Abstract
Background
Our medical center laboratory recently adapted its 24/7, two-hourly testing program to use an ARCHITECT-Multispot-viral load (AR-MS-VL) algorithm in place of a previous rapid test-immunofluorescence (RT-IF) algorithm.
Objectives
We evaluated screening test performance, acute case detection, turnaround time and ability to resolve HIV status under the new algorithm.
Study Design
We considered consecutive HIV tests from January-November 2015. AR-MS-VL results at Zuckerberg San Francisco General Hospital and Trauma Center (ZSFG) were compared with RT-IF results at ZSFG and also with AR-MS-VL results in the recently completed CDC Screening Targeted Populations to Interrupt On-going Chains of HIV Transmission with Enhanced Partner Notification (STOP) Study for targeted testing of MSM at publicly funded testing sites in San Francisco.
Results
Among 21,985 HIV tests performed at ZSFG, 16,467 were tested by RT-IF and 5,518 by AR-MS-VL. There were 321 HIV infections detected, of which 274 (84%) were known HIV+ cases, and 47 were newly identified HIV infections (prevalence 0.22%). Under the AR-MS-VL algorithm, turnaround times for screening and full algorithm results were 3 and 21 hours; status-unresolved cases were reduced (from 47% to 22%) compared with the RT-IF algorithm. The positive predictive value (PPV) of a new-positive AR screening test was low (0.44) at ZSFG, where no acute infections were detected. At STOP Study sites where acute infection was more common, the AR PPV was higher (0.93). All 24 false-positive AR screening tests at ZSFG had a signal/cutoff (S/CO) ratio of <15 and all 88 true-positive tests had S/CO ratio >15. Of 62 acute infections in the STOP Study, 23 (37%) had an S/CO<15.
Discussion
An AR-MS-VL algorithm is feasible and can return rapid results in a large medical center. In this setting, reactive 4th generation assay tests that are negative for HIV antibodies are typically false-positive with low S/CO ratios.
Keywords: HIV, diagnostics, assay evaluation, 4th-generation, ARCHITECT
Background
A decade since The Centers for Disease Control (CDC) issued recommendations to expand HIV testing as part of routine medical care, US hospitals and medical centers continue to develop procedures to scale-up HIV testing [1,2]. Recent policy changes and laboratory advances have produced a new HIV testing algorithm [3]. The algorithm was designed to improve screening for acute HIV infections, minimize turnaround time, and reduce the number of tested patients with unresolved infection status. However, little is known about how the algorithm might affect care delivery in a US major medical center with high demand for HIV testing.
HIV testing in medical settings involves challenges and considerations that are distinct from testing in other settings. There are unique opportunities to test patients who visit the emergency department or urgent care but might not otherwise seek testing [4-7], and hospital-wide routine HIV screening has been shown to be a valuable tool for identifying new infections and preventing missed opportunities to diagnose individuals of unknown status [8,9]. These testing opportunities can be driven by symptoms, which may indicate acute HIV infection [10] or by the patient care situation (e.g., pregnancy) [11,12]. In caring for patients who are sick, ready for discharge, or in labor, there can be a premium on rapid turnaround time. Additionally, clinicians use HIV testing to document HIV status in medical settings, even when patients report they have already been diagnosed with HIV infection. Because hospital labs combine all these disparate modes of clinical testing, it can be particularly difficult to study clinical test performance in the medical center laboratory.
The Zuckerberg San Francisco General Hospital and Trauma Center (ZSFG) is a major provider of emergency/trauma, inpatient, and primary and specialty outpatient care. It is the largest provider of ambulatory/drop-in care, indigent care, and primary HIV care in the city. In the past decade, ZSFG has mounted an aggressive expansion of HIV testing; integration of expanded HIV testing with care has been facilitated by a central laboratory-based, 24/7, 2-hourly batched testing model innovated at ZSFG allowing high throughput with the expectation of rapid (2-3 hour) turnaround [4]. A key component of this expanded HIV testing program is involvement of a dedicated, multidisciplinary clinical team (the Positive Health Access to Services and Treatment, or PHAST team) who assist clinicians with interpretation of results, track outcomes of confirmatory testing, assist with disclosure of positive results [4], facilitate initiation of immediate antiretroviral treatment [13], and provide linkage to HIV primary care.
Based on new testing recommendations from the CDC [3], the central laboratory at ZSFG switched their standard HIV testing algorithm from their existing rapid testing algorithm (Uni-Gold Recombigen® HIV Rapid Test (Trinity Biotech, Co. Wicklow, Ireland(RT)) for screening followed by immunofluorescence (IF) confirmation) to a new algorithm with an HIV antigen/antibody combo screening test (ARCHITECT® HIV Ag/AB Combo, Abbot Laboratories, Abbot Park, IL (AR)) and a HIV-1/2 Ab differentiation assay for confirmation (Multispot HIV-1/HIV-2 Rapid Test, Bio-Rad Laboratories, Redmond, WA (MS)); a nucleic acid or RNA test is recommended as needed for resolution of discrepant results (Abbott RealTime HIV-1 assay, Abbott Molecular Inc., Des Plaines, IL (VL)). (Note: The viral load test available at ZSFG (Abbott RealTime HIV-1 assay) is not validated for HIV diagnosis, and results are appended with a comment explaining this. Clinicians may use the viral load results, along with the AR and MS results, as a supplemental test to guide their patient care, and may continue to follow-up with additional diagnostic testing for confirmation.) The switch from “RT-IF” to “AR-MS-VL” algorithm took place in September, 2015, and the frequency of confirmatory testing also increased from three times per week (IF) to six times per week (MS) at this time. Screening tests were performed on batched specimens every two hours under both algorithms, with STAT testing available only for specimens from labor and delivery. A similar AR-MS-VL algorithm was used and results reported by the Screening Targeted Populations to Interrupt On-Going Chains of HIV Transmission with Enhanced Partner Notification (STOP) Study at HIV testing venues in New York City, New York, North Carolina, and San Francisco, California [14, 15].
For this study, we capitalized on the unique infrastructure at ZSFG for high-throughput clinical testing (at the Clinical Laboratory) and for timely and comprehensive follow-up of individual cases (by the PHAST team) to prospectively evaluate the impact of the new HIV testing algorithm on delivery of HIV testing services in a large, well-resourced, US medical center laboratory.
Methods
Objectives
The aim of this study was to determine the real-world performance of the new HIV-testing algorithm (AR-MS-VL) in a medical setting. Components of the algorithm evaluated were detection of acute infections, turn-around time from sample collection to preliminary and final results, ability of the algorithm to resolve HIV status, and performance of the screening assay. The study was designed to evaluate algorithm performance using clinical samples in the laboratory. The laboratory at ZSFG receives and tests many specimens from individuals who are already known to be HIV-positive. Therefore, in order to understand the impact of the algorithm on clinical care and testing program performance, we specifically investigated its testing performance among patients of unknown HIV status.
Study Design
This clinical laboratory performance evaluation used cross-sectional data from electronic medical records, laboratory administrative data, and PHAST program data from ZSFG Hospital and associated medical clinics whose HIV testing was performed in the ZSFG central laboratory. We reviewed all consecutive HIV tests at ZSFG from January to November 2015, before and after rollout of the new algorithm. Acute infection in the prior algorithm was defined as RT-positive with IF-negative or IF-indeterminate results for individuals with confirmed HIV infection on subsequent tests. In the new algorithm, acute infection was defined as an AR-reactive with MS-negative or MS-indeterminate results for individuals with a detectable viral load. Prior HIV testing status was defined based on test results in the electronic medical record (EMR) data and detailed chart review for every HIV-positive test result. Prior status was assigned as unknown for all first tests with negative results. For analyses grouping testers by prior status, repeat tests by patients with any positive or indeterminate test result were considered as belonging to previously HIV-positive testers. Final HIV infection status was defined by the algorithm result and any follow-up testing. For patients who screened RT-positive with the prior RT-IF algorithm, algorithm resolution was defined as either an IF-positive (true positive) or IF-negative result (false positive). IF-indeterminate results were sent out to the San Francisco Department of Public Health (SFDPH) for additional testing not yet available at ZSFG (ARCHITECT® HIV Ag/AB Combo, Multispot HIV-1/HIV-2 Rapid Test, and VL validated for diagnosis), and were considered unresolved by the algorithm, even if final infection status was subsequently determined. For patients who screened AR-reactive with the new AR-MS-VL algorithm, algorithm resolution was defined as either an MS-positive result (true positive), or an MS-positive with a VL-detected (acute HIV) or VL-undetected result (false-positive). Time to result was measured from the point at which the laboratory received the specimen. AR-reactive tests were repeated for confirmation before results were reported, and the repeat testing was included in turn-around-time calculations. Primary analyses compared the ZSFG results obtained using the new testing algorithm (AR-MS-VL: September – November, 2015) with 1) the ZSFG results obtained using the prior algorithm (RT-IF: January – August, 2015) and 2) results obtained with the new algorithm (AR-MS-VL) from the CDC STOP Study at San Francisco Department of Public Health (SFDPH) targeted testing sites for men who have sex with men (MSM) (2010-2013) [14, 15]. For the purpose of this second comparison, we excluded previous positive individuals from the ZSFG data in order to be consistent with the STOP Study population, where individuals known to be HIV-positive were excluded from the study. We also investigated the possible use of a suggested signal-to-cutoff (S/CO) ratio of 15 in distinguishing between likely true-positive and likely false-positive results [16] on the screening ARCHITECT assay, in both ZSFG and STOP Study populations.
In order to explore the utilization of HIV testing in evaluating patients for acute HIV infection on the hospital campus, we collected additional laboratory information on patients tested for group A streptococcus, influenza, or infectious mononucleosis testing as indicators of having viral syndrome-like illness. The algorithm results and HIV status were determined separately for those who were tested for one of these viral-syndrome-like illnesses during the study period.
The study protocol for the clinical laboratory performance evaluation was approved by the UCSF Committee on Human Subjects Research (UCSF CHR 12-10141). HIV test result data from the STOP Study were not personally identifiable and therefore did not constitute research with human subjects.
Results
21,985 HIV tests were performed at ZSFG during the study period, 16,467 using the RT-IF antibody algorithm and 5,518 using the AR-MS-VL algorithm (Table 1). A total of 321 HIV infections were identified by various clinics and hospital wards, for an overall HIV prevalence of 1.5%. However, 274 (85.4%) of the HIV infections identified during this study period were among individuals who had a previous HIV-positive test result. Considering only the 21,690 testers who were presumed HIV-negative or unknown status at testing, there were 47 newly confirmed HIV infections (0.22% prevalence). No antibody-negative or antibody-indeterminate acute HIV infections were identified by either algorithm during the study period.
Table 1. HIV-positive final results tested at Zuckerberg San Francisco General Hospital (ZSFG), January -November 2015.
| Total (January – Nov, 2015) | RT-IF1 (Jan – Aug, 2015) | AR-MS-VL2 (Sep – Nov, 2015) | |||||
|---|---|---|---|---|---|---|---|
| All Testers (N) | Previous HIV+ Testers (N) | HIV- /Unknown Testers (N) | New HIV Infection Prevalence3 (95%CI) | Previous HIV+ Testers (N) | HIV- /Unknown Testers (N) | New HIV Infection Prevalence3 (95%CI) | |
| Total tests done | 21,985 | 197 | 16,260 | 77 | 5,430 | ||
| HIV+ Cases Identified | 321 | 197 | 36 | 0.22% (0.16-0.30) | 77 | 11 | 0.20% (0.11-0.36) |
| Outpatient | 15 | 4 | 3 | 0.11% (0.04-0.32) | 5 | 3 | 0.30% (0.10-0.88) |
| OB | 6 | 3 | 3 | 0.16% (0.05-0.47) | 0 | 0 | 0% (0-0) |
| Jail | 12 | 4 | 5 | 0.28% (0.12-0.66) | 1 | 2 | 0.30% (0.08-1.08) |
| Inpatient | 49 | 36 | 7 | 0.51% (0.25-1.05) | 6 | 0 | 0% (0-0) |
| ED | 34 | 25 | 6 | 0.45% (0.21-0.98) | 2 | 1 | 0.23% (0.04-1.31) |
| Drop In | 69 | 39 | 12 | 0.17% (0.10-0.30) | 13 | 5 | 0.23% (0.10-0.54) |
| HIV clinic | 163 | 86 | 0 | 0% (0-0) | 77 | 0 | 0% (0-0) |
Uni-Gold HIV Rapid Test (RT) for screening followed by immunofluorescence (IF) confirmation
ARCHITECT® HIV Ag/AB Combo (AR) for screening, followed by Multispot HIV-1/HIV-2 Rapid Test (MS) confirmation, and Abbott RealTime HIV-1 assay (VL) as needed for resolution of discrepant results
Prevalence is measured among those with HIV-negative or unknown status at the time of testing
Turn-around time using different algorithms
Using the new algorithm, median time to an HIV-negative and HIV-positive screening result, respectively, increased from 1.88 (IQR: 1.35, 2.45) and 1.98 hours (IQR: 1.37, 2.65) using the Uni-Gold RT to 2.15 (IQR: 1.62, 2.78) and 3.4 hours (IQR: 2.86, 3.98) with the ARCHITECT. Under the new algorithm MS testing was considered simpler, faster, and less labor intensive than the previous IF which required microscope set-up, processing, and extra laboratory space, and the ZSFG lab increased the frequency of confirmatory testing from three days per week to six days per week. As a result, the median time from initial test to a positive confirmatory result decreased from 41 hours (IQR: 20, 57) for IF to 20 hours (IQR: 18, 23) for MS (Table 2). In cases where further testing was required after the confirmatory result, median time from initial screening test to final resolution was 323 hours (IQR: 264, 405) after indeterminate IF, and 65 hours (IQR: 49, 115) to a VL test result after a discrepant AR-reactive/MS-negative result. Overall, for all patients with a reactive screening test the median time to completion of the new algorithm (AR-MS-VL) was substantially shorter than time to completion of the prior algorithm (RT-IF) (21 vs. 44 hours).
Table 2. Comparison of algorithm characteristics for previous HIV-positive testers and previous HIV-negative/unknown testers at Zuckerberg San Francisco General Hospital (ZSFG), January -November 2015.
| RT-IF1 (Jan – Aug, 2015) | AR-MS-VL2 (Sep – Nov, 2015) | |||
|---|---|---|---|---|
| All testers | HIV- /Unknown Testers only | All testers | HIV- /Unknown Testers only | |
| Total # samples tested, N | 16,467 | 16,260 | 5,518 | 5,430 |
| Screened HIV+, N (%) | 292 (1.77) | 85 (0.52) | 115 (2.08) | 27 (0.50) |
| Confirmatory/Supplemental Ab Test, N (%) | ||||
| HIV+ | 217 (74.32) | 35 (41.18) | 88 (76.52) | 11 (40.74) |
| HIV- | 10 (3.42) | 10 (11.76) | 23 (20.00) | 16 (59.26) |
| Indeterminate | 46 (15.75) | 37 (43.53) | 1 (0.87) | 0 (0) |
| Unknown | 19 (6.51) | 3 (3.53) | 3 (2.61) | 0 (0) |
| Final Result,3 N (%) | ||||
| HIV+ | 233 (79.79) | 36 (42.35) | 88 (76.52) | 11 (40.74) |
| HIV- | 56 (27.05) | 46 (54.12) | 25 (21.74) | 14 (51.85) |
| Unknown | 3 (1.03) | 3 (3.53) | 2 (1.74) | 2 (7.41) |
| Status Unresolved by Algorithm,4 N (%) | 65 (22.26) | 40 (47.06) | 11 (9.57) | 6 (22.22)5 |
| Screening PPV, % (95% CI) | 0.81 (0.75-0.85) | 0.44 (0.33-0.55) | 0.78 (0.69-0.85) | 0.44 (0.25-0.65) |
| Screening Specificity, % (95% CI) | 99.65% (99.55-99.74) | 99.72% (99.62-99.79) | 99.54% (99.31-99.69) | 99.74% (99.55-99.85) |
| Hours from first test to result, Median (IQR) | ||||
| Screening test result | 1.98 (1.37,2.65) | 1.9 (1.37,2.6) | 3.43 (2.86,3.98) | 3.4 (2.89,3.99) |
| Confirmatory test result | 40.47 (20.16,56.96) | 41.47 (20.03, 57.60) | 20.30 (17.61, 22.83) | 18.4 (16, 23.15) |
| Final result | 44.47 (21.65, 69.52) | 70.56 (38.35, 300.35) | 20.77 (18.33, 24.11) | 25.67 (18.37, 110.62) |
Uni-Gold HIV Rapid Test (RT) for screening followed by immunofluorescence (IF) confirmation
ARCHITECT® HIV Ag/AB Combo (AR) for screening, followed by Multispot HIV-1/HIV-2 Rapid Test (MS) confirmation, and Abbott RealTime HIV-1assay (VL) as needed for resolution of discrepant results
Final HIV infection status was defined by the algorithm result and any follow-up testing.
For patients who screened RT+, algorithm resolution was defined as either an IF-positive or IF-negative result; IF-indeterminate results required send-out testing were considered unresolved by the algorithm. For patients who screened AR-reactive, algorithm resolution was defined as either an MS-positive result, or an MS-negative with a VL-detected or VL-undetected result; discrepant AR-reactive/MS-negative results without a VL result were considered unresolved by the algorithm.
Additional VL testing confirmed 4/6 to be uninfected
Resolution of HIV status among patients with negative or unknown HIV status at testing
Of the 21,690 patients with negative or unknown status, there were 85 reactive Uni-Gold RT results and 27 reactive ARCHITECT screening results during the study period (Table 2). Of the 85 RT reactive tests, 35 (41%) were IF-reactive, 10 (12%) were IF-nonreactive, and 40 (47%) were IF-indeterminate, requiring additional send-out testing for resolution. By contrast, there were no indeterminate MS confirmatory results for any of the 27 positive screening tests with the new algorithm (Figure 1a). Sixteen (59.3%) patients had a negative MS confirmatory result and, therefore, required VL testing for confirmation under the new algorithm. However, status remained unresolved for six (22%) at completion of the testing encounter because a sample for viral load testing had not been obtained. Four of those patients received follow-up at a later date and were confirmed to be HIV-negative. Two patients did not receive follow-up and their final status was not resolved. Overall, the proportion of cases unresolved at the completion of the testing algorithm was reduced from 47% (40/85) to 22% (6/27) after the implementation of AR-MS-VL testing (Table 2).
Figure 1. Panel A. AR-MS-VL1 Flow at Zuckerberg San Francisco General Hospital (ZSFG), Sept – Nov, 2015.
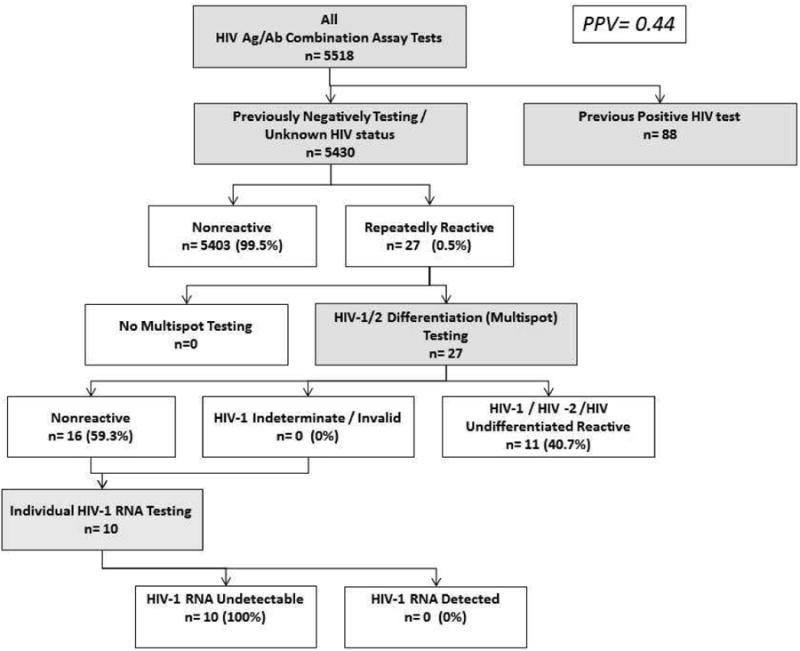

1ARCHITECT® HIV Ag/AB Combo (AR) for screening, followed by Multispot HIV-1/HIV-2 Rapid Test (MS) confirmation, and Abbott RealTime HIV-1 assay (VL) as needed for resolution of discrepant results
Panel B. AR-MS-VL1 Flow at STOP Study2, 2010-2013
1ARCHITECT® HIV Ag/AB Combo (AR) for screening, followed by Multispot HIV-1/HIV-2 Rapid Test (MS) confirmation, and Abbott RealTime HIV-1 assay (VL) as needed for resolution of discrepant results
2CDC Screening Targeted Populations to Interrupt On-going Chains of HIV Transmission with Enhanced Partner Notification (STOP) Study for targeted testing of MSM; data from publicly funded testing sites in San Francisco
Diagnostic performance of screening assays at ZSFG
Interpreting a new reactive screening result is a major challenge in medical settings. Under the observational study design we were able to assess the positive predictive value and specificity of initial screening test results under RT-IF and AR-MS-VL algorithms, considering previously HIV-negative or unknown status testers as the clinically relevant population. Under the older RT-IF algorithm, 36 out of 85 RT-reactive screening results were confirmed as HIV infected, for a positive predictive value (PPV) of 0.44 (95%CI: 0.33,0.55) (Table 2). Under the new algorithm, 11 out of 27 AR-reactive screening results confirmed as HIV infected for a similar PPV of 0.44 (95%CI: 0.25, 0.65). The proportion of false-positive screening results was similar for RT and AR (54.12% vs. 51.85%). The specificity of both assays was high (99.7%), suggesting the low prevalence of disease rather than assay failure as the main reason for the low PPV (Table 2).
(Note: The apparent positive predictive values of a reactive RT and AR result appeared better when patients who had a previous HIV+ test result were not excluded: RT PPV was 0.81 (95%CI: 0.75, 0.85) and AR PPV was 0.78 (95%CI: 0.69, 0.85). Thus, from the laboratory perspective rather than the testing program perspective, the proportion of true positive results would seem much better.)
Comparison of the AR-MS-VL algorithm at ZSFG and STOP Study sites
The positive predictive value of AR-positive screening results was substantially better in the local risk-based STOP Study population of unknown-status testers compared to the ZSFG population of unknown-status testers (PPV=0.93 vs. 0.44). Sixty three (13.8%) of 455 initially reactive results in the STOP Study were antibody-negative (AR-reactive/MS-negative/VL-detected) or antibody-indeterminate (AR-reactive/MS-indeterminate/VL-detected) acute infections, and the PPV for AR-reactive/MS-negative results was 0.62. No acute infections were identified at ZSFG during the study period. Specificity in the STOP Study was 99.86%, compared to 99.7% at ZSFG.
ARCHITECT S/CO values
In the ZSFG population, the S/CO value of 15 seemed clearly to separate false from true-positive results: PPV of a reactive ARCHITECT with S/CO result<15 for HIV infection was 0/24 (0.00; 95%CI: 0.00, 0.17), while for results with S/CO>15 PPV was 88/88 (1.00; 95%CI: 0.95,1.00) (Figure 2). In the high risk STOP Study population, however, there were many acute HIV infections in which lower values could still connote true infection. Of 62 acute infections in the STOP Study, 23 (37%) had an S/CO<15. Of 357 non-acute infections, 20 (5.6%) had an S/CO<15. In this higher-risk population, the PPV of an S/CO<15 was 0.60 (0.47,0.71), as compared to the PPV for an S/CO value >15 (0.99; 95% CI: 0.98, 1.00).
Figure 2. Distribution of signal cut-off ratios for specimens reactive on ARCHITECT screening assay at Zuckerberg San Francisco General (ZSFG) (Sept-Nov, 2015) and at the STOP Study1 (2010-2013).
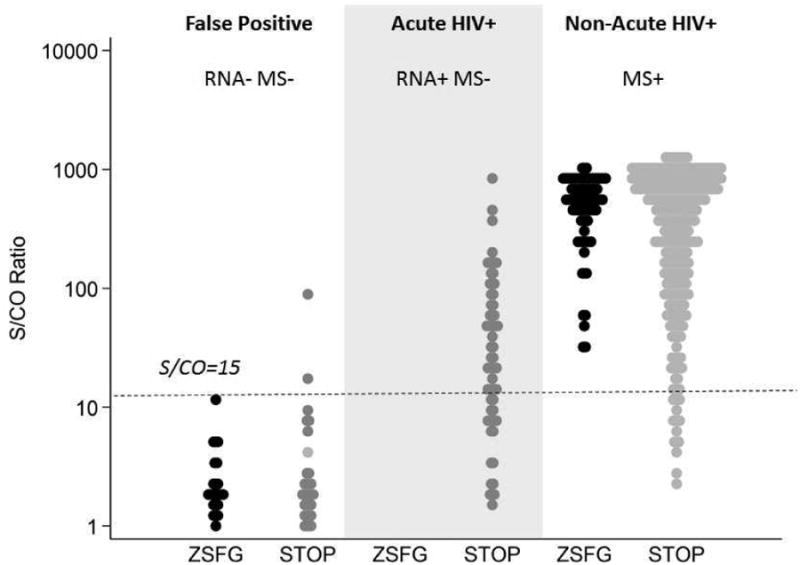
Distribution of signal cut-off ratio values are shown for specimens classified as false-positive or true-positive HIV infections, based on completion of the full AR-MS-VL testing algorithm. The dotted horizontal line illustrates the S/CO ratio of 15 previously suggested [16] as an upper bound on the distribution of false-positive, AR reactive results. There were no acute infections(Multispot indeterminate or Multispot nonreactive specimens that were VL positive) in the ZSFG testing population.
1CDC Screening Targeted Populations to Interrupt On-going Chains of HIV Transmission with Enhanced Partner Notification (STOP) Study for targeted testing of MSM; data from publicly funded testing sites in San Francisco
HIV testing of patients with symptoms of acute viral illness
We were surprised by the low proportion of acute HIV infections in our testing population and examined HIV testing and test outcomes among 2762 individuals tested for infectious mononucleosis, group A streptococcus, or influenza at ZSFG during the study period. Of those patients, only 277 (10%) had an HIV screening test (AR or RT) done within one day, among whom one (0.4%) was a new case and seven (2.5%) were previous positive cases. However, among those not tested at the time of this viral illness and who had an unknown HIV status, 201 had an HIV test done at a later date during the study period: one (0.5%) was a new case and one was unresolved and had a previously negative HIV test. Of the 277 patients who had an HIV test on the same day they were tested for acute viral illness, 136 (4.9%) included either AR or VL testing capable of detecting acute infection.
Discussion
In this implementation study, we found that it was feasible to incorporate a recommended algorithm, including 4th generation HIV Ag/Ab combo screening and supplemental HIV 1/2 antibody differentiation, into routine practice in a high throughput hospital laboratory in which clinicians expected 2-3 hour turnaround for initial screening results. However, switching from a manually performed rapid test to an automated 4th generation assay did not reduce the rate of false-positive screening test results or increase the detection of acute HIV. Indeed, the positive predictive value (PPV) of a newly positive 4th generation screening test result was unexpectedly low compared with data from the CDC STOP Study also conducted in San Francisco (PPV 44% vs. 93%). The higher prevalence of acute HIV in the STOP Study (13.8% vs. 0) as well as the similarity in assay specificity between the two populations suggests that the differences in positive predictive values were related to the low prevalence of undiagnosed disease in the ZSFG medical setting rather than test characteristics.
Overall, the new algorithm functioned relatively well with regard to the goals of reducing overall turnaround time, and resolving more cases without the need for a reference lab. Using the combination of HIV 1/2 differentiation antibody testing and viral load, the need for send-out testing to resolve the final infection status was reduced dramatically compared with the previous immunofluorescence-based approach to confirmation that produced many indeterminate results and required follow-up testing at the SFDPH, which often had prolonged turn-around-times of several weeks . However, the ability to resolve status by the new algorithm was hindered in this initial experience by the inability in some clinical circumstances to obtain a sample for viral load testing, which required a separate blood draw. The turnaround for viral load confirmation also remained about three days, owing to the need for batch viral load testing. These observations point out the potentially important role of rapid viral load diagnostic assays in improving HIV testing in medical settings in the developed world and the shortcomings of current HIV RNA assays in serving this need. They also highlight the benefit of being able to use the same type of specimen for viral load testing required for potential confirmation of the HIV screening test. This could enable reflex testing on the original specimen without the complication of obtaining a second sample from patients who do not stay or fail to return.
One of the key issues raised by the new algorithm is the ability to diagnose acute HIV infections, which one might expect to be especially important in medical settings. We found that the relative prevalence of acute infection was much lower in this hospital study than it was in the STOP Study, which used risk-based testing. In low HIV prevalence settings, a reactive 4th generation HIV Ag/Ab combo screening assay with a negative supplemental antibody result is likely a false-positive screening result, but clinicians must still be aware of the possibility of acute HIV infection and perform HIV RNA testing to resolve the discordant result. We note also that only a small number of patients with possible acute retroviral symptoms (4.9%) were tested for acute HIV, and among those screened afterward, there was an appreciable rate of new HIV diagnosis (consistent with seroconversion).
Together, the issues of low screening assay predictive value, low acute infection prevalence and prolonged time-to-resolution of status are very challenging in an era when there is increasing emphasis on immediate initiation of treatment [13, 17, 18]—particularly in possible cases of acute HIV infection [19-21]. We found that nearly all (100% at ZSFG, 99% in STOP) specimens that had S/CO>15 were truly HIV infected. However, S/CO ratios<15 were more difficult to interpret, with none of the ZSFG specimens but 60% of STOP Study specimens in this range being truly HIV infected. This difference was attributable to the large number of acute infections in the STOP Study population, which had S/CO ratios in the low range. Thus, S/CO ratios will not distinguish acute from false-positive specimens. There were also numerous non-acute (MS-positive) infections with S/CO rations below 15 in the stop study. Several recent analyses [22-24] have suggested that ARCHITECT S/CO ratios in this range likely represent recent HIV infection, such as would be expected in a high-risk population like the STOP study. This emphasizes the need for rapid nucleic acid-based diagnostics to permit rapid decision making for low S/CO values that may be acute or recent infections.
This study reflected early experience with the new algorithm and was limited by the short period of observation under the new algorithm (3 months) and by the inability to detect acute infections during the study period. This highlights an additional limitation in that because we did not test all samples for HIV RNA, we were unable to assess the sensitivity of the new algorithm for acute HIV infections in medical settings (as was done in the STOP Study). One strength of this study was the ability of the ZSFG PHAST team to do extensive case review for every patient that screened HIV-positive during the study period. This allowed us to differentiate algorithm performance for patients who were newly diagnosed vs. performance for the entire hospital testing population, of which the majority of HIV-positive results came from patients who were already known to be infected.
In summary, we have demonstrated that the new HIV algorithm can be implemented in a high-throughput medical center laboratory setting without impeding rapid return of results or diagnostic test performance. Our experience with the new algorithm suggests that expanding testing for acute HIV infection and implementation of rapid viral load diagnostic assays may both play a role in improving the effectiveness of HIV testing.
Highlights.
HIV testing by AR-MS-VL algorithm is feasible and rapid in a large medical center.
Low PPV of the 4th gen. test is due to low prevalence of HIV vs. assay specificity.
4thgeneration tests with a low S/C ratio may be false-positive or acute HIV results.
Rapid nucleic acid tests are needed to identify and quickly treat acute HIV cases.
Acknowledgments
Funding: This work was supported by the National Institutes of Health [5R34MH096606] and by a cooperative agreement between the Centers for Disease Control and Prevention (CDC) and the San Francisco Department of Public Health [5U01PS001564].
Footnotes
Competing interests: The authors have declared no competing interests.
Ethical approval: The study protocol for the clinical laboratory performance evaluation was approved by the UCSF Committee on Human Subjects Research (UCSF CHR 12-10141). HIV test result data from the STOP Study were not personally identifiable and therefore did not constitute research with human subjects.
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the Centers for Disease Control and Prevention, or the author affiliated institutions.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17. [PubMed] [Google Scholar]
- 2.Satcher Johnson A, Heitgerd J, Koenig LJ, Van Handel M, Branson BM, Connelly E, et al. Vital signs: HIV testing and diagnosis among adults—United States, 2001–2009. MMWR Morb Mortal Wkly Rep. 2010;59(47):1550–5. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) National HIV Testing Day and new testing recommendations. MMWR Morb Mortal Wkly Rep. 2014;63(25):537. [PMC free article] [PubMed] [Google Scholar]
- 4.Christopoulos KA, Zetola NM, Klausner JD, Haller B, Louie B, Hare CB, et al. Leveraging a rapid, round-the-clock HIV testing system to screen for acute HIV infection in a large urban public medical center. J Acquir Immune Defic Syndr. 2013;62(2):e30–8. doi: 10.1097/QAI.0b013e31827a0b0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Signer D, Peterson S, Hsieh YH, Haider S, Saheed M, Neira P, et al. Scaling up HIV testing in an academic emergency department: An integrated testing model with rapid fourth-generation and point-of-care testing. Public Health Rep. 2016;131(Suppl 1):82–9. doi: 10.1177/00333549161310S110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geren KI, Lovecchio F, Knight J, Fromm R, Moore E, Tomlinson C, et al. Identification of acute HIV infection using fourth-generation testing in an opt-out emergency department screening program. Ann Emerg Med. 2014;64:537–46. doi: 10.1016/j.annemergmed.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Moschella PC, Hart KW, Ruffner AH, Lindsell CJ, Wayne DB, Sperling MI, et al. Prevalence of undiagnosed acute and chronic HIV in a lower-prevalence urban emergency department. Am J Public Health. 2014;104:1695–9. doi: 10.2105/AJPH.2014.301953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rucker MG, Eavou R, Allgood KL, Sinclair D, Lawal R, Tobin A, et al. Implementing routine HIV screening in three Chicago hospitals: Lessons learned. Public Health Rep. 2016;131(Suppl 1):121–9. doi: 10.1177/00333549161310S114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liddicoat RV, Horton NJ, Urban R, Maier E, Christiansen D, Samet JH. Assessing missed opportunities for HIV testing in medical settings. J Gen Intern Med. 2004;19:349–356. doi: 10.1111/j.1525-1497.2004.21251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Facente SN, Pilcher CD, Hartogensis WE, Klausner JD, Philip SS, Louie B, et al. Performance of risk-based criteria for targeting acute HIV screening in San Francisco. PLoS One. 2011;6(7):e21813. doi: 10.1371/journal.pone.0021813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care setttings. MMWR Morb Mortal Wkly Rep. 2006;59:1–17. [PubMed] [Google Scholar]
- 12.Podhurst LS, Storm DS, Dolgonos S. Women's opinions about routine HIV testing during pregnancy: Implications for the opt-out approach. AIDS Patient Care and STDs. 2009;23(5):331–37. doi: 10.1089/apc.2008.0186. [DOI] [PubMed] [Google Scholar]
- 13.Pilcher CD, Ospina-Norvell C, Dasgupta A, Jones D, Hartogensis W, Torres S, et al. The effect of same-day observed initiation of antiretroviral therapy on HIV viral load and treatment outcomes in a U.S. Public Health setting. J Acquir Immune Defic Syndr. 2016 Jul 16; doi: 10.1097/QAI.0000000000001134. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geren K, Moore E, Tomlinson C, Hobohm D, Gardner A, Reardon-Maynard D, et al. Detection of acute HIV infection in two evaluations of a new HIV diagnostic testing algorithm –United States, 2011-2013. MMWR Morb Mortal Wkly Rep. 2013;62(24):489–94. [PMC free article] [PubMed] [Google Scholar]
- 15.Peters PJ, Westheimer E, Cohen S, Hightow-Weidman LB, Moss N, Tsoi B, et al. Screening yield of HIV antigen/antibody combination and pooled HIV RNA testing for acute HIV infection in a high-prevalence population. JAMA. 2016;315(7):682–90. doi: 10.1001/jama.2016.0286. [DOI] [PubMed] [Google Scholar]
- 16.Ramos EM, Harb S, Dragavon J, Swenson P, Stekler JD, Coombs RW. Performance of an alternative HIV diagnostic algorithm using the ARCHITECT HIV Ag/Ab Combo assay and potential utility of sample-to-cutoff ratio to discriminate primary from established infection. J Clin Virol. 2013;58(Suppl 1):e38–42. doi: 10.1016/j.jcv.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joint United Nations Programme on HIV/AIDS (UNAIDS) 90-90-90: An ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS; 2014. [Google Scholar]
- 18.National Institutes of Health (US) National Institute of Allergy and Infectious Diseases. [Accessed 25 Jul 2016];Starting antiretroviral treatment early improves outcomes for HIV-infected individuals. https://www.nih.gov/news-events/news-releases/starting-antiretroviral-treatment-early-improves-outcomes-hiv-infected-individuals.
- 19.Sullivan PS, Lyons MS, Czarnogorski M, Branson BM. Routine screening for HIV infection in medical care settings: A decade of progress and next opportunities. Public Health Rep. 2016;131(Suppl 1):1–4. doi: 10.1177/00333549161310S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le T, Wright EJ, Smith DM, He W, Catano G, Okulicz JF, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med. 2013;368:218–30. doi: 10.1056/NEJMoa1110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller WC, Rosenberg NE, Rutstein SE, Powers KA. The role of acute and early HIV infection in the sexual transmission of HIV. Curr Opin HIV AIDS. 2010;5:277–82. doi: 10.1097/COH.0b013e32833a0d3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramos EM, Ortega J, Daza G, Harb S, Dragavon J, Coombs RW. Distribution of the ARCHITECT sample to cutoff ratio (S/CO) by Fiebig Stage of HIV-1 infection. HIV Diagnostics Conference; March 21-24, 2016; Atlanta GA, U.S.A. [Google Scholar]
- 23.Grebe E, Murphy G, Pilcher CD, Keating S, Facente S, Marson K, et al. Unmodified diagnostic assay provides similar performance to avidity modification for surveillance and clinical recency staging applications. HIV Diagnostics Conference; March 21-24, 2016; Atlanta GA, U.S.A. [Google Scholar]
- 24.Grebe E, Welte A, Hall J, Busch MP, Facente S, Keating S, et al. Recency staging of HIV infections through routine diagnostic testing. Conference on Retroviruses and Opportunistic Infections; February 13-15, 2017; Seattle WA, U.S.A. [Google Scholar]


