Abstract
Cancers are an extraordinarily heterogeneous collection of diseases with distinct genetic profiles and biological features that directly influence response patterns to various treatment strategies as well as clinical outcomes. Nevertheless, our growing understanding of cancer cell biology and tumor progression is gradually leading towards rational, tailored medical treatments designed to destroy cancer cells by exploiting the unique cellular pathways that distinguish them from normal healthy counterparts. Recently, inhibition of the activity of phosphodiesterase type 5 (PDE5) is emerging as a promising approach to restore normal intracellular cyclic guanosine monophosphate (cGMP) signalling, and thereby resulting into the activation of various downstream molecules to inhibit proliferation, motility and invasion of certain cancer cells. In this review, we present an overview of the experimental and clinical evidences highlighting the role of PDE5 in the pathogenesis and prevention of various malignancies. Current data are still not sufficient to draw conclusive statements for cancer patient management, but could provide further rational for testing PDE5-targeting drugs as anticancer agents in clinical settings.
Keywords: phosphodiesterase, cancer, targeted therapy, biomarkers, chemoprevention
INTRODUCTION
The current global demographic, epidemiologic and nutritional transitions signal an enormous cancer burden on society in countries of all income levels. The incidence of cancer cases is on continuing growth also because of an increasing prevalence of established risk factors, such as tobacco use, excess body weight, physical inactivity, infection and changes in the reproductive patterns connected with urbanization and economic development. Based on data from GLOBOCAN 2012, an estimated 14.1 million new cancer cases and 8.2 million deaths were recorded in 2012 globally [1]. Breast and lung cancers represent the most frequently diagnosed cancers and the leading causes of cancer death in women and men, respectively, in the world and in less developed countries. Other frequently diagnosed cancers worldwide include those of the prostate, liver, stomach, and colorectum among males and those of the stomach, cervix uteri, and colorectum among females. A substantial proportion of cases can be prevented by broadly adoption of effective measures, such as smoking control, vaccination (for liver and cervical cancers), early detection, and promotion of healthy behaviors. Moreover, the burden of suffering and premature deaths can be decreased through the use of an appropriate therapy and palliative care [2]. Surgery, chemotherapy and radiotherapy are currently the three major treatments to prolong the survival of the majority of cancer patients, but clinical improvements are often associated to undesirable side effects on normal cells or tissues. Thus, as the biology of cancer has become progressively understood on a molecular level, therapeutic research has mainly shifted its focus from cytotoxic oncology drugs to newer target-based agents able to inhibit specifically tumor outgrowth and progression mechanisms or enhance host immune responses against cancer cells. On the other hand, clinical trials and meta-analyses have demonstrated that simultaneous or sequential multi-modal therapies may improve patient outcome, may have acceptable tolerability profiles and may be active against a variety of tumor types as compared with a single-modality therapy [3–6]. In the search of molecularly targeted cancer therapy, tremendous interest has been given to the expression and regulation of the phosphodiesterase type 5 (PDE5) as an important signaling modulator involved in diverse aspects of tumor cell function. In the last decade, a significant number of studies have reported an increased expression of PDE5 in several human cancers compared to normal or surrounding non-neoplastic tissues [7–10]. Concomitantly, PDE5 inhibitors have been examined in multiple malignancies and cancer cell lines for their direct anticancer activities, for their efficacy as chemo-sensitizers and for cancer chemoprevention (reviewed in [11, 12]). In this review, we will highlight the emerging knowledge and our recent findings showing the role of PDE5 as a tumor biomarker as well as a potential target for therapeutic strategies aimed at controlling and eventually curing malignant diseases. First, we will briefly discuss the structure and the biological function of PDE5. Next, we will summarize the significance of PDE5 expression on different cancer types in clinical settings as well as in experimental cellular and animal models. The benefit of targeting PDE5 in cancer prevention or treatment will be also discussed because of the numerous advantages of PDE5 inhibitors.
The superfamily of PDEs
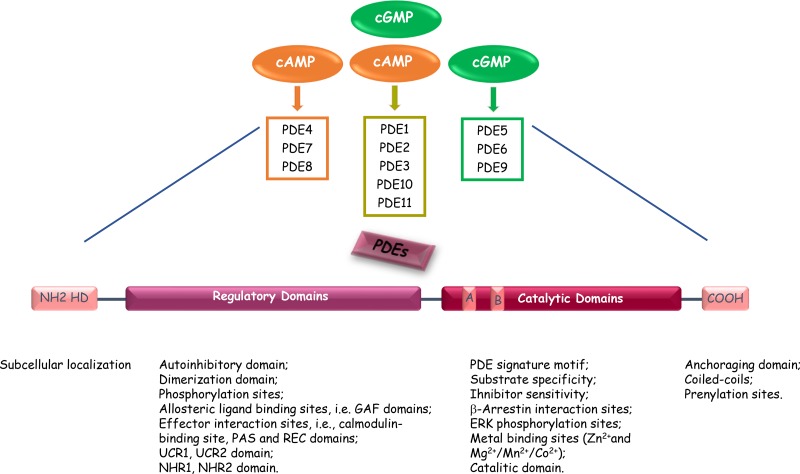
Mammalian cyclic nucleotide phosphodiesterases (PDEs) constitute a large and complex family of ubiquitously distributed hydrolases that have the unique function of catalysing the hydrolytic breakdown of cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) into the biologically inactive derivates 5′-AMP and 5′-GMP, respectively. The PDE superfamily contains 11 distinct gene families (PDEs 1 to 11), that encode at least 100 distinct PDE isoforms through alternative mRNA splicing, multiple promoters and transcription start sites in human, rat, and mouse [13]. The 11 PDEs can be grouped into three broad categories based on their sequence homology as well as substrate specificity and selectivity. PDE4, PDE7 and PDE8 are specific for cAMP hydrolysis; PDE5, PDE6 and PDE9 are specific for cGMP hydrolysis; PDE1, PDE2, PDE3, PDE10 and PDE11 exhibit dual specificity, acting on cAMP and cGMP with different affinities depending on the isoform (Figure 1 and Table 1) [13–72]. The different PDEs are modular proteins sharing the following common structural organization from N-terminus to C-terminus (Figure 1): 1) a highly divergent regulatory domain in the N-terminal portion; 2) a conserved catalytic core of approximately 270 amino acids (~ 35–50% sequence homology); 3) a region of undetermined function that can be prenylated (PDE6) or phosphorylated (PDE4) in the carboxyl terminus [19]. The N-terminal portions of PDE molecules contain structural determinants and specific amino acid sequences that are responsible for localization of individual PDE isoforms to specific intracellular sites, organelles and membranes as well as for their incorporation into particular multimolecular regulatory complexes or signalosomes. The regulatory regions contain domains that can be subjected to diverse types of modification (e.g., phosphorylation by various protein kinases), or sites that may interact with allosteric ligands (e.g., cGMP binding sites), selective effectors (e.g., Ca2+/calmodulin), protein partners (e.g., RAF1), or molecular scaffolds (e.g., caveolin). In addition, N-terminal regulatory regions include dimerization domains and autoinhibitory modules as several PDEs exist as asymmetric homo- or heterodimers (e.g., in PDEs 1, 4, and 5). Informations from the crystal structures of isolated catalytic cores of PDEs have revealed that these domains exhibit the same topography, composed of aminoacids folded into 16 helices [73]. The active site forms a deep hydrophobic pocket that includes a histidine-rich PDE signature sequence motif and consensus metal binding domains, represented by two Zn2+ binding motifs (motifs A and B – HX3HXnE/D) and an additional binding site whose metals could be Mg2+, Mn2+ or Co2+ [73, 74]. In addition to the conserved portions important for cyclic nucleotide and inhibitor bindings, the catalytic domain also encompasses variable determinants that are responsible of PDE family-related substrate affinities and selectiveness [75].
Figure 1. Common structure of the different PDE enzymes.
The PDE superfamily contains 11 structurally-related gene families. Some PDEs are specific for cAMP or cGMP, and some exhibit dual substrate specificity. The N-terminal portion of the PDEs contains sequences important for cellular localization. The regulatory domain contains PDE-family specific sequences responsible of modulation of PDE enzymatic activities. The catalytic domain is present in the carboxy-terminal part of PDEs and is highly conserved. A and B indicate the two Zn2 + -binding motifs (HX3HXnE/D) that include invariant histidines and are critical to catalysis. HD, Hydrophobic domains.
Table 1. PDE families and inhibitors.
| Isozime Family | Gene Members | Substrate Specificity | Regulation | Major Tissue and Cellular Expression | Subcellular Localization | Functions | Inhibitors | References |
|---|---|---|---|---|---|---|---|---|
| PDE1 | A, B, C | cAMP/ cGMP |
Ca2+/ calmodulin |
Lung, heart, brain, smooth muscle, testis, sperm, macrophages, lymphocytes | Cytosolic/ perinuclear |
Vascular smooth muscle contraction, sperm function (PDE1A); Dopaminergic signaling, immune cell activation, and survival (PDE1B) Vascular smooth muscle cell proliferation, sperm function, neuronal signaling (PDE1C) |
Vinpocetine, IC224, IC86.340, SCH51866, 8-MeoM-IBMX, ITI-214 | [14–24] |
| PDE2 | A | cAMP/ cGMP |
cGMP-stimulated | Adrenal cortex, lung, liver, platelets, heart, brain, macrophages, endothelium | Membrane-bound or cytosolic, mitochondria | Regulates aldosterone secretion, phosphorylation of calcium channel in heart, cGMP in neurons; endothelial cell function under inflammatory conditions | EHNA, BAY60–7550, IC933, PDP, OXIDOLE | [16, 21, 25–29] |
| PDE3 | A, B | cAMP/ cGMP |
Phosphorylation/cGMP-inhibited | Lung, heart, adipose tissue, adipocytes, liver, smooth muscle, kidney, hepatocytes, pancreatic beta cells, immune cells, platelets | Membrane-bound or cytosolic | Cardiac contractility, platelet aggregation, vascular smooth muscle contraction, oocyte maturation, renin release (PDE3A) Insulin signaling, cell cycle, proliferation (PDE3B) |
Milrinone, Tolafentrine, Enoximone, K-134, Cilostazol, Cilostamide, Trequinsin, OPC-33540 | [21, 22, 29, 30–35] |
| PDE4 | A, B, C, D | cAMP | Phosphorylation/cAMP-specfic cGMP-insensitive |
Broad, cardiovascular, neural, immune and inflammatory systems | Membrane-bound or cytosolic | Brain function, monocyte and macrophage activation, neutrophil infiltration, vascular smooth muscle proliferation, fertility, vasodilatation, cardiac contractility | Cilomilast, Rolipram, Ro20–1724, Roflumilast, AWD12281, V11294A, SCH35159, GSK256066, Denbufylline, Arofylline, Apremilast | [21, 22, 29, 32, 36–46] |
| PDE5 | A | cGMP | Phosphorylation/cGMP-specific cGMP-activated |
Broad, lung, cerebellum, heart, brain, platelets, vascular myocytes, cardiac myocytes, gastrointestinal tissues and penis | Cytosolic | Vascular smooth muscle contraction, platelet aggregation, cGMP signaling in brain | Sildenafil, Taldanafil, DA8159, Exisulind, E402, Vardenafil, Zaprinast, DMPPO, Dipyridamole, Mirodenafil | [16, 21, 29, 47–57] |
| PDE6 | A, B, C, D, G | cGMP | Phosphorylation/cGMP-specific | Retina and pineal gland | Cytosolic | Phototransduction | Avanafil, Udenafil, Zaprinast | [29, 58–61] |
| PDE7 | A, B | cAMP | Transduction activated/ cAMP-specific | Heart, liver, kidney, brain, pancreas, testis, spleen, skeletal muscle, immune cells | Cytosolic | Immune cell activation (PDE7A) Memory function and excreted T (PDE7B) |
ASB16165, BRL50481, IC242, Dipyridamole, BMS-586353, Thiadiazoles | [21, 22, 29] |
| PDE8 | A, B | cAMP | cAMP-specfic Rolipram/IBMX insensitive |
Broad, testis, liver, heart, kidney, brain, skeletal muscle, thyroid, spleen, colon, ovary, immune cells | Membrane-bound or cytosolic, mitochondria | T-cell activation, sperm or Leydig cell function, T4 and T3 production (PDE8A) | Dipyridamole, PF-04957325 | [29, 40, 62–64] |
| PDE9 | A | cGMP | cGMP-specific IBMX/insensitive |
Broad, kidney, liver, lung, brain, spleen, prostate, heart | Cytosolic or nuclear | NO-cGMP signaling in brain | BAY 73–6691, SCH-51866, WYQ C28L, PF-04447943 | [21, 29, 65, 66] |
| PDE10 | A | cAMP/ cGMP |
Unknown | Brain, heart, thyroid, testis | Cytosolic or particulate | Learning and memory | Papaverine, Dipyridamole, PQ-10, TP-10, MP-10 | [16, 29, 67, 68] |
| PDE11 | A | cAMP/ cGMP |
Unknown | Liver, prostate, testis, salivary and pituitary gland | Cytosolic | Sperm development and function | None selective | [62–72] |
As expected from their complex genomic organization, multiple PDE isoforms are expressed in almost all cells (Table 1) [75, 76]. However, some cells are relatively enriched in specific PDEs (e.g., photoreceptor PDE6 exclusively expressed in retina rods and cones and in the pineal gland) as well as some PDE alterations are tightly connected to different pathological conditions (e.g., PDE4B abnormalities have been linked to schizophrenia [77]) [75, 76, 78]. Recently, genetic alterations or overexpression in PDE genes were described to be associated with tumor development. Polymorphisms in the genes encoding PDE8A and PDE11A have been associated with a predisposition to developing certain adrenocortical [79], testicular [69], and prostatic [70] cancers. More importantly, PDE5 overexpression has been reported in several types of cancers [7–10].
PDE5 structure, regulation, distribution, signalling and function
The human PDE5A gene is located on chromosome 4q264,5 and contains about 23 exons spanning approximately 100 kilobases, with the first three exons being alternative exons encoding the isoform-specific 5’-ends of the PDE5 sequences [80, 81]. Cloning and sequencing of the PDE5A gene showed that these alternative exons are arranged in the order of A1-A3-A2 and are separated by an intron of 434 bp between A1 and A3 and by another intron of 361 bp between A3 and A2 [82, 83]. The human PDE5A gene promoter, located upstream of the three isoform-specific first exons, consists of a 139 bp core with full basal activity, a 308 bp upstream regulatory region, and a 156 bp downstream regulatory region. Each of these two regulatory regions could independently convey the responsiveness of cAMP and cGMP to the core promoter and contained multiple consensus sites for several transcription factors, including Sp1 [83, 84]. The weaker PDE5A2 promoter is 182 bp in length and contains one AP2- and three Sp1-binding sequences [84]. The upstream PDE5A promoter is expected to direct the expression of all three PDE5 isoforms, while the intronic PDE5A2 promoter can only control the expression of the A2 isoform [85].
Human PDE5A1 and A2 transcripts are found to be expressed in almost all tissues, with PDE5A2 being more common, and identifiable in almost all cells cultured from aortic smooth muscle, bladder smooth muscle, urethra smooth muscle, penile smooth muscle, penile endothelium, aortic endothelium, etc. On the contrary, the distribution of human PDE5A3 appears to be restricted to tissues with smooth and/or cardiac muscle component [81]. The three PDE5A isoforms share similar cGMP-catalytic activities and differ only in the N-terminal domain, in which no biochemical or physiological function has been identified. The N-terminal part of PDE5 contains two regulatory GAF domains named as GAF A and B (domains originally found to be present in cGMP-regulated cyclic nucleotide PDEs, certain adenylyl cyclases and the bacterial transcription factor FhlA [86]). The identified functions of these regions are cGMP-mediated allosteric regulation and dimerization of GAF-containing PDEs. Allosteric binding of cGMP facilitates phosphorylation of human PDE5 by protein kinase G (PKG) on serine 102 and this phosphorylation seems to play a role in stabilizing the enzyme in its cGMP-bound active state [87], increasing both its catalytic activity and cGMP-binding affinity [87–90]. In addition of functioning as negative feedback for cGMP signalling by activating the cGMP-specific PDE5, cGMP levels may influence also the activity of non-selective PDE isoenzymes (e.g., PDE2 or PDE3) and thereby modulate the crosstalk between cyclic nucleotide pathways [91]. Modulation of cGMP concentrations is accomplished by cAMP- and cGMP-dependent activation of PDE2 and cGMP-dependent activation of PDE5 [92–94]. cAMP may also increase cGMP levels by inhibiting cGMP-degrading activities of PDE1 and PDE3 [92]. Recently, Zhao et al. demonstrated that amongst all PDEs, PDE2 and PDE5 compensate most strongly for the reduced activity of each other, an event that was indicated as strong coupling [95]. Indeed, NO/cGMP/PKG activity potentiated by PDE5 inhibition is partially compensated by PDE2 and reciprocally, compensatory increase in PDE5 cGMP rates is also the greatest upon PDE2 inhibition. PDE5 inhibition indirectly also leads to a decrease in PDE3 cAMP hydrolysis rates [95]. It was also reported that cGMP is able to directly activate PDE5 without phosphorylation in response to sustained nitric oxide (NO) in the platelet [96]. Moreover, small molecular mass proteins immunologically related to the gamma subunit of PDE6 may prevent PKA-mediated activation of PDE5 in airway smooth muscle [97]. More recently, an elegant study revealed, for the first time in mouse, the existence of three different PDE5A isoforms with similar biochemical features and different distribution patter and highlighted their potential role in the induction of hypertrophy [98]. The authors demonstrated that exogenous overexpression of each variant induced a sustained cell cycle progression in cardiomyocytes and fibroblasts transfected cells, with PDE5A3 isoform being more efficient in the modulation of hypertrophic markers respect to the other mPDE5A isoforms.
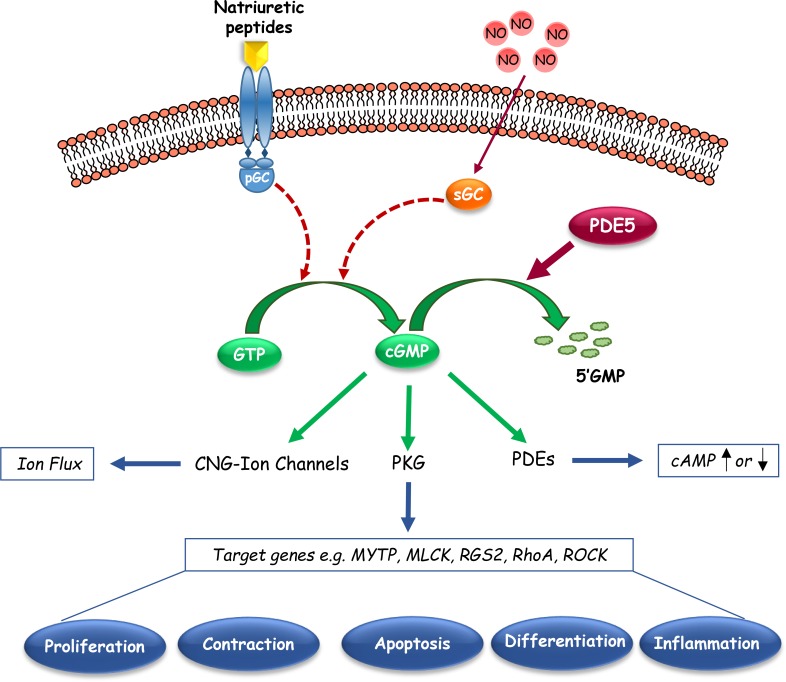
In catalysing the hydrolysis of cGMP, PDE5 plays critical roles in controlling its intracellular levels, the compartmentalization of its signalling pathways and its downstream biological responses. cGMP signalling is schematically shown in Figure 2. Briefly, cGMP activates different pathways, resulting into the activation of cGMP-dependent protein kinase G (PKG), cyclic nucleotide-gated (CNG) ion channels, or certain cGMP-binding PDEs, which lead to protein phosphorylation, ion fluxes, or cyclic nucleotide hydrolysis to affect gene expression or other aspects of cellular activity [99]. An essential player in cGMP signaling is considered the serine/threonine protein kinase PKG, whose downstream substrates are implicated in a variety of biological processes, including calcium homeostasis, platelet activation and adhesion, smooth muscle contraction, cardiac function, vasodilation, cell differentiation, proliferation, and apoptosis [100, 101]. The most recognized function of PDE5A is the modulation of vascular tone via regulation of intracellular cGMP and calcium levels, particularly in the lung and penis [75]. Moreover, cGMP-PKG signalling pathway activation increases cell proliferation and permeability in the vascular endothelium [102–104]; whereas it negatively impacts hypertrophy and contractility in cardiac myocardium [105, 106]. PDE5 has been also involved in the regulation of platelet aggregation [107], and in the improvement of learning and memory processes [108].
Figure 2. Schematic representation of cyclic guanosine monophosphate (cGMP) signaling pathways.
This graphic shows the basic synthetic, regulatory and downstream signalings that mediate the effects of endogenous cGMP in cells. Cyclic nucleotide phosphodiesterase type 5 (PDE5), which catalyzes the hydrolytic breakdown of cGMP into its biologically inactive derivative, regulates the amplitude and the duration of cGMP signalling. pGC, particulate Guanylyl Cyclase; sGC, soluble Guanylyl Cyclase; CNG-Ion channels, cyclic nucleotide-gated Ion channels; PKG, cGMP-dependent Protein Kinase or Protein Kinase G; MYTP, Myosin Phosphatase Targeting Subunit; MLCK, Myosin Light Chain Kinase; RGS2, Regulatory of G-coupled Signaling 2; ROCK, Rho-kinase.
PDE5 inhibitors: selectivity and adverse effects
Since the discovery and characterization of PDE5, a collection of molecules able to inhibit its enzymatic activity have been conceived. The first drugs, such as IBMX (3-isobutyl-1-methylxanthine), dipyridamole and coffee, have been shown to be potent but non selective inhibitors. Later, zaprinast was described as a more specific molecule [109], but it did not find its application into clinical practice. However, its chemical structure was used to design compounds with higher potency and selectivity, leading to the identification in 1989 of sildenafil (1-[4-ethoxy-3-(6, 7-dihydro-1-methyl–7-oxo-3-propyl-1H-pyrazolo [4, 3-d]pyrimidin-5-yl) phenylsulphonyl]-4-methylpiperazine). Sildenafil, developed and marketed as Viagra by Pfizer, showed a higher selectivity (> 1000 fold) for human PDE5 over PDE2, PDE3 and PDE4 and moderate selectivity (> 80 fold) over PDE1 [110]. On the contrary, this drug was shown to be only ~ 10 fold more effective for PDE5 than retinal PDE6, explaining some blue tinting of vision reported as adverse effects after its use [111]. As a consequence, many companies designed other selective PDE5 inhibitors with reduced inhibitory effects towards PDE6, including vardenafil (Levitra, Bayer-GSK), and tadalafil (Cialis, IC351, Lilly-ICOS), which is 1000 fold less potent towards PDE6 [112]. These compounds also interact with PDE11, with tadalafil being the most potent of them in respect to vardenafil (IC50 = 73 nM and IC50 = 840 nM, respectively), but the functional consequences of these effects are still unknown [113]. Compared to sildenafil and vardenafil, tadalafil also provides a longer therapeutic effect due to its extended half-life [114]. A low incidence of some PDE-related adverse events was then obtained with avanafil (Stendra, Vivus inc.) [115]. Indeed, this molecule, which acts more rapidly than other PDE5 inhibitors, showed a higher selectivity against non-PDE5 isozymes as following: i) ~ 6.5 fold greater than sildenafil and vardenafil for PDE6; ii) more than 27 fold compared to sildenafil for PDE1; iii) ~ 760 fold higher than tadalafil for PDE11. Other PDE5 inhibitors, including udenafil, mirodenafil and lodenafil carbonate are currently under clinical investigation [116–118]. It is important to underline that some PDE5 inhibitors may also affect non-PDE proteins. Indeed, it has been shown that vardenafil was able to block calcium channels in rabbit pulmonary arteries and human platelets [119], and sildenafil interacted with multidrug resistance protein 1 (MDR1; also known as ABCB1) and antigen peptide transporter 1 (APT1; also known as ABCB2) to block drug extrusion from cells [120]. This latter off-target effect could be significant in reducing ABCB1- and ABCB2-mediated drug resistance and thus improving the efficacy of some chemotherapeutic agents, most probably independently of PDE5 inhibition [120, 121].
Data from reports using selective PDE5 inhibitors have been essential for a robust understanding of the cellular functions that are regulated by PDE5 in physiological states as well as under pathological conditions. For instance, sildenafil, originally designed as an antihypertensive molecule or a coronary vasodilator, was shown to induce responses in off-target tissues, such as in penis, changing the focus of this agent to erectile dysfunction (ED) and proposing PDE5 as a target for the treatment of ED. In addition, the improvement of pulmonary vascular physiology observed in in vitro and in vivo models of pulmonary hypertension following PDE5 inhibition has provided the rationale to recommend also PDE5 as a target for the treatment of pulmonary hypertension and respiratory distress [122, 123].
At the present time, the orally administrated PDE5 inhibitors sildenafil and tadalafil have Food and Drug Administration approval for the treatment of ED as well as pulmonary arterial hypertension (PAH); whereas vardenafil and avanafil are approved only for ED. However, a great number of papers published over the last decade highlighted the potential clinical use of PDE5 inhibitors in other applications, for which current therapies are limited and in which the mechanism of action does not necessarily rely on their known vasodilatatory effects [124]. Table 2 summarizes the medical conditions other than ED and PAH that have shown consistent benefits from treatment with these pharmacologic agents [124–196]. Importantly, reported adverse effects are generally mild, i.e. flushing, headache, backache, dyspepsia, and nasal congestion. Some users of sildenafil and vardenafil, that, as mentioned earlier, slightly inhibit photoreceptor PDE6, may also experience temporary and reversible minimal visual disturbances. Therefore, as reported in various reviews of pharmacotherapy, they represent a class of relative benign molecules in terms of safety and tolerability [197–199].
Table 2. Proposed novel applications of PDE5 inhibitors.
| Applications | Conditions | Agents | References |
|---|---|---|---|
| Male genitourinary dysfunctions | Benign prostatic hyperplasia and lower urinary tract symptoms Peyronie's disease Priapism Premature ejaculation, inability to ejaculate Low sperm count or motility |
Sildenafil, Tadalafil, Vardenafil, UK-369, 003 Sildenafil Sildenafil Tadalafil, Sildenafil, Vardenafil Tadalafil, Sildenafil, Vardenafil |
[125–130] [131–134] [135–137] [138–140] [141–145] |
| Neurologic dysfunctions | Neurogenesis and recovery from stroke Cognitive functions |
Tadalafil, Sildenafil Sildenafil |
[146–149] [150–153] |
| Tissue and organ protection | Antineoplastic agent toxicity Gastrointestinal damage |
Sildenafil Sildenafil |
[157–157] [158–160] |
| Cutaneous Ulcerations | Antiphospholipid syndrome Scleroderma Refractory Raynaud's phenomenon Systemic sclerosis |
Sildenafil Sildenafil Sildenafil Sildenafil |
[161] [162, 163] [163–165] [166, 167] |
| Transplant and reconstructive surgery | Heart transplant Liver transplant Renal transplant Lung transplant Reconstructive surgery |
Sildenafil Sildenafil Sildenafil Sildenafil Sildenafil |
[168–170] [171–174] [175] [176] [177–178] |
| Female genital dysfunctions | Fertility Pre-eclampsia |
Sildenafil Sildenafil |
[179–184] [185–187] |
| Diabetes | Neuropathy and vasculopathy Endothelium damage |
Sildenafil Sildenafil, Tadalafil |
[188–191] [192–196] |
Links between PDE5 and selected cancers
There has been a remarkable interest in identifying new clinical use of PDE5 inhibitors as potent anticancer drugs with a novel mechanism of action (Figure 3). Since 2000, more than 150 papers have been reported on the connection of PDE5 and different kind of tumors. Indeed, increased expression of PDE5 in various human malignancies and the lack of such expression in normal cells have been described. In addition, PDE5 inhibitors have been examined for their direct inhibitory effects on tumor cell lines, for their ability to act as a sensitizers of cancer cells to chemotherapeutic agents, and as cancer chemopreventive agents [12]. However, much of the research is preclinical, and only few clinical trials have been completed or are ongoing so far (http://www.clinicaltrials.gov). The list of the studies focused on the effects of PDE5 inhibition in cancer patients is reported in Table 3.
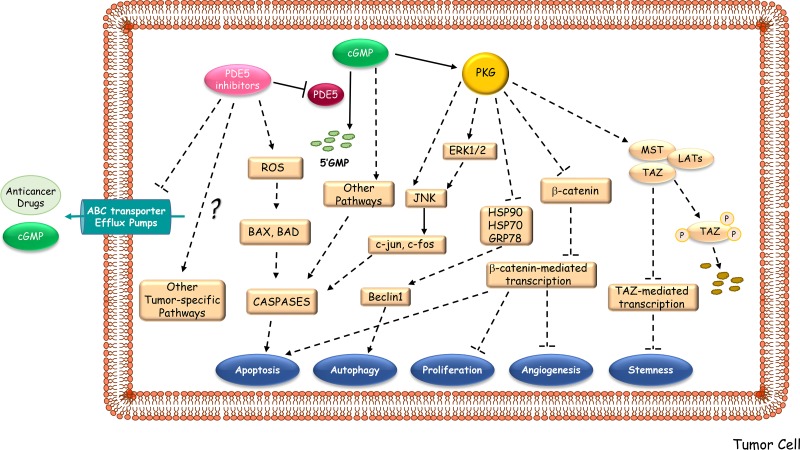
Figure 3. Proposed mechanisms underlying the anti-cancer activities of PDE5 inhibitors.
PDE5 inhibitors may hamper tumor progression by activating downstream signaling pathways, mainly PKG-mediated ones, which induce apoptosis, autophagy, growth suppression, inhibition of angiogenesis and of stemness. PDE5 inhibitors may also enhance the therapeutic effectiveness of multiple anti-neoplastic agents by increasing intracellular accumulation of drugs and cGMP levels through the block of the substrate efflux function of ABC multidrug-resistant transporters. ABC transporter Efflux Pumps, ATP-binding cassette transporter Efflux Pumps; ROS, Radical Oxygen Species; HSP, Heat Shock Protein; GRB78, Glucose-Regulated Protein; JNK, c-Jun N-terminal kinases; ERK, Extracellular Signal-regulated Kinases; MST, Mammalian Ste20-like Protein Kinase; LAT, Large Tumor Suppressor Kinase; TAZ, Transcription Regulator Protein-1.
Table 3. List of the studies on PDE5 inhibitors and cancer at www.clinicaltrials.gov*.
| Inhibitor | Indication | Status | ClinicalTrials.gov# |
|---|---|---|---|
| Sildenafil | Pancreatic Cancer, Cholangiocarcinoma Advanced Solid Tumors Bladder Cancer Kidney Cancer Breast, Gastrointestinal, Genitourinary, Gynecological Cancers Non-small Cell Lung Cancer High-grade Glioma Waldenstrom's Macroglobulinemia |
Phase 1 (recruiting) Phase 1 (recruiting) Phase 2 (recruiting) Phase 2 (completed) Phase 1 (active, not recruiting) Phase 2, Phase 3 (completed) Phase 2 (recruiting) Phase 2 (completed) |
NCT02106871 NCT02466802 NCT02422277 NCT01950923 NCT01375699 NCT00752115 NCT01817751 NCT00165295 |
| Tadalafil | Head and Neck Squamous Cell Carcinoma Head and Neck Cancer Head and Neck Cancer Head and Neck Squamous Cell Carcinoma Multiple Myeloma Multiple Myeloma Prostate Cancer Pancreatic Cancer Pancreatic Cancer Primary Abdominal Malignancy |
Phase 1 (recruiting) Phase 2 (recruiting) Phase 3 (completed) Phase 2 (completed) Phase 2 (recruiting) Phase 2 (terminated) Phase 3 (completed) Phase 1 (active, not recruiting) Phase 1 (active, not recruiting) Phase 1 (not yet recruiting) |
NCT02544880 NCT02544880 NCT00843635 NCT00894413 NCT01858558 NCT01374217 NCT00931528 NCT01342224 NCT01903083 NCT02998736 |
| Vardenafil | Glioma, Brain Neoplasms, Brain Metastasis | Early Phase 1 (recruiting) | NCT02279992 |
| Udenafil | Sigmoid Colon and Rectal Cancers | Phase 2 (completed) | NCT00607282 |
Lung cancer
Lung cancer has emerged as the most common cancer in the world, both in terms of new cases and deaths (1.82 million diagnoses and 1.6 million deaths recorded in 2012) with the highest rates in Central/Eastern Europe and Eastern Asia [2]. Non-small cell lung cancers (NSCLCs) represent more than 80% of all lung cancers [200]. Despite current advances in chemotherapy treatment regimens, response rates are still < 50%, and complete remissions remain rare, highlighting the need of agents with novel mechanisms of action to improve clinical outcomes.
Over the last years, the apoptotic and growth inhibition activities of PDE5 inhibitors have been demonstrated in numerous lung cancer cell lines. Most of the experiments were performed by using exisulind (sulindac sulfone), a sulfone metabolite of the nonsteroidal anti-inflammatory drug (NSAID) sulindac. Exisulind, which lacks the hallmark cyclooxygenase inhibitory activities of NSAIDs, acts as a cGMP PDE inhibitors, causing a persistent increase in cellular cGMP, and inducing PKG [201–203]. It was previously demonstrated that the combination of exisulind with cytotoxic drugs resulted in a synergistic inhibition of human lung cancer cell growth in culture [204]. In orthotopic lung cancer model systems, exisulind in association with docetaxel significantly induced apoptosis, reduced tumor growth and metastasis, and increased survival [10, 205, 206]. PDE5 inhibitors also improved the chemosensitivity of anti-cancer agents by increasing endocytosis-mediated cellular drug uptake in lung cancer cells; for instance, oral administration of the PDE5 inhibitor vardenafil significantly increases the accumulation and enhances the anti-tumor effect of trastuzumab in a xenograft mouse model of lung cancer [207]. Another study showed that sildenafil was able to enhance the anti-tumor effects of the standard of care drug pemetrexed in NSCLCs. In two models of human NSCLC cells growing in athymic mice, it was found that pemetrexed and sildenafil interacted in an additive fashion to suppress tumor growth and this effect was further enhanced in vivo by co-treatment with the mTOR inhibitor temsirolimus [208]. The complex molecular mechanisms by which these drug combinations induce lung cancer cell death were via increasing toxic autophagosome formation, and through the activation of extant death receptors. More recently, Booth et al. have evidenced how sildenafil enhanced the lethality of pemetrexed and sorafenib, a potent inhibitor of chaperone ATPase activities, in multiple genetically diverse lung cancer cell lines and in xenografts of lung cancer in athymic nude mice [208].
However, the clinical evidences do not support experimental findings. In 2006, a phase I/II study was designed to evaluate the safety and efficacy of exisulind in combination with gemcitabine as second-line therapy in NSCLC patients whose disease progressed more than 3 months from completion of first-line chemotherapy [209]. Although the primary endpoint of improving time to progression was met, the overall survival outcome of patients treated with the two drugs appears only slightly better than other phase II studies of single-agent gemcitabine. This may be likely due to suboptimal dosing of exisulind, or suboptimal scheduling or the lack of functional interaction between exisulind and cytotoxic drugs despite the preclinical observations. Other phase II studies using exisulind in combination with chemotherapy in advanced NSCLCs show no objective benefits regarding both overall survival and time to progression compared with historic controls treated with chemotherapy alone [210–212]. Disappointing results were also reported for the combination of carboplatin, etoposide, and exisulind as initial therapy for patients with newly diagnosed extensive stage small cell lung cancers [213].
Prostate cancer
Prostate cancer represents the second most frequently diagnosed solid malignancy among men worldwide, accounting for about 1.1 million cases in 2012 [1]. Fortunately, due to early detection and the nerve-sparing prostatectomy, relative death rates have been decreasing and men have been given hope for recovery of bladder and erectile function. Penile rehabilitation after prostatectomy often includes treatment with PDE5 inhibitors and accordingly, association between the effects of these drugs and cancer recurrence have been examined in different experimental and clinical studies.
In support of PDE5 inhibitors’ ability to affect prostate carcinogensis, it was reported that exisulind suppressed the growth of metastatic, human prostate cancer cells in a nude mouse xenograft model by increasing apoptosis [214]. In contrast, Qian et al. noted that incontinuous oral administration of sildenafil citrate was not able to influence primary tumor growth and metastasis in an orthotopic prostate cancer model, most probably because cGMP elevation were only transient [215]. It was shown that sildenafil enhanced apoptosis and antitumor efficacy of doxorubicin in mice bearing prostate tumor xenografts, while attenuating its cardiotoxic effects (i.e. left ventricular dysfunction) [216]. Next, the same research group investigated the mechanism by which sildenafil may sensitize prostate cancer cell to doxorubicin-mediated apoptosis, showing CD95 (death receptor Fas)/FLIP (Fas-associated death domain FADD) as key regulators of this event [217]. In 2016, by in vitro cell culture and in vivo xenograft approaches, it was clearly demonstrated that PDE5/cGMP/PKG signal targets to Hippo/TAZ pathway in maintaining stemness of prostate cancer stem cells, evidencing a novel role of PDE5 in governing stem cell features [218]. Moreover, PDE5 was found to be mainly located in the stromal compartment of the prostate, and accordingly tadalafil reduced proliferation of primary prostate stromal cells at a greater extent than of primary prostate basal epithelial cells [219]. Tadalafil also attenuated TGFβ1-induced fibroblast-to-myofibroblast trans-differentiation, suggesting a potential value of PDE5 inhibitors in preventing stromal enlargement and treating benign prostatic hyperplasia ([219] and reviewed in [220]).
A retrospective analysis including a total of 4974 men revealed that the use of PDE5 inhibitors over a 7-year period was associated to a decreased incidence rate of prostate cancer among men with ED compared to men with ED of the same age and with similar risk factors who were not treated with these agents [221]. It was hypothesized that a higher ejaculation frequency can protect against prostate cancer development or alternatively vasodilation induced by PDE5 inhibition may counteract hypoxia and thereby thwart the emergence of more aggressive cancer phenotypes [222–224]. Another group also evaluated the safety and efficacy of exisulind (twice daily for 12 months) in delaying disease progression in men with rising prostate specific antigen (PSA) levels after radical prostatectomy and found that exisulind inhibited the increase in PSA overall and prolonged PSA doubling time in high-risk patients compared with placebo [225]. Contrary to these findings, using a large clinical database of patients with prostate cancer (n = 4752) with a median follow-up of 60.3 months, it was shown that the use of PDE5 inhibitors after radical prostatectomy may adversely impact biochemical recurrence (BCR), defined as a PSA of 0.2 ng/ml or greater and increasing after radical prostatectomy [226]. However, there are several limitations to this study, such as the lack of information on the type of the drug, the dose, the exact duration and frequency of use. Other authors retrospectively performed a similar analysis of BCR in 2579 patients treated with bilateral nervesparing radical prostatectomy and showed that there was no association between PDE5 inhibitor use and an increased risk of BCR, regardless of the therapeutic regimen used [227].
On the basis of these findings, there is no strong evidence to modify current clinical practice, and therapy with PDE5 inhibitors still remains the more recommended option for many postprostatectomy patients for penile rehabilitation programs [228].
Breast cancer
Breast carcinoma is the most common malignancy and the leading cause of cancer-related death in women worldwide. Breast cancer is a complex and highly heterogeneous disease classified on the basis of global gene expression analyses into at least five biologically different intrinsic subtypes (i.e. luminal A, luminal B, human epidermal growth factor receptor 2 (HER2)-enriched, basal-like, and normal-like) with distinct morphologic features, variable clinical outcomes and disparate therapeutic responses [229].
Increased PDE5 expression has also been reported in various cell lines deriving from breast cancer (MCF-7, HTB-26, MDA-MB-468) [54], giving the rational to assess the anticancer effects of PDE5 inhibition. Indeed, the PDE5 inhibitor exisulind selectively exerted, in various breast cancer cells, pro-apoptotic and anti-proliferative effects concomitantly with elevation of cGMP and activation of PKG, without effects on human mammary normal epithelial cells [230]. Inhibition of PDE5 and activation of PKG by exisulind was associated with reduced oncogenic Wnt/β-catenin-mediated transcriptional activity and subsequent downregulation of target genes, including cyclin D1 and survivin [231]. Stable PDE5 silencing in the aggressive human breast cancer cell line MDA-MB-231T lead to a reduction of cell motility in vitro and of lung metastasis formations in an experimental metastasis assay in vivo [232]. This well fits with our recent data showing that stable overexpression of PDE5 in MCF-7 breast cancer cells significantly increased motility and invasion of all the stable PDE5- transfected clones tested compared to parental cells [7]. In addition to the direct anti-cancer activities, it was demonstrated that PDE5 inhibitors may act as potential cancer chemopreventive agents, due to their ability to suppress 1-methyl-1-nitrosourea (MNU)-induced mammary carcinogenesis [233].
In breast cancer clinical samples, increased PDE5 expression was verified by both RT-PCR and immunohistochemistry analyses, and was correlated with tumor grade stage and lymph node involvement [9, 234]. More recently, we demonstrated that PDE5 was differentially expressed among breast cancer molecular subtypes, with higher levels in HER2-enriched and triple-negative subtypes compared to the more favourable estrogen receptor (ER)-positive Luminal B- and the Luminal A subtypes [7]. Importantly, high PDE5 expression predict a worse prognosis for a cohort of 1,988 patients at 8-year median follow-up [7]. Significant difference was also found between overall survival for ER-positive patients having high or low PDE5 levels, highlighting a role for PDE5 in predicting disease progression in ER-positive tumors that according to our immunohistochemistry analysis may have lower levels of the enzyme compared with ER-negative cases. In addition, since PDE5 retained its significance when performing a multivariate analysis including PDE5 expression, ER, HER2, and lymph node status in the entire database, it is tempting to speculate that high PDE5 levels may independently predict poor outcome among patients with breast cancer. However, up to now, only one study was conducted to clinically evaluate the safety and activity of PDE5 inhibitor in breast cancer. In particular, it was demonstrated that administration of exisulind in combination with capecitabine was well tolerated in a small number of patients with metastatic breast cancer (n = 35), but the synergism between these two drugs at the doses tested appears to be modest [9].
Colorectal cancer
Colorectal cancer is the third most commonly diagnosed cancer in men and the second in women worldwide, with an estimated 1.4 million cases and 693,900 deaths occurring in 2012 [1]. In contrast to incidence trends, decreasing colorectal cancer mortality rates have been observed in a large number of countries and are most likely attributed to colorectal cancer screening, reduced prevalence of risk factors, and/or improved therapies. However, when widespread malignancy is encountered, these cases are not responsive to curative treatments.
In several colon tumor cell lines (e.g. HT29, T84, and HCT116), exisulind and analogs/derivatives were able to inhibit the oncogenic activity of β-catenin through a direct suppression of its transcription or increased proteosomal degradation, thereby promoting cell death [202, 235–238]. It was also reported that sulindac metabolites inhibit the mitogen-activated protein/extracellular signal-regulated kinase kinase (MEK/ERK) signaling cascade in colorectal cancer cell lines at doses that induce apoptosis, as additional molecular mechanisms by which Sulindac inhibits tumor cell growth [239, 240]. In addition, treatment of human colorectal cancer cells with sildenafil resulted in cell proliferation inhibition, cell cycle arrest and apoptosis accompanied by increased intracellular reactive oxidative specie levels in vitro and caused the reduction of xenograft tumor growth in nude mice [241]. Recently, combined inhibition of PDE5 and PDE10 by treatment with PDE isozyme-selective inhibitors, or by siRNA knockdown was shown to suppresses β-catenin, and the levels of its downstream targets, thereby suppressing proliferation and inducing apoptosis in colon tumor cells [242].
In clinical studies, exisulind prevented colorectal polyp formation in patients with familial adenomatous polyposis (FAP) over 24 months [243, 244]. Moreover, it has been demonstrated that exisulind inhibited azoxymethane-induced colon carcinogenesis in rats [201] and sildenafil suppressed polyp formation and inflammation in mice treated with azoxymethane/dextrane sulfate sodium [245], highlighting the chemopreventive role of PDE5 inhibition [246].
Brain cancer
Cancers of the brain and central nervous system accounted for 256,000 new cases and 189,000 deaths in 2012, with the highest incidence and mortality rates in more developed regions [2].
In both neuroblastoma N18TG2 and hybrid neuroblastoma-glioma NG108–15 cells, the presence and regulation of PDE5 mRNA during cell differentiation was previously demonstrated [247]. In medulloblastoma cells, PDE5 inhibitors interacted in a greater than additive fashion with vincristine/etoposide/cisplatin to cause cell death. PDE5 inhibitors promoted autophagy and enhanced chemotherapy-induced DNA damage in a nitric oxide synthase-dependent fashion [248]. Pharmacologic modulation of cGMP signaling is emerging as a novel approach for enhancing therapeutic agent permeability across the blood-brain tumor barrier, thereby increasing delivery to brain tumors and metastases. Oral administration of sildenafil and vardenafil selectively improved tumor capillary permeability in 9L gliosarcoma-bearing rats, without changes in normal brain capillaries [249]. Importantly, tumor-bearing rats treated with the chemotherapy agent, adriamycin, in combination with vardenafil exhibited a survival significantly longer than rats treated with adriamycin alone [249]. The combination of OSU-03012/sildenafil synergized with low concentrations of sorafenib to kill glioblastoma cells in vitro and in vivo [250]. PDE5 inhibitors enhanced transport and therapeutic efficacy of trastuzumab in hard-to-treat brain metastases from different primary tumors [251]. Tadalafil can also enhance the treatment efficacy of the chimeric anti-CD20 monoclonal antibody Rituximab by improving the microvascular permeability in intracranial brain lymphoma mice model [252]. In contrast to these findings, it was shown that genetic and pharmacological inhibition of PDE5 activity strongly enhanced cell motility and invasiveness in human glioblastoma T98G cells, whereas PDE5 overexpression in PDE5-negative U87G cells significantly inhibited their invasive potential and interfered with DNA damage repair and cell survival following irradiation [253].
Analysis of a cohort of 69 patients affected by glioblastoma multiforme (GBM) who underwent chemotherapy and radiotherapy following surgical resection of the tumor revealed that PDE5 was strongly expressed in 50% of cancer cases [253]. Retrospective analysis indicated that high PDE5 expression significantly correlated with increased overall survival of patients, identifying this enzyme as a favourable prognostic marker for GBM, which negatively affects cell invasiveness and survival [253].
Thyroid cancer
Carcinoma of the thyroid is the most common malignancy of endocrine organs, representing 2.1% of the new cancer cases (about 230,000 among women and 70,000 among men in 2012) [254]. Over the last few decades, a steady increase of thyroid cancer has been appreciably observed in most areas of the world, and if current trends are maintained, thyroid cancer may become the fourth most common cancer by 2030. More than 95% are well-differentiated papillary or follicular tumors that derive from follicular epithelial cells and can be effectively managed by surgical resection with or without radioctive iodine ablation. A minority of thyroid cancers are medullary thyroid carcinomas that derive from the neuroendocrine C cells of the thyroid or anaplastic malignancies that are the rarest but the most lethal subtype [255]. Treatment for about two thirds of patients with progressive metastatic papillary and follicular thyroid cancers as well as for patients with less differentiated tumors is often of limited benefit, due to their inability to concentrate radioiodine or to the development of therapy resistance [256, 257]. Thus, there is a pressing need for innovative treatments in patients having high risk of disease-related death.
The role of cGMP levels and PDE5 in thyroid cancer are still not well defined. Analysis of the mRNA expression of members of the 11 known families of PDEs has revealed the expression of PDE4, PDE5, PDE7 and PDE8 subtypes in normal thyroid tissues [258]. Previous studies have also reported the presence of PDE4 in toxic adenomas characterized by mutations in the TSH receptor (TSH-R) gene [259]. In 2015, Sponziello et al. showed, for the first time, higher mRNA and protein expression levels of PDE5 in a series of human papillary thyroid carcinomas belonging to two independent cohorts compared to non-tumor tissues [260]. Interestingly, tumors presenting BRAF V600E mutation, that is the most frequent genetic alteration and also a marker of aggressiveness [261], exhibited a marked upregulation of PDE5 respect to those without mutation. Increased PDE5 transcripts were also associated with a reduction of the expression of TSH-R, thyroglobulin (Tg), thyroid peroxidase (TPO), sodium/iodide symporter (NIS), important differentiation markers implicated in intra-thyroidal iodine metabolism and thyroid hormone synthesis. More recently, a second study reported higher intracellular cGMP levels and cGMP-PDE activity in thyroid malignant (papillar and follicular) carcinomas than in controls and benign pathologies (i.e. benign struma, adenomatous hyperplasia, chronic thyroiditis, benign adenoma) [262]. However, the cGMP-PDE expression was elevated in papillary carcinomas without lymph node involvement (N-) in respect to those with lymph node invasion (N±), while cGMP levels displayed an inverse trend. These events may represent an autophagic defence mechanism of the body against the cancer that is expanding and invading other tissues and organs. Although these findings are promising, further studies are needed to draw definitive conclusions.
In papillary and anaplastic thyroid cancer cells in vitro, sildenafil and tadalafil determined a reduction of proliferation and at lower doses, they were also able to reduce cellular migration [260]. The effects of PDE5 inhibitors were stronger in the cancer cell lines carrying the BRAF mutation, suggesting that these tumors could be a preferential subtype for the action of PDE5-targeted drugs. A significant decrease of the drug dosages necessary to achieve an anticancer effect was obtained with the encapsulation of these compounds within nanoliposomes [263], that may represent, if confirmed in vivo, a valuable novel formulation for the treatment of thyroid carcinomas and other types of cancers.
Melanoma
Cutaneous melanoma accounted for almost 5% of all new diagnosed cancer cases, with a reported mortality of approximately 2%, making it the deadliest form of skin cancer. Although early detection carries an excellent prognosis, with surgical excision often being curative, the long-term survival rate for patients with metastatic melanoma is only 5% [264]. The disease derived from genetically altered epidermal melanocytes that arises because of complex interactions between genetic alterations, such as RAS pathway mutations and environmental factors, especially exposure to UV radiation [265]. The role of PDE5 on pathogenesis or progression of melanoma remains still an area of debate. Murata et al. have shown PDE5 activity and expression in malignant melanoma cells, and reported an important function for this enzyme in regulating melanoma progression as two PDE5 inhibitors inhibited cell growth [266]. However, the first mechanistic insights on the link between PDE5 and melanoma was suggested by Arozarena et al. in 2011 [267]. Indeed, it was demonstrated that in melanoma cells oncogenic BRAF, through the transcription factor BRN2, was able to suppress PDE5A expression. PDE5 down-regulation, in turn, leads to enhanced cGMP levels, which induce an increase in cytosolic Ca2+, a stimulation of actin-myosin contractility and a subsequent increase in cell invasion in vitro and in vivo [267]. Accordingly, immunohistochemistry analysis in a tissue microarray consisting of triplicate cores of 28 primary and 29 metastatic malignant melanoma cases revealed a statistically significant downregulation of PDE5A in metastatic tumors [267]. More recently, it was reported that the growth-promoting cGMP signaling could be potentiated pharmacologically by treatment with sildenafil in murine and human melanoma cells, suggesting that possible skin adverse effects of PDE5 inhibitors should be better considered [268].
Clinically, in a prospective cohort study, men who used sildenafil for ED had a statistically significant elevated risk of developing melanoma, without affecting the risk of squamous or basal cell carcinomas, and this correlation was maintained in the models controlling for the major host characteristics, such as age, body mass index, family history, sun exposure, and UV index in the state of residence [269]. A subsequent nationwide, population-based, nested case-control study in Sweden, including 4065 melanoma cases diagnosed from 2006 through 2012, showed that the use of PDE5 inhibitors was associated with a modest but statistically significant increased risk of malignant melanoma, but the pattern of association (e.g. the lack of association with multiple filled prescriptions) raises questions about the causality of this relationship [270]. Later, a large UK-based primary care database, with 145,104 men who were prescribed a PDE5 inhibitor and 560,933 matched controls, was examined, but the findings showed no evidence of a positive association between PDE5 inhibitor exposure and melanoma risk after matching or adjusting for key potential confounders [271]. More recently, a meta-analysis of 5-population-based observational studies revealed an increased risk of malignant melanoma in users of PDE5 inhibitors for ED [272]. Although a large number of patients and a long follow-up were included in this report, population selective bias, the lack of dose-response analysis and in general the weakness inherent in observational studies should be acknowledged. Thus, collectively, the present findings are insufficient to alter current clinical recommendation.
Other cancers
A possible role of PDE5 inhibition has been suggested also in the management of other cancers. Immunohistochemistry showed that PDE5 was overexpressed in human squamous and transitional cell carcinomas compared with normal urothelium and accordingly, exisulind exhibited antineoplastic activity in vivo in a model of rat urinary bladder tumorigenesis [8]. The addition of PDE5 inhibitors to multiple existing treatment regimens, including doxorubicin, mitomycin C, gemcitabine, cisplatin and paclitaxel, significantly enhanced chemotherapy lethality by stimulating the extrinsic apoptosis pathway via CD95 and by promoting autophagy through RIP-1 (receptor interacting protein 1) in bladder and pancreatic cancer cell lines [156]. In human renal carcinoma cell lines, suppression of PDE5 gene expression by PDE5 siRNA reduced cell proliferation and induced apoptosis through cGMP-PKG activation [273]. Sildenafil in combination with C-type natriuretic peptide synergistically inhibited proliferation of rhabdomyosarcoma cells and suppressed tumor growth in vivo [274]. Sildenafil and vardenafil were also able to induce apoptosis in peripheral blood mononuclear cells isolated from fourteen patients with chronic lymphocytic leukemia through a caspase 3-dependent pathway [275]. Recently, it was shown that vardenafil potentiates the killing effect of the green tea polyphenol (–) epigallocatechin-O-3-gallate on leukaemia and multiple myeloma cells, without affecting normal cells [276, 277]. Interestingly, in a patient with end-stage relapsed/refractory multiple myeloma, the addition of tadalafil reduced myeloid-derived suppressor cell function, that was associated to anti-myeloma immune responses and clinical benefit [278]. In 2015, a randomized, prospective, double blinded, placebo controlled, phase II clinical trial to determine the activity of PDE5 inhibitors on immune function in head and neck squamous cell carcinoma (HNSCC) patients was conducted [279]. Results showed that tadalafil can reverse tumor-specific immune suppression in these patients, with important therapeutic potential.
Concluding remarks
Over the last decades, researchers have elucidated the roles that impairment of cGMP signaling pathway by PDE5 activity inhibition plays in the regulation of tumor development, and progression. As a consequence, our knowledge on the link between PDE5 inhibitors and cancer biology has expanded, holding great promise for future use of these agents in several cancers. Although evident clinical controversial data come from patients affected by melanoma and glioblastoma multiforme, studies discussed in this literature review show that PDE5 inhibition could be associated with a decreased risk of cancer development and suppression of tumor progression in several malignancies including those of the lung, prostate, breast and colorectum. PDE5 inhibitors may also provide an additional antitumor immune response in patients affected by myeloma and head and neck squamous cell carcinomas. In addition, a synergistic effect with current chemotherapeutic regimens and monoclonal antibodies has been reported. However, this research suffered from the weakness of the clinical studies conducted until now that make difficult to draw a general conclusion. First, it would be important to distinguish class effects of PDE5 inhibitors versus effects unique to selective agents within the class, also in relation to non-specific inhibition of other PDEs as well as to potential off-target effects that can both positively and negatively influence the risk-benefit profile of PDE5 inhibitors. Secondly, we will need information concerning the optimal dosages of these molecules in the various applications, their monitoring, and the potential interactions with other agents regularly used in each patient population. Moreover, major clinical benefits will come from more definitive specifications of individual patient variables that may point toward the use of PDE5 inhibitors in general and each specific drug of this class, providing antitumor therapy with reduced adverse effects. Certainly, the clarification of additional molecular processes of potential oncogenic function of PDE5 as well as further clinical trials including PDE5 inhibitors will help to facilitate better applications of PDE5 targeting drugs in the area of cancer treatment.
Footnotes
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
GRANT SUPPORT
This work was supported by PROGRAMMA “FUTURO IN RICERCA” Anno 2012 (#RBFR12FI27) and MFAG #16899 to I. Barone.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends - an update. Cancer Epidemiol Biomarkers Prev. 2016;25:16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 3.Bourdeanu L, Liu EA. Systemic treatment for breast cancer: Chemotherapy and biotherapy agents. Semin Oncol Nurs. 2015;31:156–162. doi: 10.1016/j.soncn.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Byron E, Pinder-Schenck M. Systemic and targeted therapies for early-stage lung cancer. Cancer Control. 2014;21:21–31. doi: 10.1177/107327481402100104. [DOI] [PubMed] [Google Scholar]
- 5.Gomaa AI, Waked I. Recent advances in multidisciplinary management of hepatocellular carcinoma. World J Hepatol. 2015;7:673–687. doi: 10.4254/wjh.v7.i4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiota M, Eto M. Current status of primary pharmacotherapy and future perspectives toward upfront therapy for metastatic hormone-sensitive prostate cancer. Int J Urol. 2016;23:360–369. doi: 10.1111/iju.13091. [DOI] [PubMed] [Google Scholar]
- 7.Catalano S, Campana A, Giordano C, Gyorffy B, Tarallo R, Rinaldi A, Bruno G, Ferraro A, Romeo F, Lanzino M, Naro F, Bonofiglio D, Andò S, et al. Expression and function of phosphodiesterase type 5 in human breast cancer cell lines and tissues: Implications for targeted therapy. Clin Cancer Res. 2016;22:2271–2282. doi: 10.1158/1078-0432.CCR-15-1900. [DOI] [PubMed] [Google Scholar]
- 8.Piazza GA, Thompson WJ, Pamukcu R, Alila HW, Whitehead CM, Liu L, Fetter JR, Gresh WE, Jr, Klein-Szanto AJ, Farnell DR, Eto I, Grubbs CJ. Exisulind, a novel proapoptotic drug, inhibits rat urinary bladder tumorigenesis. Cancer Res. 2001;61:3961–3968. [PubMed] [Google Scholar]
- 9.Pusztai L, Zhen JH, Arun B, Rivera E, Whitehead C, Thompson WJ, Nealy KM, Gibbs A, Symmans WF, Esteva FJ, Booser D, Murray JL, Valero V, et al. Phase I and II study of exisulind in combination with capecitabine in patients with metastatic breast cancer. J Clin Oncol. 2003;21:3454–3461. doi: 10.1200/JCO.2003.02.114. [DOI] [PubMed] [Google Scholar]
- 10.Whitehead CM, Earle KA, Fetter J, Xu S, Hartman T, Chan DC, Zhao TL, Piazza G, Klein-Szanto AJ, Pamukcu R, Alila H, Bunn PA, Jr, Thompson WJ. Exisulind-induced apoptosis in a non-small cell lung cancer orthotopic lung tumor model augments docetaxel treatment and contributes to increased survival. Mol Cancer Ther. 2003;2:479–488. [PubMed] [Google Scholar]
- 11.Savai R, Pullamsetti SS, Banat GA, Weissmann N, Ghofrani HA, Grimminger F, Schermuly RT. Targeting cancer with phosphodiesterase inhibitors. Expert Opin Investig Drugs. 2010;19:117–131. doi: 10.1517/13543780903485642. [DOI] [PubMed] [Google Scholar]
- 12.Barone I, Giordano C, Bonofiglio D, Catalano S, Andò S. Phosphodiesterase type 5 as a candidate therapeutic target in cancers. Curr Pathobiol Rep. 2015;3:193–201. [Google Scholar]
- 13.Azevedo MF, Faucz FR, Bimpaki E, Horvath A, Levy I, de Alexandre RB, Ahmad F, Manganiello V, Stratakis CA. Clinical and molecular genetics of the phosphodiesterases (PDEs) Endocr Rev. 2014;35:195–233. doi: 10.1210/er.2013-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lefievre L, de Lamirande E, Gagnon C. Presence of cyclic nucleotide phosphodiesterases PDE1A, existing as a stable complex with calmodulin, and PDE3a in human spermatozoa. Biol Reprod. 2002;67:423–430. doi: 10.1095/biolreprod67.2.423. [DOI] [PubMed] [Google Scholar]
- 15.Kanda N, Watanabe S. Regulatory roles of adenylate cyclase and cyclic nucleotide phosphodiesterases 1 and 4 in interleukin-13 production by activated human T cells. Biochem Pharmacol. 2001;62:495–507. doi: 10.1016/s0006-2952(01)00688-8. [DOI] [PubMed] [Google Scholar]
- 16.Deng C, Wang D, Bugaj-Gaweda B, De Vivo M. Assays for cyclic nucleotide-specific phosphodiesterases (PDEs) in the central nervous system (PDE1, PDE2, PDE4, and PDE10) Curr Protoc Neurosci. 2007 doi: 10.1002/0471142301.ns0721s38. Chapter 7: Unit 7 21. [DOI] [PubMed] [Google Scholar]
- 17.Nagel DJ, Aizawa T, Jeon KI, Liu W, Mohan A, Wei H, Miano JM, Florio VA, Gao P, Korshunov VA, Berk BC, Yan C. Role of nuclear Ca2+/calmodulin-stimulated phosphodiesterase 1A in vascular smooth muscle cell growth and survival. Circ Res. 2006;98:777–784. doi: 10.1161/01.RES.0000215576.27615.fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rybalkin SD, Rybalkina I, Beavo JA, Bornfeldt KE. Cyclic nucleotide phosphodiesterase 1c promotes human arterial smooth muscle cell proliferation. Circ Res. 2002;90:151–157. doi: 10.1161/hh0202.104108. [DOI] [PubMed] [Google Scholar]
- 19.Keravis T, Lugnier C. Cyclic nucleotide phosphodiesterases (PDE) and peptide motifs. Curr Pharm Des. 2010;16:1114–1125. doi: 10.2174/138161210790963760. [DOI] [PubMed] [Google Scholar]
- 20.Dunkern TR, Hatzelmann A. Characterization of inhibitors of phosphodiesterase 1c on a human cellular system. FEBS J. 2007;274:4812–4824. doi: 10.1111/j.1742-4658.2007.06001.x. [DOI] [PubMed] [Google Scholar]
- 21.Reneerkens OA, Rutten K, Steinbusch HW, Blokland A, Prickaerts J. Selective phosphodiesterase inhibitors: a promising target for cognition enhancement. Psychopharmacology (Berl) 2009;202:419–443. doi: 10.1007/s00213-008-1273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Essayan DM. Cyclic nucleotide phosphodiesterase (PDE) inhibitors and immunomodulation. Biochem Pharmacol. 1999;57:965–973. doi: 10.1016/s0006-2952(98)00331-1. [DOI] [PubMed] [Google Scholar]
- 23.Chan S, Yan C. PDE1 isozymes, key regulators of pathological vascular remodeling. Curr Opin Pharmacol. 2011;11:720–724. doi: 10.1016/j.coph.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medina AE. Therapeutic utility of phosphodiesterase type I inhibitors in neurological conditions. Front Neurosci. 2011;5:21. doi: 10.3389/fnins.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez L, Breitenbucher JG. PDE2 inhibition: Potential for the treatment of cognitive disorders. Bioorg Med Chem Lett. 2013;23:6522–6527. doi: 10.1016/j.bmcl.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Mongillo M, Tocchetti CG, Terrin A, Lissandron V, Cheung YF, Dostmann WR, Pozzan T, Kass DA, Paolocci N, Houslay MD, Zaccolo M. Compartmentalized phosphodiesterase-2 activity blunts beta-adrenergic cardiac inotropy via an NO/cGMP-dependent pathway. Circ Res. 2006;98:226–234. doi: 10.1161/01.RES.0000200178.34179.93. [DOI] [PubMed] [Google Scholar]
- 27.Sadhu K, Hensley K, Florio VA, Wolda SL. Differential expression of the cyclic GMP-stimulated phosphodiesterase PDE2a in human venous and capillary endothelial cells. J Histochem Cytochem. 1999;47:895–906. doi: 10.1177/002215549904700707. [DOI] [PubMed] [Google Scholar]
- 28.Spiessberger B, Bernhard D, Herrmann S, Feil S, Werner C, Luppa PB, Hofmann F. cGMP-dependent protein kinase II and aldosterone secretion. FEBS J. 2009;276:1007–1013. doi: 10.1111/j.1742-4658.2008.06839.x. [DOI] [PubMed] [Google Scholar]
- 29.Keravis T, Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: Benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br J Pharmacol. 2012;165:1288–1305. doi: 10.1111/j.1476-5381.2011.01729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Degerman E, Belfrage P, Manganiello VC. Structure, localization, and regulation of cGMP-inhibited phosphodiesterase (PDE3) J Biol Chem. 1997;272:6823–6826. doi: 10.1074/jbc.272.11.6823. [DOI] [PubMed] [Google Scholar]
- 31.Murata T, Shimizu K, Hiramoto K, Tagawa T. Phosphodiesterase 3 (PDE3): Structure, localization and function. Cardiovasc Hematol Agents Med Chem. 2009;7:206–211. doi: 10.2174/187152509789105453. [DOI] [PubMed] [Google Scholar]
- 32.Beca S, Ahmad F, Shen W, Liu J, Makary S, Polidovitch N, Sun J, Hockman S, Chung YW, Movsesian M, Murphy E, Manganiello V, Backx PH. Phosphodiesterase type 3A regulates basal myocardial contractility through interacting with sarcoplasmic reticulum calcium atpase type 2A signaling complexes in mouse heart. Circ Res. 2013;112:289–297. doi: 10.1161/CIRCRESAHA.111.300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Begum N, Shen W, Manganiello V. Role of PDE3a in regulation of cell cycle progression in mouse vascular smooth muscle cells and oocytes: Implications in cardiovascular diseases and infertility. Curr Opin Pharmacol. 2011;11:725–729. doi: 10.1016/j.coph.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sallstrom J, Jensen BL, Skott O, Gao X, Persson AE. Neuronal nitric oxide synthase supports renin release during sodium restriction through inhibition of phosphodiesterase 3. Am J Hypertens. 2010;23:1241–1246. doi: 10.1038/ajh.2010.153. [DOI] [PubMed] [Google Scholar]
- 35.Uckert S, Oelke M. Phosphodiesterase PDE inhibitors in the treatment of lower urinary tract dysfunction. Br J Clin Pharmacol. 2011;72:197–204. doi: 10.1111/j.1365-2125.2010.03828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ochiai K, Takita S, Eiraku T, Kojima A, Iwase K, Kishi T, Fukuchi K, Yasue T, Adams DR, Allcock RW, Jiang Z, Kohno Y. Phosphodiesterase inhibitors. Part 3: Design, synthesis and structure-activity relationships of dual PDE3/4-inhibitory fused bicyclic heteroaromatic-dihydropyridazinones with anti-inflammatory and bronchodilatory activity. Bioorg Med Chem. 2012;20:1644–1658. doi: 10.1016/j.bmc.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 37.Sakakibara H, Conti M, Weiner RI. Role of phosphodiesterases in the regulation of gonadotropin- releasing hormone secretion in GT1 cells. Neuroendocrinology. 1998;68:365–373. doi: 10.1159/000054386. [DOI] [PubMed] [Google Scholar]
- 38.Diamant Z, Spina D. PDE4-inhibitors: A novel, targeted therapy for obstructive airways disease. Pulm Pharmacol Ther. 2011;24:353–360. doi: 10.1016/j.pupt.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 39.Jin SL, Ding SL, Lin SC. Phosphodiesterase 4 and its inhibitors in inflammatory diseases. Chang Gung Med J. 2012;35:197–210. doi: 10.4103/2319-4170.106152. [DOI] [PubMed] [Google Scholar]
- 40.Vang AG, Basole C, Dong H, Nguyen RK, Housley W, Guernsey L, Adami AJ, Thrall RS, Clark RB, Epstein PM, Brocke S. Differential expression and function of PDE8 and PDE4 in effector t cells: Implications for PDE8 as a drug target in inflammation. Front Pharmacol. 2016;7:259. doi: 10.3389/fphar.2016.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richter W, Menniti FS, Zhang HT, Conti M. PDE4 as a target for cognition enhancement. Expert Opin Ther Targets. 2013;17:1011–1027. doi: 10.1517/14728222.2013.818656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richter W, Xie M, Scheitrum C, Krall J, Movsesian MA, Conti M. Conserved expression and functions of PDE4 in rodent and human heart. Basic Res Cardiol. 2011;106:249–262. doi: 10.1007/s00395-010-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujita M, Hines CS, Zoghbi SS, Mallinger AG, Dickstein LP, Liow JS, Zhang Y, Pike VW, Drevets WC, Innis RB, Zarate CA., Jr Downregulation of brain phosphodiesterase type IV measured with 11c-(r)-rolipram positron emission tomography in major depressive disorder. Biol Psychiatry. 2012;72:548–554. doi: 10.1016/j.biopsych.2012.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Houslay MD. The long and short of vascular smooth muscle phosphodiesterase-4 as a putative therapeutic target. Mol Pharmacol. 2005;68:563–567. doi: 10.1124/mol.105.015719. [DOI] [PubMed] [Google Scholar]
- 45.McKenna SD, Pietropaolo M, Tos EG, Clark A, Fischer D, Kagan D, Bao B, Chedrese PJ, Palmer S. Pharmacological inhibition of phosphodiesterase 4 triggers ovulation in follicle-stimulating hormone-primed rats. Endocrinology. 2005;146:208–214. doi: 10.1210/en.2004-0562. [DOI] [PubMed] [Google Scholar]
- 46.Page CP, Spina D. Phosphodiesterase inhibitors in the treatment of inflammatory diseases. Handb Exp Pharmacol. 2011:391–414. doi: 10.1007/978-3-642-17969-3_17. [DOI] [PubMed] [Google Scholar]
- 47.Santos-Silva AJ, Cairrao E, Morgado M, Alvarez E, Verde I. PDE4 and PDE5 regulate cyclic nucleotides relaxing effects in human umbilical arteries. Eur J Pharmacol. 2008;582:102–109. doi: 10.1016/j.ejphar.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 48.Reneerkens OA, Rutten K, Akkerman S, Blokland A, Shaffer CL, Menniti FS, Steinbusch HW, Prickaerts J. Phosphodiesterase type 5 (PDE5) inhibition improves object recognition memory: Indications for central and peripheral mechanisms. Neurobiol Learn Mem. 2012;97:370–379. doi: 10.1016/j.nlm.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 49.Kulkarni SK, Patil CS. Phosphodiesterase 5 enzyme and its inhibitors: Update on pharmacological and therapeutical aspects. Methods Find Exp Clin Pharmacol. 2004;26:789–799. doi: 10.1358/mf.2004.26.10.872561. [DOI] [PubMed] [Google Scholar]
- 50.d’Esterre CD, Lee TY. Effect of dipyridamole during acute stroke: Exploring antithrombosis and neuroprotective benefits. Ann N Y Acad Sci. 2010;1207:71–75. doi: 10.1111/j.1749-6632.2010.05801.x. [DOI] [PubMed] [Google Scholar]
- 51.Forrest CM, Stoy N, Stone TW, Harman G, Mackay GM, Oxford L, Darlington LG. Adenosine and cytokine levels following treatment of rheumatoid arthritis with dipyridamole. Rheumatol Int. 2006;27:11–17. doi: 10.1007/s00296-006-0212-6. [DOI] [PubMed] [Google Scholar]
- 52.Spano D, Marshall JC, Marino N, De Martino D, Romano A, Scoppettuolo MN, Bello AM, Di Dato V, Navas L, De Vita G, Medaglia C, Steeg PS, Zollo M. Dipyridamole prevents triple-negative breast-cancer progression. Clin Exp Metastasis. 2013;30:47–68. doi: 10.1007/s10585-012-9506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Puzzo D, Sapienza S, Arancio O, Palmeri A. Role of phosphodiesterase 5 in synaptic plasticity and memory. Neuropsychiatr Dis Treat. 2008;4:371–387. doi: 10.2147/ndt.s2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu B, Strada SJ. The novel functions of cGMP-specific phosphodiesterase 5 and its inhibitors in carcinoma cells and pulmonary/cardiovascular vessels. Curr Top Med Chem. 2007;7:437–454. doi: 10.2174/156802607779941198. [DOI] [PubMed] [Google Scholar]
- 55.Kukreja RC, Salloum FN, Das A, Koka S, Ockaili RA, Xi L. Emerging new uses of phosphodiesterase-5 inhibitors in cardiovascular diseases. Exp Clin Cardiol. 2011;16:e30–35. [PMC free article] [PubMed] [Google Scholar]
- 56.Das A, Durrant D, Salloum FN, Xi L, Kukreja RC. PDE5 inhibitors as therapeutics for heart disease, diabetes and cancer. Pharmacol Ther. 2015;147:12–21. doi: 10.1016/j.pharmthera.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: Results of a 1-year, prospective, randomized, placebo-controlled study. Circ Heart Fail. 2011;4:8–17. doi: 10.1161/CIRCHEARTFAILURE.110.944694. [DOI] [PubMed] [Google Scholar]
- 58.Kolandaivelu S, Chang B, Ramamurthy V. Rod phosphodiesterase-6 (PDE6) catalytic subunits restore cone function in a mouse model lacking cone PDE6 catalytic subunit. J Biol Chem. 2011;286:33252–33259. doi: 10.1074/jbc.M111.259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cote RH. Characteristics of photoreceptor PDE (PDE6): Similarities and differences to PDE5. Int J Impot Res. 2004;16:S28–33. doi: 10.1038/sj.ijir.3901212. [DOI] [PubMed] [Google Scholar]
- 60.Muradov KG, Granovsky AE, Artemyev NO. Mutation in rod PDE6 linked to congenital stationary night blindness impairs the enzyme inhibition by its gamma-subunit. Biochemistry. 2003;42:3305–3310. doi: 10.1021/bi027095x. [DOI] [PubMed] [Google Scholar]
- 61.Christiansen JR, Ramamurthy V. Greasing the protein biosynthesis machinery of photoreceptor neurons: Role for postprenylation processing of proteins. Cell Logist. 2012;2:15–19. doi: 10.4161/cl.19804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dong H, Osmanova V, Epstein PM, Brocke S. Phosphodiesterase 8 (PDE8) regulates chemotaxis of activated lymphocytes. Biochem Biophys Res Commun. 2006;345:713–719. doi: 10.1016/j.bbrc.2006.04.143. [DOI] [PubMed] [Google Scholar]
- 63.Vasta V, Shimizu-Albergine M, Beavo JA. Modulation of leydig cell function by cyclic nucleotide phosphodiesterase 8A. Proc Natl Acad Sci U S A. 2006;103:19925–19930. doi: 10.1073/pnas.0609483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arnaud-Lopez L, Usala G, Ceresini G, Mitchell BD, Pilia MG, Piras MG, Sestu N, Maschio A, Busonero F, Albai G, Dei M, Lai S, Mulas A, et al. Phosphodiesterase 8B gene variants are associated with serum TSH levels and thyroid function. Am J Hum Genet. 2008;82:1270–1280. doi: 10.1016/j.ajhg.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Su T, Zhang T, Xie S, Yan J, Wu Y, Li X, Huang L, Luo HB. Discovery of novel PDE9 inhibitors capable of inhibiting Aβ aggregation as potential candidates for the treatment of Alzheimer's disease. Sci Rep. 2016;6:21826. doi: 10.1038/srep21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andreeva SG, Dikkes P, Epstein PM, Rosenberg PA. Expression of cGMP-specific phosphodiesterase 9A mRNA in the rat brain. J Neurosci. 2001;21:9068–9076. doi: 10.1523/JNEUROSCI.21-22-09068.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reneerkens OA, Rutten K, Bollen E, Hage T, Blokland A, Steinbusch HW, Prickaerts J. Inhibition of phoshodiesterase type 2 or type 10 reverses object memory deficits induced by scopolamine or MK-801. Behav Brain Res. 2013;236:16–22. doi: 10.1016/j.bbr.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 68.Jager R, Russwurm C, Schwede F, Genieser HG, Koesling D, Russwurm M. Activation of PDE10 and PDE11 phosphodiesterases. J Biol Chem. 2012;287:1210–1219. doi: 10.1074/jbc.M111.263806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Horvath A, Korde L, Greene MH, Libe R, Osorio P, Faucz FR, Raffin-Sanson ML, Tsang KM, Drori-Herishanu L, Patronas Y, Remmers EF, Nikita ME, Moran J, et al. Functional phosphodiesterase 11A mutations may modify the risk of familial and bilateral testicular germ cell tumors. Cancer Res. 2009;69:5301–5306. doi: 10.1158/0008-5472.CAN-09-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Faucz FR, Horvath A, Rothenbuhler A, Almeida MQ, Libe R, Raffin-Sanson ML, Bertherat J, Carraro DM, Soares FA, Molina Gde C, Campos AH, Alexandre RB, Bendhack ML, et al. Phosphodiesterase 11A (PDE11A) genetic variants may increase susceptibility to prostatic cancer. J Clin Endocrinol Metab. 2011;96:E135–140. doi: 10.1210/jc.2010-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pomara G, Morelli G. Inhibition of phosphodiesterase 11 (PDE11) impacts on sperm quality. Int J Impot Res. 2005;17:385–386. doi: 10.1038/sj.ijir.3901304. author reply 387. [DOI] [PubMed] [Google Scholar]
- 72.Seftel AD. Phosphodiesterase 11 (PDE11) regulation of spermatozoa physiology. J Urol. 2005;174:1043–1044. doi: 10.1016/s0022-5347(01)68504-5. [DOI] [PubMed] [Google Scholar]
- 73.Ke H, Wang H, Ye M. Structural insight into the substrate specificity of phosphodiesterases. Handb Exp Pharmacol. 2011:121–134. doi: 10.1007/978-3-642-17969-3_4. [DOI] [PubMed] [Google Scholar]
- 74.Francis SH, Turko IV, Corbin JD. Cyclic nucleotide phosphodiesterases: Relating structure and function. Prog Nucleic Acid Res Mol Biol. 2001;65:1–52. doi: 10.1016/s0079-6603(00)65001-8. [DOI] [PubMed] [Google Scholar]
- 75.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 76.Francis SH, Blount MA, Corbin JD. Mammalian cyclic nucleotide phosphodiesterases: Molecular mechanisms and physiological functions. Physiol Rev. 2011;91:651–690. doi: 10.1152/physrev.00030.2010. [DOI] [PubMed] [Google Scholar]
- 77.Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, Malloy MP, Chubb JE, Huston E, Baillie GS, Thomson PA, Hill EV, Brandon NJ, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- 78.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: Essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 79.Horvath A, Mericq V, Stratakis CA. Mutation in PDE8B, a cyclic AMP-specific phosphodiesterase in adrenal hyperplasia. N Engl J Med. 2008;358:750–752. doi: 10.1056/NEJMc0706182. [DOI] [PubMed] [Google Scholar]
- 80.Yanaka N, Kotera J, Ohtsuka A, Akatsuka H, Imai Y, Michibata H, Fujishige K, Kawai E, Takebayashi S, Okumura K, Omori K. Expression, structure and chromosomal localization of the human cGMP-binding cGMP-specific phosphodiesterase PDE5A gene. Eur J Biochem. 1998;255:391–399. doi: 10.1046/j.1432-1327.1998.2550391.x. [DOI] [PubMed] [Google Scholar]
- 81.Lin CS. Tissue expression, distribution, and regulation of PDE5. Int J Impot Res. 2004;16:S8–S10. doi: 10.1038/sj.ijir.3901207. [DOI] [PubMed] [Google Scholar]
- 82.Lin CS, Lau A, Tu R, Lue TF. Identification of three alternative first exons and an intronic promoter of human PDE5A gene. Biochem Biophys Res Commun. 2000;268:596–602. doi: 10.1006/bbrc.2000.2186. [DOI] [PubMed] [Google Scholar]
- 83.Lin CS, Chow S, Lau A, Tu R, Lue TF. Identification and regulation of human PDE5A gene promoter. Biochem Biophys Res Commun. 2001;280:684–692. doi: 10.1006/bbrc.2000.4220. [DOI] [PubMed] [Google Scholar]
- 84.Lin CS, Chow S, Lau A, Tu R, Lue TF. Regulation of human PDE5A2 intronic promoter by cAMP and cGMP: Identification of a critical sp1-binding site. Biochem Biophys Res Commun. 2001;280:693–699. doi: 10.1006/bbrc.2000.4221. [DOI] [PubMed] [Google Scholar]
- 85.Lin CS, Chow S, Lau A, Tu R, Lue TF. Human PDE5A gene encodes three PDE5 isoforms from two alternate promoters. Int J Impot Res. 2002;14:15–24. doi: 10.1038/sj.ijir.3900802. [DOI] [PubMed] [Google Scholar]
- 86.Aravind L, Ponting CP. The gaf domain: An evolutionary link between diverse phototransducing proteins. Trends Biochem Sci. 1997;22:458–459. doi: 10.1016/s0968-0004(97)01148-1. [DOI] [PubMed] [Google Scholar]
- 87.Corbin JD, Turko IV, Beasley A, Francis SH. Phosphorylation of phosphodiesterase-5 by cyclic nucleotide-dependent protein kinase alters its catalytic and allosteric cGMP-binding activities. Eur J Biochem. 2000;267:2760–2767. doi: 10.1046/j.1432-1327.2000.01297.x. [DOI] [PubMed] [Google Scholar]
- 88.Zoraghi R, Bessay EP, Corbin JD, Francis SH. Structural and functional features in human PDE5a1 regulatory domain that provide for allosteric cGMP binding, dimerization, and regulation. J Biol Chem. 2005;280:12051–12063. doi: 10.1074/jbc.M413611200. [DOI] [PubMed] [Google Scholar]
- 89.Turko IV, Francis SH, Corbin JD. Binding of cGMP to both allosteric sites of cGMP-binding cGMP-specific phosphodiesterase (PDE5) is required for its phosphorylation. Biochem J. 1998;329:505–510. doi: 10.1042/bj3290505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Francis SH, Bessay EP, Kotera J, Grimes KA, Liu L, Thompson WJ, Corbin JD. Phosphorylation of isolated human phosphodiesterase-5 regulatory domain induces an apparent conformational change and increases cGMP binding affinity. J Biol Chem. 2002;277:47581–47587. doi: 10.1074/jbc.M206088200. [DOI] [PubMed] [Google Scholar]
- 91.Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res. 2007;100:309–327. doi: 10.1161/01.RES.0000256354.95791.f1. [DOI] [PubMed] [Google Scholar]
- 92.Kass DA, Takimoto E, Nagayama T, Champion HC. Phosphodiesterase regulation of nitric oxide signaling. Cardiovasc Res. 2007;75:303–314. doi: 10.1016/j.cardiores.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 93.Zhang M, Koitabashi N, Nagayama T, Rambaran R, Feng N, Takimoto E, Koenke T, O’Rourke B, Champion HC, Crow MT, Kass DA. Expression, activity, and pro-hypertrophic effects of PDE5A in cardiac myocytes. Cell Signal. 2008;20:2231–2236. doi: 10.1016/j.cellsig.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Castro LR, Schittl J, Fischmeister R. Feedback control through cGMP-dependent protein kinase contributes to differential regulation and compartmentation of cGMP in rat cardiac myocytes. Circ Res. 2010;107:1232–1240. doi: 10.1161/CIRCRESAHA.110.226712. [DOI] [PubMed] [Google Scholar]
- 95.Zhao CY, Greenstein JL, Winslow RL. Roles of phosphodiesterases in the regulation of the cardiac cyclic nucleotide cross-talk signaling network. J Mol Cell Cardiol. 2016;91:215–227. doi: 10.1016/j.yjmcc.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mullershausen F, Friebe A, Feil R, Thompson WJ, Hofmann F, Koesling D. Direct activation of PDE5 by cGMP: Long-term effects within NO/cGMP signaling. J Cell Biol. 2003;160:719–727. doi: 10.1083/jcb.200211041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lochhead A, Nekrasova E, Arshavsky VY, Pyne NJ. The regulation of the cGMP-binding cGMP phosphodiesterase by proteins that are immunologically related to gamma subunit of the photoreceptor cGMP phosphodiesterase. J Biol Chem. 1997;272:18397–18403. doi: 10.1074/jbc.272.29.18397. [DOI] [PubMed] [Google Scholar]
- 98.Campolo F, Zevini A, Cardarelli S, Monaco L, Barbagallo F, Pellegrini M, Cornacchione M, Di Grazia A, De Arcangelis V, Gianfrilli D, Giorgi M, Lenzi A, Isidori AM, et al. Identification of murine phosphodiesterase 5a isoforms and their functional characterization in hl-1 cardiac cell line. J Cell Physiol. 2018;233:325–337. doi: 10.1002/jcp.25880. [DOI] [PubMed] [Google Scholar]
- 99.Rehmann H, Wittinghofer A, Bos JL. Capturing cyclic nucleotides in action: Snapshots from crystallographic studies. Nat Rev Mol Cell Biol. 2007;8:63–73. doi: 10.1038/nrm2082. [DOI] [PubMed] [Google Scholar]
- 100.Hofmann F, Bernhard D, Lukowski R, Weinmeister P. cGMP regulated protein kinases (CGK) Handb Exp Pharmacol. 2009:137–162. doi: 10.1007/978-3-540-68964-5_8. [DOI] [PubMed] [Google Scholar]
- 101.Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010;62:525–563. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hood J, Granger HJ. Protein kinase g mediates vascular endothelial growth factor-induced RAF-1 activation and proliferation in human endothelial cells. J Biol Chem. 1998;273:23504–23508. doi: 10.1074/jbc.273.36.23504. [DOI] [PubMed] [Google Scholar]
- 103.Smolenski A, Poller W, Walter U, Lohmann SM. Regulation of human endothelial cell focal adhesion sites and migration by cGMP-dependent protein kinase i. J Biol Chem. 2000;275:25723–25732. doi: 10.1074/jbc.M909632199. [DOI] [PubMed] [Google Scholar]
- 104.Kook H, Itoh H, Choi BS, Sawada N, Doi K, Hwang TJ, Kim KK, Arai H, Baik YH, Nakao K. Physiological concentration of atrial natriuretic peptide induces endothelial regeneration in vitro. Am J Physiol Heart Circ Physiol. 2003;284:H1388–1397. doi: 10.1152/ajpheart.00414.2002. [DOI] [PubMed] [Google Scholar]
- 105.Nagendran J, Archer SL, Soliman D, Gurtu V, Moudgil R, Haromy A, St Aubin C, Webster L, Rebeyka IM, Ross DB, Light PE, Dyck JR, Michelakis ED. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation. 2007;116:238–248. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 106.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 107.Dunkern TR, Hatzelmann A. The effect of sildenafil on human platelet secretory function is controlled by a complex interplay between phosphodiesterases 2, 3 and 5. Cell Signal. 2005;17:331–339. doi: 10.1016/j.cellsig.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 108.Prickaerts J, Sik A, van Staveren WC, Koopmans G, Steinbusch HW, van der Staay FJ, de Vente J, Blokland A. Phosphodiesterase type 5 inhibition improves early memory consolidation of object information. Neurochem Int. 2004;45:915–928. doi: 10.1016/j.neuint.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 109.Lugnier C, Schoeffter P, Le Bec A, Strouthou E, Stoclet JC. Selective inhibition of cyclic nucleotide phosphodiesterases of human, bovine and rat aorta. Biochem Pharmacol. 1986;35:1743–1751. doi: 10.1016/0006-2952(86)90333-3. [DOI] [PubMed] [Google Scholar]
- 110.Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88–95. doi: 10.1161/CIRCULATIONAHA.110.944603. [DOI] [PubMed] [Google Scholar]
- 111.Gresser U, Gleiter CH. Erectile dysfunction: Comparison of efficacy and side effects of the PDE-5 inhibitors sildenafil, vardenafil and tadalafil - review of the literature. Eur J Med Res. 2002;7:435–446. [PubMed] [Google Scholar]
- 112.Daugan A, Grondin P, Ruault C, Le Monnier de Gouville AC, Coste H, Linget JM, Kirilovsky J, Hyafil F, Labaudiniere R. The discovery of tadalafil: A novel and highly selective PDE5 inhibitor 2: 2, 3, 6, 7, 12, 12a-hexahydropyrazino[1′, 2′:1, 6]pyrido[3,4-b]indole-1,4-dione analogues. J Med Chem. 2003;46:4533–4542. doi: 10.1021/jm0300577. [DOI] [PubMed] [Google Scholar]
- 113.Weeks JL, Zoraghi R, Beasley A, Sekhar KR, Francis SH, Corbin JD. High biochemical selectivity of tadalafil, sildenafil and vardenafil for human phosphodiesterase 5A1 (PDE5) over PDE11A4 suggests the absence of PDE11A4 cross-reaction in patients. Int J Impot Res. 2005;17:5–9. doi: 10.1038/sj.ijir.3901283. [DOI] [PubMed] [Google Scholar]
- 114.Porst H, Padma-Nathan H, Giuliano F, Anglin G, Varanese L, Rosen R. Efficacy of tadalafil for the treatment of erectile dysfunction at 24 and 36 hours after dosing: A randomized controlled trial. Urology. 2003;62:121–125. doi: 10.1016/s0090-4295(03)00359-5. discussion 125–126. [DOI] [PubMed] [Google Scholar]
- 115.Kotera J, Mochida H, Inoue H, Noto T, Fujishige K, Sasaki T, Kobayashi T, Kojima K, Yee S, Yamada Y, Kikkawa K, Omori K. Avanafil, a potent and highly selective phosphodiesterase-5 inhibitor for erectile dysfunction. J Urol. 2012;188:668–674. doi: 10.1016/j.juro.2012.03.115. [DOI] [PubMed] [Google Scholar]
- 116.Kim BH, Lim HS, Chung JY, Kim JR, Lim KS, Sohn DR, Cho JY, Yu KS, Shin SG, Paick JS, Jang IJ. Safety, tolerability and pharmacokinetics of udenafil, a novel PDE-5 inhibitor, in healthy young korean subjects. Br J Clin Pharmacol. 2008;65:848–854. doi: 10.1111/j.1365-2125.2008.03107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee HS, Park EJ, Ji HY, Kim SY, Im GJ, Lee SM, Jang IJ. Identification of cytochrome p450 enzymes responsible for n-dealkylation of a new oral erectogenic, mirodenafil. Xenobiotica. 2008;38:21–33. doi: 10.1080/00498250701708521. [DOI] [PubMed] [Google Scholar]
- 118.Toque HA, Teixeira CE, Lorenzetti R, Okuyama CE, Antunes E, De Nucci G. Pharmacological characterization of a novel phosphodiesterase type 5 (PDE5) inhibitor lodenafil carbonate on human and rabbit corpus cavernosum. Eur J Pharmacol. 2008;591:189–195. doi: 10.1016/j.ejphar.2008.06.055. [DOI] [PubMed] [Google Scholar]
- 119.Toque HA, Teixeira CE, Priviero FB, Morganti RP, Antunes E, De Nucci G. Vardenafil, but not sildenafil or tadalafil, has calcium-channel blocking activity in rabbit isolated pulmonary artery and human washed platelets. Br J Pharmacol. 2008;154:787–796. doi: 10.1038/bjp.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shi Z, Tiwari AK, Shukla S, Robey RW, Singh S, Kim IW, Bates SE, Peng X, Abraham I, Ambudkar SV, Talele TT, Fu LW, Chen ZS. Sildenafil reverses ABCB1- and ABCG2-mediated chemotherapeutic drug resistance. Cancer Res. 2011;71:3029–3041. doi: 10.1158/0008-5472.CAN-10-3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shi Z, Tiwari AK, Patel AS, Fu LW, Chen ZS. Roles of sildenafil in enhancing drug sensitivity in cancer. Cancer Res. 2011;71:3735–3738. doi: 10.1158/0008-5472.CAN-11-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Henrie AM, Nawarskas JJ, Anderson JR. Clinical utility of tadalafil in the treatment of pulmonary arterial hypertension: An evidence-based review. Core Evid. 2015;10:99–109. doi: 10.2147/CE.S58457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Butrous G. The role of phosphodiesterase inhibitors in the management of pulmonary vascular diseases. Glob Cardiol Sci Pract. 2014;2014:257–290. doi: 10.5339/gcsp.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kloner RA, Comstock G, Levine LA, Tiger S, Stecher VJ. Investigational noncardiovascular uses of phosphodiesterase-5 inhibitors. Expert Opin Pharmacother. 2011;12:2297–2313. doi: 10.1517/14656566.2011.600306. [DOI] [PubMed] [Google Scholar]
- 125.Kaplan SA, Gonzalez RR, Te AE. Combination of alfuzosin and sildenafil is superior to monotherapy in treating lower urinary tract symptoms and erectile dysfunction. Eur Urol. 2007;51:1717–1723. doi: 10.1016/j.eururo.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 126.Liguori G, Trombetta C, De Giorgi G, Pomara G, Maio G, Vecchio D, Ocello G, Ollandini G, Bucci S, Belgrano E. Efficacy and safety of combined oral therapy with tadalafil and alfuzosin: An integrated approach to the management of patients with lower urinary tract symptoms and erectile dysfunction. Preliminary report. J Sex Med. 2009;6:544–552. doi: 10.1111/j.1743-6109.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 127.Tuncel A, Nalcacioglu V, Ener K, Aslan Y, Aydin O, Atan A. Sildenafil citrate and Tamsulosin combination is not superior to monotherapy in treating lower urinary tract symptoms and erectile dysfunction. World J Urol. 2010;28:17–22. doi: 10.1007/s00345-009-0484-z. [DOI] [PubMed] [Google Scholar]
- 128.Bechara A, Romano S, Casabe A, Haime S, Dedola P, Hernandez C, Rey H. Comparative efficacy assessment of Tamsulosin vs. Tamsulosin plus tadalafil in the treatment of LUTS/BPH. Pilot study. J Sex Med. 2008;5:2170–2178. doi: 10.1111/j.1743-6109.2008.00940.x. [DOI] [PubMed] [Google Scholar]
- 129.Roehrborn CG, Kaminetsky JC, Auerbach SM, Montelongo RM, Elion-Mboussa A, Viktrup L. Changes in peak urinary flow and voiding efficiency in men with signs and symptoms of benign prostatic hyperplasia during once daily tadalafil treatment. BJU Int. 2010;105:502–507. doi: 10.1111/j.1464-410X.2009.08822.x. [DOI] [PubMed] [Google Scholar]
- 130.Cellek S, Cameron NE, Cotter MA, Fry CH, Ilo D. Microvascular dysfunction and efficacy of PDE5 inhibitors in LUTS/BPH. Nat Rev Urol. 2014;11:231–241. doi: 10.1038/nrurol.2014.53. [DOI] [PubMed] [Google Scholar]
- 131.Gonzalez-Cadavid NF, Rajfer J. Treatment of peyronie's disease with PDE5 inhibitors: An antifibrotic strategy. Nat Rev Urol. 2010;7:215–221. doi: 10.1038/nrurol.2010.24. [DOI] [PubMed] [Google Scholar]
- 132.Backhaus BO, Muller SC, Albers P. Corporoplasty for advanced peyronie's disease using venous and/or dermis patch grafting: New surgical technique and long-term patient satisfaction. J Urol. 2003;169:981–984. doi: 10.1097/01.ju.0000052403.11923.51. [DOI] [PubMed] [Google Scholar]
- 133.Goyal NK, Kumar A, Das SK, Pandey AK, Sharma GK, Trivedi S, Dwivedi US, Singh PB. Experience with plaque excision and dermal grafting in the surgical treatment of Peyronie's disease. Singapore Med J. 2008;49:805–808. [PubMed] [Google Scholar]
- 134.Schwarzer JU, Muhlen B, Schukai O. Penile corporoplasty using tunica albuginea free graft from proximal corpus cavernosum: A new technique for treatment of penile curvature in Peyronie's disease. Eur Urol. 2003;44:720–723. doi: 10.1016/s0302-2838(03)00378-6. [DOI] [PubMed] [Google Scholar]
- 135.Levey HR, Segal RL, Bivalacqua TJ. Management of priapism: An update for clinicians. Ther Adv Urol. 2014;6:230–244. doi: 10.1177/1756287214542096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Burnett AL, Bivalacqua TJ, Champion HC, Musicki B. Feasibility of the use of phosphodiesterase type 5 inhibitors in a pharmacologic prevention program for recurrent priapism. J Sex Med. 2006;3:1077–1084. doi: 10.1111/j.1743-6109.2006.00333.x. [DOI] [PubMed] [Google Scholar]
- 137.Rajfer J, Gore JL, Kaufman J, Gonzalez-Cadavid N. Case report: Avoidance of palpable corporal fibrosis due to priapism with upregulators of nitric oxide. J Sex Med. 2006;3:173–176. doi: 10.1111/j.1743-6109.2005.00090.x. [DOI] [PubMed] [Google Scholar]
- 138.McMahon CG, McMahon CN, Leow LJ, Winestock CG. Efficacy of type-5 phosphodiesterase inhibitors in the drug treatment of premature ejaculation: A systematic review. BJU Int. 2006;98:259–272. doi: 10.1111/j.1464-410X.2006.06290.x. [DOI] [PubMed] [Google Scholar]
- 139.Mattos RM, Marmo Lucon A, Srougi M. Tadalafil and fluoxetine in premature ejaculation: Prospective, randomized, double-blind, placebo-controlled study. Urol Int. 2008;80:162–165. doi: 10.1159/000112607. [DOI] [PubMed] [Google Scholar]
- 140.Hosseini MM, Yarmohammadi H. Effect of fluoxetine alone and in combination with sildenafil in patients with premature ejaculation. Urol Int. 2007;79:28–32. doi: 10.1159/000102909. [DOI] [PubMed] [Google Scholar]
- 141.Dimitriadis F, Tsambalas S, Tsounapi P, Kawamura H, Vlachopoulou E, Haliasos N, Gratsias S, Watanabe T, Saito M, Miyagawa I, Sofikitis N. Effects of phosphodiesterase-5 inhibitors on Leydig cell secretory function in oligoasthenospermic infertile men: A randomized trial. BJU Int. 2010;106:1181–1185. doi: 10.1111/j.1464-410X.2010.09243.x. [DOI] [PubMed] [Google Scholar]
- 142.Glenn DR, McVicar CM, McClure N, Lewis SE. Sildenafil citrate improves sperm motility but causes a premature acrosome reaction in vitro. Fertil Steril. 2007;87:1064–1070. doi: 10.1016/j.fertnstert.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 143.Francis SH. Phosphodiesterase 11 (PDE11): Is it a player in human testicular function? Int J Impot Res. 2005;17:467–468. doi: 10.1038/sj.ijir.3901377. [DOI] [PubMed] [Google Scholar]
- 144.Pomara G, Morelli G, Canale D, Turchi P, Caglieresi C, Moschini C, Liguori G, Selli C, Macchia E, Martino E, Francesca F. Alterations in sperm motility after acute oral administration of sildenafil or tadalafil in young, infertile men. Fertil Steril. 2007;88:860–865. doi: 10.1016/j.fertnstert.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 145.Mostafa T. Tadalafil as an in vitro sperm motility stimulant. Andrologia. 2007;39:12–15. doi: 10.1111/j.1439-0272.2006.00752.x. [DOI] [PubMed] [Google Scholar]
- 146.Zhang L, Zhang Z, Zhang RL, Cui Y, LaPointe MC, Silver B, Chopp M. Tadalafil, a long-acting type 5 phosphodiesterase isoenzyme inhibitor, improves neurological functional recovery in a rat model of embolic stroke. Brain Res. 2006;1118:192–198. doi: 10.1016/j.brainres.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 147.Zhang RL, Zhang Z, Zhang L, Wang Y, Zhang C, Chopp M. Delayed treatment with sildenafil enhances neurogenesis and improves functional recovery in aged rats after focal cerebral ischemia. J Neurosci Res. 2006;83:1213–1219. doi: 10.1002/jnr.20813. [DOI] [PubMed] [Google Scholar]
- 148.Ding G, Jiang Q, Li L, Zhang L, Zhang ZG, Ledbetter KA, Panda S, Davarani SP, Athiraman H, Li Q, Ewing JR, Chopp M. Magnetic resonance imaging investigation of axonal remodeling and angiogenesis after embolic stroke in sildenafil-treated rats. J Cereb Blood Flow Metab. 2008;28:1440–1448. doi: 10.1038/jcbfm.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Silver B, McCarthy S, Lu M, Mitsias P, Russman AN, Katramados A, Morris DC, Lewandowski CA, Chopp M. Sildenafil treatment of subacute ischemic stroke: A safety study at 25-mg daily for 2 weeks. J Stroke Cerebrovasc Dis. 2009;18:381–383. doi: 10.1016/j.jstrokecerebrovasdis.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 150.Cuadrado-Tejedor M, Hervias I, Ricobaraza A, Puerta E, Perez-Roldan JM, Garcia-Barroso C, Franco R, Aguirre N, Garcia-Osta A. Sildenafil restores cognitive function without affecting β-amyloid burden in a mouse model of Alzheimer's disease. Br J Pharmacol. 2011;164:2029–2041. doi: 10.1111/j.1476-5381.2011.01517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Puzzo D, Staniszewski A, Deng SX, Privitera L, Leznik E, Liu S, Zhang H, Feng Y, Palmeri A, Landry DW, Arancio O. Phosphodiesterase 5 inhibition improves synaptic function, memory, and amyloid-beta load in an Alzheimer's disease mouse model. J Neurosci. 2009;29:8075–8086. doi: 10.1523/JNEUROSCI.0864-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Goff DC, Cather C, Freudenreich O, Henderson DC, Evins AE, Culhane MA, Walsh JP. A placebo-controlled study of sildenafil effects on cognition in Schizophrenia. Psychopharmacology (Berl) 2009;202:411–417. doi: 10.1007/s00213-008-1278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rutten K, Basile JL, Prickaerts J, Blokland A, Vivian JA. Selective PDE inhibitors rolipram and sildenafil improve object retrieval performance in adult cynomolgus macaques. Psychopharmacology (Berl) 2008;196:643–648. doi: 10.1007/s00213-007-0999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Koka S, Das A, Zhu SG, Durrant D, Xi L, Kukreja RC. Long-acting phosphodiesterase-5 inhibitor tadalafil attenuates doxorubicin-induced cardiomyopathy without interfering with chemotherapeutic effect. J Pharmacol Exp Ther. 2010;334:1023–1030. doi: 10.1124/jpet.110.170191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fisher PW, Salloum F, Das A, Hyder H, Kukreja RC. Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation. 2005;111:1601–1610. doi: 10.1161/01.CIR.0000160359.49478.C2. [DOI] [PubMed] [Google Scholar]
- 156.Booth L, Roberts JL, Cruickshanks N, Conley A, Durrant DE, Das A, Fisher PB, Kukreja RC, Grant S, Poklepovic A, Dent P. Phosphodiesterase 5 inhibitors enhance chemotherapy killing in gastrointestinal/genitourinary cancer cells. Mol Pharmacol. 2014;85:408–419. doi: 10.1124/mol.113.090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee KW, Jeong JY, Lim BJ, Chang YK, Lee SJ, Na KR, Shin YT, Choi DE. Sildenafil attenuates renal injury in an experimental model of rat cisplatin-induced nephrotoxicity. Toxicology. 2009;257:137–143. doi: 10.1016/j.tox.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 158.Medeiros JV, Gadelha GG, Lima SJ, Garcia JA, Soares PM, Santos AA, Brito GA, Ribeiro RA, Souza MH. Role of the NO/cGMP/K(ATP) pathway in the protective effects of sildenafil against ethanol-induced gastric damage in rats. Br J Pharmacol. 2008;153:721–727. doi: 10.1038/sj.bjp.0707605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kato N, Mashita Y, Kato S, Mitsufuji S, Yoshikawa T, Takeuchi K. Sildenafil, an inhibitor of phosphodiesterase subtype 5, prevents indomethacin-induced small-intestinal ulceration in rats via a NO/cGMP-dependent mechanism. Dig Dis Sci. 2009;54:2346–2356. doi: 10.1007/s10620-008-0646-7. [DOI] [PubMed] [Google Scholar]
- 160.Soydan G, Sokmensuer C, Kilinc K, Tuncer M. The effects of sildenafil on the functional and structural changes of ileum induced by intestinal ischemia-reperfusion in rats. Eur J Pharmacol. 2009;610:87–92. doi: 10.1016/j.ejphar.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 161.Gertner E. Treatment with sildenafil for the healing of refractory skin ulcerations in the antiphospholipid syndrome. Lupus. 2003;12:133–135. doi: 10.1191/0961203303lu257cr. [DOI] [PubMed] [Google Scholar]
- 162.Colglazier CL, Sutej PG, O’Rourke KS. Severe refractory fingertip ulcerations in a patient with scleroderma: Successful treatment with sildenafil. J Rheumatol. 2005;32:2440–2442. [PubMed] [Google Scholar]
- 163.Impens AJ, Phillips K, Schiopu E. PDE-5 inhibitors in scleroderma Raynaud phenomenon and digital ulcers: Current status of clinical trials. Int J Rheumatol. 2011;2011:392542. doi: 10.1155/2011/392542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Roustit M, Blaise S, Allanore Y, Carpentier PH, Caglayan E, Cracowski JL. Phosphodiesterase-5 inhibitors for the treatment of secondary Raynaud's phenomenon: Systematic review and meta-analysis of randomised trials. Ann Rheum Dis. 2013;72:1696–1699. doi: 10.1136/annrheumdis-2012-202836. [DOI] [PubMed] [Google Scholar]
- 165.Fries R, Shariat K, von Wilmowsky H, Bohm M. Sildenafil in the treatment of raynaud's phenomenon resistant to vasodilatory therapy. Circulation. 2005;112:2980–2985. doi: 10.1161/CIRCULATIONAHA.104.523324. [DOI] [PubMed] [Google Scholar]
- 166.Brueckner CS, Becker MO, Kroencke T, Huscher D, Scherer HU, Worm M, Burmester G, Riemekasten G. Effect of sildenafil on digital ulcers in systemic sclerosis: Analysis from a single centre pilot study. Ann Rheum Dis. 2010;69:1475–1478. doi: 10.1136/ard.2009.116475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Young A, Khanna D. Systemic sclerosis: A systematic review on therapeutic management from 2011 to 2014. Curr Opin Rheumatol. 2015;27:241–248. doi: 10.1097/BOR.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Botha P, Parry G, Dark JH, Macgowan GA. Acute hemodynamic effects of intravenous sildenafil citrate in congestive heart failure: Comparison of phosphodiesterase type-3 and -5 inhibition. J Heart Lung Transplant. 2009;28:676–682. doi: 10.1016/j.healun.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 169.Maruszewski M, Zakliczynski M, Przybylski R, Kucewicz-Czech E, Zembala M. Use of sildenafil in heart transplant recipients with pulmonary hypertension may prevent right heart failure. Transplant Proc. 2007;39:2850–2852. doi: 10.1016/j.transproceed.2007.08.077. [DOI] [PubMed] [Google Scholar]
- 170.De Santo LS, Mastroianni C, Romano G, Amarelli C, Marra C, Maiello C, Galdieri N, Della Corte A, Cotrufo M, Caianiello G. Role of sildenafil in acute posttransplant right ventricular dysfunction: Successful experience in 13 consecutive patients. Transplant Proc. 2008;40:2015–2018. doi: 10.1016/j.transproceed.2008.05.055. [DOI] [PubMed] [Google Scholar]
- 171.Austin MJ, McDougall NI, Wendon JA, Sizer E, Knisely AS, Rela M, Wilson C, Callender ME, O’Grady JG, Heneghan MA. Safety and efficacy of combined use of sildenafil, bosentan, and iloprost before and after liver transplantation in severe portopulmonary hypertension. Liver Transpl. 2008;14:287–291. doi: 10.1002/lt.21310. [DOI] [PubMed] [Google Scholar]
- 172.Cadden IS, Greanya ED, Erb SR, Scudamore CH, Yoshida EM. The use of sildenafil to treat portopulmonary hypertension prior to liver transplantation. Ann Hepatol. 2009;8:158–161. [PubMed] [Google Scholar]
- 173.Gough MS, White RJ. Sildenafil therapy is associated with improved hemodynamics in liver transplantation candidates with pulmonary arterial hypertension. Liver Transpl. 2009;15:30–36. doi: 10.1002/lt.21533. [DOI] [PubMed] [Google Scholar]
- 174.Hemnes AR, Robbins IM. Sildenafil monotherapy in portopulmonary hypertension can facilitate liver transplantation. Liver Transpl. 2009;15:15–19. doi: 10.1002/lt.21479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lasaponara F, Sedigh O, Pasquale G, Bosio A, Rolle L, Ceruti C, Timpano M, Negro CL, Paradiso M, Abbona A, Segoloni GP, Fontana D. Phosphodiesterase type 5 inhibitor treatment for erectile dysfunction in patients with end-stage renal disease receiving dialysis or after renal transplantation. J Sex Med. 2013;10:2798–2814. doi: 10.1111/jsm.12038. [DOI] [PubMed] [Google Scholar]
- 176.Pizanis N, Heckmann J, Wendt D, Tsagakis K, Jakob H, Kamler M. Improvement of pulmonary microcirculation after lung transplantation using phosphodiesterase-5 inhibitor modified preservation solution. Eur J Cardiothorac Surg. 2009;35:801–806. doi: 10.1016/j.ejcts.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 177.Tsai JW, Ayubi FS, Hart KL, Baur DA, Parham MA, Moon JK, Vazquez R, Chasen AB, Zhang Z, Pizarro JM. Evaluation of the effect of sildenafil and vascular endothelium growth factor combination treatment on skin flap survival in rats. Aesthetic Plast Surg. 2008;32:624–631. doi: 10.1007/s00266-008-9166-2. [DOI] [PubMed] [Google Scholar]
- 178.Kaya B, Cerkez C, Isilgan SE, Gokturk H, Yigman Z, Serel S, Can B, Ergun H. Comparison of the effects of systemic sildenafil, tadalafil, and vardenafil treatments on skin flap survival in rats. J Plast Surg Hand Surg. 2015;49:358–362. doi: 10.3109/2000656X.2015.1041024. [DOI] [PubMed] [Google Scholar]
- 179.Mayer M, Stief CG, Truss MC, Uckert S. Phosphodiesterase inhibitors in female sexual dysfunction. World J Urol. 2005;23:393–397. doi: 10.1007/s00345-005-0015-5. [DOI] [PubMed] [Google Scholar]
- 180.Check JH, Graziano V, Lee G, Nazari A, Choe JK, Dietterich C. Neither sildenafil nor vaginal estradiol improves endometrial thickness in women with thin endometria after taking oral estradiol in graduating dosages. Clin Exp Obstet Gynecol. 2004;31:99–102. [PubMed] [Google Scholar]
- 181.Jerzak M, Kniotek M, Mrozek J, Gorski A, Baranowski W. Sildenafil citrate decreased natural killer cell activity and enhanced chance of successful pregnancy in women with a history of recurrent miscarriage. Fertil Steril. 2008;90:1848–1853. doi: 10.1016/j.fertnstert.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 182.El-Far M, El-Motwally Ael G, Hashem IA, Bakry N. Biochemical role of intravaginal sildenafil citrate as a novel antiabortive agent in unexplained recurrent spontaneous miscarriage: First clinical study of four case reports from Egypt. Clin Chem Lab Med. 2009;47:1433–1438. doi: 10.1515/CCLM.2009.311. [DOI] [PubMed] [Google Scholar]
- 183.Sher G, Fisch JD. Vaginal sildenafil (viagra): A preliminary report of a novel method to improve uterine artery blood flow and endometrial development in patients undergoing IVF. Hum Reprod. 2000;15:806–809. doi: 10.1093/humrep/15.4.806. [DOI] [PubMed] [Google Scholar]
- 184.Sher G, Fisch JD. Effect of vaginal sildenafil on the outcome of in vitro fertilization (ivf) after multiple ivf failures attributed to poor endometrial development. Fertil Steril. 2002;78:1073–1076. doi: 10.1016/s0015-0282(02)03375-7. [DOI] [PubMed] [Google Scholar]
- 185.George EM, Granger JP. Mechanisms and potential therapies for preeclampsia. Curr Hypertens Rep. 2011;13:269–275. doi: 10.1007/s11906-011-0204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Turgut NH, Temiz TK, Bagcivan I, Turgut B, Gulturk S, Karadas B. The effect of sildenafil on the altered thoracic aorta smooth muscle responses in rat pre-eclampsia model. Eur J Pharmacol. 2008;589:180–187. doi: 10.1016/j.ejphar.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 187.Samangaya RA, Mires G, Shennan A, Skillern L, Howe D, McLeod A, Baker PN. A randomised, double-blinded, placebo-controlled study of the phosphodiesterase type 5 inhibitor sildenafil for the treatment of preeclampsia. Hypertens Pregnancy. 2009;28:369–382. doi: 10.3109/10641950802601278. [DOI] [PubMed] [Google Scholar]
- 188.Hackett G. PDE5 inhibitors in diabetic peripheral neuropathy. Int J Clin Pract. 2006;60:1123–1126. doi: 10.1111/j.1742-1241.2006.01087.x. [DOI] [PubMed] [Google Scholar]
- 189.Wang L, Chopp M, Szalad A, Liu Z, Bolz M, Alvarez FM, Lu M, Zhang L, Cui Y, Zhang RL, Zhang ZG. Phosphodiesterase-5 is a therapeutic target for peripheral neuropathy in diabetic mice. Neuroscience. 2011;193:399–410. doi: 10.1016/j.neuroscience.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang L, Chopp M, Zhang ZG. PDE5 inhibitors promote recovery of peripheral neuropathy in diabetic mice. Neural Regen Res. 2017;12:218–219. doi: 10.4103/1673-5374.200804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Patil CS, Singh VP, Singh S, Kulkarni SK. Modulatory effect of the PDE-5 inhibitor sildenafil in diabetic neuropathy. Pharmacology. 2004;72:190–195. doi: 10.1159/000080104. [DOI] [PubMed] [Google Scholar]
- 192.Desouza C, Parulkar A, Lumpkin D, Akers D, Fonseca VA. Acute and prolonged effects of sildenafil on brachial artery flow-mediated dilatation in type 2 diabetes. Diabetes Care. 2002;25:1336–1339. doi: 10.2337/diacare.25.8.1336. [DOI] [PubMed] [Google Scholar]
- 193.Aversa A, Bruzziches R, Francomano D, Natali M, Lenzi A. Testosterone and phosphodiesterase type-5 inhibitors: New strategy for preventing endothelial damage in internal and sexual medicine? Ther Adv Urol. 2009;1:179–197. doi: 10.1177/1756287209344992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Aversa A, Vitale C, Volterrani M, Fabbri A, Spera G, Fini M, Rosano GM. Chronic administration of sildenafil improves markers of endothelial function in men with type 2 diabetes. Diabet Med. 2008;25:37–44. doi: 10.1111/j.1464-5491.2007.02298.x. [DOI] [PubMed] [Google Scholar]
- 195.Pelliccione F, D’Angeli A, D’Andrea S, Barbonetti A, Pezzella A, Necozione S, Falone S, Amicarelli F, Francavilla F, Francavilla S. Tadalafil treatment had a modest effect on endothelial cell damage and repair ability markers in men with erectile dysfunction and vascular risk. Asian J Androl. 2014;16:290–294. doi: 10.4103/1008-682X.122347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Grover-Paez F, Villegas Rivera G, Guillen Ortiz R. Sildenafil citrate diminishes microalbuminuria and the percentage of A1C in male patients with type 2 diabetes. Diabetes Res Clin Pract. 2007;78:136–140. doi: 10.1016/j.diabres.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 197.Palit V, Eardley I. An update on new oral PDE5 inhibitors for the treatment of erectile dysfunction. Nat Rev Urol. 2010;7:603–609. doi: 10.1038/nrurol.2010.165. [DOI] [PubMed] [Google Scholar]
- 198.Eardley I, Donatucci C, Corbin J, El-Meliegy A, Hatzimouratidis K, McVary K, Munarriz R, Lee SW. Pharmacotherapy for erectile dysfunction. J Sex Med. 2010;7:524–540. doi: 10.1111/j.1743-6109.2009.01627.x. [DOI] [PubMed] [Google Scholar]
- 199.Nehra A. Erectile dysfunction and cardiovascular disease: Efficacy and safety of phosphodiesterase type 5 inhibitors in men with both conditions; Mayo Clin Proc; 2009; pp. 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship; Mayo Clin Proc; 2008; pp. 584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Piazza GA, Alberts DS, Hixson LJ, Paranka NS, Li H, Finn T, Bogert C, Guillen JM, Brendel K, Gross PH, Sperl G, Ritchie J, Burt RW, et al. Sulindac sulfone inhibits azoxymethane-induced colon carcinogenesis in rats without reducing prostaglandin levels. Cancer Res. 1997;57:2909–2915. [PubMed] [Google Scholar]
- 202.Thompson WJ, Piazza GA, Li H, Liu L, Fetter J, Zhu B, Sperl G, Ahnen D, Pamukcu R. Exisulind induction of apoptosis involves guanosine 3’,5’-cyclic monophosphate phosphodiesterase inhibition, protein kinase g activation, and attenuated beta-catenin. Cancer Res. 2000;60:3338–3342. [PubMed] [Google Scholar]
- 203.Soh JW, Mao Y, Kim MG, Pamukcu R, Li H, Piazza GA, Thompson WJ, Weinstein IB. Cyclic GMP mediates apoptosis induced by sulindac derivatives via activation of c-JUN NH2-terminal kinase 1. Clin Cancer Res. 2000;6:4136–4141. [PubMed] [Google Scholar]
- 204.Soriano AF, Helfrich B, Chan DC, Heasley LE, Bunn PA, Jr, Chou TC. Synergistic effects of new chemopreventive agents and conventional cytotoxic agents against human lung cancer cell lines. Cancer Res. 1999;59:6178–6184. [PubMed] [Google Scholar]
- 205.Chan DC, Earle KA, Zhao TL, Helfrich B, Zeng C, Baron A, Whitehead CM, Piazza G, Pamukcu R, Thompson WJ, Alila H, Nelson P, Bunn PA. Exisulind in combination with docetaxel inhibits growth and metastasis of human lung cancer and prolongs survival in athymic nude rats with orthotopic lung tumors. Clin Cancer Res. 2002;8:904–912. [PubMed] [Google Scholar]
- 206.Bunn PA, Jr, Chan DC, Earle K, Zhao TL, Helfrich B, Kelly K, Piazza G, Whitehead CM, Pamukcu R, Thompson W, Alila H. Preclinical and clinical studies of docetaxel and exisulind in the treatment of human lung cancer. Semin Oncol. 2002;29:87–94. doi: 10.1053/sonc.2002.31529. [DOI] [PubMed] [Google Scholar]
- 207.Li Q, Shu Y. Pharmacological modulation of cytotoxicity and cellular uptake of anti-cancer drugs by PDE5 inhibitors in lung cancer cells. Pharm Res. 2014;31:86–96. doi: 10.1007/s11095-013-1134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Booth L, Roberts JL, Poklepovic A, Gordon S, Dent P. PDE5 inhibitors enhance the lethality of pemetrexed through inhibition of multiple chaperone proteins and via the actions of cyclic GMP and nitric oxide. Oncotarget. 2017;8:1449–1468. doi: 10.18632/oncotarget.13640. http://doi.org/18632/oncotarget.13640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hoang T, Kim K, Merchant J, Traynor AM, McGovern J, Oettel KR, Sanchez FA, Ahuja HG, Hensing TA, Larson M, Schiller JH. Phase I/II study of gemcitabine and exisulind as second-line therapy in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2006;1:218–225. doi: 10.1016/s1556-0864(15)31571-9. [DOI] [PubMed] [Google Scholar]
- 210.Jones SF, Kuhn JG, Greco FA, Raefsky EL, Hainsworth JD, Dickson NR, Thompson DS, Willcutt NT, White MB, Burris HA., 3rd A phase I/II study of exisulind in combination with docetaxel/carboplatin in patients with metastatic non-small-cell lung cancer. Clin Lung Cancer. 2005;6:361–366. doi: 10.3816/clc.2005.n.016. [DOI] [PubMed] [Google Scholar]
- 211.Masters GA, Li S, Dowlati A, Madajewicz S, Langer C, Schiller J, Johnson D. A phase II trial of carboplatin and gemcitabine with exisulind (IND #65,056) in patients with advanced non-small cell lung cancer: an Eastern Cooperative Oncology Group Study (E1501) J Thorac Oncol. 2006;1:673–678. [PubMed] [Google Scholar]
- 212.Weiss GJ, Vokes EE, Bunn PA, Jr, Magree L, Rusk J, Albert D, Kelly K. Docetaxel and exisulind in previously treated non-small cell lung cancer (NSCLC) patients: A multicenter, phase II clinical trial. J Thorac Oncol. 2007;2:933–938. doi: 10.1097/JTO.0b013e3181462051. [DOI] [PubMed] [Google Scholar]
- 213.Govindan R, Wang X, Baggstrom MQ, Burdette-Radoux S, Hodgson L, Vokes EE, Green MR Cancer Leukemia Group B. A phase II study of carboplatin, etoposide, and exisulind in patients with extensive small cell lung cancer: CALGB 30104. J Thorac Oncol. 2009;4:220–226. doi: 10.1097/JTO.0b013e3181951eb0. [DOI] [PubMed] [Google Scholar]
- 214.Goluboff ET, Shabsigh A, Saidi JA, Weinstein IB, Mitra N, Heitjan D, Piazza GA, Pamukcu R, Buttyan R, Olsson CA. Exisulind (sulindac sulfone) suppresses growth of human prostate cancer in a nude mouse xenograft model by increasing apoptosis. Urology. 1999;53:440–445. doi: 10.1016/s0090-4295(98)00513-5. [DOI] [PubMed] [Google Scholar]
- 215.Qian CN, Takahashi M, Kahnoski R, Teh BT. Effect of sildenafil citrate on an orthotopic prostate cancer growth and metastasis model. J Urol. 2003;170:994–997. doi: 10.1097/01.ju.0000080321.99119.df. [DOI] [PubMed] [Google Scholar]
- 216.Das A, Durrant D, Mitchell C, Mayton E, Hoke NN, Salloum FN, Park MA, Qureshi I, Lee R, Dent P, Kukreja RC. Sildenafil increases chemotherapeutic efficacy of doxorubicin in prostate cancer and ameliorates cardiac dysfunction. Proc Natl Acad Sci U S A. 2010;107:18202–18207. doi: 10.1073/pnas.1006965107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Das A, Durrant D, Mitchell C, Dent P, Batra SK, Kukreja RC. Sildenafil (viagra) sensitizes prostate cancer cells to doxorubicin-mediated apoptosis through CD95. Oncotarget. 2016;7:4399–4413. doi: 10.18632/oncotarget.6749. http://doi.org/10.18632/oncotarget.6749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Liu N, Mei L, Fan X, Tang C, Ji X, Hu X, Shi W, Qian Y, Hussain M, Wu J, Wang C, Lin S, Wu X. Phosphodiesterase 5/Protein Kinase G signal governs stemness of prostate cancer stem cells through hippo pathway. Cancer Lett. 2016;378:38–50. doi: 10.1016/j.canlet.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 219.Zenzmaier C, Sampson N, Pernkopf D, Plas E, Untergasser G, Berger P. Attenuated proliferation and trans-differentiation of prostatic stromal cells indicate suitability of phosphodiesterase type 5 inhibitors for prevention and treatment of benign prostatic hyperplasia. Endocrinology. 2010;151:3975–3984. doi: 10.1210/en.2009-1411. [DOI] [PubMed] [Google Scholar]
- 220.Wang C. Phosphodiesterase-5 inhibitors and benign prostatic hyperplasia. Curr Opin Urol. 2010;20:49–54. doi: 10.1097/MOU.0b013e328333ac68. [DOI] [PubMed] [Google Scholar]
- 221.Chavez AH, Scott Coffield K, Hasan Rajab M, Jo C. Incidence rate of prostate cancer in men treated for erectile dysfunction with phosphodiesterase type 5 inhibitors: Retrospective analysis. Asian J Androl. 2013;15:246–248. doi: 10.1038/aja.2012.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Khandrika L, Lieberman R, Koul S, Kumar B, Maroni P, Chandhoke R, Meacham RB, Koul HK. Hypoxia-associated p38 mitogen-activated protein kinase-mediated androgen receptor activation and increased HIF-1 alpha levels contribute to emergence of an aggressive phenotype in prostate cancer. Oncogene. 2009;28:1248–1260. doi: 10.1038/onc.2008.476. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 223.Movsas B, Chapman JD, Greenberg RE, Hanlon AL, Horwitz EM, Pinover WH, Stobbe C, Hanks GE. Increasing levels of hypoxia in prostate carcinoma correlate significantly with increasing clinical stage and patient age: an Eppendorf pO(2) study. Cancer. 2000;89:2018–2024. doi: 10.1002/1097-0142(20001101)89:9<2018::aid-cncr19>3.3.co;2-p. [DOI] [PubMed] [Google Scholar]
- 224.Hamilton TK, Hu N, Kolomitro K, Bell EN, Maurice DH, Graham CH, Siemens DR. Potential therapeutic applications of phosphodiesterase inhibition in prostate cancer. World J Urol. 2013;31:325–330. doi: 10.1007/s00345-012-0848-7. [DOI] [PubMed] [Google Scholar]
- 225.Goluboff ET, Prager D, Rukstalis D, Giantonio B, Madorsky M, Barken I, Weinstein IB, Partin AW, Olsson CA, Network UOR. Safety and efficacy of exisulind for treatment of recurrent prostate cancer after radical prostatectomy. J Urol. 2001;166:882–886. [PubMed] [Google Scholar]
- 226.Michl U, Molfenter F, Graefen M, Tennstedt P, Ahyai S, Beyer B, Budaus L, Haese A, Heinzer H, Oh SJ, Salomon G, Schlomm T, Steuber T, et al. Use of phosphodiesterase type 5 inhibitors may adversely impact biochemical recurrence after radical prostatectomy. J Urol. 2015;193:479–483. doi: 10.1016/j.juro.2014.08.111. [DOI] [PubMed] [Google Scholar]
- 227.Gallina A, Bianchi M, Gandaglia G, Cucchiara V, Suardi N, Montorsi F, Briganti A. A detailed analysis of the association between postoperative phosphodiesterase type 5 inhibitor use and the risk of biochemical recurrence after radical prostatectomy. Eur Urol. 2015;68:750–753. doi: 10.1016/j.eururo.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 228.Wang X, Wang X, Liu T, He Q, Wang Y, Zhang X. Systematic review and meta-analysis of the use of phosphodiesterase type 5 inhibitors for treatment of erectile dysfunction following bilateral nerve-sparing radical prostatectomy. PLoS One. 2014;9:e91327. doi: 10.1371/journal.pone.0091327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 230.Tinsley HN, Gary BD, Keeton AB, Zhang W, Abadi AH, Reynolds RC, Piazza GA. Sulindac sulfide selectively inhibits growth and induces apoptosis of human breast tumor cells by phosphodiesterase 5 inhibition, elevation of cyclic GMP, and activation of protein kinase G. Mol Cancer Ther. 2009;8:3331–3340. doi: 10.1158/1535-7163.MCT-09-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tinsley HN, Gary BD, Keeton AB, Lu W, Li Y, Piazza GA. Inhibition of PDE5 by sulindac sulfide selectively induces apoptosis and attenuates oncogenic Wnt/β-catenin-mediated transcription in human breast tumor cells. Cancer Prev Res (Phila) 2011;4:1275–1284. doi: 10.1158/1940-6207.CAPR-11-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Marino N, Collins JW, Shen C, Caplen NJ, Merchant AS, Gokmen-Polar Y, Goswami CP, Hoshino T, Qian Y, Sledge GW, Jr, Steeg PS. Identification and validation of genes with expression patterns inverse to multiple metastasis suppressor genes in breast cancer cell lines. Clin Exp Metastasis. 2014;31:771–786. doi: 10.1007/s10585-014-9667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Thompson HJ, Jiang C, Lu J, Mehta RG, Piazza GA, Paranka NS, Pamukcu R, Ahnen DJ. Sulfone metabolite of sulindac inhibits mammary carcinogenesis. Cancer Res. 1997;57:267–271. [PubMed] [Google Scholar]
- 234.Karami-Tehrani F, Moeinifard M, Aghaei M, Atri M. Evaluation of PDE5 and PDE9 expression in benign and malignant breast tumors. Arch Med Res. 2012;43:470–475. doi: 10.1016/j.arcmed.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 235.Liu L, Li H, Underwood T, Lloyd M, David M, Sperl G, Pamukcu R, Thompson WJ. Cyclic GMP-dependent protein kinase activation and induction by exisulind and CP461 in colon tumor cells. J Pharmacol Exp Ther. 2001;299:583–592. [PubMed] [Google Scholar]
- 236.Whitt JD, Li N, Tinsley HN, Chen X, Zhang W, Li Y, Gary BD, Keeton AB, Xi Y, Abadi AH, Grizzle WE, Piazza GA. A novel sulindac derivative that potently suppresses colon tumor cell growth by inhibiting cGMP phosphodiesterase and β-catenin transcriptional activity. Cancer Prev Res (Phila) 2012;5:822–833. doi: 10.1158/1940-6207.CAPR-11-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Li N, Xi Y, Tinsley HN, Gurpinar E, Gary BD, Zhu B, Li Y, Chen X, Keeton AB, Abadi AH, Moyer MP, Grizzle WE, Chang WC, et al. Sulindac selectively inhibits colon tumor cell growth by activating the cGMP/PKG pathway to suppress Wnt/β-catenin signaling. Mol Cancer Ther. 2013;12:1848–1859. doi: 10.1158/1535-7163.MCT-13-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Tinsley HN, Gary BD, Thaiparambil J, Li N, Lu W, Li Y, Maxuitenko YY, Keeton AB, Piazza GA. Colon tumor cell growth-inhibitory activity of sulindac sulfide and other nonsteroidal anti-inflammatory drugs is associated with phosphodiesterase 5 inhibition. Cancer Prev Res (Phila) 2010;3:1303–1313. doi: 10.1158/1940-6207.CAPR-10-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Rice PL, Goldberg RJ, Ray EC, Driggers LJ, Ahnen DJ. Inhibition of extracellular signal-regulated kinase 1/2 phosphorylation and induction of apoptosis by sulindac metabolites. Cancer Res. 2001;61:1541–1547. [PubMed] [Google Scholar]
- 240.Rice PL, Beard KS, Driggers LJ, Ahnen DJ. Inhibition of extracellular-signal regulated kinases 1/2 is required for apoptosis of human colon cancer cells in vitro by sulindac metabolites. Cancer Res. 2004;64:8148–8151. doi: 10.1158/0008-5472.CAN-04-1517. [DOI] [PubMed] [Google Scholar]
- 241.Mei XL, Yang Y, Zhang YJ, Li Y, Zhao JM, Qiu JG, Zhang WJ, Jiang QW, Xue YQ, Zheng DW, Chen Y, Qin WM, Wei MN, et al. Sildenafil inhibits the growth of human colorectal cancer in vitro and in vivo. Am J Cancer Res. 2015;5:3311–3324. [PMC free article] [PubMed] [Google Scholar]
- 242.Li N, Chen X, Zhu B, Ramirez-Alcantara V, Canzoneri JC, Lee K, Sigler S, Gary B, Li Y, Zhang W, Moyer MP, Salter EA, Wierzbicki A, et al. Suppression of β-catenin/TCF transcriptional activity and colon tumor cell growth by dual inhibition of PDE5 and 10. Oncotarget. 2015;6:27403–27415. doi: 10.18632/oncotarget.4741. http://doi.org/10.18632/oncotarget.4741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Stoner GD, Budd GT, Ganapathi R, DeYoung B, Kresty LA, Nitert M, Fryer B, Church JM, Provencher K, Pamukcu R, Piazza G, Hawk E, Kelloff G, et al. Sulindac sulfone induced regression of rectal polyps in patients with familial adenomatous polyposis. Adv Exp Med Biol. 1999;470:45–53. doi: 10.1007/978-1-4615-4149-3_5. [DOI] [PubMed] [Google Scholar]
- 244.van Stolk R, Stoner G, Hayton WL, Chan K, DeYoung B, Kresty L, Kemmenoe BH, Elson P, Rybicki L, Church J, Provencher K, McLain D, Hawk E, et al. Phase I trial of exisulind (sulindac sulfone, FGN-1) as a chemopreventive agent in patients with familial adenomatous polyposis. Clin Cancer Res. 2000;6:78–89. [PubMed] [Google Scholar]
- 245.Islam BN, Sharman SK, Hou Y, Bridges AE, Singh N, Kim S, Kolhe R, Trillo-Tinoco J, Rodriguez PC, Berger FG, Sridhar S, Browning DD. Sildenafil suppresses inflammation-driven colorectal cancer in mice. Cancer Prev Res (Phila) 2017;10:377–388. doi: 10.1158/1940-6207.CAPR-17-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Piazza GA. Validation of PDE5 as a chemoprevention target. Cancer Prev Res (Phila) 2017;10:373–376. doi: 10.1158/1940-6207.CAPR-17-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Giordano D, Giorgi M, Sette C, Biagioni S, Augusti-Tocco G. cAMP-dependent induction of PDE5 expression in murine neuroblastoma cell differentiation. FEBS Lett. 1999;446:218–222. doi: 10.1016/s0014-5793(99)00227-6. [DOI] [PubMed] [Google Scholar]
- 248.Roberts JL, Booth L, Conley A, Cruickshanks N, Malkin M, Kukreja RC, Grant S, Poklepovic A, Dent P. PDE5 inhibitors enhance the lethality of standard of care chemotherapy in pediatric CNS tumor cells. Cancer Biol Ther. 2014;15:758–767. doi: 10.4161/cbt.28553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Black KL, Yin D, Ong JM, Hu J, Konda BM, Wang X, Ko MK, Bayan JA, Sacapano MR, Espinoza A, Irvin DK, Shu Y. PDE5 inhibitors enhance tumor permeability and efficacy of chemotherapy in a rat brain tumor model. Brain Res. 2008;1230:290–302. doi: 10.1016/j.brainres.2008.06.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Booth L, Roberts JL, Tavallai M, Nourbakhsh A, Chuckalovcak J, Carter J, Poklepovic A, Dent P. OSU-03012 and Vagra treatment inhibits the activity of multiple chaperone proteins and disrupts the blood-brain barrier: Implications for anti-cancer therapies. J Cell Physiol. 2015;230:1982–1998. doi: 10.1002/jcp.24977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Hu J, Ljubimova JY, Inoue S, Konda B, Patil R, Ding H, Espinoza A, Wawrowsky KA, Patil C, Ljubimov AV, Black KL. Phosphodiesterase type 5 inhibitors increase herceptin transport and treatment efficacy in mouse metastatic brain tumor models. PLoS One. 2010;5:e10108. doi: 10.1371/journal.pone.0010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wang R, Chen W, Zhang Q, Liu Y, Qiao X, Meng K, Mao Y. Phosphodiesterase type 5 inhibitor tadalafil increases rituximab treatment efficacy in a mouse brain lymphoma model. J Neurooncol. 2015;122:35–42. doi: 10.1007/s11060-014-1690-0. [DOI] [PubMed] [Google Scholar]
- 253.Cesarini V, Martini M, Vitiani LR, Gravina GL, Di Agostino S, Graziani G, D’Alessandris QG, Pallini R, Larocca LM, Rossi P, Jannini EA, Dolci S. Type 5 phosphodiesterase regulates glioblastoma multiforme aggressiveness and clinical outcome. Oncotarget. 2017;8:13223–13239. doi: 10.18632/oncotarget.14656. http://doi.org/10.18632/oncotarget.14656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in Globocan 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 255.Sherman SI. Thyroid carcinoma. Lancet. 2003;361:501–511. doi: 10.1016/s0140-6736(03)12488-9. [DOI] [PubMed] [Google Scholar]
- 256.Schlumberger M, Lacroix L, Russo D, Filetti S, Bidart JM. Defects in iodide metabolism in thyroid cancer and implications for the follow-up and treatment of patients. Nat Clin Pract Endocrinol Metab. 2007;3:260–269. doi: 10.1038/ncpendmet0449. [DOI] [PubMed] [Google Scholar]
- 257.Qin C, Cau W, Zhang Y, Mghanga FP, Lan X, Gao Z, An R. Correlation of clinicopathological features and expression of molecular markers with prognosis after 131I treatment of differentiated thyroid carcinoma. Clin Nucl Med. 2012;37:e40–46. doi: 10.1097/RLU.0b013e31823905e4. [DOI] [PubMed] [Google Scholar]
- 258.Lakics V, Karran EH, Boess FG. Quantitative comparison of phosphodiesterase mRNA distribution in human brain and peripheral tissues. Neuropharmacology. 2010;59:367–374. doi: 10.1016/j.neuropharm.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 259.Persani L, Lania A, Alberti L, Romoli R, Mantovani G, Filetti S, Spada A, Conti M. Induction of specific phosphodiesterase isoforms by constitutive activation of the cAMP pathway in autonomous thyroid adenomas. J Clin Endocrinol Metab. 2000;85:2872–2878. doi: 10.1210/jcem.85.8.6712. [DOI] [PubMed] [Google Scholar]
- 260.Sponziello M, Verrienti A, Rosignolo F, De Rose RF, Pecce V, Maggisano V, Durante C, Bulotta S, Damante G, Giacomelli L, Di Gioia CR, Filetti S, Russo D, et al. PDE5 expression in human thyroid tumors and effects of PDE5 inhibitors on growth and migration of cancer cells. Endocrine. 2015;50:434–441. doi: 10.1007/s12020-015-0586-x. [DOI] [PubMed] [Google Scholar]
- 261.Caronia LM, Phay JE, Shah MH. Role of braf in thyroid oncogenesis. Clin Cancer Res. 2011;17:7511–7517. doi: 10.1158/1078-0432.CCR-11-1155. [DOI] [PubMed] [Google Scholar]
- 262.Spoto G, Esposito A, Santoleri F, Rubini C, Rutjes AW, Fioroni M, Ferrante M, Petrini M. Does cyclic guanosine monophosphate induce autophagy in thyroid malignant carcinoma through down-regulation of cyclic guanosine monophosphate phosphodiesterase? J Biol Regul Homeost Agents. 2016;30:599–604. [PubMed] [Google Scholar]
- 263.De Rose RF, Celano M, Maggisano V, Vero A, Di Francesco M, Paolino D, Russo D. PDE5 inhibitors-loaded nanovesicles: Physico-chemical properties and in vitro antiproliferative activity. Nanomaterials. 2016;6:1–12. doi: 10.3390/nano6050092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Cummins DL, Cummins JM, Pantle H, Silverman MA, Leonard AL, Chanmugam A. Cutaneous malignant melanoma; Mayo Clin Proc; 2006; pp. 500–507. [DOI] [PubMed] [Google Scholar]
- 265.Sulaimon SS, Kitchell BE. The basic biology of malignant melanoma: Molecular mechanisms of disease progression and comparative aspects. J Vet Intern Med. 2003;17:760–772. doi: 10.1111/j.1939-1676.2003.tb02513.x. [DOI] [PubMed] [Google Scholar]
- 266.Murata T, Shimizu K, Watanabe Y, Morita H, Sekida M, Tagawa T. Expression and role of phosphodiesterase 5 in human malignant melanoma cell line. Anticancer Res. 2010;30:355–358. [PubMed] [Google Scholar]
- 267.Arozarena I, Sanchez-Laorden B, Packer L, Hidalgo-Carcedo C, Hayward R, Viros A, Sahai E, Marais R. Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A. Cancer Cell. 2011;19:45–57. doi: 10.1016/j.ccr.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 268.Dhayade S, Kaesler S, Sinnberg T, Dobrowinski H, Peters S, Naumann U, Liu H, Hunger RE, Thunemann M, Biedermann T, Schittek B, Simon HU, Feil S, et al. Sildenafil potentiates a cGMP-dependent pathway to promote melanoma growth. Cell Rep. 2016;14:2599–2610. doi: 10.1016/j.celrep.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 269.Li WQ, Qureshi AA, Robinson KC, Han J. Sildenafil use and increased risk of incident melanoma in US men: a prospective cohort study. JAMA Intern Med. 2014;174:964–970. doi: 10.1001/jamainternmed.2014.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Loeb S, Folkvaljon Y, Lambe M, Robinson D, Garmo H, Ingvar C, Stattin P. Use of phosphodiesterase type 5 inhibitors for erectile dysfunction and risk of malignant melanoma. JAMA. 2015;313:2449–2455. doi: 10.1001/jama.2015.6604. [DOI] [PubMed] [Google Scholar]
- 271.Matthews A, Langan SM, Douglas IJ, Smeeth L, Bhaskaran K. Phosphodiesterase type 5 inhibitors and risk of malignant melanoma: matched cohort study using primary care data from the UK clinical practice research datalink. PLoS Med. 2016;13:e1002037. doi: 10.1371/journal.pmed.1002037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Wang J, Shen Y, Wang J, Xue Y, Liao L, Thapa S, Ji K. Relation of phosphodiesterase type 5 inhibitors and malignant melanoma: A meta-analysis and systematic review. Oncotarget. 2017;8:46461–46467. doi: 10.18632/oncotarget.17518. http://doi.org/10.18632/oncotarget.17518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ren Y, Zheng J, Yao X, Weng G, Wu L. Essential role of the cGMP/PKG signaling pathway in regulating the proliferation and survival of human renal carcinoma cells. Int J Mol Med. 2014;34:1430–1438. doi: 10.3892/ijmm.2014.1925. [DOI] [PubMed] [Google Scholar]
- 274.Zenitani M, Nojiri T, Uehara S, Miura K, Hosoda H, Kimura T, Nakahata K, Miyazato M, Okuyama H, Kangawa K. C-type natriuretic peptide in combination with sildenafil attenuates proliferation of rhabdomyosarcoma cells. Cancer Med. 2016;5:795–805. doi: 10.1002/cam4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sarfati M, Mateo V, Baudet S, Rubio M, Fernandez C, Davi F, Binet JL, Delic J, Merle-Beral H. Sildenafil and vardenafil, types 5 and 6 phosphodiesterase inhibitors, induce caspase-dependent apoptosis of B-chronic lymphocytic leukemia cells. Blood. 2003;101:265–269. doi: 10.1182/blood-2002-01-0075. [DOI] [PubMed] [Google Scholar]
- 276.Kumazoe M, Kim Y, Bae J, Takai M, Murata M, Suemasu Y, Sugihara K, Yamashita S, Tsukamoto S, Huang Y, Nakahara K, Yamada K, Tachibana H. Phosphodiesterase 5 inhibitor acts as a potent agent sensitizing acute myeloid leukemia cells to 67-kDa laminin receptor-dependent apoptosis. FEBS Lett. 2013;587:3052–3057. doi: 10.1016/j.febslet.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 277.Kumazoe M, Tsukamoto S, Lesnick C, Kay NE, Yamada K, Shanafelt TD, Tachibana H. Vardenafil, a clinically available phosphodiesterase inhibitor, potentiates the killing effect of EGCG on CLL cells. Br J Haematol. 2015;168:610–613. doi: 10.1111/bjh.13135. [DOI] [PubMed] [Google Scholar]
- 278.Noonan KA, Ghosh N, Rudraraju L, Bui M, Borrello I. Targeting immune suppression with PDE5 inhibition in end-stage multiple myeloma. Cancer Immunol Res. 2014;2:725–731. doi: 10.1158/2326-6066.CIR-13-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Califano JA, Khan Z, Noonan KA, Rudraraju L, Zhang Z, Wang H, Goodman S, Gourin CG, Ha PK, Fakhry C, Saunders J, Levine M, Tang M, et al. Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21:30–38. doi: 10.1158/1078-0432.CCR-14-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]