Abstract
Converging evidence from studies with animal models and humans suggests that early developmental exposure to polychlorinated biphenyls (PCBs) leads to deficits in cognitive flexibility and inhibitory control. These processes are mediated to a large extent by the prefrontal cortex, thus we examined the effects of PCB exposure during adolescence—a period of robust prefrontal cortical development—on both processes. Specifically, we used operant set-shifting and differential reinforcement of low rates of responding (DRL) tasks to assess cognitive flexibility and response inhibition, respectively. One male and one female pup from each of 14 litters were assigned to each of three treatment groups: 0, 3 or 6 mg PCB/kg/day. Rats were dosed orally from postnatal day (PND) 27–50 to capture the whole period of adolescence in rats. At approximately PND 90, they began testing in the set-shifting task which included an initial visual cue discrimination, an extra-dimensional shift to a position discrimination and a reversal of the position discrimination. There were no statistically significant group differences in errors to criterion on visual cue discrimination or on the shift from visual to position discrimination in either males or females. During the position reversal, the 6 mg/kg PCB males made significantly fewer errors to reach criterion than control males. The 3 mg/kg PCB males showed a trend in the same direction, but this did not reach statistical significance. Interestingly, error analysis revealed that PCB-exposed males made significantly fewer perseverative errors than controls in this phase. No group differences were observed in females. These results suggest a male-specific effect of adolescent PCB-exposure on the reversal phase of the set-shifting task. Following set-shifting, rats progressed to the DRL task in which they were required to withhold responding for a specified period of time (15 seconds) in order to receive a reinforcer. There were no exposure-related group differences in total presses or efficiency ratio in males or in females. In summary, there were subtle sex-specific effects of adolescent PCB exposure on the reversal phase of a set-shifting task, but no effects of exposure on performance on a DRL15 task, suggesting an effect on cognitive flexibility but not response inhibition.
Keywords: Polychlorinated biphenyls, adolescence, cognition, executive function, set-shifting, response inhibition
1. Introduction
Polychlorinated biphenyls (PCBs) are widespread environmental contaminants formerly used as lubricants and dielectric fluids in capacitors and transformers as well as in the production of carbonless copy paper, caulking material and fluorescent light ballasts (Ross, 2004). PCBs are also inadvertently produced as a byproduct of the manufacture of paint pigments (Grossman, 2013). In this way, PCBs can contaminate, and have recently been detected in, indoor and outdoor air and sediment to which humans can be exposed (Koh et al, 2015). Furthermore, older buildings still containing PCBs in caulking and fluorescent light ballasts will continue to contribute PCBs to ecosystems as they are remediated or demolished (Hornbuckle & Robertson, 2010). Thus, PCBs are likely to remain persistent in our environment for the foreseeable future.
PCBs can cross the placenta and are released into breast milk during lactation (Jacobson et al, 1984). Because of this, the potential health effects of perinatal exposure to PCBs have been the topic of much research over the last four decades. Developmental PCB exposure has been associated with impairments of executive function in humans and animals (reviewed in Eubig et al, 2010). In particular, deficits in cognitive flexibility have been seen in rats and monkeys perinatally exposed to PCBs (reviewed in Sable & Schantz, 2006). Response inhibition is also disrupted in rats (Sable et al, 2009), monkeys (Rice & Hayward, 1997; Rice, 1998) and children (Stewart et al, 2006) developmentally exposed to PCBs.
Although extensive research has been carried out evaluating the effects of perinatal PCB exposure on cognitive functioning in humans and animals, very little research has assessed the effects of PCB exposure during adolescence. During this period, the frontal lobes are undergoing marked plasticity and maturation. Specifically, there is marked synaptic remodeling occurring in this region (reviewed in Lenroot et al, 2007; Selemon, 2013). These changes likely underlie the cognitive improvements that emerge during or after adolescence (Brenhouse & Andersen, 2011). For instance, adult rats perform better than adolescent rats on tasks engaging the prefrontal cortex, including tests of response inhibition (Andrezejewski et al, 2011) and behavioral set-shifting, a task of cognitive flexibility (Newman & McGaughy, 2011). Similarly, studies in humans have shown age-related improvements in executive functioning. One study in children aged 9 to 18 years found that increasing age from preadolescence (ages 9–12) through early adolescence (ages 13–15) to late adolescence (ages 16–19) was significantly associated with better performance on measures of strategy set-shifting and response inhibition (Rosso et al, 2004). Another study found that performance on tasks of response inhibition was poor in childhood but steadily improved with age, reaching adult levels of performance in mid to late adolescence (Luna et al, 2010). Thus, given the previous research indicating that perinatal PCB exposure results in deficits in response inhibition and cognitive flexibility (reviewed in Eubig et al, 2010) and that these cognitive abilities are further developing during adolescence, it was hypothesized that adolescence would be a critical period when PCB exposure could result in deficits in these aspects of executive function. To address this question, operant tests of set-shifting and response inhibition (differential reinforcement of low rates of responding, DRL) were administered to adult rats exposed to an environmentally-relevant PCB mixture throughout adolescence.
Most previous PCB studies have used individual PCB congeners or commercial PCB mixtures, but these approaches do not accurately represent what is in the environment and what human populations are exposed to. In this study, we used an experimental PCB mixture formulated to mimic the PCB congener profile found in walleye (a popular sport-caught fish) in the Fox River in northeastern Wisconsin (Kostyniak et al, 2005), a body of water from which the human cohort we have also studied consumed sport-caught fish (Monaikul et al, in preparation). Thus, this study was designed to address not only the paucity of research on the effects of adolescent PCB exposure on cognitive functioning in adulthood but also to use an environmentally relevant mixture that more accurately models the mixture of PCBs to which human populations are exposed.
2. Methods
2.1 Animals
Twenty-one nulliparous female and 21 male Long-Evans rats, approximately 70 days of age, were purchased from Harlan (Indianapolis, IN). Animals used in this study were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). Rats were individually housed in standard plastic shoebox cages with beta-chip (virgin hardwood) bedding, in a temperature- and humidity-controlled room (22°C, 40–55% humidity) and were maintained on a 12-hour reverse light-dark cycle (lights off at 0830 h). Standard rat chow and water were available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Illinois at Urbana-Champaign and were in accordance with the guidelines of the Public Health Service Policy on Humane Care and Use of Laboratory Animals (2015) and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (2003).
2.2 Exposure
Male and female rats were individually paired for breeding for 8 days. Only litters with 7 pups or greater were kept, and larger litters were culled to 8–10 pups per litter on postnatal day (PND) 2. At weaning (PND 21), 3 male and 3 female pups from each litter were retained for cognitive testing. One male and 1 female pup from each litter were randomly assigned to each of 3 treatment groups: 0, 3 and 6 mg/kg/day PCBs (n=14, n=13, and n=14 male-female littermate pairs, respectively). The PCB mixture used in this study was formulated to mimic the congener profile found in walleye taken from the Fox River in northeast Wisconsin, thereby closely mimicking human PCB exposure from fish consumption. The mixture consisted of 35% Aroclor 1242 (Monsanto Lot KB 05-415), 35% Aroclor 1248 (AccuStandards Lot F-110), 15% Aroclor 1254 (Monsanto Lot KB 05-612), and 15% Aroclor 1260 (AccuStandards Lot 021-020). The mixture was found to have relatively low aryl hydrocarbon receptor (AhR) activity, but high ryanodine receptor (RyR) activity (Kostyniak et al, 2005). The chemicals were dissolved in corn oil to yield the dosing solutions.
At the time this study was designed, few studies existed in the literature regarding adolescent PCB exposure, especially not using a mixture comparable to the PCB mixture used here. As such, doses were chosen based on previous perinatal studies conducted in our lab using 0, 3 and 6 mg/kg/day of the Fox River PCB mixture that were shown to affect cognitive and behavioral function. These doses used in previous perinatal exposure studies were physiologically relevant because offspring born to dams given these doses appear phenotypically similar to children born to mothers with moderate to high PCB body burdens (Fein et al, 1984; Stewart et al, 2006). In particular, at these doses offspring have been shown to weigh less than control pups at birth and at weaning (Kostyniak et al, 2005) and have shown deficits in inhibitory control (Sable et al, 2009). Thus, as this study was the first to use this mixture in adolescent rats, we chose these dose levels that have had effects on cognition and behavior after perinatal exposure as a starting point to explore our hypotheses.
Dosing began at PND 27 and continued daily through PND 50. This age range was chosen initially based on reviews by Spear (2000; 2007) that describe age-specific behavioral “discontinuities” that are evident between younger and older animals. Overall, however, the literature is inconclusive in characterizing a definitive time frame for adolescence; thus, we chose a time frame (P27-P50) that captured a broad window of adolescence in both male and female rats. Pups were weighed daily through the dosing period, doses were adjusted daily to account for weight gain, and the appropriate amount of dosing solution was pipetted directly into the mouth of the pup. Beginning on PND 90, rats were weighed daily and access to food was restricted to 85% of the rats’ free-feeding weight in order to keep the animals motivated to work for food rewards in the operant chambers. Prior to food restriction, mean female weight (± SEM) was 226.8 ± 2.1g. On the first day of operant testing, mean female weight was 191.1 ± 1.9g. At the end of operant testing, mean female weight was 225.5 ± 1.9g. For males, mean weight (± SEM) was 360.8 ± 4.2g prior to food restriction. On the first day of operant testing, mean male weight was 319.1 ± 4.5g. At the end of operant testing, mean male weight was 328.9 ± 3.4g. Food restriction has been routinely used in our lab, and there is no evidence that it confounds PCB-mediated effects.
2.3 Apparatus
Behavioral testing was conducted in 24 automated operant conditioning chambers (Med Associates; St. Albans, VT) housed in sound attenuated cubicles, each ventilated by a fan. All operant chambers contained a stimulus cue light above each of the two retractable response levers, which were located symmetrically on both sides of the pellet trough approximately 5.5 centimeters above the floor. A white-noise generator masked extraneous sounds, and a sonalert speaker was used to signal reinforcement. The experimental contingencies were programmed using Medstate Notation behavioral programming language (Med Associates; St. Albans, VT).
2.4 Procedure
2.4.1 Set-Shifting
Programs described herein for the set-shifting task were modified from a procedure initially described by Floresco and colleagues (Floresco et al, 2008; Butts et al, 2013).
2.4.1.1 Pretraining
Rats were first trained to lever press using a fixed ratio (FR) 1 schedule of reinforcement in which a reward (TestDiet AIN-76A 45 mg pellets; St. Louis, MO) was delivered for each single lever press. During this pretraining phase, the house light was illuminated, the stimulus lights above the levers were not illuminated and one lever was presented and remained extended during the entire session. The rat had to make 50 presses in a session in order to progress to the next phase. After the rat successfully completed the first phase of FR1, the next phase required the rat to make 50 presses in one session on the opposite lever. Once the rat successfully completed this phase, it moved on to retractable lever press training for 5 sessions. In this phase, trials began with the illumination of the house light and one of the two levers extended. The lever retracted once a response was made, and after a 20 second inter-trial interval, one of the two levers extended again. The rat had to press the lever within 10 seconds of its insertion; otherwise, the lever retracted and the trial was counted as an “omission”. The house light extinguished after a response or an omission was made and levers retracted. Rats received 90 such trials in a daily session. Immediately following the last session of retractable lever press training (in the same day), side bias was determined for each rat. In this short session, each trial began with the illumination of the house light, and both levers were inserted into the chamber. Stimulus lights above the levers were not illuminated. A press on either of the levers resulted in the delivery of a food pellet and both levers retracting. Twenty seconds later, the house light was illuminated and both levers were extended again; if the rat chose the same lever as before, the levers retracted without dispensing a pellet. This continued until the rat made a press on the opposite lever. Thus, the trial did not end until both levers were pressed. The house light extinguished after a lever press, regardless of which was pressed. The program continued until 7 trials were completed. The rat’s side preference was determined by where the majority of first lever choices (left or right lever) were made.
2.4.1.2 Visual Cue Discrimination
After pretraining was completed, rats were trained on visual-cue discrimination where illumination of the stimulus light predicted reward. Prior to this visual-cue discrimination phase, the stimulus lights had not been illuminated. At the beginning of each trial, one of the stimulus lights located above either the right or left lever was illuminated, then both levers were extended into the chamber and the house light was illuminated. Once the trial began, the rat had 10 seconds to respond on a lever followed by a 10 second inter-trial interval. A press on the lever below the illuminated cue light resulted in the delivery of one reward pellet and retraction of both levers. If a rat failed to respond within 10 seconds or responded on the incorrect lever, no reward pellet was delivered and both levers retracted. The house light extinguished once a response (or omission) was made and the levers retracted. Each trial was 20 seconds in length (10 second choice period followed by a 10 second inter-trial interval), regardless of outcome (i.e., correct, incorrect or omission). After each 20 second trial, a new trial was initiated with the levers again extended and the house light again illuminated. This continued for 160 trials per session. To proceed to the next phase, the rat had to perform at 65% correct or better in one session to ensure that rats were performing above chance in this phase before moving to the next testing phase. Data were analyzed for number of errors made to reach a criterion of 8 consecutive correct responses.
2.4.1.3 Position discrimination (Set-shift)
Once rats performed at 65% or better in a session of visual-cue discrimination, they began the strategy-shift (set-shift) phase of the experiment where they had to disengage from the previously learned visual-cue strategy and shift to a new egocentric response strategy that predicted reward. A rat’s ability to shift from a previously relevant strategy to a new strategy is an index of its cognitive flexibility. The position discrimination strategy required the rat to press the lever opposite its side bias in order to obtain reward. The lit cue light no longer predicted reward and became an irrelevant dimension. To evaluate how well the rat remembered what it had learned previously, in the first 20 trials of this session rats continued to be reinforced for pressing the lever associated with the lit cue light. Beginning with the 21st trial, the lever opposite the rat’s side bias was reinforced, forcing the animal to shift to a new strategy in order to obtain a food reinforcer. After a response was made, the levers retracted. As in the visual cue discrimination phase, the house light illuminated when the levers extended at the beginning of each trial and extinguished when a response (or omission) was made and the levers retracted. Like the previous phase, each trial was 20 seconds in length (10 second choice period followed by 10 second inter-trial interval) regardless of outcome. After a 20 second trial, the levers extended again. This continued for 160 trials per session. Like the visual cue discrimination phase, to ensure performance above chance, sessions continued until the rat performed at 65% correct or better in a session. Data were analyzed for number of errors made to reach a criterion of 8 consecutive correct responses.
2.4.1.4 Reversal Learning
After they reached the 65% criterion in a session of position discrimination, rats moved on to the position reversal. In this phase, rats were required to respond on the lever opposite that rewarded during the initial position discrimination in order to earn a reinforcer. As in the position discrimination phase, the stimulus lights continued to illuminate individually above the levers in each trial but were an irrelevant cue. After a response was made, the levers retracted. Like in the previous phases, each trial was 20 seconds in length (10 second choice period followed by 10 second inter-trial interval) regardless of outcome. After a 20 second trial, the levers extended again. This continued for 160 trials. Reversal learning was tested in only one session, and data were analyzed for number of errors made to reach a criterion of 8 consecutive correct responses.
2.4.1.5 Error Analysis
We used an error analysis modified from the operant set-shifting paradigm in Floresco et al (2008) and Butts et al (2013). Errors committed during the position discrimination (i.e., the set-shift) phase were divided into three error subtypes: perseverative, regressive, and never-reinforced errors. During the position discrimination, a perseverative error was scored when a rat responded incorrectly by pressing the lever with the stimulus light illuminated above it, i.e., the rat was using the strategy that was correct in the previous visual cue discrimination when it should have been using position cues. Error data were analyzed in blocks of 16 trials; 8 out of these 16 trials had the old rule (press the lever below the light) and new rule (press a lever in one position) in conflict. Once a rat was using the original strategy less than 75% of the time in a block (i.e., rats made 4 or fewer perseverative-type errors in a block of 16 trials), all subsequent errors of this type were scored as regressive errors. Never-reinforced errors occurred during position discrimination when the cue light, though an irrelevant cue in this phase, happened to be lit above the correct lever position, but the rat pressed the opposite, incorrect lever. Hence, the rat made a response on a lever that would not have yielded a reward during either the visual cue discrimination or the position discrimination phases. Errors committed during the position reversal phase were divided into two error subtypes: perseverative errors and regressive errors. In the reversal phase, a perseverative error was scored during the early parts of the phase when the rat erroneously pressed the lever that was correct during the previous position discrimination phase. Regressive errors were scored when rats made 4 or fewer perseverative errors within a block of 16 trials in the reversal phase. Perseverative errors can be used as a measure of a rat’s ability to transition from the previously learned strategy. Regressive errors may be used as an index of a rat’s ability to maintain a new strategy after perseveration has ceased.
2.4.2 Differential Reinforcement of Low Rates of Responding (DRL)
2.4.2.1 DRL Training
After rats completed set-shifting and reversal learning testing, they were tested on a DRL schedule. During DRL testing, only the right lever was used, and it remained extended during the entire test session. During the first phase, a 1-second inter-response time (IRT) (DRL1) was required in order to obtain a reinforcer. The first training phase lasted for 2 sessions regardless of performance. During the second and third phases, the IRT required for reinforcement was increased to 5 seconds (DRL5) for 2 sessions and then 10 seconds (DRL10) for 2 sessions. During each training phase, animals were rewarded for the first lever press occurring after the specified time interval had elapsed. Responses occurring before the required IRT had elapsed reset the timer, requiring the animal to wait another full interval before a response would result in reinforcement. All training sessions terminated after 200 reinforcers were delivered or 90 minutes had elapsed, whichever occurred first.
2.4.2.2 DRL Testing
Following DRL training, rats were given 30 daily sessions that required a 15-second IRT in order to obtain a reinforcer (DRL15). Similar to the training phases, the first response after 15 seconds elapsed resulted in a reinforcer. Responses made before the 15 seconds elapsed reset the timer and delayed reinforcement. Daily sessions terminated after 200 reinforcers were delivered or 90 min had elapsed, whichever occurred first. After 30 sessions on DRL15, the rats moved on to DRL extinction for 3 days in which they were no longer reinforced for lever presses. Each extinction session terminated after 90 minutes.
2.5 Statistical Analyses
2.5.1 General Statistical Method
Data are reported as the mean ± SEM. All statistical analyses were conducted using SPSS for MS Windows (version 22.0, SPSS Inc.; Chicago, IL) with statistical significance set at p<0.05. If sphericity assumptions were violated, a Greenhouse-Geisser correction was used to reduce the risk of a Type 1 error (Rogan et al, 1979). Analyses requiring this correction are reported using the appropriately adjusted degrees of freedom rounded to the nearest integer. Because of previously reported sex differences in PCB-related effects on a set-shifting task (Widholm et al, 2001), male and female data were analyzed separately for the cognitive tasks in this study. In the interest of brevity, only significant exposure-related main effects and interactions are reported. Additional post-hoc analyses (Tukey’s honestly significant difference, HSD, test) were conducted as appropriate to determine the nature of significant effects that were detected via the initial omnibus analyses.
2.5.2 Set-Shifting Analysis
Group differences in response latencies, number of omissions, number of errors to reach criterion in each phase, and in the number of errors in the first 20 trials of position discrimination were determined using a one-way ANOVA with exposure (0, 3 or 6 mg/kg) as a between-subjects variable. For the position discrimination phase, group differences in perseverative, regressive and never-reinforced errors were analyzed using one-way ANOVAs with exposure (0, 3 or 6 mg/kg) as a between-subjects variable. For the reversal phase, group differences in perseverative and regressive errors were analyzed using one-way ANOVAs with exposure as a between-subjects variable.
2.5.3 DRL Analysis
Group differences in total number of lever presses and ratio of reinforced:non-reinforced responses from DRL1, DRL5 and DRL10 were analyzed in 3 separate ANOVAs using 3 (exposure) x 2 (day) mixed ANOVAs with testing day as a repeated-measures factor. For DRL15, data were averaged across five-day blocks to yield 6 testing blocks. The primary measures assessed were total presses and the ratio of reinforced:non-reinforced responses (efficiency ratio). These dependent measures were analyzed separately using a 3 (exposure) x 6 (block) mixed ANOVA with testing block as a repeated-measures factor. Each response made during DRL15 was also categorized into one of eight 2.5 second inter-response time (IRT) bins. The proportion of responses falling within each IRT bin was calculated and averaged across the 5 days in the first testing block (acquisition) and sixth testing block (steady state). Each of these was analyzed separately using a 3 (exposure) x 8 (bin) mixed ANOVA with IRT bin as a repeated-measures factor.
3. Results
3.1 Set-Shifting
Two rats did not complete or did not meet criteria on the set-shifting task and their data dropped from analyses. In the FR1 pretraining phase, rats took on average 3 to 4 sessions to complete this single lever-press training. The next pretraining phase, retractable lever-press training on both levers, took rats 5 sessions on average to complete. For each test phase (visual cue discrimination, position discrimination, position reversal), rats took 1 session on average to complete each phase with no apparent diminution in performance across each 160 trial session.
3.1.1. Omissions and Response Latencies
Mean trial omissions across all phases of the set-shifting task are presented in Table 1. During visual cue discrimination, the omission rates were very low, yet there was a significant effect of exposure on trial omissions in males [F(2, 38)=4.630, p=0.016]. Post-hoc analysis (Tukey’s HSD) revealed that males of the 6 mg/kg group had significantly more omissions than males of the 0 mg/kg (p=0.027) and 3 mg/kg (p=0.036) groups. There were no significant differences in omissions across exposures in males in the position discrimination or position reversal phases (all Fs<0.96, n.s.). There were also no significant differences in trial omissions across exposures in females in any of the phases of the set-shifting task (all Fs<1.36, n.s.) with all females making very few to no omissions.
Table 1.
Average trial omissions in set-shifting task
| Visual Cue Discrimination | Position Discrimination | Position Reversal | |
|---|---|---|---|
| Males | |||
| 0 mg/kg/day | 0.143±0.097 | 0.143±0.143 | 0.071±0.071 |
| 3 mg/kg/day | 0.167±0.103 | 0.083±0.077 | 0.000±0.000 |
| 6 mg/kg/day | *1.083±0.399 | 0.167±0.142 | 0.000±0.000 |
| Females | |||
| 0 mg/kg/day | 0.429±0.291 | 0.214±0.114 | 0.071±0.071 |
| 3 mg/kg/day | 0.333±0.142 | 0.250±0.179 | 0.417±0.229 |
| 6 mg/kg/day | 0.000±0.000 | 0.000±0.000 | 0.538±0.291 |
Effects of PCB exposure on trial omissions in the set-shifting task. There was a significant difference in omissions in males during visual cue discrimination (p=0.016) with males of the 6 mg/kg group having significantly more omissions than males of the 0 mg/kg (p=0.027) and 3 mg/kg (p=0.036) groups. Females did not differ significantly in trial omissions across any of the phases of the set-shifting task. Results are reported as mean ± SEM for all measures.
“*” denotes statistical significance (p<0.05)
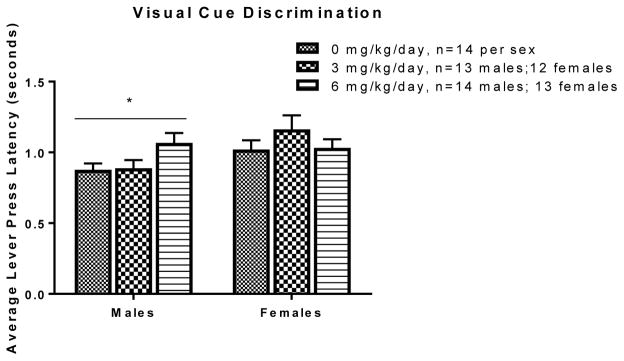
In males, there was a significant main effect of exposure on lever press latency in the visual cue discrimination phase only [F(2, 38)=3.43, p=0.043] with males of the 6 mg/kg group appearing to take longer to initiate a response, though post-hoc analysis (Tukey’s HSD) revealed no significant difference between exposure groups. Average lever press latency in the visual cue discrimination phase of the set-shifting task is represented in Figure 1. There were no significant differences across exposures for lever-press latency in females (all Fs<0.45, n.s. data not shown) suggesting that females had similar response times in each phase
Figure 1.
Effects of PCB exposure on average lever press latency of males and females during the visual cue discrimination phase of the set-shifting task. In this phase, there was a significant difference (p=0.043) in lever press latency across exposure groups in males, but not females, with males of the 6 mg/kg group appearing to take longer to initiate a response. Results are reported as mean ± SEM for all measures.
3.1.2 Visual Cue Discrimination
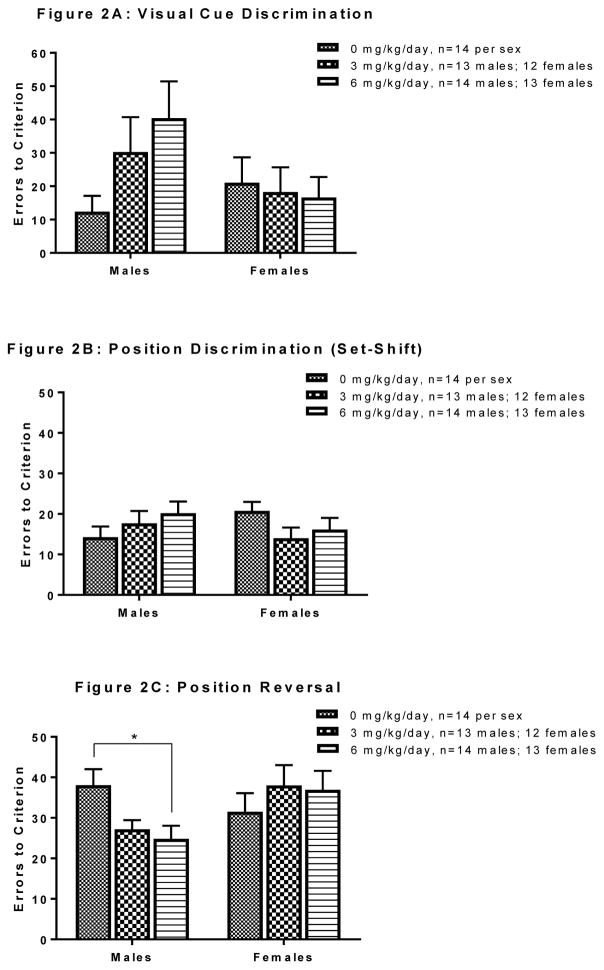
Mean errors to criterion for visual cue discrimination are represented in Figure 2A. There were no significant differences across exposures for errors to criterion in either males or females (all Fs<2.48, n.s.), although males in the 6 mg/kg PCB exposure group did appear to make more errors to reach criterion than the other two groups.
Figure 2.
Effects of PCB exposure on errors to criterion (8 consecutive correct responses) during the set-shifting task. During visual cue discrimination, there were no significant differences in performance across exposure groups in males or in females (Panel A). In the set-shift to position discrimination, there were no significant differences in performance across exposure groups in males or in females (Panel B). During the position reversal phase, males of the 6 mg/kg group made fewer errors before reaching criterion than males of the 0 mg/kg group (p=0.017). No significant difference in performance was observed across groups in females (Panel C). Results are reported as mean ± SEM for all measures. [“*” denotes a significant p-value of p<0.05]
3.1.3 Shift to Position Discrimination
There were no significant main effects of exposure in number of errors in males or females in the first 20 trials of this phase with all exposure groups performing similarly (all Fs<2.17, n.s.; data not shown). Mean errors to criterion in the position discrimination (set-shift) phase are presented in Figure 2B. There were no significant main effects of exposure in males or females in this phase with all exposure groups making a similar number of errors to reach criterion (all Fs<1.85, n.s.).
3.1.4 Response Reversal
Mean errors to criterion for the response reversal phase are presented in Figure 2C. A significant effect of exposure was found in males [F(2, 38) = 4.76; p=0.014]. Post-hoc analysis (Tukey’s HSD) revealed that males of the 6 mg/kg group made significantly fewer errors to reach criterion than males in the control group (p=0.017). A similar pattern of performance was seen with males of the 3 mg/kg group making fewer errors to reach criterion than males of the 0 mg/kg group, but this did not reach statistical significance (p=0.062). Males of the 3 mg/kg group and the 6 mg/kg group did not differ significantly from one another. In contrast, no significant differences in performance were observed across exposures in females with females of all groups making a similar number of errors to reach criterion (F<0.55, n.s.).
3.1.5 Error Analysis
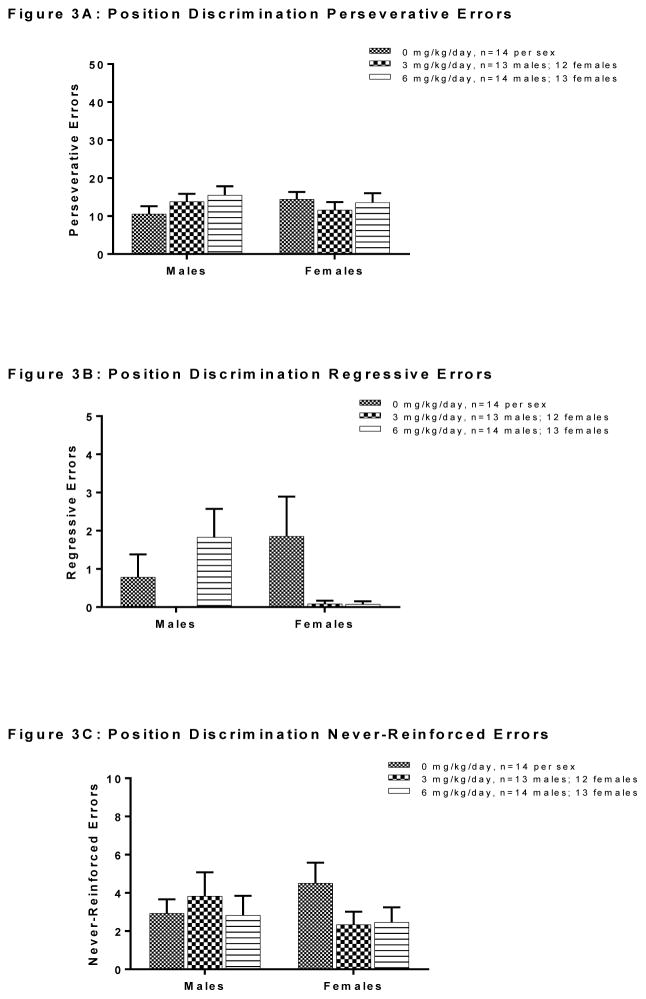
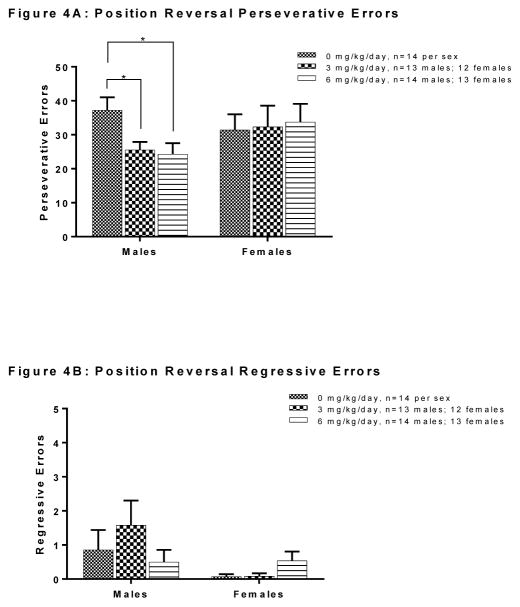
Analysis of error subtypes during the position discrimination phase is depicted in Figure 3. There was no significant main effect of exposure in males or in females on number of perseverative errors, regressive errors or never-reinforced errors (all Fs<2.654, n.s) in this phase. Analysis of error subtypes during the position reversal phase is depicted in Figure 4. In this phase, the effects of the treatment in males appeared to be driven by a reduction in the number of perseverative errors [F(2, 38)=5.040, p=0.011], with post-hoc analysis (Tukey HSD) revealing that males of the 3 and 6 mg/kg group made significantly fewer perseverative errors than controls (ps=0.038 and 0.017, respectively; Figure 4A). On the other hand, there were no differences between exposure groups in the number of perseverative errors made during the reversal phase in female rats, nor any differences in number of regressive errors in either males or females (all Fs<2.546, n.s.; Figures 4A and 4B, respectively)
Figure 3.
Effects of PCB exposure on types of errors made during the position discrimination phase of the set-shifting task. In this phase, there was no significant effect of exposure in males or in females on number of perseverative errors (Panel A), regressive errors (Panel B) or never-reinforced errors (Panel C). Results are reported as mean ± SEM for all measures.
Figure 4.
Effects of PCB exposure on types of errors made during the reversal phase of the set-shifting task. In this phase, there was a significant effect of exposure in males, but not in females, on number of perseverative errors (p=0.011) with males of the 3 mg/kg and 6 mg/kg groups making fewer perseverative errors than males of the 0 mg/kg group (Panel A). There was no significant effect of exposure in males or in females on number of regressive errors (Panel B). Results are reported as mean ± SEM for all measures. [“*” denotes a significant p-value of p<0.05]
3.2 DRL
3.2.1 Training Sessions
For the first training phase, DRL1 (data not shown), there were no significant main effects of exposure or session on total presses or efficiency ratio in males or in females, nor were there significant session by exposure interactions in males or females (all Fs<3.393, n.s.). This indicates that performance did not improve across the two sessions, and that there were no exposure-related differences in performance across sessions.
For both total presses and efficiency ratio in DRL5 (data not shown), there were no significant main effects of exposure (all Fs<0.888, n.s.), but there were significant main effects of session for both measures in males and in females (all Fs ≥ 12.230, all p<0.001) with performance improving across sessions. There were no significant session by exposure interactions in males or females (all Fs<1.195, n.s.) suggesting that there were no exposure-related differences in performance across DRL5 sessions.
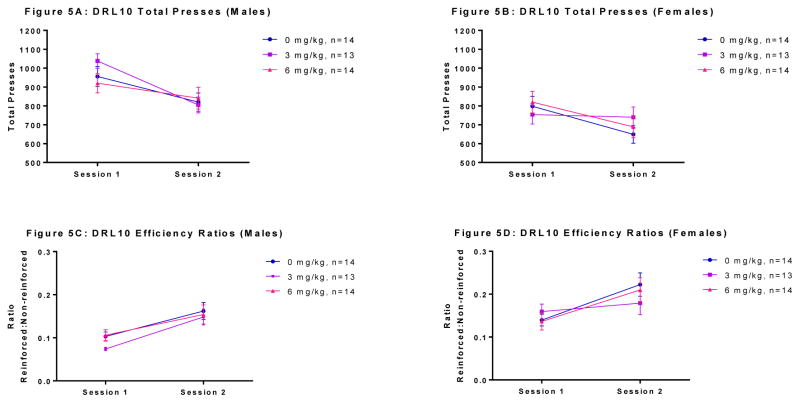
Total presses in DRL10 for males and females are presented in Figure 5 (5A and 5B, respectively). There was no significant main effect of session or exposure in males (all Fs<3.859, n.s.), but there was a significant session x exposure interaction [F(2, 38) = 4.095; p=0.025]. Post-hoc analysis (Tukey’s HSD) revealed a significant difference in total presses between sessions in males of the 3 mg/kg group (p=0.021). This effect resulted from the fact that the decline in total presses in the 3 mg/kg males between these 2 sessions was steeper than in the other two groups. More specifically, males of the 3 mg/kg group lever pressed more in the first session but were similar to other groups by the second session. The 0 mg/kg and 6 mg/kg males showed no significant difference in total presses across sessions. Females showed a similar pattern of results for total presses in DRL10. There was no significant main effect of session or exposure in females, but there was a significant session x exposure interaction [F(2, 38) = 5.264; p=0.010]. However, unlike in the males, post-hoc analysis showed no significant differences within groups across sessions.
Figure 5.
Effects of PCB exposure on DRL10 total presses and efficiency ratio. For total presses, there was no significant main effect of exposure in males (Panel A), but there was a significant session x exposure interaction (p=0.025). Post-hoc analysis revealed a significant difference in total presses between sessions in males of the 3 mg/kg group (p=0.021) only. Females showed a similar pattern of results for total presses in DRL 10 (Panel B). There was no significant main effect of exposure in females. There was a significant session x exposure interaction (p=0.010), but the post-hoc analyses were not significant. For efficiency ratio, there was no significant main effect of exposure or a significant session x exposure interaction in males (Panel C). In females, there was no significant main effect of exposure. There was a significant session x exposure interaction (p=0.032) (Panel D), but the post-hoc analyses were not significant. Results are reported as mean ± SEM for all measures.
Efficiency ratios for males and females are presented in Figure 5 (5C and 5D, respectively). There was no significant main effect of session or exposure in males, and there was no significant session x exposure interaction. Thus, males of all groups were performing with a similar pattern of efficiency across sessions. In females, there was no significant main effect of exposure, but, as with total presses, there was a significant session x exposure interaction [F(2, 38) = 3.801; p=0.032]. However, post-hoc analysis showed no significant differences within groups across sessions.
3.2.2 DRL15 Testing
Despite subtle differences during the DRL10 training phase, there were no significant exposure related effects or block x exposure interactions on either total presses or efficiency ratio (reinforced:non-reinforced presses) in males or females during the 5-session blocks of DRL15 testing (data not shown). All groups improved their performance across blocks of testing as indicated by decreases in total presses [F(2, 85) = 32.666, p<0.0001 in males; F(2, 78) =33.065, p<0.0001 in females] and increases in efficiency ratios [F(3, 117) = 62.636, p<0.0001 in males; [F(3, 116) = 41.938, p<0.0001 in females].
Responses were also analyzed in 2.5 second IRT bins across the 15 second period at the beginning of training (first block of sessions) and at the end of training (last block of sessions). There were no significant differences between groups at either time point. All groups shifted their pattern of responding to make fewer responses in the earlier IRT bins and more responses in the later bins, another indication that they learned the contingencies of the task.
3.2.3 DRL Extinction
There was no significant main effect of exposure and no exposure by session interaction for males or females (all Fs<1.743, n.s.). All groups decreased their responding across sessions at equivalent rates.
4. Discussion
In this study, we assessed cognitive flexibility and response inhibition in adult male and female rats exposed to PCBs during adolescence. In the set-shifting task, we saw a sex-specific effect of PCBs. Males, but not females, exposed to PCBs during adolescence made more omissions and had longer latencies to respond on the initial visual cue discrimination, but later made fewer errors on the reversal of a position discrimination. While there were subtle differences during the training phase of the DRL task, there were no effects of PCB exposure during adolescence on the actual DRL15 task.
4.1 Set-Shifting
The results on the set-shifting task were not exactly as hypothesized, but the data did reveal interesting PCB-related effects on performance in exposed males. Males of the 6 mg/kg group showed a marked increase in errors in the visual cue discrimination phase, though this effect was highly variable and thus did not reach statistical significance. This difference in performance may have been related to the longer response latencies and greater number of omissions observed in males of this group. Taken together, these findings suggest a disruption in performance in the 6 mg/kg males in learning the initial visual cue discrimination.
Adolescent PCB exposure did not impair learning of the set-shift (position discrimination), contrary to our hypothesis, and the PCB-exposed males in both dose groups actually seemed to acquire the position reversal more readily than controls. This apparent facilitation of reversal learning may have been due to PCB-exposed males making significantly fewer perseverative errors than the control males, suggesting perhaps that the PCB-exposed males may have not formed a response bias to the previously learned strategy, thus allowing these animals to acquire the position reversal more easily. Although these results were contrary to our hypothesis, they do suggest that adolescent PCB exposure may be disrupting the cognitive processes involved in reversal learning. Interestingly, a study performed by Thai and colleagues (2013) found a similar pattern of performance on the same operant set-shifting task in rats exposed to acute stress. In that study there were no significant differences in performance due to stress on the visual cue discrimination or the position discrimination. In the position reversal phase, however, stressed rats took fewer trials to reach criterion than controls and made fewer perseverative errors than controls. Thus, as was seen in our study, acute stress seemed to facilitate performance on reversal learning. The authors conjectured that acute stress may have biased the rats toward a strategy other than the spatial strategy that should have been acquired in the position discrimination phase.
Our approach to compensating for lever bias in the set-shifting task may also have contributed to the seemingly improved performance of the PCB-exposed males on the reversal phase. In our task, prior to the testing phases, all rats received a session in which side bias was determined. We then chose the lever opposite to each rat’s side bias as the lever that would be reinforced in the position discrimination (set-shift) phase. In the position reversal phase that followed the set-shift, the correct lever was the lever opposite to the lever reinforced in the previous phase, and was the lever for which the rat demonstrated a side bias. Learning the position discrimination and the reversal would normally engage spatial response learning (i.e., learning that the lever located in one position in the operant box was associated with the reward), but because the reversal phase involved pressing on an already favored-lever, it is possible that PCB-exposed rats may have reverted to an unextinguished habit over learning a new strategy. These results may indicate the possibility that learning of the position discrimination during the set shift was less stable in adolescent PCB-exposed rats relative to controls, and these PCB-exposed rats were thus able to more quickly revert to habit (i.e., their lever bias) to perform the reversal in fewer trials than the controls.
Because the operant set-shifting task, as many other operant tasks, can be adapted in different ways for individual studies, there are certain aspects of each iteration of the task to consider. For instance, the order of the test sessions may affect performance on the set-shifting task. In our study, after pre-training, during which the rats learned to respond to a single lever at a time, the first phase was visual cue discrimination in which both levers were presented at the same time, and rats were introduced to the illuminated cue light for the first time. This was followed by position discrimination in the next phase (both levers presented but cue lights become irrelevant). However, the opposite shift could have also been tested instead such that animals perform the position discrimination first followed by the visual cue discrimination. A study done by Floresco et al (2008) compared performance and phase order of visual cue discrimination and position discrimination and found that control rats took a greater number of trials (~80) to shift from visual cue to the position strategy than the opposite shift (position strategy to visual cue, ~40 trials). Thus, we chose to run all animals on the visual cue-to-position shift as it would be more likely than the opposite shift to pull out subtle differences in performance in our rats. Another aspect of our set-shifting task that could affect performance is the criteria used to proceed from one session to the next. Rats had to perform at 65% correct or better across a 160 trial session, and data were analyzed for trials to reach a criterion of 8 consecutive correct in a session. We performed a pilot study with this task which revealed that individual differences in trials to criterion between rats became much more pronounced as the criterion became more rigorous. We ultimately chose to try to strike a balance between making the criteria rigorous enough to be able to detect a difference between groups and reducing the interindividual differences within groups, as interindividual differences could obscure differences between groups. All in all, though there are several ways to customize this operant task, the adaptations made for the present study were chosen to optimize the detection of even subtle differences between groups.
A sex difference in performance on operant set-shifting has been reported previously in a study assessing the effect of gestational exposure to a viral mimetic on cognition in young adulthood (Zhang et al, 2012). In this study, performance on the task was disrupted in treated males and not females, though the authors reported that females did have longer response latencies than males on average throughout the task. Similarly, in our study, PCB exposure disrupted performance on the operant set-shifting task in males and not females. Furthermore, the sex-specific effect of PCB exposure is not unexpected as PCBs are known endocrine disruptors (Crinnion, 2011). Some animal studies have reported sex-specific effects in rats exposed to PCBs. For instance, a previous study done in our lab found deficits on DRL15 performance in response to amphetamine following perinatal PCB exposure, and this effect seemed to be driven by poorer performance in males (Sable et al, 2009). The authors hypothesized that this sex difference may be due to PCB-induced reductions in aromatase activity (Hany et al, 1999), an enzyme responsible for the conversion of testosterone to estradiol, which may influence proper sexual differentiation of the brain as well as the proper development of the dopaminergic system in the prefrontal cortex of the developing male rat (Stewart & Rajabi, 1994). Taken together, if these PCB-induced changes occurred in our animals, they may have contributed to sex-specific differences in performance seen in our study, but this warrants further investigation in future studies.
4.2 DRL
In the DRL task, we saw no significant effect of PCB exposure on performance on DRL15, contrary to our hypothesis. These DRL findings are inconsistent with what has been found with early postnatal PCB exposure in monkeys. Although the monkey studies reported deficits on DRL performance associated with PCB exposure (Rice 1998; Rice 1999), these studies used a PCB mixture with a different congener profile than the Fox River PCB mixture used here. The monkeys were also exposed in the early postnatal period and not as adolescents. Furthermore, these studies used a DRL30 in which monkeys had to withhold responses for 30 seconds in order to earn a reinforcer. It is possible that deficits in DRL performance would emerge in the PCB-exposed rats in our study if they were required to withhold responding for a longer period of time. Alternatively, our findings may be an indication that adolescence is not a sensitive period for the effects of PCB exposure on DRL performance.
The lack of an effect of exposure on DRL15 was not completely unexpected, however, because PCB-related deficits in performance have been somewhat inconsistent across studies. In one study using DRL15, rats exposed perinatally to the Fox River PCB mixture showed a lower ratio of reinforced to non-reinforced responses suggesting an impairment on this task (Sable et al, 2009). However, an earlier study found no significant effect of perinatal PCB treatment on DRL15 performance, although PCB-exposed rats did not extinguish lever pressing as readily as controls when tested on an extinction phase (Sable et al, 2006). These studies used the same PCB mixture and doses; however, an important difference between these two studies was that the rats that showed a deficit in performance were only tested on the DRL task (Sable et al, 2009), whereas in the study that did not see an effect of exposure rats were tested on a different operant task prior to DRL testing (Sable et al, 2006). Thus, it is possible that a transfer of experience occurred such that rats exposed to another operant task prior to DRL testing tended to perform the task more efficiently than rats for which the DRL task was their first exposure to operant training. In the current study, our rats were tested on another operant task (set-shifting) prior to DRL testing, and this prior experience could have moderated PCB-related effects on the DRL task.
4.3 Conclusions
We report a sex-specific effect of PCB exposure on an operant set-shifting task where exposed males showed better performance on reversal learning compared to controls, but we saw no effect of exposure on a DRL task of response inhibition. Our data suggest that there were some differences in cognition associated with adolescent PCB exposure. In the future, it would be useful to explore PCB-related effects on other executive functions mediated by the PFC, such as working memory, given that research has suggested PCB-related deficits exist on tasks of working memory in both humans and animals exposed perinatally (reviewed in Eubig et al, 2010). Adolescence is a period of multidimensional growth and maturation. As individuals are exploring their environments more independently, innumerable environmental influences are shaping brain, cognition, and behavior, thus making research on the effects of neurotoxicants such as PCBs and other environmental factors a valuable contribution to our understanding of this critical period of development.
Highlights.
Potential effects of adolescent exposure to an environmentally relevant PCB mixture on cognitive function in adulthood were tested; the executive functions tested (i.e., cognitive flexibility and response inhibition) are among those thought to be maturing during adolescence.
There was a subtle sex-specific effects of adolescent PCB exposure on the reversal phase of the operant set-shifting task.
There we no effects of exposure on performance on the DRL15 task.
Our results suggest an effect of adolescent PCB-exposure on cognitive flexibility but not response inhibition in adulthood.
Acknowledgments
Funding: This work was supported by NIEHS R01ES015687 and NIEHS K08ES017045 grants.
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrzejewski ME, Schochet TL, Feit EC, Harris R, McKee BL, Kelley AE. A comparison of adult and adolescent rat behavior in operant learning, extinction, and behavioral inhibition paradigms. Behavioral Neuroscience. 2011;125(1):93–105. doi: 10.1037/a0022038. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neuroscience Biobehavioral Reviews. 2011;35(8):1687–703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts KA, Floresco SB, Phillips AG. Acute stress impairs set-shifting but not reversal learning. Behavioural Brain Research. 2013;252:222–229. doi: 10.1016/j.bbr.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Crinnion WJ. Polychlorinated biphenyls: Persistent pollutants with immunological, neurological, and endocrinological consequences. Alternative Medicine Review: A Journal of Clinical Therapeutic. 2011;16(1):5–13. doi: 10.1093/toxsci/kfl018. [DOI] [PubMed] [Google Scholar]
- Eubig PA, Aguiar A, Schantz SL. Lead and PCBs as risk factors for Attention Deficit/Hyperactivity Disorder. Environmental Health Perspectives. 2010;118(12):1654–1667. doi: 10.1289/ehp.0901852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein GG, Jacobson JL, Jacobson SW, Schwartz PM, Dowler JK. Prenatal exposure to polychlorinated biphenyls: effects on birth size and gestational age. The Journal of Pediatrics. 1984;105(2):315–320. doi: 10.1016/s0022-3476(84)80139-0. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behavioural Brain Research. 2008;190(1):85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Grossman E. Nonlegacy PCBs: pigment manufacturing by-products get a second look. Environmental Health Perspectives. 2013;121(3):A86–93. doi: 10.1289/ehp.121-a86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hany J, Lilienthal H, Sarasin A, Roth-Harer A, Fastabend A, et al. Developmental exposure of rats to a reconstituted PCB mixture or aroclor 1254: effects on organ weights, aromatase activity, sex hormone levels, and sweet preference behavior. Toxicology and Applied Pharmacology. 1999;158(3):231–243. doi: 10.1006/taap.1999.8710. [DOI] [PubMed] [Google Scholar]
- Hornbuckle K, Robertson L. Polychlorinated biphenyls (PCBs): sources, exposures, toxicities. Environmental Science and Technolology. 2010;44(8):2749–51. doi: 10.1021/es100801f. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Fein GG, Jacobson SW, Schwartz PM, Dowler JK. The transfer of polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs) across the human placenta and into maternal milk. American Journal of Public Health. 1984;74(4):378–379. doi: 10.2105/AJPH.74.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh WX, Hornbuckle KC, Thorne PS. Human serum from urban and rural adolescents and their mothers shows exposure to polychlorinated biphenyls not found in commercial mixtures. Environmental Science and Technology. 2015;49(13):8105–8112. doi: 10.1021/acs.est.5b01854. doi:10:1021/acs.est.5b01854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyniak PJ, Hansen LG, Widholm JJ, Fitzpatrick RD, Olson JR, Helferich JL, et al. Formulation and characterization of an experimental PCB mixture designed to mimic human exposure from contaminated fish. Toxicological Sciences: An Official Journal of the Society of Toxicology. 2005;88(2):400–411. doi: 10.1093/toxsci/kfi338. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36(4):1065–73. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain and Cognition. 2010;72(1):101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaikul S, Monaikul N, Gardiner J, Schantz S. Polychlorinated biphenyls, polybrominated diphenyl ethers and cognitive performance in adolescent children of sports anglers in northeastern Wisconsin. 2016 Manuscript in preparation. [Google Scholar]
- National Research Council (US) Committee on Guidelines for the Use of Animals in Neuroscience and Behavioral Research. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. Washington (DC): National Academies Press (US); 2003. [DOI] [PubMed] [Google Scholar]
- Newman LA, McGaughy J. Adolescent rats show cognitive rigidity in a test of attentional set shifting. Developmental Psychobiology. 2011;53(4):391–401. doi: 10.1002/dev.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health Service. Policy on Humane Care and Use of Laboratory Animals. US Department of Health and Human Services; Washington, DC, USA: 2015. Available online: http://grants.nih.gov/grants/olaw/references/phspolicylabanimals.pdf. [Google Scholar]
- Rice DC. Effect of postnatal exposure to a PCB mixture in monkeys on multiple fixed interval-fixed ratio performance. Neurotoxicology and Teratology. 1997;19(6):429–434. doi: 10.1016/s0892-0362(97)87364-3. [DOI] [PubMed] [Google Scholar]
- Rice DC. Effects of postnatal exposure of monkeys to a PCB mixture on spatial discrimination reversal and DRL performance. Neurotoxicology and Teratology. 1998;20(4):391–400. doi: 10.1016/s0892-0362(97)00134-7. [DOI] [PubMed] [Google Scholar]
- Rice DC. Behavioral impairment produced by low-level postnatal PCB exposure in monkeys. Environmental Research. 1999;80(2 Pt 2):S113–S121. doi: 10.1006/enrs.1998.3917. [DOI] [PubMed] [Google Scholar]
- Rice DC. Effect of exposure to 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) throughout gestation and lactation on development and spatial delayed alternation performance in rats. Neurotoxicology and Teratology. 1999;21(1):59–69. doi: 10.1016/s0892-0362(98)00031-2. [DOI] [PubMed] [Google Scholar]
- Rice DC, Hayward S. Effects of postnatal exposure to a PCB mixture in monkeys on nonspatial discrimination reversal and delayed alternation performance. Neurotoxicology. 1997;18(2):479–494. [PubMed] [Google Scholar]
- Rogan WJ, Brown SM. Some fundamental aspects of epidemiology: A guide for laboratory scientists. Federation Proceedings. 1979;38(5):1875–1879. [PubMed] [Google Scholar]
- Ross G. The public health implications of polychlorinated biphenyls (PCBs) in the environment. Ecotoxicology and Environmental Safety. 2004;59(3):275–291. doi: 10.1016/j.ecoenv.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Rosso IM, Young AD, Femia LA, Yurgelun-Todd DA. Cognitive and emotional components of frontal lobe functioning in childhood and adolescence. Annals of the New York Academy of Sciences. 2004;1021:355–362. doi: 10.1196/annals.1308.045. [DOI] [PubMed] [Google Scholar]
- Sable HJ, Eubig PA, Powers BE, Wang VC, Schantz SL. Developmental exposure to PCBs and/or MeHg: Effects on a differential reinforcement of low rates (DRL) operant task before and after amphetamine drug challenge. Neurotoxicology and Teratology. 2009;31(3):149–158. doi: 10.1016/j.ntt.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sable HJ, Powers BE, Wang VC, Widholm JJ, Schantz SL. Alterations in DRH and DRL performance in rats developmentally exposed to an environmental PCB mixture. Neurotoxicology and Teratology. 2006;28(5):548–556. doi: 10.1016/j.ntt.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Sable HJK, Schantz SL. Executive function following developmental exposure to polychlorinated biphenyls (PCBs): What animal models have told us. In: Levin ED, Buccafusco JJ, editors. Animal models of cognitive impairment. Boca Raton (FL): Taylor & Francis Group, LLC; 2006. [PubMed] [Google Scholar]
- Selemon LD. A role for synaptic plasticity in the adolescent development of executive function. Translational Psychiatry. 2013;3:e238. doi: 10.1038/tp.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Assessment of adolescent neurotoxicity: rationale and methodological considerations. Neurotoxicology and Teratology. 2007;29(1):1–9. doi: 10.1016/j.ntt.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J, Rajabi H. Estradiol derived from testosterone in prenatal life affects the development of catecholamine systems in the frontal cortex in the male rat. Brain Research. 1994;646(1):157–160. doi: 10.1016/0006-8993(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Stewart PW, Sargent DM, Reihman J, Gump BB, Lonky E, Darvill T, et al. Response inhibition during differential reinforcement of low rates (DRL) schedules may be sensitive to low-level polychlorinated biphenyl, methylmercury, and lead exposure in children. Environmental Health Perspectives. 2006;114(12) doi: 10.1289/ehp.9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai CA, Zhang Y, Howland JG. Effects of acute restraint stress on set-shifting and reversal learning in male rats. Cognitive, Affective, and Behavioral Neuroscience. 2013;13(1):164–73. doi: 10.3758/s13415-012-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm JJ, Clarkson GB, Strupp BJ, Crofton KM, Seegal RF, Schantz SL. Spatial reversal learning in Aroclor 1254-exposed rats: Sex-specific deficits in associative ability and inhibitory control. Toxicology and Applied Pharmacology. 2001;174:188–198. doi: 10.1006/taap.2001.9199. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cazakoff BN, Thai CA, Howland JG. Prenatal exposure to a viral mimetic alters behavioural flexibility in male, but not female, rats. Neuropharmacology. 2012;62(3):1299–1307. doi: 10.1016/j.neuropharm.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]