Abstract
Tongue squamous cell carcinoma (TSCC) is the most common oral squamous cell carcinoma. Despite significant advances in combined therapies, the 5-year survival rate of patients with TSCC has not notably improved; this is due to regional recurrences and lymph node metastasis. Grape seed proanthocyanidins (GSPs) are consumed as dietary supplements worldwide and possess anticancer activity against several different types of cancer. However, their effect on TSCC and the underlying mechanisms by which they function remain unclear. In the present study, it was identified that GSPs significantly inhibited the viability and induced the apoptosis of Tca8113 cells in a dose-dependent manner. This was associated with a significantly increased expression of the pro-apoptosis regulator BAX protein and a significantly decreased expression of the anti-apoptosis regulator Bcl-2 protein at 100 µg/ml GSPs. In addition, at non-toxic concentrations GSPs significantly inhibited the secretion of matrix metalloproteinase-2 (MMP-2) and MMP-9 from Tca8113 cells, as well as their migration and invasion. Furthermore, it was demonstrated that GSPs significantly inhibited the phosphorylation of protein kinase B (Akt) and IκB kinase, as well as the translocation of nuclear factor-κB (NF-κB) into the nucleus of Tca8113 cells. Taken together, these results suggest that GSPs inhibit the proliferation, migration and invasion of Tca8113 cells through suppression of the Akt/NF-κB signaling pathway. This indicates that GSPs may be developed as a novel potential chemopreventive agent against TSCC.
Keywords: grape seed proanthocyanidins, tongue squamous cell carcinoma, proliferation, apoptosis, migration, invasion, protein kinase B, nuclear factor-κB
Introduction
Oral cancer is the most prevalent type of cancer affecting the head and neck (1). Worldwide in 2012 there were 300,400 new cases of oral cancer and 145,400 individuals succumbed to the disease (2). It was estimated that in 2016 there would be 48,330 new cases of oral cancer in the USA and 9,570 individuals would succumb to the disease (3). Chen et al (4) projected that in 2015 in China, there would be ~48,000 newly diagnosed cases of oral cancer and ~22,000 individuals would suffer mortality as a result. These estimates proved accurate. The majority of cases of oral cancer (~90%) are comprised of squamous cell carcinomas; the tongue is the most common site for oral squamous cell carcinomas (1). Although notable advancements in treatment have been achieved through combined therapies, including surgery, radiotherapy and neo-adjuvant chemotherapy, the prognosis and 5-year survival rate for individuals with tongue squamous cell carcinoma (TSCC) have not been significantly improved over the past several decades, remaining at ~50% (5). Treatment failure is primarily due to frequent local and regional recurrences, and lymph node metastases (6). Previous epidemiological studies have suggested that there has been an increase in the incidence of TSCC worldwide (7,8). Therefore, the identification of novel effective chemotherapeutic agents is required.
A previous epidemiological study has indicated that the dietary intake of fresh fruits and vegetables reduces the risk of oral cancer (9). This preventive action has been attributed to the polyphenols contained in fruits and vegetables (10,11). A number of polyphenolic compounds, including curcumin (12,13), green tea polyphenol epigallocatechin-3-gallate (14) and resveratrol (15) have previously demonstrated promising chemopreventive efficacy on oral cancer. Proanthocyanidins are the principal polyphenols in grapes and are abundant in grape seeds (16,17). Grape seed proanthocyanidins (GSPs) have been revealed to possess chemopreventive and chemotherapeutic potential against several types of cancer in vitro and in vivo (18–20). Studies have demonstrated that GSPs may inhibit the growth and invasiveness of oral tumor cells (21–25). A recent study by Shrotriya et al (26) revealed that grape seed extract and resveratrol significantly inhibited tumor promotion and progression in a 4-nitroquinoline 1-oxide-induced oral tumorigenesis model in mice. However, the chemopreventive potential and the underlying mechanisms of GSPs against TSCC are not well understood.
In the present study, the effects of GSPs on the proliferation, migration and invasion, and matrix metalloproteinase-2 (MMP-2) and MMP-9 secretion of TSCC Tca8113 cells was investigated. In addition, the underlying mechanisms by which GSPs function was also examined. The present study aimed to provide scientific evidence supporting GSPs as chemopreventive and chemotherapeutic agents against TSCC.
Materials and methods
Materials
GSPs containing ≥95% proanthocyanidins, ≥1.8% proanthocyanidins B2 and ≥60% oligomers were obtained from Tianjin Jianfeng Natural Product R&D Co., Ltd. (Tianjin, China). Dulbecco's modified Eagle's medium (DMEM) was obtained from Gibco, Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Fetal bovine serum (FBS) was purchased from National HyClone Bio-Engineering Co., Ltd. (Lanzhou, China). Sulforhodamine B (SRB) and gelatin from porcine skin were obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit was purchased from MultiSciences Biotech Co., Ltd. (Hangzhou, China). RNAiso Plus and the PrimeScript reverse transcription-polymerase chain reaction (RT-PCR) kit were purchased from Takara Bio, Inc. (Otsu, Japan). Millicell Cell Culture Inserts were obtained from EMD Millipore (Billerica, MA, USA). Matrigel was purchased from BD Biosciences (Franklin Lakes, NJ, USA). Primary antibodies directed against protein kinase B (Akt; cat. no. sc-8312), phosphorylated (p) Akt (cat. no. sc-7985-R) and β-actin (cat. no. sc-130656) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Primary antibodies directed against IκB kinase (IKK; cat. no. ab178870) and pIKK (cat. no. ab55341) were purchased from Abcam (Cambridge, MA, USA). Horseradish peroxidase-conjugated goat anti-rabbit IgG (cat. no. ZB-2301) was purchased from OriGene Technologies, Inc. (Beijing, China). The nuclear factor-κB (NF-κB). Activation, nuclear translocation assay kit (cat. no. SN368) was purchased from Beyotime Institute of Biotechnology (Haimen, China).
Cell culture
Human TSCC Tca8113 cells were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China), and cultured in DMEM supplemented with 10% FBS at 37°C and 5% CO2.
Cell viability assay
The viability of Tca8113 cells was determined using an SRB assay as previously described (27), with certain modifications. Tca8113 cells were plated into 96-well plates at a density of 5×103 cells/well in 100 µl DMEM. Following incubation at 37°C overnight, the cells were treated with GSPs at varying concentrations (0–200 µg/ml) for 24, 48 or 72 h. Subsequently, the cultures were fixed with cold 10% trichloroacetic acid at 4°C for 1 h and washed with water. Next, the plates were air-dried and the fixed cells were stained with 0.4% SRB at room temperature for 10 min and washed repeatedly with 0.1% acetic acid to remove the unbound dye. The bound SRB was dissolved in 1% Tris (pH 10.5). The optical density was measured at 515 nm using a microplate reader.
Annexin V-FITC/PI staining
The amount of apoptotic Tca8113 cells was determined using an Annexin V-FITC/PI apoptosis detection kit according to the manufacturer's protocol. Following treatment with varying concentrations (0–200 µg/ml) of GSPs for 24 h, the Tca8113 cells were collected and washed twice with cold phosphate-buffered saline (PBS). Subsequently, ~5×105 cells were resuspended in binding buffer, and stained with Annexin V-FITC and PI. The stained cells were detected by flow cytometry using CellQuest software (version 3.3; BD Biosciences).
RT-semi-qPCR (RT-sqPCR) analysis
Tca8113 cells were treated with different concentrations (0–100 µg/ml) of GSPs for 24 h. Total RNA was prepared using RNAiso Plus according to the manufacturer's protocol. RT-PCR was performed using the PrimeScript RT-PCR kit according to the manufacturer's protocol. The primers used were as follows: Apoptosis regulator BAX (Bax) forward, 5′-CCC TTT TGC TTC AGG GTT TC-3′ and reverse, 5′-GCC ACT CGG AAA AAG ACC TC-3′; apoptosis regulator Bcl-2 (Bcl-2) forward, 5′-TGT TGG CCT TCT TTG AGT TCG-3′ and reverse, 5′-TCA CTT GTG GCC CAG ATA GG-3′; β-actin forward, 5′-CCA CAC CTT CTA CAA TGA GC-3′ and reverse, 5′-TGA GGT AGT CAG TCA GGT CC-3′. The thermocycling conditions were as follows: 30 cycles of 98°C for 10 sec, 55°C for 30 sec and 72°C for 1 min, followed by incubation at 72°C for 5 min. β-actin was used as the internal control. The PCR products were run on 2% agarose gels and visualized by ethidium bromide staining. Images were captured under ultraviolet light. Densitometric analysis was performed using Adobe Photoshop CS5 (Adobe Systems, Inc., San Jose, CA, USA). The values of target mRNA expression were normalized to that of the β-actin mRNA expression.
Cell migration assay
Cell migration was assayed in Millicell Cell Culture Inserts as previously described (28). Tca8113 cells were trypsinized and suspended in serum-free DMEM. A total of 2×105 cells in 0.4 ml medium were seeded into the cell culture inserts in the upper chamber, with various concentrations of GSPs (0–50 µg/ml). The culture inserts were placed into 24-well plates filled with 0.6 ml DMEM supplemented with 20% FBS as a chemoattractant. Following incubation at 37°C for 24 h, the non-migrated cells on the upper surface of the membrane were wiped off with a cotton swab. Cells that had crossed the inserts were fixed with methanol for 30 min and then stained with 0.1% crystal violet for 10 min at room temperature. Images were captured using light microscopy (magnification, ×100). The number of cells that had migrated was counted using ImageJ software (version 1.51j8; National Institutes of Health, Bethesda, MD, USA).
Cell invasion assay
A cell invasion assay was performed as previously described (29). Briefly, the upper chamber of Millicell Cell Culture Inserts was coated with 50 µl Matrigel diluted 1:8 with PBS. Subsequently, 4×105 Tca8113 cells in 0.4 ml serum-free DMEM with or without GSPs (0–50 µg/ml) were added to the upper chamber. The lower chamber was filled with 0.6 ml DMEM supplemented with 20% FBS as a chemoattractant to induce invasion. Following incubation at 37°C for 24 h, the culture inserts were removed and the non-invasive cells on the upper surface of culture inserts were scraped away with a cotton swab. The cells that invaded through the Matrigel were fixed with methanol for 30 min and then stained with 0.1% crystal violet for 10 min at room temperature. Images were captured by light microscopy (magnification, ×100) and the number of cells was counted using ImageJ software.
Gelatin zymography
The enzymatic activities of MMP-2 and MMP-9 were examined by gelatin zymography as described previously (28). Subconfluent Tca8113 cells were treated with GSPs (0–50 µg/ml) for 24 h in serum-free DMEM. Following treatment, the conditioned medium was collected and centrifuged at 300 x g for 10 min at 4°C to remove cellular debris. The supernatants were subjected to 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10 µg protein/lane) in a gel containing 1% gelatin. Following electrophoresis, the gels were washed with a washing buffer [50 mM Tris-HCl (pH 7.5), 100 mM NaCl and 2.5% Triton X-100] and incubated in a reaction buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl and 10 mM CaCl2] at 37°C for 36 h. Subsequently, the gels were stained with 0.25% Coomassie Blue R250 for 30 min, and destained for 10 min with 25% methanol and 7.5% acetic acid at room temperature. Enzyme activity was visualized as a bright band on a blue background. The band intensities were measured using Adobe Photoshop CS5 software.
Western blot analysis
Tca8113 cells were treated with GSPs (0–100 µg/ml) for 24 h and lysed using radioimmunoprecipitation assay buffer (cat. no. P0013B; Beyotime Institute of Biotechnology). Total protein was collected and then quantified using the Bradford assay. Protein was separated via 10% SDS-PAGE (40 µg protein/lane). Following electrophoresis, the separated proteins were transferred to polyvidyline fluoride membranes. The membranes were subsequently probed with the primary antibodies directed against AKT (1:800), pAKT (1:800), IKK (1:1,000), pIKK (1:1,000) and β-actin (1:400) at 4°C overnight. Following this, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibodies at 4°C for 2 h. The secondary antibodies were diluted 1:8,000 for AKT, 1:8,000 for p-AKT, 1:10,000 for IKK, 1:10,000 for p-IKK and 1:4,000 for β-actin. Immunoreactivity was detected using SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Bands were quantified using Adobe Photoshop CS5.
NF-κB activation and nuclear translocation assay
NF-κB activation and nuclear translocation were examined using a NF-κB. Activation, nuclear translocation assay kit according to the manufacturer's protocol. Briefly, Tca8113 cells were treated with various concentrations of GSPs (0–100 µg/ml) for 24 h. Using the reagents provided in the kit, the cells were washed and fixed, and then incubated with blocking solution for 1 h at room temperature. Cells were incubated with rabbit anti-NF-κB p65 antibodies (provided in the kit) overnight at 4°C. Following washing, the cells were further incubated with Cy3-conjugated secondary antibodies (provided in the kit) for 1 h and 4′,6-diamidino-2-phenylindole (DAPI) for 5 min at room temperature. Images were captured by fluorescence microscopy (magnification, ×200).
Statistical analysis
Data are expressed as the mean ± standard error. Statistical significance between the control and the GSP treated groups was determined by one-way analysis of variance followed by a post hoc Dunnett's test using SPSS software (version 12; SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
GSPs decrease the viability of Tca8113 cells
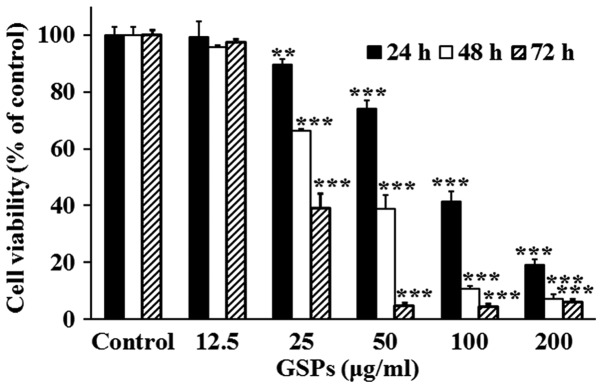
The effect of GSPs on the viability of Tca8113 cells was evaluated using an SRB assay (Fig. 1). The results demonstrated that treatment with 25–200 µg/ml GSPs significantly inhibited the viability of Tca8113 cells compared with the control group in a dose-dependent manner. The half maximal inhibitory concentration values were 86.36, 43.65 and 31.17 µg/ml for 24, 48 and 72 h, respectively.
Figure 1.
GSPs decrease the viability of Tca8113 cells. Following treatment with varying concentrations of GSPs for 24, 48 or 72 h, cell viability was measured using a sulforhodamine B assay. Results are representative of three independent experiments. **P<0.01 and ***P<0.001 vs. the control group. GSP, grape seed proanthocyanidins.
GSPs induce the apoptosis of Tca8113 cells
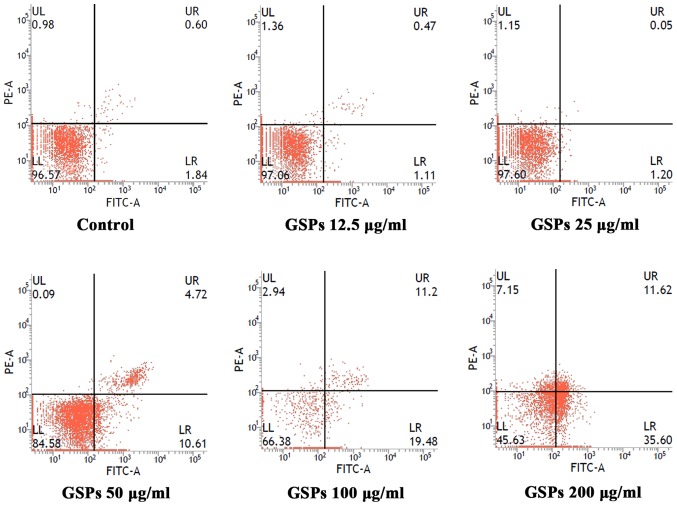
To determine whether the growth inhibitory effect of GSPs was associated with apoptosis, the effect of GSPs on the apoptosis of Tca8113 cells was analyzed by Annexin V-FITC/PI double staining (Fig. 2). Apoptotic cells may be divided into early-stage apoptotic cells and late-stage apoptotic cells. The Annexin V+ and PI− cells in the lower right quadrant of flow cytometry histograms are early-stage apoptotic cells, while the Annexin V+ and PI+ cells in the upper right quadrant of the histograms are late-stage apoptotic cells. Treatment with low concentrations (12 and 25 µg/ml) of GSP had no notable effect on early or late-stage apoptosis. When the GSP concentration was increased, early and late-stage apoptosis were induced. The total apoptotic rates for cells treated with GSPs at concentrations of 0, 12.5, 25, 50, 100 and 200 µg/ml were 2.64, 1.58, 1.25, 15.33, 30.68 and 47.22%, respectively.
Figure 2.
GSPs induce the apoptosis of Tca8113 cells. Tca8113 cells were stained with Annexin V-FITC/propidium iodide and the apoptotic rates were analyzed by flow cytometry following treatment with varying concentrations of GSPs for 24 h. The LR quadrant of the histograms indicates the early apoptotic cells, and the UR quadrant indicates the late apoptotic cells. GSP, grape seed proanthocyanidins; FITC, fluorescein isothiocyanate; UL, upper left; LL, lower left; UR, upper right; LR, lower right; PE, phycoerythrin.
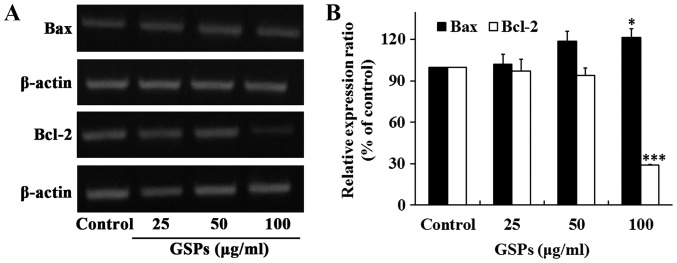
The proteins Bax and Bcl-2 serve crucial roles in the regulation of apoptosis. To determine whether these two proteins were associated with the GSP-induced apoptosis of Tca8113 cells, the expression of Bax and Bcl-2 was examined by RT-sqPCR. The results revealed that the treatment of Tca8113 cells with 100 µg/ml GSPs for 24 h significantly increased the expression of Bax, whereas it significantly decreased the expression of Bcl-2 (Fig. 3). No notable differences were observed when lower concentrations of GSP were administered.
Figure 3.
GSPs significantly increase the expression of Bax and significantly decrease the expression of Bcl-2 in Tca8113 cells. Following the treatment of Tca8113 cells with increasing concentrations of GSPs for 24 h, (A) the expression of Bax and Bcl-2 mRNA were examined by reverse transcription-semi-quantitative polymerase chain reaction analysis. (B) The relative expression of Bax and Bcl-2 were analyzed based on β-actin expression. *P<0.05 and ***P<0.001 vs. the control group. GSP, grape seed proanthocyanidins; Bax, apoptosis regulator BAX; Bcl-2, apoptosis regulator Bcl-2.
GSPs inhibit the migration of Tca8113 cells
The migration of cancer cells and their invasion through the extracellular matrix are important steps during the process of cancer metastasis. The effects of GSPs on the migration and invasion, and MMP-2 and MMP-9 secretion of Tca8113 cells were examined.
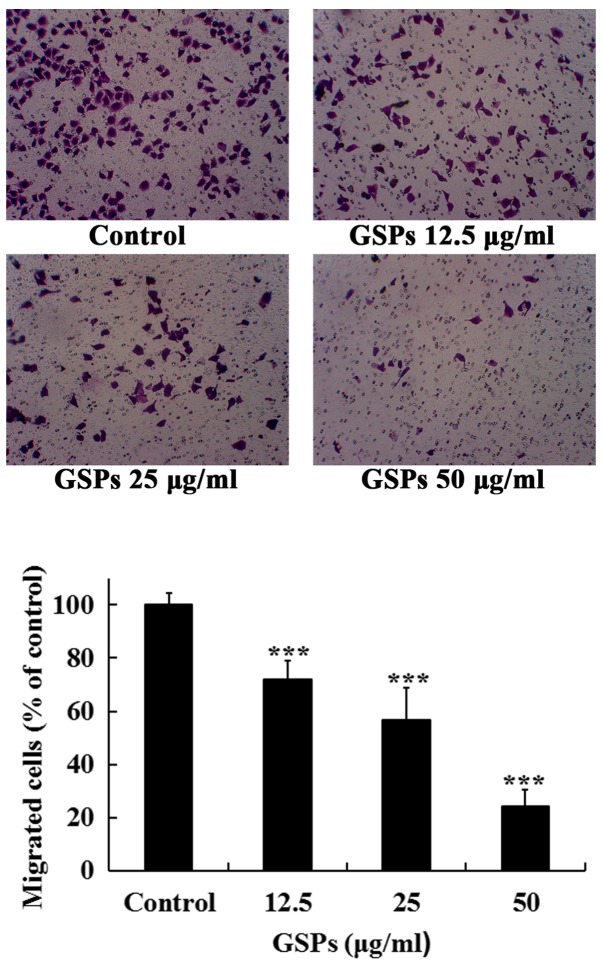
As treatment with 50 µg/ml GSPs for 24 h induced an apoptotic rate of 15.33%, the maximal concentration of 50 µg/ml was selected to detect whether low concentrations of GSPs decreased the migration and invasion, and secretion of MMP-2 and MMP-9 in Tca8113 cells. As revealed in Fig. 4, treatment with GSPs significantly inhibited the serum-induced migration of Tca8113 cells compared with the control group in a dose-dependent manner. The migration inhibition rates of GSPs at 12.5, 25 and 50 µg/ml were 28.0, 43.4 and 75.9%, respectively.
Figure 4.
GSPs inhibit the cell migration of Tca8113 cells. Following incubation with different concentrations of GSPs for 24 h in the upper chamber of the culture inserts, the Tca8113 cells that passed through the membrane were fixed, stained and counted. Images are representative of four fields from three independent experiments (magnification, ×100). ***P<0.001 vs. the control group. GSP, grape seed proanthocyanidins.
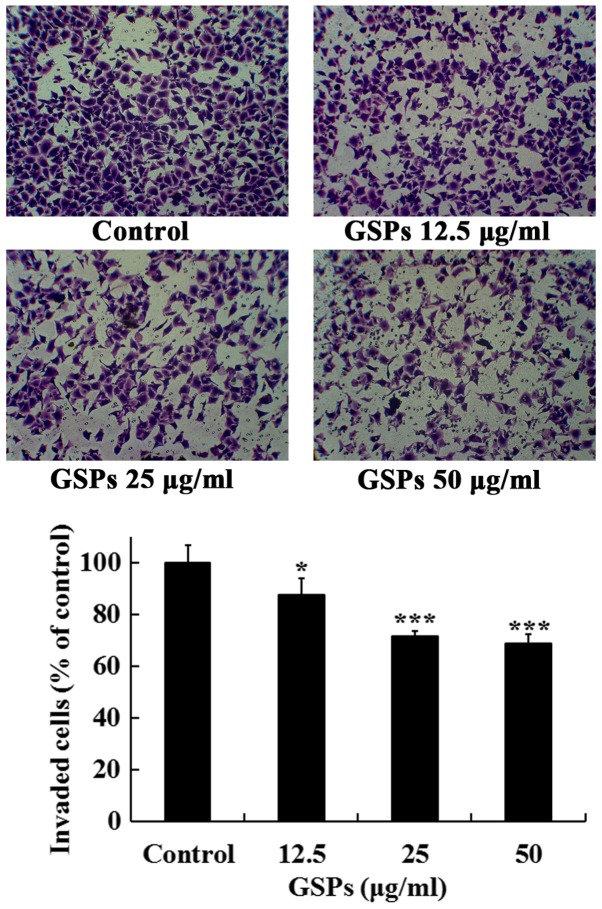
GSPs inhibit the invasion of Tca8113 cells
The effects of GSPs on the invasive capacity of Tca8113 cells were further investigated. As revealed in Fig. 5, treatment of the Tca8113 cells with different concentrations of GSPs (10–50 µg/ml) for 24 h significantly inhibited the serum-induced invasion of Tca8113 cells compared with the control group. The invasion inhibition rates of GSPs at 12.5, 25 and 50 µg/ml were 12.5, 28.4 and 31.3%, respectively.
Figure 5.
GSPs inhibit the invasion of Tca8113 cells. Following treatment with varying concentrations of GSPs for 24 h in the upper chamber of the culture inserts, the Tca8113 cells that invaded through the coated Matrigel were fixed, stained and counted. Images are representative of four fields from three independent experiments (magnification, ×100). *P<0.05 and ***P<0.001 vs. the control group. GSP, grape seed proanthocyanidins.
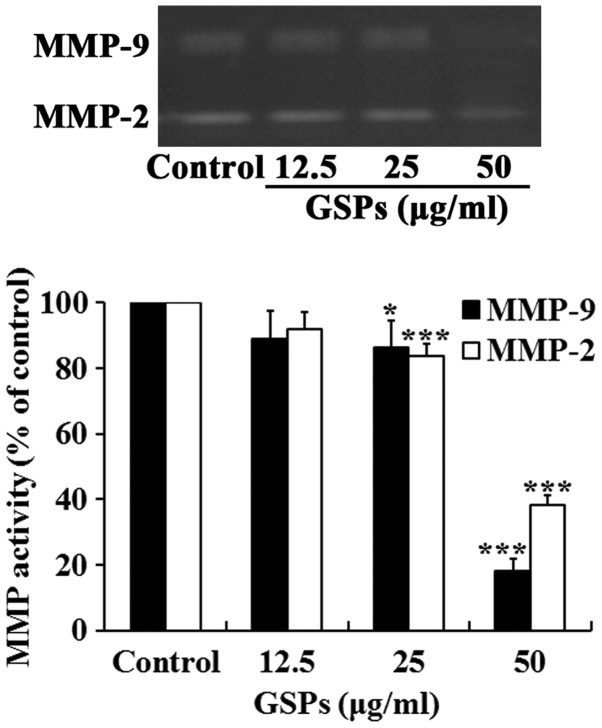
GSPs inhibit the secretion of MMP-2 and MMP-9 by Tca8113 cells
Degradation of the extracellular matrix by MMP-2 and MMP-9 is a key event in the process of tumor metastasis. The effect of GSP on the secretion of MMP-2 and MMP-9 by Tca8113 cells was examined. As demonstrated in Fig. 6, treatment with GSPs reduced the secretion of MMP-2 and MMP-9 in a dose-dependent manner. Treatment with 25 and 50 µg/ml GSPs significantly reduced the secretion of MMP-2 and MMP-9 compared with the control group.
Figure 6.
GSPs inhibit the secretion of MMP-2 and MMP-9 by Tca8113 cells. Subconfluent Tca8113 cells were treated with different concentrations of GSPs in serum-free Dulbecco's modified Eagle's medium for 24 h. The activities of MMP-2 and MMP-9 in conditioned medium were detected by gelatin zymography and quantified by measuring the band intensities. Representative images are presented. *P<0.05 and ***P<0.001 vs. the control group. GSP, grape seed proanthocyanidins; MMP, matrix metalloproteinase.
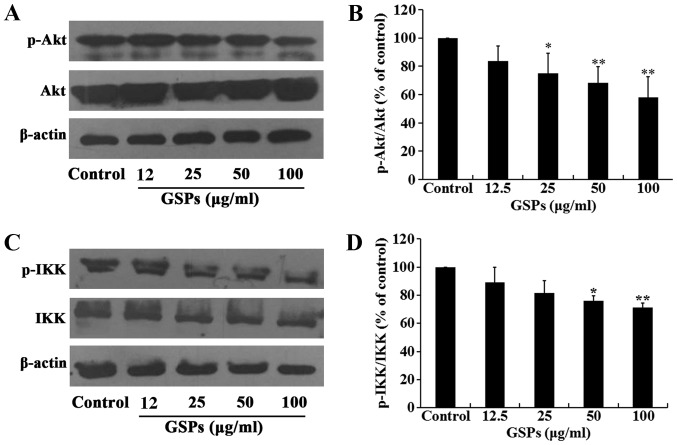
GSPs inhibit the phosphorylation of Akt in Tca8113 cells
The Akt signaling pathway regulates a number of cellular processes, including the proliferation, survival and metastasis of cancer cells. The effect of GSPs on the expression and phosphorylation of Akt in Tca8113 cells was investigated by western blot analysis. The results demonstrated that treatment of Tca8113 cells with 25–100 µg/ml GSPs significantly inhibited the phosphorylation of Akt compared with the control group in a dose-dependent manner, but did not affect the total Akt expression level (Fig. 7A and B).
Figure 7.
GSPs inhibit the phosphorylation of Akt and IKK in Tca8113 cells. Tca8113 cells were treated with various concentrations of GSPs for 24 h. (A) The phosphorylation of Akt was analyzed by western blotting and (B) densitometric analysis demonstrated the relative ratios of pAkt/Akt. (C) The phosphorylation of IKK was analyzed by western blotting and (D) densitometric analysis demonstrated the relative ratios of pIKK/IKK (D). *P<0.05 and **P<0.01 vs. the control group. GSP, grape seed proanthocyanidins; Akt, protein kinase B; IKK, IκB kinase; p, phosphorylated.
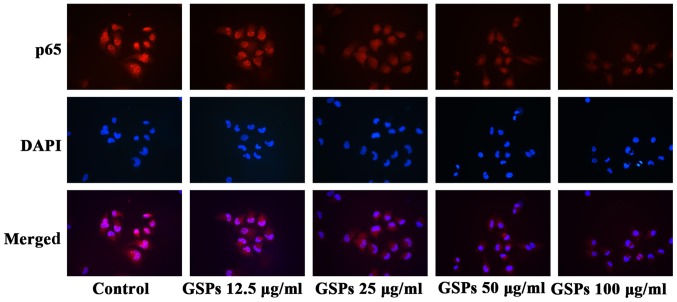
GSPs inhibit NF-κB activation and nuclear translocation in Tca8113 cells
The NF-κB signaling pathway is one of the downstream targets of activated Akt. The effect of GSPs on the expression and phosphorylation of IKK in Tca8113 cells was examined. As demonstrated in Fig. 7C and D, GSPs administered at a concentration of 50 and 100 µg/ml significantly inhibited the phosphorylation of IKK compared with the control group. However, there was no clear effect on the total IKK expression levels. The results of the NF-κB activation and nuclear translocation assay (Fig. 8) further indicated that GSPs inhibited the translocation of NF-κB into the nucleus in a dose-dependent manner in Tca8113 cells, as when higher concentrations of GSP were administered there was less NF-κB p65 visible in the nucleus.
Figure 8.
GSPs inhibit NF-κB activation and translocation into the nucleus in Tca8113 cells. Following treatment with GSPs for 24 h, Tca8113 cells were incubated with rabbit anti-NF-κB p65 antibodies, and subsequently stained with fluorescent secondary antibodies and DAPI. The activation and translocation of NF-κB were examined under a fluorescence microscope (magnification, ×200). GSP, grape seed proanthocyanidins; NF, nuclear factor.
Discussion
TSCC is the most common type of oral cancer (1). Despite significant advances in diagnosis and therapy, the 5-year survival rate of patients with TSCC remains poor due to the frequent local and regional recurrences, and neck lymph node metastases (30). Therefore, innovative and more effective treatment strategies are required for the prevention and treatment of TSCC. Previous studies have revealed that GSPs have anticancer effects on various types of human cancer whilst exhibiting no apparent toxicity in vivo (31,32). In the present study, the antitumor activity of GSPs on TSCC was investigated and their underlying mechanisms of action were explored. The results revealed that the treatment of Tca8113 cells with GSPs decreased viability and induced apoptosis in a dose-dependent manner. GSPs also inhibited the migration, invasion and MMP secretion of Tca8113 cells at non-cytotoxic concentrations. The mechanisms of action of these effects were associated with the inhibition of the Akt/NF-κB signaling pathway.
Bcl-2 and Bax are key regulators of cell growth and apoptosis. Overexpression of Bcl-2 inhibits cell apoptosis, whereas overexpression of Bax promotes cell death (33,34). In the present study, GSPs were revealed to downregulate the expression of Bcl-2 and upregulate the expression of Bax in Tca8113 cells, suggesting that Bcl-2 and Bax serve roles in the GSP-induced apoptosis of Tca8113 cells.
The secretion of MMP for extracellular matrix degradation, allowing the migration and invasion of cancer cells, is key for tumor metastasis (35). The results of the present study revealed that GSPs inhibited the migration, invasion and MMP secretion of Tca8113 cells when administered at low concentrations. These results suggest that GSPs may inhibit the metastatic capabilities of Tca8113 cells.
The phosphoinositide 3-kinase/Akt pathway is a major signaling pathway that controls cell proliferation, apoptosis, angiogenesis and metastasis during cancer progression (36). This pathway is hyperactivated in the majority of types of cancer (37). Akt is a serine/threonine kinase; once activated through phosphorylation by other kinases, Akt proceeds to phosphorylate a series of protein substrates that are important to cell proliferation, migration and invasion, including Bcl-2-associated agonist of cell death, glycogen synthase kinase 3β and IKK (38,39). NF-κB is a heterodimer composed of a p65 and a p50 subunit. Typically, NF-κB is sequestered in the cytoplasm by an inhibitor named IκB. Upon activation by the phosphorylation by a variety of receptor signals, including Akt, IKK phosphorylates IκB, resulting in the ubiquitination and degradation of IκB. Therefore, NF-κB is liberated from the control of IκB (40). NF-κB subsequently migrates into the nucleus and proceeds to activate the expression of a number of genes that are important for cell survival and mobility, such as Bcl-2 and MMP (41). In oral squamous cell carcinoma, NF-κB, IKK-α and Akt have been identified to be constitutively activated, which is associated with their malignant behavior and antiapoptotic activity (42). In the present study, treatment with GSPs inhibited the phosphorylation of Akt and IKK, as well as the translocation of NF-κB into the nucleus in Tca8113 cells.
The results of the present study indicate that GSPs inhibit the viability, migration and invasion of Tca8113 cells through inhibition of the Akt/NF-κB signaling pathway. These results suggest that GSPs may be developed as a potential chemopreventive agent against TSCC.
Acknowledgments
The present study was supported by the National Natural Science Foundation of China (grant nos. 30700142 and 81260330) and the Program of Innovation and Entrepreneurship for Talents of Lanzhou City (grant no. 2015-RC-26).
References
- 1.Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma–an update. CA Cancer J Clin. 2015;65:401–421. doi: 10.3322/caac.21293. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 5.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–316. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Sano D, Myers JN. Metastasis of squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev. 2007;26:645–662. doi: 10.1007/s10555-007-9082-y. [DOI] [PubMed] [Google Scholar]
- 7.Ng JH, Iyer NG, Tan MH, Edgren G. Changing epidemiology of oral squamous cell carcinoma of the tongue: A global study. Head Neck. 2017;39:297–304. doi: 10.1002/hed.24589. [DOI] [PubMed] [Google Scholar]
- 8.Patel SC, Carpenter WR, Tyree S, Couch ME, Weissler M, Hackman T, Hayes DN, Shores C, Chera BS. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J Clin Oncol. 2011;29:1488–1494. doi: 10.1200/JCO.2010.31.7883. [DOI] [PubMed] [Google Scholar]
- 9.Pavia M, Pileggi C, Nobile CG, Angelillo IF. Association between fruit and vegetable consumption and oral cancer: A meta-analysis of observational studies. Am J Clin Nutr. 2006;83:1126–1134. doi: 10.1093/ajcn/83.5.1126. [DOI] [PubMed] [Google Scholar]
- 10.Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: Progress and promise. Antioxid Redox Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 11.Weng CJ, Yen GC. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: Phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat Rev. 2012;38:76–87. doi: 10.1016/j.ctrv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Gonçalves VP, Ortega AA, Guimarães MR, Curylofo FA, Rossa Junior C, Ribeiro DA, Spolidorio LC. Chemopreventive activity of systemically administered curcumin on oral cancer in the 4-nitroquinoline 1-oxide model. J Cell Biochem. 2015;116:787–796. doi: 10.1002/jcb.25035. [DOI] [PubMed] [Google Scholar]
- 13.Zlotogorski A, Dayan A, Dayan D, Chaushu G, Salo T, Vered M. Nutraceuticals as new treatment approaches for oral cancer: I. Curcumin. Oral Oncol. 2013;49:187–191. doi: 10.1016/j.oraloncology.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Iriti M, Varoni EM. Chemopreventive potential of flavonoids in oral squamous cell carcinoma in human studies. Nutrients. 2013;5:2564–2576. doi: 10.3390/nu5072564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zlotogorski A, Dayan A, Dayan D, Chaushu G, Salo T, Vered M. Nutraceuticals as new treatment approaches for oral cancer: II. Green tea extracts and resveratrol. Oral Oncol. 2013;49:502–506. doi: 10.1016/j.oraloncology.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Silva RC, Rigaud J, Cheynier V, Chemina A. Procyanidin dimers and trimers from grape seeds. Phytochemistry. 1991;30:1259–1264. doi: 10.1016/S0031-9422(00)95213-0. [DOI] [Google Scholar]
- 17.Prieur C, Rigaud J, Cheynier V, Moutounet M. Oligomeric and polymeric procyanidins from grape seeds. Phytochemistry. 1994;36:781–789. doi: 10.1016/S0031-9422(00)89817-9. [DOI] [Google Scholar]
- 18.Nandakumar V, Singh T, Katiyar SK. Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett. 2008;269:378–387. doi: 10.1016/j.canlet.2008.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaur M, Agarwal C, Agarwal R. Anticancer and cancer chemopreventive potential of grape seed extract and other grape-based products. J Nutr. 2009;139:1806S–1812S. doi: 10.3945/jn.109.106864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katiyar SK, Athar M. Grape seeds: Ripe for cancer chemoprevention. Cancer Prev Res (Phila) 2013;6:617–621. doi: 10.1158/1940-6207.CAPR-13-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chatelain K, Phippen S, McCabe J, Teeters CA, O'Malley S, Kingsley K. Cranberry and grape seed extracts inhibit the proliferative phenotype of oral squamous cell carcinomas. Evid Based Complement Alternat Med. 2011;2011:467691. doi: 10.1093/ecam/nen047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin YS, Chen SF, Liu CL, Nieh S. The chemoadjuvant potential of grape seed procyanidins on p53-related cell death in oral cancer cells. J Oral Pathol Med. 2012;41:322–331. doi: 10.1111/j.1600-0714.2011.01103.x. [DOI] [PubMed] [Google Scholar]
- 23.Aghbali A, Hosseini SV, Delazar A, Gharavi NK, Shahneh FZ, Orangi M, Bandehagh A, Baradaran B. Induction of apoptosis by grape seed extract (Vitis vinifera) in oral squamous cell carcinoma. Bosn J Basic Med Sci. 2013;13:186–191. doi: 10.17305/bjbms.2013.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Q, Prasad R, Rosenthal E, Katiyar SK. Grape seed proanthocyanidins inhibit the invasiveness of human HNSCC cells by targeting EGFR and reversing the epithelial-to-mesenchymal transition. PLoS One. 2012;7:e31093. doi: 10.1371/journal.pone.0031093. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Yen CY, Hou MF, Yang ZW, Tang JY, Li KT, Huang HW, Huang YH, Lee SY, Fu TF, Hsieh CY, et al. Concentration effects of grape seed extracts in anti-oral cancer cells involving differential apoptosis, oxidative stress, and DNA damage. BMC Complement Altern Med. 2015;15:94. doi: 10.1186/s12906-015-0621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shrotriya S, Tyagi A, Deep G, Orlicky DJ, Wisell J, Wang XJ, Sclafani RA, Agarwal R, Agarwal C. Grape seed extract and resveratrol prevent 4-nitroquinoline 1-oxide induced oral tumorigenesis in mice by modulating AMPK activation and associated biological responses. Mol Carcinog. 2015;54:291–300. doi: 10.1002/mc.22099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 28.Huang S, Yang N, Liu Y, Hu L, Zhao J, Gao J, Li Y, Li C, Zhang X, Huang T. Grape seed proanthocyanidins inhibit angiogenesis via the downregulation of both vascular endothelial growth factor and angiopoietin signaling. Nutr Res. 2012;32:530–536. doi: 10.1016/j.nutres.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, McEwan RN. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987;47:3239–3245. [PubMed] [Google Scholar]
- 30.Dogan E, Cetinayak HO, Sarioglu S, Erdag TK, Ikiz AO. Patterns of cervical lymph node metastases in oral tongue squamous cell carcinoma: Implications for elective and therapeutic neck dissection. J Laryngol Otol. 2014;128:268–273. doi: 10.1017/S0022215114000267. [DOI] [PubMed] [Google Scholar]
- 31.Fraga CG, Oteiza PI. Dietary flavonoids: Role of (−)-epicatechin and related procyanidins in cell signaling. Free Radic Biol Med. 2011;51:813–823. doi: 10.1016/j.freeradbiomed.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Bagchi D, Swaroop A, Preuss HG, Bagchi M. Free radical scavenging, antioxidant and cancer chemoprevention by grape seed proanthocyanidin: An overview. Mutat Res. 2014;768:69–73. doi: 10.1016/j.mrfmmm.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Delbridge AR, Grabow S, Strasser A, Vaux DL. Thirty years of BCL-2: Translating cell death discoveries into novel cancer therapies. Nat Rev Cancer. 2016;16:99–109. doi: 10.1038/nrc.2015.17. [DOI] [PubMed] [Google Scholar]
- 34.Siddiqui WA, Ahad A, Ahsan H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: an update. Arch Toxicol. 2015;89:289–317. doi: 10.1007/s00204-014-1448-7. [DOI] [PubMed] [Google Scholar]
- 35.Wan L, Pantel K, Kang Y. Tumor metastasis: Moving new biological insights into the clinic. Nat Med. 2013;19:1450–1464. doi: 10.1038/nm.3391. [DOI] [PubMed] [Google Scholar]
- 36.Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E. PI3K/AKT signaling pathway and cancer: An updated review. Ann Med. 2014;46:372–383. doi: 10.3109/07853890.2014.912836. [DOI] [PubMed] [Google Scholar]
- 37.Tokunaga E, Oki E, Egashira A, Sadanaga N, Morita M, Kakeji Y, Maehara Y. Deregulation of the Akt pathway in human cancer. Curr Cancer Drug Targets. 2008;8:27–36. doi: 10.2174/156800908783497140. [DOI] [PubMed] [Google Scholar]
- 38.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 40.Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Arch. 2005;446:475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 41.Oeckinghaus A, Hayden MS, Ghosh S, Matias-Guiu X. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 42.Nakayama H, Ikebe T, Beppu M, Shirasuna K. High expression levels of nuclear factor kappaB, IkappaB kinase alpha and Akt kinase in squamous cell carcinoma of the oral cavity. Cancer. 2001;92:3037–3044. doi: 10.1002/1097-0142(20011215)92:12<3037::AID-CNCR10171>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]