Abstract
Background
Intraoperative hypothermia is common in patients undergoing general anesthesia during arthroscopic hip surgery. In the present study, we assessed the effect of heating and humidifying the airway with a heated wire humidification circuit (HHC) to attenuate the decrease of core temperature and prevent hypothermia in patients undergoing arthroscopic hip surgery under general anesthesia.
Methods
Fifty-six patients scheduled for arthroscopic hip surgery were randomly assigned to either a control group using a breathing circuit connected with a heat and moisture exchanger (HME) (n = 28) or an HHC group using a heated wire humidification circuit (n = 28). The decrease in core temperature was measured from anesthetic induction and every 15 minutes thereafter using an esophageal stethoscope.
Results
Decrease in core temperature from anesthetic induction to 120 minutes after induction was lower in the HHC group (–0.60 ± 0.27℃) compared to the control group (–0.86 ± 0.29℃) (P = 0.001). However, there was no statistically significant difference in the incidence of intraoperative hypothermia or the incidence of shivering in the postanesthetic care unit.
Conclusions
The use of HHC may be considered as a method to attenuate intraoperative decrease in core temperature during arthroscopic hip surgery performed under general anesthesia and exceeding 2 hours in duration.
Keywords: Arthroscopy, Body temperature, General anesthesia, Hypothermia
Introduction
In patients undergoing general anesthesia, intraoperative hypothermia is common because of the cool operating room environment and the loss of thermoregulatory control due to anesthetics. When heat loss is twice that of metabolic heat production, body temperatures may decrease up to approximately 1℃/hour [1]. During general anesthesia, additional heat loss through administration of cold intravenous fluids and surgical incisions places patients at higher risk of hypothermia [1].
Irrigation fluids used during arthroscopic surgery have a large impact on temperature regulation. Kim et al. [2] reported that when room temperature irrigation fluids are utilized, body temperature decreases in proportion to the amount of irrigation fluids. Moreover, Parodi et al. [3] reported that prolonged surgical time and cold irrigation fluids resulted in inevitable progression to hypothermia during arthroscopic hip surgery. In that study, perioperative core temperature decreased at a rate of 0.19℃/hour. In addition, 2.7% of patients experienced profound hypothermia, which was defined as core temperature below 35.0℃. The incidence of a decrease in body temperature greater than 0.5℃ at the end of the surgery was 68.22%. Therefore, efforts to prevent hypothermia are required in arthroscopic hip surgery.
Among methods of patient warming, cutaneous heating through forced air warming is recommended as one of the most effective techniques. Forced air warming completely eliminates heat loss from the skin surface [4,5]. Furthermore, forced air warming usually maintains normothermia even during the longest operations. However, the use of forced air warmers is associated with the risk of air-borne contamination of the sterile surgical field [6,7]. As an alternative to forced air warmers, heating and humidification through the trachea can be considered. There have been reports that reduction of core temperature can be attenuated from 75 minutes after anesthetic induction by the warming of anesthetic gas during arthroscopic shoulder surgery under general anesthesia [8]. It has also been reported that airway heating and humidification is even more effective in children than in adults [9].
However, since heat loss through respiration is very low, some researchers argue that heating and humidifying the respiratory tract does not affect the core temperature [10]. Heating and humidification of the respiratory tract in abdominal surgery with a long surgical duration [11], or in burn patients with large surface exposure [12], did not show a significant difference when compared to unheated breathing circuits. In one study, while the use of a heated wire humidification circuit (HHC) during spinal surgery did not reduce hypothermia, it was nevertheless associated with decreased blood loss [13]. Controversy remains regarding whether HHC prevents hypothermia during general anesthesia compared to typical circuits.
We hypothesized that the use of HHC would attenuate the decrease of core temperature in patients undergoing arthroscopic hip surgery exceeding 2 hours in duration. We investigated the effect of heating and humidification of inhalation gas on the reduction of core temperature and the incidence of perioperative hypothermia and postoperative shivering in patients undergoing arthroscopic hip surgery under general anesthesia.
Materials and Methods
Materials
After receiving Institutional Review Board approval from the hospital's ethics committee (IRB File No. 2014-06-016-005), the study was conducted by initially obtaining informed consent from patients undergoing general anesthesia for arthroscopic hip surgery. Patients aged 20 to 65, with American Society of Anesthesiologists physical status 1 or 2 were selected. Exclusion criteria included body temperature above 37.5℃ or below 36.0℃ measured 1 hour before surgery, uncontrolled hypertension or diabetes, conversion to open hip surgery, or previously diagnosed pulmonary disease. The decrease in core temperature was measured every 15 minutes starting from anesthetic induction to 120 minutes after anesthetic induction.
Patients were randomly allocated into two groups: a group that used a breathing circuit connected with a heat and moisture exchanger (HME) (TUORen, Henan Tuoren Medical Device Co., Ltd., Beijing, China) (control group, n = 28), and a group that used an HHC (Uni Heated Circuit, Unimedics Co., Seoul, Korea) (HHC group, n = 28) set to 39.0℃. HHC allows temperature settings in the range from 36℃ to 42℃, and the humidifier is a single-limb double-lumen type.
Anesthetic method and measurement
All patients were premedicated with glycopyrrolate 0.2 mg intramuscular one hour before anesthetic induction. To reduce the effects of anesthetic gas, total intravenous anesthesia was performed. Anesthesia was induced with propofol and remifentanil. The Schneider model was used for propofol target-controlled infusion and the Minto model for remifentanil. Effect site concentrations of 3.5–4 µg/ml for propofol and 4 ng/ml for remifentanil were set for induction. Neuromuscular blockade was induced with rocuronium 0.6 mg/kg. After endotracheal intubation, mechanical ventilation was provided with tidal volume 8–10 ml/kg, inspired oxygen concentration 40%, fresh gas flow 3 L/min, and end-tidal carbon dioxide concentration 30–35 mmHg set with respiratory rate adjusted at 8–12 breaths/min. Propofol was continuously infused at a rate maintaining bispectral index values between 40 and 60. One orthopedic surgeon performed all the procedures. In the supine position, patients were exposed from chest to knee without a surgical drape. Immediately after anesthetic induction, an esophageal stethoscope (Esophageal stethoscope, Sewoon Medical Co., Cheonan, Korea) was placed 35–37 cm from the upper incisors within the lower 1/3 of the esophagus to continuously monitor core temperature. Noninvasive blood pressure, pulse oximetry, and electrocardiography were measured. The operating room temperature was set to 23–24℃, and the irrigation solution was stored in the operation room.
Demographics including patient age, sex, height, weight, and body mass index were recorded. Operating time, total fluid infused (ml), and total articular irrigation fluid (ml) were also measured. Core temperature measured immediately after anesthetic induction was considered as baseline. The accumulated decrease of core temperature, defined as a decline of temperature from baseline, was assessed every 15 minutes. The incidence and magnitude of hypothermia, as well as shivering in the postanesthetic care unit (PACU), were also recorded. If the core temperature fell below 35.0℃, warming measures were taken, such as administration of pre-warmed intravenous fluid and forced air warming (Bair Hugger Model 505, Arizant Healthcare Inc., Eden Prairie, MN, USA).
Statistical analysis
On the hypothesis that the use of HHC can attenuate the decrease of core temperature in patients undergoing arthroscopic hip surgery, a pilot study was conducted in which 20 patients were assigned to either a group using a breathing circuit with HME or a group using HHC, and the decrease in core temperature was compared from the anesthetic induction to 120 minutes after induction. Total changes in core temperature were analyzed with the independent t test, and P values < 0.05 were regarded as statistically significant. The pilot study showed that the decrease in core temperature was lower in the HHC group (P = 0.001). The sample size was calculated based on this pilot study, with an average core temperature decrease of 0.62℃, standard deviation of 0.27 in the HHC group, α of 0.05, and 80% power (1-β) calculated using the MedCalc program, and each group was designated to include 28 patients.
Statistical analysis was conducted using R, and the normality of the data was assessed with the Kolmogorov-Smirnov test. For comparison between the two groups, if the normality was satisfied, we used the independent t test, and the results were expressed as mean ± SD. When the normality was not satisfied, the Mann-Whitney U test was used to compare the values of the two groups, and variables were expressed as the median (quartile). Patient sex was compared using the chi-square test. P values < 0.05 were regarded as statistically significant. Total changes in core temperature were analyzed with repeated measures analysis of variance (ANOVA). Post hoc analysis was performed at each interval using the independent t test. The Bonferroni method was applied for correction of the significance level, and P values < 0.00625 were regarded as statistically significant. Temperature change trends between the two groups were compared using linear mixed-effects modeling to numerically express core temperature change according to time. Because measured values can be analyzed despite missing data, we did not necessarily need to use linear regression. Logistic regression analysis was used to examine the relationship between core temperature at the end of the operation and shivering in the PACU, with P values < 0.05 regarded as statistically significant. The degree of association of each factor was expressed as an odds ratio with 95% CI.
Results
There were no statistically significant differences in demographics between the two groups (Table 1). Patients in both groups showed a gradual temperature decrease according to time. However, the magnitude of decrease in core temperature at 2 hours after anesthetic induction was significantly smaller in the HHC group (–0.60 ± 0.27℃) compared to the control group (–0.86 ± 0.29℃) (P = 0.001). The initial temperature during anesthetic induction was 36.63 ± 0.33℃ in the control group and 36.60 ± 0.40℃ in the HHC group, with no statistically significant difference between the two groups (P = 0.799). Two hours after anesthetic induction, the core temperatures of the control and HHC group were 35.77 ± 0.44℃ and 36.00 ± 0.55℃, respectively. There was no statistically significant difference between the two groups (P = 0.093) (Table 2). Comparison of the accumulated decrease in core temperature based on time between the two groups using repeated measures ANOVA showed a statistically significant difference between the two groups at 120 minutes after anesthetic induction (Table 3).
Table 1. Patient Characteristics and Induction Profiles.
| Control (n = 28) | HHC (n = 28) | P value | |
|---|---|---|---|
| Age (yr) | 38.4 ± 11.5 | 41.6 ± 9.7 | 0.263 |
| Sex (M/F) | 19/9 | 18/10 | 0.778 |
| Height (cm) | 167.8 ± 7.6 | 169.3 ± 8.5 | 0.496 |
| Weight (kg) | 67.5 ± 10.8 | 70.4 ± 19.0 | 0.489 |
| Body mass index (kg/m2) | 23.9 ± 3.2 | 24.4 ± 5.4 | 0.690 |
| Operation time (min) | 149 ± 23 | 153 ± 26 | 0.494 |
| Total intravenous fluid (ml) | 1059 ± 399 | 1000 ± 312 | 0.540 |
| Total irrigation fluid (ml) | 17144 ± 4126 | 17271 ± 5791 | 0.925 |
Values are mean ± SD, and numbers of patients. Control: a group that used a breathing circuit connected to a heat and moisture exchanger, HHC: a group that used a heated wire humidification breathing circuit.
Table 2. Comparison of Body Temperature at Two Hours after Anesthesia Induction and Incidence of Intraoperative Hypothermia and Shivering in the PACU.
| Control (n = 28) | HHC (n = 28) | P value | |
|---|---|---|---|
| Body temperature at anesthesia induction (℃) | 36.63 ± 0.33 | 36.60 ± 0.40 | 0.799 |
| Body temperature at 2 hours after anesthesia induction (℃) | 35.77 ± 0.44 | 36.00 ± 0.55 | 0.093 |
| Hypothermia (< 36.0℃) | 18 (64.3%) | 15 (53.6%) | 0.415 |
| Mild (35.5–35.9℃) | 12 (42.8%) | 8 (28.6%) | |
| Moderate (35.0–35.4℃) | 5 (17.9%) | 6 (21.4%) | |
| Profound (34.5–34.9℃) | 1 (3.6%) | 1 (3.6%) | |
| Shivering in PACU | 11 (39.3%) | 6 (21.4%) | 0.146 |
Values are mean ± SD, or numbers of patients (%). Control: patients treated using an unheated respiratory circuit, HHC: patient treated using a humidified and electrically heated circuit. PACU: postanesthetic care unit.
Table 3. Comparison of Accumulated Decrease of Core Temperature between Two Groups.
| Control (n = 28) | HHC (n = 28) | P value | |
|---|---|---|---|
| Δ℃/15 min | 0.12 ± 0.07 | 0.08 ± 0.06 | 0.035 |
| Δ℃/30 min | 0.21 ± 0.12 | 0.17 ± 0.12 | 0.221 |
| Δ℃/45 min | 0.30 ± 0.18 | 0.25 ± 0.14 | 0.247 |
| Δ℃/60 min | 0.39 ± 0.19 | 0.31 ± 0.17 | 0.093 |
| Δ℃/75 min | 0.51 ± 0.21 | 0.38 ± 0.22 | 0.024 |
| Δ℃/90 min | 0.64 ± 0.24 | 0.47 ± 0.24 | 0.010 |
| Δ℃/105 min | 0.73 ± 0.27 | 0.54 ± 0.28 | 0.013 |
| Δ℃/120 min | 0.86 ± 0.29 | 0.60 ± 0.27 | 0.001 |
Values are mean %± SD. Control: patients treated using an unheated respiratory circuit, HHC: patients treated using a humidified and electrically heated circuit. Δ℃: core temperature decrease from anesthetic induction to each measured point every 15 minutes. There was a significant difference in the accumulated decrease in core temperature between the two groups at 120 minutes after anesthesia induction (P < 0.00625).
The correlation between core temperature (temp) and demographics using linear mixed effect modeling can be expressed as Temp = 36.75 + time (min) × (−0.007) + sex × (−0.2244) + group × (0.002) (sex = 1: male, 0: female, group = 1: HHC, 0: control). The difference in temperature change per minute was 0.002℃ greater in the control group than in the HHC group. In addition, when excluding time and comparing only sex, temperatures in men were 0.2244℃ lower than those in women (Fig. 1). We did not check the assumptions of regression, referring to the statement by Bates and Pinheiro in Mixed-Effects Models in S and S-Plus, page 174, on the assumptions of linear mixed-effects models [14]. Distribution of the residuals was symmetric with mean zero, whether analysis was performed by sex, group, or total. There was no significant difference in variance between the two groups according to sex on standardized residual analysis. Also, because not only were the fitted values, but also the profile zeta plot, linear with the observed value (temp), the model of statistics can be considered as valid. The “Chisq,” the change of deviance, can be found in the ANOVA table, 99.0599 for time, 4.4460 for sex, and 0.9985 for group, and the interaction between time and group was 8.1933. Therefore, the level of contribution can be delineated as follows: Time > time: Group > sex > group (Table 4).
Fig. 1. Changes in core temperature (temp) in patients using an unheated respiratory circuit (●) or an electrically heated circuit (●) during hip arthroscopy. Linear mixed effect modeling: Temp = 36.75 + time (min) × (-0.007) + sex × (-0.2244) + group × (0.002) (sex = 1: male, 0: female, group = 1: HHC, 0: control).
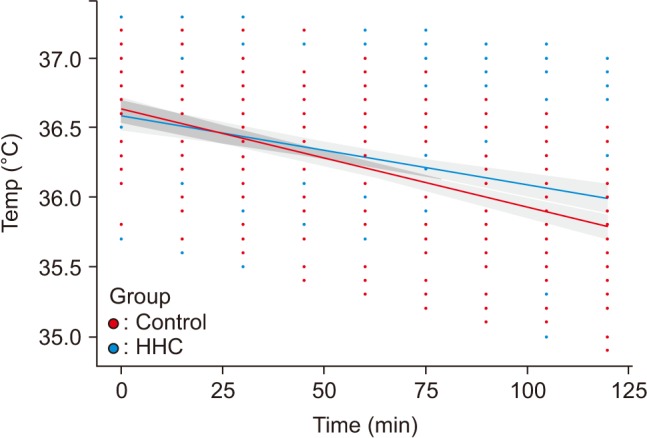
Table 4. ANOVA Table of Changes in Core Temperature in Patients using a Breathing Circuit Connected with HME or a Heated Wire Humidification Circuit during Hip Arthroscopy.
| Df | AIC | BIC | logLik | Deviance | Chisq | |
|---|---|---|---|---|---|---|
| Temp | 5 | −709.05 | −687.93 | 359.52 | −719.05 | |
| Time | 6 | −806.11 | −780.77 | 409.05 | −818.11 | 99.0599 |
| Sex | 7 | −808.55 | −778.99 | 411.28 | −822.55 | 4.4460 |
| Group | 8 | −807.55 | −773.77 | 411.78 | −823.55 | 0.9985 |
| Time: Group | 9 | −813.74 | −775.74 | 415.87 | −831.74 | 8.1933 |
ANOVA: analysis of variance, HME: heat and moisture exchanger, Df: degree of freedom, AIC: Akaike information criterion, BIC: Bayesian information criterion.
Nineteen patients (67.9%) in the control group and 16 patients (57.1%) in the HHC group were found to be hypothermic (below 36.0℃) in the PACU. Using logistic regression analysis to examine the correlation between body temperature and shivering in the PACU, it was determined that the odds (95% CI) of the incidence of shivering decreased 0.222 (0.063–0.786) times with a 1℃ increase in core temperature (P = 0.020). Shivering was improved with conventional treatment such as administration of pre-warmed intravenous fluid and forced air warming.
Discussion
General anesthesia interferes with homeostatic thermoregulation of the hypothalamus and eventually results in the decline of body temperature. Hypothermia during general anesthesia decreases metabolic oxygen requirements to protect the brain and heart from ischemic insults [15]. However, cardiovascular complications such as arrhythmia and myocardial infarction increase by more than three times, and hypothermia results in the limitation of oxygen delivery to tissues through increased peripheral vascular resistance and left shifting of the hemoglobin oxygen saturation curve [16,17]. These phenomena also cause problems such as coagulopathy from platelet dysfunction [18], increased postoperative catabolism and stress response, delayed healing at the surgical site, and increased risk of infection [19,20]. Because hypothermia is closely related to increases in morbidity and mortality [17], efforts should be made to prevent hypothermia.
There have been many reports of hypothermia less than 36.0℃ in patients undergoing arthroscopic surgery under general anesthesia, especially when large amounts of cold fluid are used despite short operating times [2,8,21]. It has been reported that during arthroscopic hip surgery, body temperature tends to decline and the incidence of hypothermia below 35.0℃ was 2.7%. The decrease in body temperature is influenced by the duration of surgery, cold irrigation fluid, and intraoperative hypotension [3]. To improve the surgical view, deliberate hypotension is also common in arthroscopic hip surgery [22]. However, efforts to prevent hypothermia, such as using heated irrigation fluids, resulted in increased intra-articular cavity bleeding, complicating the surgical view [23].
In the present study, the use of HHC resulted in statistically significant differences in the accumulated decrease in core temperature between the two groups at 120 minutes after anesthetic induction. Moreover, the temperature change per minute in the HCC group was 0.002℃ less than that in the control group. Since the difference in the rate of decrease of core temperature is not large enough, it is difficult to say that HHC is effective in a brief surgery. However, it can have a meaningful effect in longer operation, over 120 minutes in duration. Unfortunately, HHC did not decrease the frequency of intraoperative hypothermia or postoperative shivering in the present study. When excluding factors other than sex, men had a lower core temperature by 0.2244℃ than that of women. However, this finding is only a result of the formula derived by linear effects modeling, and it is difficult to say that it is a statistically significant value. This is because the initial temperatures in men and women were not considered.
Jo et al. [8] reported that core temperatures were significantly higher from 75 minutes to 120 minutes after anesthetic induction with HHC use in arthroscopic shoulder surgery than in a group using an unheated conventional respiratory circuit, but there was no significant difference in hypothermia below 36.0℃ between the two groups. There was a significant difference in core temperature reduction between the two groups, as was found in our study.
Because hypothermia during general anesthesia occurs in nearly 50–70% of patients [24], if active warming methods are not performed, core temperatures will fall 1–2℃ during the first hour of anesthesia (phase 1), and even more during the next 3 to 4 hours (phase 2) [10]. After 4 hours, there is no change in core temperature, and this will remain at a plateau (phase 3) [25]. During phase 1, heat redistributes high body temperatures from the central compartment of the body (chest, abdomen) to the peripheral compartments (arms, legs) through vasodilation induced by anesthetic agents. During this phase, heat loss from the patient's body to the environment is minimal. During phase 2, there is continuous heat loss from the patient's body to the environment, but the rate of temperature decrease is slower than during phase 1. During phase 3, the rates of metabolic temperature production and temperature loss become parallel [15,16].
Based on this principle of core temperature decrease, many intervention methods have been proposed to avert perioperative hypothermia. Warming the patient 30 minutes before surgery can effectively decrease the difference between central and peripheral temperatures of the body, blocking heat distribution in phase 1 and preventing temperature decrease. There are various ways to reduce the temperature decrease of phase 2, including the use of a forced air warming blanket [26], an electrically heated circuit [9], or warm intravenous and irrigation fluids [27], or increasing operating room temperatures [28]. The use of blankets for insulation is restricted because the whole body cannot be covered owing to the exposure of the operating site. Regardless of the operating site, a method that can be applied is the heating of inspired gas. Heating inspired gas can be used with a closed circuit, a heat and moisture exchanger, or a humidified electrically heated circuit.
HHC has several advantages besides the maintenance of body temperature. There are many reports that the use of HHC during surgery under general anesthesia attenuates the degree of core temperature reduction and is also effective in shoulder arthroscopic surgery [8] as well as in spine surgery, and that the use of HHC in spine surgery is effective in reducing intraoperative hemorrhage [13]. In addition, the effects of heated humidification of the upper airway are lost during ventilation through intubation. However, using an HHC unit can facilitate the function of ciliated cells of the airway mucosa and decrease the accumulation of airway discharge [29].
When the inhalation gas is warmed, the esophageal temperature is measured to be 0.35℃ higher than the tympanic membrane [30]. It can be influenced more by the presence of the esophagus relatively close to the trachea. Therefore, in our study, one limitation is the fact that the measurement of core temperature in the esophagus may have affected the lower core temperature decrease in the HHC group than in the control group. This is because core temperature was measured in the esophagus without measuring the temperature at other locations such as the tympanic membrane. This measurement should be supplemented in a future study.
In conclusion, use of HHC may be considered as a method to attenuate intraoperative decrease in core temperatures during arthroscopic hip surgery under general anesthesia exceeding 2 hours in duration. However, decrease in the incidence of perioperative hypothermia and shivering in the PACU is unfortunately insignificant.
References
- 1.Sessler DI. Temperature regulation and monitoring. In: Miller RD, Eriksson LI, Fleisher LA, Wiener-Kronish JP, Cohen NH, Young WL, editors. Miller's Anesthesia. 8th ed. Philadelphia: Elsevier Health Sciences; 2015. pp. 1642–1645. [Google Scholar]
- 2.Kim YS, Lee JY, Yang SC, Song JH, Koh HS, Park WK. Comparative study of the influence of room-temperature and warmed fluid irrigation on body temperature in arthroscopic shoulder surgery. Arthroscopy. 2009;25:24–29. doi: 10.1016/j.arthro.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Parodi D, Tobar C, Valderrama J, Sauthier E, Besomi J, López J, et al. Hip arthroscopy and hypothermia. Arthroscopy. 2012;28:924–928. doi: 10.1016/j.arthro.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Sessler DI, Moayeri A. Skin-surface warming: heat flux and central temperature. Anesthesiology. 1990;73:218–224. [PubMed] [Google Scholar]
- 5.Giesbrecht GG, Ducharme MB, McGuire JP. Comparison of forced-air patient warming systems for perioperative use. Anesthesiology. 1994;80:671–679. doi: 10.1097/00000542-199403000-00026. [DOI] [PubMed] [Google Scholar]
- 6.McGovern PD, Albrecht M, Belani KG, Nachtsheim C, Partington PF, Carluke I, et al. Forced-air warming and ultra-clean ventilation do not mix: an investigation of theatre ventilation, patient warming and joint replacement infection in orthopaedics. J Bone Joint Surg Br. 2011;93:1537–1544. doi: 10.1302/0301-620X.93B11.27124. [DOI] [PubMed] [Google Scholar]
- 7.Albrecht M, Gauthier RL, Belani K, Litchy M, Leaper D. Forced-air warming blowers: an evaluation of filtration adequacy and airborne contamination emissions in the operating room. Am J Infect Control. 2011;39:321–328. doi: 10.1016/j.ajic.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Jo YY, Kim HS, Chang YJ, Yun SY, Kwak HJ. The effect of warmed inspired gases on body temperature during arthroscopic shoulder surgery under general anesthesia. Korean J Anesthesiol. 2013;65:14–18. doi: 10.4097/kjae.2013.65.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bissonnette B, Sessler DI. Passive or active inspired gas humidification increases thermal steady-state temperatures in anesthetized infants. Anesth Analg. 1989;69:783–787. [PubMed] [Google Scholar]
- 10.Hynson JM, Sessler DI. Intraoperative warming therapies: a comparison of three devices. J Clin Anesth. 1992;4:194–199. doi: 10.1016/0952-8180(92)90064-8. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg ME, Epstein R, Rosenblum F, Larijani GE, Marr A, Lessin J, et al. Do heated humidifiers and heat and moisture exchangers prevent temperature drop during lower abdominal surgery? J Clin Anesth. 1992;4:16–20. doi: 10.1016/0952-8180(92)90113-f. [DOI] [PubMed] [Google Scholar]
- 12.Kwak IS, Choi DY, Lee TH, Bae JY, Lim TW, Kim KM. The effect of heated breathing circuit on body temperature and humidity of anesthetic gas in major burns. Korean J Anesthesiol. 2013;64:6–11. doi: 10.4097/kjae.2013.64.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee HK, Jang YH, Choi KW, Lee JH. The effect of electrically heated humidifier on the body temperature and blood loss in spinal surgery under general anesthesia. Korean J Anesthesiol. 2011;61:112–116. doi: 10.4097/kjae.2011.61.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. New York: Springer; 2000. p. 174. [Google Scholar]
- 15.Leslie K, Sessler DI. Perioperative hypothermia in the high-risk surgical patient. Best Pract Res Clin Anaesthesiol. 2003;17:485–498. doi: 10.1016/s1521-6896(03)00049-1. [DOI] [PubMed] [Google Scholar]
- 16.Hart SR, Bordes B, Hart J, Corsino D, Harmon D. Unintended perioperative hypothermia. Ochsner J. 2011;11:259–270. [PMC free article] [PubMed] [Google Scholar]
- 17.Frank SM, Beattie C, Christopherson R, Norris EJ, Perler BA, Williams GM, et al. Unintentional hypothermia is associated with postoperative myocardial ischemia. The Perioperative Ischemia Randomized Anesthesia Trial Study Group. Anesthesiology. 1993;78:468–476. doi: 10.1097/00000542-199303000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Schmied H, Kurz A, Sessler DI, Kozek S, Reiter A. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet. 1996;347:289–292. doi: 10.1016/s0140-6736(96)90466-3. [DOI] [PubMed] [Google Scholar]
- 19.Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334:1209–1215. doi: 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- 20.Lenhardt R, Marker E, Goll V, Tschernich H, Kurz A, Sessler DI, et al. Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology. 1997;87:1318–1323. doi: 10.1097/00000542-199712000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Yoo HS, Park SW, Yi JW, Kwon MI, Rhee YG. The effect of forced-air warming during arthroscopic shoulder surgery with general anesthesia. Arthroscopy. 2009;25:510–514. doi: 10.1016/j.arthro.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 22.Degoute CS. Controlled hypotension: a guide to drug choice. Drugs. 2007;67:1053–1076. doi: 10.2165/00003495-200767070-00007. [DOI] [PubMed] [Google Scholar]
- 23.Parodi D, Valderrama J, Tobar C, Besomi J, López J, Lara J, et al. Effect of warmed irrigation solution on core body temperature during hip arthroscopy for femoroacetabular impingement. Arthroscopy. 2014;30:36–41. doi: 10.1016/j.arthro.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 24.Frank SM, Shir Y, Raja SN, Fleisher LA, Beattie C. Core hypothermia and skin-surface temperature gradients. Epidural versus general anesthesia and the effects of age. Anesthesiology. 1994;80:502–508. doi: 10.1097/00000542-199403000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Sessler DI, Olofsson CI, Rubinstein EH. The thermoregulatory threshold in humans during nitrous oxide-fentanyl anesthesia. Anesthesiology. 1988;69:357–364. doi: 10.1097/00000542-198809000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Kurz A, Kurz M, Poeschl G, Faryniak B, Redl G, Hackl W. Forced-air warming maintains intraoperative normothermia better than circulating-water mattresses. Anesth Analg. 1993;77:89–95. doi: 10.1213/00000539-199307000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Campbell G, Alderson P, Smith AF, Warttig S. Warming of intravenous and irrigation fluids for preventing inadvertent perioperative hypothermia. Cochrane Database Syst Rev. 2015;(4):CD009891. doi: 10.1002/14651858.CD009891.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duryea EL, Nelson DB, Wyckoff MH, Grant EN, Tao W, Sadana N, et al. The impact of ambient operating room temperature on neonatal and maternal hypothermia and associated morbidities: a randomized controlled trial. Am J Obstet Gynecol. 2016;214:505.e1–505.e7. doi: 10.1016/j.ajog.2016.01.190. [DOI] [PubMed] [Google Scholar]
- 29.Williams R, Rankin N, Smith T, Galler D, Seakins P. Relationship between the humidity and temperature of inspired gas and the function of the airway mucosa. Crit Care Med. 1996;24:1920–1929. doi: 10.1097/00003246-199611000-00025. [DOI] [PubMed] [Google Scholar]
- 30.Bissonnette B, Sessler DI, LaFlamme P. Intraoperative temperature monitoring sites in infants and children and the effect of inspired gas warming on esophageal temperature. Anesth Analg. 1989;69:192–196. [PubMed] [Google Scholar]


