Abstract
Metallothioneins (MT) constitute a superfamily of small cytosolic proteins that are able to bind metal cations through numerous cysteine (Cys) residues. Like other organisms the ciliate Tetrahymena thermophila presents several MT isoforms, which have been classified into two subfamilies (Cd- and Cu-metallothioneins). The main aim of this study was to examine the specific functions and transcriptional regulation of the five MT isoforms present in T. thermophila, by using several strains of this ciliate. After a laboratory evolution experiment over more than two years, three different T. thermophila strains adapted to extreme metal stress (Cd2+, Cu2+ or Pb2+) were obtained. In addition, three knockout and/or knockdown strains for different metallothionein (MT) genes were generated. These strains were then analyzed for expression of the individual MT isoforms. Our results provide a strong basis for assigning differential roles to the set of MT isoforms. MTT1 appears to have a key role in adaptation to Cd. In contrast, MTT2/4 are crucial for Cu-adaptation and MTT5 appears to be important for Pb-adaptation and might be considered as an “alarm” MT gene for responding to metal stress. Moreover, results indicate that likely a coordinated transcriptional regulation exists between the MT genes, particularly among MTT1, MTT5 and MTT2/4. MTT5 appears to be an essential gene, a first such report in any organism of an essential MT gene.
Introduction
Metals are natural components of the Earth's crust, and some are essential in low concentrations for cellular metabolism and growth. Examples include metal co-factors in enzymatic reactions (e.g., metalloenzymes), and stabilization of some biological molecules. On the other hand, many non-essential metals are known as the most abundant, persistent and toxic inorganic pollutants on our planet [1]. Metal toxicity can arise from interaction with biomolecules, such as proteins or nucleic acids, whose native structure and function may be thereby altered. Moreover, metal toxicity often produces oxidative stress that generates reactive oxygen species, which can perturb protein structures or enzymatic functions, impair DNA repair, and inhibit cell proliferation and differentiation processes, in some cases leading to necrotic or apoptotic cell death [2–4].
Organisms have evolved a range of mechanisms to reduce metal toxicity. One of the most universal cellular detoxification processes is the chelation of metal cations by specific oligopeptides (glutathione, phytochelatins) or proteins (metallothioneins). This mechanism allows the sequestration of metallic ions by the -SH groups of the cysteine residues and their intracellular storage in vacuoles or cytoplasmic inclusions. Then, these metal-protein complexes are expelled from the cell as non-toxic or rarely toxic compounds [5].
Metallothioneins (MTs) constitute a superfamily of small cytosolic proteins that are able to bind metal cations through numerous cysteine (Cys) residues [5–6]. These proteins have been reported in a wide variety of organisms: prokaryotic and eukaryotic microorganisms, plants, invertebrates and vertebrates. The range of specific functions of these proteins is not yet clear. Among the proposed functions are the regulation of essential-metal homeostasis [7], metal detoxification [8], protection against oxidative stress [9], and regulation of cell proliferation and apoptosis [10]. In mammals, MTs function in protection against neurodegenerative diseases [11] and in several cellular differentiation processes [12]. Broadly speaking, MTs have been considered as multifunctional proteins [10, 13] and as key elements of the cellular integrated stress response program [14]. The majority of MT gene expression studies, carried out in many different organisms, have reported that these genes are regulated primarily at the transcriptional level [10, 15], although some evidence of regulation at translational level exists [16]. That is, MT genes are often expressed constitutively but are up-regulated upon exposure to metals or other environmental stressors [17–23]. MTs have not been reported to be essential, but they are likely to provide survival advantages under some stress conditions [24, 25].
Ciliate MTs have unique features with respect to MTs from other organisms. The proteins are longer and richer in Cys residues, conferring a larger metal binding capacity compared to classic MTs [6]. The MTs in T. thermophila belong to family 7, which can be resolved into two well-characterized subfamilies. Subfamily 7a corresponds to CdMT (cadmium-metallothioneins), while 7b corresponds to CuMT (copper-metallothioneins). These two subfamilies differ mainly in their distributions of conserved cysteines, and in their preferential transcriptional induction by cadmium or copper, respectively [5, 6, 26]. T. thermophila has five MT isoforms: three CdMT (MTT1, MTT3 and MTT5) and two CuMT (MTT2 and MTT4) [20]. Previously, it was found that the individual CdMT genes have different induction patterns, suggesting functional diversification [20]. One basis for this differential transcription may be found in the promoter regions: the five T. thermophila MT genes have different numbers of a conserved MTCM1 motif, which is relevant for regulation by AP-1 transcription factors (bZIP) [6, 20]. The MTT5 promoter has 13 MTCM1 motifs and this gene shows strongest induction after metal exposure, while the MTT3 promoter has 2 MTCM1 motifs and is the most weakly induced T. thermophila MT gene [20]. The gene expression results [20] were corroborated by Espart and colleagues [27] who analysed the metal binding preferences of the T. thermophila MTs. MTT1 and MTT5 isoforms preferentially bind Cd2+ ions, while MTT3 preferentially binds Zn2+. Both MTT2 and MTT4 isoforms mainly form homometallic Cu-complexes [27].
Microorganisms can acquire stress tolerance and novel metabolic abilities when they are exposed to selective pressure [28]. An experimental approach to study this phenomenon has been called "evolutionary engineering" [29]. Studies in which cells are forced to adapt to increasing levels of specific stressors can provide insights into the physiological and genetic mechanisms involved in cellular responses to environmental stressors. As the stressor intensity is progressively increased, the cells’ deployment of protective mechanisms against toxicity is likely to involve enhancing a set of mechanisms involved in the normal cellular response, which can thus be more easily detected and studied through such studies. Experiments of this type have been carried out in diverse organisms to artificially evolve resistance to abiotic stressors including high salt [30], copper, cadmium [28, 31] or alcohols [32]. There are also examples of adapted microorganisms that have developed new properties with industrial applications, an example being yeasts able to ferment xylose [33] or lactose [34].
Knockout (KO) strains are basic tools for assessing the function and relevance of specific genes [35]. Some genes are designated essential, because their total knockout is incompatible with cell viability. In such cases, gene function can be studied by reducing gene copy number or expression to generate a knockdown (KD) strain, using specific interference RNAs or other methods.
Even in well-studied model organisms, there remain many unanswered questions concerning differential expression of MT isoforms and the genetic bases of adaptation to severe metal stress. The ciliate T. thermophila is a very useful model for addressing these issues in eukaryotic microorganisms [5]. The main aim of this study was to examine the specific functions and transcriptional regulation of the five MT isoforms. In particular, we used qRT-PCR (quantitative reverse transcription polymerase chain reaction) to analyze the expression patterns of the five MT genes in a set of T. thermophila strains: the reference strain SB1969, two strains engineered to over-express MTT1 or MTT5 gene [36], three metal-adapted strains (adapted to extreme Cd2+, Cu2+ or Pb2+ concentrations) and three KO and/or KD strains targeting MTT1 and/or MTT5.
Materials and methods
Strains and culture conditions
Tetrahymena thermophila strain CU428 (mpr1-1/mpr1-1; pm-S, mp-S, mt VII) was used to obtain the KO and/or KD strains. SB1969 (chx1-1/chx1-1, mpr1-1/mpr1-1; pm-S, cy-S, mt II), kindly supplied by Dr. E. Orias (University of California, Santa Barbara, USA), was used as a control in gene expression studies, and was also used to obtain the metal-adapted strains. Micronuclear genotypes of these strains are homozygous mpr1-1 (6-methyl-purine resistant) or chx1-1 (cycloheximide resistant), respectively. Their macronuclear phenotypes are pm-S (paromomycin sensitive), mp-S (6-methyl-purine sensitive) or cy-S (cycloheximide sensitive) and their mating types (mt) are VII or II. Strains GFPMTT1 and GFPMTT5 harbour multi-copy plasmids bearing the constructs PMTT1::GFP::MTT1 or PMTT1::GFP::MTT5, which over-express MTT1 or MTT5, respectively [36].
Cells were axenically grown in PP210 medium [2% w/v proteose peptone (Pronadisa), supplemented with 10 μM FeCl3 and 250 μg/ml of streptomycin sulphate (Calbiochem) and penicillin G (Sigma)] or SPPA medium [2% proteose peptone (Difco), 0.1% yeast extract (Difco), 0.2% glucose (Sigma), 0.003% Fe-EDTA (Sigma), supplemented with 250 μg/ml of streptomycin sulphate, 250 μg/ml of penicillin G (Sigma) and 0.25 μg/ml of amphotericin B (Sigma)], and maintained at a constant temperature of 30 ± 1°C. We added 12 μg/ml of paromomycin sulphate (Sigma) in the GFPMTT1 and GFPMTT5 cultures to maintain the multi-copy plasmid.
Extreme metal-adaptation of T. thermophila strains
Three T. thermophila metal-adapted strains were generated: Cd-adap (cadmium-adapted strain), Cu-adap (copper-adapted strain) and Pb-adap (lead-adapted strain). These metal-adapted strains were obtained after exposing strain SB1969 in PP210 medium to increasing metal concentrations [Cd2+ (CdCl2, Sigma), Cu2+ (CuSO4 ∙5 H2O, Sigma) or Pb2+ (Pb(NO3)2, Sigma)]. The adaptation process consisted on increasing 10 μM the metal concentration every week, and during about 15 months of increasing metal concentrations the survivor cells were selected until achieving their maximum tolerated concentration (MTC). Then, cells were maintained to this MTC during more than two years to complete their metal adaptation. The MTC was achieved at different time periods depending on type of metal. Metal-adapted strains were permanently maintained in PP210 medium with the MTC of the relevant metal (indicated as MTC cultures).
Isolating MT knockout / knockdown strains
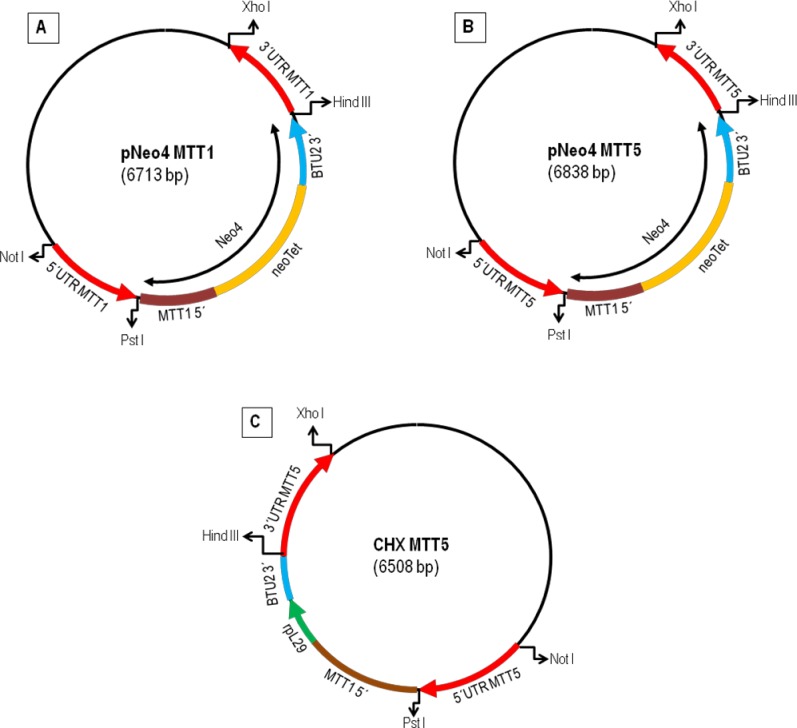
Constructs in Fig 1 were obtained by the directional cloning of the 5' and 3' UTRs of the T. thermophila MTT1 and MTT5 genes in the pNeo4 and rpl29 vectors. First, we amplified the 5' and 3' UTRs regions of the MTT1 and MTT5 genes using primers 5UTRMTT1A/1B and 3UTRMTT1A/1B for MTT1 and 5UTRMTT5A/5B and 3UTRMTT5A/5B for MTT5 (S1 Table, Fig 1). To obtain single MTT1 or MTT5 knockouts, we introduced each amplified UTR into pNeo4 after cutting with NotI HF and PstI (New England Biolabs) for the 5' UTRs and HindIII and XhoI (New England Biolabs) for the 3' UTRs, to obtain pNeo4::MTT1 and pNeo4::MTT5. These contain the neomycin/paromomycin resistance genes under the control of the cadmium-inducible MTT1 promoter (neo4 cassette) [37] and both flanked by the UTR regions of MTT1 or MTT5, respectively (Fig 1). To isolate a double KO (MTT1KO + MTT5KO) beginning with a complete MTT1KO, we prepared a MTT5 knockout construct, pCHXMTT5, using the rpl29 vector. The UTR regions of MTT5 were incorporated into the rpl29 vector using NotI HF and PstI for the 5' UTR and HindIII and XhoI for the 3' UTR. The rpl29 vector contains the cycloheximide resistance gene (rpl29) under the control of the MTT1 promoter and both were flanked by the MTT5 UTRs (Fig 1). Before transformation, the correct constructions were verified by sequencing.
Fig 1. Schematic representation of the plasmid constructs used to obtain knockout and/or knockdown strains.
(A): plasmid construct (pNeo4MTT1) used for MTT1KO strain. (B): plasmid construct (pNeo4MTT5) used for MTT5KD. (C): plasmid construct (pCHXMTT5) used for obtaining MTT1KO + MTT5KD. neoTet: neomycin/paromomycin resistance gene. rpl29: cycloheximide resistance gene. BTU2 3': part of the 3'UTR region of the β-tubulin 2 gene.
Plasmids were introduced by biolistic transformation. Gold bombardment particles (Seashell Technology) at 30 mg/ml concentration were coated, immediately before use, with 5 μg of DNA/1 mg gold particles. CU428 (3 x 105 cells/ml) were starved for 16-18h (TrisHCl buffer 0.01M pH 6.8). The starved cells (30 ml) were centrifuged at 1,100g for 1 min and resuspended in 1 ml of Tris HCl buffer. Then, 1ml cell sample was evenly spread on a sterile circular filter paper and immediately bombarded with the DNA coated gold particles at 900 psi using the DuPont Biolistic PDS-1000/He, Particle Delivery System (Biorad) [38]. Cells were then resuspended in 50 ml of 2% proteose peptone (PP2) medium and maintained with shaking at 30°C for 4-5h. Transformants were selected with 120 μg/ml of paromomycin with 1 μg/ml CdCl2, which drives neo4 expression from the MTT1 promoter. For selecting double knockouts, transformant cells were selected with 12 μg/ml of cycloheximide with 1 μg/ml CdCl2. Cells were transferred daily during at least three weeks under increasing concentrations of paromomycin or cycloheximide, while reducing CdCl2 to drive phenotypic assortment. By this procedure, MTT1 or MTT5 genes were progressively substituted by the resistance cassette, initially integrated at a single site by homologous recombination, until all the MTT1 or MTT5 macronuclear gene copies were eliminated (knockout fixation).
We checked that knockout was complete by maintaining the putative KO cells without the antibiotic selective agent for one week. We then isolated total RNA, reverse-transcribed it to obtain the corresponding cDNAs, and carried out standard PCR to check if there were remaining copies of MTT1 or MTT5 in the macronuclear genome. The MTT1KO was complete but in the putative MTT5KO strain it was not possible to obtain cells lacking all copies of MTT5 gene; thus we consider it as a knockdown (MTT5KD) strain. Similarly, we generated a MTT1KO + MTT5KD strain. Both knockdown strains are inherently unstable, because without selective pressure the gene copy number of MTT5 can increase. To prevent this, we maintained the cells in 800 μg/ml of paromomycin (for MTT5KD) or 60 μg/ml of cycloheximide (for MTT1KO + MTT5KD).
Metal stress treatments
Cells were exposed for 1 or 24h to Cd2+, Cu2+ or Pb2+. Control, KO and KD strains were treated with 44.5 μM (Cd2+), 315 μM (Cu2+) or 965 μM (Pb2+), while metal-adapted, GFPMTT1 and GFPMTT5 strains were exposed to the MTC for each metal: 115 μM Cd2+, 4 mM Cu2+ or 5.5 mM Pb2+. In some experiments with the metal adapted strains, cells were maintained for 24h in PP210 without any added metal and then were exposed for 1 or 24h to the MTC of each metal. The MTC cultures were obtained by continuously culturing cells at their maximum tolerated metal concentration. To study the persistence of the metal adaptation process, we grew the metal adapted strains for 1 or 6 months in PP210 medium in the absence of added metal. Following these periods, cells were again incubated for 1 or 24h in the presence of the MTC of each metal.
Total RNA isolation and cDNA synthesis
Cultures (1–3 x 105 cells/ml) of the different T. thermophila strains were harvested by centrifugation at 2,800 rpm for 3 min. Total RNA samples were isolated from exponential cell cultures by using the TRI Reagent method (Molecular Research Center, MRC). RNA samples were treated with DNase I (Roche) for 30 min at 37°C and visualized following agarose gel electrophoresis. RNA concentrations were determined using NanoDrop 1000 (Thermo Scientific). MultiScribe Reverse Transcriptase 50 units/μl (Life Technologies) and oligo(dT)-adaptor primer (Roche) were used to synthesize the cDNAs from 3.5 μg of total RNA samples.
Quantitative RT-PCR (qRT-PCR)
cDNA samples were amplified in duplicate in 96 microtiter plates. Each qPCR reaction (20 μl) contained: 10 μl of SBYR Green (Takara), 0.4 μl of ROX as passive reference dye (Takara), 1 μl of each primer (at 400 nM final concentration), 3.6 μl of ultrapure sterile water (Roche) and 4 μl of a 10−1 dilution of cDNA. PCR primers (S1 Table) were designed using the "Primer Quest and Probe Design" online-application of IDT (Integrated DNA Technologies). β-actin was used as an endogenous control or a normalizer gene. Melting curves were obtained and primers specificity was tested by confirming each PCR product by gel electrophoresis and sequencing. Real-time PCR reactions were carried out in an iQ5 real-time PCR apparatus (Bio-Rad) and the thermal cycling protocol was as follows: 5 min at 95°C, 40 cycles (30 sec at 95°C, 30 sec at 55°C and 20 sec at 72°C), 1 min at 95°C and 1 min at 55°C. All controls (no template control and RT minus control) were negative. Amplification efficiency (E) was measured by using 10-fold serial dilutions of a positive control PCR template. The efficiency requirement was met for all the tested genes in all the used strains (S2 Table). Results were finally processed by the standard-curve method [39] and were corroborated with at least two independent experiments, each performed in duplicate. We compared the basal expression levels of different genes using the formula: 2(Ct1-Ct2), being Ct1 and Ct2 the cycle threshold (Ct) values of both genes under a control situation (no metal exposure).
Statistical analysis
Gene expression differences were tested for statistical significance by Student’s t test using the program Statgraphics Centurion XVI (16.1.15 version). P-value was fixed in ≤ 0.05.
Results
Comparative MT gene expression analysis among different strains of T. thermophila
Over a period of more than two years, we adapted three T. thermophila cultures to high concentrations of metals. These were named Cd-adap, Cu-adap and Pb-adap. The maximum tolerated concentrations (MCT) were 115 μM Cd2+, 4 mM Cu2+, and 5.5 mM Pb2+. These MTC values are substantially higher than the LC50 values (lethal concentration killing 50% of cell population) previously determined for the parental SB1969 strain in PP210 medium (24 h exposure): ≈ 2.5x the LC50 for Cd2+ (44.5 μM), ≈ 12.7x the LC50 for Cu2+ (315 μM), and ≈ 5.7x the LC50 for Pb2+ (965 μM) [20]. In the course of constructing KO strains, we found that it was impossible to disrupt all macronuclear copies of MTT5, indicating that the gene is essential. Therefore, the strain that we obtained should be considered a knockdown (MTT5KD). In total, we obtained a MTT1KO strain, a double mutant MTT1KO+MTT5KD, and the MTT5KD strain.
We compared the gene expression of MTT1, MTT3, MTT5 and MTT2/MTT4 between different T. thermophila strains; the SB1969 control strain, the three metal-adapted strains (Cd-adap, Cu-adap and Pb-adap), the three knockout (KO) and/or knockdown (KD) strains described above, and GFPMTT1 and GFPMTT5 that over-express MTT1 or MTT5, respectively [36]. MTT2 and MTT4 are 98% identical [20] so it is not possible to design specific primers to distinguish them by qRT-PCR. Therefore, we refer to them collectively as MTT2/4, because we evaluated the expression of both genes together using primers MTT2QA and MTT2QB (S1 Table).
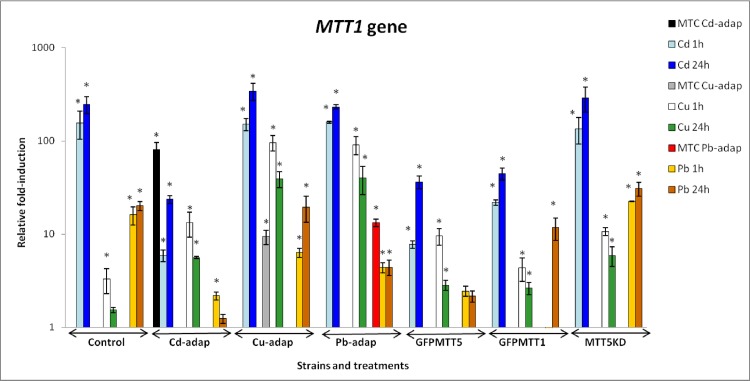
Relative fold-induction values for MTT1 are shown in Fig 2 and S3 Table. MTT1 responds preferably to Cd2+ in all studied strains and, in general, 24h Cd2+ or Pb2+ exposures result in higher relative induction than 1h. However, the opposite effect is observed for Cu2+, which induces a stronger MTT1 induction after 1h than after 24h (Fig 2). Moreover, in Cd-adap and Pb-adap strains, MTT1 induction of MTC cultures were higher than those after 1 or 24h treatments. On the other hand, in the Cu-adap strain the MTC culture induced MTT1 less strongly than in cultures treated for 1 or 24h (Fig 2). In general, MTT1 fold-induction values from control, GFPMTT1 and MTT5KD strains show the following ranking: Cd > Pb > Cu. For Cd-adap, Cu-adap, Pb-adap and GFPMTT5 strains, this ranking changes to: Cd > Cu > Pb (Fig 2). MTT1 is not induced (fold-induction < 2) in GFPMTT1 after 1h Pb2+ treatment, but it is induced (around 11-fold) in this strain after 24h treatment at the same Pb2+ concentration. Likewise, this gene is not induced in control and Cd-adap strains at 24h Cu2+ or Pb2+ treatments (Fig 2, S3 Table).
Fig 2. Comparison of relative fold-induction of MTT1 in different T. thermophila strains.
MTC: maximum tolerated concentration (115 μM Cd2+, 4 mM Cu2+ or 5.5 mM Pb2+). β-actin was used as the normalizer gene. Each histogram bar represents an average value ± standard deviation (see S3 Table) from two or three independent experiments. Relative induction values are represented in a logarithmic scale. Asterisks indicate significant differences from the control with p ≤ 0.05.
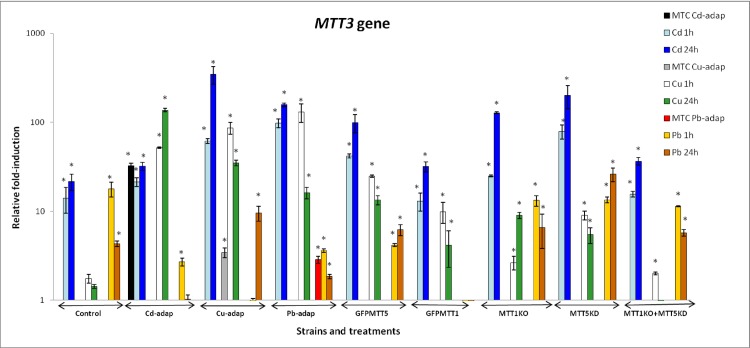
Relative fold-induction for MTT3 is reported in Fig 3 and S3 Table. Like MTT1, MTT3 preferentially responds to Cd2+ in almost all strains. The exception is the Cd-adap strain, in which stronger induction occurs after Cu2+ treatments than after Cd2+ (Fig 3). In general, 24h Cd2+ treatments cause higher relative MTT3 induction than after 1h, while for Cu2+ or Pb2+ treatments the highest induction depends on the strain rather than the metal exposure time. In Cd-adap and Pb-adap strains, the induction of MTT3 in MTC cultures was very similar to that obtained after 1 or 24h. In contrast, in the Cu-adap strain, MTT3 induction in MTC cultures was lower than after 1 or 24h (Fig 3). In control, MTT1KO, MTT5KD and MTT1KO+MTT5KD strains, MTT3 shows the following fold-induction ranking: Cd > Pb > Cu, whereas in Cu-adap, Pb-adap, GFPMTT5 and GFPMTT1 strains the ranking for MTT3 is: Cd > Cu > Pb. Finally, for the Cd-adap strain, MTT3 induction shows the following ranking: Cu > Cd > Pb (Fig 3). Following 1h of Cd2+ exposure, MTT3 gene induction values in MTT1KO and MTT5KD were considerably larger than in control strains (≈ 2- and ≈ 6-fold, respectively), and the same was true after 24h exposure (≈ 6- and ≈ 9-fold, respectively). However, in the MTT1KO + MTT5KD strain, MTT3 maintains similar induction values after Cd2+ exposures to those seen in control strain (Fig 3, S3 Table). Finally, MTT3 showed no induction in the following cases: in GFPMTT1 after 1 or 24h Pb2+ treatment; in the control strain after 1 or 24h Cu2+ treatments; in the Cd-adap (24h Pb2+); in the Cu-adap (1h Pb2+) and; in the MTT1KO + MTT5KD strain (24h Cu2+) (Fig 3, S3 Table).
Fig 3. Comparison of relative fold-induction of MTT3 in different T. thermophila strains.
MTC: maximum tolerated concentration (115 μM Cd2+, 4 mM Cu2+ or 5.5 mM Pb2+). β-actin was used as the normalizer gene. Each histogram bar represents an average value ± standard deviation (see S3 Table) from two or three independent experiments. Relative induction values are represented in a logarithmic scale. Asterisks indicate significant differences from the control with p ≤ 0.05.
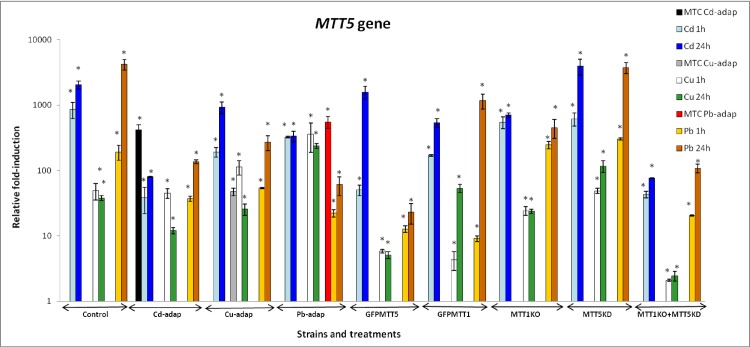
MTT5 shows the highest fold-induction among the five T. thermophila MT genes, independent of the analyzed strain. Unlike MTT1 and MTT3, it is induced under all the assayed metal treatments in all strains (S3 Table). MTT5 preferentially responds to Cd2+ and Pb2+ and shows strongest induction after 24h treatments (Fig 4, S3 Table). In the majority of the strains, the MTT5 fold-induction ranking is: Pb ≥ Cd > Cu. The exception is the Pb-adap strain, where the ranking is: Cd = Cu ≥ Pb (Fig 4). In metal-adapted strains, the MTC cultures have higher MTT5 induction values than those reported at 1 or 24h for Cd2+ and Pb2+ exposures, while for Cu2+ treatments the gene induction values in MTC cultures are lower than after 1h treatments. After Cd2+ treatments (1 or 24h), the MTT1KO and the MTT1KO + MTT5KD strains have lower MTT5 induction values than the control strain, while these values are higher (≈ 2x) in the MTT5KD strain after 24h Cd2+ treatments, compared to the control strain. After 1h Pb2+ exposures, MTT1KO and MTT5KD have higher MTT5 induction values than the control strain, while after 24h Pb2+ treatments these values are lower (in MTT1KO) or similar (in MTT5KD strain) relative to the control strain (Fig 4, S3 Table).
Fig 4. Comparison of relative fold-induction of MTT5 in different T. thermophila strains.
MTC: maximum tolerated concentration (115 μM Cd2+, 4 mM Cu2+ or 5.5 mM Pb2+). β-actin was used as the normalizer gene. Each histogram bar represents an average value ± standard deviation (see S3 Table) from two or three independent experiments. Relative induction values are represented in a logarithmic scale. Asterisks indicate significant differences from the control with p ≤ 0.05.
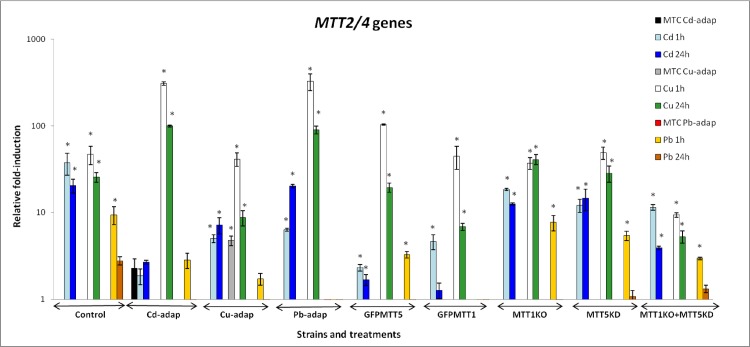
MTT2/4 responds most strongly to Cu2+, showing the highest fold-induction values after 1h treatment with this metal in all strains (Fig 5). These genes are also significantly induced upon Cd2+ exposures in most strains, but do not respond to 24h Pb2+ treatments. Moreover, MTT2/4 are only significantly induced after 1h Pb2+ exposures in control, GFPMTT5, MTT1KO, MTT5KD and MTT1KO + MTT5KD strains. In all strains the fold- induction values for MTT2/4 follow this ranking: Cu > Cd > Pb. MTT1KO and MTT5KD strains show similar MTT2/4 induction to the control under Cu2+ stress. However, the MTT1KO + MTT5KD strain has considerably weaker MTT2/4 induction than the control strain (S3 Table). MTT2/4 are not induced in the following strains and treatments: control strain (24h Pb2+); Cd-adap (all Cd2+ and Pb2+ exposures); Cu-adap (1 and 24h Pb2+); Pb-adap and GFPMTT1 strains (all Pb2+ exposures); GFPMTT5, MTT1KO, MTT5KD and MTT1KO + MTT5KD strains (1h Pb2+). Therefore, MTT2/4 show the weakest induction among the MT genes, under the conditions tested (Fig 5, S3 Table).
Fig 5. Comparison of relative fold-induction of MTT2/4 in different T. thermophila strains.
MTC: maximum tolerated concentration (115 μM Cd2+, 4 mM Cu2+ or 5.5 mM Pb2+). β-actin was used as the normalizer gene. Each histogram bar represents an average value ± standard deviation (see S3 Table) from two or three independent experiments. Relative induction values are represented in a logarithmic scale. Asterisks indicate significant differences from the control with p ≤ 0.05.
Comparative MT gene expression analysis of metal-adapted strains from reversible adaptive metal resistance experiments
The three metal-adapted strains, generated as described in Materials and Methods, were transferred and maintained for 1 or 6 months in PP210 without any added metal (indicated as -1M or -6M strains). Afterward, they were again exposed to the MTC of the metal to which they had been adapted, for 1 or 24h, and MT gene induction was measured. The three T. thermophila pre-adapted strains were able to survive in the presence of the relevant metal MTC, and no significant cell mortality was detected by microscopy.
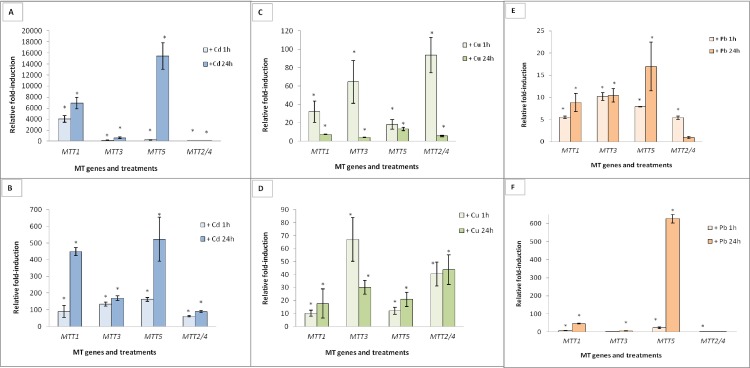
Gene induction values are shown in Fig 6. The Cd-adap strain maintained the same ranking of relative gene induction after 1 or 6 months of culture in the absence of Cd2+: MTT5 > MTT1 > MTT3 > MTT2/4 (Fig 6A, S3 Table). However, the absolute induction levels in the -1M culture were higher than seen previously, most notably for MTT5 and MTT1 (Fig 6A, S3 Table). The fold-induction decreased in the -6M sample, compared to -1M, but remained higher than the induction seen in the founder Cd-adap culture (Fig 6B). For MTT1, MTT3 and MTT5, 24h Cd2+ addition to -1M or -6M cultures generated higher fold-induction values than 1h addition. For MTT2/4, the highest expression levels were achieved after 1h Cd2+ treatments (Fig 6).
Fig 6. Relative fold-induction of the five T. thermophila MT genes in metal-adapted strains.
These strains were maintained for 1 or 6 months without the metal treatment and then re-exposed (1 or 24h) to the relevant MTC. (A): Cd-adap after 1 month without metal (-1M). (B): Cd-adap after 6 months without metal (-6M). (C): Cu-adap (-1M). (D): Cu-adap (-6M). (E): Pb-adap (-1M). (F): Pb-adap (-6M). β-actin was used as the normalizer gene. Each histogram bar represents an average value ± standard deviation (see S3 Table) from two or three independent experiments. Asterisks indicate significant differences from the control with p ≤ 0.05.
For the Cu-adap strain, the induction ranking of the founder culture was MTT5 > MTT1 >MTT3 >MTT2/4, as described above. This induction ranking changed considerably, after 1 month culture in medium lacking added Cu2+, to (MTT2/4 > MTT3 > MTT1 > MTT5), and again after 6 months to (MTT3 > MTT2/4 > MTT5 ≈ MTT1) (Fig 6C and 6D). In the Cu-adap (-1M) culture, induction (mainly for MTT2/4 and MTT3) is considerably higher after 1h Cu2+ treatments than after 24h Cu2+ or than induction in the founder Cu-adap strain (MTC samples) (Fig 6C, S3 Table). However, the Cu-adap (-6M) culture generally showed stronger induction after 24h treatment than after 1h, the exception being MTT3 (Fig 6D).
For the Pb-adap strain, the induction ranking of the founder culture was MTT5 > MTT1 > MTT3 > MTT2/4, as described above. The Pb-adap (-1M) and (-6M) strains maintained a very similar induction ranking (MTT5 > MTT1 ≥ MTT3 > MTT2/4) (Fig 6E and 6F, S3 Table). In the -1M culture, MTT1, MTT3 and MTT2/4 induction values were higher than those in the founder culture; however, MTT5 induction was lower than the in the founder (Fig 6E, S3 Table). In the -6M culture, MTT5 was enormously induced after 24h Pb2+ exposure, similar to that seen in the founder culture (S3 Table). MTT1 also responded under these conditions, mainly after 24h Pb2+ exposure, while MTT2/4 and MTT3 showed less induction than in the Pb-adap (-1M) strain (Fig 6E and 6F). In general, 24h treatments resulted in similar or stronger induction compared to induction after 1h metal exposure, for Pb-adap (-1M) and (-6M) cultures (Fig 6E and 6F).
Comparative analysis of constitutive MT gene expression levels among the different T. thermophila strains
T. thermophila MT genes show constitutive expression in wildtype cells under non-stressful conditions [20]. For the strains generated in the present study, we compared constitutive (basal) expression levels using the Ct values obtained in the absence of added metal.
In qPCR (quantitative polymerase chain reaction) or qRT-PCR studies, the threshold is defined as the level of signal that reflects a statistically significant increase over the calculated baseline signal. It is set to distinguish a relevant amplification signal from the background. The threshold cycle (Ct) value is the cycle number at which the fluorescent signal of the reaction crosses the threshold, and is inversely related to the amount of starting template. For example, low Ct values correspond with high levels of transcripts (qRT-PCR templates) or DNA copy number (qPCR templates). The Ct values corresponding to basal gene expression levels are reported in S4 Table. We used these data to compare basal Ct values of different MT genes within the same strain (S5 Table), or of individual MT genes between the different T. thermophila strains (S6 Table). In particular, we calculated 2 (Ct1-Ct2), where Ct1 and Ct2 are the Ct values of two different samples.
As shown in S4 Table and S1 Fig, MTT1 has the highest basal expression in most strains (SB1969, Cd-adap, Pb-adap, GFPMTT1, GFPMTT5, MTT5KD, -1M and - 6M metal-adapted cultures). MTT2/4 shows highest basal expression in Cu-adap, Cu-adap (-1M) and MTT1KO strains. MTT5 shows the highest basal values only in the MTT1KO + MTT5KD and Pb-adap (-1M) culture. Therefore, in general, MTT5 and MTT3 have the lowest basal expression, ranked third or fourth in most strains (SB1969, Cd-adap, Cu-adap, Cd-adap (-1M and -6M), Cu-adap (-1M and -6M), GFPMTT1, MTT1KO and MTT5KD) (S1 Fig).
The MT basal expression ranking in the SB1969 control strain is MTT1 > MTT3 > MTT2/4 > MTT5. The absolute level of MTT1 expression is about 1.6x MTT3, ≈ 8x MTT2/4 and ≈ 36x MTT5. For MTT3, expression is about 5x MTT2/4 and 22x MTT5. MTT2/4 expression is ≈ 4x MTT5 (S5 Table). In the Cd-adap strain, MTT1 ≈ 30x MTT3, ≈ 68x MTT5 and ≈118x MTT2/4, while MTT3 ≈ 2x MTT5 and ≈ 4x MTT2/4. MTT5 expression ≈ 2x MTT2/4 (S5 Table). While MTT1 in this Cd-adap culture, like in the control strain, shows the highest basal expression, the differences between basal expression levels of MTT1 vs the other MT genes are greater in the adapted culture: 2-fold for MTT5, ≈ 15-fold for MTT2/4, and ≈19-fold for MTT3 (S5 Table). MTT2/4 showed highest basal expression levels in the Cu-adap strain, being ≈ 27x MTT5 and 16x MTT3. For the Cu-adap (-1M) culture, MTT2/4 = ≈ 11x MTT5 and 6.5x MTT3 (S5 Table). MTT1 and MTT2/4 show similar high basal expression. The ranking of relative expression levels is modified in the Cu-adap (- 6M) culture: MTT1 > MTT2/4 > MTT5 > MTT3 (S1 Fig, S5 Table). MTT1 is ranked first for basal expression in the Pb-adap and Pb-adap (-6M) cultures, but this gene is ranked second in the Pb-adap (-1M) culture (MTT5 > MTT1 > MTT2/4 > MTT3) (S1 Fig, S5 Table).
In the GFPMTT1 and GFPMTT5 strains, MTT1 also shows the highest basal expression levels, with the following rank: MTT1 ≈ 35x MTT5 and 4x MTT3 in GFPMTT1 and ≈ 3x MTT5 and 22x MTT3 in GFPMTT5 (S5 Table). This last ratio (MTT1 vs. MTT3) is considerably higher than that in SB1969 (S5 Table). In MTT1KO and MTT1KO + MTT5KD strains, MTT2/4 and MTT5 (respectively) show the highest basal expression levels (S1 Fig). The ranking in MTT1KO is MTT2/4 ≈ 4x MTT5. For MTT1KO + MTT5KD, the ranking is MTT5 ≈ 6x MTT3 and MTT2/4 ≈ 4.6x MTT3 (S5 Table). For MTT5KD, MTT1 has the highest basal expression level, and MTT1 ≈ 2x MTT5 and 34x MTT3 (S5 Table).
Overall, the highest basal expression for MTT1 and MTT3 are in the Cd-adap culture, while MTT2/4 are expressed most strongly in the Cu-adap culture. MTT5 is most strongly expressed in GFPMTT5 (S6 Table). We also compared the basal expression levels to those in the control strain. MTT2/4 expression levels are considerably increased in GFPMTT1 (≈ 7x control). In GFPMTT5, the basal expression levels of MTT5 is increased ≈ 64x compared to the control strain, and the corresponding increases for MTT1 ≈ 4.5x, and for MTT2/4 ≈ 6.5x (S6 Table).
Similarly, in the metal-adapted cultures MTT1 and MTT5 genes show increased basal expression; ≈ 28x and 15x, respectively, in the Cd-adap strain, while MTT2/4 basal expression is augmented ≈ 17.5x in Cu-adap strain. MTT5 shows increased basal expression in the Pb-adap strain (≈ 7.5x) (S6 Table).
Several changes occurred in basal expression of the metal-adapted strains after they were maintained for 1 or 6 months without added metal. MTT1 expression returned to the level of the control strain in the Cd-adap (-1M) culture, but the same cells maintained higher basal expression of MTT2/4 (≈ 4x) and MTT5 (≈ 6x) relative to the control. This higher MTT5 basal expression is also maintained in the Cd-adap (-6M) strain (S6 Table).
MTT2/4 basal expression was considerably reduced in the Cu-adap (-1M) culture, but are nonetheless still higher (≈ 4x) compared to the control strain. MTT2/4 expression returned to control levels in the Cu-adap (-6M) strain, but there was an increase in MTT5 basal expression (≈ 8.5x) (S6 Table). MTT5 basal expression increased more dramatically in Pb-adap (-1M) and (- 6M), to ≈ 168x and 21x the control strain levels (S6 Table). Finally, the MTT1KO and MTT1KO + MTT5KD strains both showed increased basal expression compared to the control strain of MTT5 (≈ 8.5x and ≈ 60x, respectively) and MTT2/4 (≈ 8x and ≈ 10x, respectively) (S6 Table). The basal expression ranking in MTT5KD was similar to that of GFPMTT1 (S1 Fig).
Discussion
In the work described in this manuscript, we have focused on a set of unanswered questions regarding the regulation and specific functions of the individual T. thermophila MT isoforms. By comparing T. thermophila MT gene expression under different metal stresses and using the metal-adapted (Cd-adap, Cu-adap and Pb-adap), knockout and/or knockdown (MTT1KO, MTT5KD and MTT1 + MTT5KD) and other strains (SB1969 control, GFPMTT1 and GFPMTT5), we obtained a better understanding of the specific roles of each MT isoform.
One approach we have taken is to adapt Tetrahymena cultures over extended periods to high metal concentrations. Such adaptation programs, in which organisms are increasingly exposed to a specific external stress, can reveal the cellular mechanisms and pathways that underlie the normal stress response. In such an experimental evolution experiment, the continuous adaptation to a specific stress can serve to magnify and reveal cellular efforts to counteract any harmful effects. One mechanism of adaptation is modulation of gene expression ("gene expression plasticity"), which can produce new adaptive phenotypes in the face of a specific stress [40].
In our experiments, different T. thermophila cultures were cultured in the presence of increasing metal (Cd2+, Cu2+ or Pb2+) concentrations. We could thus define a maximum tolerated concentration (MTC). These MTC values were considerably higher than the corresponding LC50 values in this species previously determined for each metal [20]. The relative increase in MTC values (i.e., between the adapted vs non adapted cultures) was inversely correlated with the toxicity of the specific metals: Cu2+ (≈ 12.7x) > Pb2+ (≈ 5.7x) > Cd2+ (≈ 2.5x). We studied cultures that were maintained at their respective MTCs (Cd-adap, Cu-adap or Pb-adap) for extended periods.
The MTT1 isoform: A gene with a relevant and still unknown constitutive function
MTT1 is most strongly induced by Cd2+ in all strains, as previously reported in the wild-type 20]. In addition, the MTT1 protein showed the highest affinity for Cd2+ among the T. thermophila CdMTs [27]. However, MTT1 also responded to Cu2+ or Pb2+, with different expression patterns depending on the metal. With Cd2+ or Pb2+, MTT1 was induced by 1h but the highest induction values were achieved after 24h. With Cu2+, the highest MTT1 induction occurred after 1h. A difference in induction by Cd2+/Pb2+ vs Cu2+ was also observed in the metal-adapted strains. Both Cd-adap and Pb-adap strains showed strongest MTT1 induction under continuous exposure to the MTC, corroborating that the persistent presence of these metals is required to maintain highest MTT1 expression. In contrast, the strongest MTT1 induction in the Cu-adap strain occurred after growth for 24h without any added metal, and then re-exposing the cells to Cu2+ for 1h.
Among the T. thermophila MT proteins, MTT1 shows the lowest affinity for Cu2+ [27] and the MTT1 gene shows weakest induction by this metal. Unlike Cd2+ or Pb2+, Cu2+ is an essential metal and is comparatively less toxic, which may account for the difference in the MTT1 transcriptional response to this metal. MTT1 expression occurred soon after exposure to Cu2+, and then decreased over time. In contrast, the induction in response to Cd2+ or Pb2+ was more persistent. These metals may be increasingly toxic with long exposure, and therefore require more prolonged strong MTT1 induction. A similar disparity between the effects of Cd2+ or Cu2+ stress on transcription was reported for MTT3, MTT5 and MTT2/4, as well as on MT gene transcription of other Tetrahymena species [6]. In general, two different MT gene expression patterns can be distinguished in Tetrahymena. In response to Cu2+, the high initial transcript levels subsequently decrease over time. In contrast, the transcriptional response to Cd2+ or Pb2+ increases over time. These differences in the transcriptional responses are correlated with the essential vs. non-essential nature of the metals, and their levels of toxicity, but the underlying molecular or physiological mechanisms are not yet known.
MTT1 constitutive expression differed significantly between strains, with the following relative ranking: Cd-adap > GFPMTT5 > GFPMTT1 > Control strain > Cu-adap ≈ Pb-adap > MTT5KD. Compared to the control, expression was ≈ 28x higher in Cd-adap, ≈ 5x higher in GFPMTT5 and 2x higher in GFPMTT1. This is probably the reason why MTT1 gene induction levels in the GFPMTT1 strain were not higher than those in the control strain (Fig 2). Based on these results, we can conclude the following points. First, Cd-adaptation involves an increase of basal MTT1 expression. Similar phenomena have been reported in other organisms. For instance, an increase in constitutive MT expression plays an important function when the arthropod Orchesella cincta is chronically exposed to Cd2+ [41]. Likewise, in the domestic fly (Musca domestica), a cytochrome P450 isoform that confers insecticide resistance shows 9-fold higher constitutive expression in a resistant strain compared to a non-resistant one [42]. In some freshwater snail species, like Biomphalaria glabrata, constitutive expression of a MT gene seems to confer tolerance to the snails against Cd2+ exposure [43].
GFPMTT1 contains multiple copies of the recombinant plasmid pVGFMTT1 with the construct PMTT1::GFP::MTT1 (complete MTT1 ORF under its own MTT1 promoter) [36]. Therefore, MTT1 is over-expressed, including the expected increase in basal expression. In GFPMTT5, MTT5 is driven by the MTT1 promoter and its constitutive expression levels are ≈ 49x higher than in the control. The MTT1 promoter driving the heterologous construct is more active under basal conditions than the endogenous MTT5 promoter, accounting for the observed increase in MTT5 basal expression. Interestingly, MTT1 basal expression is also considerably higher in this strain compared to the wildtype (≈ 5x) and to the GFPMTT1 strain (≈ 2x). In conclusion, Cd-adap, GFPMTT1 and GFPMTT5 show increased MTT1 basal expression. This increase can be explained by a greater MTT1 copy number in GFPMTT1, and this may also be true in the Cd-adap strain (unreported results).
In GFPMTT5, the increase of MTT1 basal expression may be due to higher MTT5 basal expression driven by the MTT1 promoter. This can be explained if basal expression is coordinated between MT genes, in this case MTT1 and MTT5. Van Straalen et al. [44] have pointed out that both cis and trans-regulatory mechanisms can contribute, in a combinatorial fashion, to adaptive evolution in response to a stress. We detected more hints of coordinated gene regulation in the knockout (KO) and/or knockdown (KD) strains. MTT1KO and MTT1KO + MTT5KD, lacking all copies of MTT1, show increased MTT5 basal expression (≈ 8.5x control for MTT1KO and ≈ 59x for MTT1KO + MTT5KD). MTT5 may be upregulated to compensate for the absent MTT1 activity in these strains. It appears that while MTT1 is not essential, its activity is important in stress but also non-stress conditions, where basal expression may contribute to cellular homeostasis. Consistent with this idea, basal MTT1 expression is the highest among the MT isoforms in all strains except the Cu-adap culture (S5 Table). The ranking of MT basal expression in GFPMTT1 is MTT1 > MTT2/4 > MTT3 > MTT5, which is very similar to the control (MTT1 > MTT3 > MTT2/4 > MTT5). However, in the GFPMTT5 strain, MTT5 is second in basal expression (MTT1 > MTT5 > MTT2/4 > MTT3), and a similar ranking was found for the Pb-adap strain (MTT1 > MTT5 > MTT2/4 > MTT3) (Fig 7, S5 Table).
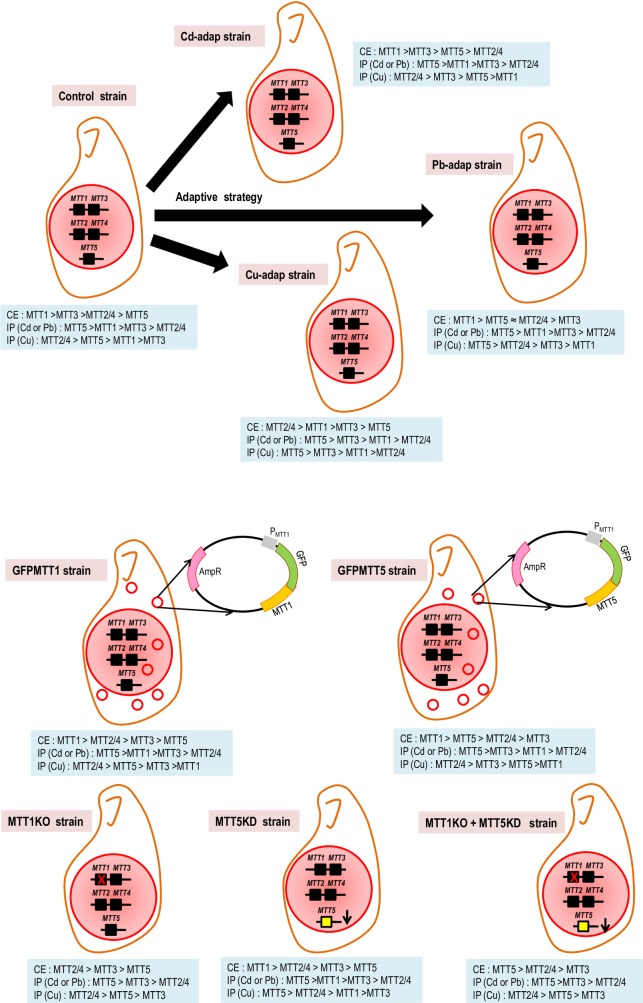
Fig 7. Schematic representation of the genomic characteristics and the MT gene expression levels from the different T. thermophila strains used in this study.
The constitutive MT gene expression ranking (CE) and the MT gene induction patterns (IP) obtained under Cd2+/ Pb2+ or Cu2+ treatments are shown for each strain.
The MTT5 isoform: An essential "alarm" MT gene
Based on previous studies, MTT5 was considered the main MT gene involved in metal detoxification [5, 26]. This protein represents one of the best Cd-thionein after MTT1 due to its high affinity for this metal cation [27]. These conclusions are extended by our present results. MTT5 is the most strongly induced MT gene for any metal treatment and in all strains. The consensus MT gene induction pattern following exposure to Cd2+ or Pb2+ is MTT5 > MTT1 / MTT3 > MTT2/4 (Fig 7). This includes MTT5KD which has a considerably reduced MTT5 copy number (unreported results). Moreover, although the MTT5 gene product has a low affinity for Cu2+ [27], it is the most highly induced MT following Cu2+ treatment in three strains (Cu-adap, Pb-adap and MTT5KD) (Fig 7). This strong induction under different metal stress treatments may be explained by the presence of a 416 bp duplicated sequence in its promoter region [20], which includes more putative binding sites for AP-1 transcription factors than are found in other T. thermophila MT isoforms [5, 6, 20, 26].
Basal expression of MTT5 is much lower than that of other MTs in several strains (control, Cu-adap, GFPMTT1, MTT1KO and MTT5KD) (Fig 7). In SB1969, basal expression of MTT1, MTT3 and MTT2/4 are ≈ 36x, 23x, and 4x the expression of MTT5, respectively. In Cd-adap and Pb-adap strains, MTT5 is ranked third and second in basal expression, respectively (Fig 7). However, MTT5 is the most highly expressed MT in the MTT1KO + MTT5KD strain, where MTT1 is absent and MTT5 gene copy number is drastically reduced (Fig 7). Basal MTT5 expression in MTT1KO is ≈1,000x that in the MTT5KD strain, a difference which is due to the reduced MTT5 copy number in MTT5KD and likely compensation in MTT1KO for the absence of MTT1. Taken together, our data suggest that the balance of the MTT5 and MTT1 activities may regulate the expression of both genes.
Unlike MTT1, which shows high basal expression, MTT5 does not seem to be required in non-stress conditions, judging by its low basal expression. Expression increases very quickly, to high levels, under stress conditions. In this sense, MTT5 might be considered an "alarm” MT gene, while at the same time it is essential for cell viability. As an essential gene, MTT5 may be required to induce other MT isoforms (e.g., MTT1 or MTT2/4), which may be needed depending on the specific stressor and the total available MT pool. GFPMTT5 shows ≈ 49-fold increased basal expression for this "alarm” MT gene. This might be interpreted by these cells as a stress situation, accounting for the increased expression of other MTs: a ≈ 5x increase for MTT1 and ≈ 6.5x increase for MTT2/4. The MTT1KO + MTT5KD strain must respond to a distinct genetic stress: loss of MTT1 and drastically reduced MTT5 copy number. Facing this situation, this strain shows ≈ 59x over-expression of MTT5 and ≈ 10x over-expression of MTT2/4. These results are consistent with the idea that expression of T. thermophila MT genes is coordinated in response to a stress situation.
MTT5 is essential for T. thermophila viability, in contrast with all MT genes described in a variety of other organisms that appear to be non-essential. For example, Caenorhabditis elegans nematodes with knockouts of either of the two MT genes, as well as with both genes disrupted, are still viable [45]. Mice with MT knockouts show normal development and reproduction, but are more vulnerable to oxidative stress [15, 46]. Similarly, knockout strains for all four Drosophila melanogaster MT genes are viable but are hypersensitive to Cu2+, Cd2+ or Zn2+ [47].
In T. thermophila, MTT5 may also have a key role in Pb2+ detoxification and adaptation processes. The Pb-affinity of MTT5 has not been determined, but the induction of MTT5 at 24h Pb2+ in the majority of strains is higher than that of other MT isoforms. In addition, basal MTT5 expression in the Pb-adap strain is increased about 7-fold relative to the control. After 6 months culturing without Pb2+, re-exposure for 24h to the Pb2+ MTC resulted in higher fold-induction of MTT5 than of the other MTs.
The MTT3 isoform: A still little defined MT
MTT3 is located adjacent to MTT1 (at 1.7 Kb) on the same macronuclear chromosome and with the same orientation. The two genes (486 bp in length, 85% nucleotide sequence identity) encode similar polypeptides (76% amino acid identity) and they probably originated by paralogous duplication [5, 20, 26]. The sequence changes may have resulted in drastic changes in their metal binding abilities and functions. Moreover, variations in their promoter regions and in their cis-regulatory motifs correlate with their differential expression, which may have been an early step in their functional divergence [48]. One difference between their promoter regions is the different number of putative binding sites for AP-1 transcription factors: the MTT1 promoter contains 6 putative binding sites while the MTT3 promoter has only 2 [20]. This difference may contribute to the lower gene expression levels of MTT3 with respect to MTT1 or MTT5, which has 13 putative AP-1 binding sites [6, 26].
MTT3 is classified as a CdMT because its pattern of Cys residues is very similar to that of other Tetrahymena CdMTs [5, 6, 26]. Its expression is mainly induced by Cd2+ (after 24h treatments) in almost all strains. However, it is also strongly induced by Cu2+ (after 1 or 24h treatment) in the metal-adapted strains, as well as in the Cu-adap (-1M) and (-6M) strains re-exposed to the Cu2 MTC + for 1h. MTT3 basal expression is quite low, ranked 2nd or 3rd depending on the strain. In the MTT5KD, basal MTT3 expression was lower than that of the other strains (e.g., 42-fold lower than the control). Although MTT3 has previously been classified as a CdMT and part of the Tetrahymena 7a subfamily [6, 20], we would now argue that it may be specialized for Cd2+ or Cu2+, and may not in fact be particularly well-suited for the coordination of Cd2+ [27]. Therefore, the specificity of this MT still remains to be fully defined [27]. The lack of a strong metal preference may provide plasticity that allows MTT3 to perform diverse physiological roles, binding Cd2+, Cu2+ and/or Zn2+ depending on the environment [27].
Unlike any of the other T. thermophila CdMTs, the MTT3 protein sequence includes 2 histidine residues, while a single histidine is found in a MT from T. patula [6]. Histidines enhance, through their imidazole rings, the affinity for Zn2+ in comparison with Cd2+. Histidines are the most frequent Zn2+-liganded residues in metalloenzymes [49] and they stabilize the formation of metal-protein complexes in MT proteins [50]. As previously reported, MTT3 is most strongly induced after Zn2+ treatment (1h) [20]. Therefore, this gene may play a role in intracellular homeostasis of essential metals, Zn2+ and/or Cu2+.
The MTT2/4 isoforms: Two better than one
MTT2 and MTT4, which are tandemly clustered in the right arm of the micronuclear chromosome 4, share 98% identity at the nucleotide level and 99% at the amino acid level. Their promoter regions are also quite similar (76% identity) and each bears two putative binding motifs for AP-1 transcription factors [6]. Following the duplication that gave rise to the gene pair, there appears to have been little diversification. We do not yet know if the genes are differentially expressed or if both copies are jointly expressed, an increase in gene dosage that could enhance the cellular stress response to metals. Neither seems to be a pseudogene, and the MTT2 upstream region functions as a copper-inducible promoter [51].
MTT2 and MTT4 isoforms are clearly CuMTs as they show highest affinity to copper (MTT2 > MTT4) among all T. thermophila MT isoforms, and moreover do not form stable complexes with Cd2+ [27]. In almost all the T. thermophila strains, MTT2/4 expression is preferentially induced by Cu2+, mainly after 1h exposure. The consensus ranking for induction following Cu2+ stress is MTT2/4 > MTT5 ≈ MTT3 > MTT1. An exception is found in the Cu-adap strain, in which MTT2/4 genes are ranked last in induction (Fig 7). This is explained by the very high basal expression of MTT2/4 in that culture compared to the other MT genes: MTT2/4 basal expression is 4 times higher than MTT1, ≈ 16x MTT3 and ≈ 27x MTT5. This elevated MTT2/4 basal expression may obviate the need to further induce MTT2/4 upon Cu2+ stress. Our results suggest that MTT2/4 may play a key role in Cu-adaptation.
MTT2/4 basal expression considerably increases in the KO and/or KD strains. In MTT1KO, MTT2/4 basal expression levels are increased ≈ 8-fold. In MTT1KO+MTT5KD, the basal expression is increased about 10-fold compared to the control, or 5-fold compared to MTT5KD. These results support the idea of coordinated regulation of MT genes that may include MTT1, MTT5 and MTT2/4. In this way, higher MTT1 basal expression in GFPMTT1 (≈ 2x) may be linked to higher MTT2/4 basal expression (≈ 7x). Likewise, in GFPMTT5, increased MTT5 basal expression (≈ 49x) may upregulate basal expression of MTT1 (≈ 5x) and MTT2/4 (≈ 6x).
Overall, our results obtained with T. thermophila strains and particularly with metal-adapted strains clearly indicate differential roles for the T. thermophila MT isoforms. Similarly, the four MT isoforms in Drosophila melanogaster do not contribute equally to metal detoxification [52]. Most simply, MT functional differentiation can be due to both differential gene expression and to differences in metal binding specificities. We found evidence for inter-connected transcriptional co-regulation of the T. thermophila MT genes, particularly MTT1, MTT5 and MTT2/4. One possibility is that the genes share trans-acting regulatory factors, which may be directly or indirectly controlled by the MTs themselves, leading to activation or repression of MT gene expression. In recent years, the traditional view that MTs are exclusively involved in metal detoxification is being replaced by the idea that they are dynamically involved in a range of phenomena including gene regulation, neurotransmission, control of neurodegenerative and neoplastic disorders, and tumor progression [53]. These functions may depend upon the roles of MTs in pathways including intracellular transport, signaling, essential metal homeostasis, enzymatic and transcriptional regulation, as well as metal detoxification [53]. These roles may depend on the formation of protein complexes that include MTs. Mammalian MTs have been reported to directly interact with proteins including Rab3A GTPase [54], LPR-receptors [55], bovine serum albumin [56] and p53 and NF-kB transcription factors [57]. Moreover, MTs can also interact indirectly with other proteins, swapping essential ions (Zn, Cu or Fe) with proteins including ferritin [58], Zn-dependent enzymes [59] and Zn-finger transcription factors [60, 61].
Finally, it is important to note that MTs are not uniquely responsible for metal detoxification. Other cellular mechanisms are involved in the stress response, such as active transport by metal efflux-pumps. Interestingly, the T. thermophila genome includes 485 genes that encode putative membrane transporters for inorganic cations [62], and some may have important functions in metal detoxification.
Conclusions
1- After carrying out a comparative MT gene expression analysis using different T. thermophila strains, we can distinguish differential roles among the five MT isoforms of this ciliate. a)- MTT1 protein is the T. thermophila MT with the highest affinity for Cd2+, and MTT1 is primarily induced by Cd2+. Under no-stress conditions, MTT1 shows the highest basal expression in all strains except for the Cu-adap. It can be considered as a MT gene probably involved in metal cell homeostasis, but also with an important detoxification role, because it is ranked second or third in the consensus pattern of MT gene expression induction under Cd2+ or Pb2+ stress. This gene is not essential, but cells lacking MTT1 show higher sensitivity to Cd2+. b)- MTT5 basal expression is the lowest in several of the T. thermophila strains. However, under metal stress, it is the MT gene with the highest induction. When MTT5 is induced, the rest of the MT gene isoforms are over-expressed as well, suggesting that there may be coordination between the MTT5 product and induction of other MT genes under metal stress. MTT5 might be considered as an "alarm” MT gene, that is over-expressed under metal stress (mainly Cd2+ or Pb2+) and promotes the expression of other MT genes. MTT5 is essential because it was not possible to isolate a stable knockout strain. Therefore, this is the first time that a MT gene appears to be essential. c)- MTT3 is preferably induced by Cd2+ and Cu2+ and the protein has an ambiguous affinity for these two metals. We consider it to be an "undefined" MT [27] and the lack of a specific metal preference may maintain plasticity, allowing it to develop diverse physiological roles, binding Cd2+, Cu2+ or Zn2+ depending on the environmental conditions. In addition, a possible role for this MT isoform could be the intracellular homeostasis of essential metals. d)- MTT2 and MTT4 are almost identical and they encode CuMTs with high affinity to Cu2+. Moreover, these genes are over-expressed under Cu2+ treatments in almost all strains. A significant increase in basal MTT2/4 expression is detected in the Cu-adap, as well as when some other MT genes have been disrupted or knocked down. The maintenance of two nearly identical genes may represent an adaptation to increase the total levels of cytoplasmic CuMTs.
2- T. thermophila MT genes (primarily MTT1, MTT5 and MTT2/4) may be connected in a transcriptional regulatory network. The details of this putative network are unknown but may involve interactions between AP-1 transcription factors, metal ions and MT proteins.
3- Cell adaptation to Cd2+ leads to up-regulation of MTT1, while Cu2+ or Pb2+ adaptation involves up-regulation of MTT2/4 or MTT5 expression, respectively. This is consistent with the three genes having specific roles in adaptation to different metals.
Supporting information
The predominant consensus pattern is shown.
(TIF)
(*): MTT2QA and MTT2QB primers were used to amplify MTT2 and MTT4 CuMT genes indistinctly because both have very similar nucleotide sequences (98% identity) and it was not possible to design specific primers for each of them.
(DOCX)
(*): correlation coefficient. Efficiency (E) is calculated from the slope value of the standard curve: E = 10(-1/slope)-1.
(DOCX)
(-): Data not obtained. Normalization of the gene expression was carried out using the β-actin as an endogenous control gene. We show the average value ± standard deviation of two or three independent experiments. (-1M) or (-6M): metal adapted strains after 1 or 6 months in growth medium without metal exposure.
(DOCX)
β-actin gene was used as an endogenous control and it was considered a reference for neutralizing the variability of the qPCR technique. (*) Ct values that are considerably lower than those obtained in the control SB1969 strain. (-): not applicable, because the MTT1KO and the MTT1KO + MTT5KD strains have lost all the copies of the MTT1 gene. (-1M) or (-6M): these parameters were calculated after maintaining metal adapted strains 1 or 6 months in growth medium without metal exposure.
(DOCX)
Differences among basal expression levels for the different MT genes in each T. thermophila strain were calculated using the following formula: 2(Ct1-Ct2), being Ct1 and Ct2 the Ct values under a control situation (no metal exposure) between two MT genes in the same strain. We compared in each strain all MT gene basal expression levels by twos, distinguishing them by two colours: red and green. For each comparison, results are indicated in red or green depending on the MT gene that has a higher basal expression level in the same strain. Comparison values higher than 4 (Ct value differences higher than 2 cycles) are shaded in grey. (-): not applicable. (-1M) or (-6M): these parameters were calculated after maintaining metal adapted strains 1 or 6 months in growth medium without metal exposure.
(DOCX)
Differences among basal expression levels for each MT gene among different T. thermophila strains were calculated using the following formula: 2(Ct1-Ct2), being Ct1 and Ct2 the Ct values under a control situation (no metal exposure) for the same MT gene into two different strains. We compared all the T. thermophila analyzed strains by twos, distinguishing them by two colours: red and green. For each comparison, results are indicated in red or green depending on the strain that has a higher basal expression level for the same MT gene. Comparison values which are higher than 4 (Ct value differences higher than 2 cycles) are shaded in grey. (-): not applicable. (-1M) or (-6M): these parameters were calculated after maintaining metal adapted strains 1 or 6 months in growth medium without metal exposure.
(DOCX)
Acknowledgments
A PhD scholarship was awarded to PdF by the Spanish Ministry of Education (FPU12/02789).
Data Availability
All relevant data are within the paper and its supporting information files.
Funding Statement
This work was supported by MINECO CGL2016-75494-R; NIH GM-105783; A PhD scholarship was awarded to PdF by the Spanish Ministry of Education (FPU12/02789). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gadd GM. Heavy metal pollutants: environmental and biotechnological aspects In: Lederberg J., editor. Encyclopedia of Microbiology. New Yok: Academic Press; 2000. pp. 607–617. [Google Scholar]
- 2.Leonard SS, Harris GK, Shi X. Metal-induced oxidative stress and signal transduction. Free Radic Biol Med. 2004;12: 1921–1942. [DOI] [PubMed] [Google Scholar]
- 3.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12: 1161–1208. [DOI] [PubMed] [Google Scholar]
- 4.Wysocki R, Tamás MJ. How Saccharomyces cerevisiae copes with toxic metals and metalloids. FEMS Microbiol Rev. 2010;34: 925–951. doi: 10.1111/j.1574-6976.2010.00217.x [DOI] [PubMed] [Google Scholar]
- 5.Gutiérrez JC, Amaro F, Díaz S, de Francisco P, Cubas LL, Martín-González A. Ciliate metallothioneins: unique microbial eukaryotic heavy-metal-binder molecules. J Biol Inorg Chem. 2011;16: 1025–1034. doi: 10.1007/s00775-011-0820-9 [DOI] [PubMed] [Google Scholar]
- 6.De Francisco P, Melgar LM, Díaz S, Martín-González A, Gutiérrez JC. The Tetrahymena metallothionein gene family: twenty-one new cDNAs, molecular characterization, phylogenetic study and comparative analysis of the gene expression under different abiotic stressors. BMC Genomics. 2016;17: 1–23. doi: 10.1186/s12864-015-2294-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakulsak N. Metallothionein: An overview on its metal homeostatic regulation in mammals. Int J Morphol. 2012;30: 1007–1012. [Google Scholar]
- 8.Liu J, Klaassen CD. Absorption and distribution of cadmium in metallothionein-I transgenic mice. Fund Appl Toxicol. 1969;29: 294–300. [DOI] [PubMed] [Google Scholar]
- 9.Viarengo A, Burlando B, Ceratto N, Panfoli I. Antioxidant role of metallothioneins: a comparative overview. Cell Mol Biol. 2000;46: 407–417. [PubMed] [Google Scholar]
- 10.Miles AT, Hawksworth GM, Beattie JH, Rodilla V. Induction, regulation, degradation, and biological significance of mammalian metallothioneins. Crit Rev Biochem Mol Biol. 2000;35: 35–70. doi: 10.1080/10409230091169168 [DOI] [PubMed] [Google Scholar]
- 11.Penkowa M, Carrasco J, Giralt M, Molinero A, Hernández J, Campbell IL, et al. Altered central nervous system cytokine-growth factor expression profiles and angiogenesis in metallothionein-I + II deficient mice. J Cereb Blood Flow Metab. 2000;20: 1174–1189. doi: 10.1097/00004647-200008000-00003 [DOI] [PubMed] [Google Scholar]
- 12.Vidal F, Hidalgo J. Effect of zinc and copper on preimplantation mouse embryo development in vitro and metallothionein levels. Zygote. 1993;1: 225–229. [DOI] [PubMed] [Google Scholar]
- 13.Coyle P, Philcox JC, Carey LC and Rofe AM. Metallothionein: the multipurpose protein. Cell Mol Life Sci. 2002;59: 627–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huggins CJ, Mayekar MK, Martin N, Saylor KL, Gonit M, Jailwala P, et al. C/EBPγ is a critical regulator of cellular stress response networks through heterodimerization with ATF4. Mol Cell Biol. 2016;36: 693–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klaassen CD, Liu J. Induction of metallothionein as an adaptive mechanism affecting the magnitude and progression of toxicological injury. Environ Health Perspect. 1998;106: 297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedrini-Martha V, Schenegg R, Baurand PE, deVaufleury A, Dallinger R. The physiological role and toxicological significance of the non-metal-selective cadmium/copper-metallothionein isoform differ between embryonic and adult helicid snails. Comp Biochem Phisiol C. 2017;199: 38–47. [DOI] [PubMed] [Google Scholar]
- 17.Amaro F, de Lucas MP, Martín-González A, Gutiérrez JC. Two new members of the Tetrahymena multi-stress-inducible metallothionein family: Characterization and expression analysis of T. rostrata Cd/Cu metallothionein genes. Gene. 2008;423: 85–91. doi: 10.1016/j.gene.2008.07.002 [DOI] [PubMed] [Google Scholar]
- 18.Boldrin F, Santovito G, Irato P, Piccinni E. Metal interaction and regulation of Tetrahymena pigmentosa metallothionein genes. Protist. 2002;153: 283–291. doi: 10.1078/1434-4610-00105 [DOI] [PubMed] [Google Scholar]
- 19.Cheung A, Pok L, Vincent KLL, King MC. Tilapia metallothionein genes: PCR-cloning and gene expression studies. Biochim Biophys Acta. 2005;1731: 191–201. doi: 10.1016/j.bbaexp.2005.09.006 [DOI] [PubMed] [Google Scholar]
- 20.Díaz S, Amaro F, Rico D, Campos V, Benitez L, Martín-González A, et al. Tetrahymena metallothioneins fall into two discrete subfamilies. PloS One. 2007;2: e291 doi: 10.1371/journal.pone.0000291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dondero F, Cavaletto M, Chezzi AR, La Terza A, Banni M, Viarengo A. Biochemical characterization and quantitative gene expression analysis of the multi-stress inducible metallothionein from Tetrahymena thermophila. Protist. 2004;155: 157–168. doi: 10.1078/143446104774199565 [DOI] [PubMed] [Google Scholar]
- 22.Fu C, Miao W. Cloning and characterization of a new multi-stress inducible metallothionein gene in Tetrahymena pyriformis. Protist. 2006;157: 193–203. doi: 10.1016/j.protis.2006.02.006 [DOI] [PubMed] [Google Scholar]
- 23.Guo L, Fu C, Miao W. Cloning, characterization, and gene expression analysis of a novel cadmium metallothionein gene in Tetrahymena pigmentosa. Gene. 2008;423: 29–35. doi: 10.1016/j.gene.2008.04.023 [DOI] [PubMed] [Google Scholar]
- 24.Hughes S, Sturzenbaum SR. Single and double metallothionein knockout in the nematode C. elegans reveals cadmium dependent and independent toxic effects on life history traits. Environ Pollut. 2007;145: 395–400. doi: 10.1016/j.envpol.2006.06.003 [DOI] [PubMed] [Google Scholar]
- 25.Masters BA, Kelly EJ, Quaife CJ, Brinster RL, Palmiter RD. Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc Natl Acad Sci USA. 1994;91: 548–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gutiérrez JC, Amaro F, Martín-González A. From heavy metal-binders to biosensors: Ciliate metallothioneins discussed. BioEssays. 2009;31: 805–816. doi: 10.1002/bies.200900011 [DOI] [PubMed] [Google Scholar]
- 27.Espart A, Marín M, Gil-Moreno S, Palacios O, Amaro F, Martín-González A, et al. Hints for metal-preference protein sequence determinants: different metal binding features of the five Tetrahymena thermophila metallothioneins. Int J Biol Sci. 2015;11: 456–471. doi: 10.7150/ijbs.11060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adamo GM, Brocca S, Passolunghi S, Salvato B, Lotti M. Laboratory evolution of copper tolerant yeast strains. Microb Cell Fact. 2012;11: 1–11. doi: 10.1186/1475-2859-11-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butler PR, Brown M, Oliver SG. Improvement of antibiotic titers from Streptomyces bacteria by interactive continuous selection. Biotechnol Bioeng. 1996;49: 185–196. doi: 10.1002/(SICI)1097-0290(19960120)49:2<185::AID-BIT7>3.0.CO;2-M [DOI] [PubMed] [Google Scholar]
- 30.Kumar G, Kuswaha HR, Panjabi-Sabharwal V, Kumari S, Joshi R, Karan R, et al. Clustered metallothionein genes are co-regulated in rice and ectopic expression of OsMT1e-P confers multiple abiotic stress tolerance in tobacco via ROS scavenging. BMC Plant Biology. 2012; 12: 107 doi: 10.1186/1471-2229-12-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costa D, Mariën J, Janssens TKS, van Gestel CAM, Driessen G, Sousa JP, et al. Influence of adaptive evolution of cadmium tolerance on neutral and functional genetic variation in Orchesella cincta. Ecotoxicology. 2012;21: 2078–2087. doi: 10.1007/s10646-012-0961-9 [DOI] [PubMed] [Google Scholar]
- 32.Minty JJ, Lesnefsky AA, Lin F, Chen Y, Zaroff TA, Veloso AB, et al. Evolution combined with genomic study elucidates genetic bases of isobutanol tolerance in Escherichia coli. Microb Cell Fact. 2011; 10: 18 doi: 10.1186/1475-2859-10-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Maris AJ, Winkler AA, Kuyper M, de Laat WT, van Dijken JP, Pronk JT. Development of efficient xylose fermentation in Saccharomyces cerevisiae: xylose isomerase as a key component. Adv Biochem Eng Biotechnol. 2007;108: 179–204. doi: 10.1007/10_2007_057 [DOI] [PubMed] [Google Scholar]
- 34.Guimaraes PM, Francois J, Parrou JL, Teixeira JA, Domingues L. Adaptive evolution of a lactose-consuming Saccharomyces cerevisiae recombinant. Appl Environ Microbiol. 2008;74: 1748–1756. doi: 10.1128/AEM.00186-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capecchi MR. Altering the genome by homologous recombination. Science. 1989;244: 1288–1292. [DOI] [PubMed] [Google Scholar]
- 36.Amaro F, Turkewitz AP, Martín-González A., Gutiérrez JC. Functional GFP-metallothionein fusion protein from Tetrahymena thermophila: a potential whole-cell biosensor for monitoring heavy metal pollution and a cell model to study metallothionein overproduction effects. Biometals. 2014;27: 195–205. doi: 10.1007/s10534-014-9704-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kataoka K, Schoeberl UE and Mochizuki K. Modules for C-terminal epitope tagging of Tetrahymena genes. J Microbiol Methods. 2010;82: 342–346. doi: 10.1016/j.mimet.2010.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cassidy-Hanley D, Bowen J, Lee JH, Cole E, VerPlank LA, Gaertig J, et al. Germline and somatic transformation of mating Tetrahymena thermophila by particle bombardment. Genetics. 1997;146: 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larionov A, Krause A, Miller W. A standard curve based method for relative real time PCR data processing. BMC Bioinform. 2005;6: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang Y, Agrawal AF. Experimental evolution of gene expression and plasticity in alternative selective regimes. PloS Genet. 2016;12: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Timmermans MJ, Ellers J, Roelofs D, van Straalen NM. Metallothionein mRNA expression and cadmium tolerance in metal-stressed and reference populations of the springtail Orchesella cincta. Ecotoxicology. 2005;14: 727–739. doi: 10.1007/s10646-005-0020-x [DOI] [PubMed] [Google Scholar]
- 42.Liu N, Scott JG. Increased transcription of CYP6D1 causes cytochrome P450-mediated insecticide resistance in house fly. Insect Biochem Mol Biol. 1998;28: 531–535. [DOI] [PubMed] [Google Scholar]
- 43.Niederwanger M, Dvorak M, Schnegg R, Pedrini-Martha V, Bacher K, Bidoli M, Dallinger R. Challenging the metallothionein (MT) gene of Biomphalaria glabrata: unexpected response patterns due to cadmium exposure and temperature stress. J Mol Sci. 2017;18: 1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Straalen NM, Janssens TKS, Roelofs D. Micro-evolution of toxicant tolerance: from single genes to the genome's tangled bank. Ecotoxicology. 2011;20: 574–579. doi: 10.1007/s10646-011-0631-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Höckner M, Dallinger R, Stürzenbaum SR. Nematode and snail metallothioneins. J Biol Inorg Chem. 2011;16: 1057–1065. doi: 10.1007/s00775-011-0826-3 [DOI] [PubMed] [Google Scholar]
- 46.Michalska AE, Choo KHA. Targeting and germ-line transmission of a null mutation at the metallothionein I and II loci in mouse. Proc Natl Acad Sci USA. 1993;90: 8088–8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Egli D, Yepiskoposyan H, Selvaraj A, Balamurugan K, Rajaram R, Simons A, et al. A family knockout of all four Drosophila metallothioneinas reveals a central role in copper homeostasis and detoxification. Mol Cell Biol. 2006;26: 2286–2296. doi: 10.1128/MCB.26.6.2286-2296.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li WH, Yang J, Gu X. Expression divergence between duplicated genes. Trends Genet. 2005;21: 602–607. doi: 10.1016/j.tig.2005.08.006 [DOI] [PubMed] [Google Scholar]
- 49.Daniels MJ, Turner-Cavet JS, Selkirk R, Sun H, Parkinson JA, Sadler PJ, et al. Coordination of Zn2+ (and Cd2+) by prokaryotic metallothionein. Involvement of His-Imidazole. J Biol Chem. 1998;273: 22957–22961. [DOI] [PubMed] [Google Scholar]
- 50.Blindauer CA. Metallothioneins with unusual residues: histidines as modulators of zinc affinity and reactivity. J Inorg Biochem. 2008;102: 507–21. doi: 10.1016/j.jinorgbio.2007.10.032 [DOI] [PubMed] [Google Scholar]
- 51.Boldrin F, Santovito G, Gaertig J, Wloga D, Cassidy-Hanley D, Clark TG, et al. Metallothionein gene from Tetrahymena thermophila with a copper-inducible-repressible promoter. Eukaryot Cell. 2006;5: 422–425. doi: 10.1128/EC.5.2.422-425.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Egli D, Domènech J, Selvaraj A, Balamurugan K, Hua H, Capdevila M, et al. The four members of the Drosophila metallothionein family exhibit distinct overlapping roles in heavy metal homeostasis and detoxification. Genes Cell. 2006;11: 647–658. [DOI] [PubMed] [Google Scholar]
- 53.Atrian S, Capdevila M. Metallothionein-protein interactions. BioMol Concepts. 2013;4: 143–160. doi: 10.1515/bmc-2012-0049 [DOI] [PubMed] [Google Scholar]
- 54.Knipp M, Meloni G, Roschitzki B, Vasak M. Zn7-metallothionein-3 and the synaptic vesicle cycle: interaction of metallothionein-3 with the small GTPase Rab3A. Biochemistry. 2005;44: 3159–3165. doi: 10.1021/bi047636d [DOI] [PubMed] [Google Scholar]
- 55.Ambjørn M, Asmussen JW, Lindstam M, Gotfryd K, Jacobsen C, Kiselyov VV, et al. Metallothionein and a peptide modeled after metallothionein, EmtinB, induce neuronal differentiation and survival through binding to receptors of the low-density lipoprotein receptor family. J Neurochem. 2008;104: 21–37. doi: 10.1111/j.1471-4159.2007.05036.x [DOI] [PubMed] [Google Scholar]
- 56.Quiming NS, Vergel RB, Nicolas MG, Villanueva JA. Interaction of bovine serum albumin and metallothionein. J Health Sci. 2005;51: 8–15. [Google Scholar]
- 57.Ostrakhovitch EA, Olsson PE, Jiang S, Cherian MG. Interaction of metallothionein with tumor suppressor p53 protein. FEBS Lett. 2006;580: 1235–1238. doi: 10.1016/j.febslet.2006.01.036 [DOI] [PubMed] [Google Scholar]
- 58.Orihuela R, Fernández B, Palacios O, Valero E, Atrian S, Watt RK, et al. Ferritin and metallothionein: dangerous liaisons. Chem Commun. 2011;47: 12155–12157. [DOI] [PubMed] [Google Scholar]
- 59.Maret W, Jacob C, Vallee BL, Fisher EH. Inhibitory sites in enzymes: zinc removal and reactivation by thionein. Proc Natl Acad Sci USA. 1999;96: 1936–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang M, Shaw IF, Petering DH. Interprotein metal exchange between transcription factor IIIa and apo-metallothionein. J Inorg Biochem. 2004;98: 639–648. doi: 10.1016/j.jinorgbio.2004.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeng J, Vallee BL, Kägi JHR. Zinc transfer from transcription factor IIIA fingers to thionein clusters. Proc Natl Acad Sci USA. 1991;88: 9984–9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eisen JA, Coyne RS, Wu M, Wu D, Thiagarajan M, Wortman JR, et al. Macronuclear genome sequence of the ciliate Tetrahymena thermophila, a model eukaryote. PLoS Biol, 2006;4: c286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The predominant consensus pattern is shown.
(TIF)
(*): MTT2QA and MTT2QB primers were used to amplify MTT2 and MTT4 CuMT genes indistinctly because both have very similar nucleotide sequences (98% identity) and it was not possible to design specific primers for each of them.
(DOCX)
(*): correlation coefficient. Efficiency (E) is calculated from the slope value of the standard curve: E = 10(-1/slope)-1.
(DOCX)
(-): Data not obtained. Normalization of the gene expression was carried out using the β-actin as an endogenous control gene. We show the average value ± standard deviation of two or three independent experiments. (-1M) or (-6M): metal adapted strains after 1 or 6 months in growth medium without metal exposure.
(DOCX)
β-actin gene was used as an endogenous control and it was considered a reference for neutralizing the variability of the qPCR technique. (*) Ct values that are considerably lower than those obtained in the control SB1969 strain. (-): not applicable, because the MTT1KO and the MTT1KO + MTT5KD strains have lost all the copies of the MTT1 gene. (-1M) or (-6M): these parameters were calculated after maintaining metal adapted strains 1 or 6 months in growth medium without metal exposure.
(DOCX)
Differences among basal expression levels for the different MT genes in each T. thermophila strain were calculated using the following formula: 2(Ct1-Ct2), being Ct1 and Ct2 the Ct values under a control situation (no metal exposure) between two MT genes in the same strain. We compared in each strain all MT gene basal expression levels by twos, distinguishing them by two colours: red and green. For each comparison, results are indicated in red or green depending on the MT gene that has a higher basal expression level in the same strain. Comparison values higher than 4 (Ct value differences higher than 2 cycles) are shaded in grey. (-): not applicable. (-1M) or (-6M): these parameters were calculated after maintaining metal adapted strains 1 or 6 months in growth medium without metal exposure.
(DOCX)
Differences among basal expression levels for each MT gene among different T. thermophila strains were calculated using the following formula: 2(Ct1-Ct2), being Ct1 and Ct2 the Ct values under a control situation (no metal exposure) for the same MT gene into two different strains. We compared all the T. thermophila analyzed strains by twos, distinguishing them by two colours: red and green. For each comparison, results are indicated in red or green depending on the strain that has a higher basal expression level for the same MT gene. Comparison values which are higher than 4 (Ct value differences higher than 2 cycles) are shaded in grey. (-): not applicable. (-1M) or (-6M): these parameters were calculated after maintaining metal adapted strains 1 or 6 months in growth medium without metal exposure.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its supporting information files.