotherapy as scheduled. About 86.79% (92/106) of patients completed more than six cycles of chemotherapy and 93.40% (99/106) of patients completed more than five cycles of chemotherapy. The incidence rates of acute toxicity (grade ≥3) in the CT, SRT, and CT + SRT groups were 41.23% (47/114), 34.86% (38/109), and 48.11% (51/106), respectively. The CT + SRT group had a higher incidence rate of acute toxicity (grade ≥3) than the CT group and the SRT group (p>0.05). Patients who showed a decrease in leukocyte count or absolute value of neutrophils were given leukocyte-increasing pills (Chengdu Diao Group Tianfu Pharmaceutical Co., Ltd., Sichuan Province, China) or subcutaneous granulocyte colony-stimulating factor; for patients who suffered from grade 3 acute oral mucosal toxicity, nasogastric feeding tube was used for enteral nutrition and anti-infection, and acesodyne treatments were performed on the basis of basic mouth care. No treatment-related deaths were observed in these three groups (Table 3).
Table 3.
Comparison of toxic reaction of NPC patients among the CT, SRT and CT + SRT group.
| Toxic reaction | CT | SRT | CT + SRT | |||
|---|---|---|---|---|---|---|
| Grade 3 | Grade 4 | Grade 3 | Grade 4 | Grade 3 | Grade 4 | |
| Non-hematological system | ||||||
| Mucositis | 11 (9.65) | 0 | 34 (31.19) | 0 | 25 (23.58) | 0 |
| Vomiting | 19 (16.67) | 0 | 0 | 0 | 6 (5.66) | 0 |
| Flaky skin | 4 (3.51) | 0 | 4 (3.67) | 0 | 4 (3.77) | 0 |
| Others | 0 | 0 | 0 | 0 | 0 | 0 |
| Hematological system | ||||||
| Leukocyte | 8 (7.02) | 0 | 0 | 0 | 13 (12.26) | 0 |
| Platelet | 2 (1.75) | 0 | 0 | 0 | 3 (2.83) | 0 |
| Others | 3 (2.63) | 0 | 0 | 0 | 0 | 0 |
NPC – nasopharyngeal carcinoma; CT – chemotherapy; SRT – stereotactic radiotherapy.
Comparison of the prognosis of NPC patients among the CT, SR, and CT + SRT groups
The two-year OS rates in the CT + SRT group and the CT group were 91.51% versus 76.32% (p=0.003), while those of the CT + SRT group and the SRT group were 91.51% versus 81.65% (p=0.045) (Figure 1). The two-year PFS rates in the CT + SRT group and the CT group were 90.57% versus 73.68% (p=0.002), while those of the CT + SRT group and the SRT group were 90.57% versus 78.90% (p=0.023) (Figure 2). Among the three groups, the CT + SRT group had the highest two-year OS and PFS rate, followed by the CT group and the SRT group (p<0.05).
Figure 1.
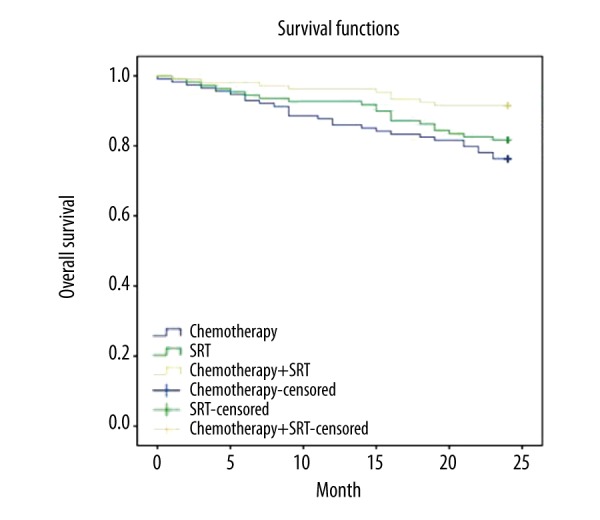
Comparison of overall survival rates of NPC patients among the CT, SRT, and CT + SRT groups. NPC – nasopharyngeal carcinoma; CT – chemotherapy; SRT – stereotactic radiotherapy.
Figure 2.
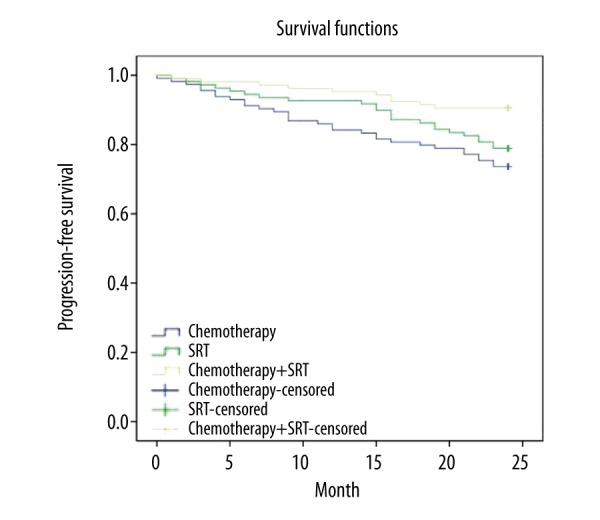
Comparison of progression free survival rates of NPC patients among the CT, SRT, and CT + SRT groups NPC – nasopharyngeal carcinoma; CT – chemotherapy; SRT – stereotactic radiotherapy.
Discussion
NPC, a radiosensitive neoplasm, has long depended on radiotherapy for its treatment [16]. However, in recent years, new treatment methods have been gaining momentum, and reporting improved outcomes in patients. On the one hand, data meta-analysis of patients with NPC has shown that the addition of chemotherapy to radiotherapy can improve patients’ OS [11]. On the other hand, SRT has been shown to be effective in eradicating a persistent local disease with improved local control (LC) as well as diminished complications [17]. However, few studies have been carried out to explore the relationship between chemotherapy combined with SRT and its efficacy for NPC. Thus it is of interest to find out whether the combined treatment has an advantage over traditional ones.
Our study revealed that patients treated with chemotherapy combined with SRT showed better outcomes than those treated with either CT or SRT alone. According to a previous report, targets in NPC, in most cases, are irregular in shape, spread along the walls of a hollow organ, and infiltrate extensively into critical soft tissues, such as the brainstem, cranial nerve, and optic pathway, making a series of complications possible [18]. One report showed that SRT may bring about an increased LC and OS as well as a decrease in complications in NPC patients in that it enables fractioned irradiation to be given right to the target rather than its adjacent organs, with mechanical precision and accuracy as well as dose conformity [19]. However, it has long been known that unevenly distributed among malignant tumors are hypoxic cells, and with the increasing accumulation of these cells, tumors gradually acquire an enhanced resistance to radiation cancer treatment, thus impeding the efficacy of SRT [20]. Fortunately, chemotherapy has been shown to possibly mitigate tumor resistance with drugs that can help enhance radiation sensitivity by regulating the activity of the DNA damage response through signaling pathways, such as mitogen-activated protein kinas/extracellular signal-regulated kinas (MAPK/ERK) and tumor necrosis factor-α/nuclear factor kappa B (TNF-α/NF-κB) [21]. Therefore, there is a high probability that chemotherapy combined with SRT can yield remarkably effective treatment results. The results of this study are also supported by similar results found in the treatment of other cancers. One study came to the conclusion that chemotherapy and SRT reaped better therapeutic effects on the treatment of cerebral glioma [22].
Furthermore, it was found in our study that the group receiving CT combined with SRT had remarkably higher two-year OS and PFS rates than the other two groups receiving either chemotherapy or SRT alone. Although few studies have been carried out that used combined treatment of chemotherapy and SRT for NPC, this treatment method has been proven to be effective with prolonged OS and PFS [23]. For example, one study concerning esophageal cancer found that combined treatment of chemotherapy and SRT was both effective and safe, and it reversed the development of the disease with better OS and PFS [24]. In addition, it was also found in the study that although toxicity occurred in all groups of patients, no statistically important difference was found in toxicity among the three groups receiving CT, SRT, and CT combined with SRT, showing that combined treatment could lead to better results without exacerbating toxicity. Also, Lo et al. [25] demonstrated that chemotherapy combined with SRT, in accordance with ours, reduced tumor size without acute radiation toxicity even after 33 months, and regarded it as a secure alternative for cancer treatment.
Conclusions
Based on the results that have been under review, we might generally arrive at the conclusion that chemotherapy, if employed for treatment coupled with SRT, can yield better outcomes with improved OS and PFS in patients with NPC compared to traditional methods, thus making it a potential alternative therapy. However, this study has limitations: little attention was paid to factors such as age and medical history, which are likely to affect outcomes. Therefore, future studies need to be conducted to exclude these and other possibly influential factors. In addition, further follow-up on the conditions of our patients is needed before a safe conclusion can be finally drawn.
Acknowledgments
We are particularly grateful to all the people who had given help for our article.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Li Y, Huang W, Pan J, et al. Rapid detection of nasopharyngeal cancer using Raman spectroscopy and multivariate statistical analysis. Mol Clin Oncol. 2015;3:375–80. doi: 10.3892/mco.2014.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee AW, Ng WT, Chan LL, et al. Evolution of treatment for nasopharyngeal cancer – success and setback in the intensity-modulated radiotherapy era. Radiother Oncol. 2014;110:377–84. doi: 10.1016/j.radonc.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Robinson M, Suh YE, Paleri V, et al. Oncogenic human papillomavirus-associated nasopharyngeal carcinoma: an observational study of correlation with ethnicity, histological subtype and outcome in a UK population. Infect Agent Cancer. 2013;8:30. doi: 10.1186/1750-9378-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wee JT, Ha TC, Loong SL, Qian CN. Is nasopharyngeal cancer really a “Cantonese cancer”? Chin J Cancer. 2010;29:517–26. doi: 10.5732/cjc.009.10329. [DOI] [PubMed] [Google Scholar]
- 5.Razak AR, Siu LL, Liu FF, et al. Nasopharyngeal carcinoma: The next challenges. Eur J Cancer. 2010;46:1967–78. doi: 10.1016/j.ejca.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Hong JS, Tian J, Han QF, Ni QY. Quality of life of nasopharyngeal cancer survivors in China. Curr Oncol. 2015;22:e142–47. doi: 10.3747/co.22.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Z, Guo Q, Lu T, et al. Pretreatment serum lactate dehydrogenase level as an independent prognostic factor of nasopharyngeal carcinoma in the intensity-modulated radiation therapy era. Med Sci Monit. 2017;23:437–45. doi: 10.12659/MSM.899531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng WT, Chang AT, Lee SW, Sze HC, Lee AW. Chemotherapy for nasopharyngeal cancer: Neoadjuvant, concomitant, and/or adjuvant. Curr Treat Options Oncol. 2015;16:44. doi: 10.1007/s11864-015-0361-5. [DOI] [PubMed] [Google Scholar]
- 9.Mori Y, Hashizume C, Kobayashi T, et al. Stereotactic radiotherapy using Novalis for skull base metastases developing with cranial nerve symptoms. J Neurooncol. 2010;98:213–19. doi: 10.1007/s11060-010-0179-8. [DOI] [PubMed] [Google Scholar]
- 10.Xiao JP, Xu GZ. Stereotactic radiotherapy – an approach to improve local control of nasopharyngeal carcinoma. Chin J Cancer. 2010;29:123–25. doi: 10.5732/cjc.009.10434. [DOI] [PubMed] [Google Scholar]
- 11.Blanchard P, Lee A, Marguet S, et al. MAC-NPC Collaborative Group. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: An update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;16:645–55. doi: 10.1016/S1470-2045(15)70126-9. [DOI] [PubMed] [Google Scholar]
- 12.Roeder F, Zwicker F, Saleh-Ebrahimi L, et al. Intensity modulated or fractionated stereotactic reirradiation in patients with recurrent nasopharyngeal cancer. Radiat Oncol. 2011;6:22. doi: 10.1186/1748-717X-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guber I, Zografos L, Schalenbourg A. Choroidal metastases in testicular choriocarcinoma, successful treatment with chemo- and radiotherapy: A case report. BMC Urol. 2011;11:24. doi: 10.1186/1471-2490-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee AW, Lau WH, Tung SY, et al. Hong Kong Nasopharyngeal Cancer Study Group. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol. 2005;23:6966–75. doi: 10.1200/JCO.2004.00.7542. [DOI] [PubMed] [Google Scholar]
- 15.Wang R, Wu F, Lu H, et al. Definitive intensity-modulated radiation therapy for nasopharyngeal carcinoma: Long-term outcome of a multicenter prospective study. J Cancer Res Clin Oncol. 2013;139:139–45. doi: 10.1007/s00432-012-1313-0. [DOI] [PubMed] [Google Scholar]
- 16.Qiu S, Lin S, Tham IW, et al. Intensity-modulated radiation therapy in the salvage of locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2012;83:676–83. doi: 10.1016/j.ijrobp.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Liu F, Xiao JP, Xu GZ, et al. Fractionated stereotactic radiotherapy for 136 patients with locally residual nasopharyngeal carcinoma. Radiat Oncol. 2013;8:157. doi: 10.1186/1748-717X-8-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King AD, Zee B, Yuen EH, et al. Nasopharyngeal cancers: which method should be used to measure these irregularly shaped tumors on cross-sectional imaging? Int J Radiat Oncol Biol Phys. 2007;69:148–54. doi: 10.1016/j.ijrobp.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 19.Wu SX, Chua DT, Deng ML, et al. Outcome of fractionated stereotactic radiotherapy for 90 patients with locally persistent and recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2007;69:761–69. doi: 10.1016/j.ijrobp.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 20.Yao Y, Kong Z. [The effect of Ixabepilone on NSCLC cells radio-sensitivity under quiescent or hypoxic condition]. Zhonghua Yi Xue Za Zhi. 2015;95:3057–60. [in Chinese] [PubMed] [Google Scholar]
- 21.Ding M, Zhang E, He R, Wang X. Newly developed strategies for improving sensitivity to radiation by targeting signal pathways in cancer therapy. Cancer Sci. 2013;104:1401–10. doi: 10.1111/cas.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hua W, Zhongmin Y, Keshuai Z. The observation of therapeutic effects of chemotherapy with fotemustine and stereotactic radiotherapy on the treatment of cerebral glioma. Anti-tumor Pharmacy. 2012:3. [Google Scholar]
- 23.Greenspoon JN, Sharieff W, Hirte H, et al. Fractionated stereotactic radiosurgery with concurrent temozolomide chemotherapy for locally recurrent glioblastoma multiforme: A prospective cohort study. Onco Targets Ther. 2014;7:485–90. doi: 10.2147/OTT.S60358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu M, Huang H, Xiong Y, et al. Combined chemotherapy plus endostar with sequential stereotactic radiotherapy as salvage treatment for recurrent esophageal cancer with severe dyspnea: A case report and review of the literature. Oncol Lett. 2014;8:291–94. doi: 10.3892/ol.2014.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo CH, Cheng SN, Lin KT, Jen YM. Successful treatment of infantile fibrosarcoma spinal metastasis by chemotherapy and stereotactic hypofractionated radiotherapy. J Korean Neurosurg Soc. 2013;54:528–31. doi: 10.3340/jkns.2013.54.6.528. [DOI] [PMC free article] [PubMed] [Google Scholar]


