Abstract
The resolution of inflammation is a highly regulated process enacted by endogenous mediators including specialized pro-resolving lipid mediators (SPMs): the lipoxins, resolvins, protectins and maresins. SPMs activate specific cellular receptors to temper the production of pro-inflammatory mediators, diminish the recruitment of neutrophils, and promote the clearance of dead cells by macrophages. These mediators also enhance host-defense and couple resolution of inflammation to subsequent phases of tissue repair. Given that unresolved inflammation plays a causal role in the development of cardiovascular diseases, an understanding of these endogenous pro-resolving processes is critical for determining why cardiovascular inflammation does not resolve. Here, we discuss the receptor-dependent actions of resolvins and related pro-resolving mediators and highlight their emerging roles in the cardiovascular system. We propose that stimulating resolution could be a novel approach for treating chronic cardiovascular inflammation without promoting immunosuppression.
Keywords: Resolution of inflammation, specialized pro-resolving mediators, atherosclerosis
1.1 Introduction
Inflammation in its simplest definition is an immune response to a given stimulus. Acute inflammation is a protective host response that has evolved to thwart pathogens and to repair tissue injury1. This protective program is self-limited which means that the inflammation is tightly coupled to a termination/resolution phase, which is now known as inflammation-resolution2. When a stimulus is either excessive or persistent or when an immune response is mounted inappropriately to non-pathogenic endogenous antigens, this once protective response can become maladaptive, which can lead to chronicity and eventual tissue damage. It was previously believed that inflammation ceased due to passive dissipation of inflammatory stimuli. We now appreciate that the resolution of inflammation is an active and highly coordinated process that involves several chemical mediators and cell types2. Some of the mediators involved in the resolution of inflammation include polyunsaturated fatty acid-derived lipoxins, resolvins, protectins and maresins (collectively referred to as specialized pro-resolving mediators or SPMs), as well as protein mediators like Annexin A1. Gaseous and nucleotide mediators are also emerging as important players in the resolution response3, 4. Importantly, the discovery that resolution of inflammation is an active process is crucial because this knowledge has the potential to lead to new approaches to selectively target a safer termination of inflammation without compromising host defense2.
Uncontrolled inflammation, and thus a failure of the inflammation-resolution response, is the underpinning of several prevalent human diseases. Atherosclerotic vascular disease, the leading cause of death worldwide, is a prominent example of failed inflammation-resolution in humans. Atherosclerosis is a disease of the vascular wall that is driven by the persistent subendothelial retention of ApoB lipoproteins5. This persistent stimulus deranges host cells in a manner that leads to arterial tissue injury6–11. Because of the chronicity of inflammation that is characteristic of atherosclerosis, clinical trials are currently testing the hypothesis that blocking inflammation could provide additional clinical benefit beyond lipid lowering strategies that are the current standard of care. Specifically, the Cardiovascular Inflammation Reduction Trial (CIRT) is utilizing low dose methotrexate to determine whether blocking inflammation will lower vascular events rates12, 13. Similarly, the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) is testing whether an interleukin 1β antibody will decrease chronic vascular inflammation and cardiovascular events without affecting cholesterol levels14, 15. While these clinical trials could provide evidence for a causal role of inflammation in atherosclerosis in humans, targeting pro-resolution pathways may offer additional advantages over traditional anti-inflammatory approaches that can be immunosupressive2. Here, we discuss the role of pro-resolving lipid mediators in the cardiovascular system and highlight recent proof-of-concept studies in rodents demonstrating that stimulating resolution has beneficial effects on atheroprogression.
1.2 Inflammation-resolution and cardiovascular diseases (CVD)
One of the first insights into the role of SPMs in atherosclerosis came from a study by Serhan and colleagues in which they reported that angioplasty increases intracoronary levels of lipoxins in humans16. Building on these observations in animal models of atherosclerosis, Chan and colleagues identified that atherogenesis is significantly thwarted in rabbits overexpressing 15-lipoxygenase (15-LOX), a key biosynthetic enzyme for SPMs17, 18. Atherogenesis is also delayed in transgenic mice overexpressing 15-LOX and crossed with ApoE−/− mice fed a chow diet, compared with wild type controls. Isolated peritoneal macrophages from the 15-LOX -ApoE−/− transgenic mice exhibited an increased production of key SPMs, compared with wild type control macrophages. Importantly, 15-LOX, can promote the biosynthesis of SPMs or pro-atherogenic oxidation of LDL18–22 and one determinant of whether these pathways promote SPM production or pro-atherogenic lipids is diet composition. Along these lines, 15-LOX -ApoE−/− transgenic mice fed a high-fat, high-cholesterol diet had worse atherosclerosis compared with wild type controls21. These studies suggest that diet is a critical determinant to whether these enzymatic pathways can promote or suppress plaque progression.
More recent studies have been focused on the therapeutic actions of SPMs in cardiovascular diseases. Several SPMs and pro-resolving proteins/peptide have been explored as therapeutics in experimental atherosclerosis. The sections below will highlight each SPM or pro-resolving protein/peptide and their roles in cardiovascular diseases, specifically atherosclerosis and myocardial infarction (Table 1).
Table 1.
Treatment with pro-resolving mediators in CVD
| CVD | Actions |
|---|---|
| Atherosclerosis | |
| RvE1 | Decreased atherogenesis (rabbit)23 Attenuated atheroprogression and further decreased atheroprogression in the presence of atorvastatin (mice)89 |
| RvD1 | Halted atheroprogression, enhanced lesional efferocytosis and lesion stability (mice); decreased RvD1 in human vulnerable plaque regions34 |
| RvD2 and Mar1 | Decreased atheroprogression and enhanced lesion stability (mice)35 |
| Aspirin triggered lipoxin A4 (ATL) | Decreased atheroprogression and enhanced lesion stability (mice)36 |
| Ac2-26 plaque-targeted nanoparticles | Decreased atheroprogression and promoted plaque stability (mice) 37 |
| Annexin A1 | Decreased leukocyte migration/atheroprogression (mice)27 Decreased atheroprogession90 |
| IL-10 plaque-targeted nanoparticles | Decreased atheroprogression and promoted plaque stability (mice)91 |
| Myocardial infraction | |
| RvE1 | Reduced infarct size (rats) 48 |
| RvD1 | Reduced PMN infiltration and fibrosis (mice)43 |
| Neointimal hyperplasia | |
| Aspirin triggered lipoxin A4 (ATL) | Reduce neointimal hyperplasia (mice)92 |
| RvD2 | Reduced neointimal hyperplasia (mice) 93, 94 |
| RvD1 | Reduced neointimal hyperplasia (rats) rats87 |
| Mar1 | Reduce neointimal hyperplasia (mice)94 |
1.2.1 SPMs and pro-resolving proteins/peptides in atherogenesis
In rabbits fed a high-fat, high-cholesterol diet, the SPM resolvin E1 (RvE1), reduces atherogenesis, which is associated with a reduction in CRP23. Importantly, RvE1 was administered at the onset of high-fat, high-cholesterol diet feeding and served as a proof-of-concept that higher levels of RvE1 early on may be important for delaying atherogenesis. In this particular study, RvE1 was given topically to the periodontium, which implies that locally administered RvE1 has systemic effects (Table 1). Importantly, there is a known link between atherosclerosis and periodontal disease24, and this study also indicated that RvE1 could thwart atherogenesis in the context of periodontal disease23. More information regarding periodontal disease and SPMs can be seen in an accompanying review in this series.
Annexin A1 is a protein-derived endogenous mediator of inflammation-resolution that has also been studied in atherosclerosis. Annexin A1 levels are high in asymptomatic plaques compared with symptomatic carotid artery plaques25. This decrease in expression at both the mRNA and protein levels in symptomatic plaques suggests that advanced, symptomatic plaques may have a general defect in inflammation-resolution programs. Interestingly, Annexin A1 expression was found to be higher in smooth muscle cells (SMCs) isolated from human asymptomatic compared with symptomatic carotid plaques26, which suggests a potential cell specific role for the generation of Annexin A1. Annexin A1 can be synthesized by several cell types, and so a deeper investigation of which cell type makes Annexin A1 in plaques would be invaluable. In other words, there could be certain cell types that are more dysregulated in their ability to synthesize Annexin A1, and this information may prove important for therapeutic intervention. In this regard, Annexin A1 and its bioactive peptide, called Ac2-26, were also recently tested in murine models of atherosclerosis. Annexin A1 plasma levels negatively correlated with lesion area27. Upon further probing, Annexin A1 was found to decrease atherogenesis and regulate key chemokines involved in leukocyte migration into plaques when given at the onset of high-fat, high-cholesterol feeding in ApoE−/− mice, largely through its ability to signal through its G-protein receptor called ALX/FPR227. These studies collectively demonstrate that increasing the levels of Annexin A1 early on is beneficial in thwarting the onset of the disease. It is important to note that some of the ligands for ALX/FPR2 also exert pro-inflammatory actions. As an example, the ALX/FPR2 ligand, CRAMP (in mice) or LL-37 (in humans) was shown to promote leukocyte extravasation into the arterial wall in an ALX/FPR2-dependent manner28. GPCRs can form dimers that affect their activation and downstream signaling29 and emerging evidence suggests that dimerization may play a key role in fine-tuning signaling pathways29. Along these lines, Cooray et al. observed that pro-resolving ligands, like Annexin A1 initiated homodimerization of ALX/FPR2, which led to a pro-resolution feed-forward circuit, and an increase in IL-1030. Ac2-26, which a has anti-inflammatory and pro-resolving properties31, initiates a heterodimer complex with ALX/FPR2 and another formyl peptide receptor called FPR1. This heterodimer complex neutralizes the actions of the ALX/FPR2 pro-inflammatory ligand called serum amyloid A (SAA)30. Therefore, the way in which ligands interact with their receptors on the plasma membrane may provide new mechanistic clues as to why several ligands can bind a common receptor and regulate distinct signaling programs.
1.2.2 SPMs and pro-resolving proteins/peptides in advanced atherosclerosis
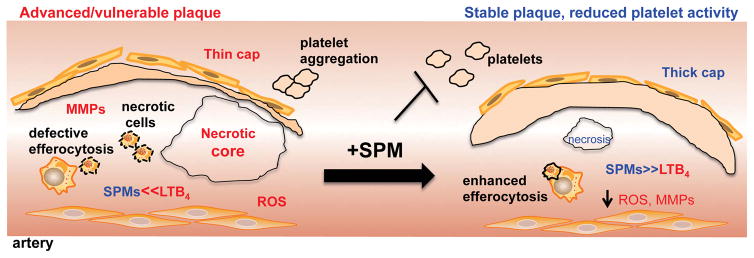
In humans, the subset of atherosclerotic plaques that are at risk for precipitating acute atherothrombotic clinical events, including myocardial infarction and stroke32, are typically referred to as vulnerable plaques. These plaques have distinct features, including heightened inflammation and oxidative stress; large areas of necrosis, which are composed of apoptotic cells not efficiently cleared by efferocytosis; and thinning of a protective layer of collagen that overlies the areas of necrosis in more stable lesions10, 32, 33. All of these features are indicative of defective inflammation-resolution and recently, we found that vulnerable plaque regions from human carotid arteries have a marked imbalance in SPMs to pro-inflammatory lipid mediators (e.g. leukotrienes), compared with stable plaque regions. We and others recapitulated these findings in mice and found that advanced plaques of Ldlr−/− or ApoE−/− mice fed a high-fat, high-cholesterol diet also exhibited a marked imbalance in the SPM:pro-inflammatory mediator ratio compared with early plaques34, 35. To prove causation, we and others administered key defective SPMs (RvD1, RvD2 and MaR1, or aspirin-triggered lipoxin A4) during the critical time period where atherosclerosis progressed to the advanced stage in mice (Table 1). In general, SPMs delayed atherosclerosis progression and promoted a more stable-like plaque phenotype34–36. One of the most striking observations of the plaques in athero-prone mice treated with SPMs was an increase in fibrous cap thickness and collagen synthesis in plaques34–37 (Fig. 1). Even though mechanisms underlying the formation of the protective cap still remain unknown, it is possible that efferocytosis can participate in the repair process. Efferocytosis stimulates the biosynthesis of SPMs and the release of other tissue reparative molecules like TGFβ10, 38–40. Furthermore, RvE1 increases collagen in the periodontal ligament post injury41, and 17R-RvD1 stimulates matrix synthesis in a model of arthritis42. Conversely, RvD1 decreases cardiac collagen in the setting of myocardial infarction43, and there other reports suggesting that SPMs exert anti-fibrotic actions44, 45. Thus, the role of SPMs in collagen synthesis and fibrosis is context-specific, and an understanding of the mechanisms whereby SPMs increase collagen synthesis in advanced atherosclerotic plaques is likely to be a fruitful area of future investigation.
Figure 1. SPMs promote features of plaque stability and temper platelet activation.
Several features of advanced plaques include large necrotic cores, thin fibrous caps, increased oxidative stress, and a deregulated activation of MMPs, defective efferocytosis, and an imbalance in the ratio of SPM:LTB4. In the event of a plaque rupture, endothelial cell erosion, or arterial injury, platelets become activated which can lead to devastating thrombotic events. Administration of SPMs potently reduces necrosis, oxidative stress, and MMPs activation and increases fibrous cap thickness, efferocytosis and the SPM:LT ratio. SPMs also prevent platelet aggregation and thrombosis.
Recent results also suggest that pro-resolving drugs can be targeted to plaques. In this regard, Ac2-26 was encapsulated into plaque-targeted nanoparticles (NPs) and administered to mice with established atherosclerosis. The Ac2-26 plaque targeted NPs delayed plaque progression and promoted several features that are characteristic of stable plaques37. Specifically, these targeted NPs enhanced the thickness of the fibrous cap and decreased lesional MMPs, which is similar to the actions of the RvD1 and aspirin-triggered lipoxin A4 (ATL) 34, 36. Importantly, RvD1, ATL and Ac2-26 bind and signal through the same receptor, ALX/FPR2. These studies used Fpr2/Fpr3−/− mice whereas the above study27 used Fpr2−/− mice. Therefore, understanding more mechanisms associated with ALX/FPR2 may be advantageous for new drug design. With that said, other SPMs like RvD2, MaR1 and RvE1 that all bind and signal through distinct receptors also exert a similar protective phenotype on advanced plaques, which is suggestive of common downstream pathways for resolution-mediators and plaque stability.
This new explosion of work is only the tip of the iceberg for the inflammation-resolution and atherosclerosis fields. Currently, little is known about the mechanisms, cell types, and signaling pathways associated with SPM-mediated protection in advanced atherosclerosis. For example, T regulatory cells (Treg) are emerging as crucial cellular players against atherosclerosis progression. Key D-series SPMs, such as those identified in human and murine plaques, have been shown to stimulate Treg in other contexts46 and therefore specific SPM in plaques may potentially play a role in regulating plaque Treg.
1.3 SPMs in ischemia and myocardial infarction
Ischemia can lead to tissue injury prompting a sterile inflammatory response. During this process, PMN rapidly infiltrate and expel reactive oxygen species (ROS) and other mediators, damaging healthy tissue47. Therefore, controlling unwarranted PMN accumulation, or promoting their removal, is important for protecting healthy tissues such as the myocardium during ischemia. Because SPMs promote both of these processes, recent studies have hypothesized that administration of SPMs could potentially improve tissue recovery during ischemic injury. Indeed, exogenous delivery of RvD1 after myocardial infarction (MI) in mice reduces accumulation of PMN and fibrosis, leading to improved cardiac function43. It was similarly shown that RvE1 decreases infarct size in a rat model of MI/reperfusion injury48. In addition to the heart, protective roles of SPM have also been described in other tissues during ischemia. For example, ischemia/reperfusion in the kidneys leads to biosynthesis of D-series resolvins and protectins. Treatment with resolvins prior to ischemia decreases PMN infiltration, and tissue fibrosis, and administration shortly after reperfusion is also organ protective49. In a model of second-organ reperfusion injury, resolvins protect against PMN recruitment into the lungs50, 51. The endogenous protective role of SPMs was demonstrated in mice lacking the Alx/Fpr2 or the Gpr18 (also known as DRV2) receptors, in which ischemia/reperfusion resulted in excessive leukocyte accumulation51, 52. In addition to well-described detrimental roles of PMN during ischemia, there is also emerging evidence that PMN participate in tissue repair53. Indeed, PMN depletion impairs the development of a tissue reparative macrophage phenotype, resulting in increased apoptotic cell accumulation in the heart after MI54. Along these lines, recent studies by Gronert et al. identified a distinct tissue resident and lymphoid homing PMN subset that produces SPM to blunt activation of Th1 and Th17 T cells in a murine model of dry eye disease55. Clearly, the roles of PMN in inflammation, resolution and tissue repair warrant further study.
Another complication from vascular inflammation and injury is neointimal hyperplasia and restinosis. This topic will be covered extensively in an accompanying review within this series but the roles of SPMs in this process are highlighted in Table 1.
1.4 Pro-resolving mediators in arteriogenesis
Arterial occlusion impairs tissue perfusion and can even lead to critical limb ischemia and limb loss in the case of peripheral atherosclerosis56, 57. Arteriogenesis (i.e. collateral vessel growth) is a process that partially compensates for defects in tissue perfusion58. Several therapeutic strategies to increase arteriogenesis have met limited success in large clinical trials for several reasons, notwithstanding the fact that most factors that promote revascularization are pro-inflammatory and can exacerbate atherosclerosis. Recent results indicate that RvD2 is generated in ischemic tissue from both humans and mice59. Importantly, RvD2 enhances tissue perfusion and arteriogenesis in mice that had undergone hind limb ischemia in a receptor (GPR18/DRV2)-dependent manner59. The improved revascularization by RvD2 was associated with decreased pro-inflammatory mediators and enhanced skeletal muscle regeneration. Chronic inflammation and metabolic disease impair the normal process of tissue revascularization and diabetic patients are particularly susceptible to defective tissue perfusion and wound healing56, 57. Importantly, RvD2 treatment to diabetic mice significantly rescued the defect in revascularization59. These studies highlight a new role for SPMs in the vasculature as well as a protective role for SPMs against skeletal muscle damage induced by ischemia.
1.5 SPMs in thrombosis and platelet activation
Myocardial infarction and stroke are the clinical manifestations of atherothrombosis60. Lipid mediators have long been recognized to play important roles in platelet activation and thrombosis, with the thromboxane/prostacyclin balance being a critical determinant of this process. A role of pro-resolution mediators in regulating platelet activation has also recently emerged. In healthy humans receiving aspirin, an epimeric aspirin-triggered form of lipoxin A4 generated from acetylated COX-2 (denoted ATL) was inversely correlated with pro-thrombotic thromboxane61, 62. Plasma levels of 15-epi-LXA4 were increased most significantly with low dose aspirin (i.e., 81mg), which is the recommended dose for patients with CVD. Interestingly, omega-3 fatty acid derived RvE1, another product of acetylated COX-2, blocks ADP and thromboxane-stimulated platelet aggregation63, 64. Further mechanistic studies revealed that RvE1 blocked ADP-initiated signals downstream of its receptor, P2Y12, and that the effects of RvE1 are mediated through its receptor called ERV1/ChemR2364. More recently, RvD2 was found to prevent thrombosis of the deep dermal vascular network and subsequent dermal necrosis in a mouse burn model65. SPM have also been shown to increase macrophage uptake of blood clots in vitro, indicating that SPM may play a role in clot remodeling during resolution66. Interestingly, platelet:PMN aggregates formed during self-limited, acute inflammation lead to SPM biosynthesis, which have been shown to reduce human platelet:PMN aggregates67, 68. Given that platelet:PMN aggregates contribute to plaque inflammation69, these results suggest that these aggregates in CVD may be deficient in their ability to generate SPMs. In this regard, new results indicate that SPMs, like Mar1, promote a pro-resolving phenotype of platelets and prevent thrombin-activated platelets from releasing several known CVD targets like soluble CD40L, thromboxanes, CD62P and platelet microparticles70. Therefore, these results collectively demonstrate that SPM may play key roles in regulating the resolution of thrombosis during inflammation, which could have important therapeutic implications for CVD.
1.6 SPMs enhance efferocytosis, an essential program for the resolution of inflammation
Efferocytosis, or the clearance of dead cells, is essential for a successful tissue resolution response. During efferocytosis, SPM (e.g., RvD1, RvD2, RvE2) production increases, which enhances further clearance of debris and apoptotic cells in an autocrine and paracrine manner38, 40. However, defective efferocytosis is observed in several chronic inflammatory diseases like asthma, obesity/diabetes, atherosclerosis, and interestingly, these conditions are also associated with defective production of several SPMs10, 71, 72. While the specific mechanisms underlying impaired SPM production and efferocytosis in these diseases are incompletely understood, the subcellular localization of 5-lipoxygenase (5-LOX) was recently shown to be a critical determinant of whether this enzyme produces pro-inflammatory leukotrienes or SPM in macrophages. Nuclear localization of 5-LOX favors the production of pro-inflammatory LTB4, while non-nuclear localization favors pro-resolving LXA473. Interestingly, RvD1 enhances this non-nuclear localization, promoting a positive feed-forward regulation of the SPM:leukotriene balance. This regulation of 5-LOX nuclear localization was found to be governed by signaling through the MER Proto-Oncogene Tyrosine Kinase (MerTK) receptor, coupling efferocytosis to SPM biosynthesis74. In mice genetically engineered to resist cleavage (and therefore inactivation) of this receptor, SPM biosynthesis is increased and resolution of acute inflammation and efferocytosis are improved74. In the context of atherosclerosis, these mice also had improved efferocytosis, decreased necrosis and increased lesional SPMs compared with controls75. In acute MI, mice deficient in efferocytosis receptors, Mertk and/or Mfge8, have decreased cardiac function and remodeling that was specifically due to abrogation of efferocytosis76, 77. Thus, defective efferocytosis in CVD could be related to defective production of SPM.
Defective efferocytosis was also reported in a mouse model of diabetic atherosclerosis. This defect, however, was reversed by a fish-oil diet rich in SPM precursors, EPA and DHA78. As mentioned above, RvD1 administration to advanced lesions showed an improvement in lesional efferocytosis and an enhancement in plaque SPMs34. Interestingly, it was also recently shown that RvD1, acting through ALX/FPR2, protects macrophages from oxidative stress-induced apoptosis during efferocytosis, in part by regulating NADPH oxidase activation and expression of apoptotic proteins, Bcl-xL and Bcl-279. In the context of atherosclerotic lesions, the ability of macrophages to survive and continue to phagocytose debris despite the highly oxidative environment is critically important.
Macrophages are highly plastic cells that adapt to their local tissue microenvironments80. It is becoming increasingly clear that the phenotype of a macrophage within an atherosclerotic plaque dictates its function81, 82. In the case of plaque regression, macrophages polarize to an M2-like phenotype83, which characteristically quells inflammation and promotes tissue repair. In another context, M2 macrophages biosynthesize SPMs more readily than M1-like macrophages, which is consistent with a tissue reparative phenotype38. Along these lines, RvD2 and MaR1 co-treatment promote a plaque tissue reparative macrophage phenotype35. How exactly SPMs regulate macrophage phenotype in plaques in is an important area of research and warrants further investigation.
1.7 Summary and future directions
It is now widely accepted that chronic inflammation plays a causal role in the development of CVD and finding new ways to target the inflammatory response is gaining traction as a new therapeutic approach to treating CVD in conjunction with the current standard of care (e.g., aspirin, statins, anti-platelet therapies)15. Unlike traditional anti-inflammatory strategies that blunt the production of inflammation “initiators” and could potentially lead to immunosuppression, pro-resolving mediators resolve inflammation without compromising host-defense2. In fact, pro-resolving mediators enhance host defense to both viral and bacterial infections and lower the threshold for antibiotic therapy84, 85 and stimulate distinct processes necessary for tissue repair and regeneration (see accompanying reviews in this series). Thus, new strategies to promote resolution of inflammation may offer unique opportunities to combat chronic inflammation associated with CVD.
In the future, it will be important to determine if SPM can be effectively targeted to sites of local inflammation (e.g., atherosclerotic plaques). Along these lines, it is interesting to point out that microparticles released from activated immune cells carry SPMs and prior work showed that humanized nanoparticles could potentially be vehicles for targeted delivery86. In the setting of vascular surgery, unidirectional delivery of SPM through biodegradable vascular wraps have been successful in animal models, preventing restenosis and local inflammation87,88. Further mechanistic studies on how resolution is perturbed in CVD, including an understanding of how SPM biosynthesis and receptor-mediated actions might be deregulated, will help to inform the development of novel pro-resolution therapeutics for CVD.
Acknowledgments
Sources of Funding
The authors acknowledge the support of NIH grants HL119587 (GF), HL106173 (MS) and GM095467 (MS); and support from the American Federation for Aging Research (AFAR), Research Grant for Junior Faculty, A16034 (GF).
Footnotes
Disclosures
None
References
- 1.Kumar V, Abbas AK, Fausto N, Robbins SL, Cotran RS. Robbins and Cotran pathologic basis of disease. 7. Philadelphia: Elsevier/Saunders; 2005. [Google Scholar]
- 2.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallace JL, Ianaro A, Flannigan KL, Cirino G. Gaseous mediators in resolution of inflammation. Semin Immunol. 2015;27:227–33. doi: 10.1016/j.smim.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Shinohara M, Serhan CN. Novel Endogenous Proresolving Molecules:Essential Fatty Acid-Derived and Gaseous Mediators in the Resolution of Inflammation. J Atheroscler Thromb. 2016;23:655–64. doi: 10.5551/jat.33928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–61. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross R, Glomset J, Harker L. Response to injury and atherogenesis. Am J Pathol. 1977;86:675–84. [PMC free article] [PubMed] [Google Scholar]
- 7.Ross R. Rous-Whipple Award Lecture. Atherosclerosis: a defense mechanism gone awry. Am J Pathol. 1993;143:987–1002. [PMC free article] [PubMed] [Google Scholar]
- 8.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 9.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–55. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Libby P, Tabas I, Fredman G, Fisher EA. Inflammation and its resolution as determinants of acute coronary syndromes. Circ Res. 2014;114:1867–79. doi: 10.1161/CIRCRESAHA.114.302699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT) J Thromb Haemost. 2009;7(Suppl 1):332–9. doi: 10.1111/j.1538-7836.2009.03404.x. [DOI] [PubMed] [Google Scholar]
- 13.Everett BM, Pradhan AD, Solomon DH, Paynter N, Macfadyen J, Zaharris E, Gupta M, Clearfield M, Libby P, Hasan AA, Glynn RJ, Ridker PM. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166:199–207. e15. doi: 10.1016/j.ahj.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, Thuren T, Group CPI. Effects of interleukin-1beta inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126:2739–48. doi: 10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]
- 15.Shapiro MD, Fazio S. From Lipids to Inflammation: New Approaches to Reducing Atherosclerotic Risk. Circ Res. 2016;118:732–49. doi: 10.1161/CIRCRESAHA.115.306471. [DOI] [PubMed] [Google Scholar]
- 16.Brezinski DA, Nesto RW, Serhan CN. Angioplasty triggers intracoronary leukotrienes and lipoxin A4. Impact of aspirin therapy. Circulation. 1992;86:56–63. doi: 10.1161/01.cir.86.1.56. [DOI] [PubMed] [Google Scholar]
- 17.Shen J, Herderick E, Cornhill JF, Zsigmond E, Kim HS, Kuhn H, Guevara NV, Chan L. Macrophage-mediated 15-lipoxygenase expression protects against atherosclerosis development. J Clin Invest. 1996;98:2201–8. doi: 10.1172/JCI119029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serhan CN, Jain A, Marleau S, Clish C, Kantarci A, Behbehani B, Colgan SP, Stahl GL, Merched A, Petasis NA, Chan L, Van Dyke TE. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol. 2003;171:6856–65. doi: 10.4049/jimmunol.171.12.6856. [DOI] [PubMed] [Google Scholar]
- 19.Poeckel D, Funk CD. The 5-lipoxygenase/leukotriene pathway in preclinical models of cardiovascular disease. Cardiovasc Res. 2010;86:243–53. doi: 10.1093/cvr/cvq016. [DOI] [PubMed] [Google Scholar]
- 20.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB Journal. 2008;22:3595–606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merched AJ, Serhan CN, Chan L. Nutrigenetic disruption of inflammation-resolution homeostasis and atherogenesis. J Nutrigenet Nutrigenomics. 2011;4:12–24. doi: 10.1159/000326890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cyrus T, Witztum JL, Rader DJ, Tangirala R, Fazio S, Linton MF, Funk CD. Disruption of the 12/15-lipoxygenase gene diminishes atherosclerosis in apo E-deficient mice. J Clin Invest. 1999;103:1597–604. doi: 10.1172/JCI5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasturk H, Abdallah R, Kantarci A, Nguyen D, Giordano N, Hamilton J, Van Dyke TE. Resolvin E1 (RvE1) Attenuates Atherosclerotic Plaque Formation in Diet and Inflammation-Induced Atherogenesis. Arterioscler Thromb Vasc Biol. 2015;35:1123–33. doi: 10.1161/ATVBAHA.115.305324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tonetti MS, Van Dyke TE working group 1 of the joint EFPAAPw. Periodontitis and atherosclerotic cardiovascular disease: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol. 2013;84:S24–9. doi: 10.1902/jop.2013.1340019. [DOI] [PubMed] [Google Scholar]
- 25.Cheuk BL, Cheng SW. Annexin A1 expression in atherosclerotic carotid plaques and its relationship with plaque characteristics. Eur J Vasc Endovasc Surg. 2011;41:364–71. doi: 10.1016/j.ejvs.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Viiri LE, Full LE, Navin TJ, Begum S, Didangelos A, Astola N, Berge RK, Seppala I, Shalhoub J, Franklin IJ, Perretti M, Lehtimaki T, Davies AH, Wait R, Monaco C. Smooth muscle cells in human atherosclerosis: proteomic profiling reveals differences in expression of Annexin A1 and mitochondrial proteins in carotid disease. J Mol Cell Cardiol. 2013;54:65–72. doi: 10.1016/j.yjmcc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Drechsler M, de Jong R, Rossaint J, Viola JR, Leoni G, Wang JM, Grommes J, Hinkel R, Kupatt C, Weber C, Doring Y, Zarbock A, Soehnlein O. Annexin A1 counteracts chemokine-induced arterial myeloid cell recruitment. Circ Res. 2015;116:827–35. doi: 10.1161/CIRCRESAHA.116.305825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doring Y, Drechsler M, Wantha S, Kemmerich K, Lievens D, Vijayan S, Gallo RL, Weber C, Soehnlein O. Lack of neutrophil-derived CRAMP reduces atherosclerosis in mice. Circ Res. 2012;110:1052–6. doi: 10.1161/CIRCRESAHA.112.265868. [DOI] [PubMed] [Google Scholar]
- 29.Filep JG. Biasing the lipoxin A4/formyl peptide receptor 2 pushes inflammatory resolution. Proc Natl Acad Sci U S A. 2013;110:18033–4. doi: 10.1073/pnas.1317798110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooray SN, Gobbetti T, Montero-Melendez T, McArthur S, Thompson D, Clark AJ, Flower RJ, Perretti M. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc Natl Acad Sci U S A. 2013;110:18232–7. doi: 10.1073/pnas.1308253110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perretti M, Leroy X, Bland EJ, Montero-Melendez T. Resolution Pharmacology: Opportunities for Therapeutic Innovation in Inflammation. Trends Pharmacol Sci. 2015;36:737–55. doi: 10.1016/j.tips.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–8. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 33.Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–61. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 34.Fredman G, Hellmann J, Proto JD, Kuriakose G, Colas RA, Dorweiler B, Connolly ES, Solomon R, Jones DM, Heyer EJ, Spite M, Tabas I. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat Commun. 2016;7:12859. doi: 10.1038/ncomms12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viola JR, Lemnitzer P, Jansen Y, Csaba G, Winter C, Neideck C, Silvestre-Roig C, Dittmar G, Doring Y, Drechsler M, Weber C, Zimmer R, Cenac N, Soehnlein O. Resolving Lipid Mediators Maresin 1 and Resolvin D2 Prevent Atheroprogression in Mice. Circ Res. 2016;119:1030–1038. doi: 10.1161/CIRCRESAHA.116.309492. [DOI] [PubMed] [Google Scholar]
- 36.Petri MH, Laguna-Fernandez A, Arnardottir H, Wheelock CE, Perretti M, Hansson GK, Back M. Aspirin-triggered lipoxin inhibits atherosclerosis progression in ApoE−/− mice. Br J Pharmacol. 2017 doi: 10.1111/bph.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fredman G, Kamaly N, Spolitu S, Milton J, Ghorpade D, Chiasson R, Kuriakose G, Perretti M, Farokhzad O, Tabas I. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci Transl Med. 2015;7:275ra20. doi: 10.1126/scitranslmed.aaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:e60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–74. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–9. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 42.Norling LV, Headland SE, Dalli J, Arnardottir HH, Haworth O, Jones HR, Irimia D, Serhan CN, Perretti M. Proresolving and cartilage-protective actions of resolvin D1 in inflammatory arthritis. JCI Insight. 2016;1:e85922. doi: 10.1172/jci.insight.85922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kain V, Ingle KA, Colas RA, Dalli J, Prabhu SD, Serhan CN, Joshi M, Halade GV. Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J Mol Cell Cardiol. 2015;84:24–35. doi: 10.1016/j.yjmcc.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borgeson E, Docherty NG, Murphy M, Rodgers K, Ryan A, O’Sullivan TP, Guiry PJ, Goldschmeding R, Higgins DF, Godson C. Lipoxin A(4) and benzo-lipoxin A(4) attenuate experimental renal fibrosis. FASEB J. 2011;25:2967–79. doi: 10.1096/fj.11-185017. [DOI] [PubMed] [Google Scholar]
- 45.Yatomi M, Hisada T, Ishizuka T, Koga Y, Ono A, Kamide Y, Seki K, Aoki-Saito H, Tsurumaki H, Sunaga N, Kaira K, Dobashi K, Yamada M, Okajima F. 17(R)-resolvin D1 ameliorates bleomycin-induced pulmonary fibrosis in mice. Physiol Rep. 2015:3. doi: 10.14814/phy2.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiurchiu V, Leuti A, Dalli J, Jacobsson A, Battistini L, Maccarrone M, Serhan CN. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci Transl Med. 2016;8:353ra111. doi: 10.1126/scitranslmed.aaf7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–6. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keyes KT, Ye Y, Lin Y, Zhang C, Perez-Polo JR, Gjorstrup P, Birnbaum Y. Resolvin E1 protects the rat heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;299:H153–64. doi: 10.1152/ajpheart.01057.2009. [DOI] [PubMed] [Google Scholar]
- 49.Duffield JS, Hong S, Vaidya VS, Lu Y, Fredman G, Serhan CN, Bonventre JV. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol. 2006;177:5902–11. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 50.Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D, Toner M, Serhan CN. Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J Immunol. 2008;181:8677–87. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiang N, Dalli J, Colas RA, Serhan CN. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J Exp Med. 2015;212:1203–17. doi: 10.1084/jem.20150225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dufton N, Hannon R, Brancaleone V, Dalli J, Patel HB, Gray M, D’Acquisto F, Buckingham JC, Perretti M, Flower RJ. Anti-inflammatory role of the murine formyl-peptide receptor 2: ligand-specific effects on leukocyte responses and experimental inflammation. J Immunol. 2010;184:2611–9. doi: 10.4049/jimmunol.0903526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colgan SP. Neutrophils and inflammatory resolution in the mucosa. Semin Immunol. 2015;27:177–83. doi: 10.1016/j.smim.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M, Weber C, Soehnlein O, Steffens S. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J. 2016 doi: 10.1093/eurheartj/ehw002. [DOI] [PubMed] [Google Scholar]
- 55.Gao Y, Min K, Zhang Y, Su J, Greenwood M, Gronert K. Female-Specific Downregulation of Tissue Polymorphonuclear Neutrophils Drives Impaired Regulatory T Cell and Amplified Effector T Cell Responses in Autoimmune Dry Eye Disease. J Immunol. 2015;195:3086–99. doi: 10.4049/jimmunol.1500610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vartanian SM, Conte MS. Surgical intervention for peripheral arterial disease. Circ Res. 2015;116:1614–28. doi: 10.1161/CIRCRESAHA.116.303504. [DOI] [PubMed] [Google Scholar]
- 57.Bonaca MP, Creager MA. Pharmacological treatment and current management of peripheral artery disease. Circ Res. 2015;116:1579–98. doi: 10.1161/CIRCRESAHA.114.303505. [DOI] [PubMed] [Google Scholar]
- 58.Chilian WM, Penn MS, Pung YF, Dong F, Mayorga M, Ohanyan V, Logan S, Yin L. Coronary collateral growth--back to the future. J Mol Cell Cardiol. 2012;52:905–11. doi: 10.1016/j.yjmcc.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang MJ, Sansbury BE, Hellmann J, Baker JF, Guo L, Parmer CM, Prenner JC, Conklin DJ, Bhatnagar A, Creager MA, Spite M. Resolvin D2 Enhances Postischemic Revascularization While Resolving Inflammation. Circulation. 2016;134:666–80. doi: 10.1161/CIRCULATIONAHA.116.021894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Foley JH, Conway EM. Cross Talk Pathways Between Coagulation and Inflammation. Circ Res. 2016;118:1392–408. doi: 10.1161/CIRCRESAHA.116.306853. [DOI] [PubMed] [Google Scholar]
- 61.Chiang N, Bermudez EA, Ridker PM, Hurwitz S, Serhan CN. Aspirin triggers antiinflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc Natl Acad Sci U S A. 2004;101:15178–83. doi: 10.1073/pnas.0405445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chiang N, Hurwitz S, Ridker PM, Serhan CN. Aspirin has a gender-dependent impact on antiinflammatory 15-epi-lipoxin A4 formation: a randomized human trial. Arterioscler Thromb Vasc Biol. 2006;26:e14–7. doi: 10.1161/01.ATV.0000196729.98651.bf. [DOI] [PubMed] [Google Scholar]
- 63.Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A, Cheng G, von Andrian UH, Serhan CN. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112:848–55. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fredman G, Van Dyke TE, Serhan CN. Resolvin E1 regulates adenosine diphosphate activation of human platelets. Arterioscler Thromb Vasc Biol. 2010;30:2005–13. doi: 10.1161/ATVBAHA.110.209908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bohr S, Patel SJ, Sarin D, Irimia D, Yarmush ML, Berthiaume F. Resolvin D2 prevents secondary thrombosis and necrosis in a mouse burn wound model. Wound Repair Regen. 2013;21:35–43. doi: 10.1111/j.1524-475X.2012.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elajami TK, Colas RA, Dalli J, Chiang N, Serhan CN, Welty FK. Specialized proresolving lipid mediators in patients with coronary artery disease and their potential for clot remodeling. FASEB J. 2016;30:2792–801. doi: 10.1096/fj.201500155R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abdulnour RE, Dalli J, Colby JK, Krishnamoorthy N, Timmons JY, Tan SH, Colas RA, Petasis NA, Serhan CN, Levy BD. Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proc Natl Acad Sci U S A. 2014;111:16526–31. doi: 10.1073/pnas.1407123111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Norris PC, Arnardottir H, Sanger JM, Fichtner D, Keyes GS, Serhan CN. Resolvin D3 multi-level proresolving actions are host protective during infection. Prostaglandins Leukot Essent Fatty Acids. 2016 doi: 10.1016/j.plefa.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gerdes N, Seijkens T, Lievens D, Kuijpers MJ, Winkels H, Projahn D, Hartwig H, Beckers L, Megens RT, Boon L, Noelle RJ, Soehnlein O, Heemskerk JW, Weber C, Lutgens E. Platelet CD40 Exacerbates Atherosclerosis by Transcellular Activation of Endothelial Cells and Leukocytes. Arterioscler Thromb Vasc Biol. 2016;36:482–90. doi: 10.1161/ATVBAHA.115.307074. [DOI] [PubMed] [Google Scholar]
- 70.Lannan KL, Spinelli SL, Blumberg N, Phipps RP. Maresin 1 induces a novel pro-resolving phenotype in human platelets. J Thromb Haemost. 2017 doi: 10.1111/jth.13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spite M, Claria J, Serhan CN. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014;19:21–36. doi: 10.1016/j.cmet.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kojima Y, Volkmer JP, McKenna K, Civelek M, Lusis AJ, Miller CL, Direnzo D, Nanda V, Ye J, Connolly AJ, Schadt EE, Quertermous T, Betancur P, Maegdefessel L, Matic LP, Hedin U, Weissman IL, Leeper NJ. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536:86–90. doi: 10.1038/nature18935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fredman G, Ozcan L, Spolitu S, Hellmann J, Spite M, Backs J, Tabas I. Resolvin D1 limits 5-lipoxygenase nuclear localization and leukotriene B4 synthesis by inhibiting a calcium-activated kinase pathway. Proc Natl Acad Sci U S A. 2014;111:14530–5. doi: 10.1073/pnas.1410851111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cai B, Thorp EB, Doran AC, Subramanian M, Sansbury BE, Lin CS, Spite M, Fredman G, Tabas I. MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proc Natl Acad Sci U S A. 2016;113:6526–31. doi: 10.1073/pnas.1524292113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cai B, Thorp EB, Doran AC, Sansbury BE, Daemen MJ, Dorweiler B, Spite M, Fredman G, Tabas I. MerTK receptor cleavage promotes plaque necrosis and defective resolution in atherosclerosis. J Clin Invest. 2017;127:564–568. doi: 10.1172/JCI90520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wan E, Yeap XY, Dehn S, Terry R, Novak M, Zhang S, Iwata S, Han X, Homma S, Drosatos K, Lomasney J, Engman DM, Miller SD, Vaughan DE, Morrow JP, Kishore R, Thorp EB. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res. 2013;113:1004–12. doi: 10.1161/CIRCRESAHA.113.301198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Howangyin KY, Zlatanova I, Pinto C, Ngkelo A, Cochain C, Rouanet M, Vilar J, Lemitre M, Stockmann C, Fleischmann BK, Mallat Z, Silvestre JS. Myeloid-Epithelial-Reproductive Receptor Tyrosine Kinase and Milk Fat Globule Epidermal Growth Factor 8 Coordinately Improve Remodeling After Myocardial Infarction via Local Delivery of Vascular Endothelial Growth Factor. Circulation. 2016;133:826–39. doi: 10.1161/CIRCULATIONAHA.115.020857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li S, Sun Y, Liang CP, Thorp EB, Han S, Jehle AW, Saraswathi V, Pridgen B, Kanter JE, Li R, Welch CL, Hasty AH, Bornfeldt KE, Breslow JL, Tabas I, Tall AR. Defective phagocytosis of apoptotic cells by macrophages in atherosclerotic lesions of ob/ob mice and reversal by a fish oil diet. Circ Res. 2009;105:1072–82. doi: 10.1161/CIRCRESAHA.109.199570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee HN, Surh YJ. Resolvin D1-mediated NOX2 inactivation rescues macrophages undertaking efferocytosis from oxidative stress-induced apoptosis. Biochem Pharmacol. 2013;86:759–69. doi: 10.1016/j.bcp.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 80.Glass CK, Natoli G. Molecular control of activation and priming in macrophages. Nat Immunol. 2016;17:26–33. doi: 10.1038/ni.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tabas I, Bornfeldt KE. Macrophage Phenotype and Function in Different Stages of Atherosclerosis. Circ Res. 2016;118:653–67. doi: 10.1161/CIRCRESAHA.115.306256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–21. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feig JE, Vengrenyuk Y, Reiser V, Wu C, Statnikov A, Aliferis CF, Garabedian MJ, Fisher EA, Puig O. Regression of atherosclerosis is characterized by broad changes in the plaque macrophage transcriptome. PLoS One. 2012;7:e39790. doi: 10.1371/journal.pone.0039790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–8. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morita M, Kuba K, Ichikawa A, Nakayama M, Katahira J, Iwamoto R, Watanebe T, Sakabe S, Daidoji T, Nakamura S, Kadowaki A, Ohto T, Nakanishi H, Taguchi R, Nakaya T, Murakami M, Yoneda Y, Arai H, Kawaoka Y, Penninger JM, Arita M, Imai Y. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell. 2013;153:112–25. doi: 10.1016/j.cell.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 86.Norling LV, Spite M, Yang R, Flower RJ, Perretti M, Serhan CN. Cutting edge: Humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J Immunol. 2011;186:5543–7. doi: 10.4049/jimmunol.1003865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu B, Mottola G, Chatterjee A, Lance KD, Chen M, Siguenza IO, Desai TA, Conte MS. Perivascular delivery of resolvin D1 inhibits neointimal hyperplasia in a rat model of arterial injury. J Vasc Surg. 2017;65:207–217. e3. doi: 10.1016/j.jvs.2016.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lance KD, Chatterjee A, Wu B, Mottola G, Nuhn H, Lee PP, Sansbury BE, Spite M, Desai TA, Conte MS. Unidirectional and sustained delivery of the proresolving lipid mediator resolvin D1 from a biodegradable thin film device. J Biomed Mater Res A. 2017;105:31–41. doi: 10.1002/jbm.a.35861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salic K, Morrison MC, Verschuren L, Wielinga PY, Wu L, Kleemann R, Gjorstrup P, Kooistra T. Resolvin E1 attenuates atherosclerosis in absence of cholesterol-lowering effects and on top of atorvastatin. Atherosclerosis. 2016;250:158–165. doi: 10.1016/j.atherosclerosis.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 90.Kusters DH, Chatrou ML, Willems BA, De Saint-Hubert M, Bauwens M, van der Vorst E, Bena S, Biessen EA, Perretti M, Schurgers LJ, Reutelingsperger CP. Pharmacological Treatment with Annexin A1 Reduces Atherosclerotic Plaque Burden in LDLR−/− Mice on Western Type Diet. PLoS One. 2015;10:e0130484. doi: 10.1371/journal.pone.0130484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kamaly N, Fredman G, Fojas JJ, Subramanian M, Choi WI, Zepeda K, Vilos C, Yu M, Gadde S, Wu J, Milton J, Carvalho Leitao R, Rosa Fernandes L, Hasan M, Gao H, Nguyen V, Harris J, Tabas I, Farokhzad OC. Targeted Interleukin-10 Nanotherapeutics Developed with a Microfluidic Chip Enhance Resolution of Inflammation in Advanced Atherosclerosis. ACS Nano. 2016;10:5280–92. doi: 10.1021/acsnano.6b01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Petri MH, Laguna-Fernandez A, Tseng CN, Hedin U, Perretti M, Back M. Aspirin-triggered 15-epi-lipoxin A(4) signals through FPR2/ALX in vascular smooth muscle cells and protects against intimal hyperplasia after carotid ligation. Int J Cardiol. 2015;179:370–2. doi: 10.1016/j.ijcard.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miyahara T, Runge S, Chatterjee A, Chen M, Mottola G, Fitzgerald JM, Serhan CN, Conte MS. D-series resolvin attenuates vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. FASEB J. 2013;27:2220–32. doi: 10.1096/fj.12-225615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Akagi D, Chen M, Toy R, Chatterjee A, Conte MS. Systemic delivery of proresolving lipid mediators resolvin D2 and maresin 1 attenuates intimal hyperplasia in mice. FASEB J. 2015;29:2504–13. doi: 10.1096/fj.14-265363. [DOI] [PMC free article] [PubMed] [Google Scholar]