Abstract
A molecular biology–based taxonomy has been proposed for pheochromocytoma and paraganglioma (PPGL). Data from the Cancer Genome Atlas revealed clinically relevant prognostic and predictive biomarkers and stratified PPGLs into three main clusters. Each subgroup has a distinct molecular–biochemical–imaging signature. Concurrently, new methods for biochemical analysis, functional imaging, and medical therapies have also become available. The research community now strives to match the cluster biomarkers with the best intervention. The concept of precision medicine has been long awaited and holds great promise for improved care. Here, we review the current and future PPGL classifications, with a focus on hereditary syndromes. We discuss the current strengths and shortcomings of precision medicine and suggest a condensed manual for diagnosis and treatment of both adult and pediatric patients with PPGL. Finally, we consider the future direction of this field, with a particular focus on how advanced molecular characterization of PPGL can improve a patient’s outcome, including cures and, ultimately, disease prevention.
The Cancer Genome Atlas proposed a molecular taxonomy of pheochromocytoma and paraganglioma that has potential to personalize genetic and biochemical screening, imaging, follow-up, and treatment.
Essential Points
There are at least 12 genetic syndromes with predisposition of PPGL
The Cancer Genome Atlas (TCGA) molecular taxonomy divides these patients into three main disease clusters: (1) pseudohypoxic, (2) Wnt-signaling, and (3) kinase-signaling
Each cluster has a unique molecular-clinical-biochemical-imaging phenotype, which can be used to personalize care; precision medicine and targeted therapies
Ongoing clinical trials investigate the hypothesized impact of PPGL biology in predicting treatment response
Improved understanding of molecular biology provides several theoretical opportunities for primary prevention
Pheochromocytomas (PCCs) and paragangliomas (PGLs), commonly denoted PPGLs, are known to physicians as the “great mimickers” because of their variable symptoms (1). PPGLs are also recognized to have the highest degree of heritability of any endocrine tumor type (2). An additional layer of complexity can be attributed to the extensive genetic heterogeneity found in between patients. There are at least 12 different genetic syndromes, 15 well-characterized PPGL driver genes, and an increasing number of potential disease-modifying genes (2–4). Together with reduced costs and increasing availability of DNA sequencing, an increasing proportion of patients with PPGL are now undergoing genetic screening and will soon ask, “What do my genetic results mean for my health?”
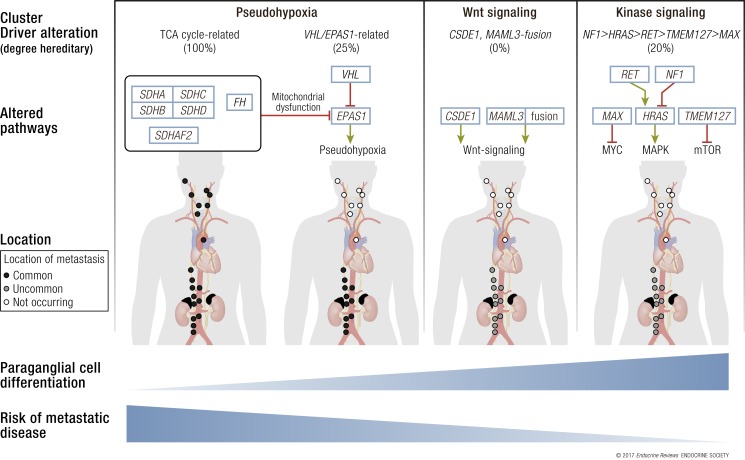
Meanwhile, new diagnostic techniques and therapies have been introduced for PPGL: biochemical workup with 3-methoxytyramine; 68Ga-DOTA somatostatin analogs used with positron emission tomography (PET)/computed tomography (CT) for localization and staging; temozolomide, sunitinib, high-potency 131I-metaiodobenzylguanidine (MIBG; Ultratrace); and 90Y- or 177Lu-coupled somatostatin analogs for treatment of nonresectable disease. At first glance, this combined clinical and biological heterogeneity increases the complexity of PPGL management, especially in patients with rare genetic syndromes. Therefore, we have placed our emphasis on the cluster approach, which divides PPGL into groups with alike pathogenesis and biology. These clusters can act as a guide for how to think about and approach PPGL personalized care and treatment. The pan-molecular characterization of the Cancer Genome Atlas (TCGA) has provided us with the most sophisticated molecular taxonomy to date (3) (Fig. 1, Table 1):
Figure 1.
Different PPGL molecular subgroups with corresponding driver mutations and a proportion of hereditary disease in the respective cluster. TCA cycle–related mutations include SDHA, SDHB, SDHC, SDHD, SDHAF2, and FH genes. MYC, c-MYC induced pathways; MAPK, mitogen-activated protein kinase cascade; mTOR, the mammalian target of rapamycin pathway. Anatomic figure was adopted and modified from Lips et al. (5).
Table 1.
PPGL Clusters and Driver Genes
| Molecular Clusters | Proportion Hereditary | Official Symbol | Official Full Name | Type | Germline/Somatic |
|---|---|---|---|---|---|
| Pseudohypoxia TCA cycle–related (10%–15% of PPGLs) | ∼100% | SDHA | Succinate dehydrogenase complex flavoprotein subunit A | TS | Germline |
| SDHB | Succinate dehydrogenase complex iron sulfur subunit B | TS | Germline | ||
| SDHC | Succinate dehydrogenase complex subunit C | TS | Germline (genetic/epigenomic) | ||
| SDHD | Succinate dehydrogenase complex subunit D | TS | Germline | ||
| SDHAF2 | Succinate dehydrogenase complex assembly factor 2 | TS | Germline | ||
| FH | Fumarate hydratase | TS | Germline | ||
| Pseudohypoxia, VHL/EPAS1-related (15%–20% of PPGLs) | 25% | VHL | Von Hippel–Lindau tumor suppressor | TS | Germline/somatic |
| EPAS1 | Endothelial PAS domain protein 1 | OG | Postzygotic/somatic | ||
| Wnt signaling (5%–10% of PPGLs)a | 0% | CSDE1 | Cold shock domain containing E1 | TS | Somatic |
| MAML3 | Mastermind like transcriptional coactivator 3 | OG, fusion | Somatic | ||
| Kinase signaling (50%–60% of PPGLs) | 20% | RET | Ret proto-oncogene | OG | Germline/somatic |
| NF1 | Neurofibromin 1 | TS | Germline/somatic | ||
| MAX | MYC-associated factor X | TS | Germline/somatic | ||
| TMEM127 | Transmembrane protein 127 | TS | Germline | ||
| HRAS | HRas proto-oncogene, GTPase | OG | Somatic |
Abbreviations: OG, oncogene; TS, tumor suppressor.
Reported only in (3).
-
1.
Pseudohypoxia group can be divided into at least two subgroups: tricarboxylic acid (TCA) cycle–related, containing germline mutations in succinate dehydrogenase subunits SDHA, SDHB, SDHC, and SDHD as well as SDHAF2 (SDHx), assembly factor of the succinate dehydrogenase complex, and FH, a second enzyme in the TCA cycle; and VHL/EPAS1-related, with somatic and germline mutations.
-
2.
Wnt signaling group includes newly recognized somatic mutations in CSDE1 as well as somatic gene fusions affecting MAML3.
-
3.
Kinase signaling group consists of germline or somatic mutations in RET, NF1, TMEM127, MAX, and HRAS.
After summarizing the recent updates in PPGL, we provide a synthesis for molecular biology, clinical, biochemical, and imaging classifications in 2017. Relevant examples of how these biomarkers can help personalize the care of patients with PPGL are provided. We are attempting to replace the vertical thinking approach to these diagnoses with a horizontal thinking approach, which looks at various analogies and parallels among all hereditary types of PPGLs. Such knowledge is then translated into personalized care and treatment. Finally, we outline future directions of the field, including how a precision medicine mindset could provide prevention of PPGL.
Background
PPGL epidemiology and heritability
The overall yearly incidence of PCC is estimated to be between 2 and 8 cases per million (6, 7). About 10% to 20% occur in pediatric patients (8, 9), with metastatic disease seen in 5%–20% of PCCs and in 15% to 35% of sympathetic PGLs (10–12). According to these numbers, we have approximated the yearly incidence of PPGLs in the United States to between 1000 and 2000 cases (13), including 100 to 200 pediatric patients and 100 to 200 with metastatic disease (14). However, these studies are outdated, and the numbers presented here should be interpreted with caution.
Recently, the proportion of patients with hereditary PPGL was estimated to be as high as 40%, reflecting a steady increase in the number of susceptibility loci. About 12% to 16% of PPGLs are expected to have SDHx or FH mutations, including mainly PGLs (22% to 70%) (15–21). The proportion of SDHx mutations among metastatic tumors is the highest among all hereditary PPGLs, being estimated at 43% to 71% in adults and 70% to 82% in pediatric patients (11, 22, 23). Between 1% and 13% of PPGLs have germline VHL mutations (18, 19, 24–26), whereas the cumulative frequency of RET, NF1, TMEM127, and MAX is reported to between 1% and 11% (18, 19, 24, 26, 27). Thus, in the United States, about 400 to 800 PPGLs could occur yearly in a heritable context (12, 16, 28–30). In total, the TCGA project identified a driver mutation or gene fusion in 73% of PPGLs: 27% as part of a genetic predisposition syndrome, harboring a germline mutation, and 46% as a somatic mutation in patients with sporadic disease (3).
Outcome
Catecholamine excess, local growth, and metastatic disease all contribute to increased morbidity and mortality in patients with PPGL (31–38). Those with sympathetic PPGLs have an almost 10 times higher incidence of cardiovascular events before their diagnosis (38). However, mortality is caused mainly by metastatic disease, which is associated with a 5-year survival rate of 40% to 95% in adults (14, 22, 38, 39) and 98% [95% confidence interval (CI) 84% to 100%] in children (12). For SDHB carriers in a metastatic setting, the 5- and 10-year survival rates were 36% to 92% and 76%, respectively (22, 28, 35, 40). For pediatric SDHB carriers, the 5- and 10-year survival rates were similarly estimated at 96% (12). Although SDHD carriers often suffer morbidity from growth and intervention of head and neck (HN) PGLs (41), mortality has not been shown to be substantially increased (37). In a study of 275 SDHD carriers, only 2 out of 18 deaths were related to PPGL, not statistically different from the control population (37). Nevertheless, the majority of glomus jugulare PGLs occur in SDHD carriers, and these tumors can be particularly problematic, causing morbidity due to a mass effect after enlarging only a few millimeters. From our experience, those that do not respond to radiotherapy or chemotherapy may cause severe morbidity or even death (41). Carriers of VHL mutations have a reduced life expectancy of 60 to 65 years. However, PPGLs were classified as the cause of death in only 2% of patients with von Hippel–Lindau (VHL) syndrome (1/67) (42). This rate is similar to that of patients with multiple endocrine neoplasia type 2 (MEN2), where PPGLs were not associated with decreased survival (43).
A three-cluster molecular taxonomy of PPGL
Researchers have long attempted to group PPGLs based on biology, and the results are uniform, with a few exceptions. We considered the results presented by TCGA as the most reliable analysis yet. But in a few instances where there are minor discrepancies with previous data, we tried to put TCGA into the context of the established literature and evidence.
Pseudohypoxic PPGL
This group of PPGL has a pathological hypoxic response due to stabilization of hypoxia-inducible factors (HIFs) under normal oxygen pressure. Two principally different mechanisms subdivide pseudohypoxic PPGLs into TCA cycle–related oncometabolite accumulation and VHL/EPAS1 related direct disturbance in HIF turnover.
Pseudohypoxic PPGL, TCA cycle–related.
This type involves genes in the TCA cycle; a majority of patients have familial PGL associated with mutations in SDHA (44), SDHB (45), SDHC (46), SDHD (47), and SDHAF2 (48), respectively. SDHA-D encodes subunits of the succinate dehydrogenase complex that catalyzes oxidation of succinate to fumarate and participates in the respiratory electron transfer chain (complex II). A small minority of pseudohypoxic PPGLs have the hereditary leiomyomatosis and renal cell cancer syndrome caused by pathogenic mutations in FH, encoding fumarate hydratase (49). This enzyme catalyzes the subsequent reaction in the TCA cycle, conversion of fumarate to malate. Constitutional mutations in MDH2, encoding malate dehydrogenase, were recently reported in a single family with PGLs (50). Malate dehydrogenase catalyzes the conversion of malate to oxaloacetate in the TCA cycle. Truncating mutations in genes encoding these three enzymes cause an oncometabolite accumulation of succinate (SDHx), fumarate (FH), and malate (MDH2). These metabolites have oncogenic effects through inhibition of enzymes involved in cell signaling and chromatin maintenance (20, 49–51).
Pseudohypoxic PPGL, VHL and EPAS1-related.
Ninety-five to 100% of patients who have VHL syndrome show pathogenic mutations in VHL (52, 53). The key pathogenic mechanism of this genetic syndrome is stabilization of HIF proteins, which results in a pseudohypoxic state (reviewed in ref 54). Activated HIF alters transcription of target genes, which results in increased angiogenesis as well as cellular proliferation and reduced apoptosis.
Patients with germline or mosaic gain-of-function mutations in EPAS1 have PPGL–somatostatinoma–polycythemia syndrome (Pacak–Zhuang syndrome) (55, 56). Similar to VHL-mutated PPGLs, those with EPAS1 mutational backgrounds have increased transcription of HIF-2α target genes (55). However, it was recently proposed that EPAS1-related PPGLs have a unique molecular signature (57). There were different activations of genes associated with HIF transcription, oxidative phosphorylation, and angiogenesis compared with VHL- and SDHx-related PPGLs (57). Therefore, we propose that VHL and EPAS1 may be subdivided as two special subgroups in the future.
Constitutional mutations in EGLN1 (57) and EGLN2 (59) occur in a small proportion of patients and are also thought to predispose patients to PPGLs. Rarely, patients with EGLN1 and EGLN2 mutations may present with polycythemia, but they do not display other characteristics of VHL syndrome. We believe that the role of these genes in the development of PPGL must be further clarified.
Wnt signaling PPGL
All patients who presented with Wnt signaling PPGL had sporadic disease, with the mutation occurring exclusively in tumor cells. It is associated with mutually exclusive somatic mutations in CSDE1 or somatic gene fusions UBTF-MAML3 that cause activation of the Wnt and Hedgehog signaling pathways (3). It was proposed that this category results in more aggressive PGGLs, because some of these patients presented with recurrence and metastasis (3).
Kinase signaling PPGL
The most common hereditary syndrome in this subgroup is MEN2, which occurs as a result of gain-of-function mutations in RET (60, 61). A minority of cases have neurofibromatosis type 1 (NF1), related to pathogenic mutations in NF1 (62), as well as familial PPGL related to TMEM127 (63) or MAX (64), These cases are linked to activation of kinase signaling pathways; RAS-RAF-MEK (RET) (65), NF1 (66), PI3K-AKT-mTOR (RET) (67), TMEM127 (63, 68), and MYC-MAX (MAX) (69). HRAS is now a validated driver event in PPGL pathogenesis, but it occurs exclusively as somatic gain-of-function mutations in sporadic cases (3, 70). A small minority of PPGLs show potential driver mutations in FGFR1 (somatic), KIF1B (germline + somatic), and MET (somatic) (3, 19, 71–73). We believe that the role of these genes in development of PPGL must be further clarified.
The TCGA effort suggested that a minority of PPGLs share a signature of cortical admixture (3). Currently, it is under debate whether this signature represents a distinct biological entity or is an artifact from impure tumor samples. Furthermore, Flynn et al. identified a MAX-like cluster, in two separate cohorts, that are not reported in the TCGA data (74). Similar to the MAX-like cluster, the Wnt signaling subgroup also has intermediate PNMT expression. The overlap between these two clusters remains to be clarified (R. Tothill, personal communication). In the TCGA article, two MAX-mutated tumors were assigned to the cortical admixture subgroup. This assignment contradicts previous findings, and we believe that it is a likely result from the contamination of nontumoral cells that became evident through histopathological and genomic analyses (74).
PPGL biology: a bird’s-eye view
Most PPGLs exhibit a low rate of point mutations, with a mean of 0.67 mutations per megabase (3). The mutation signature is predominantly age related, without traces of external mutagens (3, 71, 74). As recently identified in the TCGA data, the burden of somatic point mutations observed in PPGLs is between neuroblastoma (median 0.3 somatic mutations per Mb) and adrenocortical carcinomas (median 0.9 somatic mutations per Mb) (75, 76). However, cancers that are exposed to external mutagens, such as melanoma and lung adenocarcinoma, have a much higher mutation rate (about 20-fold) (76). A few outliers among PPGLs had a hypermutator phenotype related to defects in DNA repair (71). TCGA revealed that somatic mutation burden is associated with aggressive PPGL (3). Fifteen principal driver genes may cause PPGL through germline, postzygotic, or somatic (restricted to tumor cells) mutations. Somatic mutations in another five genes—ATRX (77), KMT2D (78), SETD2 (72), TERT (79), and TP53 (71)—have a proposed synergistic impact on PPGL tumorigenesis, some of which coexist with aggressive disease (2, 3, 80). The TCGA data set was also able to confirm previously described somatic copy number alterations, with distinct patterns depending on gene mutations: 1p deletion (pseudohypoxia TCA cycle–related and kinase signaling), 3p and 11p deletions (pseudohypoxia VHL-related), and 3q deletions (kinase signaling) (3, 71, 81). A novel finding in TCGA was PPGL with genome doubling that occurred preferentially in pseudohypoxic VHL/EPAS1-related PPGL (3).
The degree of DNA methylation among PPGLs is of great interest because it ranges from global hypomethylation to global hypermethylation and is strongly associated with disease clusters (3, 49, 82, 83). Pseudohypoxic TCA cycle–related PPGLs exhibit DNA hypermethylation, and pseudohypoxic VHL- and EPAS1-related PPGLs show intermediate DNA methylation (3, 49, 82–84). This finding is in contrast to Wnt and kinase signaling clusters that show DNA hypomethylation (3, 49, 83, 84). With varying results, these data call into question the role of hypermethylation in PPGL local aggressiveness.
”It was the distinct gene expression profiles that first led researchers to the discovery that PPGLs separate into groups with varying biology.“
However, it was the distinct gene expression profiles that first led researchers to the discovery that PPGLs separate into groups with varying biology (85, 86). The transcriptome of the pseudohypoxic cluster reflects increased activity of HIF transcription factors, with differential expression of target genes involved in glycolytic metabolism and angiogenesis (3, 26, 85). The somatostatin receptor (SSTR) 2A is also expressed heterogeneously in PPGLs, especially in those of the TCA cycle–related pseudohypoxic subtype, which have ubiquitously high expression (87–89). Similar to gastroenteropancreatic and bronchial neuroendocrine tumors, somatostatin receptor imaging first proved itself on scintigraphy that was gradually replaced by the superior 68Ga–tetraazacyclododecane tetraacetic acid–octreotate (DOTATATE) PET/CT scan (90–95). Furthermore, pseudohypoxic TCA cycle–related PPGLs show a low cell differentiation, often with a decrease or absence of key enzyme in catecholamine metabolism (96). This finding translates into purely noradrenergic or dopaminergic phenotypes together with a lower overall content of catecholamines (97–99). Unlike the other clusters, a portion of pseudohypoxic TCA cycle–related PPGLs belongs to the parasympathetic cell linage (100). Pseudohypoxic VHL-related PPGLs also have an intermediate catecholamine synthesis phenotype and are strictly noradrenergic (96, 99). Wnt and kinase signaling PPGLs both show increased neuronal differentiation and represent a more ultimate cell differentiation (3, 26). Their most prominent characteristic is expression of PNMT, which facilitates synthesis of epinephrine from norepinephrine (86). A subgroup of kinase-signaling PPGLs are linked to MAX mutations and exhibit a lower concentration of epinephrine because of the depressed expression of the PNMT enzyme (69). Similarly, PPGLs of the Wnt signaling cluster identified in the TCGA project were also characterized by depressed PNMT expression (3). Whether these differences in methylomics and transcriptomics between clusters reflect distinct disease-causing mechanisms or mirrors separate cells of origin is not understood.
Altered metabolism is a key feature of pseudohypoxic PPGLs (101). In these tumors, inhibition of the TCA cycle results in rewired glucose and amino acid metabolism and has yet to be exploited therapeutically (102). However, the pseudohypoxic phenotype (seen in both TCA cycle and VHL/EPAS1 subtypes) has been linked to the Warburg effect, with downregulation of respiration and the preferential use of glycolysis, despite normal supply in oxygen (aerobic glycolysis), to generate energy (103). This results in increased glucose consumption that can be exploited for diagnostic purposes (104, 105).
In the last 15 years, it has become evident that to fully understand PPGL, physicians and scientists need to analyze them using the cluster hypothesis (2, 4). These molecular clusters provide an essential framework for improved understanding of the different clinical characteristics of the 12 PPGL syndromes. The aim of the research community has been clear: to move beyond the restricted use of PPGL biology in genetic counseling to improve diagnosis, treatment, follow-up, and prevention.
Clinical introduction
PPGLs are clinically heterogeneous in four major attributes. The first, anatomic location, classifies cases as PCCs, originating from the adrenal medulla, or PGLs originating from extra-adrenal chromaffin cells or paraganglia (106). The symptoms caused by local growth vary widely; the most prominent are pain and nerve paralysis from HN PGLs. The second attribute comes from catecholamine synthesis and release, which are inherently linked to cell differentiation (86, 96, 107). Catecholamine release causes clinical hallmarks such as hypertension and tachycardia, as well as more diffuse symptoms that are indistinguishable from those of common stress disorders, such as headache, palpitations, and sweating (108). Location and catecholamine spectrum are used to classify PPGLs accordingly to developmental origin, into parasympathetic and sympathetic tumors (106). Sympathetic PPGLs have ubiquitous distribution from the skull base to the pelvic floor, whereas parasympathetic PGLs commonly occur in the HN region, and rarely in the thorax. There is no well-accepted grading system, and metastatic tumors are recognized by the presence of PPGL cells in nonchromaffin organs. The fourth and most constantly updated attribute is the genetic context, which shows a steadily increasing influence on both diagnostic and therapeutic algorithms (2). In the context of genetic susceptibility, PPGLs are diagnosed at an earlier age and have a higher degree of multifocal disease. The different genetic syndromes display various profiles of PPGL localization and paraganglial cell differentiation (Fig. 2, Table 2). PPGLs are associated with symptoms that occur as a direct or indirect effect of an increased synthesis and release of catecholamines (108). However, as previously discussed, among hereditary PPGLs from the pseudohypoxic cluster, the catecholamine-related symptoms are less pronounced (97, 109).
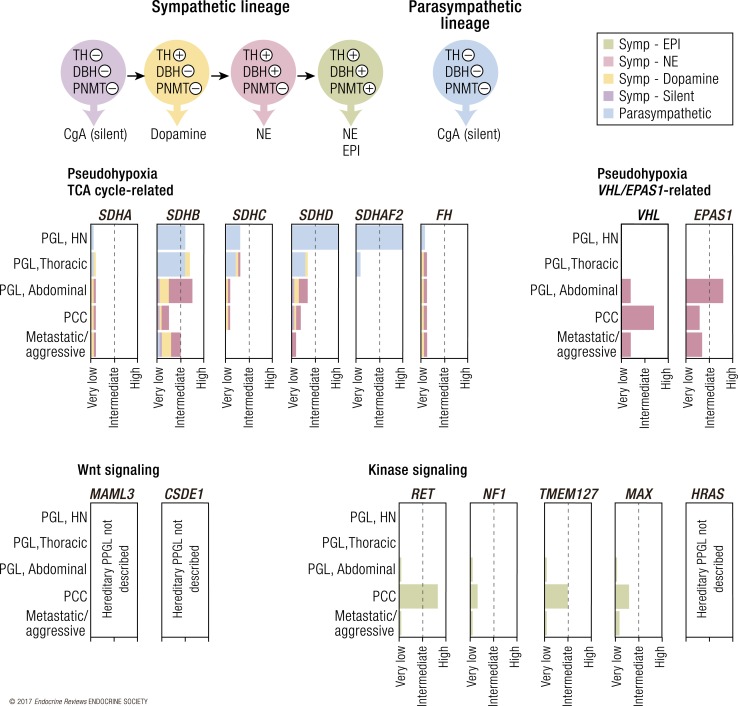
Figure 2.
Approximate frequency of PPGL in different heritable contexts, according to localization and hormone profile. Upper left shows different differentiation stages indicated by color of sympathetic PPGL, including expressed enzymes and hormone production. Bar diagrams indicate frequency of parasympathetic and sympathetic PGLs at different anatomic locations as well as PCC and metastatic disease in different mutational contexts. +, enzyme expression, −, no enzyme expression; CgA, chromogranin A; DBH, dopamine β hydroxylase; EPI, epinephrine; NE, norepinephrine; PNMT, phenylethanolamine N-methyltransferase; TH, tyrosine hydroxylase.
Table 2.
PPGL Driver Genes and Genetic Syndromes
| Gene | Proposed Syndrome Title | Current Syndrome Title | Transmission | Penetrance HN PGL | Penetrance Sympathetic PGL | Penetrance PCC | Mutation Variable Penetrance | Diagnosis | Other Manifestations |
|---|---|---|---|---|---|---|---|---|---|
| SDHA | Familial PPGL SDHA-related | Familial PGL type 5 | AD | Very low | Very low | Very low | No | Genetic | GIST, pituitary tumors, pulmonary chondroma, and RCC. |
| SDHB | Familial PPGL SDHB-related | Familial PGL type 4 | AD | Intermediate | Intermediate | Low | No | Genetic | |
| SDHCa | Familial PPGL SDHC-related | Familial PGL type 3 | AD | Low | Low | Low | No | Genetic | |
| SDHD | Familial PPGL SDHD-related | Familial PGL type 1 | AD, paternal | High (multifocal) | Low | Low | No | Genetic | |
| SDHAF2 | Familial PPGL SDHAF2-related | Familial PGL type 2 | AD | High (multifocal) | Very low | Very low | No | Genetic | |
| FH | Hereditary leiomyomatosis and renal cell cancerb | AD | Unknown | Unknown | Unknown | No | Clinical or genetic | Leiomyomatosis, RCC | |
| VHL | von Hippel–Lindau syndrome | AD | Very low | Low | High (bilateral)c | Yes | Clinical or genetic | Hemangioblastoma, RCC, epididymal cystadenoma, pancreatic neuroendocrine tumors, retinal abnormalities | |
| EPAS1 | PPGL–somatostatinoma–polycythemia syndrome (Pacak-Zhuang syndrome) | Unknown (postzygotic) | Very low | High (multifocal) | High | Yes | Clinical or genetic | Polycythemia, somatostatinoma, retinal abnormalities, organ cysts | |
| CSDE1 | No predisposition to PPGL (only somatic mutations reported) | ||||||||
| MAML3 | No predisposition to PPGL (only somatic mutations reported) | ||||||||
| RET | Multiple endocrine neoplasia type 2 | AD | Very low | Very low | High (bilateral)d | Yes | Clinical or genetic | MEN2A: Medullary thyroid carcinoma, parathyroid adenoma. MEN2B; medullary thyroid carcinoma, mucosal neuromas, dysmorphic features. | |
| NF1 | Neurofibromatosis type 1 | AD | Very low | Very low | Low (bilateral) | No | Clinical or genetic | Café-au-lait spots, Lisch nodules in the eye, neurofibromas, intellectual disability, dysmorphic features, skeletal abnormalities | |
| MAX | Familial PPGL, MAX-related | Familial PCC | AD | Unknown | Unknown | Unknown | No | Genetic | Renal oncocytoma |
| TMEM127 | Familial PPGL, TMEM127-related | Familial PCC | AD | Very low | Low | Intermediate | No | Genetic | |
| HRASe | No predisposition to PPGL (only somatic mutations reported) | ||||||||
Patients with familial PPGL related to SDHA-SDHD may present with Carney Stratakis syndrome (PGL and GIST) or Carney triad (PGL, GIST and pulmonary chondroma).
Abbreviations: AD, autosomal dominant; GIST, gastrointestinal stromal tumor; MEN2, multiple endocrine neoplasia type 2; RCC, renal cell carcinoma.
Occurs both as genomic and epigenomic mutations.
PPGL not included in clinical criteria.
High in VHL type 2, low in VHL type 1.
Genotype dependent, 50% in cysteine mutations, low in noncysteine sites.
Constitutional mutations in HRAS codons 12, and rarely 13, cause Costello syndrome. To our knowledge no cases of PPGL and germline HRAS mutation have been described in the literature. It should be noted that somatic HRAS mutations in PPGL are restricted to codon 61 and rarely codon 13.
A 2017 Classification of PPGL
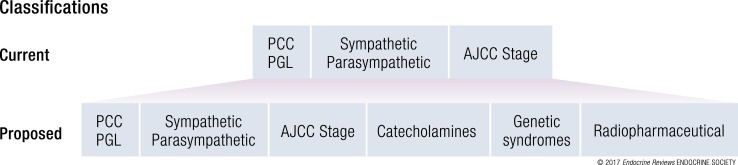
New systems for PPGL classification and staging were recently presented by the World Health Organization (WHO) and the American Joint Committee on Cancer (AJCC). We present these current classifications and propose additional disease hallmarks that greatly contribute to a precision medicine decision-making process in the diagnosis and treatment of patients with PPGL (Table 3, Fig. 3).
Table 3.
Current, Proposed, and Future PPGL Classification Systems
| Classification System | Criteria | Information | |
|---|---|---|---|
| WHO, current (2017) | Location, hormone production | PCC, adrenal | Diagnostic, prognostic, predictive |
| PGL, sympathetic | |||
| PGL, HN | |||
| Metastatic, PPGL in nonparaganglial tissue | |||
| AJCC staging, current (2017) | Primary tumor size, primary tumor location, regional lymph node, or distant metastases | T1, PCC <5 cm; T2, PCC ≥5 cm or sympathetic PGL; T3, invasion into surrounding tissues | Prognostic |
| N0/N1, absence or presence of regional lymph node metastasis | |||
| M0/M1, absence or presence of distant metastasis; further subgrouped based on location | |||
| Biochemistry, proposed | Hormone production | Sympathetic | Diagnostic, prognostic |
| Silent | |||
| 3MT, pos/neg | |||
| NMN, pos/neg | |||
| MN, pos/neg | |||
| Parasympathetic | |||
| 3MT, pos/neg | |||
| NMN, pos/neg | |||
| Silent | |||
| 3MT, pos/neg | |||
| NMN, pos/neg | |||
| Genetic syndromes, proposed | Genetics, germline DNA | SDHx, FH, VHL, EPAS1, RET, NF1, TMEM127, MAX | Diagnostic, prognostic, predictive |
| Radiopharmaceutical, proposed | Quantitative imaging parameters | 68Ga-DOTATATE PET/CT, 123I-MIBG scintigraphy | Diagnostic, predictive |
| Pathology, future potential, | Transcriptome, methylome, miRNAome, driver mutation | Pseudohypoxia, TCA cycle–related | Prognostic, predictive |
| Pseudohypoxia, VHL/EPAS1-related | |||
| Wnt signaling | |||
| Kinase signaling | |||
| Pathology, future potential | Germline + somatic mutations in driver genes | SDHx, FH, MDH2, VHL, EPAS1, EGLN1-2, CSDE1, MAML3, RET, NF1, TMEM127, MAX, HRAS, KIF1B, MET, FGFR1 | Prognostic, predictive |
| Somatic mutations in modifier genes | ATRX, SETD2, TP53, TERT, MKT2D | ||
| Histopathology and molecular pathology | Morphology, histology, cell proliferation, MGMT methylation, somatic mutation load |
For each factor, we described the criteria and how the information may be used to determine the clinical scenario. Diagnostic, detects or confirms presence of disease; prognostic, indicates the likely outcome from disease; predictive, indicates the likely outcome from interventions. TCA cycle–related include SDHx (SDHA-SDHD, SDHAF2), FH and MDH2.
Abbreviations: 3MT, 3-methoxytyramine; MN, metanephrine; NMN, normetanephrine; pos/neg, above reference limit.
Figure 3.
Current synthesis of the important clinical facts that contribute to the decision-making process in diagnosis, follow-up, and treatment of PPGL.
Current
The 2017 WHO of adrenal tumors classification recognize anatomic criteria for PPGL classification (106, 110):
PCCs originate from the adrenal medulla.
PGLs originate from extra-adrenal paraganglia. PGLs are further divided accordingly to clinical and biological behavior into:
HN PGLs that originate from parasympathetic paraganglia and therefore lack catecholamine secretion (111). All parasympathetic PGLs are denoted HN to reflect the localization of a majority of cases. Still, HN PGL can arise from the parasympathetic ganglia in the anterior and middle mediastinum along the course of the vagus nerve.
Sympathetic PGLs that originate from sympathetic paraganglia, have ubiquitous distribution from the skull base to the pelvic floor, and are biochemically positive (111). Approximately 85% arise below the diaphragm.
Metastatic disease is classified by the presence of PPGLs in nonchromaffin organs, a distinction that can sometimes be difficult to make especially for tumors along the aorta. The term benign PPGL was abolished to highlight the fact that all PPGLs should be considered to have metastatic potential.
In 2017, AJCC published the first staging system for PPGL that takes into account tumor location, size of primary tumor, and hormone secretion (112, 113).
TNM system, T, primary tumor size and location; N, lymph node metastases; and M, presence and location of distant metastases. Readers should be cautioned because this staging system does not define metastasis, and it excludes HN PGL.
Stage grouping into stage I to IV.
Proposed
In addition to the current WHO and AJCC classification systems, we suggest the following clinical attributes to contribute to a precision medicine decision-making process in the diagnosis and treatment of patients with PPGL (Table 3, Fig. 3).
Biochemistry
Elevated metanephrine, normetanephrine, and 3-methoxytyramine contribute to the diagnosis of PPGL. In addition, the spectrum of catecholamines are inherently linked to paraganglial cell differentiation and metastatic potential and may be used as prognostic markers (86, 96, 107, 114).
Genetic syndromes
Guidelines from the US Endocrine Society and the European Society of Endocrinology recommend that “all patients should be engaged in shared decision making for genetic testing” and “considered for genetic testing,” respectively (1, 115). Because of the high degree of heritability and the ever-increasing influence on both diagnostic and therapeutic algorithms, we propose that now is the time for a universal recommendation of genetic in patients with PPGL (2, 3). Diagnosis of genetic syndromes can be made based on clinical criteria (that may be confirmed by genetic testing) or by genetic data alone:
Clinical or genetic criteria: VHL syndrome, MEN2, NF1, FH
Genetic criteria: SDHx, EPAS1, TMEM127, MAX
In a strive for clarity, we recognize the possibility of using a gene-based classification to better recognize the genetic syndromes that were characterized in this millennium: SDHx, TMEM127, and MAX. For genetic diagnostic procedures, we refer to this recent consensus statement (116).
Radiopharmaceutical
Functional imaging may aid in the diagnosis and staging of PPGL. A wide range of functional imaging approaches may be used for localization and staging. Somatostatin analog and MIBG uptake are also predictive factors for targeted internal radiation therapy that can be relevant in the case of progressive metastatic or unresectable disease.
Pathology
Generally, there is no established grading system in PPGL. We argue that the best future approach may be a combined clinical–biochemical–pathological approach.
Follow-up
Follow-up is needed to detect new events, (metachronous or recurrent PPGLs). All patients should be offered surveillance after PPGL resection. Follow-up for new events is recommended in carriers of germline mutations in PPGL susceptibility genes.
Targeted Diagnostics and Therapy in Hereditary PPGL
Precision medicine strives to improve patient care by using the unique clinical and molecular genetic profile of each patient and his or her disease (117). Here, we provide a current update on precision medicine in hereditary PPGL, structured according to interpretation of biochemical results; genetic syndromes, focusing on characteristics and classification; selection of the optimal imaging technique; histopathological classification; and choice of the ideal treatment option.
We must caution clinicians and patients of the current limitations of this concept for PPGL treatment selection. This initiative is still in its early phases and is supported only by limited evidence.
Interpreting biochemical results: novel applications for old biomarkers in hereditary PPGL
Contemporary data conclude that the spectrum of catecholamine secretion reflects paraganglial cell differentiation and can be used as a prognostic biomarker. There are at least four principal secretory profiles: adrenergic, noradrenergic, dopaminergic, and silent (Fig. 3, Tables 3 and 4). Chromogranin A is also a useful biomarker, especially in silent PPGLs (118, 119). Therefore, optimal screening and follow-up should include a quartet of biomarkers consisting of metanephrine, normetanephrine, 3-methoxytyramine, and chromogranin A, as discussed below.
Table 4.
Biochemical and Imaging Approach to PPGL
| Cluster | Gene | Biochemistry | Functional Imaging | Near Future Developments |
|---|---|---|---|---|
| Pseudohypoxic, TCA cycle–related |
SDHA
SDHB SDHC SDHD SDHAF2 |
Norepinephrine/normetanephrine, 3-methoxytyramine chromogranin A | 68Ga-DOTATATE PET/CT | Metabolite profiling, MR spectroscopy |
| FH | ||||
| Pseudohypoxic VHL/EPAS1-related | VHL-related | Norepinephrine/normetanephrine | VHL-related should have similar imaging as EPAS 1 related = 18F-DOPA PET/CT | Hypoxia imaging |
| EPAS1-related | 18F-DOPA PET/CT (EPAS1) | |||
| Wnt signaling | CSDE1 | Norepinephrine/normetanephrine, epinephrine/metanephrine | Optimal tracer unknown | |
| MAML3 | ||||
| Kinase signaling |
RET
NF1 MAX |
Norepinephrine/normetanephrine, epinephrine/metanephrine | 18F-DOPA PET/CT | |
| TMEM127 | ||||
| HRAS |
Abbreviations: DOPA, dihydroxyphenylalanine; FDG, fluorodeoxyglucose.
Quantifying excess secretion of metanephrine, normetanephrine, and 3-methoxytyramine in either plasma or urine, is the diagnostic gold standard and should always be the starting point in screening or follow-up of patients with PPGL (120–123). Metanephrine or normetanephrine values three to four times above the upper reference limit are typically pathognomonic for PPGL, and a longitudinal comparison can be used to reflect relative tumor volumes. Tumor biochemistry mirrors PPGL biology and could be important for PPGL classification (114).
Through the pioneering work of G. Eisenhofer and others, we currently view the PPGL catecholaminergic spectrum as an indirect measurement of paraganglial cell differentiation regulated by HIF and MYC/MAX-related signaling (96, 98, 124–126). Under normal conditions in the adrenal medulla, epinephrine is converted from norepinephrine in the final stage of catecholamine biosynthesis (127, 128). However, pseudohypoxic PPGLs always lack the necessary enzyme PNMT. Thus, these tumors are strictly noradrenergic and can be diagnosed preferentially by normetanephrine levels (86, 129). Furthermore, pseudohypoxic TCA cycle–related PPGLs have reduced synthesis or secretion of normetanephrine. Therefore, before a patient presents with signs and symptoms of catecholamine excess, most TCA cycle–related PPGLs have generally reached a larger size. Tumors >3 cm in diameter (smaller size applies to TCA cycle–related PPGLs) possess an independent risk for metastasis (130). Thus, silent growth may cause delayed diagnosis, which could be one reason why pseudohypoxic TCA cycle–related PPGLs more often present with metastasis (22, 130). However, the low catecholamine content results in lower plasma concentrations, with mild or no peripheral adrenoceptor stimulation. This leaves the patient relatively “unharmed” from hormone-related morbidities, especially concerning the cardiovascular system, as described earlier (97).
The penultimate step in the catecholamine synthesis is the conversion of dopamine to norepinephrine (131). Up to 70% of SDHx carriers show reduced activity of the necessary catalytic enzyme dopamine-β-hydroxylase. They present with elevated dopamine (or its metabolite 3-methoxytyramine), most often in conjunction with elevated normetanephrine (99, 132, 133). Although dopamine results in negligible clinical symptoms, it represents another step down the paraganglial differentiation ladder and is associated with a negative outcome (114). Up to 70% of PGLs with elevated metoxytyramine have metastatic disease (124, 134).
Finally, and in contrast to most PPGLs, a small portion of those with TCA cycle–related sympathetic PPGLs also lack the starting and rate-limiting enzyme in catecholamine synthesis (tyrosine hydroxylase) and are therefore biochemically silent (109, 124). Unless tumor growth causes a mass effect, early detection of these cases remains a challenge. A potential solution could come from chromogranin A, which was shown to have a 22% increase in diagnostic yield compared with normetanephrine alone (118). Ultimately, the relative benefit of chromogranin A and metanephrines as well as anatomic imaging must be clarified in future studies.
In conclusion, pseudohypoxic TCA cycle–related PPGLs cause fewer catecholamine-related symptoms than other subgroups of PPGL, grow larger, reach more advanced stages, and metastasize more frequently, often before patients and their physicians are aware and able to perform the necessary investigations (125).
Genetic syndromes, focusing on characteristics and classification
Pseudohypoxia, TCA cycle–related
Mutations in SDHx cause autosomal dominantly inherited cancer syndromes with indistinct clinical presentations in between patients: HN PGL, sympathetic PGL, PCC, gastrointestinal stromal tumor (GIST), pituitary adenomas, and renal cell carcinoma, all with variable penetrance across the different syndromes (Table 2). The current Online Mendelian Inheritance in Man (OMIM) classification recognizes familial PGL types 1 to 5, which were consecutively assigned to SDHA-D and SDHAF2, following their chronological order of discovery. These syndromes lack clinical criteria and are diagnosed based on the genetic findings. In an attempt to clarify the nomenclature, we propose using a gene-related description (Table 2). In addition, Carney triad (GIST, extra-adrenal PGL, and pulmonary chondroma) and Carney–Stratakis syndrome (GIST and PGL) are two clinical syndromes in which a proportion of patients are related to mutations in SDHx (135–137). Recently, postzygotic epigenetic SDHC mutations were identified in patients with Carney triad; the significance of this finding for clinical practice remains to be established (138, 139).
Familial PPGL, SDHA-related (OMIM nomenclature familial PGL type 5), is thought to be an autosomal-dominant syndrome associated with PCC and sympathetic and parasympathetic PGLs. Among 70 patients having PPGL in the context of SDHA mutation there were 9 cases (13%) with metastatic disease (140, 141). At the National Institutes of Health (NIH), five out of seven SDHA-mutated PPGLs had metastatic disease (K. Pacak, unpublished observations). However, PPGL penetrance of SDHA mutation is estimated to be low; there is not a single known family with more than one individual affected by disease, and index patients rarely develop multiple tumors (44, 140–143).
Familial PPGL, SDHB-related (OMIM nomenclature familial PGL type 4), is an autosomal-dominant syndrome (45) that was initially thought to have almost complete penetrance but has now been adjusted to about 25% to 50% (16, 144–150). A meta-analysis found that metastatic disease occurs in 17% of patients (95% CI, 10% to 28%) (40). The mean age of diagnosis of the first PPGL is typically in the third or fourth decade (145). Penetrance in pediatric patients is low, but the precise risk is disputed, and disease may occur as early as 3 years of age (16, 144–146). Among the patients who had PPGL manifestation before 18 years of age, 25% and 12% developed a second and third PPGL, respectively (mean follow-up of 10 years) (29). Presentation of metastatic disease occurs both at diagnosis and at metachronous time points, with the mean time to metastasis being 8 years (range 0 to 25) (12).
Familial PPGL, SDHC-related (OMIM nomenclature familial PGL type 3), is a rare autosomal-dominant syndrome that manifests mainly with parasympathetic PGLs. Development of metastatic disease is uncommon, and only 25% of patients have multiple tumors (151–153).
Familial PPGL, SDHD-related (OMIM nomenclature familial PGL type 1), is an autosomal-dominant syndrome with paternal transmission (47). The most prominent feature in SDHD carriers is the HN PGL, which has an almost complete penetrance and normally presents after the patient is 30 years old (16, 144, 145). The penetrance of PCC and sympathetic PGLs is ~50% and ~30%, respectively (16, 144, 145). A meta-analysis found that the risk of metastatic disease in SDHD-related PPGLs was ~8% (95% CI, 2% to 26%) (40). Only considering the pediatric population, the mean age of diagnosis is ~14 years; however, PPGLs may occur as early as 5 years of age (29). Sixty percent of pediatric patients with SDHD with disease manifestations developed a second PPGL, and 25% of patients developed a third (mean follow-up 13 years) (20).
Familial PPGL, SDHAF2-related (OMIM nomenclature familial PGL type 2), is an autosomal-dominant syndrome that has been detected only in a few European families and shows almost complete penetrance for parasympathetic PGLs (48, 154–156). Most patients have multifocal HN PGLs, and there are no reported cases of metastatic disease.
Germline FH mutations were previously known to occur in the context of hereditary leiomyomatosis and renal cell cancer (157). Recent reports have shown germline FH mutations in a few patients with PPGLs (20, 21, 49). Occurrence of PPGL was later assessed in 182 carriers with FH mutations, identifying 2 cases (1%) of PCC (158). The phenotype of the affected patients was characterized by multifocal PPGLs and a high frequency of metastatic disease (20, 21, 49).
Pseudohypoxia, VHL/EPAS1-related
The VHL syndrome is inherited in an autosomal-dominant fashion and can be diagnosed with clinical or genetic criteria. The overall penetrance of PPGLs in VHL syndrome has been estimated at ~30% (159). However, clear genotype–phenotype correlations exist, and alleles with a high risk of PPGL are well documented and subgrouped into VHL type 2 (reviewed in ref. 160).
PPGL–somatostatinoma–polycythemia syndrome (Pacak–Zhuang syndrome) almost exclusively occurs in carriers with somatic mosaicism; only one carrier with a germline mutation (presenting without somatostatinoma) has been described in the literature (55, 161). The mosaic status of the mutation can be confirmed through analysis of leukocytes or buccal swab by using special genomic analyses, capable of detecting low allele frequencies (162). The largest report describes seven patients, and the remaining manuscripts are confined to case presentations (162, 163). Patients harboring the pathogenic allele without disease manifestations have not been identified. Interestingly, all PGLs in these patients are multiple and recurrent, and about 30% present with metastatic disease (163).
Kinase signaling
MEN2 is inherited in an autosomal-dominant fashion and diagnosed with clinical or genetic criteria. Penetrance of PCC in MEN2 is dependent on genotype; variants in the RET cysteine-rich extracellular domains (exons 10 to 11) or in codon 918 constitute a majority of MEN2 cases and have a penetrance of ~50%, whereas only a few percent of the noncysteine carriers develop PCC (164, 165).
Familial PPGL, TMEM127-related, is caused by germline mutations in TMEM127, which has an autosomal-dominant inheritance of PCC. Penetrance was estimated at 32% from a six-generation family tree with 151 individuals (166). PCCs are bilateral in one-third of patients with disease manifestation (140, 167).
Familial PPGL, MAX-related, is caused by MAX mutations and has an autosomal-dominant PPGL inheritance that has a particular predisposition for PCC, and ~50% of patients present with bilateral tumors (69, 140).
Up-to-date imaging approach imaging approach to localization and staging of hereditary PPGLs
It is known that PPGL mutation status may guide the selection of the most appropriate tracer in functional imaging (reviewed in ref. 168). In the last year, the role of SSTR imaging for improved localization and staging was confirmed in metastatic TCA cycle–related PPGLs and in HN PGLs, regardless of their genetic background. In three prospective trials, we were able to demonstrate the superior lesion detection rates of 68Ga-DOTATATE PET/CT when compared with other radiopharmaceuticals (90–92). For pseudohypoxic EPAS1-related PPGLs, we have recently showed the superior performance of 18F-FDOPA PET/CT (163, 169).
In the localization of PPGLs, anatomic imaging has almost 100% sensitivity for adrenal chromaffin tumors (170, 171). However, it has less accuracy in more complex disease scenarios, including small or multiple PCC, PGLs, recurrent, and metastatic disease (90, 170). As researchers investigated more accurate techniques, they unconsciously exploited the inherently high glucose turnover in pseudohypoxic PPGLs (103, 104). A proof of principle of 18F-FDG PET/CT in PPGL was published in 1993, followed by independent confirmatory results (105, 172). Nearly 20 years later, Timmers et al. presented a comparative study of 139 PPGLs that “dethroned” traditional imaging by demonstrating superior detection of metastatic lesions by 18F-FDG PET/CT compared with both CT/magnetic resonance imaging (MRI) and 123I-MIBG single-photon emission computed tomography (SPECT) (173). For SDHx-mutated PPGLs, the performance of 18F-FDG PET/CT turned out to be a game changer, with 100% and 92% (95% CI, 87% to 97%) detection rates for primary and metastatic tumors, respectively (173). This led to the 2014 recommendation by the US Endocrine Society Task Force for Pheochromocytoma, which stated that 18F-FDG PET/CT should be used for assessment of metastatic PPGLs (1).
Compared with other neuroendocrine tumors, imaging that targeted SSTRs was initially deemed inferior in PPGLs (94). Once more, pseudohypoxic TCA cycle–related PPGLs were shown to be unique, having a high expression of SSTR type 2 (SSTR2) that was translated into optimistic results on somatostatin receptor functional imaging (87, 174, 175). When evaluated at the NIH, the first study showed that 68Ga-DOTATATE PET/CT had an almost perfect lesion detection rate, 98.6% (95% CI, 96.5% to 99.5%), which was superior to 18F-FDG PET/CT (90). The second NIH study with HN PGLs revealed a 100% detection rate of 68Ga-DOTATATE PET/CT compared with 71% of 18F-FDG PET/CT (91). Our experience is that 68Ga-DOTATATE PET/CT provides excellent and precise diagnostic localization, especially for metastatic SDHB-related PPGL.
Based on these experiences, we recommend 68Ga-DOTATATE PET/CT for localization and staging of PPGL in patients with SDHx mutations. 68Ga-DOTATATE PET/CT is now approved by the US Food and Drug Administration for localization of neuroendocrine tumors expressing SSTRs. At the NIH, we have extended the use of 68Ga-DOTATATE PET/CT to pediatric patients with PPGL. Our preliminary results show that 68Ga-DOTATATE have a lower sensitivity for abdominal PGLs in this setting (K.P., unpublished observations).
Another decisive advantage of 68Ga-DOTATATE PET/CT is its predictive power for efficacy of peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE, which could expand the scope of theranostics based on 68Ga/ 177Lu-labeled DOTATATE for patients with PPGL (176–178). In the NETTER-1 trial, 177Lu-DOTATATE was the first therapeutic intervention to demonstrate improved survival with high evidence in small intestinal neuroendocrine tumors (179). Results on long-term safety of 177Lu-labeled DOTATATE were recently published for gastroenteropancreatic and bronchial neuroendocrine tumors; among 610 patients, acute leukemia occurred in 4 (0.7%) and myelodysplastic syndrome in 9 patients (1.5%), similar to previous studies (180, 181). Grade 3 to 4 nephrologic and hematologic toxicity occurred in 2 out of 872 (0.2%) and 70 out of 872 (8%) (180, 181). As the data from the NETTER-1 (179) mature, we will have a better idea of the summarized benefit from this therapy in neuroendocrine tumors.
Molecular pathology: closing in on a grading system?
The inability of pathology grading to identify metastatic PPGLs and a lack of prognostic factors that can exclude recurrence are two major problems in the care of patients with PPGLs. At present, each clinician must summarize information from tumor location, biochemistry, and size as well as patient age and genetic syndrome to make his or her own risk prediction. A meta-analysis showed that PGLs have a strong tendency for recurrence compared with PCCs (hazard ratios of 8.8 and 11) (182–184). Syndromic disease was the second strongest factor for recurrence compared with sporadic tumors (hazard ratios of 3.4 and 14) (182–184). Larger tumor size (hazard ratio 1.2 for each additional centimeter in tumor diameter) was also a risk factor (182, 184). However, the studies underlying this meta-analysis were performed at a time when the knowledge of the genetic background was limited. Indeed, SDHB mutation status is the strongest risk factor for metastatic disease (12, 28, 185).
Several pathology-based classification systems have been proposed. Thompson et al. described the first pathology scoring system, PCC of Adrenal Gland Scaled Score (PASS), to identify metastatic PPGL (186). However, because validation of PASS showed inconsistent results, Kimura et al. proposed the grading system for adrenal PCC and PGL (GAPP). GAPP is a clinical–biochemical–pathological grading system that separates PPGL into well-differentiated (WD), moderately differentiated (MD), and poorly differentiated (PD) (187) cases. These categories show different frequencies of metastatic disease; 4% (WD), 60% (MD), and 82% (PD), as well as different frequencies of 5-year survival 100% (WD), 67% (MD), and 22% (PD) (187). One potential refinement of GAPP could be to include measurements of O-methylated metabolites (188). As previously mentioned, data from the TCGA and other studies also suggested that tumor mutation status could be used to identify aggressive disease (3, 77). We acknowledge the potential of biochemistry and digital–molecular pathology to further refine GAPP, which could help stratify patients into groups that may benefit from different follow-up approaches.
Treatment of hereditary and sporadic PPGL
An overview with recommendations for treatment of patients with PPGL is provided in Table 5. For all patients with elevated norepinephrine or metanephrine the present-day recommendation is to offer preoperative pharmaceutical “blockade” regardless of symptoms (188–191). There has been no randomized trial for pharmaceutical blockade in the palliative situation. However, because of the limited toxicity of these agents and the potentially disastrous consequences of catecholamine secretion (31, 38, 192), it is our strong recommendation that patients in this scenario should be offered such pharmaceutical treatment (1). In the palliative scenario, such treatment has to be balanced with side effects to avoid worsening quality of life (1).
“All patients with PPGL are at risk for tumor recurrence even after complete resection without residual disease.”
Table 5.
Treatment Manual for PPGL
| Scenario | Intervention | Ref | # | |
|---|---|---|---|---|
| Staging and blockade | Elevated plasma or urine normetanephrine and/or metanephrine | a. alpha-adrenoceptor blocker, eg, doxazosin 1–2 mg, increase 2–4 mg weekly to maximum tolerated dosage for ≤30 mg/d. | (1) | 1.1 |
| b. Localization studies CT, MRI, or PET/CT. | (168) | 1.2 | ||
| Localized stage | Thoracic or abdominal/pelvic | Curative resection, if safe. | (1) | 2.1 |
| HN | Surgery, external beam radiation, locoregional therapy, or watchful waiting. If not possible, follow algorithm for malignant disease 5.3.1. | (41) | 2.2 | |
| Metastatic stage | Elevated plasma or urine normetanephrine and/or metanephrine | a. Palliative doxazosin 1–2 mg, increase 2–4 mg weekly. Balance maximum tolerated dosage to quality of life. | (191) | 3.1.1 |
| b. Before start of any treatment, doxazosin according to 1.1. | 3.1.2 | |||
| Confined disease | Surgery, external radiation, or locoregional therapy if safe and with acceptable morbidity. If not, proceed to 3.3.1. | 3.2 | ||
| Disseminated disease | Medical treatment to alleviate hormone or mass effect alternatively at disease progression. Perform 123I-MIBG scintigraphy and 68Ga-DOTATATE PET/CT. | 3.3 | ||
| First-line 131I-MIBG or 68Ga-DOTATATE positivea | 123I-MIBG = 68Ga-DOTATATE, choose 131I-MIBG. | 3.4.1 | ||
| 123I-MIBG > 68Ga-DOTATATE, choose 131I-MIBG. | 3.4.2 | |||
| 123I-MIGB < 68Ga-DOTATATE, choose 177Lu-DOTATATE. | 3.4.3 | |||
| Second-line or first-line 123I-MIBG/68Ga-DOTATATE negative | Priority I. Rechallenge 123I-MIBG or 68Ga-DOTATATE. | 3.5.1 | ||
| Priority II. CVD,a if WHO performance status >1 or wish for nonhospitalization, proceed to 3.5.3. | 3.5.2 | |||
| Priority III. Temozolomide, Tyrosine kinase inhibitor or experimental therapy. | (213, 214) | 3.5.3 |
CVD chemotherapy may be considered as first-line therapy in patients where the investigator considers the disease as rapidly progressing; recommendations are based on own experience with PPGL.
The most common regimen is alpha-adrenoceptor blockade with appropriate titrations that allow for safe surgical and medical interventions. Preoperative blockade can also be different in various hereditary tumors, and those with pseudohypoxic PPGL often need alpha-adrenoceptor blockade and less commonly addition of beta-adrenoceptor blockade. For those in the kinase signaling cluster, beta-adrenoceptor blockade are often used because of high epinephrine levels that stimulate cardiac beta-adrenoceptors, resulting in tachyarrhythmia.
Surgery has always been, and will continue to be, the preferred therapy for localized, sympathetic PPGLs (193, 194). Deciding on treatment of parasympathetic PPGLs is more delicate. There is less benefit from a curative surgical approach, which must be balanced against potentially adverse treatment-related morbidities, especially of the cranial nerves in vagal and jugular PGLs (195). However, particularly SDHB-related HN PGLs are associated with a higher risk of metastatic disease, and it can be discussed whether they should be offered more aggressive surgical approach. In cases where surgery is considered a high risk, therapeutic radiation (radiotherapy, radiosurgery) can be a less invasive venue that still offer curative-like outcomes (196, 197).
Palliative treatment should be offered to alleviate hormonal symptoms or delay tumor progression, but it must take into account the intrapatient and interpatient heterogeneity in tumor behavior. The median time to progression in therapy-naive patients with PPGL, regardless of mutation, was reported by Hescot et al. at 13.5 months, and almost 20% had ongoing stable disease at 4 years (198). Based on our own experience and recent data from Hamidi et al., a subset of patients with rapidly growing PPGLs experience a very short median time to progression (199); additional longitudinal observation studies are needed to facilitate a new grading system to distinguish indolent tumors from those in need of early therapy, because of their aggressive behavior. It has not been defined at what time point patients with metastatic PPGL benefit most from systemic therapy. In such a scenario without proper evidence, we believe that a multidisciplinary discussion may facilitate a better balancing act between treatment efficacy and side effects.
Surgery can be used in selected patients to reduce tumor volume and catecholamine burden, with subsequent improvements in blood pressure control (200).
Currently, 131I-MIBG is the most studied treatment in metastatic PPGL and has been investigated in small prospective trials (201–203). We recommend that it be used as a first-line treatment in patients who are positive on 123I-MIBG scintigraphy and have slowly growing metastatic lesions. A new preparation of 131I-MIBG, produced on the Ultratrace® platform, may increase tumor uptake and treatment efficacy (204). It is being evaluated in a phase II trial (NCT00874614) that reported a preliminary radiological partial response rate of 35% (n = 34), comparable with standard 131I-MIBG treatment (205). High-dose 131I-MIBG is associated with hematologic side effects. The spectrum of side effects on the Ultratrace® platform has yet to be presented. The effects of radiolabeled somatostatin analogs have also been demonstrated in small retrospective studies (206–209). Chemotherapy with cyclophosphamide, vincristine, and dacarbazine (CVD) remains the second most studied palliative approach and is especially used in patients who present with rapidly progressing metastatic disease (210, 211). CVD is effective in metastatic SDHB-related PPGL, a finding that was confirmed by our own retrospective data (212). Anecdotal reports have suggested several other treatment approaches, a few of which deserve further evaluation. First, monotherapy with the alkylating agent temozolomide showed a 50% partial response rate in SDHB carriers (n = 10) in a retrospective series (213). A second, retrospective study of the tyrosine kinase inhibitor sunitinib also showed three partial responses and five stable diseases (total n = 14) (214). In six hereditary PPGLs belonging to the pseudohypoxic cluster, there were one partial response, four stable, and one progressive disease (214). Sunitinib is being investigated in the first prospective, randomized, placebo-controlled trial in PPGL (FIRSTMAPPP, NCT01371201). This study demonstrated to the scientific community that large, international trials are feasible in this patient population. A second tyrosine kinase inhibitor, pazopanib, was prospectively investigated in six patients; one of the six had a partial response (215). Tyrosine kinase inhibitors axitinib (NCT01967576) and cabozantinib (NCT02302833) are also being evaluated in single-arm phase II studies.
Follow-up
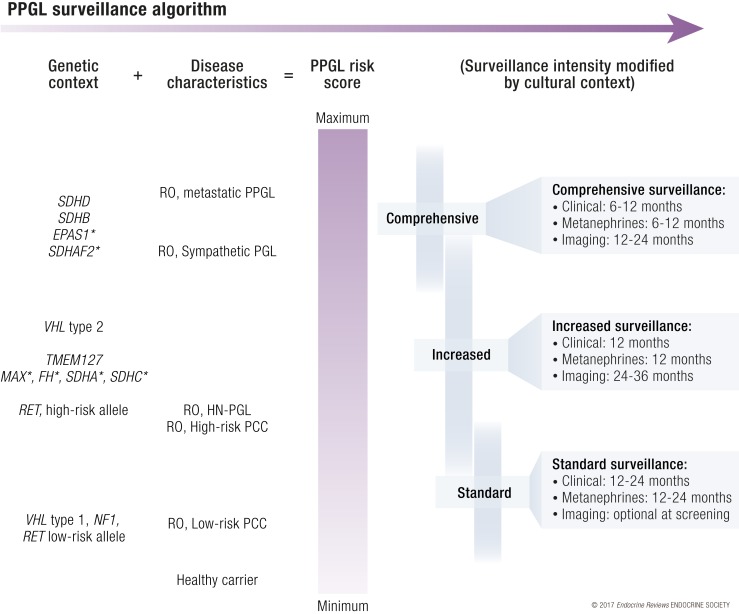
All patients with PPGL are at risk for tumor recurrence even after complete resection without residual disease, and the current WHO classification stresses that all PPGLs have a metastatic potential (110, 115). Those with a genetic predisposition are also at risk for developing new PPGLs (4). Surveillance is used with the intention to improve survival and reduce suffering by early identification of new PPGL events. It is thought that reduced time to diagnosis could result in an increased chance of curative therapy and reduced exposure to pathological levels of catecholamines. These notions are supported by limited scientific evidence. Clinical execution is made even more difficult by the pronounced interpatient heterogeneity, providing a strong rationale to offer personalized surveillance programs (1). To determine the risk of a new PPGL event, we propose using genetic mutation together with disease characteristics (Fig. 4). Characteristics of the individual patient and within families also have to be considered. Patients with presentation of disease at a young age have a high risk of new PPGL events, especially in carriers of mutations belonging to the pseudohypoxic subgroup (29). Taking these complexities together, it is inevitable that the cultural context will influence the surveillance practice. Management by multidisciplinary teams with experience from PPGL is highly encouraged (1).
Figure 4.
Algorithm for personalized follow-up of pheochromocytoma and paraganglioma patients where risk from genetic subtype is added to disease characteristics (healthy carriers assigned zero risk) to estimate a PPGL risk score that incorporates risk of new PPGL events and frequency of metastatic disease. In addition, family history of metastatic PPGL and presentation at a young age may warrant increased surveillance of other members of that particular family, especially in patients with SDHx. Proposed definition of high-risk PCC: diameter >5 cm, 3–3.5 cm for SDHB carriers, young age at diagnosis, or moderately to poorly differentiated PPGL accordingly to GAPP classification system. Detailed description of surveillance, clinical; patient history and clinical investigation to cover symptoms of catecholamine secretion in addition to relevant information for the particular genetic syndrome. Metanephrines: selection of biochemical tests can be guided by mutation subtype as well as PPGL secretory profile of that particular patient. 3-methoxytyramine is recommended for SDHx patients only. Chromogranin A is optional. Imaging should include MRI or ultrasound of relevant anatomical sites; CT scan can be used too, based on MRI results, a radiologist’s advice, or a physician’s recommendation after detailed patient evaluation. R0, absence of residual PPGL after treatment. *, indicates high uncertainties in assumption of PPGL risk score.
Current recommendations are that all patients with PPGL should be offered postoperative surveillance (1, 115). The precision medicine approach suggests that sporadic PCCs without risk factors have a low risk of recurrence, and patients could be offered a less comprehensive follow-up program (115). Patients with sporadic PCC and available risk factors as well as all PGL should be offered lifelong surveillance (1, 115). At a minimum, such follow-up should include clinical examination and metanephrine measurements. The role of anatomical imaging remains to be defined, and we suggest that such practice is warranted only in patients considered to have a very high risk of recurrence.
Endocrine Society guidelines recommend that patients with hereditary PPGL should be offered a mutation-tailored surveillance program (1). Such recommendations for screening and follow-up have been published for VHL, MEN2, and NF1 (Table 6) (4, 160, 200, 216, 217). More recently, consensus statements on SDHx, TMEM127, and MAX syndromes became available (200, 218). As a general rule, all patients should have clinical examination, biochemistry testing, and anatomical imaging at screening. Those considered to have a low risk of new PPGL events may be offered a follow-up consisting of clinical examination or measurement of metanephrines as well as 3-methoxytyramine in relevant cases. Those with a higher risk should also be offered periodical anatomic imaging, especially TCA cycle–related PPGLs, which often have less biochemically active PPGLs (219). MRI is preferred to minimize radiation exposure. Whole-body examination with a diffusion-weighted imaging protocol is proposed as a cost-effective method with high sensitivity to detect both PPGL and other tumors related to relevant genetic syndromes (219). If PCC is suspected from patient symptoms or biochemistry, CT scans have the best performance for localization (1). Contrast-enhanced ultrasound has attracted interest, but there are insufficient data to recommend it for PPGL screening (200). The need to perform complementary radionuclide imaging and the choice of optimal radiopharmaceuticals should be tailored to individualized situations and decided by an experienced multidisciplinary team. Ultimately, large prospective studies are needed to adequately estimate the benefit from surveillance for new PPGL events in the various subtypes of patients.
Table 6.
Consensus Statements on Surveillance for PPGL
| Author | On Behalf Of | Scenario | Reference |
|---|---|---|---|
| Lenders et al. | Endocrine Society | R0 PPGL | (1) |
| Plouin et al. | European Society of Endocrinology | R0 PPGL | (115) |
| Brandi et al. | International Consensus Group | RET | (217) |
| Rednam et al. | North American Consensus Group | VHL, SDHx, TMEM127, MAX | (200) |
| Nielsen et al. | International Consensus Group | VHL | (160) |
Recommended in-depth reading for follow-up of patients with PPGL.
Abbreviations: R0, absence of residual PPGL after treatment.
Future Classifications and Tailored Therapies
The unique biology related to different PPGL mutations can thus be translated into improved diagnostic, imaging, and prognostic biomarkers. Although progress in treatment of PPGL has developed more slowly, the imminent pharmacological pipeline is indeed promising. Several drugs targeting a broad range of features unique to PPGL are in different developmental stages.
Genetic instability is the force driving a majority of cancers, and DNA is the ultimate target for chemotherapy and radiotherapy. An improved understanding of how cancer cells maintain their genome has enabled researchers to target features unique to cancer cells, using both established and novel drugs. These include chemotherapy, beta-emitting radiotherapy, ataxia telangiectasia and PARP inhibitors, which are all context specific (220–222). Genes involved in chromatin maintenance include ATRX, TP53, and SETD2, all of which have been identified with recurrent mutations in PPGL (3, 71, 72). The prognostic and predictive impact of these genes is currently being investigated.
Pseudohypoxic TCA cycle–related tumors harbor a unique methylome that is characterized by global hypermethylation, a discovery initially described by Letouzé et al. (49, 82). The demethylating agents azacytidine and decitabine are undergoing evaluation in SDHx-mutated gastrointestinal stromal tumors, and we expect that the NIH will open a trial investigating SGI-110 in SDHx-related tumors including PPGLs in 2017.
Another promising vulnerability in pseudohypoxic TCA cycle–related PPGL is its unique metabolome. Because succinate dehydrogenase–deficient cells rely on hijacking alternative metabolomics pathways to support their survival, they will become vulnerable to a pharmaceutical blockade, which in theory should leave healthy cells untouched (102). With the invention of MRI spectroscopy, metabolic effects of such interventions could possibly be followed in vivo and in real time (223, 224). A potential metabolic drug target includes glutamine synthesis. The glutaminase inhibitor CB-839 is undergoing phase I evaluation (NCT02071862) and will include succinate dehydrogenase–deficient tumors. This research exemplifies how basket trials, collecting rare tumors with shared molecular alterations, may facilitate more comprehensive trials.
Mechanisms underlying tumorigenesis in pseudohypoxic PPGLs converge on the HIF-signaling pathway, and several investigators are looking into how this hypoxiome may be exploited for therapeutics. Chemotherapy (225) and inhibition of vascular endothelial growth factor signaling, HIF-2α (226), or the succinate receptor (227) all represent possible future avenues for a drugging the hypoxia signaling pathways. In addition to the ongoing prospective, randomized, placebo-controlled phase II trial of sunitinib (NCT01371201), a novel HIF-2α inhibitor, PT2385, is being evaluated in an ongoing phase I trial (NCT02293980) in metastatic renal cell carcinoma. The relationship between inactivation of VHL, in both sporadic and syndromic cases, and outcome of treatment with tyrosine kinase inhibitors has shown contradictory findings; clarifying results from prospective phase II studies are awaited shortly (228, 229).
As already mentioned, PRRT with either 90Y- or 177Lu-coupled somatostatin analogs can be guided to PPGL cells through their increased expression of SSTR2s (230). We expect that a prospective phase II trial of 177Lu-DOTATATE in PPGL will open at the NIH in 2017. Further developments of this concept are also being investigated; SSTR2 antagonists may increase tumor uptake compared with normal tissues, and 213Bi-DOTATOC, an alpha particle emitter, has a stronger source of radioactive energy and therefore has greater cytotoxic effect (231, 232). In addition to these receptor-targeted agents, other radiopharmaceuticals based on affinity for bone matrix can be used in patients who develop bone metastases. 223Ra-dichloride has demonstrated effect in prostate carcinoma (233). Given the high incidence of metastatic bone disease that accumulates bone-seeking radiopharmaceuticals in PPGLs, this effect would allow the use of Ra-223 dichloride (an alpha particle emitter) in a subset of patients with a positive bone scans.
SSTR2s can also be targeted by long-acting somatostatin analogs that have shown long-term disease stabilization in closely related gastroenteropancreatic neuroendocrine tumors (234). Somatostatin analogs have few side effects (234). Recent data from animal models indicate that long-term treatment can stabilize endocrine neoplasms and increase survival in the related hereditary syndrome multiple endocrine neoplasia type 1 (235). Thus, a prospective phase II trial using the somatostatin analog lanreotide should be launched for metastatic PPGL. Deregulated cell signaling as a consequence of mutations in RET and NF1 may render these cases sensitive to tyrosine kinase inhibitors (RET) or MEK inhibitors (NF1) and has shown promising results in other tumors, but it is still unknown whether these results may be extrapolated to PPGL (236, 237).
The publisher of Science magazine declared cancer immunotherapy the breakthrough of the year in 2013. This treatment modality has resulted in improved treatment of multiple cancers, including melanoma and lung cancer, that demonstrated unprecedented, durable responses (238, 239). It appears that cancers with high mutation loads are particularly susceptible to the immune system. One likely explanation is that more nonsynonymous and insertion deletion mutations will generate neoantigens that will make cancer cells different compared with normal cells and therefore visible to the immune system (240, 241). Although the genomes of PPGLs are relatively intact, the paraganglial cell is a specialized entity with a unique set of transcripts. This finding could have a potential use for increasing immune cell recognition, either through already-registered immune checkpoint inhibitors (CTLA4 and PD-1 antibodies) or newer approaches, such as vaccines, immune cells, or microbe-based therapies (242, 243). Intriguingly, there is a case report of a PPGL that responded to treatment with interferon (244).
Future role of precision medicine and targeted therapies in hereditary PPGL
The future pipeline for treatments of PPGLs looks promising. However, until appropriate data are available, it is recommended that experimental molecular profiling be used to guide clinical decision making within research protocols (245). Although it may be tempting to extrapolate experiences from other diseases, predictive factors and drug targets are strictly dependent on tissue context, well exemplified by the variable experiences from targeting BRAF V600 mutations in different tumors (246). Second, as a consequence of tumor evolution, biomarkers may not always occur in a ubiquitous manner (81, 247, 248). Thus, although rare cancers can benefit from learned experiences in other diseases, they should be used only to generate research hypotheses and not affect clinical care. Despite ongoing celebrations and hope with regard to precision medicine–related therapies, clinicians must be cautious about rushing into experimental or off-label treatments, which could result in harming rather than healing the patient.
Propositions to maximize tumor response while minimizing toxicity must recognize the infinite possibilities of empirical tests. Future directions must focus on building mathematical models describing tumor growth during therapies and implementing modeling tools for the personalization of treatment schedules and sequencing. Predictive biomarkers of treatment responses, such and molecular imaging and circulating DNA (249), can also help physicians determine which patients will benefit the most from treatment (250). The response evaluation based on response evaluation criteria in solid tumors has many limitations, especially for monitoring response to targeted therapies and PRRT. PET imaging, as well as high-field and ultra-high-field MRI with the use of multiparametric image postprocessing and analysis, can provide reliable biomarkers of response to therapies. These imaging techniques must be evaluated in the setting of clinical trials in comparison with classic follow-up (251).
As we start to generate “big data” for each individual patient, transforming information in a resource-effective manner to deliver evidence-based recommendations will be a major challenge. Here, artificial intelligence or machine learning has been recognized as a potential solution (250, 252) and may ultimately disrupt the way traditional health care is performed, by democratizing information and interpretations, shifting power from providers to the patients.
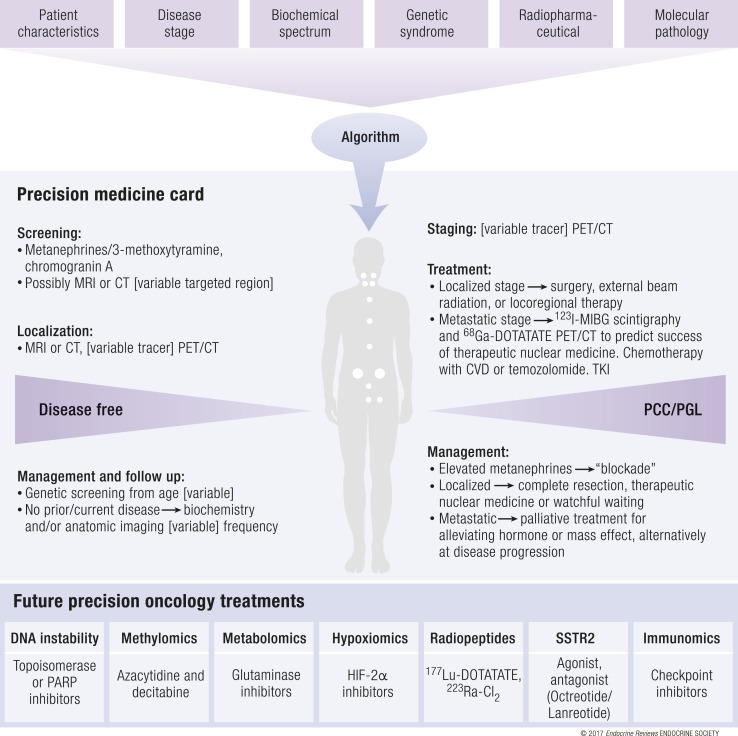
Over time, when more data and experience are available, we are optimistic that precision therapy may provide a path toward increased therapeutic efficacy in PPGL. Each patient could have his or her own precision medicine card (physical or cloud based), which carries the information that is necessary to personalize his or her care (Fig. 5).
Figure 5.
Schematic of a future precision medicine workflow. Top: Relevant factors for disease assessment. Middle: Recommendations presented on a precision medicine card based on whether the patient is disease free. Bottom: Future potential treatments for systemic disease. PARP, poly [ADP-ribose] polymerase; TKI; tyrosine kinase inhibitor.
Disease Prevention: Transforming the Lives of Patients With Hereditary PPGL
Precision medicine is most commonly associated with diagnosis and treatment. However, insights from the underlying biology can also be used to design tailored interventions to completely avert disease: (primary) precision prevention. Current ideas on how such a preventive approach of hereditary manifestations could succeed are scarce. For example, there were no actionable clues from PPGL genomic landscapes, because they did not reveal any traces of external (read preventable) mutagens (3, 71, 74). Yet because pseudohypoxic TCA cycle–related PPGL is fundamentally a metabolic disease, it is tempting to imagine that we could use tailored environmental, dietary, or pharmaceutical strategies to reduce the overall penetrance. Potential mechanisms are succinate dehydrogenase deficiency, accelerated removal of mutated protein by the proteasome, pseudohypoxia, and hypermethylation. One of the most interesting concepts come from B. Baysal, who built on the initial observation by Arias-Stella et al. that showed a positive correlation between high altitude and carotid body size and a correlation between high altitude and increased HN PGL penetrance in SDHD carriers (253, 254). The concept of hypoxic vulnerability in patients with SDHx mutations is also supported by the increased incidence of HN PGLs among sporadic patients at high altitudes and in disorders with chronic hypoxia, such as congenital cyanotic heart disease (255, 256). Experimental data suggest synergism between hypoxia and succinate accumulation, increasing stabilization of HIFs and methylation of histones and DNA (257). Although these data should not be used in clinical decision making, it may prove to be a potential avenue for future research. The concept of succinate/fumarate ration may also be of interest in the future to monitor therapeutic responses (258). These insights end this review where we started, by highlighting the importance of understanding key disease mechanisms to improve care of patients with PPGL.
Conclusions
The precision medicine approach to PPGLs can now be used for screening, diagnosis, staging, and follow-up in both adult and pediatric patients. Precision therapeutics is the next crucial step, and it has tremendous future potential for transforming the lives of patients with these tumors. While keeping a realistic mindset, it is not hard to be sparked by optimism when there is an exponential increase in therapeutic agents undergoing prospective phase II trials. This is undoubtedly a new era in the care of these patients. Finally, our vision of precision prevention is tightly linked with understanding the biology of the disease, host, and environmental factors. Though still in need of hard work, precision medicine offers the prospect of reaching the ultimate goal: no deaths due to PPGL.
Acknowledgments
We thank Ms. Katherine Wolf for carefully reviewing the manuscript, and we are grateful to all the patients who inspired us throughout the writing process. This review was conceived and partially written during an exchange between the US National Institutes of Health by J.C., which was generously supported by the Paradifference Foundation (http://www.paradifference.org).
Financial Support: This work was supported in part by the Eunice Kennedy Shriver National Institutes of Child Health and Human Development of the National Institutes of Health in Bethesda, Maryland. J.C.’s research position was funded by Akademiska Sjukhuset, Uppsala, Sweden.
Author contributions: J.C., D.T., and K.P. made substantial contributions to the content of this article, wrote the manuscript, and reviewed or edited all drafts before submission.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AJCC
- American Joint Committee on Cancer
- CI
- confidence interval
- CT
- computed tomography
- CVD
- cyclophosphamide, vincristine, and dacarbazine
- DOTATATE
- tetraazacyclododecane tetraacetic acid–octreotate
- GAPP
- grading system for adrenal PCC and PGL
- GIST
- gastrointestinal stromal tumor
- HIF
- hypoxia-inducible factor
- HN
- head and neck
- MD
- moderately differentiated
- MEN2
- multiple endocrine neoplasia type 2
- MIBG
- metaiodobenzylguanidine
- MRI
- magnetic resonance imaging
- NF1
- neurofibromatosis type 1
- NIH
- National Institutes of Health
- OMIM
- Online Mendelian Inheritance in Man
- PASS
- PCC of Adrenal Gland Scaled Score
- PCC
- pheochromocytoma
- PD
- poorly differentiated
- PET
- positron emission tomography
- PPGL
- pheochromocytoma and paraganglioma
- PGL
- paraganglioma
- PRRT
- peptide receptor radionuclide therapy
- SPECT
- single-photon emission computed tomography
- SSTR
- somatostatin receptor
- TCA
- tricarboxylic acid
- TCGA
- The Cancer Genome Atlas
- VHL
- von Hippel–Lindau
- WD
- well-differentiated
- WHO
- World Health Organization.
References
- 1.Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, Pacak K, Young WF Jr; Endocrine Society . Pheochromocytoma and paraganglioma: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915–1942. [DOI] [PubMed] [Google Scholar]
- 2.Dahia PL. Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity. Nat Rev Cancer. 2014;14(2):108–119. [DOI] [PubMed] [Google Scholar]
- 3.Fishbein L, Leshchiner I, Walter V, Danilova L, Robertson AG, Johnson AR, Lichtenberg TM, Murray BA, Ghayee HK, Else T, Ling S, Jefferys SR, de Cubas AA, Wenz B, Korpershoek E, Amelio AL, Makowski L, Rathmell WK, Gimenez-Roqueplo AP, Giordano TJ, Asa SL, Tischler AS, Pacak K, Nathanson KL, Wilkerson MD; Cancer Genome Atlas Research Network . Comprehensive molecular characterization of pheochromocytoma and paraganglioma. Cancer Cell. 2017;31(2):181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Favier J, Amar L, Gimenez-Roqueplo AP. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nat Rev Endocrinol. 2015;11(2):101–111. [DOI] [PubMed] [Google Scholar]
- 5.Lips C, Lentjes E, Hoppener J, Luijt R, Moll F.. Familial paragangliomas. Hered Cancer Clin Pract. 2006;4:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beard CM, Sheps SG, Kurland LT, Carney JA, Lie JT. Occurrence of pheochromocytoma in Rochester, Minnesota, 1950 through 1979. Mayo Clin Proc. 1983;58(12):802–804. [PubMed] [Google Scholar]
- 7.Stenström G, Svärdsudd K. Pheochromocytoma in Sweden 1958–1981. An analysis of the National Cancer Registry Data. Acta Med Scand. 1986;220(3):225–232. [PubMed] [Google Scholar]
- 8.Barontini M, Levin G, Sanso G. Characteristics of pheochromocytoma in a 4- to 20-year-old population. Ann N Y Acad Sci. 2006;1073:30–37. [DOI] [PubMed] [Google Scholar]
- 9.Beltsevich DG, Kuznetsov NS, Kazaryan AM, Lysenko MA. Pheochromocytoma surgery: epidemiologic peculiarities in children. World J Surg. 2004;28(6):592–596. [DOI] [PubMed] [Google Scholar]
- 10.O’Riordain DS, Young WF Jr, Grant CS, Carney JA, van Heerden JA. Clinical spectrum and outcome of functional extraadrenal paraganglioma. World J Surg. 1996;20(7):916–921, discussion 922. [DOI] [PubMed] [Google Scholar]
- 11.Amar L, Bertherat J, Baudin E, Ajzenberg C, Bressac-de Paillerets B, Chabre O, Chamontin B, Delemer B, Giraud S, Murat A, Niccoli-Sire P, Richard S, Rohmer V, Sadoul JL, Strompf L, Schlumberger M, Bertagna X, Plouin PF, Jeunemaitre X, Gimenez-Roqueplo AP. Genetic testing in pheochromocytoma or functional paraganglioma. J Clin Oncol. 2005;23(34):8812–8818. [DOI] [PubMed] [Google Scholar]
- 12.King KS, Prodanov T, Kantorovich V, Fojo T, Hewitt JK, Zacharin M, Wesley R, Lodish M, Raygada M, Gimenez-Roqueplo AP, McCormack S, Eisenhofer G, Milosevic D, Kebebew E, Stratakis CA, Pacak K. Metastatic pheochromocytoma/paraganglioma related to primary tumor development in childhood or adolescence: significant link to SDHB mutations. J Clin Oncol. 2011;29(31):4137–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pacak K, Chrousos G, Koch C, Lenders J, Eisenhofer G.. Pheochromocytoma: progress in diagnosis, therapy, and genetics. In Adrenal disorders. New York, NY: Springer; 2001;1:479–523. [Google Scholar]
- 14.Goffredo P, Sosa JA, Roman SA. Malignant pheochromocytoma and paraganglioma: a population level analysis of long-term survival over two decades. J Surg Oncol. 2013;107(6):659–664. [DOI] [PubMed] [Google Scholar]
- 15.Gill AJ, Benn DE, Chou A, Clarkson A, Muljono A, Meyer-Rochow GY, Richardson AL, Sidhu SB, Robinson BG, Clifton-Bligh RJ. Immunohistochemistry for SDHB triages genetic testing of SDHB, SDHC, and SDHD in paraganglioma-pheochromocytoma syndromes. Hum Pathol. 2010;41(6):805–814. [DOI] [PubMed] [Google Scholar]
- 16.Neumann HP, Pawlu C, Peczkowska M, Bausch B, McWhinney SR, Muresan M, Buchta M, Franke G, Klisch J, Bley TA, Hoegerle S, Boedeker CC, Opocher G, Schipper J, Januszewicz A, Eng C; European-American Paraganglioma Study Group . Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA. 2004;292(8):943–951. [DOI] [PubMed] [Google Scholar]
- 17.Mannelli M, Castellano M, Schiavi F, Filetti S, Giacchè M, Mori L, Pignataro V, Bernini G, Giachè V, Bacca A, Biondi B, Corona G, Di Trapani G, Grossrubatscher E, Reimondo G, Arnaldi G, Giacchetti G, Veglio F, Loli P, Colao A, Ambrosio MR, Terzolo M, Letizia C, Ercolino T, Opocher G; Italian Pheochromocytoma/Paraganglioma Network . Clinically guided genetic screening in a large cohort of Italian patients with pheochromocytomas and/or functional or nonfunctional paragangliomas. J Clin Endocrinol Metab. 2009;94(5):1541–1547. [DOI] [PubMed] [Google Scholar]
- 18.Crona J, Nordling M, Maharjan R, Granberg D, Stålberg P, Hellman P, Björklund P. Integrative genetic characterization and phenotype correlations in pheochromocytoma and paraganglioma tumours. PLoS One. 2014;9(1):e86756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welander J, Andreasson A, Juhlin CC, Wiseman RW, Bäckdahl M, Höög A, Larsson C, Gimm O, Söderkvist P. Rare germline mutations identified by targeted next-generation sequencing of susceptibility genes in pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2014;99(7):E1352–E1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castro-Vega LJ, Buffet A, De Cubas AA, Cascón A, Menara M, Khalifa E, Amar L, Azriel S, Bourdeau I, Chabre O, Currás-Freixes M, Franco-Vidal V, Guillaud-Bataille M, Simian C, Morin A, Letón R, Gómez-Graña A, Pollard PJ, Rustin P, Robledo M, Favier J, Gimenez-Roqueplo AP. Germline mutations in FH confer predisposition to malignant pheochromocytomas and paragangliomas. Hum Mol Genet. 2014;23(9):2440–2446. [DOI] [PubMed] [Google Scholar]
- 21.Clark GR, Sciacovelli M, Gaude E, Walsh DM, Kirby G, Simpson MA, Trembath RC, Berg JN, Woodward ER, Kinning E, Morrison PJ, Frezza C, Maher ER. Germline FH mutations presenting with pheochromocytoma. J Clin Endocrinol Metab. 2014;99(10):E2046–E2050. [DOI] [PubMed] [Google Scholar]
- 22.Turkova H, Prodanov T, Maly M, Martucci V, Adams K, Widimsky J Jr., Chen CC, Ling A, Kebebew E, Stratakis C, Fojo T, Pacak K. Characteristics and outcomes of metastatic SDHB and sporadic pheochromocytoma/paraganglioma: an NIH study. Endocr Pract. 2015;22:302–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayala-Ramirez M, Feng L, Johnson MM, Ejaz S, Habra MA, Rich T, Busaidy N, Cote GJ, Perrier N, Phan A, Patel S, Waguespack S, Jimenez C. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab. 2011;96:717–725. [DOI] [PubMed] [Google Scholar]
- 24.Cascón A, Pita G, Burnichon N, Landa I, López-Jiménez E, Montero-Conde C, Leskelä S, Leandro-García LJ, Letón R, Rodríguez-Antona C, Díaz JA, López-Vidriero E, González-Neira A, Velasco A, Matias-Guiu X, Gimenez-Roqueplo AP, Robledo M. Genetics of pheochromocytoma and paraganglioma in Spanish patients. J Clin Endocrinol Metab. 2009;94(5):1701–1705. [DOI] [PubMed] [Google Scholar]
- 25.Cotesta D, Petramala L, Serra V, Pergolini M, Crescenzi E, Zinnamosca L, De Toma G, Ciardi A, Carbone I, Massa R, Filetti S, Letizia C. Clinical experience with pheochromocytoma in a single centre over 16 years. High Blood Press Cardiovasc Prev. 2009;16(4):183–193. [DOI] [PubMed] [Google Scholar]
- 26.Burnichon N, Vescovo L, Amar L, Libé R, de Reynies A, Venisse A, Jouanno E, Laurendeau I, Parfait B, Bertherat J, Plouin PF, Jeunemaitre X, Favier J, Gimenez-Roqueplo AP. Integrative genomic analysis reveals somatic mutations in pheochromocytoma and paraganglioma. Hum Mol Genet. 2011;20(20):3974–3985. [DOI] [PubMed] [Google Scholar]
- 27.Muth A, Abel F, Jansson S, Nilsson O, Ahlman H, Wängberg B. Prevalence of germline mutations in patients with pheochromocytoma or abdominal paraganglioma and sporadic presentation: a population-based study in western Sweden. World J Surg. 2012;36(6):1389–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gimenez-Roqueplo AP, Favier J, Rustin P, Rieubland C, Crespin M, Nau V, Khau Van Kien P, Corvol P, Plouin PF, Jeunemaitre X; COMETE Network . Mutations in the SDHB gene are associated with extra-adrenal and/or malignant phaeochromocytomas. Cancer Res. 2003;63(17):5615–5621. [PubMed] [Google Scholar]
- 29.Bausch B, Wellner U, Bausch D, Schiavi F, Barontini M, Sanso G, Walz MK, Peczkowska M, Weryha G, Dall’igna P, Cecchetto G, Bisogno G, Moeller LC, Bockenhauer D, Patocs A, Rácz K, Zabolotnyi D, Yaremchuk S, Dzivite-Krisane I, Castinetti F, Taieb D, Malinoc A, von Dobschuetz E, Roessler J, Schmid KW, Opocher G, Eng C, Neumann HP. Long-term prognosis of patients with pediatric pheochromocytoma. Endocr Relat Cancer. 2013;21(1):17–25. [DOI] [PubMed] [Google Scholar]
- 30.Ciftci AO, Tanyel FC, Senocak ME, Büyükpamukçu N. Pheochromocytoma in children. J Pediatr Surg. 2001;36(3):447–452. [DOI] [PubMed] [Google Scholar]
- 31.Timmers HJ, Brouwers FM, Hermus AR, Sweep FC, Verhofstad AA, Verbeek AL, Pacak K, Lenders JW. Metastases but not cardiovascular mortality reduces life expectancy following surgical resection of apparently benign pheochromocytoma. Endocr Relat Cancer. 2008;15(4):1127–1133. [DOI] [PubMed] [Google Scholar]
- 32.Khorram-Manesh A, Jansson S, Wängberg B, Nilsson O, Tisell LE, Ahlman H. Mortality associated with pheochromocytoma: increased risk for additional tumors. Ann N Y Acad Sci. 2006;1073:444–448. [DOI] [PubMed] [Google Scholar]
- 33.Brouwers FM, Eisenhofer G, Lenders JW, Pacak K. Emergencies caused by pheochromocytoma, neuroblastoma, or ganglioneuroma. Endocrinol Metab Clin North Am. 2006;35(4):699–724, viii. [DOI] [PubMed] [Google Scholar]
- 34.Netterville JL, Jackson CG, Miller FR, Wanamaker JR, Glasscock ME. Vagal paraganglioma: a review of 46 patients treated during a 20-year period. Arch Otolaryngol Head Neck Surg. 1998;124(10):1133–1140. [DOI] [PubMed] [Google Scholar]
- 35.Amar L, Baudin E, Burnichon N, Peyrard S, Silvera S, Bertherat J, Bertagna X, Schlumberger M, Jeunemaitre X, Gimenez-Roqueplo AP, Plouin PF. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab. 2007;92:3822–3828. [DOI] [PubMed] [Google Scholar]
- 36.van Hulsteijn LT, Louisse A, Havekes B, Kaptein AA, Jansen JC, Hes FJ, Smit JW, Corssmit EP. Quality of life is decreased in patients with paragangliomas. Eur J Endocrinol. 2013;168(5):689–697. [DOI] [PubMed] [Google Scholar]
- 37.van Hulsteijn LT, Heesterman B, Jansen JC, Bayley JP, Hes FJ, Corssmit EP, Dekkers OM. No evidence for increased mortality in SDHD variant carriers compared with the general population. Eur J Hum Genet. 2015;23(12):1713–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stolk RF, Bakx C, Mulder J, Timmers HJ, Lenders JW. Is the excess cardiovascular morbidity in pheochromocytoma related to blood pressure or to catecholamines? J Clin Endocrinol Metab. 2013;98(3):1100–1106. [DOI] [PubMed] [Google Scholar]
- 39.Nomura K, Kimura H, Shimizu S, Kodama H, Okamoto T, Obara T, Takano K. Survival of patients with metastatic malignant pheochromocytoma and efficacy of combined cyclophosphamide, vincristine, and dacarbazine chemotherapy. J Clin Endocrinol Metab. 2009;94(8):2850–2856. [DOI] [PubMed] [Google Scholar]
- 40.van Hulsteijn LT, Dekkers OM, Hes FJ, Smit JW, Corssmit EP. Risk of malignant paraganglioma in SDHB-mutation and SDHD-mutation carriers: a systematic review and meta-analysis. J Med Genet. 2012;49(12):768–776. [DOI] [PubMed] [Google Scholar]
- 41.Taïeb D, Kaliski A, Boedeker CC, Martucci V, Fojo T, Adler JR Jr, Pacak K. Current approaches and recent developments in the management of head and neck paragangliomas. Endocr Rev. 2014;35(5):795–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Binderup ML, Jensen AM, Budtz-Jørgensen E, Bisgaard ML. Survival and causes of death in patients with von Hippel–Lindau disease. J Med Genet. 2017;54(1):11–18. [DOI] [PubMed] [Google Scholar]
- 43.Thosani S, Ayala-Ramirez M, Palmer L, Hu MI, Rich T, Gagel RF, Cote G, Waguespack SG, Habra MA, Jimenez C. The characterization of pheochromocytoma and its impact on overall survival in multiple endocrine neoplasia type 2. J Clin Endocrinol Metab. 2013;98(11):E1813–E1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burnichon N, Brière JJ, Libé R, Vescovo L, Rivière J, Tissier F, Jouanno E, Jeunemaitre X, Bénit P, Tzagoloff A, Rustin P, Bertherat J, Favier J, Gimenez-Roqueplo AP. SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet. 2010;19(15):3011–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Astuti D, Latif F, Dallol A, Dahia PL, Douglas F, George E, Sköldberg F, Husebye ES, Eng C, Maher ER. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet. 2001;69(1):49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niemann S, Müller U. Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat Genet. 2000;26(3):268–270. [DOI] [PubMed] [Google Scholar]
- 47.Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, van der Mey A, Taschner PE, Rubinstein WS, Myers EN, Richard CW III, Cornelisse CJ, Devilee P, Devlin B. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287(5454):848–851. [DOI] [PubMed] [Google Scholar]
- 48.Hao HX, Khalimonchuk O, Schraders M, Dephoure N, Bayley JP, Kunst H, Devilee P, Cremers CW, Schiffman JD, Bentz BG, Gygi SP, Winge DR, Kremer H, Rutter J. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science. 2009;325:1139–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Letouzé E, Martinelli C, Loriot C, Burnichon N, Abermil N, Ottolenghi C, Janin M, Menara M, Nguyen AT, Benit P, Buffet A, Marcaillou C, Bertherat J, Amar L, Rustin P, De Reyniès A, Gimenez-Roqueplo AP, Favier J. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell. 2013;23(6):739–752. [DOI] [PubMed] [Google Scholar]
- 50.Cascón A, Comino-Méndez I, Currás-Freixes M, de Cubas AA, Contreras L, Richter S, Peitzsch M, Mancikova V, Inglada-Pérez L, Pérez-Barrios A, Calatayud M, Azriel S, Villar-Vicente R, Aller J, Setién F, Moran S, Garcia JF, Río-Machín A, Letón R, Gómez-Graña Á, Apellániz-Ruiz M, Roncador G, Esteller M, Rodríguez-Antona C, Satrústegui J, Eisenhofer G, Urioste M, Robledo M. Whole-exome sequencing identifies MDH2 as a new familial paraganglioma gene. J Natl Cancer Inst. 2015;107(5):107. [DOI] [PubMed] [Google Scholar]
- 51.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7(1):77–85. [DOI] [PubMed] [Google Scholar]
- 52.Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML, Stackhouse T, Kuzmin I, Modi W, Geil L, et al. . Identification of the von Hippel–Lindau disease tumor suppressor gene. Science. 1993;260(5112):1317–1320. [DOI] [PubMed] [Google Scholar]
- 53.Hes FJ, van der Luijt RB, Janssen AL, Zewald RA, de Jong GJ, Lenders JW, Links TP, Luyten GP, Sijmons RH, Eussen HJ, Halley DJ, Lips CJ, Pearson PL, van den Ouweland AM, Majoor-Krakauer DF. Frequency of Von Hippel–Lindau germline mutations in classic and non-classic Von Hippel–Lindau disease identified by DNA sequencing, Southern blot analysis and multiplex ligation-dependent probe amplification. Clin Genet. 2007;72(2):122–129. [DOI] [PubMed] [Google Scholar]
- 54.Jochmanová I, Yang C, Zhuang Z, Pacak K. Hypoxia-inducible factor signaling in pheochromocytoma: turning the rudder in the right direction. J Natl Cancer Inst. 2013;105(17):1270–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhuang Z, Yang C, Lorenzo F, Merino M, Fojo T, Kebebew E, Popovic V, Stratakis CA, Prchal JT, Pacak K. Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. N Engl J Med. 2012;367:922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pacak K, Jochmanova I, Prodanov T, Yang C, Merino MJ, Fojo T, Prchal JT, Tischler AS, Lechan RM, Zhuang Z. New syndrome of paraganglioma and somatostatinoma associated with polycythemia. J Clin Oncol. 2013;31(13):1690–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fliedner SM, Shankavaram U, Marzouca G, Elkahloun A, Jochmanova I, Daerr R, Linehan WM, Timmers H, Tischler AS, Papaspyrou K, Brieger J, de Krijger R, Breza J, Eisenhofer G, Zhuang Z, Lehnert H, Pacak K. Hypoxia-inducible factor 2α mutation-related paragangliomas classify as discrete pseudohypoxic subcluster. Neoplasia. 2016;18(9):567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ladroue C, Carcenac R, Leporrier M, Gad S, Le Hello C, Galateau-Salle F, Feunteun J, Pouysségur J, Richard S, Gardie B. PHD2 mutation and congenital erythrocytosis with paraganglioma. N Engl J Med. 2008;359(25):2685–2692. [DOI] [PubMed] [Google Scholar]
- 59.Yang C, Zhuang Z, Fliedner SM, Shankavaram U, Sun MG, Bullova P, Zhu R, Elkahloun AG, Kourlas PJ, Merino M, Kebebew E, Pacak K. Germ-line PHD1 and PHD2 mutations detected in patients with pheochromocytoma/paraganglioma-polycythemia. J Mol Med (Berl). 2015;93(1):93–104. [DOI] [PubMed] [Google Scholar]
- 60.Mulligan LM, Kwok JB, Healey CS, Elsdon MJ, Eng C, Gardner E, Love DR, Mole SE, Moore JK, Papi L, et al. . Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature. 1993;363(6428):458–460. [DOI] [PubMed] [Google Scholar]
- 61.Santoro M, Carlomagno F, Romano A, Bottaro DP, Dathan NA, Grieco M, Fusco A, Vecchio G, Matoskova B, Kraus MH, et al. . Activation of RET as a dominant transforming gene by germline mutations of MEN2A and MEN2B. Science. 1995;267(5196):381–383. [DOI] [PubMed] [Google Scholar]
- 62.Wallace MR, Marchuk DA, Andersen LB, Letcher R, Odeh HM, Saulino AM, Fountain JW, Brereton A, Nicholson J, Mitchell AL, et al. . Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science. 1990;249(4965):181–186. [DOI] [PubMed] [Google Scholar]
- 63.Qin Y, Yao L, King EE, Buddavarapu K, Lenci RE, Chocron ES, Lechleiter JD, Sass M, Aronin N, Schiavi F, Boaretto F, Opocher G, Toledo RA, Toledo SP, Stiles C, Aguiar RC, Dahia PL. Germline mutations in TMEM127 confer susceptibility to pheochromocytoma. Nat Genet. 2010;42(3):229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Comino-Méndez I, Gracia-Aznárez FJ, Schiavi F, Landa I, Leandro-García LJ, Letón R, Honrado E, Ramos-Medina R, Caronia D, Pita G, Gómez-Graña A, de Cubas AA, Inglada-Pérez L, Maliszewska A, Taschin E, Bobisse S, Pica G, Loli P, Hernández-Lavado R, Díaz JA, Gómez-Morales M, González-Neira A, Roncador G, Rodríguez-Antona C, Benítez J, Mannelli M, Opocher G, Robledo M, Cascón A. Exome sequencing identifies MAX mutations as a cause of hereditary pheochromocytoma. Nat Genet. 2011;43(7):663–667. [DOI] [PubMed] [Google Scholar]
- 65.Califano D, Rizzo C, D’Alessio A, Colucci-D’Amato GL, Cali G, Bartoli PC, Santelli G, Vecchio G, de Franciscis V. Signaling through Ras is essential for ret oncogene-induced cell differentiation in PC12 cells. J Biol Chem. 2000;275(25):19297–19305. [DOI] [PubMed] [Google Scholar]
- 66.Martin GA, Viskochil D, Bollag G, McCabe PC, Crosier WJ, Haubruck H, Conroy L, Clark R, O’Connell P, Cawthon RM, et al. . The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell. 1990;63(4):843–849. [DOI] [PubMed] [Google Scholar]
- 67.Segouffin-Cariou C, Billaud M. Transforming ability of MEN2A-RET requires activation of the phosphatidylinositol 3-kinase/AKT signaling pathway. J Biol Chem. 2000;275(5):3568–3576. [DOI] [PubMed] [Google Scholar]
- 68.Qin Y, Deng Y, Ricketts CJ, Srikantan S, Wang E, Maher ER, Dahia PL. The tumor susceptibility gene TMEM127 is mutated in renal cell carcinomas and modulates endolysosomal function. Hum Mol Genet. 2014;23(9):2428–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burnichon N, Cascón A, Schiavi F, Morales NP, Comino-Méndez I, Abermil N, Inglada-Pérez L, de Cubas AA, Amar L, Barontini M, de Quirós SB, Bertherat J, Bignon YJ, Blok MJ, Bobisse S, Borrego S, Castellano M, Chanson P, Chiara MD, Corssmit EP, Giacchè M, de Krijger RR, Ercolino T, Girerd X, Gómez-García EB, Gómez-Graña A, Guilhem I, Hes FJ, Honrado E, Korpershoek E, Lenders JW, Letón R, Mensenkamp AR, Merlo A, Mori L, Murat A, Pierre P, Plouin PF, Prodanov T, Quesada-Charneco M, Qin N, Rapizzi E, Raymond V, Reisch N, Roncador G, Ruiz-Ferrer M, Schillo F, Stegmann AP, Suarez C, Taschin E, Timmers HJ, Tops CM, Urioste M, Beuschlein F, Pacak K, Mannelli M, Dahia PL, Opocher G, Eisenhofer G, Gimenez-Roqueplo AP, Robledo M. MAX mutations cause hereditary and sporadic pheochromocytoma and paraganglioma. Clin Cancer Res. 2012;18(10):2828–2837. [DOI] [PubMed] [Google Scholar]
- 70.Crona J, Delgado Verdugo A, Maharjan R, Stålberg P, Granberg D, Hellman P, Björklund P. Somatic mutations in H-RAS in sporadic pheochromocytoma and paraganglioma identified by exome sequencing. J Clin Endocrinol Metab. 2013;98(7):E1266–E1271. [DOI] [PubMed] [Google Scholar]
- 71.Castro-Vega LJ, Letouzé E, Burnichon N, Buffet A, Disderot PH, Khalifa E, Loriot C, Elarouci N, Morin A, Menara M, Lepoutre-Lussey C, Badoual C, Sibony M, Dousset B, Libé R, Zinzindohoue F, Plouin PF, Bertherat J, Amar L, de Reyniès A, Favier J, Gimenez-Roqueplo AP. Multi-omics analysis defines core genomic alterations in pheochromocytomas and paragangliomas. Nat Commun. 2015;6:6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Toledo RA, Qin Y, Cheng ZM, Gao Q, Iwata S, Silva GM, Prasad ML, Ocal IT, Rao S, Aronin N, Barontini M, Bruder J, Reddick RL, Chen Y, Aguiar RC, Dahia PL. Recurrent mutations of chromatin-remodeling genes and kinase receptors in pheochromocytomas and paragangliomas. Clin Cancer Res. 2016;22(9):2301–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schlisio S, Kenchappa RS, Vredeveld LC, George RE, Stewart R, Greulich H, Shahriari K, Nguyen NV, Pigny P, Dahia PL, Pomeroy SL, Maris JM, Look AT, Meyerson M, Peeper DS, Carter BD, Kaelin WG Jr. The kinesin KIF1Bbeta acts downstream from EglN3 to induce apoptosis and is a potential 1p36 tumor suppressor. Genes Dev. 2008;22(7):884–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Flynn A, Benn D, Clifton-Bligh R, Robinson B, Trainer AH, James P, Hogg A, Waldeck K, George J, Li J, Fox SB, Gill AJ, McArthur G, Hicks RJ, Tothill RW. The genomic landscape of phaeochromocytoma. J Pathol. 2015;236(1):78–89. [DOI] [PubMed] [Google Scholar]
- 75.Zheng S, Cherniack AD, Dewal N, Moffitt RA, Danilova L, Murray BA, Lerario AM, Else T, Knijnenburg TA, Ciriello G, Kim S, Assie G, Morozova O, Akbani R, Shih J, Hoadley KA, Choueiri TK, Waldmann J, Mete O, Robertson AG, Wu HT, Raphael BJ, Shao L, Meyerson M, Demeure MJ, Beuschlein F, Gill AJ, Sidhu SB, Almeida MQ, Fragoso MC, Cope LM, Kebebew E, Habra MA, Whitsett TG, Bussey KJ, Rainey WE, Asa SL, Bertherat J, Fassnacht M, Wheeler DA, Hammer GD, Giordano TJ, Verhaak RG; Cancer Genome Atlas Research Network . Comprehensive pan-genomic characterization of adrenocortical carcinoma [published correction appears in Cancer Cell. 2016;30(2):363]. Cancer Cell. 2016;29(5):723–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lawrence MS, Stojanov P, Mermel CH, Robinson JT, Garraway LA, Golub TR, Meyerson M, Gabriel SB, Lander ES, Getz G. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505(7484):495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fishbein L, Khare S, Wubbenhorst B, DeSloover D, D’Andrea K, Merrill S, Cho NW, Greenberg RA, Else T, Montone K, LiVolsi V, Fraker D, Daber R, Cohen DL, Nathanson KL. Whole-exome sequencing identifies somatic ATRX mutations in pheochromocytomas and paragangliomas. Nat Commun. 2015;6:6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Juhlin CC, Stenman A, Haglund F, Clark VE, Brown TC, Baranoski J, Bilguvar K, Goh G, Welander J, Svahn F, Rubinstein JC, Caramuta S, Yasuno K, Günel M, Bäckdahl M, Gimm O, Söderkvist P, Prasad ML, Korah R, Lifton RP, Carling T. Whole-exome sequencing defines the mutational landscape of pheochromocytoma and identifies KMT2D as a recurrently mutated gene. Genes Chromosomes Cancer. 2015;54(9):542–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Papathomas TG, Oudijk L, Zwarthoff EC, Post E, Duijkers FA, van Noesel MM, Hofland LJ, Pollard PJ, Maher ER, Restuccia DF, Feelders RA, Franssen GJ, Timmers HJ, Sleijfer S, de Herder WW, de Krijger RR, Dinjens WN, Korpershoek E. Telomerase reverse transcriptase promoter mutations in tumors originating from the adrenal gland and extra-adrenal paraganglia. Endocr Relat Cancer. 2014;21(4):653–661. [DOI] [PubMed] [Google Scholar]
- 80.Björklund P, Pacak K, Crona J. Precision medicine in pheochromocytoma and paraganglioma: current and future concepts. J Intern Med. 2016;280(6):559–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Crona J, Backman S, Maharjan R, Mayrhofer M, Stålberg P, Isaksson A, Hellman P, Björklund P. Spatiotemporal heterogeneity characterizes the genetic landscape of pheochromocytoma and defines early events in tumorigenesis. Clin Cancer Res. 2015;21(19):4451–4460. [DOI] [PubMed] [Google Scholar]
- 82.Killian JK, Kim SY, Miettinen M, Smith C, Merino M, Tsokos M, Quezado M, Smith WI Jr, Jahromi MS, Xekouki P, Szarek E, Walker RL, Lasota J, Raffeld M, Klotzle B, Wang Z, Jones L, Zhu Y, Wang Y, Waterfall JJ, O’Sullivan MJ, Bibikova M, Pacak K, Stratakis C, Janeway KA, Schiffman JD, Fan JB, Helman L, Meltzer PS. Succinate dehydrogenase mutation underlies global epigenomic divergence in gastrointestinal stromal tumor. Cancer Discov. 2013;3(6):648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de Cubas AA, Korpershoek E, Inglada-Pérez L, Letouzé E, Currás-Freixes M, Fernández AF, Comino-Méndez I, Schiavi F, Mancikova V, Eisenhofer G, Mannelli M, Opocher G, Timmers H, Beuschlein F, de Krijger R, Cascon A, Rodríguez-Antona C, Fraga MF, Favier J, Gimenez-Roqueplo AP, Robledo M. DNA methylation profiling in pheochromocytoma and paraganglioma reveals diagnostic and prognostic markers. Clin Cancer Res. 2015;21(13):3020–3030. [DOI] [PubMed] [Google Scholar]
- 84.Backman S, Maharjan R, Falk-Delgado A, Crona J, Cupisti K, Stålberg P, Hellman P, Björklund P. Global DNA methylation analysis identifies two discrete clusters of pheochromocytoma with distinct genomic and genetic alterations. Sci Rep. 2017;7:44943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dahia PL, Ross KN, Wright ME, Hayashida CY, Santagata S, Barontini M, Kung AL, Sanso G, Powers JF, Tischler AS, Hodin R, Heitritter S, Moore F, Dluhy R, Sosa JA, Ocal IT, Benn DE, Marsh DJ, Robinson BG, Schneider K, Garber J, Arum SM, Korbonits M, Grossman A, Pigny P, Toledo SP, Nosé V, Li C, Stiles CDA. A HIF1alpha regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 2005;1(1):72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eisenhofer G, Huynh TT, Pacak K, Brouwers FM, Walther MM, Linehan WM, Munson PJ, Mannelli M, Goldstein DS, Elkahloun AG. Distinct gene expression profiles in norepinephrine- and epinephrine-producing hereditary and sporadic pheochromocytomas: activation of hypoxia-driven angiogenic pathways in von Hippel–Lindau syndrome. Endocr Relat Cancer. 2004;11(4):897–911. [DOI] [PubMed] [Google Scholar]
- 87.Elston MS, Meyer-Rochow GY, Conaglen HM, Clarkson A, Clifton-Bligh RJ, Conaglen JV, Gill AJ. Increased SSTR2A and SSTR3 expression in succinate dehydrogenase-deficient pheochromocytomas and paragangliomas. Hum Pathol. 2015;46(3):390–396. [DOI] [PubMed] [Google Scholar]
- 88.Epelbaum J, Bertherat J, Prevost G, Kordon C, Meyerhof W, Wulfsen I, Richter D, Plouin PF. Molecular and pharmacological characterization of somatostatin receptor subtypes in adrenal, extraadrenal, and malignant pheochromocytomas. J Clin Endocrinol Metab. 1995;80(6):1837–1844. [DOI] [PubMed] [Google Scholar]
- 89.Reubi JC, Waser B, Khosla S, Kvols L, Goellner JR, Krenning E, Lamberts S. In vitro and in vivo detection of somatostatin receptors in pheochromocytomas and paragangliomas. J Clin Endocrinol Metab. 1992;74(5):1082–1089. [DOI] [PubMed] [Google Scholar]
- 90.Janssen I, Blanchet EM, Adams K, Chen CC, Millo CM, Herscovitch P, Taieb D, Kebebew E, Lehnert H, Fojo AT, Pacak K. Superiority of [68Ga]-DOTATATE PET/CT to other functional imaging modalities in the localization of SDHB-associated metastatic pheochromocytoma and paraganglioma. Clin Cancer Res. 2015;21(17):3888–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Janssen I, Chen CC, Taieb D, Patronas NJ, Millo CM, Adams KT, Nambuba J, Herscovitch P, Sadowski SM, Fojo AT, Buchmann I, Kebebew E, Pacak K. 68Ga-DOTATATE PET/CT in the localization of head and neck paragangliomas compared with other functional imaging modalities and CT/MRI. J Nucl Med. 2016;57:186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Janssen I, Chen CC, Millo CM, Ling A, Taieb D, Lin FI, Adams KT, Wolf KI, Herscovitch P, Fojo AT, Buchmann I, Kebebew E, Pacak K. PET/CT comparing (68)Ga-DOTATATE and other radiopharmaceuticals and in comparison with CT/MRI for the localization of sporadic metastatic pheochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2016;43(10):1784–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hofman MS, Kong G, Neels OC, Eu P, Hong E, Hicks RJ. High management impact of Ga-68 DOTATATE (GaTate) PET/CT for imaging neuroendocrine and other somatostatin expressing tumours. J Med Imaging Radiat Oncol. 2012;56(1):40–47. [DOI] [PubMed] [Google Scholar]
- 94.Mundschenk J, Unger N, Schulz S, Höllt V, Schulz S, Steinke R, Lehnert H. Somatostatin receptor subtypes in human pheochromocytoma: subcellular expression pattern and functional relevance for octreotide scintigraphy. J Clin Endocrinol Metab. 2003;88(11):5150–5157. [DOI] [PubMed] [Google Scholar]
- 95.van der Harst E, de Herder WW, Bruining HA, Bonjer HJ, de Krijger RR, Lamberts SW, van de Meiracker AH, Boomsma F, Stijnen T, Krenning EP, Bosman FT, Kwekkeboom DJ. [(123)I]metaiodobenzylguanidine and [(111)In]octreotide uptake in benign and malignant pheochromocytomas. J Clin Endocrinol Metab. 2001;86(2):685–693. [DOI] [PubMed] [Google Scholar]
- 96.Eisenhofer G, Pacak K, Huynh TT, Qin N, Bratslavsky G, Linehan WM, Mannelli M, Friberg P, Grebe SK, Timmers HJ, Bornstein SR, Lenders JW. Catecholamine metabolomic and secretory phenotypes in phaeochromocytoma. Endocr Relat Cancer. 2010;18(1):97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Timmers HJ, Kozupa A, Eisenhofer G, Raygada M, Adams KT, Solis D, Lenders JW, Pacak K. Clinical presentations, biochemical phenotypes, and genotype-phenotype correlations in patients with succinate dehydrogenase subunit B-associated pheochromocytomas and paragangliomas. J Clin Endocrinol Metab. 2007;92(3):779–786. [DOI] [PubMed] [Google Scholar]
- 98.Eisenhofer G, Goldstein DS, Sullivan P, Csako G, Brouwers FM, Lai EW, Adams KT, Pacak K. Biochemical and clinical manifestations of dopamine-producing paragangliomas: utility of plasma methoxytyramine. J Clin Endocrinol Metab. 2005;90(4):2068–2075. [DOI] [PubMed] [Google Scholar]
- 99.Eisenhofer G, Lenders JW, Timmers H, Mannelli M, Grebe SK, Hofbauer LC, Bornstein SR, Tiebel O, Adams K, Bratslavsky G, Linehan WM, Pacak K. Measurements of plasma methoxytyramine, normetanephrine, and metanephrine as discriminators of different hereditary forms of pheochromocytoma. Clin Chem. 2011;57:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Badenhop RF, Jansen JC, Fagan PA, Lord RS, Wang ZG, Foster WJ, Schofield PR. The prevalence of SDHB, SDHC, and SDHD mutations in patients with head and neck paraganglioma and association of mutations with clinical features. J Med Genet. 2004;41(7):e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jochmanova I, Pacak K. Pheochromocytoma: the first metabolic endocrine cancer. Clin Cancer Res. 2016;22(20):5001–5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lussey-Lepoutre C, Hollinshead KE, Ludwig C, Menara M, Morin A, Castro-Vega LJ, Parker SJ, Janin M, Martinelli C, Ottolenghi C, Metallo C, Gimenez-Roqueplo AP, Favier J, Tennant DA. Loss of succinate dehydrogenase activity results in dependency on pyruvate carboxylation for cellular anabolism. Nat Commun. 2015;6:8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Favier J, Brière JJ, Burnichon N, Rivière J, Vescovo L, Benit P, Giscos-Douriez I, De Reyniès A, Bertherat J, Badoual C, Tissier F, Amar L, Libé R, Plouin PF, Jeunemaitre X, Rustin P, Gimenez-Roqueplo AP. The Warburg effect is genetically determined in inherited pheochromocytomas. PLoS One. 2009;4(9):e7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Berkel A, Rao JU, Kusters B, Demir T, Visser E, Mensenkamp AR, van der Laak JA, Oosterwijk E, Lenders JW, Sweep FC, Wevers RA, Hermus AR, Langenhuijsen JF, Kunst DP, Pacak K, Gotthardt M, Timmers HJ. Correlation between in vivo 18F-FDG PET and immunohistochemical markers of glucose uptake and metabolism in pheochromocytoma and paraganglioma. J Nucl Med. 2014;55:1253–1259. [DOI] [PubMed] [Google Scholar]
- 105.Timmers HJ, Kozupa A, Chen CC, Carrasquillo JA, Ling A, Eisenhofer G, Adams KT, Solis D, Lenders JW, Pacak K. Superiority of fluorodeoxyglucose positron emission tomography to other functional imaging techniques in the evaluation of metastatic SDHB-associated pheochromocytoma and paraganglioma. J Clin Oncol. 2007;25(16):2262–2269. [DOI] [PubMed] [Google Scholar]
- 106.Lam AK. Update on adrenal tumours in 2017 World Health Organization (WHO) of endocrine tumours. Endocr Pathol. 2017. [DOI] [PubMed]
- 107.Eisenhofer G, Huynh TT, Hiroi M, Pacak K. Understanding catecholamine metabolism as a guide to the biochemical diagnosis of pheochromocytoma. Rev Endocr Metab Disord. 2001;2(3):297–311. [DOI] [PubMed] [Google Scholar]
- 108.Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366(9486):665–675. [DOI] [PubMed] [Google Scholar]
- 109.Eisenhofer G, Walther MM, Huynh TT, Li ST, Bornstein SR, Vortmeyer A, Mannelli M, Goldstein DS, Linehan WM, Lenders JW, Pacak K. Pheochromocytomas in von Hippel–Lindau syndrome and multiple endocrine neoplasia type 2 display distinct biochemical and clinical phenotypes. J Clin Endocrinol Metab. 2001;86(5):1999–2008. [DOI] [PubMed] [Google Scholar]
- 110.Lloyd RV, Osamura RY, Kloppel G, Rosai J. WHO Classification of Tumours: Pathology and Genetics of Tumours of Endocrine Organs. 4th ed. Lyon, France: IARC; 2017. [Google Scholar]
- 111.DeLellis R, Lloyd R, Heitz P, Eng C. Pathology and Genetics of Tumours of Endocrine Organs (IARC/World Health Organization Classification of Tumours). Lyon, France: IARC Press; 2004. [Google Scholar]
- 112.Jimenez C, Libutti SK, Landry CS, Lloyd RV, McKay RR, Rohren E, Seethala RR, Wang TS, Chen H, Perrier ND, Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR, eds. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2016:919–927. [Google Scholar]
- 113.Roman-Gonzalez A, Jimenez C. Malignant pheochromocytoma–paraganglioma: pathogenesis, TNM staging, and current clinical trials. Curr Opin Endocrinol Diabetes Obes. 2017;24(3):174–183. [DOI] [PubMed] [Google Scholar]
- 114.Eisenhofer G, Peitzsch M. Laboratory evaluation of pheochromocytoma and paraganglioma. Clin Chem. 2014;60(12):1486–1499. [DOI] [PubMed] [Google Scholar]
- 115.Plouin PF, Amar L, Dekkers OM, Fassnacht M, Gimenez-Roqueplo AP, Lenders JW, Lussey-Lepoutre C, Steichen O; Guideline Working Group . European Society of Endocrinology Clinical Practice Guideline for long-term follow-up of patients operated on for a phaeochromocytoma or a paraganglioma. Eur J Endocrinol. 2016;174(5):G1–G10. [DOI] [PubMed] [Google Scholar]
- 116.Toledo RA, Burnichon N, Cascon A, Benn DE, Bayley JP, Welander J, Tops CM, Firth H, Dwight T, Ercolino T, Mannelli M, Opocher G, Clifton-Bligh R, Gimm O, Maher ER, Robledo M, Gimenez-Roqueplo AP, Dahia PL. Consensus Statement on next-generation-sequencing-based diagnostic testing of hereditary phaeochromocytomas and paragangliomas. Nat Rev Endocrinol. 2017;13(4):233–247. [DOI] [PubMed] [Google Scholar]
- 117.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372(9):793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zuber S, Wesley R, Prodanov T, Eisenhofer G, Pacak K, Kantorovich V. Clinical utility of chromogranin A in SDHx-related paragangliomas. Eur J Clin Invest. 2014;44(4):365–371. [DOI] [PubMed] [Google Scholar]
- 119.Hsiao RJ, Parmer RJ, Takiyyuddin MA, O’Connor DT. Chromogranin A storage and secretion: sensitivity and specificity for the diagnosis of pheochromocytoma. Medicine (Baltimore). 1991;70(1):33–45. [PubMed] [Google Scholar]
- 120.Weise M, Merke DP, Pacak K, Walther MM, Eisenhofer G. Utility of plasma free metanephrines for detecting childhood pheochromocytoma. J Clin Endocrinol Metab. 2002;87(5):1955–1960. [DOI] [PubMed] [Google Scholar]
- 121.Lenders JW, Pacak K, Walther MM, Linehan WM, Mannelli M, Friberg P, Keiser HR, Goldstein DS, Eisenhofer G. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287(11):1427–1434. [DOI] [PubMed] [Google Scholar]
- 122.Eisenhofer G, Lenders JW, Linehan WM, Walther MM, Goldstein DS, Keiser HR. Plasma normetanephrine and metanephrine for detecting pheochromocytoma in von Hippel–Lindau disease and multiple endocrine neoplasia type 2. N Engl J Med. 1999;340(24):1872–1879. [DOI] [PubMed] [Google Scholar]
- 123.Pacak K, Ilias I, Adams KT, Eisenhofer G. Biochemical diagnosis, localization and management of pheochromocytoma: focus on multiple endocrine neoplasia type 2 in relation to other hereditary syndromes and sporadic forms of the tumour. J Intern Med. 2005;257(1):60–68. [DOI] [PubMed] [Google Scholar]
- 124.Timmers HJ, Pacak K, Huynh TT, Abu-Asab M, Tsokos M, Merino MJ, Baysal BE, Adams KT, Eisenhofer G. Biochemically silent abdominal paragangliomas in patients with mutations in the succinate dehydrogenase subunit B gene. J Clin Endocrinol Metab. 2008;93(12):4826–4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Haissaguerre M, Courel M, Caron P, Denost S, Dubessy C, Gosse P, Appavoupoulle V, Belleannée G, Jullié ML, Montero-Hadjadje M, Yon L, Corcuff JB, Fagour C, Mazerolles C, Wagner T, Nunes ML, Anouar Y, Tabarin A. Normotensive incidentally discovered pheochromocytomas display specific biochemical, cellular, and molecular characteristics. J Clin Endocrinol Metab. 2013;98(11):4346–4354. [DOI] [PubMed] [Google Scholar]
- 126.Qin N, de Cubas AA, Garcia-Martin R, Richter S, Peitzsch M, Menschikowski M, Lenders JW, Timmers HJ, Mannelli M, Opocher G, Economopoulou M, Siegert G, Chavakis T, Pacak K, Robledo M, Eisenhofer G. Opposing effects of HIF1alpha and HIF2alpha on chromaffin cell phenotypic features and tumor cell proliferation: insights from MYC-associated factor X. Int J Cancer. 2014;135:2054–2064. [DOI] [PubMed] [Google Scholar]
- 127.Huber K. The sympathoadrenal cell lineage: specification, diversification, and new perspectives. Dev Biol. 2006;298(2):335–343. [DOI] [PubMed] [Google Scholar]
- 128.Axelrod J. Methylation reactions in the formation and metabolism of catecholamines and other biogenic amines. Pharmacol Rev. 1966;18(1):95–113. [PubMed] [Google Scholar]
- 129.Sue M, Martucci V, Frey F, Lenders JM, Timmers HJ, Peczkowska M, Prejbisz A, Swantje B, Bornstein SR, Arlt W, Fassnacht M, Beuschlein F, Robledo M, Pacak K, Eisenhofer G. Lack of utility of SDHB mutation testing in adrenergic metastatic phaeochromocytoma. Eur J Endocrinol. 2015;172(2):89–95. [DOI] [PubMed] [Google Scholar]
- 130.Schovanek J, Martucci V, Wesley R, Fojo T, Del Rivero J, Huynh T, Adams K, Kebebew E, Frysak Z, Stratakis CA, Pacak K. The size of the primary tumor and age at initial diagnosis are independent predictors of the metastatic behavior and survival of patients with SDHB-related pheochromocytoma and paraganglioma: a retrospective cohort study. BMC Cancer. 2014;14:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Blaschko H. The specific action of L-dopa decarboxylase. J Physiol. 1939;96:50P–51P. [Google Scholar]
- 132.Feldman JM, Blalock JA, Zern RT, Shelburne JD, Gaede JT, Farrell RE, Wells SA Jr. Deficiency of dopamine-beta-hydroxylase. A new mechanism for normotensive pheochromocytomas. Am J Clin Pathol. 1979;72(2):175–185. [DOI] [PubMed] [Google Scholar]
- 133.Eisenhofer G, Keiser H, Friberg P, Mezey E, Huynh TT, Hiremagalur B, Ellingson T, Duddempudi S, Eijsbouts A, Lenders JW. Plasma metanephrines are markers of pheochromocytoma produced by catechol-O-methyltransferase within tumors. J Clin Endocrinol Metab. 1998;83(6):2175–2185. [DOI] [PubMed] [Google Scholar]
- 134.Eisenhofer G, Lenders JW, Siegert G, Bornstein SR, Friberg P, Milosevic D, Mannelli M, Linehan WM, Adams K, Timmers HJ, Pacak K. Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer. 2012;48:1739–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Carney JA, Sheps SG, Go VL, Gordon H. The triad of gastric leiomyosarcoma, functioning extra-adrenal paraganglioma and pulmonary chondroma. N Engl J Med. 1977;296(26):1517–1518. [DOI] [PubMed] [Google Scholar]
- 136.McWhinney SR, Pasini B, Stratakis CA; International Carney Triad and Carney-Stratakis Syndrome Consortium . Familial gastrointestinal stromal tumors and germ-line mutations. N Engl J Med. 2007;357(10):1054–1056. [DOI] [PubMed] [Google Scholar]
- 137.Boikos SA, Pappo AS, Killian JK, LaQuaglia MP, Weldon CB, George S, Trent JC, von Mehren M, Wright JA, Schiffman JD, Raygada M, Pacak K, Meltzer PS, Miettinen MM, Stratakis C, Janeway KA, Helman LJ. Molecular subtypes of KIT/PDGFRA wild-type gastrointestinal stromal tumors: a report from the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol. 2016;2(7):922–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Killian JK, Miettinen M, Walker RL, Wang Y, Zhu YJ, Waterfall JJ, Noyes N, Retnakumar P, Yang Z, Smith WI Jr, Killian MS, Lau CC, Pineda M, Walling J, Stevenson H, Smith C, Wang Z, Lasota J, Kim SY, Boikos SA, Helman LJ, Meltzer PS. Recurrent epimutation of SDHC in gastrointestinal stromal tumors. Sci Transl Med. 2014;6(268):268ra177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Haller F, Moskalev EA, Faucz FR, Barthelmeß S, Wiemann S, Bieg M, Assie G, Bertherat J, Schaefer IM, Otto C, Rattenberry E, Maher ER, Ströbel P, Werner M, Carney JA, Hartmann A, Stratakis CA, Agaimy A. Aberrant DNA hypermethylation of SDHC: a novel mechanism of tumor development in Carney triad. Endocr Relat Cancer. 2014;21(4):567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bausch B, Schiavi F, Ni Y, Welander J, Patocs A, Ngeow J, Wellner U, Malinoc A, Taschin E, Barbon G, Lanza V, Söderkvist P, Stenman A, Larsson C, Svahn F, Chen JL, Marquard J, Fraenkel M, Walter MA, Peczkowska M, Prejbisz A, Jarzab B, Hasse-Lazar K, Petersenn S, Moeller LC, Meyer A, Reisch N, Trupka A, Brase C, Galiano M, Preuss SF, Kwok P, Lendvai N, Berisha G, Makay Ö, Boedeker CC, Weryha G, Racz K, Januszewicz A, Walz MK, Gimm O, Opocher G, Eng C, Neumann HP; European-American-Asian Pheochromocytoma-Paraganglioma Registry Study Group . Clinical characterization of the pheochromocytoma and paraganglioma susceptibility genes SDHA, TMEM127, MAX, and SDHAF2 for gene-informed prevention. JAMA Oncol. 2017. [DOI] [PMC free article] [PubMed]
- 141.Tufton N, Ghelani R, Srirangalingam U, Kumar AV, Drake WM, Iacovazzo D, Skordilis K, Berney D, Al-Mrayat M, Khoo B, Akker SA. SDHA mutated paragangliomas may be at high risk of metastasis. Endocr Relat Cancer. 2017;24(7):L43–L49. [DOI] [PubMed] [Google Scholar]
- 142.Korpershoek E, Favier J, Gaal J, Burnichon N, van Gessel B, Oudijk L, Badoual C, Gadessaud N, Venisse A, Bayley JP, van Dooren MF, de Herder WW, Tissier F, Plouin PF, van Nederveen FH, Dinjens WN, Gimenez-Roqueplo AP, de Krijger RR. SDHA immunohistochemistry detects germline SDHA gene mutations in apparently sporadic paragangliomas and pheochromocytomas. J Clin Endocrinol Metab. 2011;96(9):E1472–E1476. [DOI] [PubMed] [Google Scholar]
- 143.Pantaleo MA, Astolfi A, Indio V, Moore R, Thiessen N, Heinrich MC, Gnocchi C, Santini D, Catena F, Formica S, Martelli PL, Casadio R, Pession A, Biasco G. SDHA loss-of-function mutations in KIT-PDGFRA wild-type gastrointestinal stromal tumors identified by massively parallel sequencing. J Natl Cancer Inst. 2011;103:983–987. [DOI] [PubMed] [Google Scholar]
- 144.Benn DE, Gimenez-Roqueplo AP, Reilly JR, Bertherat J, Burgess J, Byth K, Croxson M, Dahia PL, Elston M, Gimm O, Henley D, Herman P, Murday V, Niccoli-Sire P, Pasieka JL, Rohmer V, Tucker K, Jeunemaitre X, Marsh DJ, Plouin PF, Robinson BG. Clinical presentation and penetrance of pheochromocytoma/paraganglioma syndromes. J Clin Endocrinol Metab. 2006;91(3):827–836. [DOI] [PubMed] [Google Scholar]
- 145.Ricketts CJ, Forman JR, Rattenberry E, Bradshaw N, Lalloo F, Izatt L, Cole TR, Armstrong R, Kumar VK, Morrison PJ, Atkinson AB, Douglas F, Ball SG, Cook J, Srirangalingam U, Killick P, Kirby G, Aylwin S, Woodward ER, Evans DG, Hodgson SV, Murday V, Chew SL, Connell JM, Blundell TL, Macdonald F, Maher ER. Tumor risks and genotype–phenotype–proteotype analysis in 358 patients with germline mutations in SDHB and SDHD. Hum Mutat. 2010;31(1):41–51. [DOI] [PubMed] [Google Scholar]
- 146.Schiavi F, Milne RL, Anda E, Blay P, Castellano M, Opocher G, Robledo M, Cascón A. Are we overestimating the penetrance of mutations in SDHB? Hum Mutat. 2010;31(6):761–762. [DOI] [PubMed] [Google Scholar]
- 147.Rijken JA, Niemeijer ND, Corssmit EP, Jonker MA, Leemans CR, Menko FH, Hensen EF. Low penetrance of paraganglioma and pheochromocytoma in an extended kindred with a germline SDHB exon 3 deletion. Clin Genet. 2016;89(1):128–132. [DOI] [PubMed] [Google Scholar]
- 148.Solis DC, Burnichon N, Timmers HJ, Raygada MJ, Kozupa A, Merino MJ, Makey D, Adams KT, Venisse A, Gimenez-Roqueplo AP, Pacak K. Penetrance and clinical consequences of a gross SDHB deletion in a large family. Clin Genet. 2009;75(4):354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rijken JA, Niemeijer ND, Jonker MA, Eijkelenkamp K, Jansen JC, van Berkel A, Kunst HPM, Bisschop P, Kerstens MN, Dreijerink KMA, van Dooren MF, van der Horst-Schrivers ANA, Hes FJ, Rene Leemans C, Corssmit EPM, Hensen EF. The penetrance of paraganglioma and pheochromocytoma in SDHB germline mutation carriers. Clin Genet. 2017. [DOI] [PubMed]
- 150.Jochmanova I, Wolf KI, King KS, Nambuba J, Wesley R, Martucci V, Raygada M, Adams KT, Prodanov T, Fojo AT, Lazurova I, Pacak K. SDHB-related pheochromocytoma and paraganglioma penetrance and genotype-phenotype correlations. J Cancer Res Clin Oncol. 2017;143(8):1421–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Niemann S, Steinberger D, Müller U. PGL3, a third, not maternally imprinted locus in autosomal dominant paraganglioma. Neurogenetics. 1999;2(3):167–170. [DOI] [PubMed] [Google Scholar]
- 152.Hensen EF, van Duinen N, Jansen JC, Corssmit EP, Tops CM, Romijn JA, Vriends AH, van der Mey AG, Cornelisse CJ, Devilee P, Bayley JP. High prevalence of founder mutations of the succinate dehydrogenase genes in the Netherlands. Clin Genet. 2012;81:284–288. [DOI] [PubMed] [Google Scholar]
- 153.Mannelli M, Ercolino T, Giachè V, Simi L, Cirami C, Parenti G. Genetic screening for pheochromocytoma: should SDHC gene analysis be included? J Med Genet. 2007;44(9):586–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.van Baars F, Cremers C, van den Broek P, Geerts S, Veldman J. Genetic aspects of nonchromaffin paraganglioma. Hum Genet. 1982;60(4):305–309. [DOI] [PubMed] [Google Scholar]
- 155.Bayley JP, Kunst HP, Cascon A, Sampietro ML, Gaal J, Korpershoek E, Hinojar-Gutierrez A, Timmers HJ, Hoefsloot LH, Hermsen MA, Suárez C, Hussain AK, Vriends AH, Hes FJ, Jansen JC, Tops CM, Corssmit EP, de Knijff P, Lenders JW, Cremers CW, Devilee P, Dinjens WN, de Krijger RR, Robledo M. SDHAF2 mutations in familial and sporadic paraganglioma and phaeochromocytoma. Lancet Oncol. 2010;11(4):366–372. [DOI] [PubMed] [Google Scholar]
- 156.Casey R, Garrahy A, Tuthill A, O’Halloran D, Joyce C, Casey MB, O’Shea P, Bell M. Universal genetic screening uncovers a novel presentation of an SDHAF2 mutation. J Clin Endocrinol Metab. 2014;99(7):E1392–E1396. [DOI] [PubMed] [Google Scholar]
- 157.Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D, Leigh I, Gorman P, Lamlum H, Rahman S, Roylance RR, Olpin S, Bevan S, Barker K, Hearle N, Houlston RS, Kiuru M, Lehtonen R, Karhu A, Vilkki S, Laiho P, Eklund C, Vierimaa O, Aittomäki K, Hietala M, Sistonen P, Paetau A, Salovaara R, Herva R, Launonen V, Aaltonen LA; Multiple Leiomyoma Consortium . Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30(4):406–410. [DOI] [PubMed] [Google Scholar]
- 158.Muller M, Ferlicot S, Guillaud-Bataille M, Le Teuff G, Genestie C, Deveaux S, Slama A, Poulalhon N, Escudier B, Albiges L, Soufir N, Avril MF, Gardie B, Saldana C, Allory Y, Gimenez-Roqueplo AP, Bressac-de Paillerets B, Richard S, Benusiglio PR. Reassessing the clinical spectrum associated with hereditary leiomyomatosis and renal cell carcinoma syndrome in French FH mutation carriers. Clin Genet. 2017. [DOI] [PubMed]
- 159.Aufforth RD, Ramakant P, Sadowski SM, Mehta A, Trebska-McGowan K, Nilubol N, Pacak K, Kebebew E. Pheochromocytoma screening initiation and frequency in von Hippel–Lindau syndrome. J Clin Endocrinol Metab. 2015;100(12):4498–4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nielsen SM, Rhodes L, Blanco I, Chung WK, Eng C, Maher ER, Richard S, Giles RH. Von Hippel–Lindau disease: genetics and role of genetic counseling in a multiple neoplasia syndrome. J Clin Oncol. 2016;34(18):2172–2181. [DOI] [PubMed] [Google Scholar]
- 161.Taïeb D, Yang C, Delenne B, Zhuang Z, Barlier A, Sebag F, Pacak K. First report of bilateral pheochromocytoma in the clinical spectrum of HIF2A-related polycythemia–paraganglioma syndrome. J Clin Endocrinol Metab. 2013;98(5):E908–E913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Buffet A, Smati S, Mansuy L, Ménara M, Lebras M, Heymann MF, Simian C, Favier J, Murat A, Cariou B, Gimenez-Roqueplo AP. Mosaicism in HIF2A-related polycythemia–paraganglioma syndrome. J Clin Endocrinol Metab. 2014;99(2):E369–E373. [DOI] [PubMed] [Google Scholar]
- 163.Därr R, Nambuba J, Del Rivero J, Janssen I, Merino M, Todorovic M, Balint B, Jochmanova I, Prchal JT, Lechan RM, Tischler AS, Popovic V, Miljic D, Adams KT, Prall FR, Ling A, Golomb MR, Ferguson M, Nilubol N, Chen CC, Chew E, Taïeb D, Stratakis CA, Fojo T, Yang C, Kebebew E, Zhuang Z, Pacak K. Novel insights into the polycythemia–paraganglioma–somatostatinoma syndrome. Endocr Relat Cancer. 2016;23(12):899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mukherjee S, Zakalik D. RET codon 804 mutations in multiple endocrine neoplasia 2: genotype–phenotype correlations and implications in clinical management. Clin Genet. 2011;79(1):1–16. [DOI] [PubMed] [Google Scholar]
- 165.Ponder BA. The phenotypes associated with ret mutations in the multiple endocrine neoplasia type 2 syndrome. Cancer Res. 1999;59:1736s–1741s; discussion 1742s. [PubMed] [Google Scholar]
- 166.Toledo SP, Lourenço DM Jr, Sekiya T, Lucon AM, Baena ME, Castro CC, Bortolotto LA, Zerbini MC, Siqueira SA, Toledo RA, Dahia PL. Penetrance and clinical features of pheochromocytoma in a six-generation family carrying a germline TMEM127 mutation. J Clin Endocrinol Metab. 2015;100(2):E308–E318. [DOI] [PubMed] [Google Scholar]
- 167.Yao L, Schiavi F, Cascon A, Qin Y, Inglada-Pérez L, King EE, Toledo RA, Ercolino T, Rapizzi E, Ricketts CJ, Mori L, Giacchè M, Mendola A, Taschin E, Boaretto F, Loli P, Iacobone M, Rossi GP, Biondi B, Lima-Junior JV, Kater CE, Bex M, Vikkula M, Grossman AB, Gruber SB, Barontini M, Persu A, Castellano M, Toledo SP, Maher ER, Mannelli M, Opocher G, Robledo M, Dahia PL. Spectrum and prevalence of FP/TMEM127 gene mutations in pheochromocytomas and paragangliomas. JAMA. 2010;304(23):2611–2619. [DOI] [PubMed] [Google Scholar]
- 168.Castinetti F, Kroiss A, Kumar R, Pacak K, Taieb D. 15 years of paraganglioma: imaging and imaging-based treatment of pheochromocytoma and paraganglioma. Endocr Relat Cancer. 2015;22(4):T135–T145. [DOI] [PubMed] [Google Scholar]
- 169.Janssen I, Chen CC, Zhuang Z, Millo CM, Wolf K, Ling A, Lin FI, Adams KT, Herscovitch P, Feelders RA, Fojo AT, Taieb D, Kebebew E, Pacak K. Functional imaging signature of patients presenting with polycythemia/paraganglioma syndromes. J Nucl Med. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jalil ND, Pattou FN, Combemale F, Chapuis Y, Henry JF, Peix JL, Proye CA. Effectiveness and limits of preoperative imaging studies for the localisation of pheochromocytomas and paragangliomas: a review of 282 cases. French Association of Surgery (AFC), and The French Association of Endocrine Surgeons (AFCE). Eur J Surg. 1998;164:23–28. [DOI] [PubMed] [Google Scholar]
- 171.Ganguly A, Henry DP, Yune HY, Pratt JH, Grim CE, Donohue JP, Weinberger MH. Diagnosis and localization of pheochromocytoma. Detection by measurement of urinary norepinephrine excretion during sleep, plasma norepinephrine concentration and computerized axial tomography (CT-scan). Am J Med. 1979;67(1):21–26. [DOI] [PubMed] [Google Scholar]
- 172.Shulkin BL, Koeppe RA, Francis IR, Deeb GM, Lloyd RV, Thompson NW. Pheochromocytomas that do not accumulate metaiodobenzylguanidine: localization with PET and administration of FDG. Radiology. 1993;186(3):711–715. [DOI] [PubMed] [Google Scholar]
- 173.Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Eisenhofer G, King KS, Rao JU, Wesley RA, Adams KT, Pacak K. Staging and functional characterization of pheochromocytoma and paraganglioma by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography. J Natl Cancer Inst. 2012;104(9):700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Naji M, Zhao C, Welsh SJ, Meades R, Win Z, Ferrarese A, Tan T, Rubello D, Al-Nahhas A. 68Ga-DOTA-TATE PET vs. 123I-MIBG in identifying malignant neural crest tumours. Mol Imaging Biol. 2011;13(4):769–775 [DOI] [PubMed] [Google Scholar]
- 175.Gimenez-Roqueplo AP, Caumont-Prim A, Houzard C, Hignette C, Hernigou A, Halimi P, Niccoli P, Leboulleux S, Amar L, Borson-Chazot F, Cardot-Bauters C, Delemer B, Chabolle F, Coupier I, Libe R, Peitzsch M, Peyrard S, Tenenbaum F, Plouin PF, Chatellier G, Rohmer V; EVA Investigators . Imaging work-up for screening of paraganglioma and pheochromocytoma in SDHx mutation carriers: a multicenter prospective study from the PGL.. J Clin Endocrinol Metab. 2013;98(1):E162–E173. [DOI] [PubMed] [Google Scholar]
- 176.Oberg K. Molecular imaging radiotherapy: theranostics for personalized patient management of neuroendocrine tumors (NETs). Theranostics. 2012;2(5):448–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Krenning EP, Valkema R, Kooij PP, Breeman WA, Bakker WH, de Herder WW, van Eijck CH, Kwekkeboom DJ, de Jong M, Jamar F, Pauwels S. The role of radioactive somatostatin and its analogues in the control of tumor growth. Recent Results Cancer Res. 2000;153:1–13. [DOI] [PubMed] [Google Scholar]
- 178.Krenning EP, de Jong M, Kooij PP, Breeman WA, Bakker WH, de Herder WW, van Eijck CH, Kwekkeboom DJ, Jamar F, Pauwels S, Valkema R. Radiolabelled somatostatin analogue(s) for peptide receptor scintigraphy and radionuclide therapy. Ann Oncol. 1999;10(suppl 2):S23–29. [DOI] [PubMed] [Google Scholar]
- 179.Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D, O’Dorisio TM, Baum RP, Kulkarni HR, Caplin M, Lebtahi R, Hobday T, Delpassand E, Van Cutsem E, Benson A, Srirajaskanthan R, Pavel M, Mora J, Berlin J, Grande E, Reed N, Seregni E, Öberg K, Lopera Sierra M, Santoro P, Thevenet T, Erion JL, Ruszniewski P, Kwekkeboom D, Krenning E; NETTER-1 Trial Investigators . Phase 3 trial of (177)Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376(2):125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Brabander T, van der Zwan WA, Teunissen JJM, Kam BLR, Feelders RA, de Herder WW, van Eijck CHJ, Franssen GJH, Krenning EP, Kwekkeboom DJ. Long-term efficacy, survival, and safety of [177Lu-DOTA0,Tyr3]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin Cancer Res. doi:10.1158/1078-0432.CCR-16-2743 [DOI] [PubMed]
- 181.Bodei L, Kidd M, Paganelli G, Grana CM, Drozdov I, Cremonesi M, Lepensky C, Kwekkeboom DJ, Baum RP, Krenning EP, Modlin IM. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. 2015;42(1):5–19. [DOI] [PubMed] [Google Scholar]
- 182.Amar L, Servais A, Gimenez-Roqueplo AP, Zinzindohoue F, Chatellier G, Plouin PF. Year of diagnosis, features at presentation, and risk of recurrence in patients with pheochromocytoma or secreting paraganglioma. J Clin Endocrinol Metab. 2005;90:2110–2116. [DOI] [PubMed] [Google Scholar]
- 183.Obara T, Kanbe M, Okamoto T, Ito Y, Yamashita T, Ito K, Hirose K, Yamazaki K, Hagihara J, Kusakabe K, et al. . Surgical strategy for pheochromocytoma: emphasis on the pledge of flank extraperitoneal approach in selected patients. Surgery. 1995;118(6):1083–1089. [DOI] [PubMed] [Google Scholar]
- 184.Amar L, Lussey-Lepoutre C, Lenders JW, Djadi-Prat J, Plouin PF, Steichen O. Management of endocrine disease: recurrence or new tumors after complete resection of pheochromocytomas and paragangliomas: a systematic review and meta-analysis. Eur J Endocrinol. 2016;175(4):R135–R145. [DOI] [PubMed] [Google Scholar]
- 185.Assadipour Y, Sadowski SM, Alimchandani M, Quezado M, Steinberg SM, Nilubol N, Patel D, Prodanov T, Pacak K, Kebebew E. SDHB mutation status and tumor size but not tumor grade are important predictors of clinical outcome in pheochromocytoma and abdominal paraganglioma. Surgery. 2017;161(1):230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Thompson LD. Pheochromocytoma of the Adrenal Gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26(5):551–566. [DOI] [PubMed] [Google Scholar]
- 187.Kimura N, Takayanagi R, Takizawa N, Itagaki E, Katabami T, Kakoi N, Rakugi H, Ikeda Y, Tanabe A, Nigawara T, Ito S, Kimura I, Naruse M; Phaeochromocytoma Study Group in Japan . Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2014;21(3):405–414. [DOI] [PubMed] [Google Scholar]
- 188.Eisenhofer G, Tischler AS. Neuroendocrine cancer. Closing the GAPP on predicting metastases. Nat Rev Endocrinol. 2014;10(6):315–316. [DOI] [PubMed] [Google Scholar]
- 189.Plouin PF, Duclos JM, Soppelsa F, Boublil G, Chatellier G. Factors associated with perioperative morbidity and mortality in patients with pheochromocytoma: analysis of 165 operations at a single center. J Clin Endocrinol Metab. 2001;86(4):1480–1486. [DOI] [PubMed] [Google Scholar]
- 190.Goldstein RE, O’Neill JA Jr., Holcomb GW III, Morgan WM III, Neblett WW III, Oates JA, Brown N, Nadeau J, Smith B, Page DL, Abumrad NN, Scott HW Jr. Clinical experience over 48 years with pheochromocytoma. Ann Surg. 1999;229:755–764; discussion 764–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92(11):4069–4079. [DOI] [PubMed] [Google Scholar]
- 192.Majtan B, Zelinka T, Rosa J, Petrák O, Krátká Z, Štrauch B, Tuka V, Vránková A, Michalský D, Novák K, Wichterle D, Widimský J Jr, Holaj R. Long-term effect of adrenalectomy on cardiovascular remodeling in patients with pheochromocytoma. J Clin Endocrinol Metab. 2017;102(4):1208–1217. [DOI] [PubMed] [Google Scholar]
- 193.Castinetti F, Qi XP, Walz MK, Maia AL, Sansó G, Peczkowska M, Hasse-Lazar K, Links TP, Dvorakova S, Toledo RA, Mian C, Bugalho MJ, Wohllk N, Kollyukh O, Canu L, Loli P, Bergmann SR, Biarnes Costa J, Makay O, Patocs A, Pfeifer M, Shah NS, Cuny T, Brauckhoff M, Bausch B, von Dobschuetz E, Letizia C, Barczynski M, Alevizaki MK, Czetwertynska M, Ugurlu MU, Valk G, Plukker JT, Sartorato P, Siqueira DR, Barontini M, Szperl M, Jarzab B, Verbeek HH, Zelinka T, Vlcek P, Toledo SP, Coutinho FL, Mannelli M, Recasens M, Demarquet L, Petramala L, Yaremchuk S, Zabolotnyi D, Schiavi F, Opocher G, Racz K, Januszewicz A, Weryha G, Henry JF, Brue T, Conte-Devolx B, Eng C, Neumann HP. Outcomes of adrenal-sparing surgery or total adrenalectomy in phaeochromocytoma associated with multiple endocrine neoplasia type 2: an international retrospective population-based study. Lancet Oncol. 2014;15(6):648–655. [DOI] [PubMed] [Google Scholar]
- 194.Walz MK, Alesina PF, Wenger FA, Deligiannis A, Szuczik E, Petersenn S, Ommer A, Groeben H, Peitgen K, Janssen OE, Philipp T, Neumann HP, Schmid KW, Mann K. Posterior retroperitoneoscopic adrenalectomy: results of 560 procedures in 520 patients. Surgery. 2006;140(6):943–948, discussion 948–950. [DOI] [PubMed] [Google Scholar]
- 195.Moore MG, Netterville JL, Mendenhall WM, Isaacson B, Nussenbaum B. Head and neck paragangliomas: an update on evaluation and management. Otolaryngol Head Neck Surg. 2016;154(4):597–605. [DOI] [PubMed] [Google Scholar]
- 196.Dupin C, Lang P, Dessard-Diana B, Simon JM, Cuenca X, Mazeron JJ, Feuvret L. Treatment of head and neck paragangliomas with external beam radiation therapy. Int J Radiat Oncol Biol Phys. 2014;89(2):353–359. [DOI] [PubMed] [Google Scholar]
- 197.Vogel J, Atanacio AS, Prodanov T, Turkbey BI, Adams K, Martucci V, Camphausen K, Fojo AT, Pacak K, Kaushal A. External beam radiation therapy in treatment of malignant pheochromocytoma and paraganglioma. Front Oncol. 2014;4:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hescot S, Leboulleux S, Amar L, Vezzosi D, Borget I, Bournaud-Salinas C, de la Fouchardiere C, Libé R, Do Cao C, Niccoli P, Tabarin A, Raingeard I, Chougnet C, Giraud S, Gimenez-Roqueplo AP, Young J, Borson-Chazot F, Bertherat J, Wemeau JL, Bertagna X, Plouin PF, Schlumberger M, Baudin E; French group of Endocrine and Adrenal Tumors (Groupe des Tumeurs Endocrines-Reseau National des Tumeurs Endocrines and Cortico-Medullo Tumeurs Endocrines networks) . One-year progression-free survival of therapy-naive patients with malignant pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2013;98(10):4006–4012. [DOI] [PubMed] [Google Scholar]
- 199.Hamidi O, Young WF Jr, Iñiguez-Ariza NM, Kittah NE, Gruber L, Bancos C, Tamhane S, Bancos I. Malignant pheochromocytoma and paraganglioma: 272 patients over 55 years. J Clin Endocrinol Metab. [DOI] [PMC free article] [PubMed]
- 200.Rednam SP, Erez A, Druker H, Janeway KA, Kamihara J, Kohlmann WK, Nathanson KL, States LJ, Tomlinson GE, Villani A, Voss SD, Schiffman JD, Wasserman JD. Von Hippel–Lindau and hereditary pheochromocytoma/paraganglioma syndromes: clinical features, genetics, and surveillance recommendations in childhood. Clin Cancer Res. 2017;23(12):e68–e75. [DOI] [PubMed] [Google Scholar]
- 201.Gonias S, Goldsby R, Matthay KK, Hawkins R, Price D, Huberty J, Damon L, Linker C, Sznewajs A, Shiboski S, Fitzgerald P. Phase II study of high-dose [131I]metaiodobenzylguanidine therapy for patients with metastatic pheochromocytoma and paraganglioma. J Clin Oncol. 2009;27(25):4162–4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Krempf M, Lumbroso J, Mornex R, Brendel AJ, Wemeau JL, Delisle MJ, Aubert B, Carpentier P, Fleury-Goyon MC, Gibold C, et al. . Treatment of malignant pheochromocytoma with [131I]metaiodobenzylguanidine: a French multicenter study. J Nucl Biol Med. 1991;35:284–287. [PubMed] [Google Scholar]
- 203.Loh KC, Fitzgerald PA, Matthay KK, Yeo PP, Price DC. The treatment of malignant pheochromocytoma with iodine-131 metaiodobenzylguanidine (131I-MIBG): a comprehensive review of 116 reported patients. J Endocrinol Invest. 1997;20(11):648–658. [DOI] [PubMed] [Google Scholar]
- 204.Barrett JA, Joyal JL, Hillier SM, Maresca KP, Femia FJ, Kronauge JF, Boyd M, Mairs RJ, Babich JW. Comparison of high-specific-activity ultratrace 123/131I-MIBG and carrier-added 123/131I-MIBG on efficacy, pharmacokinetics, and tissue distribution. Cancer Biother Radiopharm. 2010;25(3):299–308. [DOI] [PubMed] [Google Scholar]
- 205.Jimenez C, Pryma DA, Sullivan DC, Schwarz JK, Noto RB, Stambler N, Armor T, Jensen JD, Israel RJ 2015 long term follow-up of a pivotal phase 2 study of Ultratrace® Iobenguane I-131 (AZEDRA TM) in patients with malignant relapsed/refractory pheochromocytoma (pheo)/paraganglioma (para). In: Adrenal Tumors: Clinical Implications of the Recent Molecular and Genetic Findings; Endocrine Society’s 97th Annual Meeting and Expo; March 5–8, 2015; San Diego, CA. [Google Scholar]
- 206.van Essen M, Krenning EP, Kooij PP, Bakker WH, Feelders RA, de Herder WW, Wolbers JG, Kwekkeboom DJ. Effects of therapy with [177Lu-DOTA0, Tyr3]octreotate in patients with paraganglioma, meningioma, small cell lung carcinoma, and melanoma. J Nucl Med. 2006;47:1599–1606. [PubMed] [Google Scholar]
- 207.Zovato S, Kumanova A, Demattè S, Sansovini M, Bodei L, Di Sarra D, Casagranda E, Severi S, Ambrosetti A, Schiavi F, Opocher G, Paganelli G. Peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE in individuals with neck or mediastinal paraganglioma (PGL). Horm Metab Res. 2012;44(5):411–414. [DOI] [PubMed] [Google Scholar]
- 208.Forrer F, Riedweg I, Maecke HR, Mueller-Brand J. Radiolabeled DOTATOC in patients with advanced paraganglioma and pheochromocytoma. Q J Nucl Med Mol Imaging. 2008;52(4):334–340. [PubMed] [Google Scholar]
- 209.Kong G, Grozinsky-Glasberg S, Hofman MS, Callahan J, Meirovitz A, Maimon O, Pattison DA, Gross DJ, Hicks RJ. Efficacy of peptide receptor radionuclide therapy (PRRT) for functional metastatic paraganglioma and phaeochromocytoma. J Clin Endocrinol Metab. [DOI] [PubMed]
- 210.Niemeijer ND, Alblas G, van Hulsteijn LT, Dekkers OM, Corssmit EP. Chemotherapy with cyclophosphamide, vincristine and dacarbazine for malignant paraganglioma and pheochromocytoma: systematic review and meta-analysis. Clin Endocrinol (Oxf). 2014;81(5):642–651. [DOI] [PubMed] [Google Scholar]
- 211.Averbuch SD, Steakley CS, Young RC, Gelmann EP, Goldstein DS, Stull R, Keiser HR. Malignant pheochromocytoma: effective treatment with a combination of cyclophosphamide, vincristine, and dacarbazine. Ann Intern Med. 1988;109(4):267–273. [DOI] [PubMed] [Google Scholar]
- 212.Huang H, Abraham J, Hung E, Averbuch S, Merino M, Steinberg SM, Pacak K, Fojo T. Treatment of malignant pheochromocytoma/paraganglioma with cyclophosphamide, vincristine, and dacarbazine: recommendation from a 22-year follow-up of 18 patients. Cancer. 2008;113(8):2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hadoux J, Favier J, Scoazec JY, Leboulleux S, Al Ghuzlan A, Caramella C, Deandreis D, Borget I, Loriot C, Chougnet C, Letouze E, Young J, Amar L, Bertherat J, Libe R, Dumont F, Deschamps F, Schlumberger M, Gimenez-Roqueplo AP, Baudin E. SDHB mutations are associated with response to temozolomide in patients with metastatic pheochromocytoma or paraganglioma. Int J Cancer. 2014; 135:2711–2720. [DOI] [PubMed] [Google Scholar]
- 214.Ayala-Ramirez M, Chougnet CN, Habra MA, Palmer JL, Leboulleux S, Cabanillas ME, Caramella C, Anderson P, Al Ghuzlan A, Waguespack SG, Deandreis D, Baudin E, Jimenez C. Treatment with sunitinib for patients with progressive metastatic pheochromocytomas and sympathetic paragangliomas. J Clin Endocrinol Metab. 2012; 97:4040–4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jasim S, Suman VJ, Jimenez C, Harris P, Sideras K, Burton JK, Worden FP, Auchus RJ, Bible KC. Phase II trial of pazopanib in advanced/progressive malignant pheochromocytoma and paraganglioma. Endocrine. 2017;57(2):220–225. [DOI] [PubMed] [Google Scholar]
- 216.Gutmann DH, Aylsworth A, Carey JC, Korf B, Marks J, Pyeritz RE, Rubenstein A, Viskochil D. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. JAMA. 1997;278(1):51–57. [PubMed] [Google Scholar]
- 217.Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, Conte-Devolx B, Falchetti A, Gheri RG, Libroia A, Lips CJ, Lombardi G, Mannelli M, Pacini F, Ponder BA, Raue F, Skogseid B, Tamburrano G, Thakker RV, Thompson NW, Tomassetti P, Tonelli F, Wells SA Jr, Marx SJ. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86(12):5658–5671. [DOI] [PubMed] [Google Scholar]
- 218.Nationella rekommendationer för genetisk utredning av paragangliom/ feokromocytom samt kontrollprogram för friska anlagsbärare av ett anlag i SDHx-, TMEM127 och MAX-generna. http://sfmg.se/download/riktlinjer/Cancergenetik/Arftligt-feokromocytom-paragangliom_170627.pdf.
- 219.Jasperson KW, Kohlmann W, Gammon A, Slack H, Buchmann L, Hunt J, Kirchhoff AC, Baskin H, Shaaban A, Schiffman JD. Role of rapid sequence whole-body MRI screening in SDH-associated hereditary paraganglioma families. Fam Cancer. 2014;13(2):257–265. [DOI] [PubMed] [Google Scholar]
- 220.Koschmann C, Calinescu AA, Nunez FJ, Mackay A, Fazal-Salom J, Thomas D, Mendez F, Kamran N, Dzaman M, Mulpuri L, Krasinkiewicz J, Doherty R, Lemons R, Brosnan-Cashman JA, Li Y, Roh S, Zhao L, Appelman H, Ferguson D, Gorbunova V, Meeker A, Jones C, Lowenstein PR, Castro MG. ATRX loss promotes tumor growth and impairs nonhomologous end joining DNA repair in glioma. Sci Transl Med. 2016;8(328):328ra28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, Nava Rodrigues D, Robinson D, Omlin A, Tunariu N, Boysen G, Porta N, Flohr P, Gillman A, Figueiredo I, Paulding C, Seed G, Jain S, Ralph C, Protheroe A, Hussain S, Jones R, Elliott T, McGovern U, Bianchini D, Goodall J, Zafeiriou Z, Williamson CT, Ferraldeschi R, Riisnaes R, Ebbs B, Fowler G, Roda D, Yuan W, Wu YM, Cao X, Brough R, Pemberton H, A’Hern R, Swain A, Kunju LP, Eeles R, Attard G, Lord CJ, Ashworth A, Rubin MA, Knudsen KE, Feng FY, Chinnaiyan AM, Hall E, de Bono JS. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Flynn RL, Cox KE, Jeitany M, Wakimoto H, Bryll AR, Ganem NJ, Bersani F, Pineda JR, Suvà ML, Benes CH, Haber DA, Boussin FD, Zou L. Alternative lengthening of telomeres renders cancer cells hypersensitive to ATR inhibitors. Science. 2015;347(6219):273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lussey-Lepoutre C, Bellucci A, Morin A, Buffet A, Amar L, Janin M, Ottolenghi C, Zinzindohoué F, Autret G, Burnichon N, Robidel E, Banting B, Fontaine S, Cuenod CA, Benit P, Rustin P, Halimi P, Fournier L, Gimenez-Roqueplo AP, Favier J, Tavitian B. In vivo detection of succinate by magnetic resonance spectroscopy as a hallmark of SDHx mutations in paraganglioma. Clin Cancer Res. 2016;22(5):1120–1129. [DOI] [PubMed] [Google Scholar]
- 224.Imperiale A, Moussallieh FM, Roche P, Battini S, Cicek AE, Sebag F, Brunaud L, Barlier A, Elbayed K, Loundou A, Bachellier P, Goichot B, Stratakis CA, Pacak K, Namer IJ, Taïeb D. Metabolome profiling by HRMAS NMR spectroscopy of pheochromocytomas and paragangliomas detects SDH deficiency: clinical and pathophysiological implications. Neoplasia. 2015;17(1):55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Schovanek J, Bullova P, Tayem Y, Giubellino A, Wesley R, Lendvai N, Nölting S, Kopacek J, Frysak Z, Pommier Y, Kummar S, Pacak K. Inhibitory effect of the noncamptothecin topoisomerase I inhibitor LMP-400 on female mice models and human pheochromocytoma cells. Endocrinology. 2015;156(11):4094–4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Scheuermann TH, Li Q, Ma HW, Key J, Zhang L, Chen R, Garcia JA, Naidoo J, Longgood J, Frantz DE, Tambar UK, Gardner KH, Bruick RK. Allosteric inhibition of hypoxia inducible factor-2 with small molecules. Nat Chem Biol. 2013;9(4):271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sapieha P, Sirinyan M, Hamel D, Zaniolo K, Joyal JS, Cho JH, Honoré JC, Kermorvant-Duchemin E, Varma DR, Tremblay S, Leduc M, Rihakova L, Hardy P, Klein WH, Mu X, Mamer O, Lachapelle P, Di Polo A, Beauséjour C, Andelfinger G, Mitchell G, Sennlaub F, Chemtob S. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat Med. 2008;14(10):1067–1076. [DOI] [PubMed] [Google Scholar]
- 228.Oudard S, Elaidi R, Brizard M, Le Rest C, Caillet V, Deveaux S, Benoit G, Corréas JM, Benoudiba F, David P, Gaudric A, Hammel P, Joly D, Timsit MO, Méjean A, Richard S. Sunitinib for the treatment of benign and malignant neoplasms from von Hippel–Lindau disease: a single-arm, prospective phase II clinical study from the PREDIR group. Oncotarget. 2016;7(51):85306–85317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Choueiri TK, Fay AP, Gagnon R, Lin Y, Bahamon B, Brown V, Rosenberg JE, Hutson TE, Baker-Neblett KL, Carpenter C, Liu Y, Pandite L, Signoretti S. The role of aberrant VHL/HIF pathway elements in predicting clinical outcome to pazopanib therapy in patients with metastatic clear-cell renal cell carcinoma. Clin Cancer Res. 2013;19(18):5218–5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Van Essen M, Krenning EP, De Jong M, Valkema R, Kwekkeboom DJ. Peptide receptor radionuclide therapy with radiolabelled somatostatin analogues in patients with somatostatin receptor positive tumours. Acta Oncol. 2007;46(6):723–734. [DOI] [PubMed] [Google Scholar]
- 231.Nicolas G, Kaul F, Mena R, Bouterfa H, Fani M, Wild D. First clinical data on 68Ga-labeled somatostatin receptor antagonists: a phase I/II study comparing 68Ga-OPS202 with 68Ga-DOTATOC PET/CT. J Nucl Med. 2015;56(3):266. [Google Scholar]
- 232.Kratochwil C, Giesel FL, Bruchertseifer F, Mier W, Apostolidis C, Boll R, Murphy K, Haberkorn U, Morgenstern A. 213Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: a first-in-human experience. Eur J Nucl Med Mol Imaging. 2014;41(11):2106–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M, Widmark A, Johannessen DC, Hoskin P, Bottomley D, James ND, Solberg A, Syndikus I, Kliment J, Wedel S, Boehmer S, Dall’Oglio M, Franzén L, Coleman R, Vogelzang NJ, O’Bryan-Tear CG, Staudacher K, Garcia-Vargas J, Shan M, Bruland OS, Sartor O; ALSYMPCA Investigators . Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–223. [DOI] [PubMed] [Google Scholar]
- 234.Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P; CLARINET Investigators . Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224–233. [DOI] [PubMed] [Google Scholar]
- 235.Walls GV, Stevenson M, Soukup BS, Lines KE, Grossman AB, Schmid HA, Thakker RV. Pasireotide therapy of multiple endocrine neoplasia type 1–associated neuroendocrine tumors (NETs) in female mice deleted for an Men1 allele improves survival and reduces tumor progression. Endocrinology. 2016;157(5):1789–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Dombi E, Baldwin A, Marcus LJ, Fisher MJ, Weiss B, Kim A, Whitcomb P, Martin S, Aschbacher-Smith LE, Rizvi TA, Wu J, Ershler R, Wolters P, Therrien J, Glod J, Belasco JB, Schorry E, Brofferio A, Starosta AJ, Gillespie A, Doyle AL, Ratner N, Widemann BC. Activity of selumetinib in neurofibromatosis type 1–related plexiform neurofibromas. N Engl J Med. 2016;375(26):2550–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sherman SI, Clary DO, Elisei R, Schlumberger MJ, Cohen EE, Schöffski P, Wirth LJ, Mangeshkar M, Aftab DT, Brose MS. Correlative analyses of RET and RAS mutations in a phase 3 trial of cabozantinib in patients with progressive, metastatic medullary thyroid cancer. Cancer. 2016;122(24):3856–3864. [DOI] [PubMed] [Google Scholar]
- 238.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor D, Salama AK, Taylor M, Ott PA, Rollin LM, Horak C, Gagnier P, Wolchok JD, Hodi FS. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Arén Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Leja J, Yu D, Nilsson B, Gedda L, Zieba A, Hakkarainen T, Åkerström G, Öberg K, Giandomenico V, Essand M. Oncolytic adenovirus modified with somatostatin motifs for selective infection of neuroendocrine tumor cells. Gene Ther. 2011;18(11):1052–1062. [DOI] [PubMed] [Google Scholar]
- 243.Mandal R, Chan TA. Personalized oncology meets immunology: the path toward precision immunotherapy. Cancer Discov. 2016;6(7):703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Bahougne T, Imperiale A, Averous G, Chabrier G, Burnichon N, Gimenez-Roqueplo AP, Dali-Youcef N, Libe R, Baudin E, Roy C, Lang H, Kessler L. Successful response to pegylated interferon alpha in a patient with recurrent paraganglioma. Endocr Relat Cancer. 2017;24(2):L7–L11. [DOI] [PubMed] [Google Scholar]
- 245.Fojo T. Precision oncology: a strategy we were not ready to deploy. Semin Oncol. 2016;43(1):9–12. [DOI] [PubMed] [Google Scholar]
- 246.Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, Beijersbergen RL, Bardelli A, Bernards R. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483(7387):100–103. [DOI] [PubMed] [Google Scholar]
- 247.McGranahan N, Swanton C. Biological and therapeutic impact of intratumor heterogeneity in cancer evolution [published correction appears in Cancer Cell 2015;28(1):141] Cancer Cell. 2015;27(1):15–26. [DOI] [PubMed] [Google Scholar]
- 248.Flynn A, Dwight T, Benn D, Deb S, Colebatch AJ, Fox S, Harris J, Duncan EL, Robinson B, Hogg A, Ellul J, To H, Duong C, Miller JA, Yates C, James P, Trainer A, Gill AJ, Clifton-Bligh R, Hicks RJ, Tothill RW. Cousins not twins: intratumoural and intertumoural heterogeneity in syndromic neuroendocrine tumours. J Pathol. 2017;242(3):273–283. [DOI] [PubMed] [Google Scholar]
- 249.Neal JW, Gainor JF, Shaw AT. Developing biomarker-specific end points in lung cancer clinical trials. Nat Rev Clin Oncol. 2015;12(3):135–146. [DOI] [PubMed] [Google Scholar]
- 250.Barbolosi D, Ciccolini J, Lacarelle B, Barlési F, André N. Computational oncology: mathematical modelling of drug regimens for precision medicine. Nat Rev Clin Oncol. 2016;13(4):242–254. [DOI] [PubMed] [Google Scholar]
- 251.Herold CJ, Lewin JS, Wibmer AG, Thrall JH, Krestin GP, Dixon AK, Schoenberg SO, Geckle RJ, Muellner A, Hricak H. Imaging in the age of precision medicine: summary of the proceedings of the 10th Biannual Symposium of the International Society for Strategic Studies in Radiology. Radiology. 2016;279(1):226–238. [DOI] [PubMed] [Google Scholar]
- 252.Kantarjian H, Yu PP. Artificial intelligence, big data, and cancer. JAMA Oncol. 2015;1(5):573–574. [DOI] [PubMed] [Google Scholar]
- 253.Astrom K, Cohen JE, Willett-Brozick JE, Aston CE, Baysal BE. Altitude is a phenotypic modifier in hereditary paraganglioma type 1: evidence for an oxygen-sensing defect. Hum Genet. 2003;113(3):228–237. [DOI] [PubMed] [Google Scholar]
- 254.Arias-Stella J, Valcarcel J. The human carotid body at high altitudes. Pathol Microbiol (Basel). 1973;39(3):292–297. [DOI] [PubMed] [Google Scholar]
- 255.Arias-Stella J, Valcarcel J. Chief cell hyperplasia in the human carotid body at high altitudes; physiologic and pathologic significance. Hum Pathol. 1976;7(4):361–373. [DOI] [PubMed] [Google Scholar]
- 256.Opotowsky AR, Moko LE, Ginns J, Rosenbaum M, Greutmann M, Aboulhosn J, Hageman A, Kim Y, Deng LX, Grewal J, Zaidi AN, Almansoori G, Oechslin E, Earing M, Landzberg MJ, Singh MN, Wu F, Vaidya A. Pheochromocytoma and paraganglioma in cyanotic congenital heart disease. J Clin Endocrinol Metab. 2015;100(4):1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Her YF, Nelson-Holte M, Maher LJ III. Oxygen concentration controls epigenetic effects in models of familial paraganglioma. PLoS One. 2015;10(5):e0127471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kim E, Wright MJ, Sioson L, Novos T, Gill AJ, Benn DE, White C, Dwight T, Clifton-Bligh RJ. Utility of the succinate: fumarate ratio for assessing SDH dysfunction in different tumor types. Mol Genet Metab Rep. 2016;10:45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]