Abstract
Background
The incidence of pyogenic vertebral osteomyelitis (PVO) has increased over the past two decades. One possible cause of this increase is the aging of the population, which results in more comorbidities in high income countries.
Objective
To better characterize the clinical presentation and outcome of PVO in the elderly.
Design
We conducted a post-hoc analysis of a previously published trial that studied treatment duration in PVO and compared the presentation and outcomes according to age.
Participants
Our analysis included 351 patients among whom 85 (24%) were 75-years-old or more.
Results
There were no significant differences in the socio-demographics of the patients. Neoplasia and chronic inflammatory diseases were more common in the older group: 34% vs. 19% (p = 0.021) and 9% versus 1% (p = 0.004), respectively. There were no significant differences in clinical and radiological presentations between the groups in terms of back pain (337/351, 97%), fever (182/351, 52%), PVO localization, neurological signs and epidural abscess. Associated infective endocarditis (IE) was more frequent in the older group (37% vs. 14%, p<0.001). Streptococci were more frequently involved in infections of older patients (29% vs. 14%, p = 0.003) in contrast to Staphylococcus aureus (31% vs. 45%, p = 0.03). Older patients displayed higher mortality rates at 1 year (21% vs. 3%, p<0.001) and more adverse events related to cardiorespiratory failure (10.6% vs. 3.8%, p = 0.025), but had similar quality of life among the survivors.
Conclusion
During PVO, the clinical and radiological findings are similar in older patients. Global mortality rates are higher in older patients compared to younger patients, which could be explained by the increased frequency of neoplasia at diagnosis and higher prevalence of associated IE in the elderly.
Introduction
Pyogenic vertebral osteomyelitis (PVO) is a rare condition with an estimated annual incidence in France of 2.4/100 000 inhabitants. In patients aged 70 years or more, the incidence reached 6.5/100 000 inhabitants in 2002–2003 [1]. A recent Spanish study that analyzed the trends in bone and joint infections reported an increasing incidence of PVO in older patients (≥ 65 years) compared to younger patients [≤ 49 years over a 26 year-period (1985–2011)] [2]. Diagnosis can be difficult due to the lack of specific clinical signs and variable febrile symptoms. Back pain has been reported in 86% of PVO cases [3], but such a complaint is common and is made by up to 40% of females between 60 and 69 years of age [4]. Studies focusing on PVO in elderly cohorts are scarce and often rely on case series. The impact of age on the outcomes of PVO has differed across many studies.
In an open-label, randomized clinical trial, we demonstrated that 6 weeks of antibiotics were non-inferior to 12 weeks of treatment for patients presenting with PVO [5]. Among the 351 patients included, 85 (24%) were older than 75 years, which as one of the factors associated with treatment failure in the multivariable analysis.
In this case-control ancillary study, we aimed to describe the clinical characteristics, radiological findings, microbiological epidemiology and outcomes of PVO in this specific population of elderly patients.
Patients and methods
This post-hoc analysis was conducted using patient data extracted from the previously published multi-centric randomized controlled trial (RCT) NCT00764114, which investigated the optimal duration of antibiotics for the treatment of PVO [5]. Overall, 359 patients with PVO underwent randomization for 6 or 12 weeks of antibiotics treatment between November 15, 2006, and March 15, 2011, in 71 medical centers in France. To be included, patients had to be adults with typical radiological findings of PVO associated with microbiological documentation, a life expectancy longer than one year, and no device at the site of the infection.
The main objective of this case-control study was to describe demographic and symptom data from a large series of aged patients suffering from PVO and to compare these findings to a younger population. The secondary objective was to determine the link between age and outcome.
Patients included in the intention-to-treat (ITT) analysis of the RCT were therefore divided into two groups according to the age at diagnosis. Given the lack of a consensus definition, elderly patients were defined arbitrarily as patients aged 75 years or more. We used variables recorded in the RCT as previously described.
The comparison between the groups was performed using a univariate analysis that employed the appropriate statistical parameters according to the nature of the variables. Distributions were compared using the Chi-square test or Fisher’s exact test if necessary. Continuous quantitative variables and ordinal variables were tested using the non-parametric method of Kruskal-Wallis.
In the RCT, 160 (90.9%) of 176 patients in the 6-week group and 159 (90.9%) of those in the 12-week group met the criteria for clinical cure. The factors associated with unfavorable outcomes were determinate by a multivariable logistic regression analysis. The explanatory variables included in the model were those that had a degree of significance (p) below 5% in the univariate analysis: age (≥75 years or <75 years), infective endocarditis (IE) (yes or no), Staphylococcus aureus infection (yes or no), positive blood culture (yes or no), treatment with oral fluoroquinolone and rifampicin (yes or no), duration of intravenous antibiotic treatment (<7 days or >7 days), and allocated duration of treatment (6 weeks or 12 weeks). We examined the effects of variable exclusion on the Akaike information criterion (AIC) and chose the model with the smallest AIC. All statistical analyses were performed using R software version 3.1 [6].
The French National Agency for the Safety of Medicines and Health Products, French Data Protection Agency, and ethics committee of the Versailles University Hospital approved the study protocol. The study was performed in accordance with the ethical principles of the Declaration of Helsinki and the guidelines for Good Clinical Practice. Written informed consent for participation in the trial was obtained from all patients.
Results
Among the 351 patients included in the ITT analysis of the RCT, 85 (24%) were 75 years or older (mean age: 80.5 ± 4.2 years) and 266 (76%) were younger than 75 years of age (mean age: 55 ± 14.1 years; Fig 1). The comorbidities of patients in both groups are presented in Table 1. Significantly more patients had neoplasia and chronic inflammatory disease in the ≥75-year-old group. The duration between the onset of symptoms and diagnosis was similar (45.3 ± 43 days vs 49.9 ± 59.1 days, p = 0.929). Back pain and fever at diagnosis were present in 337 patients (96%) and 182 patients (52%), respectively, and did not significantly differ between the two groups. One week after enrollment, fever persisted more often in older patients (13.2% vs. 3%, p = 0.004), and this difference disappeared after further follow-up observations. The presence of neurological symptoms and the localization of the PVO were similar in both age groups. The main features of PVO presentation in our patients are shown in Table 2.
Fig 1. Flow-chart.
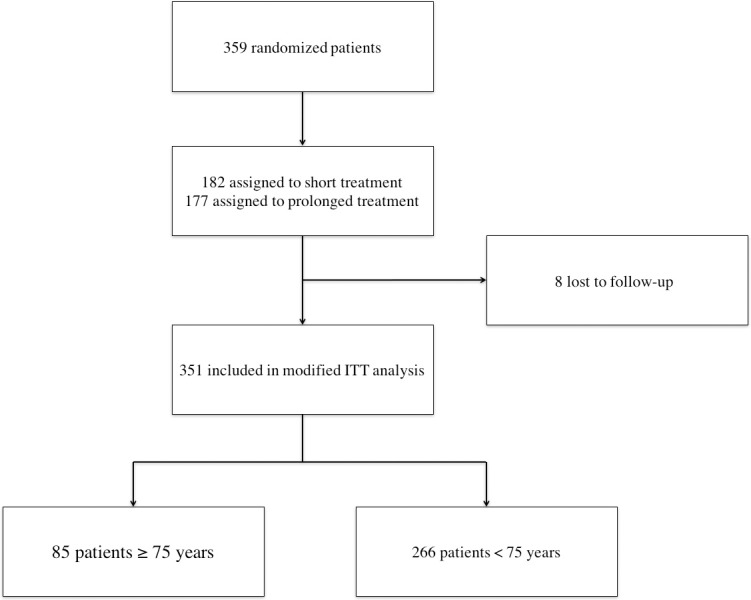
All patients were included in the Duration of treatment for Spondylodiscitis study. Short treatment: 6 weeks of antibiotic treatment. Prolonged treatment: 12 weeks of antibiotic treatment. ITT: intention to treat.
Table 1. Description of the study population.
| ≥ 75 years (n = 85) | < 75 years (n = 266) | p | |
|---|---|---|---|
| Women | 37 (43.5) | 72 (27.1) | 0.007 |
| Malnutrition | 9 (13) | 11 (5.6) | 0.083 |
| Diabetes mellitus | 13 (18.6) | 41 (20) | 0.932 |
| Alcohol abuse | 1 (1.5) | 30 (14.8) | 0.007 |
| Smokers | 11 (16.2) | 108 (48.2) | <0.001 |
| Cirrhosis | 0 (0) | 8 (4.1) | 0.206 |
| Neoplasm | 22 (33.8) | 38 (19) | 0.021 |
| Central venous catheter | 3 (4.4) | 13 (6.8) | 0.769 |
| Renal insufficiency | 1 (1.6) | 4 (2.1) | 1 |
| Respiratory insufficiency | 2 (3.1) | 7 (3.5) | 1 |
| Immunocompromised host | 5 (7.4) | 11 (5.6) | 0.567 |
| Chronic inflammatory disease | 6 (9.1) | 2(1) | 0.004 |
| Randomized in 12-weeks arm | 40 (47.1) | 135 (50.8) | 0.640 |
Numbers in brackets are the percentages of the sub-group populations. Bolded p-values are considered to be significant as they are lower than 0.05.
Table 2. Clinical and radiological presentation of vertebral osteomyelitis.
| ≥ 75 years (n = 85) | < 75 years (n = 266) | p | |
|---|---|---|---|
| Fever | 49 (57.6) | 133 (50.2) | 0.283 |
| Back pain | 80 (94.1) | 257 (96.6) | 0.34 |
| Neurological symptoms | |||
| Radiculopathy | 6 (7.1) | 33 (12.4) | 0.243 |
| Spinal cord compression | 3 (3.5) | 16 (6) | 0.582 |
| PVO localization | |||
| Multiple | 12 (14.1) | 26 (9.8) | 0.357 |
| Cervical | 9 (10.6) | 43 (16.2) | 0.278 |
| Thoracic | 28 (32.9) | 69 (25.6) | 0.984 |
| Lumbar | 59 (69.4) | 187 (70.3) | 0.086 |
| Sacral | 6 (7.1) | 39 (14.7) | 0.101 |
| Epidural Inflammation | 20 (24.7) | 91 (35.8) | 0.567 |
| Infective endocarditis | 25 (36.8) | 26 (13.8) | <0.001 |
Numbers in brackets are the percentages of the sub-group populations. Bolded p-values are considered to be significant as they are lower than 0.05. PVO: pyogenic vertebral osteomyelitis. Localization of PVO describes the spinal level affected; the sum of the values exceeds the number of total cases because of the presence of PVO in multiple localizations.
The diagnosis of IE was assessed by echocardiography in 257 (73.2%) patients (transesophageal echocardiography for 227 patients). Bacteremia was more frequent in the ≥75-year-old group (83.5% vs. 63.5%, p<0.001). In the older group, 25 patients (36.8%) met the Duke criteria for IE compared to 26 (13.8%) in the <75-year-old group (p<0.001).
Microbiological diagnosis of PVO was performed using blood cultures for 240 (68%) patients, CT-guided vertebral biopsy for 138 patients (39%) and perioperative surgical biopsy for 19 patients (5%). While Staphylococcus aureus, regardless of the susceptibility test results, was more frequent in the <75-year-old group (30.6% vs. 44.7%, p = 0.029), methicillin-resistant S. aureus (MRSA) was involved in 8/351 patients (2.3%) and more frequently in the ≥75-year-old group (33.3% vs. 5.6%, p = 0.013). Streptococcus spp. were also more likely to be isolated from older patients (29.4% vs. 14.3%, p = 0.003); the microbiological identification results did not show any significant differences for coagulase-negative Staphylococci (17.6% vs. 17.3% p = 1), Enterococcus spp (12.9% vs. 10.2% p = 0.603) or Enterobacteriaceae (3.5% vs. 3.8% p = 1).
A higher rate of severe adverse events was recorded in the ≥75-year-old group (45.9% vs. 23.3%, p<0.001), particularly with respect to cardiorespiratory failure (10.6% vs. 3.8%, p = 0.025). There were no significant differences in adverse events due to antimicrobials, Clostridium difficile associated diarrhea (1.2% vs. 1.1%, p = 1) or neurological complications (10.6% vs. 18%, p = 0.146) of the PVO. The median hospital length of stay was significantly longer in the elderly cohort, with durations of 23 days (Inter Quartile Range: IQR = 15) and 26 days (IQR = 13) for the younger and older groups (p = 0.02), respectively. Regardless of the duration of antibiotic treatment (6 or 12 weeks), age ≥ 75 years was associated with treatment failure according to a multivariable analysis (OR [95% CI]: 1.08 [1.01–1.16], p = 0.028; please see S1 Table for the other risk factors involved in failure of treatment). Death occurred in 18 (21.2%) patients in the elderly group versus 8 (3%) in the younger group (p<0.001). Regarding quality of life during the follow-up, the EQ-5D score was significantly lower in the ≥75-year-old group after 6 months (0.5 ± 0.3 vs. 0.7 ± 0.4, p = 0.007), but there was no significant differences observed at the one year follow-up.
Discussion
This post-hoc analysis of the DTS study provides interesting results and enables a better understanding of PVO occurring in elderly patients. The main strengths of this study are the sample size and homogeneity of data collected prospectively through the RCT. To our knowledge, this is the largest representative cohort of aged patients with PVO. The description of specific aspects due to advanced age is of the highest importance in these times of an increasing incidence of PVO [2,7,8] and aging population. The main limits of this study are the post-hoc characteristics of the analysis and its primary descriptive angle.
The World Health Organization’s definition of “aged” is someone who is older than 65 years of age, but this is probably not fully applicable to high income countries where aging populations represent a high proportion of residents. Of note, 65 years is also the median age of patients presenting with PVO in France, and therefore, this definition does not represent a discriminant cut-off. The choice of 75 years of age as a cut-off is somewhat arbitrary but is often chosen in daily practice, including for administrative permission for admission into retirement homes. This cut-off was chosen in the multivariable regression logistic model developed in the DTS study to identify the predictive factors of failure. Not surprisingly, the sex ratio was in favor of females in older patients suffering from PVO in the same way as the whole population of this age. Clinical symptoms did not differ significantly from younger patients, especially with respect to fever, which was detected in the same proportion of younger and older patients. However, older patients displayed poorer outcomes with higher mortality rates and more adverse events, which were not explained by a delay in management initiation or an increase in PVO-related severity of neurological complications. Neoplasia and chronic inflammatory diseases were the two conditions in the ≥75-year-old group that significantly differed from younger patients.
The microbiological results and IE diagnosis might offer some interesting hypotheses to explain these discrepancies in outcome. There are contradictory data on the influence of age on PVO outcomes. One series included patients aged from 60 to 84 years who required neurosurgical management with no lethality recorded [9]. Others reported that being under the age of 60 was an independent factor predicting favorable outcome in non-surgical patients [10]. The higher mortality rate recorded in our study for older patients is in accordance with pervious published results. In a Japanese retrospective observational study including 7118 PVO patients, in-hospital mortality increased proportionally with age and was significantly influenced by IE co-infection [11]. In the study of McHenry et al. [12] reporting the long-term outcomes of 253 patients with PVO with a 6.5 year median duration follow-up, the mortality rate was 11%. The authors compared patients under or over 50 years and did not find any differences in terms of outcome. The association with IE was not studied. The cut-off age of 50 years and fact that most of the patients underwent surgical management do not allow for a direct comparison with our study.
Unlike others, we did not find an increase in the proportion of PVO cases that were caused by Gram-negative bacilli in our aged population. It should be noted that the first reports on the abundance of such bacteria were case series or retrospective works [13–15] and, as such, they do not appear to be representative of larger populations.
Patients in the ≥75-year-old group presented more frequently with Streptococcus spp PVO and IE. If the pathophysiological determinants of those results in this specific age group remain unclear, the association between viridans Streptococci species and IE during PVO has already been reported to be significantly stronger than with S. aureus [16]. Two other studies reported an association between PVO caused by Streptococcus spp or Enterococcus spp and the diagnosis of associated IE [17,18]. Similarly, a retrospective series of 92 IE patients identified 14 with PVO. Streptococcus spp were involved in 8 of these cases compared to only one with a S. aureus infection [19]. The increased incidence of Viridans Streptococci and Streptococcus gallolyticus infections during IE in elderly compared to younger patients (<65 years) has already been reported [20]. However, cases of IE caused by Viridans Streptococci are associated with a decrease in in-hospital mortality rates compared to IE caused by other bacteria [21]. Thus, it is likely that the higher incidence of IE contributes to the unfavorable outcomes.
Conclusion
While PVO diagnosis is not easy and requires attentive physicians, it does not seem to be more difficult in the elderly. The initial presentation does not differ significantly in terms of symptoms or severity. The microbiological findings are different, with fewer Staphylococci infections but a higher prevalence of MRSA and Streptococci spp infections in the elderly. The global mortality rate at one year is higher in patients older than 75 years of age and could be explained by a higher frequency of associated IE and higher rate of comorbid conditions, such as neoplasia. In this population, looking for IE should be mandatory, especially when Streptococci spp are involved.
Supporting information
The Akaike Information Criterion (AIC) of the final model was 108.9. OR = Odds ratio. aOR = adjusted Odds ratio. CI = confidence interval.
(DOCX)
Acknowledgments
Collaborators of the DTS (Duration of Treatment for Spondylodiscitis) study group: Carlier R, Salomon J, Masse V, Gros H, Perronne C, Pellen JU, Rouveix E, Hanslik T, Fantin B, Belmatoug N, Meyer O, Leport C, Ballard M, Debord T, Rapp C, Lechevallier D, Mechai F, Salmon D, Tassadit T, Gherissi D, Lejeune C, Perrot S, Fain O, Morrin Z, Arras AS, Orcel P, Greder A, Monnier S, Manceron V, Vinceneux P, Charlier C, Lortholary O, Bourgeois P, Gandjbakhch F, Berenbaum F, Champey J, Pacanowski J, Girard PM, Patey O, Grados F, Bensalem M, Oziol E, Simorre B, Ragnaud JM, Dutronc H, Cazanave C, Abraham B, Regouby Y, Marcelli C, Verdon R, Denis A, Dargere S, Ristori JM, Poujol D, Portier H, Piroth C, Piroth L, Ornetti P, Epaulard O, Stahl JP, Devailly J, Paccou J, Weinbreck P, Doudier B, Brouqui P, Makinson A, Morel J, Combe B, Prazuck T, Niang M, Roblot F, Lemoal G, Brault R, Eschard JP, Direz G, Revest M, Coiffier G, Challes G, Cazorla C, Guglieminotti C, Lucht F, Thomas T, Amouzougan A, Chadapaud X, Marchou B, Meliani P, Massip P, Bonnet E, Bastides F, Besnier JM, Choutet P, Cottier JP, Valat JP, Griffoul I, Bevilacqua S, May T, Koumouvi M, De Bandt M, Lasbleiz S, Boissier MC, Saidenberg N, Permal S, Brazille P, Welker Y, Saraux A, Jousse-Joulin S, Grange C, Piperno M, Ziza JM, Khanine V, Lahalle S, Curlier E, Hoen B, Wyplosz B, Lechiche C, Sotto A, Simo D, Zeller V, Issartel B, Le Moing V, Lesprit P, Bru JP, Bouhour D, Dénes E, Debard A, Chirouze C, Fèvre K, Dupon M, Aegerter P, Mulleman D.
Data Availability
The data are not fully available due to legal restrictions. The signed consent forms state that only the medical team, the persons duly mandated by the research sponsor, and possibly some authorized health authorities are allowed to access the data. Requests for data accession may be sent to: Unité de Recherche Clinique – Dpt de Santé Publique – UMR-S1168, Hôpital Ambroise Paré – UFR Médecine Paris-Ile-de-France-Ouest Université Versailles St-Quentin, 9, avenue Charles de Gaulle, 92100 Boulogne (France), Tel: +33(0)149095668. The consent forms were required and approved by the French Data Protection Agency (CNIL), the institutional review board of Versailles University Hospital (authorization No. 06030) and one ethical committee (comité de protection des personnes Ile de France Paris XI, address: Comité de protection des personnes, Hôpital de Poissy Saint Germain en Laye 20, rue Armagis 78105 Saint-Germain-en-Laye France).
Funding Statement
The DTS trial was funded by French Ministry of Health through a national Hospital Program for Clinical Research to Louis Bernard. The funder of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report.
References
- 1.Grammatico L, Baron S, Rusch E, Lepage B, Surer N, Desenclos JC, et al. Epidemiology of vertebral osteomyelitis (VO) in France: analysis of hospital-discharge data 2002–2003. Epidemiol Infect. 2008;136: 653–660. doi: 10.1017/S0950268807008850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murillo O, Grau I, Lora-Tamayo J, Gomez-Junyent J, Ribera A, Tubau F, et al. The changing epidemiology of bacteraemic osteoarticular infections in the early 21st century. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2015;21: 254.e1–8. doi: 10.1016/j.cmi.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 3.Zimmerli W. Clinical practice. Vertebral osteomyelitis. N Engl J Med. 2010;362: 1022–1029. doi: 10.1056/NEJMcp0910753 [DOI] [PubMed] [Google Scholar]
- 4.Hoy D, Bain C, Williams G, March L, Brooks P, Blyth F, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64: 2028–2037. doi: 10.1002/art.34347 [DOI] [PubMed] [Google Scholar]
- 5.Bernard L, Dinh A, Ghout I, Simo D, Zeller V, Issartel B, et al. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet Lond Engl. 2015;385: 875–882. doi: 10.1016/S0140-6736(14)61233-2 [DOI] [PubMed] [Google Scholar]
- 6.R Foundation for Statistical Computing, Vienna, Austria. R: A language and environment for statistical computing. n.d.
- 7.Grammatico-Guillon L, Baron S, Gettner S, Lecuyer A-I, Gaborit C, Rosset P, et al. Bone and joint infections in hospitalized patients in France, 2008: clinical and economic outcomes. J Hosp Infect. 2012;82: 40–48. doi: 10.1016/j.jhin.2012.04.025 [DOI] [PubMed] [Google Scholar]
- 8.Doutchi M, Seng P, Menard A, Meddeb L, Adetchessi T, Fuentes S, et al. Changing trends in the epidemiology of vertebral osteomyelitis in Marseille, France. New Microbes New Infect. 2015;7: 1–7. doi: 10.1016/j.nmni.2015.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cahill DW, Love LC, Rechtine GR. Pyogenic osteomyelitis of the spine in the elderly. J Neurosurg. 1991;74: 878–886. doi: 10.3171/jns.1991.74.6.0878 [DOI] [PubMed] [Google Scholar]
- 10.Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1997;79: 874–880. [DOI] [PubMed] [Google Scholar]
- 11.Akiyama T, Chikuda H, Yasunaga H, Horiguchi H, Fushimi K, Saita K. Incidence and risk factors for mortality of vertebral osteomyelitis: a retrospective analysis using the Japanese diagnosis procedure combination database. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-002412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McHenry MC, Easley KA, Locker GA. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis Off Publ Infect Dis Soc Am. 2002;34: 1342–1350. doi: 10.1086/340102 [DOI] [PubMed] [Google Scholar]
- 13.Thompson D, Bannister P, Murphy P. Vertebral osteomyelitis in the elderly. Br Med J Clin Res Ed. 1988;296: 1309–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chelsom J, Solberg CO. Vertebral osteomyelitis at a Norwegian university hospital 1987–97: clinical features, laboratory findings and outcome. Scand J Infect Dis. 1998;30: 147–151. [DOI] [PubMed] [Google Scholar]
- 15.Belzunegui J, Intxausti JJ, De Dios JR, Del Val N, Rodríguez Valverde V, González C, et al. Haematogenous vertebral osteomyelitis in the elderly. Clin Rheumatol. 2000;19: 344–347. [DOI] [PubMed] [Google Scholar]
- 16.Murillo O, Roset A, Sobrino B, Lora-Tamayo J, Verdaguer R, Jiménez-Mejias E, et al. Streptococcal vertebral osteomyelitis: multiple faces of the same disease. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2014;20: O33–38. doi: 10.1111/1469-0691.12302 [DOI] [PubMed] [Google Scholar]
- 17.Koslow M, Kuperstein R, Eshed I, Perelman M, Maor E, Sidi Y. The unique clinical features and outcome of infectious endocarditis and vertebral osteomyelitis co-infection. Am J Med. 2014;127: 669.e9–669.e15. doi: 10.1016/j.amjmed.2014.02.023 [DOI] [PubMed] [Google Scholar]
- 18.Mulleman D, Philippe P, Senneville E, Costes C, Fages L, Deprez X, et al. Streptococcal and enterococcal spondylodiscitis (vertebral osteomyelitis). High incidence of infective endocarditis in 50 cases. J Rheumatol. 2006;33: 91–97. [PubMed] [Google Scholar]
- 19.Le Moal G, Roblot F, Paccalin M, Sosner P, Burucoa C, Roblot P, et al. Clinical and laboratory characteristics of infective endocarditis when associated with spondylodiscitis. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2002;21: 671–675. doi: 10.1007/s10096-002-0798-x [DOI] [PubMed] [Google Scholar]
- 20.Durante-Mangoni E, Bradley S, Selton-Suty C, Tripodi M-F, Barsic B, Bouza E, et al. Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med. 2008;168: 2095–2103. doi: 10.1001/archinte.168.19.2095 [DOI] [PubMed] [Google Scholar]
- 21.Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VG, Bayer AS, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169: 463–473. doi: 10.1001/archinternmed.2008.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Akaike Information Criterion (AIC) of the final model was 108.9. OR = Odds ratio. aOR = adjusted Odds ratio. CI = confidence interval.
(DOCX)
Data Availability Statement
The data are not fully available due to legal restrictions. The signed consent forms state that only the medical team, the persons duly mandated by the research sponsor, and possibly some authorized health authorities are allowed to access the data. Requests for data accession may be sent to: Unité de Recherche Clinique – Dpt de Santé Publique – UMR-S1168, Hôpital Ambroise Paré – UFR Médecine Paris-Ile-de-France-Ouest Université Versailles St-Quentin, 9, avenue Charles de Gaulle, 92100 Boulogne (France), Tel: +33(0)149095668. The consent forms were required and approved by the French Data Protection Agency (CNIL), the institutional review board of Versailles University Hospital (authorization No. 06030) and one ethical committee (comité de protection des personnes Ile de France Paris XI, address: Comité de protection des personnes, Hôpital de Poissy Saint Germain en Laye 20, rue Armagis 78105 Saint-Germain-en-Laye France).


