Abstract
Purpose
Benign prostatic hyperplasia (BPH) is strongly associated with obesity and prostatic tissue inflammation, but the molecular underpinning of this relationship is not known. Here, we examined the association between urine levels of chemokines/adipokines with histological markers of prostate inflammation, obesity and lower urinary tract symptoms LUTS in BPH patients.
Methods
Frozen urine specimens from 207 BPH/LUTS patients enrolled in Nashville Men’s Health Study were sent for blinded analysis of 11 analytes, namely sIL-1RA, CXC chemokines (CXCL-1, CXCL-8, CXCL-10), CC chemokines (CCL2, CCL3, CCL5), PDGF-BB, interleukins IL-6, IL-17 and sCD40L using Luminex™ xMAP® technology. After adjusting for age and medication use, the urine levels of analytes were correlated with the scales of obesity, prostate inflammation grade, extent, and markers of lymphocytic infiltration (CD3 and CD20) using linear regression.
Results
sIL-1RA levels were significantly raised with higher BMI, waist circumference and waist-hip ratio in BPH patients after correction for multiple testing (p=0.02). Men with greater overall extent of inflammatory infiltrates and maximal CD3 infiltration were marginally associated with CXCL-10 (p=0.054) and CCL5 (p=0.054), respectively. CCL3 in 15 patients with moderate to severe grade inflammation was marginally associated with maximal CD20 infiltration (p=0.09), whereas CCL3 was undetectable in men with mild prostate tissue inflammation. There was marginal association of sCD40L with AUA-SI scores (p=0.07).
Conclusions
Strong association of sIL-1RA in urine with greater body size supports it as a major molecular correlate of obesity in the urine of BPH patients. Increased urine levels of CXCL-10, CCL5 and CCL3 were marginally associated with the scores for prostate tissue inflammation and lymphocytic infiltration. Overall, elevated urinary chemokines support that BPH is a metabolic disorder and suggest a molecular link between BPH/LUTS and prostatic inflammation.
Keywords: urine, obesity, BPH, inflammation
Introduction
Accumulating evidence from large clinical studies including Medical Therapy Of Prostatic Symptoms (MTOPS) and Reduction by Dutasteride of Prostate Cancer Events (REDUCE) strongly suggests a role for prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia / lower urinary tract symptoms (BPH/LUTS)1. The role of inflammation in BPH was further supported by the finding of an inverse association of LUTS with daily use of non-steroidal anti-inflammatory drugs in the Olmsted County Study of Aging2.
Healthy prostate is an immunocompetent organ, populated with a small number of stromal and intraepithelial T and B lymphocytes3. Intra-prostatic lymphocytic infiltration in prostate tissue is a sign of inflammation4, which is phenotyped by grade, extent5 and the density of CD3+ and CD20+ staining. Intra prostatic infiltration in transition zone of prostate6 was positively associated with symptom scores of LUTS6, but inflammation in peripheral zone was not strongly associated with LUTS in a recent study7. Since chemokine expression temporally precedes the intra-prostatic infiltration8, chemokines have been studied as molecular signatures of inflammation in prostate tissue4 and in expressed prostatic secretions9.
Epidemiological evidence suggests that prostatic inflammation associated with BPH is most likely initiated in response to metabolic stress signals10, which may explain a modest overall increased prevalence of BPH/LUTS in obese subjects11,12. The present study seeks to evaluate whether urinary chemokines can reflect the clinical attributes of BPH/LUTS patients. The selection of the chemokines for the present study was based on the known role of following chemokines CXCL-1, CXCL-8, CXCL-10, CCL2, CCL3 and CCL5 in the chemoattraction of CD3+ and CD20+ lymphocytes13–18 and the reports on detection of other included proteins in the prostate tissue or biofluids of BPH patients or patients suffering from LUTS19,20.
Patients & Methods
Study Population
Participants were enrolled as a part of the Nashville Men’s Health Study, a multi-centered, rapid-recruitment protocol initiated in 2002 at Vanderbilt University Medical Center, the Tennessee Valley Veterans Administration Medical Center, and Urology Associates - a large community urology practice. Prostate cancer patients were excluded from enrollment and those enrolled were seeking prostate biopsy for either elevated prostate specific antigen or positive digital rectal exam or LUTS or pelvic pain. Exclusion criteria included age less than 40 years, prior prostate surgery, use of androgen supplementation, or English language insufficient for informed consent. Approximately 90% of eligible men that were approached for recruitment consented to participate in the IRB approved protocol.
Prostate Tissue Inflammation
In order to support the feasibility of analysis in large number of specimens, the analysis for prostate inflammation was focused on the mid-region of the prostate. This approach was adapted from the consensus criteria developed by Nickel, the North American Chronic Prostatitis Collaborative Research Network, and the International Prostatitis Collaborative Network5. Tissue samples were stained by hematoxylin and eosin (H&E), and images were taken to capture the entire length of each stained prostate core using the Clearview Software package from Olympus. Resultant images, were reviewed in a blinded manner by an experienced pathologist without knowledge of the patients’ prostate size or LUTS severity. Within each image, regions of inflammation (ROI) were identified for further analysis and processing. These ROI included confluent sheets of inflammatory cells, with or without tissue destruction or lymphoid nodule or follicle formation.
Immune Cell Infiltration
We measured CD3, a marker of T cell infiltration, and CD20, a marker of B cell infiltration, on an automated immunostainer in the Vanderbilt Translational Pathology Core. Percentage of tissue with immune cell infiltration was calculated as the number of positive pixels for CD3 or CD20 divided by the total number of pixels in the scorable tissue space. The level of staining was then sorted in ascending order of percent positive staining within each region across the entire population of biopsies for each patient, providing an index of each patient’s maximal CD3 or CD20 positive staining.
Urine analysis Study
A spot urine sample was collected from consented patients during the period from 2009–2012. Collected urine samples were aliquoted into cryovials and preserved at −80°C. Frozen urine aliquots were later shipped overnight on dry ice to University of Pittsburgh for blinded analysis in October 2015. Aliquots were thawed prior to analysis and 50µL of urine specimens were analyzed in duplicate for 11 proteins. CXC chemokines (CXCL-1, CXCL-8, CXCL-10), CC chemokines (CCL2, CCL3, CCL5), sIL-1ra, platelet derived growth factor –BB (PDGF-BB), interleukin IL-6, IL-17 and sCD40L were analyzed using Luminex™ xMAP® technology.
Statistical Analysis
Patient characteristics were summarized with median and quartiles for continuous variables. Urine was available for analysis from 207 study participants and tissue inflammation scores were available from 107 participants. The number (n) varies across biomarker measures due to differences in assay failure rate across chemokines (Table 2) as well as the availability of inflammatory scores. Correlation with obesity and infiltration measured was analyzed by Spearman correlation coefficient rs. Chemokine values were natural log transformed prior to analysis to meet model assumptions and minimize the leveraging effects of a small number of extreme values. The independent relationship of chemokines was ascertained by beta-coefficients (β) from the linear model after adjusting for age and current BPH medication. Statistical significance was defined as p ≤ 0.05, and marginal significance as p<0.10.
Table 2.
Urine levels of Different Chemokines
| Chemokine | Patients Analyzed (n) |
Median ( pg/mL) |
25th Percentil e (pg/mL) |
75th Percenti le (pg/mL) |
Detection Limit (pg/mL) |
|---|---|---|---|---|---|
| CXCL1 | 207 | 29.24 | 21.6 | 44.29 | 9.9 |
| CXCL8 | 207 | 8.48 | 3.52 | 20.45 | 0.4 |
| CXCL10 | 207 | 82.46 | 30.32 | 162 | 8.6 |
| sIL-1RA | 207 | 89.18 | 36.93 | 215.88 | 8.3 |
| CCL2 | 207 | 316.29 | 168.24 | 616.04 | 1.9 |
| CCL3 | 207 | 4.63 | 3.92 | 5.18 | 2.9 |
| CCL5 | 118 | 13.10 | 6.46 | 20.74 | 1.2 |
| sCD40L | 177 | 6.54 | 5.84 | 8.06 | 5.1 |
| PDGF-BB | 118 | 29.57 | 14.64 | 38.87 | 2.2 |
| IL-6 | 118 | 3.26 | 1.82 | 5.06 | 0.9 |
| IL-17 | 177 | 0.90 | 0.77 | 1.01 | 0.7 |
CCL2, CXCL-8 and sIL-1RA were above the detectable limit of assay in ~89% of enrolled patients.
Given the exploratory nature of the analysis, we did not use Bonferroni correction, which is considered by far the most conservative and so vulnerable to missing a true effect. Instead we relied on False discovery rate (FDR) method of Benjamini and Cohen21 for multiple testing of 11 urinary proteins to arrive at the corrected significance level of 0.03. FDR considers the correlations between the markers and therefore less likely to miss a potentially important association given that our sample size is fixed. All analyses were conducted with R (http://www.R-project.org/).
Results
Clinical Characteristics
Quartile distribution of the clinical characteristics for study participants is summarized in Table 1 with the median age of 65 yrs. 179 out of the 207 enrolled subjects were Caucasians and 21 subjects were of African-American ancestry. Majority of men were either overweight or obese with median BMI of 28.2. Indices of centralized fat deposition include waist circumference and waist hip ratio, with median values of 104.1 cm and 1.03, respectively. Median values for prostate volume, PSA, and AUA-SI were 49 mLs, 5 ng/mL, and 9, respectively.
Table 1.
Clinical Characteristics of 207 Patients Enrolled for Urine Analysis
| Variable | N | Media n |
25th Pctl |
75th Pctl |
|---|---|---|---|---|
| Age (yr) | 207 | 65.0 | 60.0 | 70.0 |
| BMI | 207 | 28.2 | 25.8 | 30.6 |
| Height-cm | 207 | 175.3 | 171.5 | 180.3 |
| Waist- cm | 207 | 104.1 | 95.5 | 110.5 |
| Waist Hip ratio | 207 | 1.03 | 0.98 | 1.07 |
| Prostate Volume (mls) | 207 | 49.0 | 34.7 | 66.1 |
| PSA at recruitment (ng/ml) | 205 | 5.0 | 3.8 | 7.0 |
| AUA-SI | 207 | 9.0 | 4.0 | 15.0 |
The majority of enrolled men were either overweight or obese with median age of 65 years.
Urine levels of Chemokines
Table 2 summarizes the distribution and the considerable variability in the detection of eleven chemokines. The CCL2 and sIL-1ra were present above the assay limit in 89% of enrolled patients and similar percentage showed detectable levels of CXCL-1, CXCL-8 and CXCL-10. In contrast, CCL5 and PDGF-BB were detectable in approximately one-half of participants, while levels of CCL3, IL-6, sCD40L and IL-17 in urine were consistently low and below assay limit in a majority of patients likely due to degradation during storage period of 3–6 years prior to analysis.
Association of urine levels with obesity measures
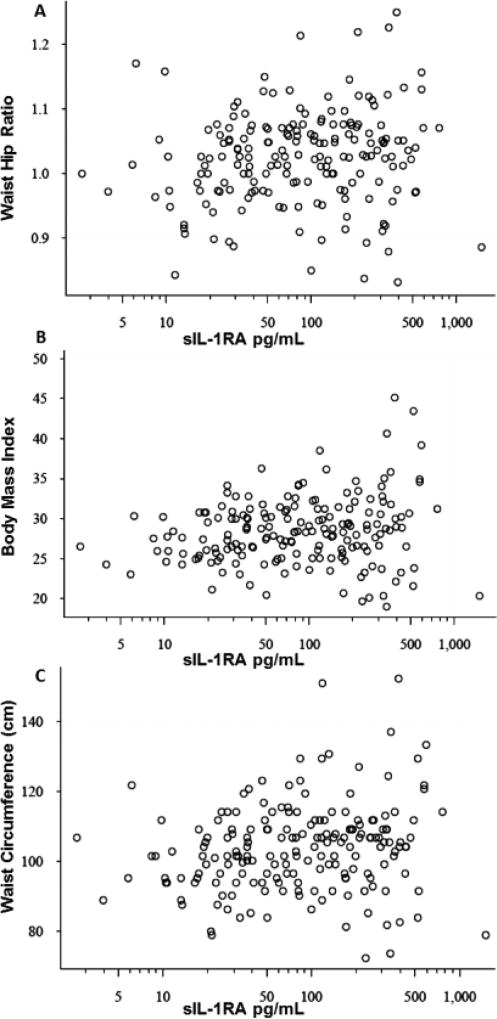
We first considered the potential interaction between obesity and urine levels of different chemokines and only sIL-1Ra was significantly associated (Fig. 1A–C) with obesity measures. The present study on 207 subjects confirmed the association of sIL-1Ra with measures of obesity noted in earlier pilot study on 30 subjects [12] with the spearman correlation coefficient rs of 0.15(p=0.02), 0.2(p=0.003) and 0.19(0.004) for BMI, waist circumference and waist-hip ratio, respectively. After controlling for age and BPH treatment on log transformed sIL-1RA values, β coefficients from regression models were calculated to assess an overall effect estimate. The β coefficients for BMI (p=0.02), waist circumference (p<0.01) and waist hip ratio (p=0.01) were 0.04, 0.016 and 3, respectively. Stratification of BPH patients on the basis of obesity parameter (Table 3) found that urine levels of sIL-1RA were elevated by 47.5pg/mL in 65 BPH patients with BMI >30 (p<0.005). Likewise, urine levels of sIL-1Ra were nearly doubled in BPH patients with waist circumference larger than 104cm (n=107; p<0.001) and those with higher waist-hip ratio (n=105; p<0.004). Furthermore, statins, non-steroidal anti-inflammatory drugs (NSAIDs) or medications for hypertension were not associated with adjusted sIL-1Ra levels. The findings with regard to the association of urinary sIL-1Ra levels with obesity measures are significant after adjustment of p values for multiple testing by the FDR approach.
Fig. 1. A–C: Scatter plot of unadjusted sIL-1RA levels in urine against waist-hip ratio (Panel A) BMI(Panel B) and waist circumference (Panel C).
The association of unadjusted urine levels of sIL-1RA with waist-hip ratio, BMI and waist circumference was statistically significant with spearman correlation coefficient rs of 0.19(p= 0.004), 0.15(p=0.02) and 0.2(p=0.003), respectively as shown in panel A,B and C. Linear regression modelling of sIL-1RA levels adjusted for age and BPH treatment determined the β coefficient of 3 for waist hip ratio (p=0.01) and 0.04 and 0.016 for BMI (p=0.02) and waist circumference (p<0.01), respectively.
Table 3.
Urine levels of sIL-1RA in BPH patients stratified by obesity measures
| Number of patients |
sIL-1RA pg/mL |
p | ||
|---|---|---|---|---|
|
|
||||
| BMI | <30 | 142 | 72.3 | 0.005* |
| >30 | 65 | 119.8 | ||
| Waist circumference | <104 cm | 100 | 61.7 | 0.001* |
| >104 cm | 107 | 113.9 | ||
| WHR | <1.02 | 102 | 66.6 | 0.004* |
| >1.02 | 105 | 107.1 | ||
Urine levels of sIL-1RA were significantly elevated in obese patients with BMI >30 (n= 65; p<0.005), waist circumference larger than 104cm (n=107; p<0.001) and higher waist-hip ratio (n=105; p<0.005) compared to less obese patients.
Prostate Tissue Immune Cell Infiltration and Urine chemokines
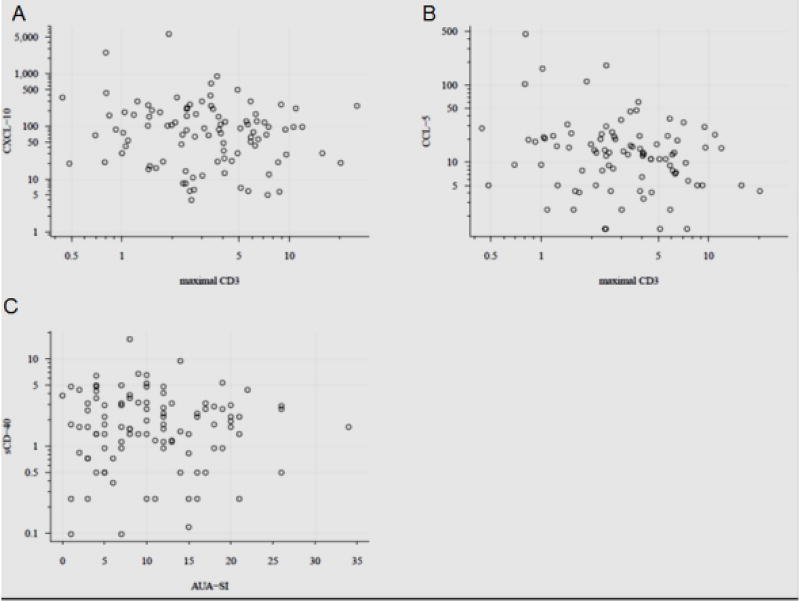
The infiltration of T and B cells in prostate biopsy was scored by staining of CD3 and CD20, respectively. The association between urine levels of chemokines within each participant was assessed with the local region of tissue with the maximal staining (Table 4&5) and the mean levels of staining across all tissues. Urine levels of CCL5 after adjustment for age and treatment showed a marginal inverse association with maximal CD3 expression (β = −0.64, p=0.054, n=89) in prostate biopsy (Table 4 and Fig. 2B). None of the other chemokines were associated with maximal CD3 expression or with mean CD3 expression (data not shown). Only the urine levels of CCL3 were significantly associated with maximal CD20 staining (β= 2.93, p=0.02; Table 5) and marginally associated with mean CD20 staining (β= 0.16, p=0.09) (data not shown). These findings require further confirmation, because tissue inflammatory scores were only available for a quarter of 60 patients with urine levels of CCL3 above the detection limit. The association of CCL5 and CCL3 with CD3 and CD20, holds biological significance because CCL5 and CCL3 are primarily chemotactic for T-lymphocytes. After adjustment to age and medications for other comorbidities, BPH and prostatic inflammation is most likely explanation for the elevated urine levels of CCL5 and CCL3.
Table 4.
Association between maximal CD3 expression in biopsy and urine levels of chemokines adjusted for age and treatment
| Variable | N | β* | p value |
|---|---|---|---|
| CCL2 | 107 | −0.03 | 0.59 |
| CCL3 | 15 | 0.13 | 0.77 |
| CCL5 | 89 | −0.07 | 0.22 |
| CXCL1 | 105 | 0.01 | 0.80 |
| CXCL8 | 105 | 0.02 | 0.72 |
| CXCL10 | 96 | −0.05 | 0.27 |
| IL6 | 74 | −0.03 | 0.60 |
| IL1RA | 107 | 0.07 | 0.12 |
| PDGF-BB | 86 | 0 | 0.97 |
| sCD40L | 46 | −0.02 | 0.81 |
Urine levels of CCL5 were associated with maximal CD3 expression.
Linear regression modelling employed CD3 as the response and the urinary chemokine as the primary covariate to calculate β coefficient adjusted for age and BPH treatment
Table 5.
Association between maximal CD20 expression in biopsy and urine levels of chemokines adjusted for age and treatment
| Variable | N | β* | p value |
|---|---|---|---|
| CCL2 | 107 | −0.09 | 0.59 |
| CCL3 | 15 | 2.93 | 0.02* |
| CCL5 | 89 | −0.03 | 0.88 |
| CXCL1 | 105 | 0.001 | 0.99 |
| CXCL8 | 105 | 0.089 | 0.55 |
| CXCL10 | 96 | 0.014 | 0.92 |
| IL6 | 74 | 0.06 | 0.75 |
| IL1RA | 107 | 0.004 | 0.97 |
| PDGF-BB | 89 | −0.11 | 0.64 |
| sCD40L | 46 | 0.29 | 0.30 |
Urine levels of CCL3 were significantly associated with maximal CD20 expression.
Linear regression modelling employed CD20 as the response and the log transformed urinary chemokine as the primary covariate to calculate β coefficient adjusted for age and BPH treatment
Fig. 2. A–C: Scatter plot of maximal CD3 expression in biopsy against unadjusted urine levels of CXCL-10 (Panel A) and CCL5 (Panel B).
The score for maximal CD3 expression is plotted on x-axis and urine levels of CXCL-10 and CCL5 on y-axis. Elevated urine levels of CXCL-10 were marginally associated with the extent of prostatic inflammation (β = 0.10, p=0.054, n=82). Panel C depicts the marginal inverse association of sCD40L levels in urine of 110 BPH/LUTS patients with their AUA-SI scores (β = −1.25, p=0.076, n=103).
Prostate Tissue Inflammation Grade and Extent
We next investigated the relationship chemokines levels in urine across the grade and extent of prostatic inflammation. Grade of inflammation was characterized by confluency of inflammatory cells in each ROI of the biopsy core, where increased grade was marginally associated with CCL3 elevation in urine (β = 0.29, p=0.09, n=38). In contrast, elevated urine levels of CXCL-10 were marginally associated with the extent of prostatic inflammation (β = 0.10, p=0.054, n=82), which was calculated as the total area of all ROI across all images for that patient divided by the total tissue area scored for that patient × 100, to yield as the percent of all analyzed cores with inflammation (Fig. 2A).
Association of urine levels with symptom scores
We next investigated the relationship between urine levels of chemokines and symptom scores. Regression models using AUA-SI scores as the response and log-transformed chemokines as the explanatory response, found no significant association. Although, a marginal inverse association was notable between AUA-SI scores (β = −1.25, p=0.076, n=103) and sCD40L levels in urine (Fig. 2C).
Discussion
Present study demonstrated that chemokines measured non-invasively in urine can reflect on the relationship of BPH/LUTS with obesity measures and prostatic inflammation. This current study restricted only to BPH patients negative for prostate cancer as determined by prostate biopsy, found that increasing urine levels of CXCL-10, CCL5 and CCL3 were marginally associated with the extent of inflammation and greater intensity of CD3 and CD20 lymphocytic infiltration in prostate tissue. Previous studies have reported that urine levels of CXCL-10, CCL3 and CCL5 in healthy male asymptomatic control subjects are either undetectable or below the median values of BPH subjects reported here and elsewhere20,22.
One of the predisposing factor for BPH is the metabolic syndrome10, whose key determinant is the increased centralized deposition of adipose tissue. We found a broad and robust relationship between a key measure of centralized adiposity called waist-hip ratio and increased urine levels of sIL-1Ra. The source elevated sIL-1Ra in urine of obese BPH patients is presumed to be elevated serum levels of sIL-1Ra, because serum levels of sIL-1ra were reported to be elevated 7 fold in morbidly obese men23. Besides, median urine levels of sIL-1Ra measured here in overweight or obese men with BPH were comparable to the published levels of sIL-1Ra in serum of lean men23,24, which lend support to the suggestion that serum elevation of sIL-1Ra precedes the elevation in urine. The larger sample size of this study was able to reproduce the association of sIL-1Ra in urine with obesity measures of BMI, waist circumference and waist-hip ratio, found earlier in a pilot study19. Therefore, sIL-1Ra is the key molecular correlate for the obesity or greater body size of BPH patients23,24 which is detectable in both serum and urine.
The cross-sectional study design used here is limited in determining temporal relationships between outcomes and hence future studies in this direction need to perform simultaneous longitudinal measurement of serum and urine specimens of same patients to investigate whether the elevation of sIL-1Ra in serum precedes the urinary elevation of sIL-1Ra in BPH patients. Besides, sIL-1Ra, several other adipokines such as leptin can also be potential molecular correlate of greater body size in BPH patients. A significant association between serum levels of leptin and prostate enlargement was recently reported from serum analysis of another cohort of this dataset11.
Stromal enlargement is the predominant histologic feature of BPH/LUTS and the CC chemokine CCL2 primarily released from stroma13 was easily detectable in ~90% of enrolled patients of our study. Other members of CC chemokine family namely CCL5 and CCL3 are chemotactic for the infiltration of CD3+ T lymphocytes and CD20 B lymphocytes, respectively14. We did not see a strong or significant relationship between CD3 or CD20 immune cell infiltration and chemokine levels in urine for a large majority of BPH patients. Intriguingly, the maximal density of CD3+ cells in prostate biopsy had an inverse association with the CCL5 levels measured in urine of BPH patients. The inverse association is intriguing considering that maximal CD3 staining in biopsy of BPH patients had an inverse association with prostate volume in another cohort of this large dataset11. Besides, urine levels of CCL5 measured here in BPH patients were thousand fold lower than the serum levels reported in BPH patients15, which did not studied the density of CD3+ cells in prostate biopsy of BPH patients. Basic research reported recently that androgen deprivation17 upregulates CCL5 expression in prostatic epithelium, which is likely to be released into serum from prostate instead of urine Therefore, future studies need to concurrently investigate the serum and urine levels of CCL5 in BPH patients treated with NSAIDS and androgen deprivation therapy to understand the inverse association with maximal CD3 density.
Tissue inflammation in biopsy specimens of BPH patient was scored by grade and extent to capture the total inflammatory effect across all immune cell types. The association of different chemokines to different inflammation parameters highlight the role of different chemokines in driving different aspects of inflammation. Here, we noted that extent of prostatic inflammation in different biopsy cores of BPH patients was associated with elevation of CXCL-10 and the grade of inflammation was marginally with elevated urine levels of CCL3. We have previously reported that elevated urine levels of CXCL-10 are associated with acute and chronic inflammation in lower urinary tract20 and CCL3 and CCL2 were found elevated in expressed prostatic secretions of prostatitis patients25. Association of CCL3 with moderate grade of inflammation in prostate biopsy of BPH patients suggests that prostatic inflammation in some BPH patients can approach the severity noted in prostatitis patients. We noted a significant association between maximal infiltration of CD20+ B lymphocytes and urine levels of CCL3, but only in a minority of patients, which is 15 out of 60 patients with detectable CCL3 levels in urine. Interestingly, infiltration of CD20+ B lymphocytes is reported in only 10–15% of patients6,26, which is consistent with the consideration that B-cell activity is a late event in BPH progression.
CD40 ligand (CD40L) is a type II membrane glycoprotein of the tumor necrosis factor (TNF) family that is expressed as a trimer on activated T cells, B cells, monocytes, macrophages and platelets. Urine levels of soluble CD40L (sCD40L) likely reflect the activation of T cells and platelets27 in a small subset of BPH/LUTS patients to explain the association with symptom score of AUA-SI. The marginal inverse association of sCD40L with AUA-SI in male patients is very intriguing, considering elevated urine levels of sCD40L in female OAB patients relative to healthy controls28. Since, most of the men included in this study had incidental BPH with mild to moderate LUTS, future comparative studies need to be performed in symptomatic BPH and age matched asymptomatic healthy patients.
Chemokines primarily produced in stroma (CCL2)16 and in prostatic epithelium (CCL5, CXCL-10)17,18 can be potential candidates for assessing the prostatic inflammation. Compared to CCL2, inflammatory proteins with relatively lower tissue expression such as IL-6 and CCL3 were undetectable in majority of urine specimens of BPH/LUTS patients. Low and undetectable urine levels of IL-6 in BPH14 are not surprising considering that serum IL-6 levels are reported to be 40 fold lower than serum levels of CCL2 and several thousand fold lower than the serum CCL5 levels in BPH patients15. Comparison of the median urine levels for CC chemokines, CCL2 and CCL5 in BPH patients with the serum levels of respective chemokines reported in BPH patients15 suggest preferential secretion of only CCL2 and not CCL5 from prostate into urine. The thousand fold high serum levels of CCL515 reported in BPH patients compared to very low urine levels measured here suggests that it is not directly secreted from prostate into urine.
Anatomic continuity of prostate with urethra facilitates the preferential drainage of overexpressed chemokines in prostate via shedding and budding of micro and multivesicular bodies from plasma membrane29. Urine is therefore biased towards detection of proteins that are overexpressed in prostate tissue such as CCL230 and PSA31, which are then secreted into detectable levels urine. In fact, 100 fold higher levels of PSA31 in urine over serum are commensurate with direct secretion from prostate into urine instead of increased excretion of a 26kDa protein32 from a properly functioning kidney of BPH patients. However, urine may not be appropriate for the detection of CCL5 secreted from the prostate. Preferential drainage from prostate into urine may explain the relatively higher median urine levels of CXC chemokines, CXCL-1, CXCL-833 and CXCL-1018 in BPH patients compared to the reported serum levels of respective chemokines in BPH patients15. Our earlier study on female interstitial cystitis patients found significant association between chemokine levels measured in urine and the chemokine levels measured in respective bladder punch biopsy34. Since prostate biopsy is sensitive to sampling error and a urinary marker would avoid inflammation induced by the biopsy procedure and may represent inflammation in prostate tissue beyond the selected biopsy selection site. Since some of the study participant were taking medications, we controlled for the BPH treatment throughout the main analysis and the analyses stratified by BPH treatment status did not suggest an effect.
Conclusions
Strong association of sIL-1Ra in urine with greater body size support it as a major molecular correlate of obesity in urine of BPH patients. Increased urine levels of CXCL-10, CCL5 and CCL3 were marginally associated with scores for prostate tissue inflammation and lymphocytic infiltration. Overall, urinary chemokines can non-invasively inform about the molecular underpinning of BPH/LUTS with obesity and prostatic inflammation.
Acknowledgments
Funding: This work was partly supported by grant RO1CA121060; RO1DK087962 and, U54 DK112079
Footnotes
Disclosure of potential conflicts of interest: The authors declare that they have no conflict of interest to report.
References
- 1.Nickel JC, Roehrborn CG, Castro-Santamaria R, Freedland SJ, Moreira DM. Chronic Prostate Inflammation Is Associated with Severity and Progression of Benign Prostatic Hyperplasia, Lower Urinary Tract Symptoms and Risk of Acute Urinary Retention. J Urol. 2016 doi: 10.1016/j.juro.2016.06.090. [DOI] [PubMed] [Google Scholar]
- 2.St Sauver JL, Jacobson DJ, McGree ME, Lieber MM, Jacobsen SJ. Protective association between nonsteroidal antiinflammatory drug use and measures of benign prostatic hyperplasia. American journal of epidemiology. 2006;164(8):760–768. doi: 10.1093/aje/kwj258. [DOI] [PubMed] [Google Scholar]
- 3.Di Carlo E, Magnasco S, D'Antuono T, Tenaglia R, Sorrentino C. The prostate-associated lymphoid tissue (PALT) is linked to the expression of homing chemokines CXCL13 and CCL21. Prostate. 2007;67(10):1070–1080. doi: 10.1002/pros.20604. [DOI] [PubMed] [Google Scholar]
- 4.Steiner GE, Stix U, Handisurya A, et al. Cytokine expression pattern in benign prostatic hyperplasia infiltrating T cells and impact of lymphocytic infiltration on cytokine mRNA profile in prostatic tissue. Lab Invest. 2003;83(8):1131–1146. doi: 10.1097/01.lab.0000081388.40145.65. [DOI] [PubMed] [Google Scholar]
- 5.Nickel JC, True LD, Krieger JN, Berger RE, Boag AH, Young ID. Consensus development of a histopathological classification system for chronic prostatic inflammation. BJU Int. 2001;87(9):797–805. doi: 10.1046/j.1464-410x.2001.02193.x. [DOI] [PubMed] [Google Scholar]
- 6.Robert G, Descazeaud A, Nicolaiew N, et al. Inflammation in benign prostatic hyperplasia: a 282 patients' immunohistochemical analysis. Prostate. 2009;69(16):1774–1780. doi: 10.1002/pros.21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulac I, Gumuskaya B, Drake CG, et al. Peripheral Zone Inflammation Is Not Strongly Associated With Lower Urinary Tract Symptom Incidence and Progression in the Placebo Arm of the Prostate Cancer Prevention Trial. Prostate. 2016;76(15):1399–1408. doi: 10.1002/pros.23224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez de Lema G, Maier H, Nieto E, et al. Chemokine expression precedes inflammatory cell infiltration and chemokine receptor and cytokine expression during the initiation of murine lupus nephritis. J Am Soc Nephrol. 2001;12(7):1369–1382. doi: 10.1681/ASN.V1271369. [DOI] [PubMed] [Google Scholar]
- 9.Penna G, Mondaini N, Amuchastegui S, et al. Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur Urol. 2007;51(2):524–533. doi: 10.1016/j.eururo.2006.07.016. discussion 533. [DOI] [PubMed] [Google Scholar]
- 10.Vignozzi L, Rastrelli G, Corona G, Gacci M, Forti G, Maggi M. Benign prostatic hyperplasia: a new metabolic disease? J Endocrinol Invest. 2014;37(4):313–322. doi: 10.1007/s40618-014-0051-3. [DOI] [PubMed] [Google Scholar]
- 11.Fowke JH, Koyama T, Fadare O, Clark PE. Does Inflammation Mediate the Obesity and BPH Relationship? An Epidemiologic Analysis of Body Composition and Inflammatory Markers in Blood, Urine, and Prostate Tissue, and the Relationship with Prostate Enlargement and Lower Urinary Tract Symptoms. PLoS One. 2016;11(6):e0156918. doi: 10.1371/journal.pone.0156918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahle SE, Chokkalingam AP, Gao YT, Deng J, Stanczyk FZ, Hsing AW. Body size and serum levels of insulin and leptin in relation to the risk of benign prostatic hyperplasia. J Urol. 2002;168(2):599–604. [PubMed] [Google Scholar]
- 13.Spary LK, Salimu J, Webber JP, Clayton A, Mason MD, Tabi Z. Tumor stroma-derived factors skew monocyte to dendritic cell differentiation toward a suppressive CD14 PD-L1 phenotype in prostate cancer. Oncoimmunology. 2014;3(9):e955331. doi: 10.4161/21624011.2014.955331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin-Tsai O, Clark PE, Miller NL, et al. Surgical intervention for symptomatic benign prostatic hyperplasia is correlated with expression of the AP-1 transcription factor network. Prostate. 2014;74(6):669–679. doi: 10.1002/pros.22785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal M, He C, Siddiqui J, Wei JT, Macoska JA. CCL11 (eotaxin-1): a new diagnostic serum marker for prostate cancer. Prostate. 2013;73(6):573–581. doi: 10.1002/pros.22597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujita K, Ewing CM, Getzenberg RH, Parsons JK, Isaacs WB, Pavlovich CP. Monocyte chemotactic protein-1 (MCP-1/CCL2) is associated with prostatic growth dysregulation and benign prostatic hyperplasia. Prostate. 2010;70(5):473–481. doi: 10.1002/pros.21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan Y, Hu S, Liu J, et al. Low intraprostatic DHT promotes the infiltration of CD8+ T cells in BPH tissues via modulation of CCL5 secretion. Mediators Inflamm. 2014;2014:397815. doi: 10.1155/2014/397815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linge HM, Collin M, Giwercman A, Malm J, Bjartell A, Egesten A. The antibacterial chemokine MIG/CXCL9 is constitutively expressed in epithelial cells of the male urogenital tract and is present in seminal plasma. J Interferon Cytokine Res. 2008;28(3):191–196. doi: 10.1089/jir.2007.0100. [DOI] [PubMed] [Google Scholar]
- 19.Tyagi P, Motley SS, Kashyap M, et al. Urine chemokines indicate pathogenic association of obesity with BPH/LUTS. Int Urol Nephrol. 2015;47(7):1051–1058. doi: 10.1007/s11255-015-0992-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyagi P, Tyagi V, Qu X, Chuang YC, Kuo HC, Chancellor M. Elevated CXC chemokines in urine noninvasively discriminate OAB from UTI. Am J Physiol Renal Physiol. 2016;311(3):F548–554. doi: 10.1152/ajprenal.00213.2016. [DOI] [PubMed] [Google Scholar]
- 21.Benjamini Y, Cohen R. Weighted false discovery rate controlling procedures for clinical trials. Biostatistics. 2017;18(1):91–104. doi: 10.1093/biostatistics/kxw030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otoni A, Teixeira AL, Voieta I, et al. Chemokine profile in the sera and urine of patients with schistosomal glomerulopathy. Am J Trop Med Hyg. 2014;90(1):48–53. doi: 10.4269/ajtmh.13-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meier CA, Bobbioni E, Gabay C, Assimacopoulos-Jeannet F, Golay A, Dayer JM. IL-1 receptor antagonist serum levels are increased in human obesity: a possible link to the resistance to leptin? J Clin Endocrinol Metab. 2002;87(3):1184–1188. doi: 10.1210/jcem.87.3.8351. [DOI] [PubMed] [Google Scholar]
- 24.Charles BA, Doumatey A, Huang H, et al. The roles of IL-6, IL-10, and IL-1RA in obesity and insulin resistance in African-Americans. J Clin Endocrinol Metab. 2011;96(12):E2018–2022. doi: 10.1210/jc.2011-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desireddi NV, Campbell PL, Stern JA, et al. Monocyte chemoattractant protein-1 and macrophage inflammatory protein-1alpha as possible biomarkers for the chronic pelvic pain syndrome. J Urol. 2008;179(5):1857–1861. doi: 10.1016/j.juro.2008.01.028. discussion 1861–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Nunzio C, Kramer G, Marberger M, et al. The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. Eur Urol. 2011;60(1):106–117. doi: 10.1016/j.eururo.2011.03.055. [DOI] [PubMed] [Google Scholar]
- 27.Langer F, Chun FK, Amirkhosravi A, et al. Plasma tissue factor antigen in localized prostate cancer: distribution, clinical significance and correlation with haemostatic activation markers. Thromb Haemost. 2007;97(3):464–470. [PubMed] [Google Scholar]
- 28.Tyagi P, Barclay D, Zamora R, et al. Urine cytokines suggest an inflammatory response in the overactive bladder: a pilot study. Int Urol Nephrol. 2010;42(3):629–635. doi: 10.1007/s11255-009-9647-5. [DOI] [PubMed] [Google Scholar]
- 29.Jia X, Chen J, Sun S, et al. Detection of aggressive prostate cancer associated glycoproteins in urine using glycoproteomics and mass spectrometry. Proteomics. 2016;16(23):2989–2996. doi: 10.1002/pmic.201500506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shibuya S, Xia Z, Sugimoto M, Ueda N, Haba R, Kakehi Y. The phytotherapeutic agent, eviprostat, suppresses stromal proliferation and inflammation even after establishment of nonbacterial prostatitis in the rat prostate. Urology. 2014;83(3):528–534. doi: 10.1016/j.urology.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 31.Bolduc S, Lacombe L, Naud A, Gregoire M, Fradet Y, Tremblay RR. Urinary PSA: a potential useful marker when serum PSA is between 2.5 ng/mL and 10 ng/mL. Canadian Urological Association journal = Journal de l'Association des urologues du Canada. 2007;1(4):377–381. doi: 10.5489/cuaj.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haraldsson B, Nystrom J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008;88(2):451–487. doi: 10.1152/physrev.00055.2006. [DOI] [PubMed] [Google Scholar]
- 33.Schauer IG, Ressler SJ, Tuxhorn JA, Dang TD, Rowley DR. Elevated epithelial expression of interleukin-8 correlates with myofibroblast reactive stroma in benign prostatic hyperplasia. Urology. 2008;72(1):205–213. doi: 10.1016/j.urology.2007.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corcoran AT, Yoshimura N, Tyagi V, Jacobs B, Leng W, Tyagi P. Mapping the cytokine profile of painful bladder syndrome/interstitial cystitis in human bladder and urine specimens. World J Urol. 2013;31(1):241–246. doi: 10.1007/s00345-012-0852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]