Abstract
In Italy the prevalence of recent HIV infection (RHI) isn’t currently monitored. Early diagnosis is crucial to allow introduction of antiretroviral therapy (cART) in the recent phase of infection. We aimed to estimate the proportion and the determinants of RHI among patients enrolled in the ICONA cohort; we explored differences in the median time from HIV diagnosis to cART initiation and in the viro-immunological response between RHI and Less Recent HIV infections (NRHI). We included antiretroviral-naïve HIV-positive patients enrolled in the cohort with documented dates of HIV-negative and positive antibodies tests, grouped in RHI (estimated date of seroconversion within 12 months of enrolment) and NRHI. Proportion of RHI and the trend of this proportion by calendar period (1996–2014) were investigated (Chi-square test). Logistic regression analysis was employed to identify factors associated with RHI. The time from seroconversion to cART initiation was compared in RHI and NRHI overall and after stratification by calendar period (survival analysis). We finally explored the time from starting cART to HIV-RNA <50 copies/mL and to CD4+ gain ≥200 cells/mmc by Cox regression. HIV seroconversion could be estimated for 2608/12,616 patients: 981/2608 (37.6%) were RHI. Proportion of RHI increased in recent calendar periods and was associated with younger age, baseline higher HIV-RNA and CD4+ count. There wasn’t difference in the 2-year estimates of cART start between RHI and NRHI, regardless of calendar period. Rates and hazards of virological response were similar in RHI versus NRHI. RHI showed a 1.5-fold higher probability of CD4+ gain, also following adjustment for calendar period and cART regimen, and for age, HCV and smoking; the difference in probability was however attenuated after further controlling for baseline HIV-RNA and CD4+ T-cells. The increased proportion of RHI over time suggests that in recent years in Italy HIV infections are more likely to be detected earlier than before. The similar rates of cART introduction and viro-immunological response in RHI and NRHI probably reflect the efficacy of the modern cART regimens. An improvement of the prevention services is warranted to allow an early cART access, also in the perspective of therapy as prevention.
Introduction
Recent HIV Infection (RHI) is defined by a negative HIV antibody test within 6/12 months of diagnosis [1–3]. Recently the CASCADE collaboration published the largest study of seroconverters cohorts from 25 countries to estimate the rates of immunological decline and survival in HIV-positive patients; they found that mean age at seroconversion was 31.1 years for 16373 patients and 6947 started cART. Lower CD4+ counts at seroconversion and higher mortality rates were reported in HIV-positive patients infected at an older age [4]. Early diagnosis is crucial to insure benefit for the individual due to early access to care and cART, especially now that immediate treatment is recommended for all patients, and to reduce HIV transmission at population level [5–7]. Two recent studies have demonstrated the health benefits of an early initiation of cART for asymptomatic HIV-infected patients with high CD4+ counts: when cART was immediately started instead of waiting until CD4+ count was <350 cells/mmc, there was a reduction of over 40% in the risk of death or AIDS defining disease [6, 8]. In particular, early treatment leads to better immune recovery [9, 10], HIV reservoir decline [11, 12] and reduction of new infections, considering the high rate of transmissions during RHI [2, 3, 13]. In Italy new HIV diagnoses are reported to the Healthcare System; public health surveillance captures new diagnoses irrespective of time of HIV infection. Given the lack of current monitoring of RHI prevalence in Italy, we aimed to use the ICONA Foundation Study cohort to estimate the proportion and determinants of HIV infections diagnosed during the recent phase over the period 1996 to 2014; RHI was defined as having an estimated date of seroconversion within 12 months from the date of enrolment in the cohort. We also explored the differences in the time from seroconversion to cART initiation and in viro-immunological response under treatment between RHI and less recent infections (non RHI, NRHI).
Materials and methods
We conducted an observational retrospective longitudinal study over 1996–2014. We included untreated HIV-positive patients with documented dates of HIV-negative and positive antibodies tests enrolled in the ICONA Foundation Study cohort. The Icona Foundation Study cohort is an observational multicentre cohort that enrolls HIV-infected individuals who are antiretroviral-naïve at the time of enrolment. A detailed description of the cohort is reported elsewhere [14]. Patients are voluntary enrolled by physicians at the different centres in Italy participating in ICONA Study after signing an informed consent. This cohort was set up in January 1997 and currently includes data on patients enrolled at 51 infectious disease units in Italy.
Participants’ date of HIV seroconversion was estimated as the midpoint between the last available HIV-negative and the first available HIV-positive test. People with such a date recorded within 1 year (since the last negative HIV serological test) were defined as RHI, according to current definition [2]. The proportion of RHI was calculated for the following calendar periods: 1996–2000, 2001–2006, 2007–2009, 2010–2014. Because the rate of enrolment in the cohort has been varying over time, the group period classification was based on the quantile of the distribution of enrolments in the cohort in order to have similar denominators in each calendar period.
AIDS defining conditions were diagnosed according to CDC revised classification system in 1993 [15]. Patients with hepatitis co-infection were defined as HCV Ab or HBsAg positive subjects. The proportion of RHI at entry in the cohort and the trend of this proportion by calendar period of enrolment were investigated using a Chi-square test. Univariable and multivariable logistic regression analysis was employed to identify factors associated with RHI. All the variables associated with the outcome with a p value <0.01 in the univariable analysis were selected for inclusion in the multivariable model; we thus included the following factors in the multivariable analysis: calendar period of seroconversion, gender, age, mode of HIV transmission, AIDS diagnosis, CD4+ T-cells and HIV-RNA (measured at enrolment), HCV co-infection, site geographical position, employment status, smoking and blood glucose. Survival analysis techniques were used to compare the time from seroconversion to cART initiation in RHI and NRHI, overall and after stratification by calendar period of enrolment (Kaplan-Meier method with log-rank test). We investigated the proportion of patients achieving a HIV-RNA ≤500 copies/mL at 6 months (time window +3; +9 months) from the date of starting cART, stratified by RHI status and calendar period (missing values of HIV-RNA were excluded from the analysis). We have chosen the threshold of 500 copies/mL, instead of 50 copies/mL, because this was the lower limit of detection of the assay used at the sites in the early calendar periods. We finally explored the determinants of probability of virological success (time from cART start to confirmed HIV-RNA <50 copies/mL) and of CD4+ count recovery (time from cART start to CD4+ gain ≥200 cells/mmc) by univariable and multivariable Cox regression models. We also performed a sensitivity analysis to explore the probability of immunological recovery according to another definition: CD4+ gain of 200 cells/mmc or reaching a single CD4+ count >350 cells/mmc, which ever came first.
The study was approved by the Ethical Committee of all the Centers participating to the ICONA Foundation Study (see acknowledgments and Ethics Statement for the full name of the ethics committees that approved the study). All patients signed written informed consent. Statistical analyses were performed using SAS software package (SAS Institute).
Results
Proportion of Recent HIV Infections in the ICONA Foundation Study cohort
Between 1996 and 2014 the date of HIV seroconversion could be estimated for 2608/12616 patients. Overall, 981/2608 (37.6%) patients were defined as RHI, with a trend for an increased proportion in latest years: from 213/578 (36.9%) in 1996–2000 up to 526/927 (56.7%) in more recent years (2010–2014; p<0.001) (Fig 1).
Fig 1. Proportion of Recent HIV Infections by calendar period of enrolment.
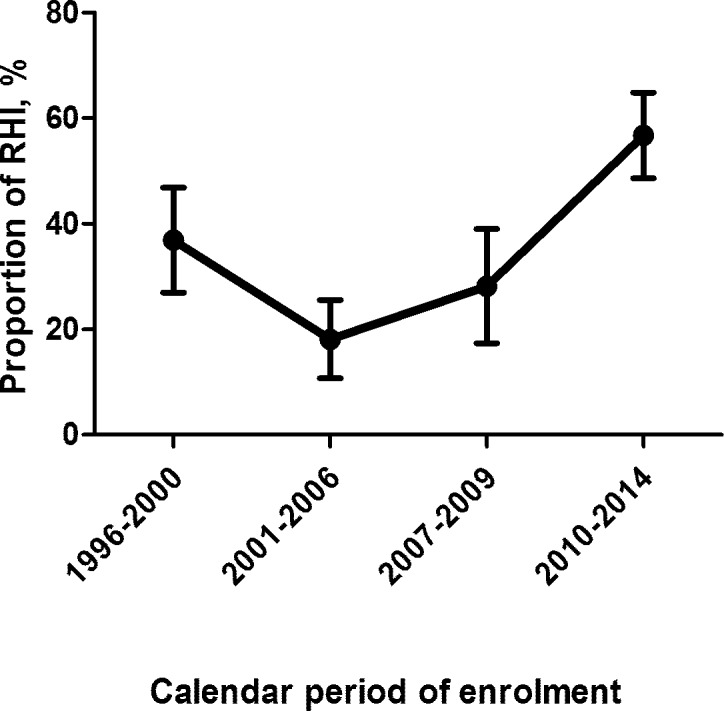
The graph illustrates the proportion of Recent HIV Infections (RHI), defined as a positive HIV serological test within 12 months since the last negative one, according to calendar period of seroconversion (1996–2000, 2001–2006, 2007–2009, 2010–2014). X-axis: calendar periods, Y-axis: proportion of RHI in percentages.
Table 1 shows the characteristics of the study population stratified by RHI status. RHI patients were younger than NRHI subjects (median age was 34, IQR 28–40 years in RHI and 36, IQR 31–42 in NRHI, p<0.001); proportion of females and risk factors for HIV transmission were similar between the two groups of patients. RHI patients also presented higher CD4+ T cells count in comparison with NRHI (CD4+ counts, cells/mmc: 493, IQR 336–667 in RHI and 452, IQR 289–632 in NRHI, p<0.001) (Table 1). Median time from the estimated date of seroconversion to entry in the cohort was 6 (IQR 4–9) in RHI and 27 (IQR 18–45) in NRHI (p<0.001).
Table 1. Characteristics of the study population according to RHI status.
| Recent seroconversion | ||||
|---|---|---|---|---|
| Characteristics | Yes (RHI) | No (NRHI) | p-value | Total |
| N 981 | N 1627 | N 2608 | ||
| Age, years, median (IQR) | 34 (28, 40) | 36 (31, 42) | <0.001 | 35 (30, 42) |
| Females, n (%) | 117 (11.9%) | 273 (16.8%) | 1.000 | 390 (15.0%) |
| Mode of HIV Transmission, n (%) | 0.501 | |||
| IDUs | 79 (8.1%) | 164 (10.1%) | 243 (9.4%) | |
| Homosexual contacts | 587 (59.9%) | 893 (55.3%) | 1480 (57.0%) | |
| Heterosexual contacts | 256 (26.1%) | 489 (30.1%) | 745 (28.6%) | |
| Other/Unknown | 58 (5.9%) | 70 (4.3%) | 128 (4.9%) | |
| Not Italian nationality, n (%) | 123 (12.5%) | 212 (13.0%) | 0.050 | 335 (12.8%) |
| AIDS diagnosis, n (%) | 26 (2.7%) | 67 (4.1%) | 0.478 | 93 (3.6%) |
| CVD diagnosis, n (%) | 3 (0.3%) | 8 (0.5%) | 0.295 | 11 (0.4%) |
| HBsAg, n (%) | 0.036 | |||
| Negative | 947 (96.5%) | 1582 (97.2%) | 2529 (97.0%) | |
| Positive | 34 (3.5%) | 45 (2.8%) | 79 (3.0%) | |
| Not tested | 309 (31.5%) | 553 (34.0%) | 862 (33.1%) | |
| HCV Ab, n (%) | 0.161 | |||
| Negative | 606 (61.8%) | 932 (57.3%) | 1538 (59.0%) | |
| Positive | 80 (8.2%) | 172 (10.6%) | 252 (9.7%) | |
| Not tested | 295 (30.1%) | 523 (32.1%) | 818 (31.4%) | |
|
CD4 count, cells/mmc, median (IQR) |
493 (336, 667) | 452 (289, 632) | <0.001 | 469 (307, 651) |
|
CD4 count nadir, cells/mmc, median (IQR) |
495 (338, 669) | 452 (293, 625) | <0.001 | 470 (309, 641) |
|
CD8 count, cells/mmc, median (IQR) |
904 (654, 1284) | 931 (688, 1283) | 0.383 | 923 (675, 1283) |
|
Viral load, log10
copies/mL, median (IQR) |
4.67 (4.06, 5.23) | 4.42 (3.81, 4.97) | <0.001 | 4.5 (3.89–5.07) |
|
Time from HIV diagnosis to date of enrolment, months, median (IQR) |
6 (4, 9) | 27 (18, 45) | <0.001 | 17 (8, 32) |
| Site geographical position, n (%) | 0.017 | |||
| North | 590 (60.1%) | 916 (56.3%) | 1506 (57.7%) | |
| Center | 313 (31.9%) | 616 (37.9%) | 929 (35.6%) | |
| South | 78 (8.0%) | 95 (5.8%) | 173 (6.6%) | |
| Diabetes, n (%) | 10 (1.0%) | 16 (1.0%) | <0.001 | 26 (1.0%) |
| Smoking, n (%) | 0.950 | |||
| No | 327 (33.3%) | 595 (36.6%) | 922 (35.4%) | |
| Yes | 289 (29.5%) | 592 (36.4%) | 881 (33.8%) | |
| Unknown | 365 (37.2%) | 440 (27.0%) | 805 (30.9%) | |
|
Total cholesterol, mg/dL, median (IQR) |
165 (143, 192) | 164 (140, 188) | 0.395 | 165 (141, 189) |
|
HDL cholesterol, mg/dL, median (IQR) |
42 (34, 48) | 41 (33, 49) | 0.673 | 41 (34, 48) |
| EGFR (CKD_Epi formula), ml/min/1.73m2, median (IQR) | 4.67 (4.04, 5.17) | 4.37 (3.63, 4.90) | <0.001 | 4.45 (3.77, 5) |
|
Blood glucose, mg/dL, median (IQR) |
86 (79, 92) | 86 (80, 95) | 0.037 | 86 (80, 94) |
| Use of statins, n (%) | 5 (0.5%) | 8 (0.5%) | 0.353 | 13 (0.5%) |
| Use of blood pressure lowering drugs, n (%) | 16 (1.6%) | 35 (2.2%) | 0.003 | 51 (2.0%) |
| Education, n (%) | <0.001 | |||
| Primary school | 44 (4.5%) | 55 (3.4%) | 99 (3.8%) | |
| Secondary school | 194 (19.8%) | 354 (21.8%) | 548 (21.0%) | |
| College | 307 (31.3%) | 580 (35.6%) | 887 (34.0%) | |
| University | 157 (16.0%) | 253 (15.6%) | 410 (15.7%) | |
| Other/Unknown | 279 (28.4%) | 385 (23.7%) | 664 (25.5%) | |
| Employment, n (%) | <0.001 | |||
| Unemployed | 128 (13.0%) | 194 (11.9%) | 322 (12.3%) | |
| Employed | 454 (46.3%) | 789 (48.5%) | 1243 (47.7%) | |
| Self-employed | 138 (14.1%) | 284 (17.5%) | 422 (16.2%) | |
| Occasional | 31 (3.2%) | 48 (3.0%) | 79 (3.0%) | |
| Student | 63 (6.4%) | 47 (2.9%) | 110 (4.2%) | |
| Retired | 15 (1.5%) | 35 (2.2%) | 50 (1.9%) | |
| Invalid | 1 (0.1%) | 2 (0.1%) | 3 (0.1%) | |
| Housewife | 20 (2.0%) | 46 (2.8%) | 66 (2.5%) | |
| Other/unknown | 131 (13.4%) | 182 (11.2%) | 313 (12.0%) | |
LEGEND: Categorical data are presented as absolute numbers (percentages); p values for comparison of proportions between RHI and NRHI are by Chi-square test. Quantitative data are presented as median (Interquartile Range, IQR); p values for comparison of medians between RHI and NRHI are by Mann Whitney test. IDUs, Intravenous Drug Users; CVD, cardiovascular diseases; EGFR, glomerular filtration rate.
As regards the characteristics of patients enrolled in the study stratified by calendar period, in 2010–2014 patients with a known date of seroconversion presented a median age of 33 years (IQR: 27–40), median CD4+ count was 479 cells/mmc (IQR: 331–629) and HIV-RNA 4.65 log10 copies/mL (IQR: 4.11–5.16). With more recent years, we observed a reduction of females, AIDS presenters, hepatitis B and C co-infected patients and intravenous drug users (IDUs), but a sharp increase in men who have sex with men (MSM) and in people from northern Italy. Higher baseline HIV-RNA has also been reported in more recent calendar periods. Conversely, no difference in age and CD4+ count at enrolment was displayed (Table 2).
Table 2. Characteristics of patients with a known date of seroconversion according to calendar period.
| Period of Seroconversion | ||||||
|---|---|---|---|---|---|---|
| Characteristics | 1996–2000 | 2001–2006 | 2007–2009 | 2010–2014 | p-value | Total |
| N 578 | N 676 | N 427 | N 927 | N 2608 | ||
| Recent HIV infection, n (%) | 213 (36.9%) | 122 (18%) | 120 (28.1%) | 526 (56.7%) | <0.001 | 981 (37.6%) |
|
Age, years– median (IQR) |
34 (30–41) | 37 (31–43) | 36 (29–42) | 33 (27–40) | <0.001 | 35 (30, 42) |
| Females, n (%) | 163 (28.2%) | 108 (16%) | 45 (10.5%) | 74 (8%) | <0.001 | 390 (15.0%) |
| Mode of HIV Transmission, n (%) | <0.001 | |||||
| IDUs | 128 (22.1%) | 62 (9.2%) | 23 (5.5%) | 30 (3.3%) | 243 (9.4%) | |
| Homosexual contacts | 185 (32.0%) | 376 (55.7%) | 264 (62.6%) | 655 (71.1%) | 1480 (57.0%) | |
| Heterosexual contacts | 239 (41.3%) | 207 (30.6%) | 119 (27.9%) | 180 (19.4%) | 745 (28.6%) | |
| Other/Unknown | 26 (4.5%) | 30 (4.4%) | 16 (3.8%) | 56 (6.1%) | 128 (4.9%) | |
| Not Italian Nationality, n (%) | 40 (6.9%) | 77 (11.4%) | 65 (15.2%) | 153 (16.5%) | 0.449 | 335 (12.8%) |
| AIDS diagnosis, n (%) | 33 (5.7%) | 28 (4.1%) | 19 (4.4%) | 13 (1.4%) | <0.001 | 93 (3.6%) |
| CVD diagnosis, n (%) | 2 (0.3%) | 2 (0.3%) | 5 (1.2%) | 2 (0.2%) | 0.073 | 11 (0.4%) |
| HBsAg, n (%) | <0.001 | |||||
| Negative | 547 (94.6%) | 659 (97.5%) | 419 (98.1%) | 904 (97.5%) | 2529 (97%) | |
| Positive | 31 (5.4%) | 17 (2.5%) | 8 (1.9%) | 23 (2.5%) | 79 (3%) | |
| Not tested | 143 (24.7%) | 244 (36.1%) | 160 (37.5%) | 315 (34%) | 862 (33.1%) | |
| HCV Ab, n (%) | <0.001 | |||||
| Negative | 306 (52.9%) | 367 (54.3%) | 258 (60.4%) | 607 (65.5%) | 1538 (59%) | |
| Positive | 141 (24.4%) | 60 (8.9%) | 25 (5.9%) | 26 (2.8%) | 252 (9.7%) | |
| Not tested | 131 (22.7%) | 249 (36.8%) | 144 (33.7%) | 294 (31.7%) | 818 (31.4%) | |
|
CD4 count, cells/mmc, median (IQR) |
480 (294, 670) |
466 (301, 655) |
448 (295, 643) |
479 (331, 629) |
0.566 | 469 (307, 651) |
|
CD4 count nadir, cells/mmc, median (IQR) |
473 (294, 664) |
460 (301, 630) |
448 (293, 617) |
481 (337, 643) |
0.079 | 470 (309, 641) |
|
CD8 count, cells/mmc, median (IQR) |
865 (657, 1196) |
942 (678, 1277) |
990 (728, 1428) |
930 (658, 1340) |
0.001 | 923 (675, 1283) |
|
Viral load, log10 copies/mL, median (IQR) |
4.35 (3.73, 5.01) |
4.42 (3.78, 5.00) |
4.57 (3.98, 5.00) |
4.65 (4.11, 5.16) |
<0.001 | 4.50 (3.89, 5.07) |
| Time from HIV diagnosis to date of enrolment, months, median (IQR) | 16 (8, 31) | 33 (17, 62) | 21 (11, 35) | 10 (5, 18) | <0.001 | 17 (8, 32) |
| Site geographical position, n (%) | <0.001 | |||||
| North | 284 (49.1%) | 357 (52.8%) | 256 (60.0%) | 609 (66%) | 1506 (57%) | |
| Center | 225 (38.9%) | 278 (41.1%) | 153 (35.8%) | 273 (29%) | 929 (37%) | |
| South | 69 (11.9%) | 41 (6.1%) | 18 (4.2%) | 45 (4.9%) | 173 (6%) | |
| Diabetes, n (%) | 5 (0.9%) | 6 (0.9%) | 9 (2.1%) | 6 (0.6%) | 0.083 | 26 (1.0%) |
| Smoking, n (%) | <0.001 | |||||
| No | 100 (17.3%) | 262 (38.8%) | 169 (39.6%) | 391 (42.2%) | 922 (35.4%) | |
| Yes | 157 (27.2%) | 265 (39.2%) | 156 (36.5%) | 303 (32.7%) | 881 (33.8%) | |
| Unknown | 321 (55.5%) | 149 (22.0%) | 102 (23.9%) | 233 (25.1%) | 805 (30.9%) | |
| Total cholesterol, mg/dL, median (IQR) | 164 (141, 195) |
162 (137, 187) |
166 (141, 190) |
165 (143, 189) |
0.380 | 165 (141, 189) |
| HDL cholesterol, mg/dL, median (IQR) | 40 (32, 48) | 41 (33, 50) | 42 (34, 48) | 41 (34, 48) | 0.785 | 41 (34, 48) |
|
EGFR (CKD_Epi formula), ml/min/1.73m2, median (IQR) |
4.20 (3.56, 5) |
4.37 (3.63, 4.99) |
4.52 (3.90, 4.91) |
4.71 (4.04, 5.17) |
0.002 | 4.45 (3.77, 5.00) |
| Blood glucose, mg/dL, median (IQR) | 87 (81, 95) | 86 (80, 93) | 87 (80, 94) | 85 (79, 93) | 0.011 | 86 (80, 94) |
| Use of statins, n (%) | 1 (0.2%) | 5 (0.7%) | 2 (0.5%) | 5 (0.5%) | 0.559 | 13 (0.5%) |
| Use of blood pressure lowering drugs, n (%) | 3 (0.5%) | 22 (3.3%) | 9 (2.1%) | 17 (1.8%) | 0.006 | 51 (2.0%) |
| Started ART over 3 months after enrolment, n (%) | 272 (47.1%) | 255 (37.7%) | 182 (42.6%) | 458 (49.4%) | <0.001 | 1167 (44.7%) |
| Education, n (%) | <0.001 | |||||
| Primary school | 49 (8.5%) | 18 (2.7%) | 14 (3.3%) | 18 (1.9%) | 99 (3.8%) | |
| Secondary school | 201 (34.8%) | 160 (23.7%) | 67 (15.7%) | 120 (12.9%) | 548 (21.0%) | |
| College | 157 (27.2%) | 247 (36.5%) | 164 (38.4%) | 319 (34.4%) | 887 (34.0%) | |
| University | 43 (7.4%) | 108 (16.0%) | 68 (15.9%) | 191 (20.6%) | 410 (15.7%) | |
| Other/Unknown | 128 (22.1%) | 143 (21.2%) | 114 (26.7%) | 279 (30.1%) | 664 (25.5%) | |
| Employment, n (%) | <0.001 | |||||
| Unemployed | 104 (18.0%) | 80 (11.8%) | 41 (9.6%) | 97 (10.5%) | 322 (12.3%) | |
| Employed | 260 (45.0%) | 360 (53.3%) | 204 (47.8%) | 419 (45.2%) | 1243 (47.7%) | |
| Self-employed | 107 (18.5%) | 101 (14.9%) | 77 (18.0%) | 137 (14.8%) | 422 (16.2%) | |
| Occasional | 25 (4.3%) | 21 (3.1%) | 11 (2.6%) | 22 (2.4%) | 79 (3.0%) | |
| Student | 13 (2.2%) | 23 (3.4%) | 13 (3.0%) | 61 (6.6%) | 110 (4.2%) | |
| Retired | 13 (2.2%) | 21 (3.1%) | 8 (1.9%) | 8 (0.9%) | 50 (1.9%) | |
| Invalid | 2 (0.3%) | 0 (0.0%) | 0 (0.0%) | 1 (0.1%) | 3 (0.1%) | |
| Housewife | 43 (7.4%) | 13 (1.9%) | 6 (1.4%) | 4 (0.4%) | 66 (2.5%) | |
| Other/unknown | 11 (1.9%) | 57 (8.4%) | 67 (15.7%) | 178 (19.2%) | 313 (12.0%) | |
LEGEND: Categorical data are presented as absolute numbers (percentages); p values for comparison of proportions among different calendar periods are by Chi-square test. Quantitative data are presented as median (Interquartile Range, IQR); p values for comparison of medians among different calendar period are by Kruskal-Wallis test. IDUs, Intravenous Drug Users–ART, HIV combination antiretroviral therapy, EGFR, glomerular filtration rate.
Factors associated with Recent HIV Infections in Italy over 1996–2014
Factors associated with RHI by fitting a multivariable logistic regression analysis were younger age at HIV diagnosis, higher baseline CD4+ T-cells and HIV-RNA. More recent calendar period (2010–2014) was also associated with a 12-fold higher probability of RHI versus 1996–2000 (Table 3).
Table 3. Parameters associated with Recent HIV Infections (RHI) by univariate and multivariate logistic regression analysis.
| Odds ratios of recent seroconversion | ||||
|---|---|---|---|---|
| Characteristic | Unadjusted OR (95% CI) |
p-value | Adjusted OR (95% CI) |
p-value |
| Calendar period of Seroconversion | ||||
| 1996–2000 | 1 | 1 | ||
| 2001–2006 | 0.38 (0.29, 0.49) | <0.001 | 1.04 (0.67, 1.61) | 0.858 |
| 2007–2009 | 0.67 (0.51, 0.88) | 0.004 | 1.48 (0.91, 2.41) | 0.115 |
| 2010–2014 | 2.25 (1.82, 2.78) | <0.001 | 12.01 (6.69, 21.57) | <0.001 |
| Gender | ||||
| Female vs. male | 0.67 (0.53, 0.85) | <0.001 | 0.66 (0.37, 1.20) | 0.175 |
| Mode of HIV Transmission | ||||
| IDUs | 1 | 1 | ||
| Homosexual contacts | 1.36 (1.02, 1.82) | 0.034 | 1.08 (0.50, 2.31) | 0.843 |
| Heterosexual contacts | 1.09 (0.80, 1.48) | 0.596 | 0.90 (0.42, 1.94) | 0.796 |
| Other/Unknown | 1.72 (1.11, 2.67) | 0.016 | 1.86 (0.65, 5.35) | 0.250 |
| Nationality | ||||
| Not Italian vs. Italian | 0.96 (0.75, 1.21) | 0.717 | ||
| AIDS diagnosis | ||||
| Yes vs. No | 0.63 (0.40, 1.00) | 0.052 | 1.56 (0.64, 3.78) | 0.328 |
| HCV Ab | ||||
| Negative | 1 | 1 | ||
| Positive | 0.72 (0.54, 0.95) | 0.021 | 0.82 (0.42, 1.61) | 0.560 |
| Not tested | 0.87 (0.73, 1.03) | 0.113 | 0.65 (0.42, 1.00) | 0.050 |
| Age, years | ||||
| per 10 years older | 0.77 (0.69, 0.86) | <0.001 | 0.78 (0.65, 0.95) | 0.011 |
| CD4 count, cells/mmc | ||||
| per 100 cells higher | 1.05 (1.02, 1.08) | 0.003 | 1.09 (1.03, 1.16) | 0.004 |
| CD8 count, cells/mmc | ||||
| per 100 cells higher | 1.00 (1.00, 1.01) | 0.208 | ||
| Viral load, log10 copies/mL | ||||
| per log copies/mL higher | 1.34 (1.22, 1.48) | <0.001 | 1.41 (1.16, 1.71) | <0.001 |
| Diabetes | ||||
| Yes vs. No | 1.04 (0.47, 2.29) | 0.928 | ||
| Smoking | ||||
| No | 1 | 1 | ||
| Yes | 0.89 (0.73, 1.08) | 0.233 | 1.25 (0.88, 1.78) | 0.219 |
| Unknown | 1.51 (1.24, 1.83) | <0.001 | 1.43 (0.84, 2.42) | 0.185 |
| Total cholesterol, mg/dL | ||||
| per 10 mg/dL higher | 1.15 (0.93, 1.44) | 0.204 | ||
| HDL cholesterol, mg/dL | ||||
| per 100 mg/dL higher | 0.72 (0.31, 1.68) | 0.447 | ||
| Use of statins | ||||
| Yes vs. No | 1.04 (0.34, 3.18) | 0.949 | ||
| Use of blood pressure lowering drugs | ||||
| Yes vs. No | 0.75 (0.42, 1.37) | 0.354 | ||
| Blood glucose, mg/dL | ||||
| per 100 mg/dL higher | 0.44 (0.22, 0.85) | 0.015 | 0.69 (0.24, 1.99) | 0.490 |
| Site geographical position | ||||
| North | 1 | 1 | ||
| Center | 0.79 (0.66, 0.94) | 0.007 | 1.02 (0.71, 1.45) | 0.930 |
| South | 1.27 (0.93, 1.75) | 0.133 | 1.24 (0.66, 2.30) | 0.505 |
| Education | ||||
| University | 1 | |||
| Primary/Secondary school | 0.94 (0.73, 1.21) | 0.622 | ||
| College | 0.85 (0.67, 1.09) | 0.199 | ||
| Other/Unknown | 1.17 (0.91, 1.50) | 0.227 | ||
| Employment | ||||
| Unemployed | 1 | 1 | ||
| Employed | 0.87 (0.68, 1.12) | 0.286 | 0.71 (0.42, 1.19) | 0.197 |
| Self-employed | 0.74 (0.54, 1.00) | 0.047 | 0.82 (0.44, 1.54) | 0.543 |
| Occasional | 0.98 (0.59, 1.62) | 0.934 | 0.90 (0.37, 2.19) | 0.814 |
| Student | 2.03 (1.31, 3.15) | 0.002 | 0.89 (0.35, 2.27) | 0.815 |
| Retired/Invalid/Housewife | 0.66 (0.42, 1.03) | 0.068 | 1.00 (0.41, 2.42) | 0.995 |
| Other/unknown | 1.09 (0.79, 1.50) | 0.590 | 0.60 (0.28, 1.30) | 0.197 |
LEGEND: Univariate and multivariate logistic regression analysis; OR: odds ratio– 95%CI, 95% confidence interval. IDUs, Intravenous Drug Users–cART, HIV combination antiretroviral therapy. Parameters included in the multivariable logistic regression model were calendar period of seroconversion, gender, mode of HIV transmission, age, HCV coinfection, AIDS diagnosis, CD4+ T-cells count and HIV-RNA (measured at enrolment), employment status, smoking, blood glucose and site geographical position.
Estimates of cART initiation and of viro-immunological success in Recent and Less Recent HIV Infections
Among all patients included in the analysis, 49% of participants started cART by 3 months from seroconversion with no differences over time: 47.1% in 1996–2000, 49.4% in 2010–2014 (Table 2). There was also no difference in the 2-year cumulative probability of cART initiation between RHI and NRHI, regardless of calendar period (74.2% in RHI, 74.1% in NRHI, p = 0.73). The reasons for non cART initiation in the study population were the following: an HIV diagnosis within 6 months of enrolment (58%), no indication for cART (25% which is likely to be CD4+ count and guidelines driven), first access to care (13%), patients’ choice (2%), physician’s choice (2%) and other/unknown (1%).
The proportion of patients achieving a HIV-RNA ≤500 copies/mL at 6 months from the date of starting cART was similar in RHI and NRHI and stratifying by time periods (2008–2009: RHI 100%, NRHI 96%; 2010–2014: RHI 97%, NRHI 97%) (Table 4).
Table 4. Proportion of patients achieving a HIV-RNA ≤500 copies/mL at 6 months from the date of starting cART.
| Calendar Period of enrolment | ||||
|---|---|---|---|---|
| 1996–2000 | 2001–2007 | 2008–2009 | 2010–2015 | |
| RHI | 6/8 (75%) | 27/30 (90%) | 20/20 (100%) | 176/182 (97%) |
| NRHI | - | 38/42 (90%) | 41/43 (96%) | 349/360 (97%) |
| Total | 6/8 (75%) | 65/72 (90%) | 61/63 (97%) | 525/542 (97%) |
LEGEND: RHI, Recent HIV Infections; NRHI, Non Recent HIV Infections. Data are presented as absolute numbers (percentages).
Rates and hazards of virological success from fitting a Cox regression analysis were also similar in RHI (9.4 events/person-years of follow-up, PYFU 95%CI 8.15–10.84) versus NRHI (9.87 events/PYFU, 95%CI 8.93–10.91; HR 0.91 of RHI versus NRHI, 95%CI 0.76–1.09).
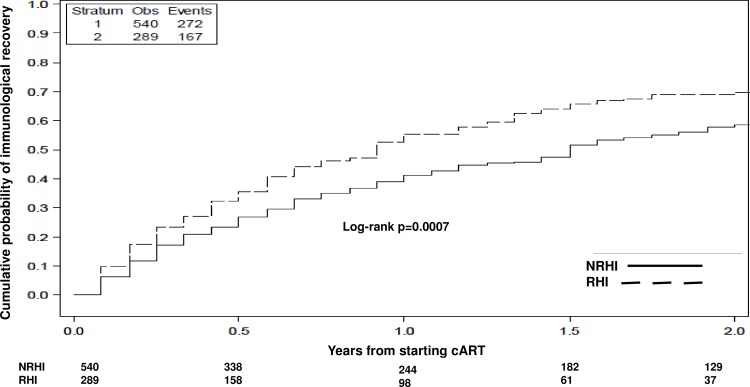
The Kaplan Meier estimates of immune recovery showed a higher probability for RHI compared to NRHI (log-rank test, p = 0.0007) (Fig 2).
Fig 2. Probability of immune recovery (time from cART start to CD4+ count gain ≥200 cells/mmc) by Kaplan Meier estimates.
Kaplan Meier estimates of the probability of achieving CD4+ T-cells count ≥200 cells/mmc from cART start according to Recent and non Recent HIV Infection; log rank test. The continuous line represents Less Recent HIV Infections (NRHI), the dot line represents Recent HIV Infections (RHI).
In the Cox regression analysis, in fact, RHI showed a 1.5-fold higher probability of CD4+ gain ≥200 cells/mmc (RHI: 6.85 events/PYFU, 95%CI 5.79–8.1; NRHI: 5.79 events/PYFU, 95%CI 5.09–6.6; HR 1.46 versus NRHI, 95%CI 1.18–1.81), also following adjustment for calendar period and type of antiretroviral regimen started (adjusted HR 1.33, 95%CI 1.05–1.69) and for age, HCV co-infection and smoking (adjusted HR 1.44, 95%CI 1.09–1.9); the difference in probability was however largely attenuated after further controlling for baseline HIV-RNA and CD4+ count (adjusted HR 1.23, 95%CI 0.98–1.54). The results were similar also exploring the probability of immunological recovery according the second definition (CD4+ gain of 200 cells/mmc or reaching a single CD4+ count >350 cells/mmc, which ever came first): RHI, in comparison with NRHI, presented a higher probability of CD4+ gain by fitting Kaplan Meier curves (log-rank test p = 0.001) and Cox regression analysis (HR 1.26 versus NRHI, 95%CI 1.08–1.47), but the difference between RHI and NRHI was not confirmed after adjustment for calendar period, type of antiretroviral regimen, age, HCV coinfection, baseline HIV-RNA and CD4+ T cells count (adjusted HR 0.98 versus NRHI, 95%CI 0.74–1.31).
Discussion
Trough the analysis of the data of untreated HIV-positive patients enrolled in an Italian cohort with documented dates of HIV-negative and positive serological tests, we tried to bridge the gap of lack of monitoring regarding the recent HIV infections. The knowledge of proportion and epidemiological features of new HIV infections is in fact essential in order to address screening services and try to control HIV epidemics. HIV test is the first step of the prevention interventions; in spite of the increased numbers of persons at risk of having HIV infection that are tested, still an unacceptable high proportion is not screened annually [16]. Since the awareness of HIV infection allows linkage to care, access to cART and virological suppression, with a reduced probability of HIV transmission, prevention measures are crucial to the reduction of HIV-related morbidity and mortality [17–19].
Among people in the ICONA cohort for whom the date of seroconversion could be accurately estimated, we hereby describe a significant trend for an increased proportion of RHI over time (57% in 2010–2014). This could be related to the increase in prevalence of MSM in recent years as well as an increase in self-awareness of risky behavior in this specific patient group [20, 21]. In our study we found no difference in mode of HIV transmission between RHI and NRHI; conversely, from 1996–2000 to most recent periods there was a decline in the proportion of IDUs and a significant increase in MSM (from 32% to 71%) in agreement with Italian data of the “Istituto Superiore di Sanità” and data of European Centre for Disease Prevention and Control (ECDC) about new HIV infections; the last report in fact described that in Italy the majority of new HIV infections were sexually acquired with 44.9% in heterosexuals and 40.6% in homosexuals [22, 23]. MSM are still vulnerable to HIV infection, as documented by the persistence of high prevalence of infection in this group, despite an overall decline of new infections in the general population [21, 24]. Data from the US Center of Disease Control and Prevention (CDC) report that 54% of estimated HIV diagnoses in United States in 2014 are in MSM, even if they represent only the 2% of the population [25]. A previous Italian study reported an increased risk of HIV seroconversion in younger MSM throughout the study period (1984–2010) [26]; similar data were confirmed in other European studies with a double risk of HIV in MSM aging 20–29 years from 2003 to 2012 [27, 28].
Our results are in keeping with the results from other European studies: the prevalence of recent infections varied from 7 to 47% due to differences in the prevalence of HIV infection in European countries, the analyzed study period and the definition of recent infection. All the other European studies described a shift in HIV risk from IDUs to sexual transmission in most recent years [29–31].
Policies of HIV prevention have changed over recent years acquiring new effective tools and targeting groups at risk in areas most affected by HIV epidemics. Our finding of an increased proportion of RHI might also reflect an increase of rate of HIV testing as a result of the introduction and implementation of ‘test and treat’ policies [32]. Currently, however, prevention services need to be monitored to identify weak areas to be improved. In fact, according to the last ECDC reports about HIV infection in the European Union, prevention interventions are still not enough to reduce the number of new HIV infections; in comparison with the UNAIDS 90:90:90 target, 15–17% of people living with HIV in Europe are estimated to have not yet been diagnosed, and among people diagnosed with HIV, nearly half are diagnosed late. Furthermore, 17% of people diagnosed with HIV are still not on treatment and the proportion of patients receving cART that are virally suppressed is around 51–95% [23].
By 3 months from seroconversion cART was started in half of the patients without significant differences between RHI and NRHI, but this analysis was performed before the results of the START trial became public [6].
The benefits of early treatment are now well recognized [33]: the 5-year risk of clinical progression is 3.2% in people starting cART immediately versus 7% in those deferring [34]. Rapid cART initiation is known to confer a significantly enhanced 2 year probability of immunological recovery, independently from baseline CD4+ count, and minimized HIV-associated inflammation [9]. These benefits translate in a reduced clinical progression in previous cohort studies [10, 11]. Similarly, retrospective studies have shown that long pretreatment waiting time in HIV-positive patients is associated with a higher risk of reduced cART adherence and subsequent higher mortality [35]. As previously demonstrated by HPTN052 trial, besides reducing AIDS and non AIDS related diseases, early cART initiation is also associated with a reduction of 96% in HIV transmission [18, 36]. Similarly, during a median follow-up of 1.3 years no cases of HIV transmission have been reported among serodifferent couples when the HIV-positive partner was under virally suppressive cART [18]. Our study reports data from 1996 to 2014; in the previous periods Italian guidelines suggested starting cART with a CD4+ count below 200 cells/mmc and then 350 cells/mmc. Most recent guidelines recommend that cART is offered to all HIV-positive patients, irrespective of CD4+ counts, also in order to reduce risk of transmission. Thus, in agreement with the UNAIDS 90:90:90 target [37], a reduction in HIV transmission is foreseeable that reflects the increasing number of HIV-positive patients on successful treatment.
Despite these data on clinical and immunologic advantages of treatment in the recent or acute phase [10, 11, 38], in our cohort we found similar rates of immune-virological response in RHI and NRHI; reasons are unclear, but it is possible that in the era of modern cART with highly effective and tolerated regimens differences are attenuated. Researchers of the Swiss HIV cohort showed that a substantial fraction of HIV transmissions can be attributed to recently infected patients, for whom the preventive effect of treatment is weaker, due to under-diagnosis and lack of patient’s awareness of their seropositive status [3]. Thus, early testing and the use of antiretroviral drugs associated with a rapid viral decay in RHI is crucial to maximize the effect of cART use as prevention and to reduce the risk of HIV transmission.
The main limitation of our analysis is the lack of differentiation between acute HIV and RHI (symptoms of acute infection are not collected in the cohort) and the potential selection bias introduced by including only cohort participants for whom the date of seroconversion could be accurately estimated; however, the increase of prevalence of MSM at enrolment has been also shown in analyses including the whole cohort. Furthermore, not all HIV-infected patients in Italy are enrolled in the ICONA cohort due to the design based upon voluntary enrolment. Finally, our finding of regional differences in the different calendar periods might reflect several changes in the centers participating to ICONA occurred in most recent years. Despite such limitations our data are in line with epidemiological reports by the “Istituto Superiore di Sanità” [22].
Conclusions
The increased proportion of RHI over time in our cohort suggests that in recent years in Italy people are diagnosed earlier with HIV and more quickly enter care after the diagnosis. National data in fact show that the incidence of new HIV infections remained stable over the study period; nevertheless, efforts to the development and implementation of effective prevention interventions should continue to guarantee broad early cART access, reduce new infections and get closer to the UNAIDS 90-90-90 target.
Acknowledgments
ICONA FOUNDATION STUDY GROUP:
BOARD OF DIRECTORS
A d’Arminio Monforte (President), A Antinori, A Castagna, F Castelli, R Cauda, G Di Perri, M Galli, R Iardino, G Ippolito, GC Marchetti, CF Perno, G Rezza, F von Schloesser, P Viale
SCIENTIFIC SECRETARY
A d’Arminio Monforte, A Antinori, A Castagna, F Ceccherini-Silberstein, A Cozzi-Lepri, E Girardi, S Lo Caputo, C Mussini, M Puoti
STEERING COMMITTEE
M Andreoni, A Ammassari, A Antinori, C Balotta, A Bandera, P Bonfanti, S Bonora, M Borderi, A Calcagno, L Calza, MR Capobianchi, A Castagna, F Ceccherini-Silberstein, A Cingolani, P Cinque, A Cozzi-Lepri, A d’Arminio Monforte, A De Luca, A Di Biagio, E Girardi, N Gianotti, A Gori, G Guaraldi, G Lapadula, M Lichtner, S Lo Caputo, G Madeddu, F Maggiolo, G Marchetti, S Marcotullio, L Monno, C Mussini, S Nozza, M Puoti, E Quiros Roldan, R Rossotti, S Rusconi, MM Santoro, A Saracino, M Zaccarelli.
STATISTICAL AND MONITORING TEAM
A Cozzi-Lepri, I Fanti, L Galli, P Lorenzini, A Rodano, M Shanyinde, A Tavelli
BIOLOGICAL BANK INMI
F Carletti, S Carrara, A Di Caro, S Graziano, F Petrone, G Prota, S Quartu, S Truffa
PARTICIPATING PHYSICIANS AND CENTERS
Italy A Giacometti, A Costantini, V Barocci (Ancona); G Angarano, L Monno, C Santoro (Bari); F Maggiolo, C Suardi (Bergamo); P Viale, V Donati, G Verucchi (Bologna); F Castelli, C Minardi, E Quiros Roldan (Brescia); T Quirino, C Abeli (Busto Arsizio); PE Manconi, P Piano (Cagliari); B Cacopardo, B Celesia (Catania); J Vecchiet, K Falasca (Chieti); A Pan, S Lorenzotti (Cremona); L Sighinolfi, D Segala (Ferrara); F Mazzotta, F Vichi (Firenze); G Cassola, C Viscoli, A Alessandrini, N Bobbio, G Mazzarello (Genova); C Mastroianni, V Belvisi (Latina); P Bonfanti, I Caramma (Lecco); A Chiodera, P Milini (Macerata); A d’Arminio Monforte, M Galli, A Lazzarin, G Rizzardini, M Puoti, A Castagna, G Marchetti, MC Moioli, R Piolini, AL Ridolfo, S Salpietro, C Tincati, (Milano); C Mussini, C Puzzolante (Modena); A Gori, G Lapadula (Monza); N Abrescia, A Chirianni, G Borgia, R Orlando, G Bonadies, F Di Martino, I Gentile, L Maddaloni (Napoli); AM Cattelan, S Marinello (Padova); A Cascio, C Colomba (Palermo); F Baldelli, E Schiaroli (Perugia); G Parruti, F Sozio (Pescara); G Magnani, MA Ursitti (Reggio Emilia); M Andreoni, A Antinori, R Cauda, A Cristaudo, V Vullo, R Acinapura, G Baldin, M Capozzi, S Cicalini, A Cingolani, L Fontanelli Sulekova, G Iaiani, A Latini, I Mastrorosa, MM Plazzi, S Savinelli, A Vergori (Roma); M Cecchetto, F Viviani (Rovigo); G Madeddu, P Bagella (Sassari); A De Luca, B Rossetti (Siena); A Franco, R Fontana Del Vecchio (Siracusa); D Francisci, C Di Giuli (Terni); P Caramello, G Di Perri, S Bonora, GC Orofino, M Sciandra (Torino); M Bassetti, A Londero (Udine); G Pellizzer, V Manfrin (Vicenza) G Starnini, A Ialungo(Viterbo).
Funding:
Icona Foundation is sponsored by unrestricted grants of BMS, Gilead, Janssen, MSD and ViiV Italy
Data Availability
All relevant data are within the paper.
Funding Statement
Icona Foundation is sponsored by unrestricted grants of BMS, Gilead, Janssen, MSD and ViiV Italy.
References
- 1.Ryom L, Boesecke C, Gisler V, Manzardo C, Rockstroh JK, Puoti M, et al. Essentials from the 2015 European AIDS Clinical Society (EACS) guidelines for the treatment of adult HIV-positive persons. HIV Med. 2016;17(2):83–8. Epub 2015/11/08. doi: 10.1111/hiv.12322 . [DOI] [PubMed] [Google Scholar]
- 2.Blaser N, Wettstein C, Estill J, Vizcaya LS, Wandeler G, Egger M, et al. Impact of viral load and the duration of primary infection on HIV transmission: systematic review and meta-analysis. AIDS. 2014;28(7):1021–9. doi: 10.1097/QAD.0000000000000135 ; PubMed Central PMCID: PMCPMC4058443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marzel A, Shilaih M, Yang WL, Böni J, Yerly S, Klimkait T, et al. HIV-1 Transmission During Recent Infection and During Treatment Interruptions as Major Drivers of New Infections in the Swiss HIV Cohort Study. Clin Infect Dis. 2016;62(1):115–22. Epub 2015/09/19. doi: 10.1093/cid/civ732 . [DOI] [PubMed] [Google Scholar]
- 4.Mangal TD, EuroCoord UWGoCPaMAHSitCCi. Joint estimation of CD4+ cell progression and survival in untreated individuals with HIV-1 infection. AIDS. 2017;31(8):1073–82. doi: 10.1097/QAD.0000000000001437 ; PubMed Central PMCID: PMCPMC5414573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Günthard HF, Saag MS, Benson CA, del Rio C, Eron JJ, Gallant JE, et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2016 Recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316(2):191–210. doi: 10.1001/jama.2016.8900 ; PubMed Central PMCID: PMCPMC5012643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373(9):795–807. Epub 2015/07/20. doi: 10.1056/NEJMoa1506816 ; PubMed Central PMCID: PMCPMC4569751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nozza S, Poli A, Ripa M, Galli L, Chiappetta S, Spagnuolo V, et al. Efficacy of elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate as treatment for primary or recent HIV infection. J Antimicrob Chemother. 2016. Epub 2016/10/17. doi: 10.1093/jac/dkw439 .27798217 [Google Scholar]
- 8.Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, Ouassa T, et al. A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. N Engl J Med. 2015;373(9):808–22. Epub 2015/07/20. doi: 10.1056/NEJMoa1507198 . [DOI] [PubMed] [Google Scholar]
- 9.Le T, Wright EJ, Smith DM, He W, Catano G, Okulicz JF, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med. 2013;368(3):218–30. doi: 10.1056/NEJMoa1110187 ; PubMed Central PMCID: PMCPMC3657555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thornhill J, Inshaw J, Kaleebu P, Cooper D, Ramjee G, Schechter M, et al. Brief Report: Enhanced Normalization of CD4/CD8 Ratio With Earlier Antiretroviral Therapy at Primary HIV Infection. J Acquir Immune Defic Syndr. 2016;73(1):69–73. doi: 10.1097/QAI.0000000000001013 ; PubMed Central PMCID: PMCPMC4981213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karris MY, Umlauf A, Vaida F, Richman D, Little S, Smith D. A randomized controlled clinical trial on the impact of CCR5 blockade with maraviroc in early infection on T-cell dynamics. Medicine (Baltimore). 2016;95(44):e5315 doi: 10.1097/MD.0000000000005315 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laanani M, Ghosn J, Essat A, Melard A, Seng R, Gousset M, et al. Impact of the Timing of Initiation of Antiretroviral Therapy During Primary HIV-1 Infection on the Decay of Cell-Associated HIV-DNA. Clin Infect Dis. 2015;60(11):1715–21. Epub 2015/03/03. doi: 10.1093/cid/civ171 . [DOI] [PubMed] [Google Scholar]
- 13.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N Engl J Med. 2016;375(9):830–9. Epub 2016/07/18. doi: 10.1056/NEJMoa1600693 ; PubMed Central PMCID: PMCPMC5049503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.d'Arminio Monforte A, Lepri AC, Rezza G, Pezzotti P, Antinori A, Phillips AN, et al. Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral naïve patients. I.CO.N.A. Study Group. Italian Cohort of Antiretroviral-Naïve Patients. AIDS. 2000;14(5):499–507. . [DOI] [PubMed] [Google Scholar]
- 15.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. . [PubMed] [Google Scholar]
- 16.Patrick R, Greenberg A, Magnus M, Opoku J, Kharfen M, Kuo I. Development of an HIV Testing Dashboard to Complement the HIV Care Continuum Among MSM, PWID, and Heterosexuals in Washington, DC, 2007–2015. J Acquir Immune Defic Syndr. 2017;75 Suppl 3:S397–S407. doi: 10.1097/QAI.0000000000001417 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen M, Eshleman S, McCauley M, Chen Y. Antiretroviral Therapy to Prevent HIV Acquisition: Limits of Estimation From a Population Cohort. Clin Infect Dis. 2016;63(12):1679–80. Epub 2016/09/29. doi: 10.1093/cid/ciw673 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, van Lunzen J, et al. Sexual Activity Without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy. JAMA. 2016;316(2):171–81. doi: 10.1001/jama.2016.5148 . [DOI] [PubMed] [Google Scholar]
- 19.Henn A, Flateau C, Gallien S. Primary HIV Infection: Clinical Presentation, Testing, and Treatment. Curr Infect Dis Rep. 2017;19(10):37 Epub 2017/09/07. doi: 10.1007/s11908-017-0588-3 . [DOI] [PubMed] [Google Scholar]
- 20.Linley L, An Q, Song R, Valverde E, Oster AM, Qian X, et al. HIV Testing Experience Before HIV Diagnosis Among Men Who Have Sex with Men—21 Jurisdictions, United States, 2007–2013. MMWR Morb Mortal Wkly Rep. 2016;65(37):999–1003. Epub 2016/09/23. doi: 10.15585/mmwr.mm6537a3 . [DOI] [PubMed] [Google Scholar]
- 21.Sargin F, Yildiz D, Aydin OA, Mete B, Gunduz A, Karaosmanoglu HK, et al. Changes in HIV demographic patterns in a low prevalence population: no evidence of a shift towards men who have sex with men. Int J Infect Dis. 2016;48:52–6. Epub 2016/05/09. doi: 10.1016/j.ijid.2016.05.006 . [DOI] [PubMed] [Google Scholar]
- 22.Notiziario dell'Istituto Superiore di Sanità, aggiornamento delle nuove diagnosi di infezione da HIV e dei casi di AIDS in Italia. http://www.iss.it/ccoa.
- 23.European Centre for Disease Prevention and Control, WHO Regional Office for Europe. HIV/AIDS surveillance in Europe 2015. Stockholm: ECDC; 2016. [Google Scholar]
- 24.Pines HA, Karris MY, Little SJ. Sexual Partner Concurrency Among Partners Reported by MSM with Recent HIV Infection. AIDS Behav. 2017. Epub 2017/07/12. doi: 10.1007/s10461-017-1855-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shadaker S, Magee M, Paz-Bailey G, Hoots BE, Group NS. Characteristics and Risk Behaviors of Men Who Have Sex With Men and Women Compared With Men Who Have Sex With Men-20 US Cities, 2011 and 2014. J Acquir Immune Defic Syndr. 2017;75 Suppl 3:S281–S7. doi: 10.1097/QAI.0000000000001403 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giuliani M, Vescio MF, Latini A, Palamara G, Pimpinelli F, Dona MG, et al. Continuous increase in HIV-1 incidence after the year 2000 among men who have sex with men in Rome: insights from a 25-year retrospective cohort study. Euro Surveill. 2014;19(47):20969 Epub 2014/11/27. . [DOI] [PubMed] [Google Scholar]
- 27.Janiec J, Haar K, Spiteri G, Likatavicius G, Van de Laar M, Amato-Gauci AJ. Surveillance of human immunodeficiency virus suggests that younger men who have sex with men are at higher risk of infection, European Union, 2003 to 2012. Euro Surveill. 2013;18(48):20644 Epub 2013/11/28. . [DOI] [PubMed] [Google Scholar]
- 28.Pharris A, Spiteri G, Noori T, Amato-Gauci AJ. Ten years after Dublin: principal trends in HIV surveillance in the EU/EEA, 2004 to 2013. Euro Surveill. 2014;19(47):20968 Epub 2014/11/27. . [DOI] [PubMed] [Google Scholar]
- 29.Rosińska M, Marzec-Bogustawska A, Janiec J, Smoleń-Dzirba J, Wąsik T, Gniewosz J, et al. High percentage of recent HIV infection among HIV-positive individuals newly diagnosed at voluntary counseling and testing sites in Poland. AIDS Res Hum Retroviruses. 2013;29(5):805–13. Epub 2013/02/26. doi: 10.1089/AID.2012.0314 ; PubMed Central PMCID: PMCPMC3636578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simmons R, Malyuta R, Chentsova N, Karnets I, Murphy G, Medoeva A, et al. HIV Incidence Estimates Using the Limiting Antigen Avidity EIA Assay at Testing Sites in Kiev City, Ukraine: 2013–2014. PLoS One. 2016;11(6):e0157179 Epub 2016/06/08. doi: 10.1371/journal.pone.0157179 ; PubMed Central PMCID: PMCPMC4898716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soodla P, Simmons R, Huik K, Pauskar M, Jõgeda EL, Rajasaar H, et al. HIV incidence in the Estonian population in 2013 determined using the HIV-1 limiting antigen avidity assay. HIV Med. 2017. Epub 2017/08/01. doi: 10.1111/hiv.12535 . [DOI] [PubMed] [Google Scholar]
- 32.Aghaizu A, Wayal S, Nardone A, Parsons V, Copas A, Mercey D, et al. Sexual behaviours, HIV testing, and the proportion of men at risk of transmitting and acquiring HIV in London, UK, 2000–13: a serial cross-sectional study. Lancet HIV. 2016;3(9):e431–40. Epub 2016/07/14. doi: 10.1016/S2352-3018(16)30037-6 . [DOI] [PubMed] [Google Scholar]
- 33.Marcellusi A, Viti R, Russo S, Andreoni M, Antinori A, Mennini FS. Early Treatment in HIV Patients: A Cost-Utility Analysis from the Italian Perspective. Clin Drug Investig. 2016;36(5):377–87. doi: 10.1007/s40261-016-0382-2 . [DOI] [PubMed] [Google Scholar]
- 34.Lodi S, Sharma S, Lundgren JD, Phillips AN, Cole SR, Logan R, et al. The per-protocol effect of immediate versus deferred antiretroviral therapy initiation. AIDS. 2016;30(17):2659–63. doi: 10.1097/QAD.0000000000001243 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su S, Li S, Gao L, Cai Y, Fu J, Guo C, et al. Gaps in the Continuum of HIV Care: Long Pretreatment Waiting Time between HIV Diagnosis and Antiretroviral Therapy Initiation Leads to Poor Treatment Adherence and Outcomes. Biomed Res Int. 2016;2016:2648923 Epub 2016/12/22. doi: 10.1155/2016/2648923 ; PubMed Central PMCID: PMCPMC5214466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grinsztejn B, Hosseinipour MC, Ribaudo HJ, Swindells S, Eron J, Chen YQ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14(4):281–90. Epub 2014/03/04. doi: 10.1016/S1473-3099(13)70692-3 ; PubMed Central PMCID: PMCPMC4144040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.UNAIDS 90:90:90 An ambitious treatment target to help end the AIDS epidemic, 2014 http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf.
- 38.Herout S, Mandorfer M, Breitenecker F, Reiberger T, Grabmeier-Pfistershammer K, Rieger A, et al. Impact of Early Initiation of Antiretroviral Therapy in Patients with Acute HIV Infection in Vienna, Austria. PLoS One. 2016;11(4):e0152910 Epub 2016/04/11. doi: 10.1371/journal.pone.0152910 ; PubMed Central PMCID: PMCPMC4827808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.