Abstract
Background
Recently, many surgeons have chosen sublobar resection for the curative treatment of lung tumors with ground-glass opacity, which is a hallmark of lepidic lung cancer. The purpose of this study was to evaluate the oncological results of sublobar resection for non-lepidic lung cancer in comparison with lobectomy.
Methods
We conducted a retrospective chart review of 328 patients with clinical N0 non-small cell lung cancer sized ≤2 cm who underwent curative surgical resection from January 2009 to December 2014. The patients were classified on the basis of their lesions into non-lepidic and lepidic groups. The survival rates following lobectomy and sublobar resection were compared within each of these 2 groups.
Results
The non-lepidic group contained a total of 191 patients. The 5-year recurrence-free survival rate was not significantly different between patients who received sublobar resection or lobectomy in the non-lepidic group (80.1% vs. 79.2%, p=0.822) or in the lepidic group (100% vs. 97.4%, p=0.283). Multivariate analysis indicated that only lymphatic invasion was a significant risk factor for recurrence in the non-lepidic group. Sublobar resection was not a risk factor for recurrence in the non-lepidic group.
Conclusion
The oncological outcomes of sublobar resection and lobectomy in small-sized non-small cell lung cancer did not significantly differ according to histological type.
Keywords: Lung neoplasms, Thoracic surgery, Pathology, Lung lobectomy, Pathology
Introduction
The standard treatment for stage I non-small cell lung cancer (NSCLC) is surgical lobectomy [1]. However, sublobar resection may provide a prognosis comparable to that of lobectomy for small-sized (≤2 cm) NSCLC. Two meta-analyses have evaluated the results of sublobar resection versus lobectomy for small-sized NSCLC, but were unable to reach a consensus recommendation [2,3]. Some studies reported similar prognoses following sublobar resection or lobectomy, whereas others reported that sublobar resection had a worse prognosis than lobectomy. Two ongoing randomized trials (Cancer and Leukemia Group B-140503 and Japan Clinical Oncology Group 0802) are investigating the hypothesis that sublobar resection is comparable to lobectomy for small-sized (≤2 cm) NSCLC [4,5].
NSCLC is characterized by several histological types, including adenocarcinoma and squamous cell carcinoma. The prognosis of sublobar resection may differ depending on the histological type. For example, lung adenocarcinoma has variable prognoses after treatment because of its histological heterogeneity [6]. The histological subtypes of adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA) both have very good prognoses after surgical resection [7], and a good prognosis is anticipated after sublobar resection. Lepidic adenocarcinoma, a subtype of invasive adenocarcinoma, has low-grade potential for malignancy, and a good prognosis is anticipated after sublobar resection [8]. Tumors with ground-glass opacity (GGO) are usually considered to be AIS, MIA, or lepidic adenocarcinoma. Many studies have reported good prognoses after the sublobar resection of GGO tumors [9–11]. The effect of sublobar resection on other NSCLCs cannot be rigorously analyzed when the sublobar resection group includes patients with AIS, MIA, or lepidic adenocarcinoma, which are known to have good prognoses following resection.
The purpose of this study was to evaluate the effects of sublobar resection in 2 groups of patients with small-sized NSCLCs: one group of patients included cases of AIS, MIA, and lepidic adenocarcinoma, and had a good postoperative prognosis, while the other group included other NSCLCs, and did not have a good postoperative prognosis. We investigated whether sublobar resection was associated with a good prognosis regardless of the histological type of small-sized NSCLC.
Methods
1) Patients
This study enrolled a total of 328 consecutive patients diagnosed with stage I NSCLC of 2 cm or less who underwent curative resection at Seoul St. Mary’s Hospital, Korea, from January 2009 to December 2014. Patients who underwent incomplete resection were excluded. Complete resection was defined as an absence of either macroscopic or microscopic residual cancer, especially in the resection margin. None of the included patients received preoperative chemotherapy or radiotherapy. The medical charts of all 328 patients were reviewed retrospectively. Surgical procedures included wedge resection, segmentectomy, lobectomy, and bilobectomy. Sublobar resection was defined as including wedge resection and segmentectomy. Sublobar resection was performed preferentially in high-risk patients with reduced pulmonary function or underlying pulmonary or heart disease. Sublobar resection was indicated for patients with pure GGO or GGO-dominant partially solid small-sized NSCLC tumors. Intentional sublobar resection was considered for patients with radiologically solid dominant cT1aN0 tumors who had adequate pulmonary function for lobectomy, unless the patient did not provide consent. Intraoperative mediastinal lymph node dissection or sampling was not a routine procedure. Instead, selective dissection or sampling was done at enlarged lymph nodes. The surgical procedures were selected depending on the surgeon’s preference and/or the patient’s decision.
All patients were classified as having lepidic or non-lepidic NSCLC according to tumor histomorphology. The clinicopathological features and survival rate after lobectomy and sublobar resection were compared in the non-lepidic and lepidic NSCLC groups. The risk factors for recurrence were analyzed in the non-lepidic NSCLC group. The study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital at the Catholic University of Korea (IRB No. KC16RISI1032) and performed in accordance with the principles of the Declaration of Helsinki. Written informed consents were obtained.
2) Histological evaluation
All clinical specimens were examined by pathologists and their observations were recorded. TNM (tumor-node-metastasis) staging was based on the seventh American Joint Committee on Cancer guidelines [12]. Each tumor was reviewed for size, location, differentiation, lymph node status, pleural invasion, lymphatic invasion, and vascular invasion. To describe the histomorphological patterns of tumors, the occupancy ratio of each component (lepidic, acinar, papillary, micropapillary, and solid) in the total tumor area was measured and recorded semiquantitively in 5% increments according to the 2015 World Health Organization classification of lung tumors [13]. AIS and MIA were defined as small (≤3 cm) and solitary adenocarcinomas characterized by a lepidic growth pattern without invasion for AIS or by ≤5 mm of invasion for MIA. Invasive adenocarcinomas were classified into several subtypes, including acinar adenocarcinoma, papillary adenocarcinoma, micropapillary adenocarcinoma, and lepidic adenocarcinoma. The lepidic NSCLC group included AIS, MIS, and lepidic adenocarcinoma. The non-lepidic NSCLC group included invasive adenocarcinoma (except for lepidic NSCLC) and other types of NSCLC.
3) Statistical analysis
The clinicopathological characteristics of lobectomy and sublobar resection were compared in both the lepidic and non-lepidic NSCLC groups using the Student t-test or the Wilcoxon rank-sum test for continuous variables and the chi-square test or Fisher exact test for categorical variables. Data for the interval between surgical resection and the last follow-up examination were analyzed using the Kaplan-Meier method, and confirmed recurrences/deaths were used to calculate recurrence-free survival (RFS) and overall survival. Survival in each group was compared using the log-rank test. The risk of recurrence was determined by multivariate analysis using the Cox proportional hazards model. All p-values <0.05 were considered to indicate statistical significance. Statistical analyses were performed with IBM SPSS ver. 19.0 (IBM Corp., Armonk, NY, USA).
Results
Among the 328 patients enrolled in this study, 191 patients had non-lepidic NSCLC (58.2%) and 137 patients had lepidic NSCLC (41.8%). The median follow-up time for all patients was 1,063 days (range, 86 to 2,584 days).
The clinical characteristics of patients treated with sublobar resection and lobectomy were compared separately in the non-lepidic and lepidic NSCLC groups (Table 1). The clinical characteristics of patients with non-lepidic NSCLC who received sublobar resection or lobectomy were compared. Age, gender, smoking status, the level of serum carcinoembryonic antigen, maximum standardized uptake value (SUVmax), clinical stage, the use of video-assisted thoracoscopic surgery (VATS), and adjuvant treatment did not significantly differ between the sublobar resection and lobectomy groups. Only tumor location and pulmonary function tests (forced expiratory volume in 1 second and diffusing capacity of the lung for carbon monoxide) differed between the sublobar resection and lobectomy patients. The incidence of postoperative complications was 8.3% and 11.5% in the sublobar resection and lobectomy groups, respectively. No cases of postoperative mortality occurred in patients with non-lepidic NSCLC. In patients with lepidic NSCLC, no clinical characteristics were significantly different between the patients who underwent sublobar resection and those who underwent lobar resection. The incidence of postoperative complications was 12.0% and 9.2% in the sublobar resection and lobectomy groups, respectively. There were no cases of postoperative mortality in patients with lepidic NSCLC.
Table 1.
Clinical characteristics of patients with non-lepidic and lepidic NSCLC who underwent sublobar resection or lobectomy
| Characteristic | Non-lepidic NSCLC | Lepidic NSCLC | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Sublobar resection (n=60) | Lobectomy (n=131) | p-value | Sublobar resection (n=50) | Lobectomy (n=87) | p-value | |
| Age (yr) | 65.5±11.7 | 62.7±11.0 | 0.103 | 60.1±12.3 | 59.9±8.4 | 0.913 |
|
| ||||||
| Gender | 0.523 | |||||
|
| ||||||
| Male | 30 (50.0) | 72 (55.0) | 21 (42.0) | 29 (33.3) | ||
|
| ||||||
| Female | 30 (50.0) | 59 (45.0) | 29 (58.0) | 58 (66.7) | 0.310 | |
|
| ||||||
| Current or former smoker | 23 (38.3) | 45 (34.4) | 0.594 | 12 (24.0) | 24 (27.6) | 0.646 |
|
| ||||||
| Serum carcinoembryonic antigen level (ng/mL) | 2.3±3.4 | 2.0±1.6 | 0.477 | 1.3±0.9 | 2.2±7.4 | 0.430 |
|
| ||||||
| Maximum standardized uptake value | 2.9±3.0 | 3.7±3.2 | 0.141 | 0.9±1.6 | 1.6±1.9 | 0.060 |
|
| ||||||
| Forced expiratory volume in 1 second (%) | 92.6±18.4 | 100.8±17.7 | 0.004 | 97.8±18.3 | 103.6±15.0 | 0.051 |
|
| ||||||
| Diffusing capacity of the lung for carbon monoxide (%) | 76.6±17.2 | 87.2±19.0 | <0.001 | 84.7±14.7 | 88.9±17.5 | 0.180 |
|
| ||||||
| Tumor location | 0.018 | 1.000 | ||||
|
| ||||||
| Central | 0 | 11 (8.4) | 0 | 1 (1.1) | ||
|
| ||||||
| Peripheral | 60 (100.0) | 120 (91.6) | 50 (100.0) | 86 (98.9) | ||
|
| ||||||
| Clinical stage | 0.463 | 0.415 | ||||
|
| ||||||
| T1aN0M0 | 56 (93.3) | 118 (90.1) | 49 (98.0) | 82 (94.3) | ||
|
| ||||||
| T2aN0M0 | 4 (6.7) | 13 (9.9) | 1 (2.0) | 5 (5.7) | ||
|
| ||||||
| Video-assisted thoracoscopic surgery | 49 (81.7) | 96 (73.3) | 0.208 | 44 (88.0) | 67 (77.0) | 0.114 |
|
| ||||||
| Open thoracotomy | 11 (18.3) | 35 (26.7) | 6 (12.0) | 20 (23.0) | ||
|
| ||||||
| Procedure | ||||||
|
| ||||||
| Wedge resection | 43 (71.7) | 33 (66.0) | ||||
|
| ||||||
| Segmentectomy | 17 (28.3) | 17 (34.0) | ||||
|
| ||||||
| Lobectomy | 129 (98.5) | 85 (97.7) | ||||
|
| ||||||
| Bilobectomy | 2 (1.5) | 2 (2.3) | ||||
|
| ||||||
| Complications | 5 (8.3) | 15 (11.5) | 0.514 | 6 (12.0) | 8 (9.2) | 0.602 |
|
| ||||||
| Postoperative mortality | 0 | 0 | 0 | 0 | ||
|
| ||||||
| Adjuvant therapy | 4 (6.7) | 4 (3.1) | 0.262 | 0 | 0 | |
Values are presented as mean±standard deviation or number (%).
NSCLC, non-small cell lung cancer.
The pathological characteristics of patients with non-lepidic NSCLC and lepidic NSCLC were compared (Table 2). Tumor differentiation, histological subtype distribution, pathological stage, and the presence of visceral pleural invasion, lymphatic invasion, and vascular invasion were not significantly different in patients with non-lepidic NSCLC who underwent sublobar resection or lobectomy. The only significant difference was the mean tumor size, which was larger in lobectomy patients than in sublobar resection patients (1.6 cm versus 1.3 cm, p<0.001). In the lepidic NSCLC group, tumor differentiation and the presence of visceral pleural invasion, lymphatic invasion, and vascular invasion did not significantly differ between patients who underwent lobectomy or sublobar resection. However, the tumor size was smaller in the sublobar resection patients (1.1 cm versus 1.5 cm, p<0.001). AIS was more common in the sublobar resection patients (p<0.001).
Table 2.
Pathological characteristics of sublobar resection and lobectomy in non-lepidic NSCLC and lepidic NSCLC
| Characteristic | Sublobar resection | Lobectomy | p-value |
|---|---|---|---|
| Non-lepidic NSCLC | |||
| N | 60 | 131 | |
| Tumor size (cm) | 1.3±0.4 | 1.6±0.3 | <0.001 |
| Differentiation | 0.967 | ||
| Well | 22 (36.7) | 47 (35.9) | |
| Moderate | 31 (51.7) | 70 (53.4) | |
| Poor | 7 (11.7) | 14 (10.7) | |
| No. of dissected lymph nodes | 4.2±6.7 | 12.1±7.4 | <0.001 |
| Histological type | 0.390 | ||
| Adenocarcinoma | 49 (81.7) | 111 (84.7) | |
| Squamous cell carcinoma | 8 (13.3) | 10 (7.6) | |
| Others | 3 (5.0) | 10 (7.6) | |
| Adenocarcinoma subtype | 0.102 | ||
| Acinar adenocarcinoma | 35 (71.4) | 78 (70.3) | |
| Papillary adenocarcinoma | 5 (10.2) | 21 (18.9) | |
| Micropapillary adenocarcinoma | 2 (4.1) | 0 | |
| Solid adenocarcinoma | 6 (12.2) | 6 (5.4) | |
| Other | 1 (2.0) | 6 (5.4) | |
| Pathological stage | 0.805 | ||
| T1aN0M0 | 50 (83.3) | 111 (84.7) | |
| T1bN0M0 | 0 | 0 | |
| T2aN0M0 | 10 (16.7) | 20 (15.3) | |
| Visceral pleural invasion | 10 (16.7) | 20 (15.3) | 0.805 |
| Lymphatic invasion | 15 (25.0) | 36 (27.5) | 0.719 |
| Vascular invasion | 5 (8.3) | 14 (10.7) | 0.614 |
| Lepidic NSCLC | |||
| N | 50 | 87 | |
| Tumor size (cm) | 1.1±0.4 | 1.5±0.4 | <0.001 |
| Differentiation | 0.448 | ||
| Well | 47 (94.0) | 82 (94.3) | |
| Moderate | 1 (2.0) | 4 (4.6) | |
| Poor | 2 (4.0) | 1 (1.1) | |
| No. of dissected lymph nodes | 3.8±5.9 | 11.2±6.7 | <0.001 |
| Subtype | <0.001 | ||
| Adenocarcinoma in situ | 22 (44.0) | 9 (10.3) | |
| Minimally invasive adenocarcinoma | 22 (44.0) | 42 (48.3) | |
| Lepidic adenocarcinoma | 6 (12.0) | 36 (41.4) | |
| Pathological stage | <0.001 | ||
| TisN0M0 | 22 (44.0) | 9 (10.3) | |
| T1aN0M0 | 27 (54.0) | 74 (85.1) | |
| T2aN0M0 | 1 (2.0) | 4 (4.6) | |
| Visceral pleural invasion | 1 (2.0) | 4 (4.6) | 0.652 |
| Lymphatic invasion | 2 (4.0) | 11 (12.6) | 0.133 |
| Vascular invasion | 0 | 1 (1.1) | 1.000 |
Values are presented as mean±standard deviation or number (%).
NSCLC, non-small cell lung cancer.
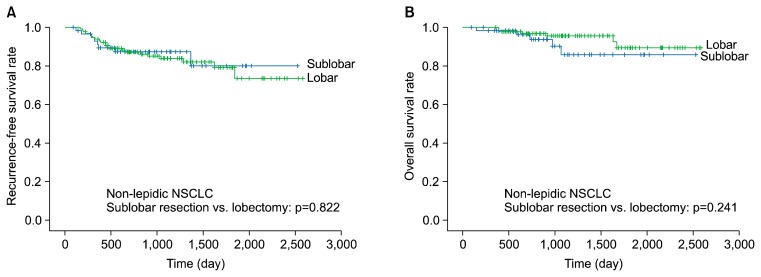
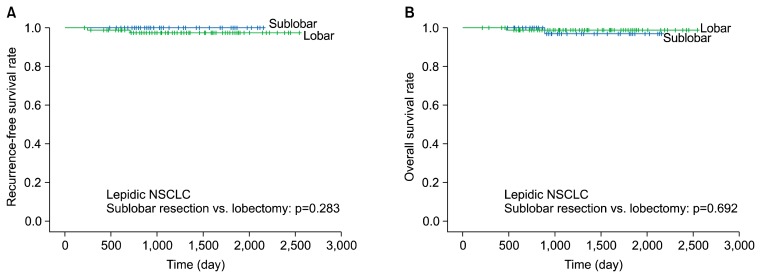
The 5-year RFS and overall survival rates were not significantly different between the patients who underwent sublobar resection or lobectomy in the non-lepidic NSCLC group (RFS, 80.1% versus 79.2%, respectively; p=0.822; overall survival, 85.8% versus 89.4%, respectively; p=0.241) (Fig. 1). The 5-year RFS and overall survival rates were likewise not significantly different between the patients who underwent sublobar resection or lobectomy in the lepidic NSCLC group (RFS, 100% versus 97.4%, respectively; p=0.283; overall survival, 96.9% versus 98.8%, respectively; p=0.692) (Fig. 2).
Fig. 1.
Five-year recurrence-free survival (A) and 5-year overall survival (B) of non-lepidic NSCLC (sublobar resection versus lobectomy). NSCLC, non-small cell lung cancer.
Fig. 2.
Five-year recurrence-free survival (A) and 5-year overall survival (B) of lepidic NSCLC (sublobar resection versus lobectomy). NSCLC, non-small cell lung cancer.
We performed univariate and multivariate analyses using the Cox proportional hazards model to identify factors associated with the recurrence of non-lepidic NSCLC measuring 2 cm or less (Table 3). Variables with p<0.1 in the univariate analysis (gender, smoking status, SUVmax, VATS, tumor size, tumor differentiation, and lymphatic invasion) were entered into the multivariate analysis. The multivariate analysis indicated that only lymphatic invasion was a significant risk factor for the recurrence of stage I non-lepidic NSCLC measuring 2 cm or less (hazard ratio, 2.671; 95% confidence interval, 1.152 to 6.190; p=0.022). Sublobar resection was not a risk factor for recurrence.
Table 3.
Univariate and multivariate analysis of factors affecting the recurrence of non-lepidic non-small cell lung cancer of 2 cm or less (Cox proportional hazard model)
| Variable | Hazard ratio (95% confidence interval) | p-value |
|---|---|---|
| Univariate analysis | ||
| Age | 1.023 (0.987–1.060) | 0.208 |
| Male | 3.278 (1.406–7.642) | 0.006 |
| Current or former smoking | 2.395 (1.169–4.910) | 0.017 |
| CEA | 0.966 (0.799–1.169) | 0.705 |
| SUVmax | 1.165 (1.057–1.285) | 0.002 |
| FEV1 (%) | 1.006 (0.986–1.027) | 0.549 |
| DLCO (%) | 1.002 (0.982–1.022) | 0.873 |
| Central location | 1.742 (0.527–5.754) | 0.363 |
| VATS | 0.411 (0.197–0.860) | 0.018 |
| Sublobar resection | 0.911 (0.405–2.050) | 0.822 |
| Adjuvant chemotherapy | 1.647 (0.390–6.957) | 0.497 |
| Tumor size | 2.660 (0.861–8.218) | 0.089 |
| Differentiation | 0.026 | |
| Well | 1 | |
| Moderate | 3.786 (1.299–11.032) | 0.015 |
| Poor | 5.371 (1.441–20.018) | 0.012 |
| No. of dissected lymph nodes | 0.960 (0.914–1.009) | 0.107 |
| Subtypes | 0.231 | |
| Acinar | 1 | |
| Papillary | 0.974 (0.281–3.380) | 0.967 |
| Micropapillary | 4.478 (0.588–34.082) | 0.148 |
| Solid | 2.897 (0.960–8.744) | 0.059 |
| Other | 1.586 (0.646–3.892) | 0.314 |
| Visceral pleural invasion | 1.085 (0.415–2.838) | 0.868 |
| Lymphatic invasion | 3.411 (1.661–7.005) | 0.001 |
| Vascular invasion | 1.500 (0.521–4.321) | 0.453 |
| Multivariate analysis | ||
| Male | 2.691 (0.918–7.885) | 0.071 |
| Current or former smoking | 0.953 (0.357–2.542) | 0.923 |
| SUVmax | 1.103 (0.964–1.263) | 0.154 |
| VATS | 0.154 (0.237–1.255) | 0.545 |
| Tumor size | 0.935 (0.250–3.501) | 0.921 |
| Differentiation | 0.831 | |
| Well | 1 | |
| Moderate | 1.220 (0.361–4.128) | 0.749 |
| Poor | 1.578 (0.346–7.192) | 0.556 |
| Lymphatic invasion | 2.671 (1.152–6.190) | 0.022 |
Continuous variables: age, CEA, SUVmax, FEV1 (%), DLCO (%), tumor size, number of dissected lymph nodes. Categorical variables: male sex, current or former smoking, central location, VATS, sublobar resection, adjuvant chemotherapy, differentiation, subtypes, visceral pleural invasion, lymphatic invasion, vascular invasion.
CEA, carcinoembryonic antigen; SUVmax, maximum standardized uptake value; FEV1, forced expiratory volume in 1 second; DLCO, diffusing capacity for carbon monoxide; VATS, video-assisted thoracoscopic surgery.
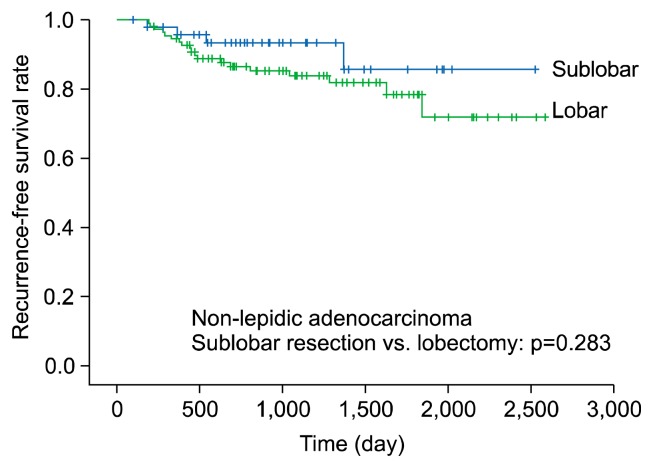
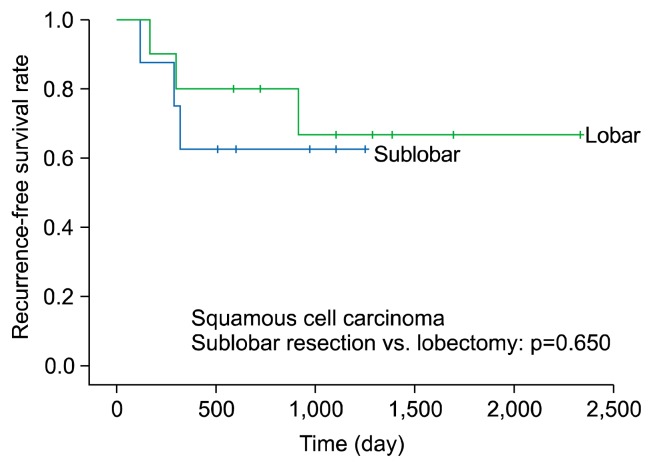
We divided non-lepidic NSCLC patients into those with non-lepidic adenocarcinoma and those with squamous cell carcinoma, and performed a subgroup analysis of survival in patients who underwent sublobar resection or lobectomy. The non-lepidic adenocarcinoma group included 49 patients who underwent sublobar resection and 111 patients who underwent lobectomy. The 5-year RFS rate of patients in the non-lepidic adenocarcinoma group did not significantly differ depending on whether they underwent sublobar resection or lobectomy (RFS, 85.6% versus 78.5%, respectively; p=0.283) (Fig. 3). The squamous cell carcinoma group included 8 patients who underwent sublobar resection and 10 patients who underwent lobectomy. The 3-year RFS rate of patients in the squamous cell carcinoma group did not significantly differ between the sublobar resection and lobectomy groups (RFS, 62.5% versus 66.7%; p=0.650) (Fig. 4).
Fig. 3.
Five-year recurrence-free survival of non-lepidic adenocarcinoma (sublobar resection versus lobectomy).
Fig. 4.
Five-year recurrence-free survival of squamous cell carcinoma (sublobar resection versus lobectomy).
Discussion
Sublobar resection has been adopted at many institutions for the surgical treatment of early-stage NSCLC, especially for low-grade malignant tumors such as AIS, MIA, or lepidic adenocarcinoma presenting with GGO. However, a number of studies have reported that the survival of patients with small tumors who received sublobar resection or lobectomy did not significantly differ, even if not all the tumors were low-grade malignancies [2,3]. The detection rate of GGO-type lung cancer is increasing in Korea and other Asian countries. Therefore, it is highly likely that any subset of patients undergoing sublobar resection for early-stage lung cancer would include a number of GGO-type lesions with a good prognosis. The overall prognosis of sublobar resection in all patients with small-sized NSCLC appears to be good, according to several studies. The aim of this study was to determine the prognosis of patients after sublobar resection for non-low-grade malignant NSCLC. The goal was to establish that sublobar resection is an applicable option for all small-sized NSCLCs, regardless of histological type.
In this study, low-grade malignant tumors (AIS, MIA, and lepidic adenocarcinoma) accounted for 41.8% of all cases of small-sized (≤2 cm) NSCLC. AIS or MIA, which showed a 100% 5-year RFS rate after sublobar resection, accounted for 29.0% of the total population. The incidence of low-grade malignant tumors was not low in this study, and most tumors were GGO, so sublobar resection was preferred. Therefore, it would not be reasonable to simply compare the survival of a sublobar resection group containing a considerable number of low-grade malignant tumors and a lobectomy group. To obtain more meaningful results, we also compared sublobar resection with lobectomy in cases of non-lepidic NSCLC, which included intermediate- to high-grade malignant tumors. In our study, sublobar resection had a comparable prognosis to that of lobectomy in patients with non-lepidic NSCLC. Sublobar resection was not a risk factor for the recurrence of non-lepidic NSCLC in the Cox proportional hazard model. Therefore, we confirmed that sublobar resection is a possible treatment option for small-sized non-lepidic NSCLC. Our multivariate analysis indicated that lymphatic invasion was an independent risk factor for the recurrence of non-lepidic NSCLC. This result supports the hypothesis that the prognosis of small-sized NSCLC is determined by the malignancy grade of the tumor, not by the surgical extent.
Tumor location is very important when choosing sublobar resection for small-sized NSCLC. All tumors treated with sublobar resection were located peripherally. Intentional sublobar resection was considered (if the patient consented) for patients with a GGO nodule or a small peripheral solid nodule located near the visceral pleura. Sublobar resection was more likely to be selected when a sufficient resection margin could be obtained. Sublobar resection was performed via wedge resection or segmentectomy according to the depth of the nodule from the lung surface. In other words, the choice depended on the feasibility of obtaining an adequate resection margin. In most cases, a satisfactory margin was obtained, with a length that was larger than the tumor diameter.
Most of the cases in this study were adenocarcinoma, which is characterized by histological heterogeneity [13,14]. In the non-lepidic adenocarcinoma group, most of the tumors were acinar adenocarcinoma or papillary adenocarcinoma. It is well known that the prognosis of acinar and papillary adenocarcinoma is better than that of micropapillary adenocarcinoma and solid adenocarcinoma [15,16]. Therefore, the effect of tumor subtype (such as acinar, papillary, solid, or micropapillary) on survival after sublobar resection was insufficiently examined in our study. Micropapillary and solid-type tumors are generally rare among cases of early-stage lung cancer. Therefore, most of the evidence indicates that sublobar resection had a good prognosis in patients with small-sized NSCLC. Sublobar resection can be considered as a possible treatment option for acinar and papillary adenocarcinoma; however, more data are needed to evaluate whether sublobar resection is a viable treatment option for micropapillary and solid adenocarcinoma. Our study also did not observe a difference in survival after sublobar resection or lobectomy in patients with squamous cell carcinoma. However, there were only 8 cases of sublobar resection and 10 cases of lobectomy in this group of patients, so future studies are necessary.
The implications of this study are as follows. Sublobar resection may have a comparable prognosis to that of lobectomy, even if the tumor type is not low-grade malignant. Although a subgroup analysis of all histological subtypes was not conducted due to the lack of data, the prognosis of small-sized NSCLC depended on the nature of the tumor itself rather than the choice of sublobar resection or lobectomy. Our results are consistent with those of a previous study [17]. More data are required to accurately identify tumors that should not be treated with sublobar resection.
A number of limitations of this study should be acknowledged. First, this was a retrospective review conducted at a single center. Second, we obtained data from a single institution, and the number of cases was relatively small. Only 60 patients with non-lepidic NSCLC underwent sublobar resection. Additionally, due to the limited sample size, the prognoses of segmentectomy and wedge resection were not compared. Because most of the sublobar procedures we performed were wedge resections, the latter at least proved viable in an oncological sense, comparing favorably with lobectomy. However, another recently conducted study has established that the oncological outcomes of stage IA lung cancer treated by segmentectomy or wedge resection did not differ [18]. A larger number of individuals is required to increase the accuracy of the results, and a comprehensive subgroup analysis according to the histological subtype should be performed. Finally, the data herein were clearly not homogeneous in the comparison between sublobar resection and lobectomy. Thus, the analytical outcomes are difficult to generalize. Nevertheless, our data can serve as a baseline and support future studies. More accurate results could be obtained if the analysis and comparison were conducted with a larger, more homogenous patient sample.
In conclusion, the prognosis after sublobar resection for non-lepidic NSCLC did not significantly differ from the prognosis after lobectomy. In other words, the oncological outcomes of sublobar resection and lobectomy in small-sized NSCLC did not significantly differ according to histological subtype. The oncological outcomes of small-sized NSCLC were determined by tumor pathology, rather than surgical extent. Further research through multicenter randomized controlled trials may more accurately characterize patient outcomes.
Acknowledgments
This study was supported by a Grant of the Samsung Vein Clinic Network (Daejeon, Anyang, Cheongju, Cheonan; Fund no. KTCS04-088).
Footnotes
Conflict of interest
No potential conflicts of interest relevant to this article are reported.
References
- 1.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–22. doi: 10.1016/0003-4975(95)00537-U. [DOI] [PubMed] [Google Scholar]
- 2.Taioli E, Yip R, Olkin I, et al. Survival after sublobar resection for early-stage lung cancer: methodological obstacles in comparing the efficacy to lobectomy. J Thorac Oncol. 2016;11:400–6. doi: 10.1016/j.jtho.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 3.Cao C, Chandrakumar D, Gupta S, Yan TD, Tian DH. Could less be more?: a systematic review and meta-analysis of sublobar resections versus lobectomy for non-small cell lung cancer according to patient selection. Lung Cancer. 2015;89:121–32. doi: 10.1016/j.lungcan.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Blasberg JD, Pass HI, Donington JS. Sublobar resection: a movement from the Lung Cancer Study Group. J Thorac Oncol. 2010;5:1583–93. doi: 10.1097/JTO.0b013e3181e77604. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L) Jpn J Clin Oncol. 2010;40:271–4. doi: 10.1093/jjco/hyp156. [DOI] [PubMed] [Google Scholar]
- 6.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–85. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eguchi T, Kadota K, Park BJ, Travis WD, Jones DR, Adusumilli PS. The new IASLC-ATS-ERS lung adenocarcinoma classification: what the surgeon should know. Semin Thorac Cardiovasc Surg. 2014;26:210–22. doi: 10.1053/j.semtcvs.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon Y, Sung SW, Lee KY, Kim YK, Park JK. The importance of the lepidic component as a prognostic factor in stage I pulmonary adenocarcinoma. World J Surg Oncol. 2016;14:37. doi: 10.1186/s12957-016-0791-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshida J, Nagai K, Yokose T, et al. Limited resection trial for pulmonary ground-glass opacity nodules: fifty-case experience. J Thorac Cardiovasc Surg. 2005;129:991–6. doi: 10.1016/j.jtcvs.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 10.Nitadori J, Bograd AJ, Morales EA, et al. Preoperative consolidation- to-tumor ratio and SUVmax stratify the risk of recurrence in patients undergoing limited resection for lung adenocarcinoma ≤2 cm. Ann Surg Oncol. 2013;20:4282–8. doi: 10.1245/s10434-013-3212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho JH, Choi YS, Kim J, Kim HK, Zo JI, Shim YM. Long-term outcomes of wedge resection for pulmonary ground-glass opacity nodules. Ann Thorac Surg. 2015;99:218–22. doi: 10.1016/j.athoracsur.2014.07.068. [DOI] [PubMed] [Google Scholar]
- 12.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York (NY): Springer; 2010. [Google Scholar]
- 13.Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–60. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 14.Moon Y, Kim KS, Sung SW, et al. Correlation of histological components with tumor invasion in pulmonary adenocarcinoma. World J Surg Oncol. 2014;12:388. doi: 10.1186/1477-7819-12-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasada S, Nakayama H, Miyata Y, et al. Comparison of malignant grade between pure and partially invasive types of early lung adenocarcinoma. Ann Thorac Surg. 2015;99:956–60. doi: 10.1016/j.athoracsur.2014.10.041. [DOI] [PubMed] [Google Scholar]
- 16.Yanagawa N, Shiono S, Abiko M, Ogata SY, Sato T, Tamura G. The correlation of the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) classification with prognosis and EGFR mutation in lung adenocarcinoma. Ann Thorac Surg. 2014;98:453–8. doi: 10.1016/j.athoracsur.2014.04.108. [DOI] [PubMed] [Google Scholar]
- 17.Koike T, Kitahara A, Sato S, et al. Lobectomy versus segmentectomy in radiologically pure solid small-sized non-small cell lung cancer. Ann Thorac Surg. 2016;101:1354–60. doi: 10.1016/j.athoracsur.2015.10.048. [DOI] [PubMed] [Google Scholar]
- 18.Altorki NK, Kamel MK, Narula N, et al. Anatomical segmentectomy and wedge resections are associated with comparable outcomes for patients with small cT1N0 non-small cell lung cancer. J Thorac Oncol. 2016;11:1984–92. doi: 10.1016/j.jtho.2016.06.031. [DOI] [PubMed] [Google Scholar]