Abstract
Aneura pinguis is a thalloid liverwort species with broad geographical distribution. It is composed of cryptic species, however, the number of cryptic species within A. pinguis is not known. Five cpDNA regions (matK, rbcL, rpoC1, trnH-psbA and trnL-trnF) and the entire nuclear ITS region were studied in 130 samples of A. pinguis from different geographical regions. The relationships between the cryptic species of A. pinguis, A. maxima and A. mirabilis were analyzed. All of the examined samples were clustered into 10 clades corresponding to 10 cryptic species of A. pinguis (marked A to J). Aneura mirabilis and A. maxima were nested among different cryptic species of A. pinguis, which indicates that A. pinguis is a paraphyletic taxon. Subgroups were found in cryptic species A, B, C and E. As single barcodes, all tested DNA regions had 100% discriminant power and fulfilled DNA barcode criteria for species identification; however, the only combination detected in all subgroups was trnL-trnF with trnH-psbA or ITS2. The distances between cryptic species were 11- to 35-fold higher than intraspecific distances. In all analyzed DNA regions, the distances between most pairs of cryptic A. pinguis species were higher than between A. maxima and A. mirabilis. All cryptic species of A. pinguis clearly differed in their habitat preferences, which suggests that habitat adaptation could be the main driving force behind cryptic speciation within this taxon.
Introduction
Taxonomy is a branch of biology concerned with the description, identification and classification of organisms and the phylogenetic relationships between them. The species is the fundamental unit in biology. The species concept and the delimitation of species have stirred much controversy since the early days of systematic biology [1]. Conflicting definitions of species have been proposed based on different criteria. According to Mayden [2], various aspects of lineage divergence arise at different times during the process of speciation [3]. The most popular definition of species is based on morphological differences [4, 5]. However, not all species can be identified based on morphological differences. In some cases, the accumulation of genetic and ecological differences is not correlated with the accumulation of morphological variations, this situation lead to appearance of cryptic species. Cryptic species are taxa which are characterized by distinctive genetic differences, different ecological preferences and the complete or nearly complete absence of morphological variations. For this reason, they cannot be identified based on the traditional morphological species concept [6, 7]. These species are difficult or impossible to identify based on their morphological traits, and they can be distinguished only with the use of biochemical or molecular methods [3, 8, 9]. DNA barcoding is a highly useful method for identifying taxonomically difficult species. The DNA barcoding concept is based on the presence of species-specific DNA sequences in one locus or multiple loci [10]. In recent years, quite a lot of new bryophyte species have been discovered by DNA barcoding [11–13]. These studies revealed that cryptic speciation in bryophytes is more common than previously thought.
Aneura pinguis (L.) Dumort. is a thalloid liverwort species with simple morphology and it is widespread in the Southern and Northern Hemispheres [14, 15]. The species is commonly found in diverse regions that extend from lowlands to high mountain zones, and it grows in various habitats, including calcareous rocks, humus, peat bogs, wet sands on lake shores and clay soils [16]. For over twenty years, it has been known that A. pinguis is a complex of cryptic species [9, 16–18]. Five cryptic species, provisionally named A. pinguis species A, B, C, D and E, have been identified to date. These species have been identified only in Europe, including four (A, B, C and E) in Central Europe and two (B and D) on the British Isles. The genetic differences among these species were as extensive as among related species, of other bryophytes and higher plants. Moreover there is no evidence to suggest recombination between these species [9, 16], i.e. they are species according to biological species concept. In cryptic species A, B and C, minor differences were found in morphological and anatomical features such as thallus and cell size, the thickness and number of cells in thallus cross-sections [19] and the size of oil bodies [20]. These differences are not sufficiently distinct and cannot be used as diagnostic features, however, they could support species identification. Wawrzyniak et al. [21] found qualitative differences in the composition of volatile compounds between cryptic species A, B, C and E of A. pinguis. Bączkiewicz et al. [22] reported differences in the environmental preferences of the analyzed species. The cryptic species of A. pinguis have never been formally described, and A. pinguis is still regarded as a taxonomically homogeneous species. However, the exact number of cryptic species within the entire geographical range of A. pinguis has not been unambiguously defined.
The main research aims of this study were to: i) analyze genetic differentiation within A. pinguis, ii) test the effectiveness of DNA barcoding (matK, rbcL, rpoC1, trnH-psbA and trnL-trnF and complete nuclear ITS) in the identification of cryptic species of A. pinguis, iii) analyze the evolutionary process of the Aneura pinguis complex, and the phylogenetic relationships between the cryptic species of Aneura pinguis and A. mirabilis and A. maxima.
Materials and methods
Plant material
Plant material consisted of 104 fresh samples and 26 herbarium specimens of A. pinguis, and 14 fresh samples of A. maxima from different geographical regions and different types of habitats (Tables 1 and 2 and S1 Table). The plants were initially identified based on morphological traits according to Schuster [23], and Buczkowska and Bączkiewicz [24]. Sequences from six DNA regions were newly generated for 70–143 specimens, depending on the region (GenBank accession numbers are listed in S1 Table). Several sequences of rbcL, trnL-trnF and ITS for the analyzed species were obtained from GenBank. The sequences of A. mirabilis, which was examined in this study for comparative purposes, Pellia endiviifolia (Dicks.) Dumort., P. neesiana (Gottsche) Limpr. and Lobatiriccardia lobata (Schiffn.) Furuki, selected as outgroups, were obtained from GenBank (Acc. No.: NC010359.1, AJ276490, AY507553.1, DQ986148.1).
Table 1. Number of studied samples from different geographical regions.
| No. of samples | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. pinguis | A. maxima | ||||||||||||||||
| Regions | A1 | A2 | A3 | B1 | B2 | B3 | C1 | C2 | D | E1 | E2 | F | G | H | I | J | |
| Poland: | |||||||||||||||||
| Wolin Island | 3 | ||||||||||||||||
| Western Pomerania | 1 | 5 | 6 | 1 | 3 | 5 | 1 | ||||||||||
| Warmia | 1 | 1 | |||||||||||||||
| Suwałki Lake District | 1 | 2 | 2 | ||||||||||||||
| Wielkopolska | 1 | 2 | 1* | 2 | |||||||||||||
| Białowieża Forest | 3 | ||||||||||||||||
| Śląsk | 1 | 1 | |||||||||||||||
| Tatra Mts | 3 | 1 | 15 | 3 | 6 | 17 | 1 | 3 | |||||||||
| Beskidy Mts | 4 | 3 | 2 | 1 | 1 | ||||||||||||
| Gorce Mts | 1 | ||||||||||||||||
| Pieniny Mts | 2 | 1 | 2 | 1 | 2 | ||||||||||||
| Góry Bialskie Mts | 1 | ||||||||||||||||
| Bieszczady Mts | 2 | 2 | 4 | 1 | 3 | 6 | 1 | 2 | |||||||||
| Great Britain, Scotland, North Ebudes, Rum** | 1 | ||||||||||||||||
| Ireland, West Galway, Broadboy, Glencorbet ** | 1 | ||||||||||||||||
| Romania | 1 | ||||||||||||||||
| Japan*** | 3 | ||||||||||||||||
| New Zealand | 1 | ||||||||||||||||
| Canada | 2 | ||||||||||||||||
| U.S.A. | 2 | ||||||||||||||||
| Total | 11 | 5 | 18 | 5 | 7 | 5 | 15 | 3 | 2 | 21 | 1 | 18 | 7 | 4 | 5 | 3 | 14 |
(leg.*P.Górski,.** D.G. Long, *** M. Itouga)
Table 2. Habitat characteristics of Aneura pinguis cryptic species and A. maxima.
| Species/ cryptic species / lineages | No. of haplotypes | Habitat preferences |
|---|---|---|
| A1 | 3 | humus over detritus flysch rocks or on humus over limestone rocks |
| A2 | 1 | humus over detritus flysch rocks or on humus over limestone rocks |
| A3 | 4 | humus over limestone rocks |
| B1 | 4 | clay soil or on humus |
| B2 | 2 | clay soil or on humus mixed with clay |
| B3 | 2 | humus |
| C1 | 2 | sandy soil or humus over limestone rocks or on humus or on rotten wood |
| C2 | 1 | sandy soil |
| D | 1 | on wet flushed rock |
| E1 | 2 | on rocks with leaking or flowing water |
| E2 | 1 | on rocks with leaking water |
| F | 4 | clay soil and on humus mixed with clay |
| G | 4 | peat bog or peat covered lake shore, among Sphagnum |
| H | 2 | humus |
| I | 2 | peat covered lake shore, among Sphagnum |
| J | 2 | on wet flushed rock |
| A.maxima | 5 | in marsh situated on the river or stream banks |
Ethics statement
The samples of A. pinguis from the Tatra, Białowieża, Pieniny, Bieszczady and Wolin National Parks were collected by AB and KB with the permission given by the Ministry of Environment in Poland and the Directors of these National Parks. For the remaining locations specific permission was not required. A. pinguis is neither an endangered nor protected species.
DNA extraction, PCR amplification and sequencing
Total genomic DNA was extracted from fresh material using the GeneJET Plant Genomic DNA Purification Mini Kit (Thermo Scientific) and from dried material using the Novabeads Plant DNA Kit (Novazym, Poland). The quality of isolated DNA was evaluated by electrophoresis in 0.8% agarose gel, and the concentration and purity of DNA samples were determined in the Epoch™ Multi-Volume Spectrophotometer System.
Six DNA regions, including five regions in the chloroplast genome (matK, rbcL, rpoC1, trnH-psbA and trnL-trnF) and the complete nuclear ITS region (ITS1-5.8S-ITS2) were analyzed. Standard barcode regions [25] were amplified for rbcL and matK. For trnHGUG-psbA, in addition to the spacer region, a fragment of the psbA gene was sequenced according to Bell et al. [26]. The trnL-trnF region contains the trnLUAA gene (5’exon, intron and 3’exon) and the trnLUAA-trnFGAA intergenic spacer [27]. Amplification and sequencing primers and PCR cycling conditions are given in S2 Table. PCR amplification was carried out according to the procedure described by Krawczyk et al. [28]. Purified PCR products of the studied DNA regions were sequenced in both directions using the same primers and the ABI BigDye 3.1 Terminator Cycle Kit (Applied Biosystems). The sequenced products were visualized using the ABI Prism 3130 Automated DNA Sequencer (Applied Biosystems). Bidirectional sequencing was applied to avoid sequencing errors.
Data analysis
Chromatograms of DNA sequences were edited and assembled in Geneious R6 (Biomatters, USA). The assembled sequences were aligned in MEGA 6.06 [29] and Muscle [30] with default settings. Regions of ambiguous alignment and incomplete data were excluded from analysis. Seven individual DNA regions, five two-locus combinations and the combined dataset were evaluated in accordance with CBOL recommendations [25, 31] concerning potential barcode loci.
To illustrate differences between the examined specimens, neighbor joining trees were computed for individual and combined DNA regions. Separate analyses were performed for ITS1 and ITS2. Neighbor joining trees were generated based on the Kimura 2-parameter model [32] to enable comparison with other studies on DNA barcoding. Next, phylogenetic trees were generated by maximum parsimony (MP), maximum likelihood (ML) and Bayesian methods. The NJ, MP and ML analyses were carried out in MEGA 6.06, Bayesian inference in MrBayes 3.2 [33]. For both maximum likelihood and Bayesian analyses, the best model of evolution for the combined dataset (GTR+G+I) was determined using maximum likelihood model testing and the Bayesian Information Criterion (BIC) in MEGA 6.06, with four categories used for modeling the discrete gamma distribution.
Maximum parsimony analyses were performed with the following tree inference options: Tree-Bisection-Reconnection (TBR) as a search method with 10 initial trees (random-addition), search level 3, and the maximum number of 100 trees retained in each step. The confidence of clades within the inferred trees was evaluated by the bootstrap method with 1000 replicates.
Bayesian analysis was run on the combined dataset for four million generations with four simultaneous Markov chains. Model parameters and trees were sampled every 1000 generation. The first 25% of trees were discarded as burn-in. Bayesian posterior probability (BPP) confidence values generated from tree saved after this initial burn-in were used for estimatimation of clade support. Values ≥0.95% were regarded as significant.
The genetic distances for the pairs of sequences between and within the studied species were calculated using K2P and uncorrected p-distances to estimate evolutionary divergence and evaluate the effectiveness of the examined barcode loci. The mean, median, 90th percentile and 95th percentile were calculated for each tested locus for intra- and interspecific distances. The significance of differences between intraspecific and interspecific K2P distances was determined in the Mann–Whitney U test. The distribution of intraspecific and interspecific K2P distances for each examined locus was presented graphically to determine the presence of barcoding gaps and assess the effectiveness of barcode loci [10, 31, 34]. The presence of a classical barcoding gap was also checked by calculating the difference between the interspecific mean and the intraspecific mean and by verifying the 10-fold rule proposed by Hebert et al. [35]. Aneura mirabilis was represented by one sample and was excluded from barcoding gap analysis.
The Automatic Barcode Gap Discovery (ABGD) software was used to split the examined specimens of A. pinguis into candidate cryptic species based on pairwise distances by detecting differences between the intraspecific and interspecific variation (i.e. barcoding gap) without a priori species hypothesis. The method automatically find the distance where the barcode gap is located and can be used even when the two distributions (intraspecific and interspecific) overlap to partition the data set into candidate species [36]. ABGD analyses were performed on a web interface (http://www.abi.snv.jussieu.fr/public/abgd/abgdweb.html) with the use of all available distance metrics: JC69 [37], K2P and the uncorrected p-distance. Default values of P (Pmin = 0.001, Pmax = 0.1) and relative gap width X = 1.5 were used, with the exception of rpoC1 where relative gap width was X = 1.2.
Haplotype networks with the MJ option (median joining; [38]) were calculated to examine variation and the relationships between the studied species. The MP option [39] was applied to identify redundant median vectors and links. Haplotype networks were developed in Network 5.0 (Fluxus Technology). The geographic location of each specimen carrying a given haplotype was coded to illustrate its distribution range. The pairwise homoplasy test (PHI) implemented in Splits-Tree 4 [40] was applied to detect possible recombination events in nrITS sequences between cryptic species of A. pinguis.
Results
Sequencing success and the characteristics of sequences
In all examined samples, high-quality DNA sequences were obtained for matK, trnL-trnF, ITS1 and ITS2. Regions rbcL, rpoC1 and trnH-psbA were amplified with 100% efficiency, but high-quality sequences were obtained only in 85.6%, 53.4% and 74.6% of the analyzed samples, respectively. Sequences of satisfactory quality were used in alignment analysis. A total of 3569 bp were aligned in the examined chloroplast regions in genus Aneura, including 509 variable sites and 460 parsimony informative sites. The nuclear ITS1-5.8S-ITS2 region was composed of 753 bp, including 207 variable sites and 195 parsimony informative sites. The lengths of the analyzed DNA sequences with variable and parsimony informative sites for the examined plastid loci and separately for nuclear loci ITS1 and ITS2 are given in Table 3. The most parsimony informative loci were ITS1 (31.15%) and ITS2 (30.50%), followed by plastid loci trnL-trnF (14.92%), matK (14.32%) and trnH-psbA (14.13%), whereas rbcL was the least parsimony informative locus (8.27%).
Table 3. The length of examined DNA regions in the studied species of Aneura.
| rbcL | matK | rpoC1 | trnL-F | trnH-pabA | ITS1 | ITS2 | ITS | |
|---|---|---|---|---|---|---|---|---|
| A. pinguis | ||||||||
| A1 | 617 | 817 | 765 | 543 | 821 | 345–346 | 254 | 743 |
| A2 | 617 | 817 | 765 | 540 | 821 | 346 | 254 | 743 |
| A3 | 617 | 817 | 765 | 543 | 817 | 346 | 254 | 742–743 |
| B1 | 617 | 817 | 765 | 545 | 794 | 349–350 | 249 | 741–742 |
| B2 | 617 | 817 | 765 | 545 | 794 | 349 | 249 | 741 |
| B3 | 617 | 817 | 765 | 545 | 796–798 | 349 | 249 | 741 |
| C1 | 617 | 817 | 765 | 543 | 803 | 348 | 249 | 740 |
| C2 | 617 | 817 | 765 | 543 | 796 | 347 | 249 | 739 |
| D | 617 | 817 | 765 | 539 | 794 | 345 | 255 | 743 |
| E1 | 617 | 817 | 765 | 543 | 801 | 345 | 254 | 742 |
| E2 | 617 | 817 | 765 | 543 | 801 | 345 | 254 | 742 |
| F | 617 | 817 | 765 | 543 | 802 | 348 | 249 | 740 |
| G | 617 | 817 | 765 | 543 | 794 | 341 | 254 | 738 |
| H | 617 | 817 | 765 | 543 | 793 | 348 | 257 | 748 |
| I | 617 | 817 | 765 | 543 | 805 | 347 | 255 | 745 |
| J | 617 | 817 | 765 | 543 | 790 | 345 | 256 | 744 |
| A. maxima | 617 | 817 | 765 | 543 | 799 | 347 | 254 | 744 |
| A. mirabilis | 616 | 817 | 765 | 552 | 817 | 347 | 254 | 744 |
| Alignment length | 617 | 817 | 765 | 555 | 828 | 351 | 259 | 753 |
| Conserved sites | 556 | 687 | 663 | 457 | 691 | 235 | 174 | 546 |
| Variable sites (V) | 61 | 130 | 102 | 89 | 137 | 115 | 85 | 207 |
| Parsi-info sites (P) | 51 | 117 | 87 | 82 | 117 | 110 | 79 | 195 |
| % Parsi-info | 8.27 | 14.32 | 11.37 | 14.77 | 14.13 | 31.15 | 30.50 | 25.90% |
| Singleton sites (S) | 10 | 13 | 15 | 7 | 20 | 5 | 6 | 12 |
DNA barcode variation in A. pinguis
Nucleotide diversity in the analyzed DNA regions of A. pinguis was determined at 2.15% to 10.32% in the K2P model. The nuclear region ITS1 was most variable. Nuclear regions ITS1 and ITS2 were more variable than chloroplast genome sequences, and average variation reached 9.49% and 3.52%, respectively. The most diverse chloroplast locus was matK, and the least diverse locus was rbcL (Table 4). The variations in the corresponding DNA regions of A. maxima were 80-fold smaller on average than in A. pinguis, and were determined at 0% in rpoC1 and trnH-psbA to 0.20% in ITS2. The average variation in A. maxima was 0.07%, and it reached 0.09% in barcode locus rbcL and 0.04% in matK (Table 4). Uncorrected p-distances were somewhat lower than K2P in all analyzed DNA regions.
Table 4. Genetic differentiation (%) in the examined DNA regions of Aneura pinguis and A. maxima based on K2P model of nucleotide substitution.
| Species | matK | rbcL | rpoC1 | trnL-trnF | trnH-psbA | combined cp | ITS1 | ITS2 | ITS | combined data set |
|---|---|---|---|---|---|---|---|---|---|---|
| A. pinguis | 4.24 | 2.15 | 3.46 | 3.94 | 3.82 | 3.55 | 10.32 | 8.66 | 7.72 | 4.23 |
| A. maxima | 0.04 | 0.09 | 0.00 | 0.08 | 0.00 | 0.04 | 0.08 | 0.20 | 0.11 | 0.06 |
Identification of cryptic species within A. pinguis
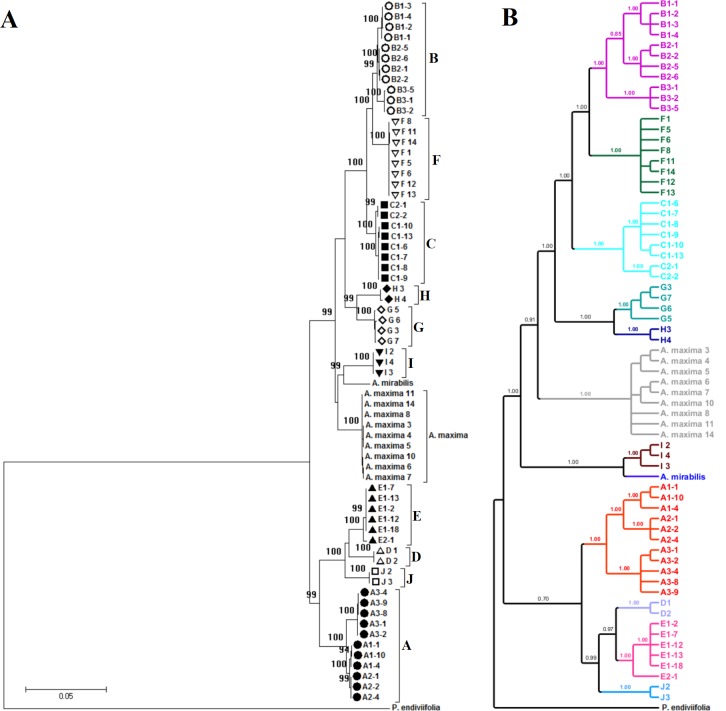
The cryptic species of the A. pinguis complex were identified in phylogenetic analyses in the first stage of the study. The analyses conducted with the use of NJ and MP methods revealed stable topology and the complex structure of A. pinguis. Maximum parsimony analyses of combined plastid loci and the nuclear ITS locus produced trees with identical topology to NJ trees. The two datasets could be combined due to the absence of differences in the topology of plastid and ITS trees. The ML analysis of the combined dataset resulted in a single optimal topology (-ln = 15136.9224) and revealed two major clades differentiated the analyzed Aneura species and resolved A. pinguis as a paraphyletic species. The same topologies were obtained from Bayesian interference of phylogeny, maximum parimony and neighbor joining analyses (Fig 1 and S1 Fig). The first major clade contained four clades of A. pinguis, and the second clade consisted of six clades of A. pinguis as well as clades of A. maxima and A. mirabilis. All of the examined samples of A. pinguis were clustered into 10 clades (marked A to J) with high bootstrap values (BS 99–100%, BPP>0.95) (Fig 1). Three of the tested loci (matK, rbcL, trnH-psbA) and two-gene combination cluster of A. pinguis into the same 10 clades with BS>80%. The remaining loci did not correctly distinguish species B (BS support <50%) but divided it into two or three clades with high BS value (S2 Fig).
Fig 1.
Phylogeny of Aneura pinguis cryptic species obtained by: Maximum likelihood (A) and Bayesian (B) methods based on a combined dataset. Aneura maxima and A. mirabilis were used for comparison. Pellia endiviifolia was used as an outgroup. Only the accessions with the sequences obtained for all loci were included in the analysis. The maximum likelihood tree with highest log likelihood (-15136.92) and Bootstrap values above 85% is shown. Bayesian posterior probabilities > 0.95redibility are given above the branches.
In the K2P model, genetic divergence between the 10 cryptic species of A. pinguis ranged from 1.45% to 7.41% for the combined dataset. The lowest genetic divergence (1.45%) was found for species pairs B-C and B-F. The highest genetic distances were observed in species pairs D-F, F-J and E-F at 7.41%, 7.38% and 7.26%, respectively (Table 5). Of the two loci considered as core barcodes for plants (rbcL and matK), greater differences between the examined cryptic species occur in the matK region, but both regions support discrimination between all cryptic species. In matK, genetic difference was highest in species pair F-J (8.30%) and lowest in pair G-H (1.24%). In rbcL, genetic difference was highest in pair F-E (4.29%) and lowest in B-C (0.78%). In combined plastid loci, genetic divergence ranged from 1.22% to 6.38%, whereas in the ITS–from 1.58% to 12.97%. In nuclear regions, genetic differences between cryptic species ranged from 1.77% to 17.97% in ITS1 (lowest for the species pair B-F and highest for E-F) and from 1.20% to 15.43% in ITS2 (lowest for the species pair C-F and highest for E-H) (S3 Table). Uncorrected p-distances between the examined cryptic species were somewhat lower than K2P. Statistically significant evidence for recombination between clades in the nrITS region was not found in the PHI test (p = 0.3275).
Table 5. Average genetic divergences (%) for Aneura pinguis (A-J) cryptic species, A. maxima and A. mirabilis, based on the combined data set K2P (below diagonal) and uncorrected p-distance (above diagonal).
| A | B | C | D | E | F | G | H | I | J | A. maxima | A. mirabilis | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | *** | 5.72 | 5.54 | 4.55 | 4.34 | 6.06 | 5.05 | 5.76 | 5.13 | 4.42 | 4.98 | 5.26 |
| B | 6.00 | *** | 1.43 | 6.49 | 6.31 | 1.43 | 3.53 | 3.56 | 4.21 | 6.60 | 3.59 | 4.13 |
| C | 5.81 | 1.45 | *** | 6.39 | 6.13 | 1.90 | 3.48 | 3.69 | 4.00 | 6.44 | 3.28 | 4.01 |
| D | 4.72 | 6.84 | 6.74 | *** | 2.57 | 7.00 | 5.84 | 6.22 | 5.68 | 3.17 | 5.39 | 5.91 |
| E | 4.50 | 6.64 | 6.44 | 2.62 | *** | 6.87 | 5.48 | 5.85 | 5.47 | 2.55 | 5.01 | 5.50 |
| F | 6.37 | 1.45 | 1.93 | 7.41 | 7.26 | *** | 3.96 | 4.15 | 4.62 | 6.97 | 3.98 | 4.62 |
| G | 5.27 | 3.63 | 3.58 | 6.13 | 5.73 | 4.09 | *** | 2.43 | 3.69 | 5.71 | 3.36 | 3.93 |
| H | 6.05 | 3.66 | 3.80 | 6.54 | 6.13 | 4.29 | 2.47 | *** | 4.16 | 6.26 | 3.62 | 4.10 |
| I | 5.35 | 4.35 | 4.13 | 5.94 | 5.72 | 4.79 | 3.79 | 4.30 | *** | 5.77 | 3.35 | 3.17 |
| J | 4.59 | 6.97 | 6.79 | 3.25 | 2.60 | 7.38 | 5.98 | 6.59 | 6.05 | *** | 5.57 | 5.80 |
| A. maxima | 5.19 | 3.70 | 3.37 | 5.64 | 5.22 | 4.11 | 3.45 | 3.73 | 3.44 | 5.84 | *** | 3.04 |
| A. mirabilis | 5.50 | 4.27 | 4.14 | 6.21 | 5.75 | 4.80 | 4.06 | 4.23 | 3.25 | 6.09 | 3.11 | *** |
Differentiation within cryptic species
All examined DNA regions within the cryptic species of A. pinguis showed intraspecific variation. The highest intraspecific variation was detected in species A and B. Sequence diversity in species A was 0.543% in the combined dataset (0.375% in plastid and 1.362% in nrITS sequences) (Table 6). In individual loci, sequence diversity ranged from 0.106% to 1.817%, and it was lowest in matK and highest in ITS1. Sequence diversity in cryptic species B was 0.482% (0.397% in plastid and 0.886 in nrITS sequences).
Table 6. K2P (%) genetic variation in the DNA sequences of studied groups of Aneura pinguis.
| combined cp loci | ITS | combined data set | |||||||
|---|---|---|---|---|---|---|---|---|---|
| cryptic species | groups | cryptic species | groups | cryptic species | groups | ||||
| 0.375 | A1 | 0.019 | 1.362 | A1 | 0.124 | 0.543 | A1 | 0.031 | |
| A | A2 | 0.000 | A2 | 0.000 | A2 | 0.000 | |||
| A3 | 0.028 | A3 | 0.087 | A3 | 0.024 | ||||
| 0.397 | B1 | 0.000 | 0.886 | B1 | 0.191 | 0.482 | B1 | 0.047 | |
| B | B2 | 0.000 | B2 | 0.091 | B2 | 0.016 | |||
| B3 | 0.019 | B3 | 0.000 | B3 | 0.016 | ||||
| C | 0.110 | C1 | 0.000 | 0.225 | C1 | 0.062 | 0.141 | C1 | 0.013 |
| C2 | 0.000 | C2 | 0.000 | C2 | 0.000 | ||||
| D | 0.000 | 0.000 | 0.000 | ||||||
| E | 0.104 | E1 | 0.000 | 0.135 | E1 | 0.064 | 0.137 | E1 | 0.016 |
| E | n/c | E2 | n/c | E2 | n/c | ||||
| F | 0.025 | 0.056 | 0.024 | ||||||
| G | 0.076 | 0.165 | 0.098 | ||||||
| H | 0.257 | 0.000 | 0.212 | ||||||
| I | 0.019 | 0.000 | 0.016 | ||||||
| J | 0.000 | 0.182 | 0.024 | ||||||
In cryptic species A and B, three well-supported (BSP 95–100%) monophyletic lineages were identified in the MP tree (Fig 1). These lineages could not be classified as separate species based on the differences in their DNA sequences, and they were regarded as different groups of cryptic species. The groups of cryptic species A were labeled A1, A2, A3, and the groups of cryptic species B–B1, B2, B3.
Two well-supported evolutionary lineages were also identified in cryptic species C and E which were labeled C1, C2 and E1, E2, respectively (Fig 1). Genetic distances based on the combined dataset ranged from 0.188% to 0.944% between the lineages of cryptic species A, from 0.377 to 0.942% between the lineages of cryptic species B, and between the lineages of species C and E were 0.314% and 0.377%, respectively. In the nuclear ITS region, the distances were greater and ranged from 0.205% to 2.625% between the lineages of cryptic species A, from 0.948% to 1.573% between the lineages of cryptic species B, and 0.622% 0.641 between the lineages of species C and E, respectively (S4 Table).
Haplotype network
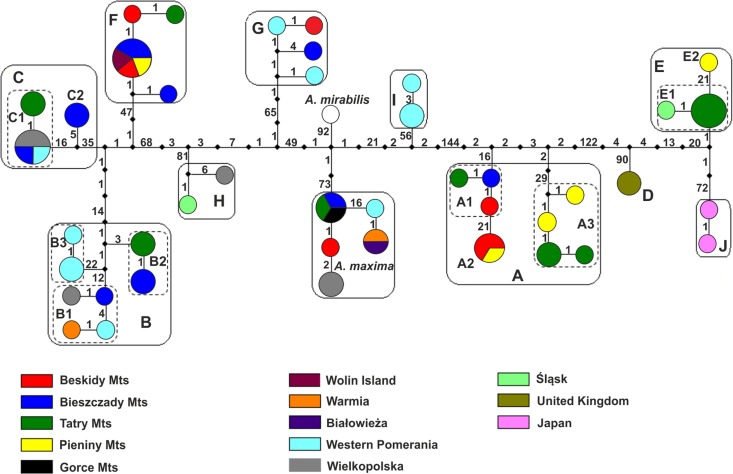
Thirty-seven haplotypes of A. pinguis were identified in the combined dataset (Table 2, Fig 2). The number of haplotypes ranged from 17 to 22 in individual chloroplast loci, and it was determined at 24 in ITS2 and 29 in ITS1. Based on the combined dataset, haplotypes were divided into 10 separate clades (A-J) corresponding to the cryptic species identified within A. pinguis using the phylogenetic tree. Haplotypes of A. maxima and A. mirabilis formed two separate clades. Individual cryptic species of A. pinguis harbored one to eight different haplotypes. The highest number of haplotypes was noted in species A and B which can be divided into three groups corresponding to lineages A1, A2, A3, and B1, B2, B3, separated by 10–52 mutation steps. Two haplotype groups separated by 21 and 15 mutation steps, respectively, were also found in cryptic species C and E (Fig 2).
Fig 2. A haplotype network of the studied Aneura samples based on the combined dataset.
Colored circles represent haplotypes. Colors represent the geographic origin of the specimens. Diameters denote the number of specimens carrying a particular haplotype, the smallest circle represents a single individual, and the largest circle represents five individuals. Black squares represent median vectors and figures–the number of mutation steps.
Intraspecific and interspecific distances and the barcoding gap
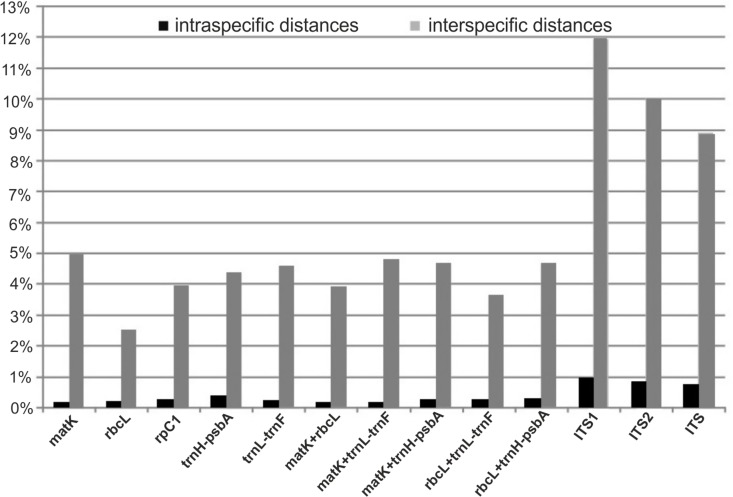
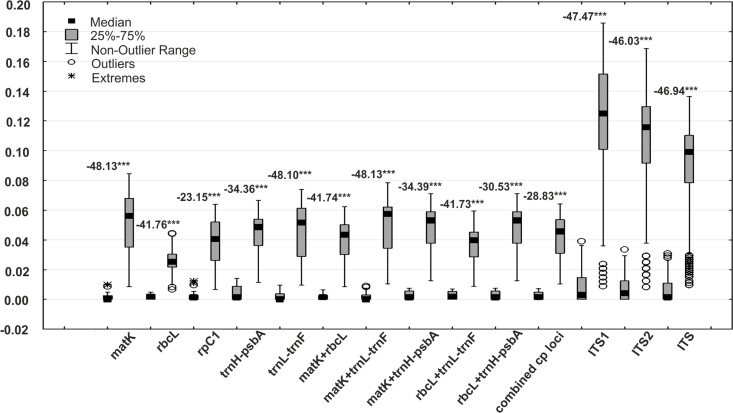
Intraspecific and interspecific variation in the analyzed loci was calculated for the set of the cryptic species which were identified within A. pinguis based on the NJ tree. The greatest mean interspecific distances were found for nuclear loci (ITS1 = 11.94%, ITS2 = 10.02%), and the smallest distance (2.51%) was determined in the rbcL barcode locus (Fig 3). In plastid loci, the greatest (4.96%) mean interspecific variation was found in the matK barcode locus, and it was the highest difference in the analyzed plastid regions (Table 7). Uncorrected p-distances were somewhat lower than K2P in all of the analyzed DNA regions. The Mann–Whitney test revealed significant differences between the mean values of intraspecific and interspecific distances for each examined DNA region (Fig 4). The ranges of intraspecific and interspecific distances, means and medians for the tested loci and their combinations are given in Table 7.
Fig 3. Mean intraspecific and interspecific K2P distances of individual loci and their combinations in Aneura pinguis.
Table 7. Parameters of intra- and interspecific variation of Aneura pinguis based on K2P (%) model of nucleotide substitution.
| DNA region | N | Mean | Mean | Median | Min | Max | Overlap1 | Percentile | Percentile | Overla2 | Percentile | Percentile | Overla3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| inter- | ||||||||||||||
| /mean intraspecific | 10% | 90% | 5% | 95% | ||||||||||
| intra | 792 | 0.14 | 0.05 | 0.00 | 0.99 | 0.00 | 0.25 | 0.00 | 0.86 | |||||
| matK | inter | 4564 | 4.96 | 35× | 5.60 | 0.86 | 8.45 | 0.13 | 1.98 | 6.95 | 0 | 1.61 | 7.21 | 0 |
| intra | 576 | 0.18 | 0.16 | 0.00 | 0.49 | 0.00 | 0.33 | 0.00 | 0.49 | |||||
| rbcL | inter | 3165 | 2.51 | 14× | 2.52 | 0.66 | 4.44 | 0 | 1.16 | 3.37 | 0 | 0.82 | 3.90 | 0 |
| intra | 148 | 0.25 | 0.14 | 0.00 | 1.23 | 0.00 | 1.08 | 0.00 | 1.09 | |||||
| rpoC1 | inter | 1077 | 3.92 | 16× | 4.04 | 0.68 | 6.39 | 0.55 | 1.49 | 5.89 | 0 | 1.09 | 6.09 | 0 |
| intra | 390 | 0.38 | 0.13 | 0.00 | 1.40 | 0.00 | 1.02 | 0.00 | 1.15 | |||||
| trnH-psbA | inter | 2536 | 4.35 | 11× | 4.85 | 1.14 | 6.66 | 0.26 | 2.31 | 5.60 | 0 | 1.66 | 5.79 | 0 |
| intra | 792 | 0.21 | 0.08 | 0.00 | 0.95 | 0.00 | 0.76 | 0.00 | 0.76 | |||||
| trnL-trnF | inter | 4564 | 4.58 | 22× | 5.16 | 0.95 | 7.39 | 0 | 2.30 | 6.35 | 0 | 1.34 | 7.16 | 0 |
| intra | 576 | 0.16 | 0.14 | 0.00 | 0.63 | 0.00 | 0.28 | 0.00 | 0.63 | |||||
| matK+rbcL | inter | 3165 | 3.91 | 24× | 4.34 | 0.85 | 6.24 | 0 | 1.77 | 5.17 | 0 | 1.41 | 6.33 | 0 |
| intra | 792 | 0.16 | 0.07 | 0.00 | 0.90 | 0.00 | 0.37 | 0.00 | 0.75 | |||||
| matK+trnL-trnF | inter | 4564 | 4.81 | 30× | 5.74 | 1.05 | 7.85 | 0 | 1.96 | 6.62 | 0 | 1.58 | 7.11 | 0 |
| intra | 390 | 0.25 | 0.12 | 0.00 | 0.75 | 0.00 | 0.62 | 0.00 | 0.69 | |||||
| matK+trnH-psbA | inter | 2536 | 4.68 | 18× | 5.30 | 1.25 | 7.11 | 0 | 2.35 | 6.17 | 0 | 1.70 | 6.62 | 0 |
| intra | 576 | 0.24 | 0.17 | 0.00 | 0.70 | 0.00 | 0.61 | 0.00 | 0.61 | |||||
| rbcL+trnL-trnF | inter | 3165 | 3.63 | 15× | 3.97 | 0.88 | 5.95 | 0 | 1.86 | 4.90 | 0 | 1.23 | 5.18 | 0 |
| intra | 390 | 0.25 | 0.12 | 0.00 | 0.75 | 0.00 | 0.62 | 0.00 | 0.69 | |||||
| rbcL+trnH-psbA | inter | 2536 | 4.68 | 12× | 5.30 | 1.25 | 7.11 | 0 | 2.02 | 6.34 | 0 | 1.70 | 6.62 | 0 |
| intra | 338 | 0.24 | 0.14 | 0.00 | 0.71 | 0.00 | 0.69 | 0.00 | 0.69 | |||||
| combined cp loci | inter | 1542 | 4.14 | 17× | 4.56 | 1.03 | 6.40 | 0 | 1.81 | 5.60 | 0 | 1.38 | 6.15 | 0 |
| intra | 792 | 0.94 | 0.29 | 0.00 | 3.91 | 0.00 | 3.29 | 0.00 | 3.60 | |||||
| ITS1 | inter | 4564 | 11.94 | 13× | 12.50 | 0.88 | 18.59 | 3.03 | 4.58 | 16.58 | 0 | 3.91 | 17.72 | 0 |
| intra | 792 | 0.80 | 0.41 | 0.00 | 3.35 | 0.00 | 2.49 | 0.00 | 2.50 | |||||
| ITS2 | inter | 4564 | 10.02 | 12× | 11.57 | 0.82 | 16.85 | 2.53 | 2.49 | 13.94 | 0 | 1.23 | 14.62 | 0.0127 |
| intra | 792 | 0.74 | 0.14 | 0.00 | 3.08 | 0.00 | 2.51 | 0.00 | 2.65 | |||||
| ITS | inter | 4564 | 8.92 | 12× | 9.91 | 0.96 | 13.64 | 2.12 | 2.80 | 12.11 | 0 | 2.37 | 12.79 | 0.0028 |
| intra | 338 | 0.31 | 0.09 | 0.00 | 1.04 | 0.00 | 0.97 | 0.00 | 0.99 | |||||
| combined data set | inter | 1542 | 4.78 | 15.4× | 5.12 | 1.28 | 7.45 | 0 | 1.96 | 6.60 | 0 | 1.47 | 6.88 | 0 |
Note: Overlap1 = Maximum of intraspecific—Minimum of interspecific distances; Overlap2 = 90% of intraspecific—10% of interspecific distances; Overlap3 = 95% of intraspecific—5% of interspecific distances.
Fig 4. The ranges of intraspecific and interspecific K2P distances in Aneura pinguis.
The ranges of intraspecific (the first box) and interspecific (the second box) distances for individual studied DNA regions and their combinations with the results of the Mann-Whitney test were compared.
A barcoding gap was detected in rbcL, trnL-trnF, in all two-gene combinations and in all combined chloroplast loci, which supported 100% discrimination of individuals. In matK, rpoC1 and trnH-psbA, certain overlaps were noted in the ranges of intraspecific and interspecific distances (Fig 4, S3 Fig). A clear barcoding gap was not determined in ITS1, ITS2 or in the entire ITS. However, mean interspecific distances were 11- to 35-fold higher than mean intraspecific distances. The greatest differences between intraspecific and interspecific means were noted in matK (35-fold) and trnL-trnF (22-fold), and the smallest differences were observed in trnH-psbA (11-fold) and ITS2 (12-fold). Median values (the preferred statistics for non-normal distribution) were even higher, and up to 112-fold differences were noted in matK (Table 7). For all loci, the overlap between the largest intraspecific distance and smallest interspecific distance did not occur at the 90th intraspecific percentile and the 10th interspecific percentile, and, with the exception of ITS2 and entire ITS, even at the 95 and 5th percentile.
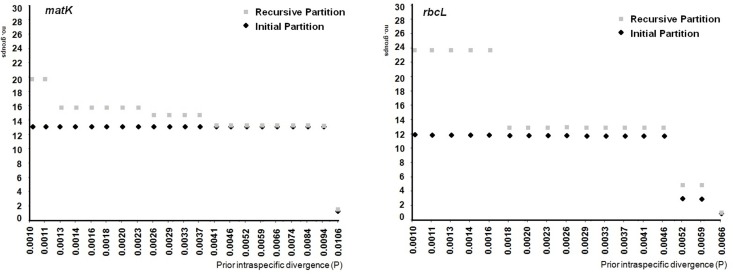
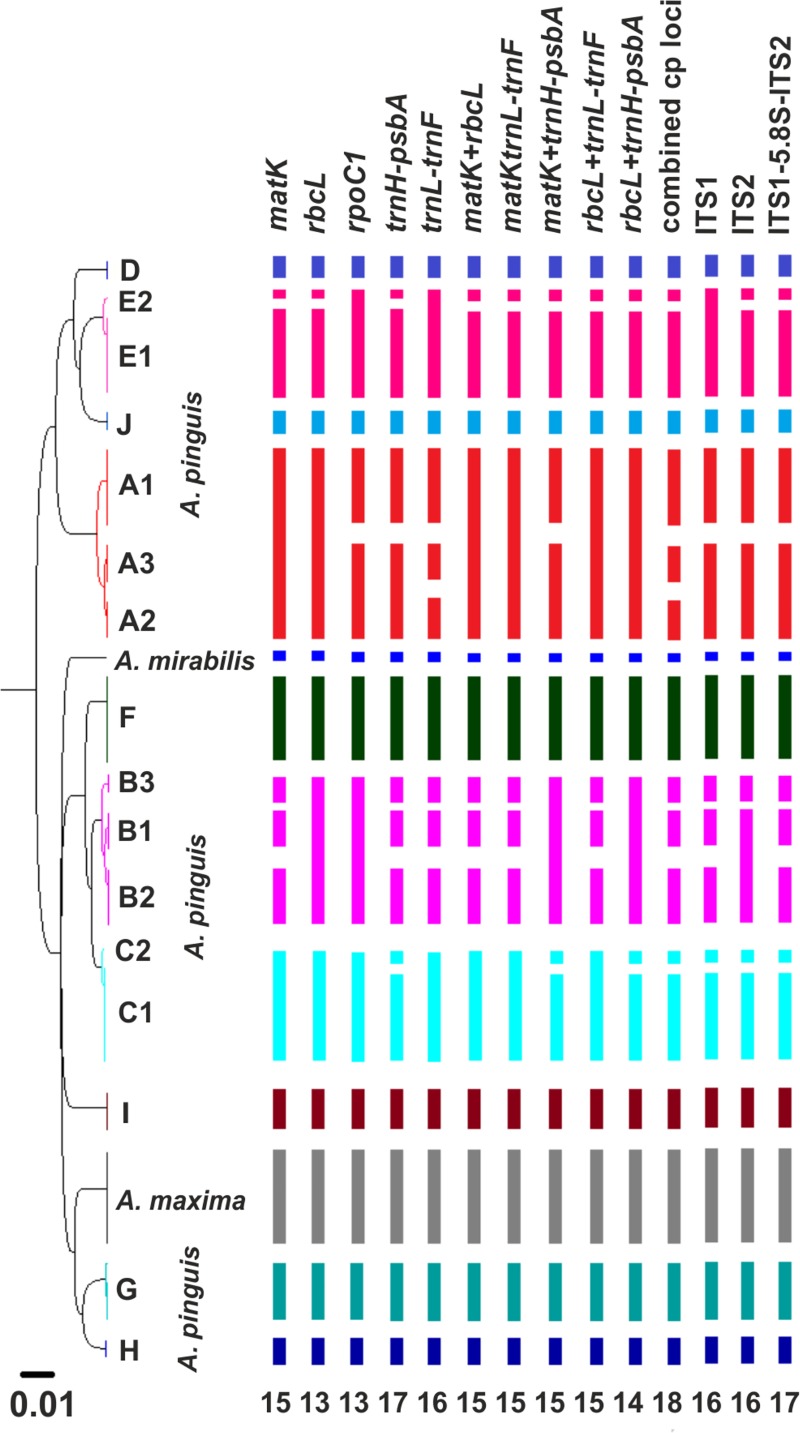
In the ABGD analysis, six to 13 groups were identified within A. pinguis as initial partitions, depending on the locus. In the K2P model, the rbcL locus, two 2-gene combinations (matK + trnH-psbA, rbcL + trnH-psbA) and combined plastid loci produced one initial partition that contained always the same 10 groups of A. pinguis (plus one group of A. maxima and one of A. mirabilis) with intraspecific values in the range of 0.46% to 0.94% (Fig 5). The groups formed by the ABGD method were congruent with the groups created on the basis of phylogenetic trees, and they corresponded to the detected cryptic species A-J (Figs 1 and 6). All A. pinguis samples were assigned to the same group that was created on the basis of phylogenetic trees. The matK locus and the rbcL + matK combination produced 11 groups of A. pinguis corresponding to cryptic species A-J, and species B were split into two groups. The highest number of groups (13) was produced by trnH-psbA which split species A, B and C into two groups. The trnL-trnF locus, matK + trnL-trnF and rbcL + trnL-trnF combinations, and both nuclear regions (ITS1, ITS2) produced nine groups as the initial partition with P values of 0.59–2.15%. The combinations of trnL-trnF, matK + trnL-trnF and rbcL + trnL-trnF did not separate cryptic species B and C, whereas ITS1 and ITS2 did not distinguish species B and F. In the JC69 model, the results of the ABGD analysis were highly similar to those in the K2P model, except for rpoC1. In the K2P model, the rpoC1 locus as the initial partition produced only 6 groups with P values of up to 1.06%, and it did not recognize species pairs B-F, E-J and G-H, whereas in the JC69 model, the rpoC1 locus produced nine groups (P = 0.74%) and did not differentiate the species pair B-C. In all tested loci, A. maxima and A. mirabilis formed separate groups in the initial partition. In the ABGD analysis, data are first divided into groups as the initial partition based on a statistically inferred barcode gap, and the same procedure is then applied to the groups obtained in the first step to form a recursive partition. In all studied loci, recursive partitions resulted in 11 (rbcL and rpoC1) to 15 (trnH-psbA) groups of A. pinguis which split cryptic species A, B, C and E into three or two groups (Fig 6). However, only combined plastid loci distinguished between all groups in cryptic species A, B, C and E with P values from 0.17%. When the uncorrected p-distance was used, the ABGD analysis produced identical groups, but the P value of prior intraspecific differences was lower than that in K2P and JC69 models.
Fig 5. Automatic partition of the studied samples of Aneura spp. based on matK and rbcL loci.
The number of groups, including A. maxima and A. mirabilis, resulted in initial and recursive partition at each given prior intraspecific divergence value were reported.
Fig 6. Ultrametric tree obtained by UPGMA analysis of the studied Aneura species generated from the combined dataset.
The cryptic species of A. pinguis and the results of the ABGD analysis for the examined individual loci and their combinations were marked in different colors. The numbers below the diagram represent the number of groups detected as recursive partitions in ABGD.
Distribution of A. pinguis cryptic species
A comparison of the sequences obtained from the studied samples (Table 1) with GenBank sequences points to a wider distribution of individual cryptic species of A. pinguis in the world (S1 Fig). Plants belonging to cryptic species A occur also in the UK (A3), Portugal (A2 haplotypes with one and two substitutions) and New Zealand. Plants corresponding to cryptic species B were noted in the USA (B1), Costa Rica (B2), UK and Germany (B3). Cryptic species C and E were observed in Canada and Germany. Haplotypes identical to species F were found in the UK and USA (haplotypes with two and three substitutions in the USA). To date, cryptic species G, H and I have been found exclusively in Poland. Moreover, GenBank sequences harbored new haplotypes which formed separate clades, not identified in the samples examined in the present study. These haplotypes were found in North America (USA), Central America (Dominican Republic), South America (Ecuador), Asian Russia and Japan.
The cryptic species of A. pinguis clearly differ across various habitats (Table 2). The lineages of species A (A1, A2, A3) grows mainly on humus developed on limestone rocks, lineages of species B (B1, B2, B3) and F occur mainly on clay soils. The lineages C1 and C2 occupies mostly wet sandy soils, including on the shores of oligotrophic lakes, river and mountain stream banks and the lineages E1 and E2 thrives on calcareous rocks in flowing water. Species G, H and I are found in peat bogs.
Discussion
Identification of cryptic species of A. pinguis by DNA barcoding
DNA barcoding revealed that the nominally cosmopolitan A. pinguis was composed of 10 cryptic species, five of which had been previously described (signet A to E) [9, 41] and five were completely new (F to J). Furthermore, intraspecific differentiation was observed within four cryptic species A, B, C, and E. We identified 3 subgroups in cryptic species A and B (A1, A2, A3 and B1, B2, B3 respectively), and two subgroups in cryptic species C and E (C1, C2 and E1, E2, respectively). A total of 16 lineages in different evolutionary stages were distinguished within A. pinguis. In our study, groups A1, B1, C1and E1 corresponded to the previously described cryptic species A, B, C and E, respectively. Greater differentiation within A. pinguis can be explained by the fact that the analyzed material originated from a larger geographic area, and that the barcoding method delivers more accurate results than isozyme electrophoresis. Each of the tested loci in phylogenetic trees and network clusters show that the cryptic species of A. pinguis and A. maxima and A. mirabilis are a monophyletic clades (Figs 1 and 2; S1 and S2 Figs).
This study confirms the high potential of DNA barcoding for resolving taxonomic problems, and it demonstrates that DNA barcoding is a useful tool that complements the classical taxonomy of liverworts. We tested the core plant barcode (rbcL + matK) and five additional loci, including promising complementary barcodes (trnH-psbA, ITS and ITS2) in the cryptic species of A. pinguis and A. maxima. We also compared the sequences of the studied species with A. mirabilis sequences from GenBank [42]. The amplification efficiency of all sequences was 100%. High quality DNA was obtained for all (matK, trnL-trnF, ITS1, ITS2) or nearly all (rbcL, rpoC1 and trnH-psbA) of the examined samples. All tested loci had 100% discriminant power to distinguish the studied species, they fulfilled the criteria of barcode DNA. None of the tested DNA regions alone had the power to detect all lineages. The combination of the trnL-trnF locus (the only locus that identified lineage A2) with trnH-psbA or ITS2 (loci that split species C and E) detected all lineages. This result was supported by the outcome of the ABGD analysis which automatically finds the distance where the barcode gap is located and splits the sequence alignment dataset into candidate species [36]. The units identified by ABGD correspond to the cryptic species and lineages of A. pinguis resolved by the NJ tree and to A. maxima and A. mirabilis. Among the examined loci, trnH-psbA, trnL-trnF, matK and both ITS regions were characterized by the highest species resolution in the ABGD analysis, whereas rbcL and rpoC1 were least effective. The ABGD analysis also revealed that trnL-trnF, which was tested with universal primers and produced high amplification and sequencing success, is also a promising candidate barcode for Aneura species. The trnH-psbA, trnL-trnF and ITS loci, together or combined with other sequences, are frequently used to resolve taxonomic problems (including cryptic species) in closely related liverworts [8, 26, 34, 43–45], and they are potentially the best DNA barcodes for this group of plants.
Genetic differentiation of A. pinguis
Interspecies divergence ranged from 1.220% to 6.377% in combined cpDNA sequences, from 1.558% to 12.973% in ITS, and from 1.45% to 7.41% in the combined dataset (Table 5). Notably, most divergence exceeded the 3% threshold typically encountered between congeneric species pairs recognized by morphological features [46]. Recently divergence of 3% or 2% is proposed in different taxa as a threshold between species [6]. However the use of arbitrary distance thresholds in taxonomy has been debated. In some cases arbitrary distance thresholds can to suffer from varying rates of false-positive and false-negative error, depending on the data [47]. For example in close relatives species the distance thresholds are often smaller than Hebert’s proposal–they can be less than 1% [48,49]. In our study, divergence was below 3%, but higher than 1.22% in only six out of 45 pairs of cryptic species, whereas more than half of the distances in pairwise comparisons were higher than 5%. Moreover, the average divergence among the cryptic species of A. pinguis exceeded intraspecific divergences 15-fold (Table 7). Hebert et al. [35] proposed the 10-fold rule as the standard sequence threshold, where the mean of interspecific distances should be more than 10-fold higher than the mean of intraspecific distances for the examined group. Our results point to clear genetic differences between the cryptic species of A. pinguis.
Phylogenetic analyses (Fig 1, S1 and S2 Figs) of the combined dataset consistently revealed that all cryptic species of A. pinguis as well as A. maxima and A. mirabilis (two taxonomically recognized species of Aneura genus) formed separate clades and that A. maxima and A. mirabilis were nested between different cryptic species of A. pinguis. These results correspond with previous molecular findings which demonstrated that A. pinguis is a paraphyletic taxon [17, 50–52]. In our study, the phylogenetic tree of Aneura was divided into two distinct clades. The first clade contained 6 cryptic species of A. pinguis (B, F, C, H, G and I) as well as A. maxima and A. mirabilis, whereas the second clade contained four cryptic species (A, D, E, J) of A. pinguis. The above suggests that the cryptic species of A. pinguis are not directly derived from one common ancestor and that their evolutionary history is more complex. Moreover, these two distinct evolutionary lines of A. pinguis had diverged before A. maxima and A. mirabilis were split. The division of A. pinguis into two major clades confirmed the results of the network analysis (Fig 2), where the two groups of cryptic species were separated from each other by at least 164 mutation steps. The analysis of K2P distances confirmed this thesis. In all analyzed DNA regions, the distances between most pairs of cryptic species of A. pinguis were greater than between A. maxima and A. mirabilis (Table 5). Wickett & Goffinet [50] postulated that A. pinguis, A. maxima and A. mirabilis could be regarded as a species complex. Indeed, this group appears to have a more complex taxonomy because A. pinguis is a complex of cryptic species and, as indicated by other authors [50,51], A. maxima is not a homogeneous taxon either.
Geographic distribution, habitat preferences and morphological diversity
A comparison of the obtained sequences (rbcL, trnL-trnF and ITS) with A. pinguis sequences from GenBank indicates that in addition to the identified haplotypes, the analyzed sequences harbored other haplotypes which could suggest the presence of additional cryptic species of A. pinguis (S2 Fig). In this study, the distribution of A. pinguis was analyzed only within a limited range, therefore other cryptic species of A. pinguis could exist. New haplotypes forming separate clades were found in the USA, Dominican Republic, Ecuador, Asian Russia and Japan. To date, five (A, D, G, H, I) cryptic species have been found exclusively in Europe, of which three have been identified only in Poland (G, H and I). Species B, which grows in Europe, North, Central and South America, was the most sampled (most sequences were found in GenBank) and widespread species.
A. pinguis species differ not only in their geographic distribution, but also in habitat preferences. Minor differences between subgroups within cryptic species were found (Table 2). The cryptic species growing in peat bogs (G, H and I) were most highly correlated with habitat type. The lineage C1 was most tolerant and occupy the most different substrata. In our opinion, diversification within A. pinguis is clearly linked to individual species ecology, and it is indicative of stabilizing selection in different habitats. Moreover, the haplotypes in the ITS region indicate that cryptic species form reproductively isolated populations, even if they are largely sympatric, such as species A, B and C. A lack of recombinants in the cryptic species of A. pinguis also revealed a previous enzymatic study [9].
Similarly to earlier studies of cryptic species A, B and C [19], we struggled to find morphological features that would identify the remaining cryptic species of A. pinguis. Unfortunately, a biometric analysis of thalli in A. pinguis cryptic species did not reveal significant qualitative morphological differences between these cryptic species. We were only able to identify minor phenotypic diversity in morphology, especially in the size of the thallus. For example, species A, B and C were larger, whereas species E, H, G, I were rather smaller. The range of variation in thallus size is high, with partial overlap between the species. Therefore, this feature cannot be the basis for the identification of A. pinguis species, and it can only be used as a supportive characteristics. This observation is consistent with the findings of Schuster [23, 53] who stated that morphological varieties within A. pinguis are “virtually inseparable”. However some sporophyte characteristics, such as: seta anatomy, capsule wall structure and thickening pattern, spores, spore wall anatomy, elater features and spermatid architecture are less variable then gametophyte characters and therefore more valuable as taxonomic markers [54–56]. Thus, sporophyte features may be helpful for delimitation at the species level within the A. pinguis species complex.
The DNA barcode of A. pinguis reveals new cryptic species. They are impossible to distinguish using morphological methods alone. Bryophytes such as A. pinguis are structurally simple plants with a limited number of morphological traits, and they frequently include morphologically indistinguishable entities. From the point of view of traditional taxonomy, cryptic species cannot be classified as classical taxonomic species because they do not have unique morphological traits that correspond to genetic differentiation; however, they conform to the species concept due to a lack of recombination [4]. The accelerated rate of cryptic species detection in DNA sequencing suggests that molecular data should be incorporated into alpha taxonomy whenever possible. Integrative taxonomy which relies on collaborative and mutually beneficial integrative applications of molecular biology, such as DNA barcoding, comparative morphology and descriptive taxonomy, is recommended for describing species [7, 57–58]. According to some authors, ecological preferences and geographical distribution should also be taken into account in the newly detected molecular species [1, 36, 59, 60]. Most of the distinguished cryptic species of A. pinguis differed in their habitat preferences and geographical distribution, which appears to be an important consideration and provides additional evidence for the presence of a new biological species in the genus Aneura.
Supporting information
*Samples from herbarium collection, a-f references for sequences from GenBank, N–number of sequences obtained in present studies.
(DOC)
(DOC)
(DOC)
(DOC)
(PDF)
Neighbor joining (A) and maximum parsimony (B) consensus trees of Aneura. pinguis cryptic species based on a combined dataset. Aneura maxima and A. mirabilis were used for comparison. Pellia endiviifolia was used as an outgroup. Only the accessions with the sequences obtained for all loci were included in the analysis. Bootstrap values above 85% are indicated above branches.
(PDF)
(PDF)
Acknowledgments
The authors would like to thank Patrycja Gonera, Mariola Rabska and Daria Ratajczak for assistance in laboratory analyses. We are grateful to the management of Tatra, Białowieża, Pieniny, Bieszczady and Wolin National Parks for their support during field work, and the Curators of NYBG, UBC, CHR, C and S herbaria for providing A. pinguis specimens. This work was done by Molecular Taxonomy and Genetics team.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Polish National Science Centre, grants no. 2011/01/B/NZ8/00364 (AB, KB, JS) and 2013/09/B/NZ8/03274 (AB, KB). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ahmadzadeh F, Flecks M, Carretero MA, Mozaffari O, Böhme W, Harris DJ, et al. Cryptic speciation patterns in Iranian rock lizards. Uncovered by integrative taxonomy. PLoS ONE. 2013; 8:e80563 doi: 10.1371/journal.pone.0080563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayden RL. A hierarchy of species concepts: the denouement in the saga of the species problem. In: Claridge MF, Dawah HA, Wilson MR, editors. The units of biodiversity. London: Chapman and Hall; 1997. pp. 381–424. [Google Scholar]
- 3.Vanderpoorten A, Shaw AJ. The application of molecular data to the phylogenetic delimitation of species in bryophytes: A note of caution. Phytotaxa. 2010; 9:229–237. [Google Scholar]
- 4.Mayr E. Systematics and the origin of species 1st ed. New York: Columbia University Press. [Google Scholar]
- 5.Wiens JJ. Species delimitations: New approaches for discovering diversity. Syst Biol 2007; 56(6):875–878. doi: 10.1080/10635150701748506 [DOI] [PubMed] [Google Scholar]
- 6.Hebert PDN, Penton EH, Burns JM, Janzen DH, Hallwachs W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc Natl Acad Sci USA. 2004; 101(41):14812–7. doi: 10.1073/pnas.0406166101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinrichs J, Kreier HP, Feldberg K, Schmidt AR, Zhu R-L, Shaw B, et al. Formalizing morphologically cryptic biological entities: new insights from DNA taxonomy, hybridization, and biogeography in the leafy liverwort Porella platyphylla (Jungermanniopsida, Porellales). Am J Bot. 2011; 98(8):1252–1262. doi: 10.3732/ajb.1100115 [DOI] [PubMed] [Google Scholar]
- 8.Heinrichs J, Hentschel J, Bombosch A, Fiebig A, Reise J, Edelmann M, et al. One species or at least eight? Delimitation and distribution of Frullania tamarisci (L.) Dumort. s.l. (Jungermanniopsida, Poreales) inferred from nuclear and chloroplast DNA markers. Mol Phylogenet Evol. 2010; 56(3):1105–14. doi: 10.1016/j.ympev.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 9.Bączkiewicz A, Buczkowska K. Differentiation and genetic variability of three cryptic species within the Aneura pinguis complex (Jungermanniidae, Marchantiophyta). Cryptogam Bryol. 2016; 37:1–16. https://doi.org/10.1515/biorc-2016-0001. [Google Scholar]
- 10.Kress WJ, Erikson DL. A two-locus global DNA barcode for land plants: the coding rbcL gene complements that non-coding trnH-psbA spacer region. PLoS ONE. 2007; 6:e508 https://doi.org/10.1371/journal.pone.0000508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramaiya M, Johnson M, Shaw B, Heinrichs J, Hentschel J, von Konrat M, et al. Morphologically cryptic biological species within the liverwort, Frullania asagrayana. Am J Bot. 2010; 97:1707–1718. doi: 10.3732/ajb.1000171 [DOI] [PubMed] [Google Scholar]
- 12.Feldberg K, Váňa J, Long DG, Shaw J, Hentschel J, Heinrichs J. A phylogeny of Adelanthaceae (Jungermanniales, Marchantiophyta) based on nuclear and chloroplast DNA markers, with comments on classification, cryptic speciation and biogeography. Mol Phylogenet Evol. 2010;55(1):293–304. doi: 10.1016/j.ympev.2009.11.009 [DOI] [PubMed] [Google Scholar]
- 13.Buczkowska K, Sawicki J, Szczecińska M, Klama H, Bączkiewicz A. Allopolyploid speciation of Calypogeia sphagnicola (Jungermanniopsida, Calypogeiaceae) based on isozyme and DNA markers. Pl Syst Evol. 2012; 298:549–560. doi: 10.1007/s00606-011-0565-5 [Google Scholar]
- 14.Damsholt K. Illustrated Flora of Nordic Liverworts and Hornworts. 1st ed. Lund: Nordic Bryological Society, 2002, pp.654–656. [Google Scholar]
- 15.Szweykowski J. An annotated checklist of Polish liverworts and hornworts In: Mirek Z, editor. Biodiversity of Poland. Vol. 4 Cracow: W. Szafer Institute of Botany, Polish Academy of Sciences; 2006. pp. 13. [Google Scholar]
- 16.Bączkiewicz A, Buczkowska K. Genetic variability of the Aneura pinguis complex (Hepaticae) in central and western Europe. Biol Lett. 2005; 42:61–72. [Google Scholar]
- 17.Wachowiak W, Bączkiewicz A, Chudzińska E, Buczkowska K. Cryptic speciation in liverworts–a Aneura pinguis complex. Bot J Linn Soc. 2007; 155:273–282. [Google Scholar]
- 18.Bączkiewicz A, Sawicki J, Buczkowska K, Polok K, Zieliński R. Aplication of different DNA markers in studies on cryptic species of Aneura pinguis (Jungermanniopsida, Metzgeriales). Cryptogam Bryol. 2008; 29(1):3–21. [Google Scholar]
- 19.Buczkowska K, Adamczak M, Bączkiewicz A. Morphological and anatomical differentiation within the Aneura pinguis complex (Metzgeriales, Hepaticae). Biol Lett. 2006; 43:51–68. [Google Scholar]
- 20.Buczkowska K, Chudzińska E, Bączkiewicz A. Differentiation of oil body characters in the Aneura pinguis complex (Hepaticae) in Poland In: Prus-Głowacki W, Pawlaczyk E, editors. Variability and Evolution. Poznań: Adam Mickiewicz University; 2005. pp. 97–106. [Google Scholar]
- 21.Wawrzyniak R, Wasiak W, Bączkiewicz A, Buczkowska K. Volatile compounds in cryptic species of the Aneura pinguis complex and Aneura maxima (Marchantiophyta, Metzgeriidae). Phytochemistry. 2014; 105:115–122. doi: 10.1016/j.phytochem.2014.06.010 [DOI] [PubMed] [Google Scholar]
- 22.Bączkiewicz A, Gonera P, Buczkowska K. Geographic distribution and new localities for cryptic species of the Aneura pinguis complex and A. maxima in Poland. Biodiv. Res. Conserv. 2016;41:1–10. https://doi.org/10.1515/biorc-2016-0001 [Google Scholar]
- 23.Schuster RM. The Hepaticae and Anthocerotae of North America. East of the Hundredth Meridian, vol. 5 Chicago: Field Museum of Natural History; 1992. [Google Scholar]
- 24.Buczkowska K, Bączkiewicz A. Aneura maxima–a liverwort new to Poland. Cryptogam Bryol. 2006; 27:1–6. [Google Scholar]
- 25.CBOL Plant Working Group, Hollingsworth PM, Forrest LL et al. A DNA barcode for land plants. Proc Natl Acad Sci USA. 2009; 106:12794–12797. doi: 10.1073/pnas.0905845106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bell D, Long DG, Forrest AD, Hollingsworth ML, Blom HH, Hollingsworth PM. DNA barcoding of European Herbertus (Marchantiopsida, Herbertaceae) and the discovery and description of a new species. Mol Ecol Resour. 2012; 12:36–47. doi: 10.1111/j.1755-0998.2011.03053.x [DOI] [PubMed] [Google Scholar]
- 27.Taberlet P, Gielly L, Pautou G, Bouvet J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol Biol. 1991; 17:1105–1109. doi: 10.1007/BF00037152 [DOI] [PubMed] [Google Scholar]
- 28.Krawczyk K, Szczecińska M, Sawicki J. Evaluation of 11 single-locus and seven multilocus DNA barcodes in Lamium L. (Lamiaceae). Mol Ecol Resour. 2013; 14(2):272–285. doi: 10.1111/1755-0998.12175 [DOI] [PubMed] [Google Scholar]
- 29.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013; 30(12):2725–2729. doi: 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004; 32(5):1792–1797. doi: 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Yan HF, Cao T, Ge XJ. Evaluation of 10 plant barcodes in Bryophyta (Mosses). J Syst Evol. 2010; 48:36–46. doi: 10.1111/j.1759-6831.2009.00063.x [Google Scholar]
- 32.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980; 16(2):111–120. doi: 10.1007/BF01731581 [DOI] [PubMed] [Google Scholar]
- 33.Huelsenbeck JP, Ronquist FR. MRBAYES: Bayesian inference of phylogeny. Bioinformatics 2001; 17: 754–755. [DOI] [PubMed] [Google Scholar]
- 34.Hollingsworth ML, Clark A, Forrest LL, Richardson J, Penningtpn RT, Long DG, et al. Selecting barcoding loci for plants: evaluation of seven candidate loci with species-level sampling in three divergent groups of land plants. Mol Ecol Resour. 2009; 9(2):439–457. doi: 10.1111/j.1755-0998.2008.02439.x [DOI] [PubMed] [Google Scholar]
- 35.Hebert PDN, Stoeckle MY, Zemlak TS, Francis CM. Identification of birds through DNA barcodes. PLoS Biology. 2004; 2:e312 https://doi.org/10.1371/journal.pbio.0020312 doi: 10.1371/journal.pbio.0020312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puillandre N, Lambert A, Brouillet S, Achaz G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol Ecol. 2012; 21(8):1864–1877. doi: 10.1111/j.1365-294X.2011.05239.x [DOI] [PubMed] [Google Scholar]
- 37.Jukes TH, Cantor CR. Evolution of protein molecules In: Munro NH, editor. Mammalian Protein Metabolism. New York: Academic Press; 1969. pp. 21–132. [Google Scholar]
- 38.Bandelt H-J, Forster P, Röhl A. Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol. 1999; 16: 37–48 [DOI] [PubMed] [Google Scholar]
- 39.Polzin T, Daneschmand SV. On Steiner trees and minimum spanning trees in hypergraphs. Operations Res Lett., 2003; 31:12–20. [Google Scholar]
- 40.Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol. 2006; 23(2):254–267. https://doi.org/10.1093/molbev/msj030 [DOI] [PubMed] [Google Scholar]
- 41.Buczkowska K, Rabska M, Gonera P Pawlaczyk EM, Wawrzyniak P, Czołpińska M, et al. Effectiveness of ISSR markers for determination of the Aneura pinguis cryptic species and Aneura maxima. Biochem Syst Ecol. 2016; 68:27–35. doi: 10.1016/j.bse.2016.06.013 [Google Scholar]
- 42.Wickett NJ, Zhang Y, Hansen SK, Roper JM, Kuehl JV, Plock SA, et al. Functional Gene Losses Occur with Minimal Size Reduction in the Plastid Genome of the Parasitic Liverwort Aneura mirabilis. J Mol Evol. 2008; 25(2):393–401. doi: 10.1093/molbev/msm267 [DOI] [PubMed] [Google Scholar]
- 43.Heinrichs J, Klugmann F, Hentschel J, Schneider H. DNA taxonomy, cryptic speciation and diversification of the Neotropical-African liverworts, Marchesinia brachiata (Lejeuneaceae, Porellales). Mol Phylogenet Evol. 2009; 53(1):113–121. https://doi.org/10.1016/j.ympev.2009.05.032 [DOI] [PubMed] [Google Scholar]
- 44.Hassel K, Segreto R, Ekrem T. Restricted variation in plant barcoding markers limits identification in closely related bryophyte species. Mol Ecol Resour. 2013; 13 (6):1047–1057. doi: 10.1111/1755-0998.12074 [DOI] [PubMed] [Google Scholar]
- 45.Stech M, Veldman S, Larraín J, Muñoz J, Quandt D, Hassel K, et al. Molecular Species Delimitation in the Racomitrium canescens Complex (Grimmiaceae) and Implications for DNA Barcoding of Species Complexes in Mosses. PLoS ON 2013; 8(1):e53134 https://doi.org/10.1371/journal.pone.0053134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith MA, Fisher BL, Hebert PDN. DNA barcoding for effective biodiversity assessment of a hyperdiverse arthropod group: the ants of Madagascar. Philos Trans R Soc Lond B Biol Sci. 2005; 360(1462):1825–1834. doi: 10.1098/rstb.2005.1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collins RA, Cruickshank RH. The seven deadly sins of DNA barcoding. Mol. Mol Ecol Resour. 2013; 13:969–975. doi: 10.1111/1755-0998.12046 [DOI] [PubMed] [Google Scholar]
- 48.Stech M, Veldman S, Larraín J, Muñoz J, Quandt D, Hassel K, et al. Molecular Species Delimitation in the Racomitrium canescens Complex (Grimmiaceae) and Implications for DNA Barcoding of Species Complexes in Mosses. PLoS ONE 2013; 8(1): e53134 doi: 10.1371/journal.pone.0053134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lang AS, Kruijer JD, Stech M. DNA barcoding of Arctic bryophytes: an example from the moss genus Dicranum (Dicranceace, Bryophyta). Polar Biol. 2014; 37:1157–1169. doi: 10.1007/s00300-014-1509-7 [Google Scholar]
- 50.Wickett NJ, Goffinet B. Origin and relationships of the myco-heterotrophic liverwort Cryptothallus mirabilis Malmb.(Metzgeriales, Marchantiophyta). Bot J Linn Soc. 2008; 156(1):1–12. doi: 10.1111/j.1095-8339.2007.00743.x [Google Scholar]
- 51.Wickett NJ, Fan Y, Lewis PO, Goffinet B. Distribution and Evolution of Pseudogenes, Gene Losses, and a Gene Rearrangement in the Plastid Genome of the Nonphotosynthetic Liverwort, Aneura mirabilis (Metzgeriales, Jungermanniopsida). J Mol Evol. 2008; 67:111–122. doi: 10.1007/s00239-008-9133-1 [DOI] [PubMed] [Google Scholar]
- 52.Preussing M, Olsson S, Schäfer-Verwimp A, Wickett NJ, Wicke S, Quandt D, et al. New insights in the evolution of the liverwort family Aneuraceae (Metzgeriales, Marchantiophyta), with on the genus Lobatiriccardia. Taxon. 2010; 59(5):1424–1440. [Google Scholar]
- 53.Schuster RM. Studies on Metzgeriales. I. North American Aneuraceae. J Hatt Bot Lab.1987;62:299–329. [Google Scholar]
- 54.Schuster RM. 1966. The Hepaticae and Anthocerotae of North America. Vol.1 Columbia University Press, New York and London [Google Scholar]
- 55.Furuki T, Long DG. Aneura crateriformis, a new liverwort species from the East Himalaya and China.
- 56.Singh D, Singh DK. Notes on sporophytic details of Aneura crateriformis (Aneuraceae, Marchantiophyta). J. Bryology 2016: 351–353. [Google Scholar]
- 57.Will KW, Mishler BD, Wheeler QD. The perils of DNA barcoding and the need for integrative taxonomy. Syst Biol. 2005; 54(5):844–851. doi: 10.1080/10635150500354878 [DOI] [PubMed] [Google Scholar]
- 58.Heinrichs J, Feldberg K, Bechteler J, Scheben A, Czumaj A, Pócs T, et al. Integrative taxonomy of Lepidolejeunea (Jungermanniopsida:Porellales): Ocelli allow the recognition of two neglected species. Taxon. 2015; 64(2):216–228. [Google Scholar]
- 59.Medina R, Lara F, Goffinet B, Garilleti R, Mazimpaka V. Integrative taxonomy successfully resolves the pseudo-cryptic complex of the disjunct epiphytic moss Orthotrichum consimile s.l. (Orthotrichaceae). Taxon. 2012; 61:1180–1198. [Google Scholar]
- 60.DeSalle R, Egan MG, Siddall M. The unholy trinity: taxonomy, species delimitation and DNA barcoding. Philos Trans R Soc Lond B Biol Sci. 2005; 360(1462):1905–1916. doi: 10.1098/rstb.2005.1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
*Samples from herbarium collection, a-f references for sequences from GenBank, N–number of sequences obtained in present studies.
(DOC)
(DOC)
(DOC)
(DOC)
(PDF)
Neighbor joining (A) and maximum parsimony (B) consensus trees of Aneura. pinguis cryptic species based on a combined dataset. Aneura maxima and A. mirabilis were used for comparison. Pellia endiviifolia was used as an outgroup. Only the accessions with the sequences obtained for all loci were included in the analysis. Bootstrap values above 85% are indicated above branches.
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.