Abstract
Fullerenes and related carbon based derivatives have shown a growing relevance in biology and medicine, mainly due to the unique electronic and structural properties that make them excellent candidates for multiple functionalization. This review focuses on the most recent developments of fullerene derivatives for different biological applications.
Graphical abstract
1. Introduction
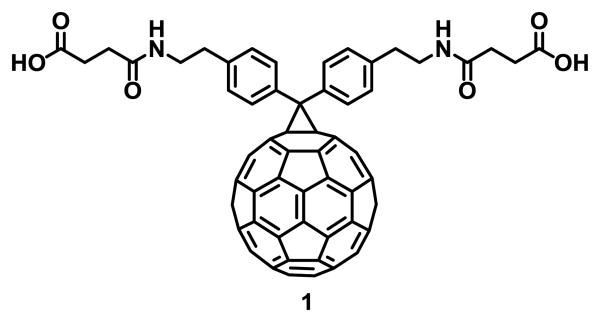
The research on fullerene C60 started in 1985 when Kroto, Curl and Smalley discovered the third allotropic form of carbon after diamond and graphite.1 The first functionalization reaction2 was reported soon after the fullerenes were synthesized in macroscopic amounts in 1990.3 Functionalized fullerenes exhibit improved solubility and different chemical and physical properties that allow applications in multiple fields.4 In 1993 Wudl and coworkers reported the first water-soluble fullerene derivative 1 (Fig. 1) that inhibited the activity of the HIV-1 Protease (HIV-1 PR), as determined from the interaction of this compound and the HIV-1 PR on an in vitro assay.5 Since then, efficient synthetic methods for fullerene functionalization have been developed.6, 7 Functionalization with bioactive compounds that exhibit affinity to certain nucleic acids, proteins or cell receptors such as peptides8-10 or saccharides,11, 12 functionalization with polar or ionic groups such as hydroxyl,13, 14 carbonyl15-17 or quaternary ammonium salts,18, 19 and encapsulation with macromolecules20, 21 are the most common approaches to synthesize water-soluble fullerene derivatives for biomedical applications.22-26
Fig. 1.
First water-soluble and biologically active fullerene derivative reported.
Several reviews have been published that summarize the biological applications of fullerene derivatives.23, 27, 28 In this review, we summarize the biological properties of active fullerene derivatives reported during the last 3 years along, with their biomedical applications.
2. Properties
The three dimensional shape of fullerenes results in unique chemical and physical properties. Chemical functionalization can be easily achieved to alter the properties of the new compounds.7, 29-31
2.1 Chemical properties
Due to the spherical shape of fullerenes, carbon atoms are highly pyramidalized and are thus highly reactive. The conjugated carbon atoms deviate from planarity by orbital rehybridization of the sp2 orbitals to sp2.27 orbitals with a p-character gain.32
C60 and C70 fullerenes can be reversibly reduced with up to six electrons.33 This high electron affinity results from the presence of triply degenerate low-lying lowest unoccupied molecular orbitals (LUMOs).34
The chemical reactivity of fullerenes is similar to that of an electron deficient olefin, yielding cycloaddition reaction products35 where the addition takes place at a 6,6 ring junction. Additions to 5,6 ring junctions have been reported as a result of rearrangements after the initial kinetic addition to a 6,6 bond.32
2.2 Physical properties
Fullerenes can resist high pressures and return to their original shape after being subjected to over 3000 atmospheres.36 It was theoretically calculated that a single C60 molecule has an effective bulk modulus of 668 GPa when compressed to 75% of its size. This property makes fullerenes harder than steel and diamond, whose bulk moduli are 160 GPa and 442 GPa, respectively.37
Fullerenes are the only known allotropes of carbon that can be dissolved in some common solvents at room temperature. Large indices of refraction, a dielectric constant of approximately 4, large molecular volumes, and Hildebrand solubility parameter equal to 10 cal1/2cm-3/2 have been reported for C60 solutions.38
3. Antiviral activity
3.1 Anti HIV-1
In the absence of an effective vaccine, antiretroviral therapy (ART) is the only therapeutic tool that can be used to treat HIV-1 infection. Usually, this protocol combines a nucleoside reverse-transcriptase inhibitor (RTI) with either a non-nucleoside RTI or a protease inhibitor (PI).39 These ARTs efficiently suppress the spread of HIV in patients; however, the emergence of multidrug resistant viruses is still a serious problem in anti-HIV therapy.39-41 Therefore, the development of new type of anti-HIV drugs that are structurally distinct from the currently used pharmaceutical agents is a critical need.
It has been demonstrated in vivo and in vitro that impairment of viral maturation is a powerful strategy to block HIV-1 replication. HIV-1 maturation can be inhibited by PIs or by maturation inhibitors (MIs). The latter can bind to Gag and affect its processing or to CA, thus impairing core assembly.42 Currently, there are no maturation inhibitors used clinically.43
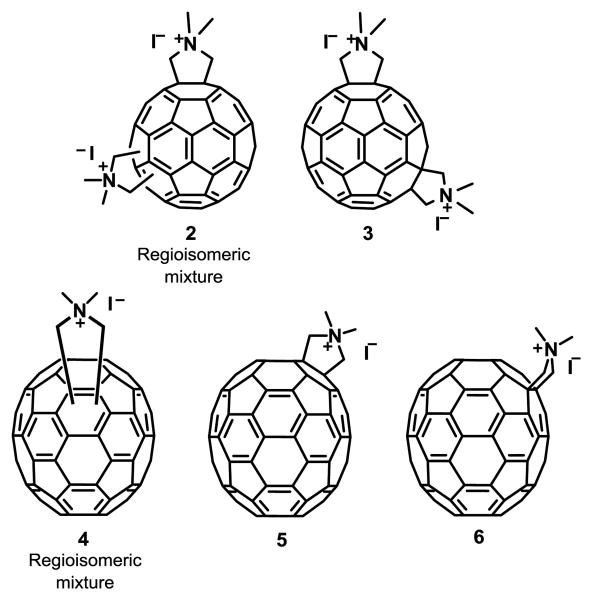
HIV PIs work by specifically binding to the active site and blocking the activity of the protease. For 24 years, it was assumed that fullerene inhibition of HIV-1 was only due to the interaction between the fullerene and the hydrophobic pocket of this enzyme, as determined by in silico predictions combined with an in vitro assay.5, 44 This paradigm was recently challenged by the fact that C60 and C70 fullerene derivatives do not inhibit the activity of HIV-1 PR at concentrations (3 μM) that potently inhibit HIV-1 infection (see structures in Fig. 2).19, 45 Additionally, fullerenes 2-6 inhibited HIV-1 viruses resistant to multiple PR and MIs. Although the mechanism of action is not known, it was suggested to be via impairment of virus maturation as a result of a strong interaction with the immature capsid in pull-down experiments.19 Fullerene derivatives 2-6 were reported to exhibit no cytotoxicity, therefore they are excellent candidates as anti HIV-1 agents.19, 45 Due to the solubility of [60]fullerene-peptide derivatives, they have also been considered as potential inhibitors of HIV-1 replication.46 Using drug screening and docking calculations, a database of PI [60]fullerene derivatives and their binding constants were predicted.47 Strom et al.48 determined that improved inhibition can be achieved with minor modifications of both the peptide and the amino acid structure.
Fig. 2. Maturation inhibitor fullerene derivatives.
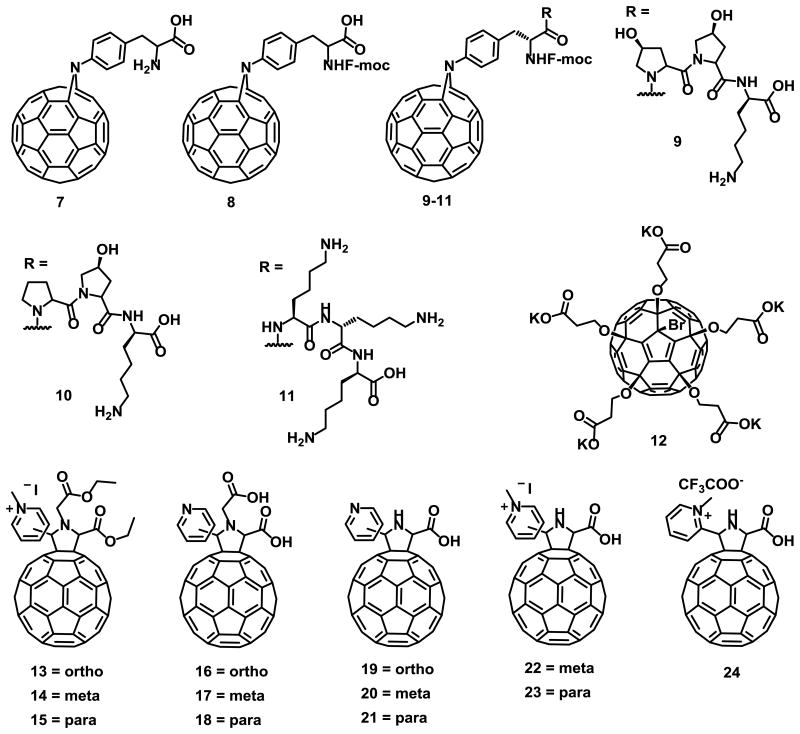
PR inhibition by [60]fullerene peptides 7-11 (Fig. 3) was predicted in silico, and binding constants were calculated experimentally using a cell-free fluorescence resonance electron transfer (FRET) assay.48 Recently Khakina et al.49 reported a new fullerene derivative 12 (Fig. 3) that exhibits low cytotoxicity (above 100 μM) and blocks the HIV-1 infection in vitro, with a minimum inhibitory concentration (EC50) of 4.6 μM. Compound 12 showed higher anti HIV-1 activity than its pentasulfide analogue C60(SC2H4COOK)5H (EC50 = 45.2 μM).50 However, no mechanism of action was proposed for this antiviral compound.
Fig. 3.
Structure of the anti HIV-1 [60]fullerene derivatives 7-24.
As previously mentioned, the HIV-1 reverse transcriptase (RT) is required for early proviral DNA synthesis and thus inhibition of this enzyme results in inhibition of the virus replication. The HIV-1 RT inhibitory activity of cationic, anionic and zwitterion fullerene derivatives have been previously reported,51 where cationic fullerenes showed lower activity. Yasuno et al.52 reported for first time that pyridine/pyridinium-type fullerene derivatives 13-15 (Fig. 3) without any carboxylic acid, inhibit the HIV-1 RT in a cell-free environment, with no cytotoxicity and clearly showing that the fullerene cage was crucial for the inhibition of HIV-RT. Compounds 16-24 (Fig. 3), were also active against HIV-RT.
3.2 Bacterial and viral inhibition via anti-adhesion to host cells
3.2.1 Anti-bacterial inhibition
Molecular recognition between carbohydrates and proteins play and essential role in many biological processes.53 One example is bacterial infection that begins with bacterial adhesion to host cells. Inhibition of this step using glycoclusters or glycodendrimers that mimic multivalent carbohydrates at the cell surface is one of the most studied mechanisms over the last decade.53 Protein-carbohydrate interactions exhibit high specificity and weak affinities toward carbohydrate ligands. It has been demonstrated that ligand multivalency increases the low binding affinities of monovalent glycosides.53
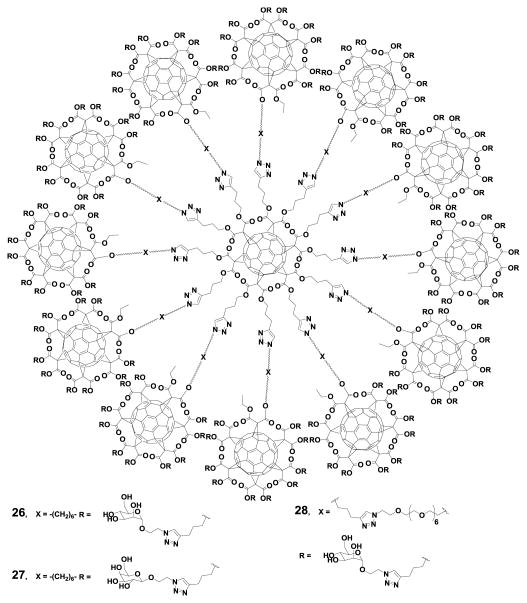
Nierengarten et al.54 have demonstrated that fullerenes are very attractive spherical scaffolds that can be functionalized with carbohydrates using a very straightforward approach. Using this synthetic approach Sánchez-Navarro et al.55 reported new hexakis adducts of C60 endowed with 24 sugars moieties. The carbohydrate recognition of these new derivatives by a lectin was investigated by isothermal titration calorimetry (ITC) experiments. The results showed that fullerene C60 is an efficient platform for the globular presentation of carbohydrates for protein-carbohydrate interactions. Buffet et al.56 reported the synthesis and the multivalent effect against two lectins, LecB and RSL (compounds 25A-25E) (Fig. 4). The results showed higher affinity for compound 25E, which contains higher valency and a longer linker between the core and the sugar. This fullerene derivative appears to be one of the best inhibitors for both lectins.
Fig. 4.
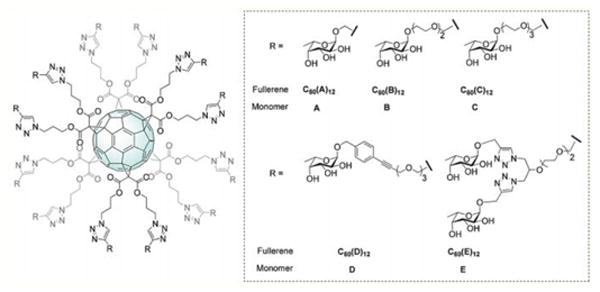
Fucofullerenes and their corresponding monomeric controls. Reproduced from Ref.56 with permission from The Royal Society of Chemistry.
3.2.2 Anti Ebola-virus
Ebola virus infection causes severe and often fatal hemorrhagic fever in humans and other mammals. The 2013-2015 Ebola virus epidemic in West Africa57 caused at least 11,310 confirmed deaths.58 The virus initiates infection by binding to host receptors through its glycoprotein surface. DC-SIGN, a C-type lectin receptor has been proposed as one of the receptors for cell entrance for several microorganisms that contain glycoconjugates on their envelope.
Due to the important role of DC-SIGN in the first step of the viral infections such as HIV and Ebola,59, 60 the discovery of new compounds with higher affinity for this receptor is of great importance.
The sugar residues in fullerenes act as solubilizing groups and induce remarkable biological properties.56, 61-64 In 2013, Luczkowiak et al.65 reported for the first time the potential application of glycodendrofullerenes as antiviral agents. In 2016 Muñoz et al.12 reported the synthesis of hexakis adducts of fullerene C60, with the most effective dendrimeric growth (compounds 26-28) (Fig. 5). Compounds 25 and 27 showed very potent antiviral activity in the range of nM and pM concentrations, respectively. The results clearly showed the dependence of the inhibition effect on mannoses, compound 26 with 120 galactoses was not active.12
Fig. 5.
Giant globular multivalent glycofullerenes. Reprinted by permission from Macmillan Publishers Ltd: Nature Chemistry, Muñoz et al.12 copyright (2016).
4. Photodynamic therapy
Photodynamic therapy (PDT) is a clinically approved therapeutic technique for the treatment of multiple diseases, mainly cancer.66 It relies on the photoexcitation of an inactive photosensitizer (PS) at a wavelength that matches the absorption of the PS, leading to localized generation of cytotoxic reactive oxygen species (ROS). ROS cause oxidative damage to nearly all types of biomolecules (proteins, lipids and nucleic acids) and tumor cell death.67 PDT has remarkably improved selectivity and fewer side effects than traditional anticancer therapies.68
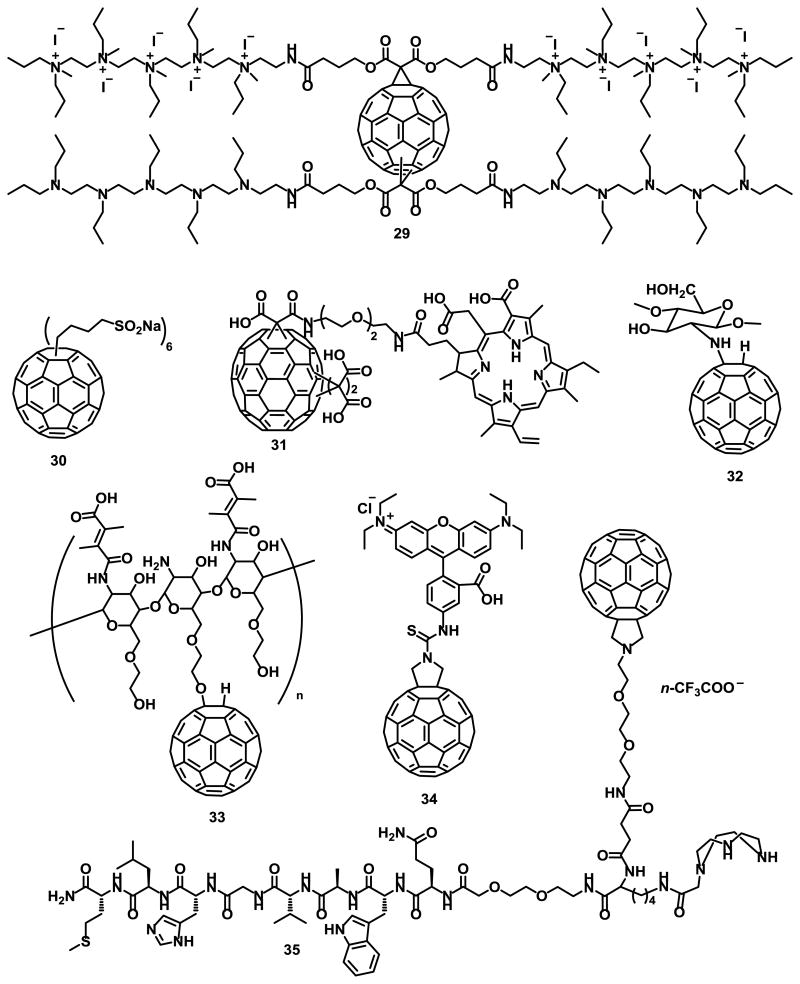
Fullerenes C60 and C70 can effectively be excited to their triplet state and can efficiently convert 3O2 to 1O2.69, 70 Hamblin and coworkers71-74 have tested electron-transfer reactions of photoexcited cationic fullerene derivatives both in vitro and in vivo on animal models with localized bacterial infections. The activity of compound 29 (Fig. 6) was significantly improved by the addition of potassium iodide (KI) salt, although how the iodide anion enhances the antibacterial effects is not completely understood.72
Fig. 6.
Fullerene derivatives for PDT applications.
Functionalization of C60 with n-butyl sulfonic acid salts to form self-assembled molecular micellar nanospheres, as with compound 30 (Fig. 6), was found to retain high efficiency in generating singlet oxygen upon irradiation with visible light.75 The mechanism was correlated to the facile intermolecular triplet energy-transfer from photoexcited 330* to molecular oxygen. Guan et al.76 reported nanovesicles of fullerene C70 tris-adducts conjugated with chlorin e6 (Ce6), compounds 31, (Fig. 6) through an oligo ethylene glycol linker. This system significantly enhanced both cellular uptake efficiency and PDT in vitro and in vivo. Biocompatibility and total body release of these particles are among the advantages of these conjugated nanovesicles.76
The production of ROS in mitochondria plays a critical role in photodynamic therapy. Li et al.77 investigated the generation of additional endogenous ROS in mitochondria using a chitosan oligosaccharide grafted fullerene conjugate (CS–C60) compound 32 (Fig.6). The ROS generated at low doses of CS-C60 was rich in mitochondria, enhancing the inhibition of human malignant melanoma (A375) cells. Rotenone Assays showed that PDT-induced endogenous ROS was generated through an electron transport chain (ETC).77 Kim et al.78 reported the synthesis of chitosan/fullerene nanogel compound 33 (Fig. 6) for efficient tumor therapy. At pH 7.4, electrostatic interactions between the pendant carboxylic acid groups of 2,3-dimethylmaleic acid (DMA) and the residual free amine groups of glycol chitosan (GC) resulted in the formation of multinanogel aggregates. Nevertheless, dissociation of the nanogel aggregates was observed at pH 5.0, resulting in improved singlet oxygen generation and elevated photodynamic tumor cell ablation.78
Franskevych et al.79 reported the cytotoxic effect of C60 in combination with visible light irradiation on leukemic L1210 cell lines, both sensitive and resistant to cisplatin. Their results suggest that C60-mediated photodynamic treatment is a good candidate for restoring drug-resistant leukemic cells induced to mitochondrial apoptosis. C60-RITC compound 34 (Fig. 6) was synthesized to monitor C60 entry into leukemic cells via confocal microscopy.79
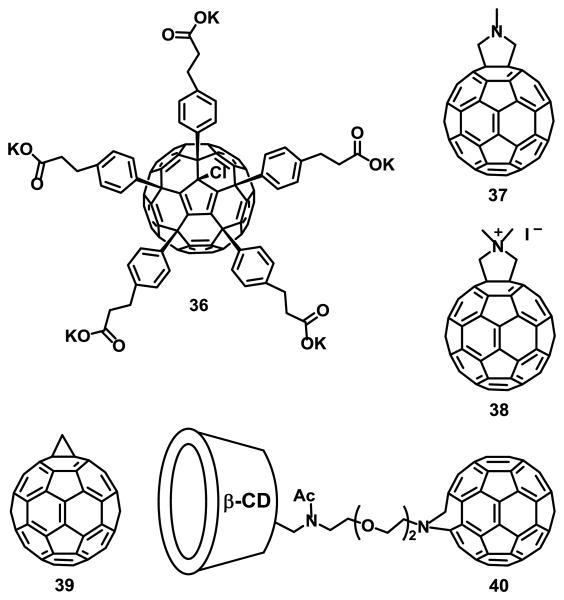
Mion et al.80 reported the synthesis of fullerene compound 35 (Fig. 6) for photodynamic therapy and imaging applications. Although it was not completely isolated, its presence was confirmed by MALDI-TOF. Ershova et al.81 studied the cytotoxicity and genotoxicity of a C60 fullerene derivative functionalized with five residues of 3-phenylpropionic acid and a chlorine atom arranged around one cyclopentadienyl unit, compound 36 (Fig. 7), in diploid human embryonic lung fibroblasts (HELFs). It was found to cause complex time-dependent changes in ROS levels. At short exposure times, when it had not yet penetrated the cytoplasm, compound 36 considerably decreased ROS levels in the medium and on the surface of the cell membrane. Long-term exposure can induce secondary oxidative stress and possibly induce pulmonary fibrosis.
Fig. 7.
Fullerene derivatives for photodynamic therapy.
Fullerene C60 derivatives 37-39 (Fig. 7) were incorporated into liposomes using a fullerene exchange method involving the transfer of fullerenes from the cavity of two γ-cyclodextrin molecules to a liposome, as illustrated in Fig. 8.20, 21
Fig. 8.
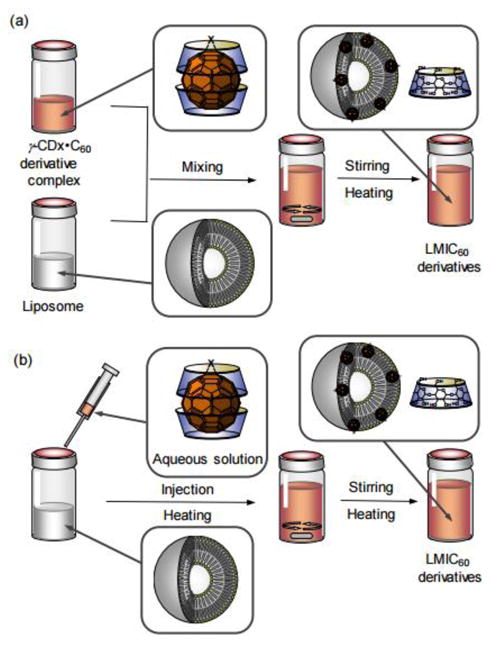
Exchange method used for the preparation of the LMIC60 derivatives. a) Rapid mixing of two solutions and b) Injection. Reproduced from Ikeda et al.21 with permission from The Royal Society of Chemistry.
The photodynamic activity of compound 38 incorporated into lipid membranes was much higher than that of compounds 37 and 39 under the same conditions. The high photodynamic activity of compound 38 was attributed to its increased 1O2 generating ability, as determined by 9,10-anthracenediyl-bis(methylene)dimalonic acid (ABDA) as a detector.82
Wang et al.83 reported the synthesis and DNA cleavage activity of a β-cyclodextrin fullerene C60, compound 40 (Fig. 7). The DNA cleaving ability was tested by using pBR322 plasmid DNA containing more than 90% supercoiled DNA, NADH and compound 40. DNA cleavage was observed after 4 h of visible light irradiation, but it was not observed under no light in the presence or absence of both NADH and compound 40. Additionally, compound 40 showed no significant cytotoxicity in human neuroblastoma SH-SY5Y cells, as determined by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.83
5. Antioxidant properties of fullerenol derivatives
Functionalization of fullerenes with polar functional groups is one of the approaches to overcome their insolubility in water, while retaining their unique properties and to enhance their biological activities.84-86 fullerenes are commonly classified as a “radical sponges”,87 due to their remarkable reactivity with free radicals.88-97
The antioxidant capacity of Fullerenols and their derivatives have been measured by DMPO-spin trap/ESR methods, and by using the β-carotene and DPPH assays.98 However, the kinetics and radical scavenging mechanisms of these compounds are still not completely understood. Density functional theory (DFT) calculations suggest that fullerenols with 6 and 12 hydroxyl groups are the most stable derivatives.99
Ueno et al.100 studied the antioxidant activity of several hydroxylated fullerenols with 8, 10, 12, 24, 26, 36, and 44 hydroxyl groups by a β-carotene bleaching assay. The relative radical scavenging rate of fullerenols toward radical species derived from linoleic acid under autoxidation conditions indicated that C60(OH)12 and C60(OH)44 reacted 1.62 and 1.54 times faster than β-carotene, respectively.
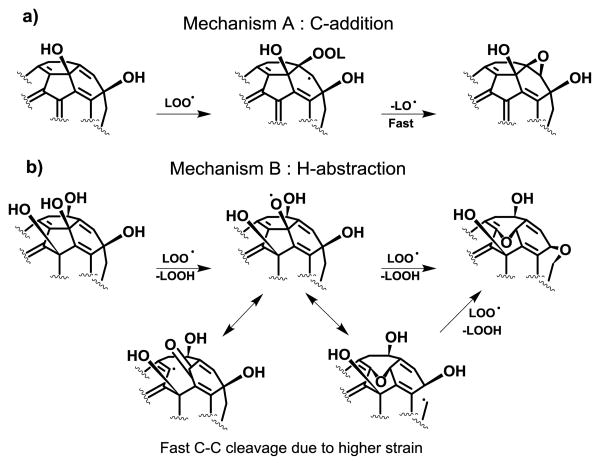
Based on the analysis of the possible products of both fullerenols and C60 with radical species generated from methyl linoleate under autoxidation conditions, the authors proposed two antioxidant mechanisms dependent on the number of hydroxyl groups (Fig. 9).100
Fig. 9.
Possible mechanisms for lipid peroxyl radical (LOO●) scavenging by fullerenols C60(OH)n: a) addition to C=C bonds and b) H-abstraction from -OH groups. Adapted from Ueno et al.100 (2014).
The C-addition, which includes the peroxyl radical addition to a conjugated C=C double bond (mechanism A), and the H-abstraction that involves hydrogen atom abstraction from –OH groups and the subsequent of the fullerene radical rearrangement to form ether bridge (mechanism B). Fullerenol C60(OH)10 with more π-conjugated double bonds undergoes the C-addition mechanism similar to C60, while fullerenol C60(OH)40 undergoes H-abstraction because of the less π-conjugated double bonds.
Grebowski et al.101 studied the rate constants of highly hydroxylated fullerene C60(OH)36 interacting with hydroxyl radicals (●OH) and hydrated electrons. The rate constant for the reaction of C60(OH)36 with ●OH was 2.0×109 dm3mol-1s-1, which was similar to that for C60(OH)18), 4.5×10-8 dm3mol-1s-1.102 These results showed that the decreased number of π bonds, due to the larger number of OH groups attached to the fullerene, did not significantly affect the rate constant for the reaction with hydroxyl radicals.
Srdjenovic et al.103 studied the influence of C60(OH)24 on Chinese hamster ovary cells (CHO-K1). Their results showed strong antioxidant properties influencing the cellular redox state and enzyme activities. Therefore, they reduce cell proliferation and were proposed as cytoprotective agents.
Improved free radical scavenger properties were reported by Awan et al.104 as a result of functionalization of C60(OH)30 on the surface of cellulose nanocrystals (CNCs). The immobilization appears to be due to the covalent interaction between the -OH groups on the CNC and π-conjugation of the fullerenol. It was proposed that this results in a reduced tendency to form clusters and greater accessibility to the free radicals (Fig. 10).
Fig. 10.
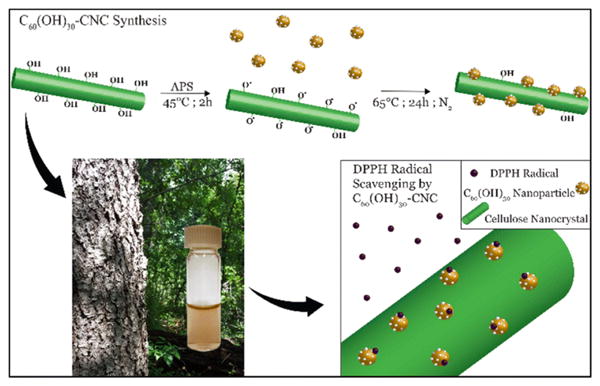
Schematic illustrating the conjugation of fullerenols on CNC. Reprinted from Awan et al.104 (2016), with permission of Springer.
Mitochondrial dysfunction is considered a crucial mechanism of nanomaterial toxicity. Yang et al.105 investigated the effects of C60(OH)44 on isolated mitochondria. The exposure of mitochondria to fullerenol not only affected the respiratory chain but also damaged the inner membrane, allowing permeability to H+ and K+ in a concentration dependent manner.
6. Anti cancer properties
Recent studies illustrate the wide range of avenues of the use of fullerenes as anticancer agents.106-113 Pyrrolidinium fullerene derivatives have been shown to induce mitochondrion- and caspase-dependent apoptosis through the generation of ROS in human promyeloleukaemia (HL-60) cells.114
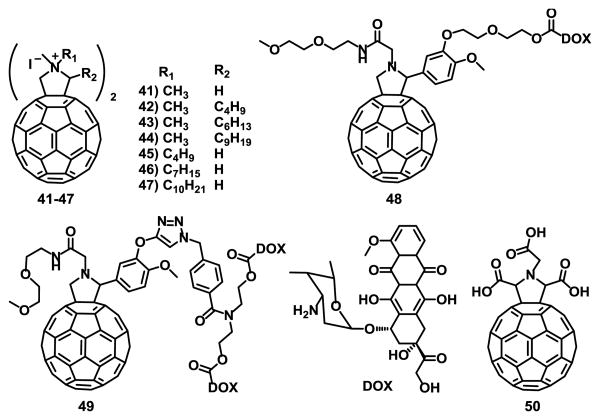
It has also been reported that pyrrolidinium fullerene compounds 41-47 (Fig. 11) induced apoptotic cell death of cells mutated on JAK2 V617F, which show resistance to multiple anti tumor drugs.115
Fig. 11.
Fullerene derivatives with anticancer properties.
The results showed that replacement of one hydrogen atom (compound 42) or the N-methyl group (compound 45) on compound 41 with a butyl group enhanced the ability of pyrrolidinium fullerenes to induce apoptosis on VF-Ba/F3 cells.115
Attachment of structural conjugate drugs to C60 is a good strategy to confer biological activity to fullerenes.116 Magoulas et al.117 studied the properties of fullerene C60 conjugated with one (48) and two (49) units of doxorubicin (DOX), a potent anticancer agent (Fig. 11). To avoid bis-adduct mixture of isomers, the authors opted for multiple arms on a mono-fulleropyrrolidine derivative (Fig. 11). These conjugates exhibited comparable, but with a more delayed onset, antiproliferative effect compared to free (non-conjugated) DOX when incubated with MCF-7 cells under the same conditions.
Probably, after conjugate entrance into the cells, the drug must be released from the conjugate to become active.
The effect of sonodynamically activated pyrrolidine tris-acid fullerene derivative 50 (Fig. 11) in isolated sarcoma-180 cells, and mice bearing ectopically-implanted colon 26 carcinoma was studied by Yumiko et al.118 The results showed a 5-fold enhanced cell damage induced by ultrasonic exposure in the presence of compound 50 (80 μM) and the growth of the implanted colon 26 carcinoma was decreased both in vitro and in vivo experiments. Histidine, a singlet oxygen and hydroxyl radical scavenger was used to determine whether reactive oxygen species participate in sonodynamically inducing cell damage. The results suggest that the enhancement was due to the generation of active oxygen. This work is the first step for clinical applications of sonodynamic therapy using fullerene derivatives.118
7. Immunological properties
It has been reported that C60 fullerene derivatives, when conjugated to bovine thyroglobulin or rabbit serum albumin, induce the production of fullerene-specific IgG antibodies in immunized mice.119, 120, 121, 122 Turabekova et al.123 reported a computational study where C60 fullerenes can act as ligands for toll-like receptors and therefore can be recognized as pathogens, inducing proinflammatory immune response.
The potential suppressive effects of compound 50 (Fig. 11) and fullerenol (C60(OH)36) was studied on many in vitro and in vivo models of inflammatory diseases by Hirai et al.124 Their results suggest that both fullerene derivatives can suppress acquired immune responses that require T cells in vitro. Additionally, compound 50 suppressed ovalbumin-specific antibody production and ovalbumin specific T cell responses in vivo.
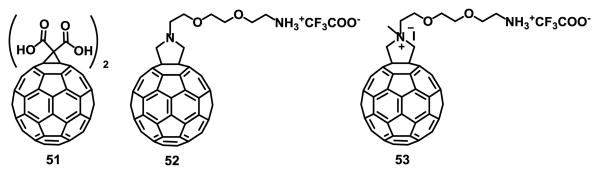
Funakoshi-Tago et at.125 demonstrated that bis-malonic acid fullerene derivative compound 51 (Fig. 12) suppressed the interleukin 33 (IL-33) induced expression of IL-6 in bone marrow-derived mast cells (BMMC). IL-33 works both intra- and extracellularly as a nuclear factor regulating gene transcription and as a traditional cytokine, respectively.126 Intracellular IL-33 is thought to be released after cell necrosis to alert the immune system of tissue damage or stress, and extracellular IL-33 coordinates immune defence and repair mechanisms while also initiating differentiation of helper T cells as the adaptive immune response is triggered.127 This has attracted attention as a new target for the treatment of inflammatory diseases.128
Fig. 12.
Fullerene derivatives with immunological properties.
Pacor et al.129 studied the interaction between fullerene derivatives 52 and 53 (Fig. 12) and cells of the immune system and their results stress the important role of fullerene derivatives in the control of inflammatory and cancer diseases.
52 showed biological properties that make it compatible with monocytes and macrophages, remarking its potential use to deliver compounds capable of modulating the immune responses or as a vehicle to deliver anticancer drugs.129 Compounds 52 and 53 were more active on neoplastic proliferating cells than on circulating monocytes. Compound 53 is more toxic than compound 52 against the tested cells. At active doses on tumor cells, 53 was found in the cell cytoplasm without altering the cell membrane permeability. It was not clear why 53 was toxic to macrophages, but it can be attributed to the high concentration to which the cells were exposed rather than a negative effect on mitochondrial function.
8. Biological applications of endohedral metallofullerenes (EMFs)
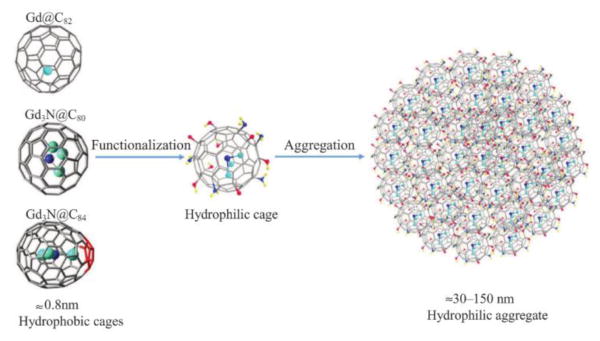
EMFs are fullerenes that encapsulate metal atoms or clusters. Some of the encapsulated species can be used in diagnostic or tumor therapy, but these properties are limited due to their high toxicity. It is well known that the carbon cages of EMFs prevent the encapsulated species from being released to or reacting with biological systems. Additionally, they retain enough reactivity for exohedral-functionalization that transforms the hydrophobic cage into more hydrophilic compounds as illustrated in Fig. 13.130 The recent progress with gadolinium (Gd) based EMFs for biomedical applications is described.
Fig. 13.
Functionalization of Gd-EMFs. Reprinted with permission from Li et al.130 Copyright (2016) Wiley-VCH and Sons.
8.1 Magnetic Resonance Imaging (MRI) Contrast Agents
Gd-based metallofullerenes (Gd-MF) have attracted special interest as MRI contrast agents due to their high paramagnetic properties and high relaxivity.131-134
Murphy et al.135 reported the application of Gd3N@C80 for in vitro and in vivo imaging and tracking of human amniotic fluid-derived stem cells within lung tissue. Gd3N@C80 was able to specifically bind to damaged lung tissue, although the imaging intensity dissipated a month after the initial dosage. This is the first reported use of a Gd-EMF to label and deliver exogenous cells to tissue and perform longitudinal MRI to evaluate the dynamics of cell homing, migration and clearance.
Zhang et al.136 reported a hydroxylated Gd3N@Cs-C84, an “egg-shaped” EMF with fused pentagons on its cage that increase the dipole moment of the compound, and thus its solubility in water. Moreover, it exhibited enhanced relaxivity. Meanwhile, Li et al.137 reported two hydroxylated derivatives, Gd@C82(O)10(OH)16 and Gd@C82(O)10(OH)22, that exhibited similar properties as those of Gd3N@Cs-C84.
Xiao et al.138 synthetized a new Gd endohedral fullerene derivative, IL-13-TAMRA-Gd3N@C80(OH)30-(CH2CH2COOH)20, functionalized with a cytokine IL-3 that increases its selectivity for IL-13Rα2 receptors. The former are important markers for Chronic Post-Traumatic Osteomyelitis, an opportunistic infection of the bone marrow. Gd-EMFs functionalized with the same IL-13 cytokine and additional positively charged amino groups (NH3+) have also been used in imaging Glioblastoma tumor cells as represented in Fig. 14.139 The positively charged groups, coupled with the IL-3 functionalization, increased selectivity for tumor U-251 cells. Li et al.140 studied the improved relaxivity of Gd-EMF derivatives deposited on graphene oxide nanosheets (GO-Gd@C82). Their results showed that physicochemical properties and structure are important factors that can affect relaxivity. Additionally, electron transfer between Gd-EMF and the GO sheet was found to aid proton relaxation. This interaction is stronger than that of water and the Gd ions.
Fig. 14.
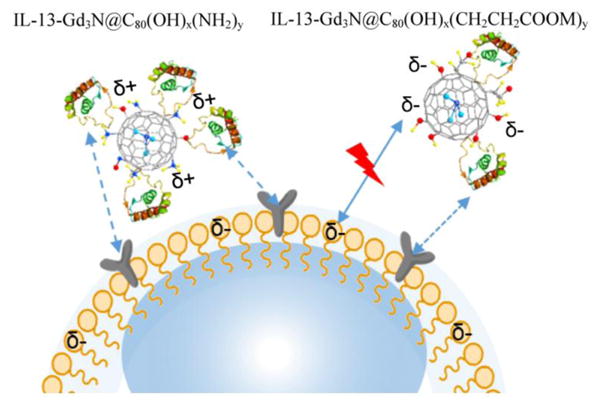
IL-13-Gd3N@C80(OH)x(NH2)y nanoparticles with positive charges illustrating facile binding to a negatively charged phospholipid bilayer cellular surfaces. Reprinted with permission from Li et al.139 Copyright (2015) American Chemical Society.
Pan et al.141 investigated the mechanism of Gd@C82(OH)22 on epigenetic regulation of human pancreatic cancer metastasis. From computational and experimental results both in vitro and in vivo, it was concluded that Gd@C82(OH)22 inhibits histone deacetylase-1 (HDAC-1) and suppresses pancreatic cancer cell invasion and metastasis.
Tang et al.142 investigated the activity of Gd@C82(OH)22 in cancer immunotherapy by regulating macrophage activity. The results showed enhanced macrophage viability, phagocytosis, and secretion of cytokines by macrophages. The activated macrophages inhibited tumor formation and metastasis in vivo, and induced cancer cell death in vitro, as represented in Fig. 15.
Fig. 15.
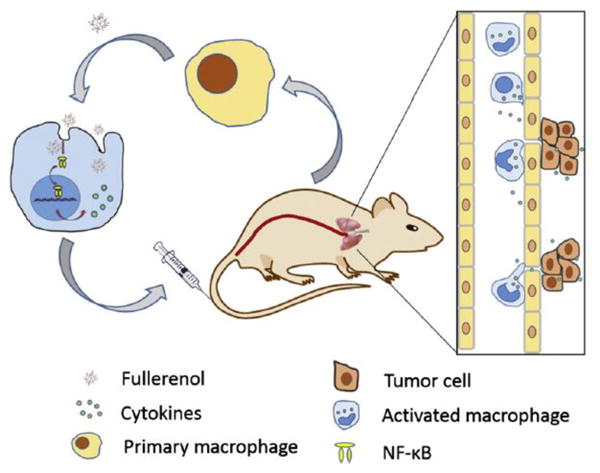
The possible mechanism by which back transfusion of macrophages activated by Gd@C82(OH)22 nanoparticles inhibit tumor metastasis. Reprinted from Tang et al.142 Copyright (2016), with permission from Elsevier.
The ability of Gd@C82(OH)22 on activating macrophages was also studied by Chen et al.143 using synchrotron-based scanning transmission X-ray microscopy (STXM) with high spatial resolution of 30 nm. Gd@C82(OH)22 significantly activated primary mouse macrophages and produced pro-inflammatory cytokines, evidencing the possible role of these fullerenols in antitumor immune response.
9. Future and perspectives
It is evident from the biological and medical applications of functionalized empty and endohedral fullerenes that many studies including toxicity and solubility should be addressed. However, many of the biological experiments are far from practical applications at this point. Most of these fullerene derivatives have yet to prove their efficacy and safety in vivo, as most have been conducted in vitro with a non-consistent variety of cell types. The compatibility of fullerene derivatives with various biological systems ought to be established before any clinical studies are attempted.
One of the biggest obstacles for the progress of fullerene-based treatments is the elevated cytotoxicity due to their hydrophobic nature, which increases their likelihood of absorbing biological species or accumulating in lipid membranes. However, multiple research studies have presented evidence against this fear, or demonstrated that it can be circumvented via modification with biocompatible moieties, as described in this review. Varied cytotoxicity levels and properties are expected for the wide breadth of fullerene derivatives presented in this summary, as their cytotoxic effects depend on different physical properties (size and shape), fabrication methods, and surface functionalization. Systematic toxicological and pharmacokinetic investigations of individual fullerene derivatives are expected to continue and become a more important topic in the field of biomedicine. Beyond studying their therapeutic effects, bioaccumulation studies are just as vital for their eventual translational use.
Conclusions
In this short review we have summarized the biological application of empty as well as of reported endohedral fullerene derivatives during the last 3 years, which include anti-viral agents, PDT, antioxidants, anticancer, immunological properties, MRI contrast agents and tumor therapy. Remarkable progress has been made on biological applications of fullerenes since the first report in 1993. To solve the almost complete insolubility in aqueous media, several synthetic approaches have been developed as discussed in this review.
Given the fast growth of these compounds for different biological applications combined with low toxicity, it is anticipated that many more biomedical applications will be forthcoming for these interesting compounds.
Supplementary Material
Acknowledgments
L.E. thanks the US National Science Foundation (NSF) for generous support of this work under the NSF-PREM program (DMR 1205302) and the CHE-1408865. The Robert A. Welch Foundation is also gratefully acknowledged for an endowed chair to L.E. (Grant AH-0033). The National Institute of General medical Sciences of the National Institutes of Health under linked Award Numbers RL5GM118969, TL4GM118971, and UL1GM118970, is also acknowledged.
References
- 1.Kroto HW, Heath JR, O'Brien SC, Curl RF, Smalley RE. Nature. 1985;318:162–163. [Google Scholar]
- 2.Hawkins JM, Lewis TA, Loren SD, Meyer A, Heath JR, Shibato Y, Saykally RJ. J Org Chem. 1990;55:6250–6252. doi: 10.1021/jo00313a009. [DOI] [PubMed] [Google Scholar]
- 3.Kratschmer W, Lamb LD, Fostiropoulos K, Huffman DR. Nature. 1990;347:354–358. [Google Scholar]
- 4.Wudl F. Acc Chem Res. 1992;25:157–161. [Google Scholar]
- 5.Sijbesma R, Srdanov G, Wudl F, Castoro JA, Wilkins C, Friedman SH, DeCamp DL, Kenyon GL. J Am Chem Soc. 1993;115:6510–6512. [Google Scholar]
- 6.Thilgen C, Diederich F. Chem Rev. 2006;106:5049–5135. doi: 10.1021/cr0505371. [DOI] [PubMed] [Google Scholar]
- 7.Tzirakis MD, Orfanopoulos M. Chem Rev. 2013;113:5262–5321. doi: 10.1021/cr300475r. [DOI] [PubMed] [Google Scholar]
- 8.Barron AR. J Enzyme Inhib Med Chem. 2016;31:164–176. doi: 10.1080/14756366.2016.1177524. [DOI] [PubMed] [Google Scholar]
- 9.Jennepalli S, Pyne SG, Keller PA. RSC Adv. 2014;4:46383–46398. [Google Scholar]
- 10.Vance SJ, Desai V, Smith BO, Kennedy MW, Cooper A. Biophys Chem. 2016;214–215:27–32. doi: 10.1016/j.bpc.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatia S, Dimde M, Haag R. Med Chem Comm. 2014;5:862–878. [Google Scholar]
- 12.Muñoz A, Sigwalt D, Illescas BM, Luczkowiak J, Rodríguez-Pérez L, Nierengarten I, Holler M, Remy JS, Buffet K, Vincent SP, Rojo J, Delgado R, Nierengarten JF, Martín N. Nat Chem. 2016;8:50–57. doi: 10.1038/nchem.2387. [DOI] [PubMed] [Google Scholar]
- 13.Grebowski J, Kazmierska P, Krokosz A. BioMed Research International. 2013;2013:9. doi: 10.1155/2013/751913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semenov KN, Charykov NA, Postnov VN, Sharoyko VV, Vorotyntsev IV, Galagudza MM, Murin IV. Prog Solid State Chem. 2016;44:59–74. [Google Scholar]
- 15.Ali SS, Hardt JI, Dugan LL. Nanomedicine: NBM. 2008;4:283–294. doi: 10.1016/j.nano.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kornev AB, Peregudov AS, Martynenko VM, Balzarini J, Hoorelbeke B, Troshin PA. Chem Commun. 2011;47:8298–8300. doi: 10.1039/c1cc12209f. [DOI] [PubMed] [Google Scholar]
- 17.Kataoka H, Ohe T, Takahashi K, Nakamura S, Mashino T. Bioor Med Chem Lett. 2016;26:4565–4567. doi: 10.1016/j.bmcl.2016.08.086. [DOI] [PubMed] [Google Scholar]
- 18.Pastorin G, Marchesan S, Hoebeke J, Da Ros T, Ehret-Sabatier L, Briand JP, Prato M, Bianco A. Org Biomol Chem. 2006;4:2556–2562. doi: 10.1039/b604361e. [DOI] [PubMed] [Google Scholar]
- 19.Castro E, Martinez ZS, Seong CS, Cabrera-Espinoza A, Ruiz M, Hernandez G, Valdez AF, Llano M, Echegoyen LA. J Med Chem. 2016;59:10963–10973. doi: 10.1021/acs.jmedchem.6b00994. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda A. Chem Rec. 2016;16:249–260. doi: 10.1002/tcr.201500249. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda A, Mae T, Ueda M, Sugikawa K, Shigeto H, Funabashi H, Kuroda A, Akiyama M. Chem Commun. 2017 doi: 10.1039/c7cc00302a. [DOI] [PubMed] [Google Scholar]
- 22.Cantalado F, Da Ros T. Springer. 2008;1:107–121. [Google Scholar]
- 23.Yang X, Ebrahimi A, Li J, Cui Q. Int J Nanomed. 2014;9:77–92. doi: 10.2147/IJN.S52829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nozdrenko DM, Zavodovskyi DO, Matvienko TY, Zay SY, Bogutska KI, Prylutskyy YI, Ritter U, Scharff P. Nanoscale Res Lett. 2017;12:115. doi: 10.1186/s11671-017-1876-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuliana PG, Igor A, Petr Z, Evgenia P, Maria M, Anna B, Vladimir C. Current Aging Science. 2017;10:56–67. [Google Scholar]
- 26.Vance SJ, Desai V, Smith BO, Kennedy MW, Cooper A. Biophys Chem. 2016;214-215:27–32. doi: 10.1016/j.bpc.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosi S, Da Ros T, Spalluto G, Prato M. Eur J Med Chem. 2003;38:913–923. doi: 10.1016/j.ejmech.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Bakry R, Vallant RM, Najam-ul-Haq M, Rainer M, Szabo Z, Huck CW, Bonn GK. Int J Nanomedicine. 2007;2:639–649. [PMC free article] [PubMed] [Google Scholar]
- 29.Hirsch A. Fullerenes and Related Structures. Vol. 199. Springer Berlin Heidelberg; 1999. pp. 1–65. ch. 1. [Google Scholar]
- 30.Yamada M, Akasaka T, Nagase S. Chem Rev. 2013;113:7209–7264. doi: 10.1021/cr3004955. [DOI] [PubMed] [Google Scholar]
- 31.Lebedeva MA, Chamberlain TW, Khlobystov AN. Chem Rev. 2015;115:11301–11351. doi: 10.1021/acs.chemrev.5b00005. [DOI] [PubMed] [Google Scholar]
- 32.Prato M. J Mater Chem. 1997;7:1097–1109. [Google Scholar]
- 33.Xie Q, Perez-Cordero E, Echegoyen L. J Am Chem Soc. 1992;114:3978–3980. [Google Scholar]
- 34.Dorn HC, Duchamp JC. In: Introduction to Nanoscale Science and Technology. Di Ventra M, Evoy S, Heflin JR, editors. Springer US; Boston, MA: 2004. pp. 119–135. [Google Scholar]
- 35.Wudl F, Hirsch A, Khemani KC, Suzuki T, Allemand PM, Koch A, Eckert H, Srdanov G, Webb HM. Fullerenes. Vol. 481. American Chemical Society; 1992. pp. 161–175. ch. 11. [Google Scholar]
- 36.Smalley RE, Curl RF. Vigyan Scientific American. 1991:32–41. [Google Scholar]
- 37.Ruoff RS, Ruoff AL. Appl Phys Lett. 1991;59:1553–1555. [Google Scholar]
- 38.Ruoff RS, Tse DS, Malhotra R, Lorents DC. J Phys Chem. 1993;97:3379–3383. [Google Scholar]
- 39.Kumarasamy N, Krishnan S. Curr Opin HIV AIDS. 2013;8:586–590. doi: 10.1097/COH.0000000000000004. [DOI] [PubMed] [Google Scholar]
- 40.Paydary K, Khaghani P, Emamzadeh-Fard S, Alinaghi SA, Baesi K. Asian Pac J Trop Biomed. 2013;3:515–522. doi: 10.1016/S2221-1691(13)60106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santoro MM, Perno CF. ISRN Microbiol. 2013;2013:20. doi: 10.1155/2013/481314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Urano E, Ablan SD, Mandt R, Pauly GT, Sigano DM, Schneider JP, Martin DE, Nitz TJ, Wild CT, Freed EO. Antimicrob Agents Chemother. 2016;60:190–197. doi: 10.1128/AAC.02121-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freed EO. Nat Rev Microbiol. 2015;13:484–496. doi: 10.1038/nrmicro3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedman SH, DeCamp DL, Sijbesma RP, Srdanov G, Wudl F, Kenyon GL. J Am Chem Soc. 1993;115:6506–6509. [Google Scholar]
- 45.Martinez ZS, Castro E, Seong CS, Ceron MR, Echegoyen L, Llano M. Antimicrob Agents Chemother. 2016;60:5731–5741. doi: 10.1128/AAC.00341-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toniolo C, Bianco A, Maggini M, Scorrano G, Prato M, Marastoni M, Tomatis R, Spisani S, Palu G, Blair ED. J Med Chem. 1994;37:4558–4562. doi: 10.1021/jm00052a015. [DOI] [PubMed] [Google Scholar]
- 47.Durdagi S, Supuran CT, Strom TA, Doostdar N, Kumar MK, Barron AR, Mavromoustakos T, Papadopoulos MG. J Chem Inf Model. 2009;49:1139–1143. doi: 10.1021/ci900047s. [DOI] [PubMed] [Google Scholar]
- 48.Strom TA, Durdagi S, Ersoz SS, Salmas RE, Supuran CT, Barron AR. J Pep Sci. 2015;21:862–870. doi: 10.1002/psc.2828. [DOI] [PubMed] [Google Scholar]
- 49.Khakina EA, Kraevaya OgA, Popova ML, Peregudov AS, Troyanov SI, Chernyak AV, Martynenko VM, Kulikov AV, Schols D, Troshin PA. Org Biomol Chem. 2017;15:773–777. doi: 10.1039/c6ob02251k. [DOI] [PubMed] [Google Scholar]
- 50.Khakina EA, Yurkova AA, Peregudov AS, Troyanov SI, Trush VV, Vovk AI, Mumyatov AV, Martynenko VM, Balzarini J, Troshin PA. Chem Commun. 2012;48:7158–7160. doi: 10.1039/c2cc32517a. [DOI] [PubMed] [Google Scholar]
- 51.Nakamura S, Mashino T. J Nippon Med Sch. 2012;79:248–254. doi: 10.1272/jnms.79.248. [DOI] [PubMed] [Google Scholar]
- 52.Yasuno T, Ohe T, Takahashi K, Nakamura S, Mashino T. Bioorg Med Chem Lett. 2015;25:3226–3229. doi: 10.1016/j.bmcl.2015.05.086. [DOI] [PubMed] [Google Scholar]
- 53.Galan MC, Dumy P, Renaudet O. Chem Soc Rev. 2013;42:4599–4612. doi: 10.1039/c2cs35413f. [DOI] [PubMed] [Google Scholar]
- 54.Nierengarten JF, Iehl J, Oerthel V, Holler M, Illescas BM, Munoz A, Martin N, Rojo J, Sanchez-Navarro M, Cecioni S, Vidal S, Buffet K, Durka M, Vincent SP. Chem Commun. 2010;46:3860–3862. doi: 10.1039/c0cc00034e. [DOI] [PubMed] [Google Scholar]
- 55.Sánchez-Navarro M, Muñoz A, Illescas BM, Rojo J, Martín N. Chem Eur J. 2011;17:766–769. doi: 10.1002/chem.201002816. [DOI] [PubMed] [Google Scholar]
- 56.Buffet K, Gillon E, Holler M, Nierengarten JF, Imberty A, Vincent SP. Org Biomol Chem. 2015;13:6482–6492. doi: 10.1039/c5ob00689a. [DOI] [PubMed] [Google Scholar]
- 57.Na W, Park N, Yeom M, Song D. Clin Exp Vaccine Res. 2015;4:17–22. doi: 10.7774/cevr.2015.4.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.World-Health-Organization. Ebola data and statistics. [accessed February 27th, 2017]; http://apps.who.int/gho/data/view.ebola-sitrep.ebola-summary-latest?lang=en.
- 59.Carette JE, Raaben M, Wong AC, Herbert AS, Obernosterer G, Mulherkar N, Kuehne AI, Kranzusch PJ, Griffin AM, Ruthel G, Cin PD, Dye JM, Whelan SP, Chandran K, Brummelkamp TR. Nature. 2011;477:340–343. doi: 10.1038/nature10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McReynolds KD, Gervay-Hague J. Chem Rev. 2007;107:1533–1552. doi: 10.1021/cr0502652. [DOI] [PubMed] [Google Scholar]
- 61.Nierengarten I, Nierengarten JF. Chem Asian J. 2014;9:1436–1444. doi: 10.1002/asia.201400133. [DOI] [PubMed] [Google Scholar]
- 62.Tikad A, Fu H, Sevrain CM, Laurent S, Nierengarten JF, Vincent SP. Chem Eur J. 2016;22:13147–13155. doi: 10.1002/chem.201602190. [DOI] [PubMed] [Google Scholar]
- 63.Abellán Flos M, García Moreno MI, Ortiz Mellet C, García Fernández JM, Nierengarten JF, Vincent SP. Chem Eur J. 2016;22:11450–11460. doi: 10.1002/chem.201601673. [DOI] [PubMed] [Google Scholar]
- 64.Stauffert F, Bodlenner A, Nguyet Trinh TM, Garcia-Moreno MI, Ortiz Mellet C, Nierengarten JF, Compain P. New J Chem. 2016;40:7421–7430. [Google Scholar]
- 65.Luczkowiak J, Muñoz A, Sánchez-Navarro M, Ribeiro-Viana R, Ginieis A, Illescas BM, Martín N, Delgado R, Rojo J. Biomacromolecules. 2013;14:431–437. doi: 10.1021/bm3016658. [DOI] [PubMed] [Google Scholar]
- 66.Gross S, Gilead A, Scherz A, Neeman M, Salomon Y. Nat Med. 2003;9:1327–1331. doi: 10.1038/nm940. [DOI] [PubMed] [Google Scholar]
- 67.Broekgaarden M, Weijer R, van Gulik TM, Hamblin MR, Heger M. Cancer Metastasis Revi. 2015;34:643–690. doi: 10.1007/s10555-015-9588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhen Z, Tang W, Guo C, Chen H, Lin X, Liu G, Fei B, Chen X, Xu B, Xie J. ACS Nano. 2013;7:6988–6996. doi: 10.1021/nn402199g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arbogast JW, Darmanyan AP, Foote CS, Diederich FN, Whetten RL, Rubin Y, Alvarez MM, Anz SJ. J Phys Chem. 1991;95:11–12. [Google Scholar]
- 70.Yamakoshi Y, Umezawa N, Ryu A, Arakane K, Miyata N, Goda Y, Masumizu T, Nagano T. J Am Chem Soc. 2003;125:12803–12809. doi: 10.1021/ja0355574. [DOI] [PubMed] [Google Scholar]
- 71.Huang L, Wang M, Dai T, Sperandio FF, Huang YY, Xuan Y, Chiang LY, Hamblin MR. Nanomedicine. 2013;9:253–266. doi: 10.2217/nnm.13.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y, Dai T, Wang M, Vecchio D, Chiang LY, Hamblin MR. Nanomedicine. 2015;10:603–614. doi: 10.2217/nnm.14.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu Z, Dai T, Huang L, Kurup DB, Tegos GP, Jahnke A, Wharton T, Hamblin MR. Nanomedicine (Lond) 2010;5:1525–1533. doi: 10.2217/nnm.10.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mroz P, Tegos GP, Gali H, Wharton T, Sarna T, Hamblin MR. Photochem Photobiol Sci. 2007;6:1139–1149. doi: 10.1039/b711141j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu C, Avci P, Canteenwala T, Chiang LY, Chen BJ, Hamblin MR. J Nanosci Nanotechnol. 2016;16:171–181. doi: 10.1166/jnn.2016.10652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guan M, Ge J, Wu J, Zhang G, Chen D, Zhang W, Zhang Y, Zou T, Zhen M, Wang C, Chu T, Hao X, Shu C. Biomaterials. 2016;103:75–85. doi: 10.1016/j.biomaterials.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 77.Li Q, Liu C, Li H. J Nanosci Nanotechnol. 2016;16:5592–5597. doi: 10.1166/jnn.2016.11717. [DOI] [PubMed] [Google Scholar]
- 78.Kim S, Lee DJ, Kwag DS, Lee UY, Youn YS, Lee ES. Carbohydr Polym. 2014;101:692–698. doi: 10.1016/j.carbpol.2013.09.108. [DOI] [PubMed] [Google Scholar]
- 79.Franskevych D, Palyvoda K, Petukhov D, Prylutska S, Grynyuk I, Schuetze C, Drobot L, Matyshevska O, Ritter U. Nanoscale Res Lett. 2017;12:40. doi: 10.1186/s11671-016-1819-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mion G, Mari C, Da Ros T, Rubbiani R, Gasser G, Gianferrara T. ChemistrySelect. 2017;2:190–200. [Google Scholar]
- 81.Ershova ES, Sergeeva VA, Chausheva AI, Zheglo DG, Nikitina VA, Smirnova TD, Kameneva LV, Porokhovnik LN, Kutsev SI, Troshin PA, Voronov II, Khakina EA, Veiko NN, Kostyuk SV. Mutat Res-Fund Mol M. 2016;805:46–57. doi: 10.1016/j.mrgentox.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 82.Miller CR, Bondurant B, McLean SD, McGovern KA, O'Brien DF. Biochemistry. 1998;37:12875–12883. doi: 10.1021/bi980096y. [DOI] [PubMed] [Google Scholar]
- 83.Wang J, Zhang Z, Wu W, Jiang X. Chin J Chem. 2014;32:78–84. [Google Scholar]
- 84.Djordjevic A, Injac R, Jovic D, Mrdjanovic J, Seke M. Advanced Carbon Materials and Technology. John Wiley & Sons, Inc.; 2014. pp. 193–271. [Google Scholar]
- 85.Caputo F, De Nicola M, Ghibelli L. Biochem Pharmacol. 2014;92:112–130. doi: 10.1016/j.bcp.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 86.Lichota A, Krokosz A. Medycyna Pracy. 2016;67:817–831. doi: 10.13075/mp.5893.00466. [DOI] [PubMed] [Google Scholar]
- 87.Xiao L, Takada H, Gan Xh, Miwa N. Bioorg Med Chem Lett. 2006;16:1590–1595. doi: 10.1016/j.bmcl.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 88.Krusic PJ, Wasserman E, Keizer PN, Morton JR, Preston KF. Science. 1991;254:1183–1185. doi: 10.1126/science.254.5035.1183. [DOI] [PubMed] [Google Scholar]
- 89.Krokosz A, Lichota A, Nowak KE, Grebowski J. Radiat Phys Chem. 2016;128:143–150. [Google Scholar]
- 90.Djordjevic A, Srdjenovic B, Seke M, Petrovic D, Injac R, Mrdjanovic J. J Nanomater. 2015;2015:15. [Google Scholar]
- 91.Li J, Guan M, Wang T, Zhen M, Zhao F, Shu C, Wang C. ACS Appl Mater Interfaces. 2016;8:25770–25776. doi: 10.1021/acsami.6b08659. [DOI] [PubMed] [Google Scholar]
- 92.Jacevic V, Djordjevic A, Srdjenovic B, Milic-Tores V, Segrt Z, Dragojevic-Simic V, Kuca K. Exp Mol Pathol. 2017;102:360–369. doi: 10.1016/j.yexmp.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 93.Ghasemzadeh P, Rezayat SM, Adeli S, Rahbar-Roshandel N. Acta Med Iran. 2016:7. [PubMed] [Google Scholar]
- 94.Vani JR, Mohammadi MT, Foroshani MS, Jafari M. EXCLI Journal. 2016;15:378–390. doi: 10.17179/excli2016-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang J, Zhou C, He J, Hu Z, Guan Wc, Liu Sh. J Huazhong Univ Sci Technolog Med Sci. 2016;36:356–363. doi: 10.1007/s11596-016-1591-x. [DOI] [PubMed] [Google Scholar]
- 96.Wakimoto T, Uchida K, Mimura K, Kanagawa T, Mehandjiev TR, Aoshima H, Kokubo K, Mitsuda N, Yoshioka Y, Tsutsumi Y, Kimura T, Yanagihara I. Am J Obstet Gynecol. 2015;213:708.e701–708.e709. doi: 10.1016/j.ajog.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 97.Yang X, Li CJ, Wan Y, Smith P, Shang G, Cui Q. Int J Nanomedicine. 2014;9:4023–4031. doi: 10.2147/IJN.S66785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kato S, Aoshima H, Saitoh Y, Miwa N. Bioor Med Chem Lett. 2009;19:5293–5296. doi: 10.1016/j.bmcl.2009.07.149. [DOI] [PubMed] [Google Scholar]
- 99.Wang Z, Chang X, Lu Z, Gu M, Zhao Y, Gao X. Chem Sci. 2014;5:2940–2948. [Google Scholar]
- 100.Ueno H, Yamakura S, Arastoo RS, Oshima T, Kokubo K. J Nanomater. 2014;2014:7. [Google Scholar]
- 101.Grebowski J, Krokosz A, Konarska A, Wolszczak M, Puchala M. Radiat Phys Chem. 2014;103:146–152. [Google Scholar]
- 102.Guldi DM, Asmus KD. Radiat Phys Chem. 1999;56:449–456. [Google Scholar]
- 103.Srđenović B, Slavić M, Stankov K, Kladar N, Jović D, Seke M, Bogdanović V. Hem Ind. 2015;69:425–431. [Google Scholar]
- 104.Awan F, Bulger E, Berry RM, Tam KC. Cellulose. 2016;23:3589–3599. [Google Scholar]
- 105.Yang LY, Gao JL, Gao T, Dong P, Ma L, Jiang FL, Liu Y. J Hazard Mater. 2016;301:119–126. doi: 10.1016/j.jhazmat.2015.08.046. [DOI] [PubMed] [Google Scholar]
- 106.Sun M, Kiourti A, Wang H, Zhao S, Zhao G, Lu X, Volakis JL, He X. Mol Pharmaceutics. 2016;13:2184–2192. doi: 10.1021/acs.molpharmaceut.5b00984. [DOI] [PubMed] [Google Scholar]
- 107.Wang H, Agarwal P, Zhao S, Yu J, Lu X, He X. Biomaterials. 2016;97:62–73. doi: 10.1016/j.biomaterials.2016.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hsieh FY, Zhilenkov AV, Voronov II, Khakina EA, Mischenko DV, Troshin PA, Hsu Sh. ACS Appl Mater Interfaces. 2017 doi: 10.1021/acsami.7b01077. [DOI] [PubMed] [Google Scholar]
- 109.Wu G, Gao XJ, Jang J, Gao X. J Mol Model. 2016;22:161. doi: 10.1007/s00894-016-3019-8. [DOI] [PubMed] [Google Scholar]
- 110.Prylutska SV, Skivka LM, Didenko GV, Prylutskyy YI, Evstigneev MP, Potebnya GP, Panchuk RR, Stoika RS, Ritter U, Scharff P. Nanoscale Res Lett. 2015;10:499. doi: 10.1186/s11671-015-1206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Raza K, Thotakura N, Kumar P, Joshi M, Bhushan S, Bhatia A, Kumar V, Malik R, Sharma G, Guru SK, Katare OP. Int J Pharm. 2015;495:551–559. doi: 10.1016/j.ijpharm.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 112.Zhang H, Hou L, Jiao X, Ji Y, Zhu X, Zhang Z. Biomaterials. 2015;37:353–366. doi: 10.1016/j.biomaterials.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 113.Watanabe T, Nakamura S, Ono T, Ui S, Yagi S, Kagawa H, Watanabe H, Ohe T, Mashino T, Fujimuro M. Biochem Biophys Res Commun. 2014;451:93–100. doi: 10.1016/j.bbrc.2014.07.068. [DOI] [PubMed] [Google Scholar]
- 114.Nishizawa C, Hashimoto N, Yokoo S, Funakoshi-Tago M, Kasahara T, Takahashi K, Nakamura S, Mashino T. Free Radic Res. 2009;43:1240–1247. doi: 10.3109/13814780903260764. [DOI] [PubMed] [Google Scholar]
- 115.Funakoshi-Tago M, Tsukada M, Watanabe T, Mameda Y, Tago K, Ohe T, Nakamura S, Mashino T, Kasahara T. Int Immunopharmacol. 2014;20:258–263. doi: 10.1016/j.intimp.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 116.Prylutska S, Grynyuk I, Matyshevska O, Prylutskyy Y, Evstigneev M, Scharff P, Ritter U. Drugs R D. 2014;14:333–340. doi: 10.1007/s40268-014-0074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Magoulas GE, Bantzi M, Messari D, Voulgari E, Gialeli C, Barbouri D, Giannis A, Karamanos NK, Papaioannou D, Avgoustakis K. Pharm Res. 2015;32:1676–1693. doi: 10.1007/s11095-014-1566-1. [DOI] [PubMed] [Google Scholar]
- 118.Yumiko I, Koji N, Junya F, Toshio F, Nagahiko Y, Toshihiko I, Fu-shin C, Yasunori M, Shin-ichiro U. Jpn J Appl Phys. 2016;55:07KF02. [Google Scholar]
- 119.Chen BX, Wilson SR, Das M, Coughlin DJ, Erlanger BF. Proc Natl Acad Sci USA. 1998;95:10809–10813. doi: 10.1073/pnas.95.18.10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shershakova N, Baraboshkina E, Andreev S, Purgina D, Struchkova I, Kamyshnikov O, Nikonova A, Khaitov M. J Nanobiotechnol. 2016;14:8. doi: 10.1186/s12951-016-0159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Braden BC, Goldbaum FA, Chen BX, Kirschner AN, Wilson SR, Erlanger BF. Proc Natl Acad Sci USA. 2000;97:12193–12197. doi: 10.1073/pnas.210396197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Petrovic D, Seke M, Srdjenovic B, Djordjevic A. J Nanomater. 2015;2015:11. [Google Scholar]
- 123.Turabekova M, Rasulev B, Theodore M, Jackman J, Leszczynska D, Leszczynski J. Nanoscale. 2014;6:3488–3495. doi: 10.1039/c3nr05772k. [DOI] [PubMed] [Google Scholar]
- 124.Hirai T, Yoshioka Y, Udaka A, Uemura E, Ohe T, Aoshima H, Gao JQ, Kokubo K, Oshima T, Nagano K, Higashisaka K, Mashino T, Tsutsumi Y. Nanoscale Res Lett. 2016;11:449. doi: 10.1186/s11671-016-1663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Funakoshi-Tago M, Miyagawa Y, Ueda F, Mashino T, Moriwaki Y, Tago K, Kasahara T, Tamura H. Int Immunopharmacol. 2016;40:254–264. doi: 10.1016/j.intimp.2016.08.031. [DOI] [PubMed] [Google Scholar]
- 126.Miller AM. J Inflamm. 2011;8:22. doi: 10.1186/1476-9255-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Martin NT, Martin MU. Nat Immunol. 2016;17:122–131. doi: 10.1038/ni.3370. [DOI] [PubMed] [Google Scholar]
- 128.Wang S, Ding L, Liu SS, Wang C, Leng RX, Chen GM, Fan YG, Pan HF, Ye DQ. J Investig Med. 2012;60:1151–1156. doi: 10.2310/JIM.0b013e31826d8fcb. [DOI] [PubMed] [Google Scholar]
- 129.Pacor S, Grillo A, Đorđević L, Zorzet S, Lucafò M, Da Ros T, Prato M, Sava G. BioMed Res Int. 2015;2015:13. doi: 10.1155/2015/915130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li T, Dorn HC. Small. 2016;13:1603152. [Google Scholar]
- 131.Kato H, Kanazawa Y, Okumura M, Taninaka A, Yokawa T, Shinohara H. J Am Chem Soc. 2003;125:4391–4397. doi: 10.1021/ja027555+. [DOI] [PubMed] [Google Scholar]
- 132.Cui R, Li J, Huang H, Zhang M, Guo X, Chang Y, Li M, Dong J, Sun B, Xing G. Nano Res. 2015;8:1259–1268. [Google Scholar]
- 133.Wang L, Zhu X, Tang X, Wu C, Zhou Z, Sun C, Deng SL, Ai H, Gao J. Chem Commun. 2015;51:4390–4393. doi: 10.1039/c5cc00285k. [DOI] [PubMed] [Google Scholar]
- 134.Zhang Y, Zou T, Guan M, Zhen M, Chen D, Guan X, Han H, Wang C, Shu C. ACS Appl Mater Interfaces. 2016;8:11246–11254. doi: 10.1021/acsami.5b12848. [DOI] [PubMed] [Google Scholar]
- 135.Murphy SV, Hale A, Reid T, Olson J, Kidiyoor A, Tan J, Zhou Z, Jackson J, Atala A. Methods. 2016;99:99–111. doi: 10.1016/j.ymeth.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang J, Ye Y, Chen Y, Pregot C, Li T, Balasubramaniam S, Hobart DB, Zhang Y, Wi S, Davis RM, Madsen LA, Morris JR, LaConte SM, Yee GT, Dorn HC. J Am Chem Soc. 2014;136:2630–2636. doi: 10.1021/ja412254k. [DOI] [PubMed] [Google Scholar]
- 137.Li J, Wang T, Feng Y, Zhang Y, Zhen M, Shu C, Jiang L, Wang Y, Wang C. Dalton Trans. 2016;45:8696–8699. doi: 10.1039/c6dt00223d. [DOI] [PubMed] [Google Scholar]
- 138.Xiao L, Li T, Ding M, Yang J, Rodríguez-Corrales J, LaConte SM, Nacey N, Weiss DB, Jin L, Dorn HC, Li X. Bioconjugate Chem. 2017;28:649–658. doi: 10.1021/acs.bioconjchem.6b00708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li T, Murphy S, Kiselev B, Bakshi KS, Zhang J, Eltahir A, Zhang Y, Chen Y, Zhu J, Davis RM, Madsen LA, Morris JR, Karolyi DR, LaConte SM, Sheng Z, Dorn HC. J Am Chem Soc. 2015;137:7881–7888. doi: 10.1021/jacs.5b03991. [DOI] [PubMed] [Google Scholar]
- 140.Li J, Cui R, Chang Y, Guo X, Gu W, Huang H, Chen K, Lin G, Dong J, Xing G, Sun B. RSC Advances. 2016;6:58028–58033. [Google Scholar]
- 141.Pan Y, Wang L, Kang Sg, Lu Y, Yang Z, Huynh T, Chen C, Zhou R, Guo M, Zhao Y. ACS Nano. 2015;9:6826–6836. doi: 10.1021/nn506782f. [DOI] [PubMed] [Google Scholar]
- 142.Tang J, Chen Z, Sun B, Dong J, Liu J, Zhou H, Wang L, Bai R, Miao Q, Zhao Y, Chen C, Liu Y. Nanomedicine: NBM. 2016;12:945–954. doi: 10.1016/j.nano.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 143.Chen Z, Liu Y, Sun B, Li H, Dong J, Zhang L, Wang L, Wang P, Zhao Y, Chen C. Small. 2014;10:2362–2372. doi: 10.1002/smll.201302825. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.