Abstract
Iron(III) salts promote the condensation of aldehydes or acetals with electron-rich phenols to generate ortho-quinone methides that undergo Diels—Alder condensations with alkenes. The reaction sequence occurs in a single vessel to afford benzopyrans in up to 95% yield. The reaction was discovered while investigating a two-component strategy using 2-(hydroxy(phenyl)methyl)phenols to access the desired ortho-quinone methide in a Diels—Alder condensation. The two-component condensation also afforded the corresponding benzopyran products in yields up to 97%. Taken together, the two- and three-component strategies using ortho-quinone methide intermediates provide efficient access to benzopyrans in good yields and selectivities.
Graphical Abstract
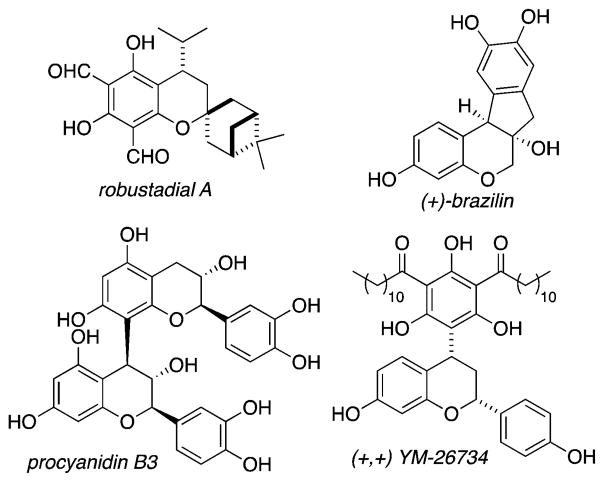
ortho-Quinone Methides (oQMs) are reactive synthetic building blocks useful for the synthesis of natural products and pharmaceutical compounds (Figure 1).1 They are short-lived intermediates, and their reactivity is understood by two canonical forms: a biradical species or a polarized zwitterion.2 These resonance structures highlight the susceptibility of oQMs to undergo nucleophilic attack at the methide carbon, with rearomatization as the thermodynamic driving force.3 In addition, oQMs react with electron-rich dienophiles in inverse-electron demand hetero-Diels—Alder reactions.4 We reported a Diels—Alder reaction of oQMs and dienophiles promoted by Fe salts.5 We postulated that we would be able to develop a multicomponent approach using oQMs as reactive intermediates in Diels—Alder reactions using phenols and aldehydes as oQM precursors. Herein we report a one-pot, multicomponent reaction (MCR) strategy, enabling convergent approaches in the synthesis of benzopyrans using oQMs as reactive intermediates.6
Figure 1.
Representative natural products and pharmaceuticals bearing substituted benzopyrans.
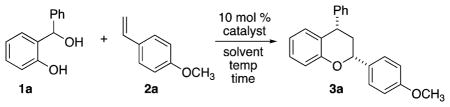
Condensation reactions between hydroxybenzyl alcohols and olefins affording benzopyrans have featured specific dienophile reaction partners catalyzed by chiral phosphoric acid (PA) catalysts.7 Schneider reported the chiral PA-catalyzed reactions with electron-rich enamines and diones,8 and Rueping described the asymmetric synthesis of chromans in the reaction of hydroxybenzyl alcohols with diones and styrene derivatives.9 Similarly, Shi examined reactions with vinyl indoles and enamides catalyzed by chiral PA catalysts.10 These two-component approaches are advantageous when the o-hydroxybenzyl alcohols are easily prepared and react well in the cycloaddition reaction. However, we wished to determine if metals could promote the reaction of hydroxybenzyl alcohols with olefins and investigated the multicomponent strategy to access oQMs in situ.
We initially designed experiments to evaluate the reactivity of o-hydroxybenzyl alcohols and p-methoxy styrene with Lewis acids (Table 1). Magnesium chloride led to a trace amount of product after 1 h (Table 1, entry 1), and Pd(TFA)2 (entry 2) resulted in a 22% yield. The reaction promoted by FeCl3·6H2O (Table 1, entry 3) afforded a 36% yield of the desired product in a 6:1 dr. Anhydrous FeCl3 afforded a 57% yield and 4:1 dr (Table 1, entry 4) after 1 h in CH2Cl2. The addition of molecular sieves slowed the reaction; FeCl3 with 4 Å MS (200 mg) in CH2Cl2 gave a 28% yield and 1:1 dr (Table 1, entry 5). Dry HCl was generated from TMSCl and MeOH and promoted the reaction in 26% yield and 4:1 dr (Table 1, entry 6). Anhydrous FeCl3 proved the most efficient at mediating the reaction, and after evaluating additional solvents, CHCl3 afforded a higher yield than CH2Cl2 (82% yield, entry 7).
Table 1.
Catalyzed Hetero-Diels–Alder of o-Quinone Methidesa
| |||||
|---|---|---|---|---|---|
| entry | catalyst | solvent | time (h) | yield (%)b | drc |
| 1 | MgCl2 | CH2Cl2 | 1 | <5 | — |
| 2 | Pd(TFA)2 | CH2Cl2 | 5 | 22 | 2:1 |
| 3 | FeCl3·6H2O | CH2Cl2 | 6 | 36 | 6:1 |
| 4 | FeCl3 | CH2Cl2 | 1 | 57 | 4:1 |
| 5d | FeCl3, 4 Å MS | CH2Cl2 | 1 | 28 | 1:1 |
| 6 | TMSCl/MeOH | CH2Cl2 | 1 | 26 | 4:1 |
| 7e | FeCl3 | CHCl3 | 1.3 | 82 | 4:1 |
Reaction conditions: diol (0.5 mmol, 1 equiv), p-methoxystyrene (2 equiv), catalyst (10 mol % with respect to diol) and 0.5 M with respect to diol, 0 °C to rt.
Isolated yields.
diastereomeric ratio determined by 1H NMR
4 Å MS (200 mg),
CHCl3 stabilized with amylenes.
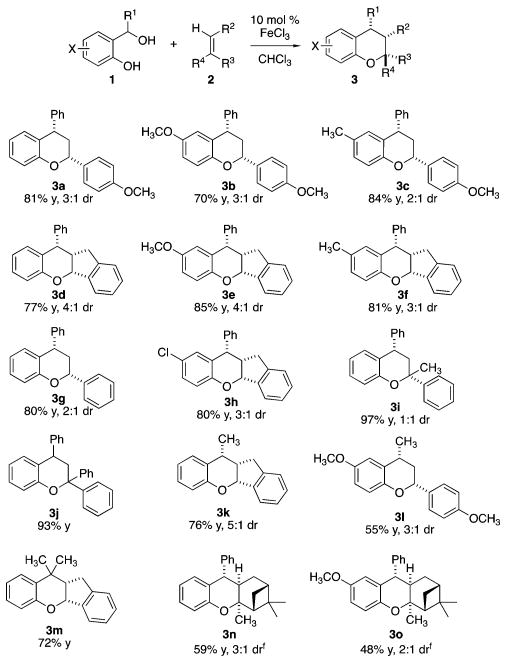
The optimized reaction conditions were used to explore the scope of the two-component hetero-Diels–Alder reaction (Figure 2). We evaluated diols featuring both electron-rich and -deficient substituents. Diols with pendant aryl rings at the methide carbon performed better than those with alkyl groups, likely due to the increased stability of the oQM from conjugation (3b vs 3l). Styrene worked well in the reaction (3g). The reaction conditions also tolerated geminal methyl substituents at the methide carbon (3m). Indene was effective, and although it does not bear electron-donating substituents, the rigidity of the fused bicycle and ring strain may have contributed to its reactivity (3d–f, 3h, 3k, 3m). 1,1′-Disubstituted olefins underwent the cycloaddition condensation reaction in good yields (3i, 3j, 3n, 3o). The geminal substitution renders the dienophile more electron-rich, which is represented in the higher isolated yields. Additionally, α-pinene underwent the condensation and was determined to proceed with exo selectivity (3n, 3o). The use of this dienophile affords a trisubstituted benzopyran with the cyclobutane intact.
Figure 2.
FeCl3 mediated cycloaddition of o-hydroxybenzyl alcohols and olefins.a a Reaction conditions: 1 (1.0 mmol), 2 (2 equiv), FeCl3 (10 mol % with respect to diol) and 0.5 M with respect to diol; diastereomeric ratio determined by 1H NMR. b Diol (0.5 mmol, 1 equiv), p-methoxystyrene (2 equiv), FeCl3 (20 mol % with respect to diol). Detailed experimental conditions are provided in the Supporting Information.
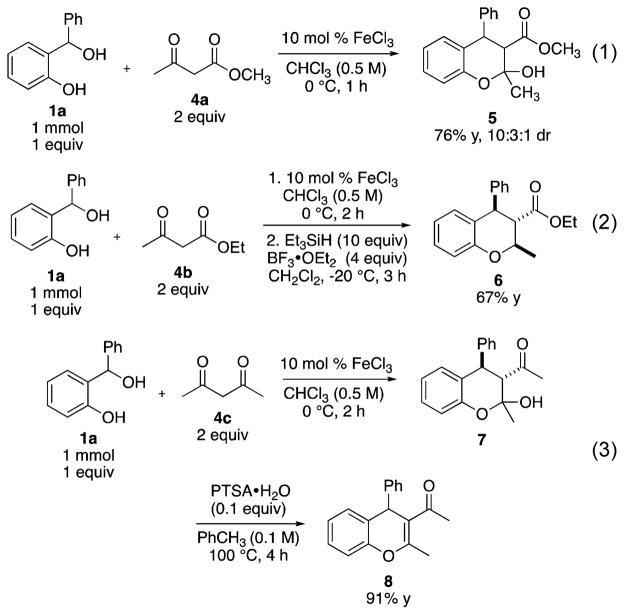
We sought to expand the type of condensation reaction that could undergo the Fe salt catalyzed process by using stabilized enolates (Scheme 1). Wang and co-workers described the reaction of oQM precursors with β-ketoesters and diones under reflux conditions to afford the corresponding 4H-chromenes in high yields.11 Under FeCl3 catalyzed conditions, the reaction between o-hydroxybenzyl alcohol 1a and methyl acetoacetate 4a afforded the trisubstituted benzopyran 5 featuring an adjacent hemiketal and ester. The hemiketal can be isolated and further functionalized. Reduction of the chroman hemiketal with a triethylsilane and BF3·OEt2 reduction to afford benzopyran 6 in 67% yield as a single diastereomer.12 In addition, 4H-chromene 8 was accessed by an acid catalyzed dehydration of hemiketal 7 in 91% yield in two steps from the o-hydroxybenzyl alcohol 1a and 2,4-pentanedione 4c.
Scheme 1.
FeCl3 Mediated Cycloaddition of Diones and β-Ketoesters To Afford Benzopyrans and 4H-Chromenes
There are a limited number of stable oQMs; most reactions that use oQMs employ precursors that undergo in situ formation of the reactive oQM.13,14 We sought to test the limits of oQM generation and reactivity and postulated a multicomponent condensation to generate oQM in situ from aldehydes and phenols. We envisaged a metal-mediated condensation reaction as a route to an oQM that would undergo a subsequent HDA reaction (Figure 3).
Figure 3.
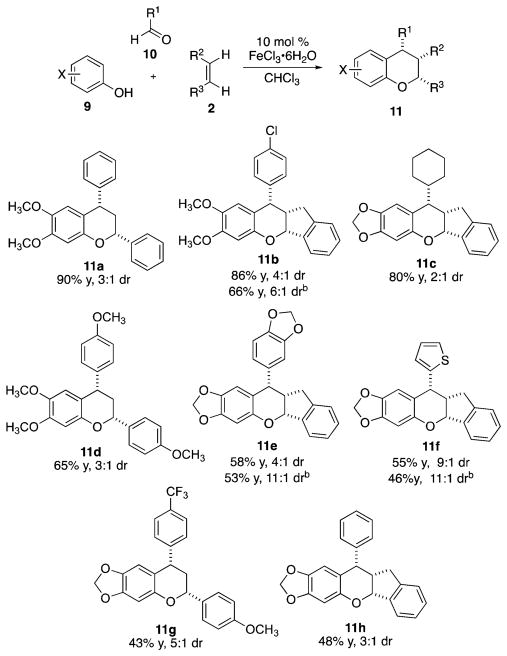
Multicomponent condensation reaction with aldehydes, phenols, and olefins.a a Reaction conditions: 2.0 mmol of phenol, 2.0 mmol of aldehdye, and 1.0 mmol of dienophile, 0.5 M with respect to dienophile and 10 mol % FeCl3·6H2O with respect to phenol; isolated yields; diastereomeric ratio determined by 1H NMR; detailed experimental conditions are provided in the Supporting Information. b Yield and dr after trituration with hot hexanes.
The reaction of 3,4-dimethoxyphenol with benzaldehyde and styrene afforded 11a in 90% yield and 3:1 dr. p-Chloro-benzaldehyde was used to afford 11b in good yields and diastereoselectivities. Cyclohexanecarboxaldehyde also underwent condensation to afford chroman 11c in good yields but in only 2:1 dr. Heteroatom-containing aldehydes underwent the transformation, albeit in modest yields (11d–f). The electron-deficient aryl aldehyde, p-(trifluoromethyl)benzaldehyde, afforded 11g in 43% yield, 5:1 dr. The reaction of sesamol with benzaldehyde and indene afforded 11h in 48% yield and 3:1 dr.
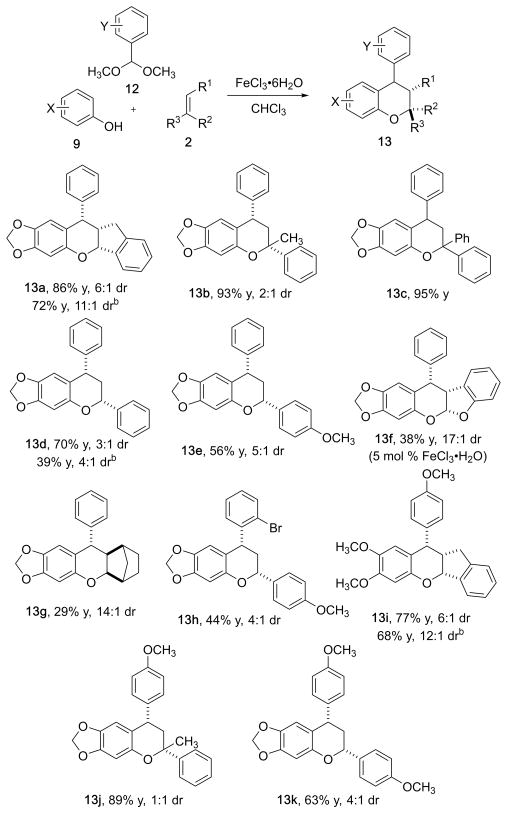
While the multicomponent reaction proceeded with aldehydes, the yields tended to be less than 70%. We hypothesized that acetals may be better electrophiles for the Friedel–Crafts reaction. Acetals react through an oxonium ion which has a lower π* than that of an aldehyde. For comparison, the reaction of benzaldehyde dimethyl acetal with sesamol and indene afforded the corresponding product 13a in 86% yield and 6:1 dr. The same product was isolated from the reaction of benzaldehyde in 48% yield and 3:1 dr (11h), providing evidence that the electrophilicity of the second component has a significant outcome on the reaction yield (Figure 4).
Figure 4.
Multicomponent condensation reaction with acetals, phenols, and olefins.a a Reaction conditions: 2.0 mmol of phenol, 2.0 mmol of acetal, and 1.0 mmol of dienophile, 0.5 M with respect to and 10 mol % FeCl3·6H2O with respect to phenol unless otherwise stated; isolated yields; diastereomeric ratio determined by 1H NMR; detailed experimental conditions are provided in the Supporting Information. b Yield and dr after trituration with hot hexanes.
Sesamol and benzaldehdye dimethyl acetal were excellent reaction partners for generating the reactive oQM to be used in the MCR. 1,1-Disubstituted dieneophiles were effective at trapping the oQM and generating a benzopyran. 1,1′-Diphenyl-ethylene and α-methylstyrene were high yielding (13b, 13c, 13j). Indene proceeded with good diastereoselectivity, most likely due to the rigidity of the fused bicycle (13a, 13i). Styrene performed better than p-methoxy styrene, despite being less electron-rich (13d).
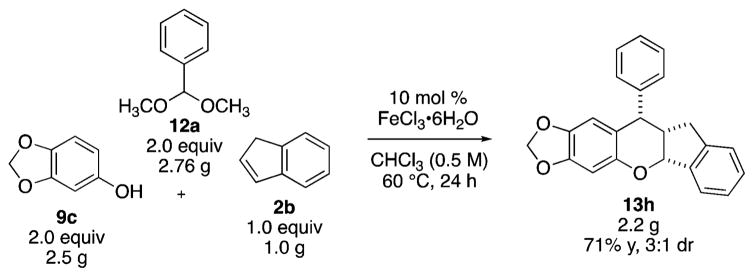
Interestingly, benzofuran afforded a fused pentacycle 13f in good diastereoselectivity (17:1 dr) but low yields. The best yields were obtained with 5 mol % FeCl3; the product undergoes further decomposition under the reaction conditions. Norborene afforded the corresponding condensation product 13g in low yield and selectivity (29% yield, 14:1 dr). The reaction conditions also tolerated substituted benzalde-hyde acetals. 2-Bromobenzaldehyde dimethyl acetal proceeded in lower yield, likely due to the steric hindrance of the ortho-Br, but still afforded 13h in 44% yield and 4:1 dr. p-Methoxybenzaldehyde dimethyl acetal performed well under the general reaction conditions (13i–k); the acetal is less electrophilic than benzaldehyde dimethyl acetal, but can facilitate the formation of the oQM. 3,4-Dimethoxyphenol also performed well in the condensation reaction with p-methoxy-benzaldehyde dimethyl acetal and indene to afford chroman 13i.
Scalability of the reaction was demonstrated with 1.0 g of dienophile. The reaction of sesamol, benzaldehyde dimethyl acetal, and indene afforded 2.2 g of the desired product 13h in 71% yield, 3:1 dr (Scheme 2). Trituration of the isolated product with boiling hexanes afforded a 64% yield and 5:1 dr.
Scheme 2.
Multicomponent Reaction Scale Up
In conclusion, we have developed two strategies to access benzopyrans using Fe(III) salts as catalysts. The two-component approach to access benzopyrans and 4H-chromenes from o-hydroxybenzyl alcohols and olefins proceeds well under Fe-catalysis. Moreover, Fe(III) salts will also mediate the one-pot MCR via an in situ generated oQM from the condensation of a phenol and an aldehyde or acetal. The MCR features the use of readily available starting materials and performs well on gram scale. Future studies will focus on the development of an asymmetric catalytic approach and use in natural product syntheses.
Supplementary Material
Acknowledgments
This research was supported by the NIH (R01 GM078240 and P50 GM067041).
Footnotes
The authors declare no competing financial interest.
Optimization, experimental procedures, compound characterization, and spectral data (PDF)
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.or-glett.7b00647.
References
- 1.(a) Beaudry CM, Malerich JP, Trauner D. Chem Rev. 2005;105:4757–4778. doi: 10.1021/cr0406110. [DOI] [PubMed] [Google Scholar]; (b) Rokita SE. Quinone Methides. Wiley; 2009. [Google Scholar]; (c) Willis NJ, Bray CD. Chem - Eur J. 2012;18:9160–9173. doi: 10.1002/chem.201200619. [DOI] [PubMed] [Google Scholar]; (d) Bai WJ, David JG, Feng ZG, Weaver MG, Wu KL, Pettus TRR. Acc Chem Res. 2014;47:3655–3664. doi: 10.1021/ar500330x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pathak TP, Sigman MS. J Org Chem. 2011;76:9210–9215. doi: 10.1021/jo201789k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.1,4-Addition: Zhang Y, Sigman MS. Org Lett. 2006;8:5557–5560. doi: 10.1021/ol062222t.Jensen KH, Pathak TP, Zhang Y, Sigman MS. J Am Chem Soc. 2009;131:17074–17075. doi: 10.1021/ja909030c.Pathak TP, Gligorich KM, Welm BE, Sigman MS. J Am Chem Soc. 2010;132:7870–7871. doi: 10.1021/ja103472a.Pathak TP, Sigman MS. Org Lett. 2011;13(10):2774–2777. doi: 10.1021/ol200913r.Jana R, Pathak TP, Jensen KH, Sigman MS. Org Lett. 2012;14(16):4074–4077. doi: 10.1021/ol3016989.Luan Y, Schaus SE. J Am Chem Soc. 2012;134:19965–199678. doi: 10.1021/ja309076g.Barbato KS, Luan Y, Ramella D, Panek JS, Schaus SE. Org Lett. 2015;17(23):5812–5815. doi: 10.1021/acs.orglett.5b02954.
- 4.[4 + 2]-Cycloaddition: Selenski C, Pettus TRR. J Org Chem. 2004;69:9196–9203. doi: 10.1021/jo048703c.Alden-Danforth E, Scerba MT, Lectka T. Org Lett. 2008;10(21):4951–4953. doi: 10.1021/ol802029e.Wenderski TA, Marsini MA, Pettus TRR. Org Lett. 2011;13(1):118–121. doi: 10.1021/ol102652t.Green JC, Brown ER, Pettus TRR. Org Lett. 2012;14:2929–2931. doi: 10.1021/ol301092w.
- 5.Luan Y, Sun H, Schaus SE. Org Lett. 2011;13(24):6480–6483. doi: 10.1021/ol202772k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robustadial: Xu R, Snyder JK, Nakanishi K. J Am Chem Soc. 1984;106:734–736.Salomon RG, Lal K, Mazza SM, Zarate EA, Youngs WJ. J Am Chem Soc. 1988;110:5213–5214.Bharate SB, Bhutani KK, Khan SI, Tekwani BL, Jacob MR, Khan IA, Singh IP. Bioorg Med Chem. 2006;14:1750–1760. doi: 10.1016/j.bmc.2005.10.027.Bharate SB, Singh IP. Tetrahedron Lett. 2006;47:7021–7024.(+)-Brazilin: Craig JC, Naik AR, Pratt R, Johnson E, Bhacca NS. J Org Chem. 1965;30:1573–1576. doi: 10.1021/jo01016a058.Huang Y, Zhang J, Pettus TRR. Org Lett. 2005;7(26):5841–5844. doi: 10.1021/o1023749.Jung Y, Kim I. J Org Chem. 2015;80(3):2001–2005. doi: 10.1021/jo502745j.Procyanidin B3: Oizumi Y, Mohri Y, Hirota M, Makabe H. J Org Chem. 2010;75(14):4884–4886. doi: 10.1021/jo1009382.YM-26734: Oslund RC, Cermak N, Verlinde CLMJ, Gelb MH. Bioorg Med Chem Lett. 2008;18(20):5415–5419. doi: 10.1016/j.bmcl.2008.09.041.
- 7.(a) Gharpure SJ, Sathiyanarayanan AM, Vuram PK. RSC Adv. 2013;3:18279–18282. [Google Scholar]; (b) Tan W, Du B-X, Li X, Zhu Xu, Shi F, Tu S-J. J Org Chem. 2014;79:4635–4643. doi: 10.1021/jo500644v. [DOI] [PubMed] [Google Scholar]; (c) Zhao JJ, Zhang YC, Xu MM, Tang M, Shi F. J Org Chem. 2015;80:10016–10024. doi: 10.1021/acs.joc.5b01613. [DOI] [PubMed] [Google Scholar]
- 8.(a) El-Sepelgy O, Haseloff S, Alamsetti SK, Schneider C. Angew Chem, Int Ed. 2014;53:7923–7927. doi: 10.1002/anie.201403573. [DOI] [PubMed] [Google Scholar]; (b) Saha S, Schneider C. Org Lett. 2015;17:648–651. doi: 10.1021/ol503662g. [DOI] [PubMed] [Google Scholar]
- 9.(a) Hsiao CC, Liao HH, Rueping M. Angew Chem, Int Ed. 2014;53:13258–13263. doi: 10.1002/anie.201406587. [DOI] [PubMed] [Google Scholar]; (b) Hsiao CC, Raja S, Liao HH, Atodiresei J, Rueping M. Angew Chem, Int Ed. 2015;54:5762–5765. doi: 10.1002/anie.201409850. [DOI] [PubMed] [Google Scholar]
- 10.Zhao JJ, Sun SB, He SH, Wu Q, Shi F. Angew Chem, Int Ed. 2015;54:5460–5464. doi: 10.1002/anie.201500215. [DOI] [PubMed] [Google Scholar]
- 11.Fan J, Wang Z. Chem Commun. 2008:5381–5383. doi: 10.1039/b812046c. [DOI] [PubMed] [Google Scholar]
- 12.Triethyl silane reduction: Zhu YH, Zhang M, Li QY, Liu Q, Zhang J, Yuan YY, Nan FJ, Wang MW. Chin Chem Lett. 2014;25:693–698.Bai WJ, Green JC, Pettus TRR. J Org Chem. 2012;77:379–387. doi: 10.1021/jo201971g.
- 13.Stable: Jurd L. Tetrahedron. 1977;33:163.Kopach ME, Harman WD. J Am Chem Soc. 1994;116:6581–6592.Amouri H, Vaissermann J. Organometallics. 2000;19:1740–1748.Amouri H, Le Bras J. Acc Chem Res. 2002;35:501–510. doi: 10.1021/ar010105m.MacIntosh AD, Yang H, Pike RD, Sweigart DA. J Organomet Chem. 2012;719:14–17.
- 14.Robust leaving groups: Chambers JD, Crawford J, Williams HWR, Dufresne C, Scheigetz J, Bernstein MA, Lau CK. Can J Chem. 1992;70(6):1717–1732.Huang Y, Pettus TRR. Synlett. 2008;2008:1353–1356. doi: 10.1055/s-2008-1072750.Marsini MA, Huang Y, Lindsey CC, Wu KL, Pettus TRR. Org Lett. 2008;10:1477–1480. doi: 10.1021/ol8003244.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.