Abstract
A general method for synthesis of 1,2-bis-trifluoromethylthioarenes has been developed. Arynes generated from silyl aryl triflates or halides react with bis(trifluoromethyl)disulfide to afford 1,2-bis-trifluoromethylthioarenes. Aryl, alkyl, ester, halide, and methoxy functionalities are compatible with reaction conditions. Use of bis(perfluoroaryl)-disulfides gave moderate yields of aryne disulfenylation or cyclization to fluorinated dibenzothiophenes.
Graphical Abstract
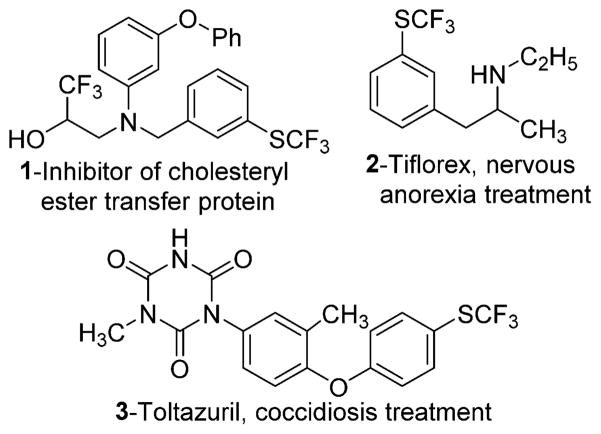
Fluorine-containing organic substances are important in medicine, agriculture, and as materials.1 Among fluorinated organics, aryl trifluoromethyl sulfides (ArSCF3) are particularly interesting because they are present in many biologically active substances (Figure 1).2 Furthermore, trifluoromethylthiolation of organic compounds is less studied than fluorination and trifluoromethylation.3 For many years, the trifluoromethylthio moiety was generated either by halogen–fluorine exchange in chloro- or bromomethyl sulfides or by trifluoromethylation of thiols.3g,h Trifluoromethylthiolation by nucleophilic substitution usually requires harsh reaction conditions and stoichiometric amounts of transition metals.4 Recently, however, efficient methods utilizing milder protocols have been developed for trifluoromethylthiolation of aryl halides, boronic acids, and sp2 C–H bonds under transition metal catalysis.5–7 Synthesis of 1,2-bis(trifluoromethylthio)arenes has been reported in a very limited number of publications, and the reactions appear to lack generality.8 For example, electrophilic aromatic substitution affords low yields of 1,2-bis(trifluoromethylthio)benzene.8a Multistep reactions involving cyclo-addition of bis(trifluoro-methylthio)acetylene have also been reported.8b Perhaps the most general method involves reaction of trifluoromethylthiocopper with aromatic halides.8d Unfortunately, trifluoromethylthiocopper is not commercially available. We report here a convenient method for the preparation of 1,2-bis(trifluoromethylthio)arenes from silyl aryl triflates or halides and commercially available bis(trifluoromethyl)disulfide.
Figure 1.
Bioactive molecules possessing trifluoromethylthioarene functionality.
A few examples of aryne reactions with trifluoromethylthio-containing nucleophiles have been reported in the literature.9 In 2008, Kolomeitsev reported one example of trifluoromethyl-thiobenzene synthesis in the reaction between trimethylsilyl-phenyl triflate, fluoride, and trifluoromethylsulfide anion.9a In 2013, Lee and co-workers disclosed silver-mediated trifluoromethylthiolation via hexadehydro Diels–Alder reaction where a trifluoromethylthio substituent is introduced by employing excess AgSCF3 reagent.9b Recently, Hu reported the reaction of in situ generated arynes with excess AgSCF3, affording aryl trifluoromethylsulfides in good yields.9c In all these examples, the source of trifluoromethylthio substituent is not commercially available, and synthesis of bis-1,2-(trifluoromethyl-thio)-arenes was not reported.
Aryne insertion in disufide S–S bonds, which is a potential route to 1,2-bis(trifluoromethylthio)arenes, has not been extensively studied.10 Aryne generation by employing strong bases is not compatible with electrophilic bis(trifluoromethyl)-disulfide.11 Consequently, the mildest conditions of aryne generation from silyl aryl triflates and silyl aryl halides were employed.12 Three differentconditions of aryne generation from silyl aryl triflates or halides were explored based on our previous work.12b
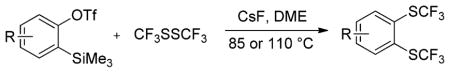
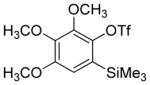
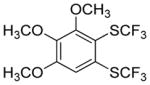
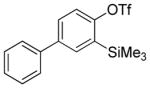
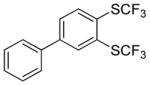
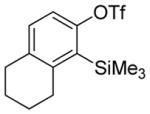
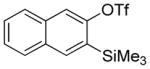
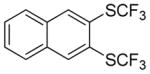
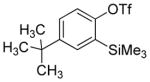
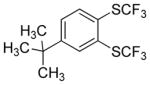
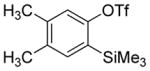
Aryne insertion in the bis(trifluormethyl)disulfide S–S bond proceeds with best yields at relatively high temperatures. Use of cesium fluoride in dimethoxyethane afforded the highest yields. Other fluoride sources, such as tetramethylammonium fluoride, and solvents such as acetonitrile gave lower yields.13 The scope of the reaction with respect to silyl aryl triflates is presented in Table 1. Substrates possessing both electron-withdrawing and electron-releasing substituents are reactive. A methoxy substituent is tolerated affording products in good yields (entries 1–3). Phenyl-substituted silyl aryl triflate is reactive as well, giving bis(trifluoromethylthio)biphenyl in 78% yield (entry 4). Alkyl-substituted substrates afford products in 64–74% yields (entries 5, 8, 10, and 12).
Table 1.
Bis(trifluoromethylthiolation) of Silyl Aryl Triflatesa
| |||
|---|---|---|---|
| entry | aryne precursor | product | yield (%) |
| 1 |
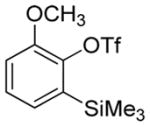
|
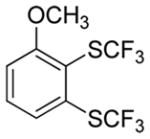
|
75 |
| 2 |
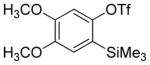
|
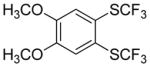
|
76 |
| 3 |
|
|
84 |
| 4 |
|
|
78 |
| 5b |
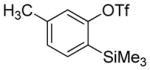
|
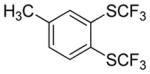
|
64 |
| 6b |
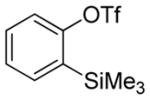
|
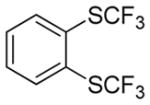
|
71 |
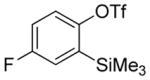
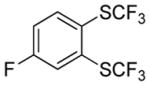
| 7b |
|
|
56 |
| 8 |
|
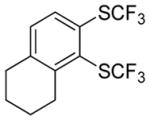
|
74 81c |
| 9 |
|
|
69 |
| 10b |
|
|
66 |
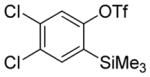
| 11 |
|
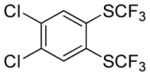
|
44 |
| 12 |
|
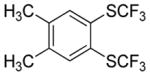
|
68 |
| 13b |
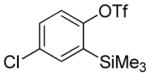
|
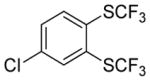
|
65 |
| 14 |
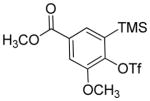
|
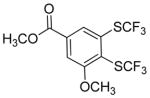
|
41 |
Reaction conditions: Aryne precursor (0.3 or 0.5 mmol), CsF (3 equiv), CF3SSCF3 (3.5 equiv), DME (2 mL), 24–48 h, 85 or 110 °C. Yields are isolated yields.
Yield determined by NMR with PhCF3 as an internal standard. DME is dimethoxyethane.
Scale: 1.0 mmol. Please see Supporting Information for details.
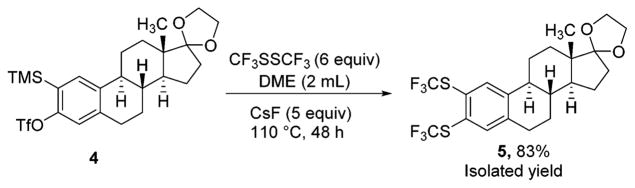
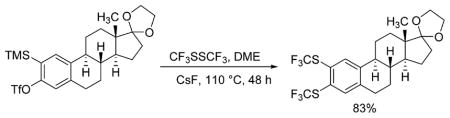
A fluorinated substrate gives bis(trifluoromethylthio)- fluorobenzene in an acceptable yield (entry 7). Chlorinated substrates provide products in 44% and 65% yields (entries 11 and 13). An ester substituent is tolerated as well, and product is obtained in 41% yield (entry 14). Several of the products are very volatile, and their NMR yields were determined (entries 5–7, 10, 13). Silyl aryl triflate 4 derived from estrone was converted to bis(trifluoromethylthio) derivative 5 in 83% yield, showing relevance of the methodology to the modification of biologically active compounds (Scheme 1).
Scheme 1.
Bis(trifluoromethylthiolation) of Estrone Derivative
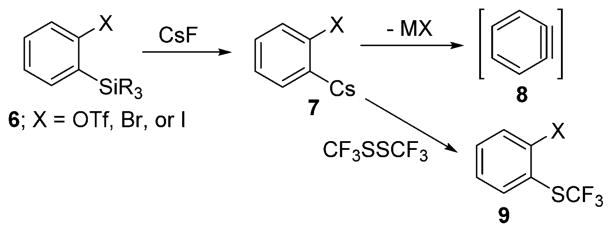
Unfortunately, only a few silyl aryl triflates are commercially available. In addition, their preparation requires lengthy synthetic procedures starting from ortho-bromophenols, which are of limited availability as well. In contrast, silyl aryl bromides and iodides can be prepared in one step from commercially available starting materials. Arynes can be generated from these compounds under conditions similar to those employed for silyl aryl triflates.12b Consequently, bis(trifluoromethyl-thiolation) of silyl aryl halides would be practical and convenient. A potential issue that could interfere with the desired reaction pathway is the trapping of arylcesium intermediate 7 by the highly electrophilic bis(trifluoromethyl)-disulfide affording monotrifluoromethylthiolation product 9 (Scheme 2). The ease of aryne formation from ortho-metalated species 7 is related to the strength of the leaving group (X−) conjugate acid. Examination of hydrogen iodide and triflic acid DMSO pKa values shows that the acidity of HI is close to that of CF3SO3H.14
Scheme 2.
Reactivity of Arylmetal Intermediate
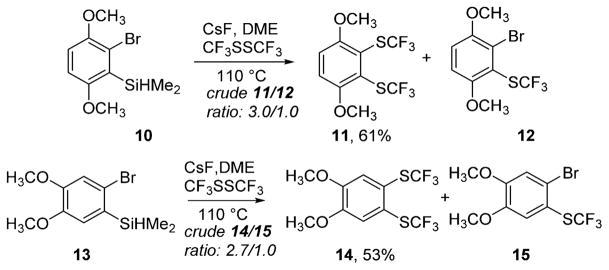
As expected, use of silyl aryl bromides was problematic due to 2-bromoarylcesium trapping with bis(trifluoromethyl)-disulfide (Scheme 3). Out of many silyl aryl bromide substrates subjected to the reaction conditions, only two gave modest yields of the desired products. Compounds 10 and 13 were reacted with bis(trifluoromethyl)disulfide to afford 61% and 53% isolated yields of 11 and 14, respectively. Crude reaction mixture NMR spectra showed that, in addition to the desired 11 and 14, substantial amounts of monotrifluoromethyl-sulfenylation products 12 and 15 were formed.
Scheme 3.
Silyl Aryl Bromide Reactions
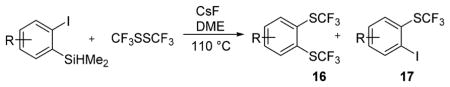
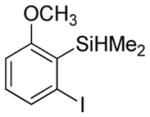
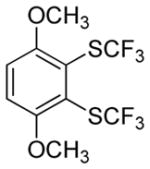
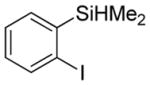
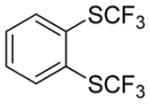
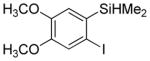
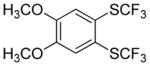
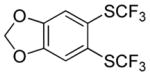
Considering the above results, silyl aryl iodides were used as aryne precursors. Silyl aryl iodide reactions with bis(trifluoromethyl)disulfide are summarized in Table 2. 1-Iodo-3-methoxy-2-(dimethylsilyl)benzene reacts with bis(trifluoromethyl)-disulfide to afford the product in 74% isolated yield (entry 1). 3,6-Dimethoxy-1,2-bis(trifluoromethylthio)benzene was synthesized in 71% yield (entry 2). 1,2-Bis(trifluoromethylthio)benzene was prepared in 58% yield (entry 3). Entries 4 and 5 show the formation of symmetric alkoxy-substituted products in 63% and 58% isolated yields, respectively. 1-Iodo-3,4,5-trimethoxy-2-(dimethylsilyl)benzene reacts with bis(trifluoromethyl)disulfide to afford the product in 82% isolated yield (entry 6). While the reactions appear to be more general than those of corresponding silyl aryl bromides, substantial amounts of monotrifluoromethyl-sulfenylation products are observed in all reaction mixtures. The bis-/monofunctionalized product crude ratios range from 2.4/1.0 (entry 3) to 4.5/1.0 (entry 6) showing the high electrophilicity of bis(trifluoromethyl)disulfide.
Table 2.
Silyl Aryl Iodide Reactionsa
| |||
|---|---|---|---|
| entry | aryne precursor | 16 | yield (%) (16/17) |
| 1 |
|
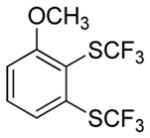
|
74 3.7:1.0 |
| 2 |
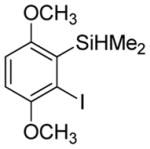
|
|
71b 3.1:1.0 |
| 3 |
|
|
58c 2.4:1.0 |
| 4 |
|
|
63 2.9:1.0 |
| 5 |
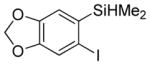
|
|
58 2.8:1.0 |
| 6 |
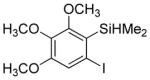
|
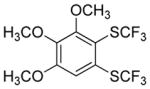
|
82 4.5:1.0 |
| 7 |
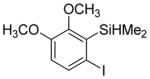
|
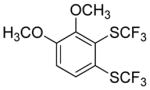
|
60 2.8:1.0 |
Reaction conditions: Aryne precursor (0.3 mmol), CsF (3 equiv), CF3SSCF3 (3.5 equiv), DME (2 mL), 37–46 h. Yields are isolated yields.
3,6-Dimethoxy-2-trifluoromethylthioiodobenzene (23%) also isolated.
Yield determined by NMR with PhCF3 as an internal standard. Please see Supporting Information for details.
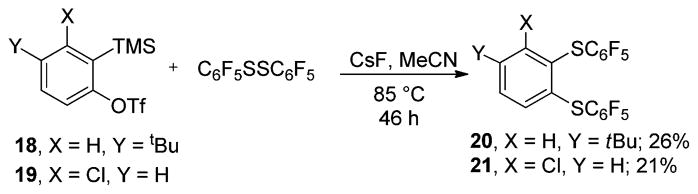
Next, silyl aryl triflate reactions with bis(perfluoroaryl)-disulfides were examined. 4-tert-Butyl and 3-chlorobenzynes generated from the corresponding silyl aryl triflates were reacted with bis(pentafluorophenyl)disulfide (Scheme 4). Isolated yields of the products were 26% and 21%, respectively. Attempts to improve reaction efficiency were not successful.
Scheme 4.
Reactions with Bis(pentafluoroaryl)disulfides
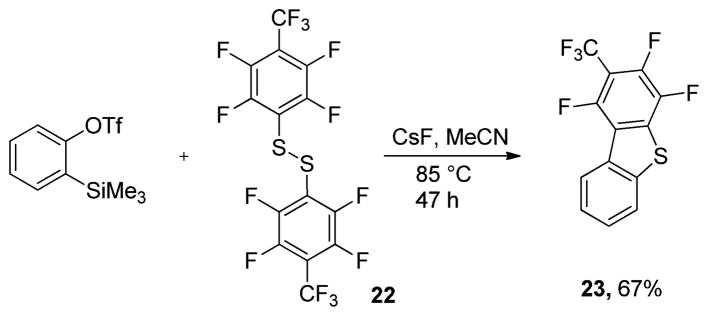
Interestingly, when bis(perfluoro-p-tolyl)disulfide was reacted with trimethylsilylphenyl triflate, a different pathway was followed (Scheme 5). Instead of aryne formal insertion into the disulfide S–S bond, a single addition of sulfide followed by intramolecular nucleophilic substitution of fluoride on the aromatic ring occurred, affording a polyfluorinated benzothiophene 23 in 67% yield. The isolation of 23 argues against a concerted aryne reaction with disulfides. Instead, initial nucleophilic attack on aryne would generate an anionic intermediate, which subsequently gives a 1,2-disulfenylation product. Alternatively, in the presence of a strongly electrophilic polyfluorinated aryl ring, internal trapping affords 23.15
Scheme 5.
Reaction with Bis(perfluorotolyl)disulfide
In summary, a general method for synthesis of 1,2-bis-trifluoromethylthioarenes has been developed. Arynes generated from silyl aryl triflates and silyl aryl halides react with bis(trifluoromethyl)disulfide to afford 1,2-bis(trifluoromethylthio)arenes. Aryl, alkyl, ester, halide, and methoxy functionalities are compatible with reaction conditions. Use of bis(perfluoroaryl)disulfide reagents gave moderate yields of disulfenylation products or, in the case of bis(perfluoro-p-tolyl)disulfide, cyclization to fluorinated dibenzothiophenes. Silyl aryl bromides and iodides gave substantial amounts of monotrifluoromethylsulfenylation products due to the high electrophilicity of bis(trifluoromethyl)disulfide.
Supplementary Material
Acknowledgments
We thank the Welch Foundation (Chair No. E-0044) and NIGMS (Grant No. R01GM077635) for supporting this research.
Footnotes
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.7b01901.
Detailed experimental procedures and characterization data for new compounds (PDF)
References
- 1.Wang J, Sánchez-Roselló M, Acenña JL, Del Pozo C, Sorochinsky AE, Fustero S, Soloshonok VA, Liu H. Chem Rev. 2014;114:2432. doi: 10.1021/cr4002879. [DOI] [PubMed] [Google Scholar]
- 2.(a) Leroux F, Jeschke P, Schlosser M. Chem Rev. 2005;105:827. doi: 10.1021/cr040075b. [DOI] [PubMed] [Google Scholar]; (b) Boiko VN. Beilstein J Org Chem. 2010;6:880. doi: 10.3762/bjoc.6.88. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Durley RC, Grapperhaus ML, Massa MA, Mischke DA, Parnas BL, Fobian YM, Rath NP, Honda DD, Zeng M, Connolly DT, Heuvelman DM, Witherbee BJ, Glenn KC, Krul ES, Smith ME, Sikorski JA. J Med Chem. 2000;43:4575. doi: 10.1021/jm000337b. [DOI] [PubMed] [Google Scholar]
- 3.(a) Truong T, Klimovica K, Daugulis O. J Am Chem Soc. 2013;135:9342. doi: 10.1021/ja4047125. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fier PS, Hartwig JF. Science. 2013;342:956. doi: 10.1126/science.1243759. [DOI] [PubMed] [Google Scholar]; (c) Ye Y, Schimler SD, Hanley PS, Sanford MS. J Am Chem Soc. 2013;135:16292. doi: 10.1021/ja408607r. [DOI] [PubMed] [Google Scholar]; (d) Zhang Q, Brusoe AT, Mascitti V, Hesp KD, Blakemore DC, Kohrt JT, Hartwig JF. Angew Chem, Int Ed. 2016;55:9758. doi: 10.1002/anie.201604793. [DOI] [PubMed] [Google Scholar]; (e) Dubinina GG, Furutachi H, Vicic DA. J Am Chem Soc. 2008;130:8600. doi: 10.1021/ja802946s. [DOI] [PubMed] [Google Scholar]; (f) Novák P, Lishchynskyi A, Grushin VV. J Am Chem Soc. 2012;134:16167. doi: 10.1021/ja307783w. [DOI] [PubMed] [Google Scholar]; (g) Nodiff EA, Lipschutz S, Craig PN, Gordon MJ. J Org Chem. 1960;25:60. [Google Scholar]; (h) Umemoto T, Ishihara S. J Am Chem Soc. 1993;115:2156. [Google Scholar]
- 4.(a) Manteau B, Pazenok S, Vors JP, Leroux FR. J Fluorine Chem. 2010;131:140. [Google Scholar]; (b) Baert F, Colomb J, Billard T. Angew Chem, Int Ed. 2012;51:10382. doi: 10.1002/anie.201205156. [DOI] [PubMed] [Google Scholar]
- 5.(a) Teverovskiy G, Surry DS, Buchwald SL. Angew Chem, Int Ed. 2011;50:7312. doi: 10.1002/anie.201102543. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhang CP, Vicic DA. J Am Chem Soc. 2012;134:183. doi: 10.1021/ja210364r. [DOI] [PubMed] [Google Scholar]
- 6.Chen C, Xie Y, Chu L, Wang RW, Zhang X, Qing FL. Angew Chem, Int Ed. 2012;51:2492. doi: 10.1002/anie.201108663. [DOI] [PubMed] [Google Scholar]
- 7.Tran LD, Popov I, Daugulis O. J Am Chem Soc. 2012;134:18237. doi: 10.1021/ja3092278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Haas A, Hellwig V. J Fluorine Chem. 1975;6:521. [Google Scholar]; (b) Haas A, Krächter HU. Chem Ber. 1988;121:1833. [Google Scholar]; (c) Scribner RM. J Org Chem. 1966;31:3671. [Google Scholar]; (d) Kondratenko NV, Kolomeytsev AA, Popov VI, Yagupolskii LM. Synthesis. 1985;1985:667. [Google Scholar]
- 9.(a) Kolomeitsev AA, Vorobyev M, Gillandt H. Tetrahedron Lett. 2008;49:449. [Google Scholar]; (b) Wang KP, Yun SY, Mamidipalli P, Lee D. Chem Sci. 2013;4:3205. [Google Scholar]; (c) Zeng Y, Hu J. Org Lett. 2016;18:856. doi: 10.1021/acs.orglett.6b00142. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama J, Tajiri T, Hoshino M. Bull Chem Soc Jpn. 1986;59:2907. [Google Scholar]
- 11.(a) Truong T, Daugulis O. J Am Chem Soc. 2011;133:4243. doi: 10.1021/ja200184b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Truong T, Daugulis O. Chem Sci. 2013;4:531. doi: 10.1039/c2sc21288a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Truong T, Mesgar M, Le KKA, Daugulis O. J Am Chem Soc. 2014;136:8568. doi: 10.1021/ja504886x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Himeshima Y, Sonoda T, Kobayashi H. Chem Lett. 1983;12:1211. [Google Scholar]; (b) Mesgar M, Daugulis O. Org Lett. 2016;18:3910. doi: 10.1021/acs.orglett.6b01952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Please see Supporting Information for optimization details.
- 14.(a) Bordwell FG. Acc Chem Res. 1988;21:456. [Google Scholar]; (b) Trummal A, Lipping L, Kaljurand I, Koppel IA, Leito IJ. J Phys Chem A. 2016;120:3663. doi: 10.1021/acs.jpca.6b02253. [DOI] [PubMed] [Google Scholar]
- 15.Travieso-Puente R, Budzak S, Chen J, Stacko P, Jastrzebski JTBH, Jacquemin D, Otten E. J Am Chem Soc. 2017;139:3328. doi: 10.1021/jacs.6b12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.