Abstract
BACKGROUND
Metabolic function is regulated by the interplay of central and peripheral factors that ultimately regulate food intake and energy expenditure. The tachykinin substance P (SP) has been identified as a novel regulator of energy balance, however, the mechanisms underlying this effect are ill-defined and conflicting data regarding the role of SP on food intake have been reported by different groups.
OBJECTIVE
To further characterize the metabolic role of the Tac1 gene products (SP and neurokinin A (NKA)) in mice through a series of genetic, metabolic and behavioral studies in Tac1 deficient mice.
RESULTS
Tac1−/− mice are leaner than controls and display reduced food intake and altered feeding circadian rhythm, supported by disrupted expression of the clock genes Cry1/2, Per1/2 in the suprachiasmatic nucleus (SCN), medio-basal hypothalamus (MBH) and liver, as well as increased Pomc expression in the MBH. Tac1 ablation induced resistance to obesity, improved glucose tolerance, prevented insulin resistance under high-fat-diet, increased activation of brown adipose tissue and improved hepatic steatosis. Moreover, deletion of Tac1 in ob/ob mice ameliorated BW gain in females only but was sufficient to decrease fat and triglyceride content in the liver of males.
CONCLUSIONS
These results provide further evidence that Tac1 controls circadian feeding behavior and metabolism in mice through mechanisms that involve the regulation of the melanocortin system. In addition, these studies suggest that the blockade of SP may offer a new method to treat metabolic syndrome.
Keywords: Tac1, substance P, circadian rhythm, metabolism, mouse
INTRODUCTION
Tac1 encodes the tachykinins substance P (SP) and neurokinin A (NKA) (1). SP is widely distributed in the brain and has been involved in a number of neurological processes such as nociception, anxiety or sleep disturbances (1). In addition, we and others have documented the role of SP in the central control of the gonadotropic axis by stimulating, at least in part, Kiss1 neurons (2–4). This action is also shared by neurokinin B (NKB), the other member of the tachykinin family of peptides. Numerous studies have characterized the role of NKB in the control of GnRH release, including its action as a conveyor of the metabolic status to the central nodes that regulate reproduction (5–7).
Reproduction and metabolism are very tightly interconnected (8), where reproduction is often halted during periods of energy deficiency as a mechanism to optimize chances of survival of the organism. Thus, neuropeptides involved in the control of metabolism often participate in the control of fertility. In this context, a metabolic role of SP has also been revealed in recent years. Studies by Karagiannides and colleagues documented that the blockade of SP signaling leads to the decrease of body weight (BW) in diet induced obese mice and leptin deficient mice, as well as to the increase in insulin sensitivity (9). Recently, Trivedi et al. identified the products of the Tac1 gene as mediators of the action of ghrelin in the regulation of energy balance by controlling the levels of adiposity (10). While there is increasing evidence supporting the role of SP in the control of energy balance, a detailed characterization of the metabolic phenotype of the absence of Tac1 and the mechanisms of action underlying the effect of SP is missing. The studies mentioned above have documented controversial data regarding the orexigenic action of SP. In some cases, it has been described to induce food intake, while no action or even inhibition of food intake has been described in others (9–11). Whether this is due to differences in the animal model (mouse versus rat) or to the dose/route of administration remains to be deciphered and evidences the need for further research to clarify this role. Furthermore, the first studies using Tac1 null mice did not show any difference in body size compared to controls (9). However, our present data contradict that study and clearly evidence a metabolic phenotype in these animals, including significantly lower body weight in all studied conditions during adulthood.
Therefore, in this study we aim to further characterize the role of SP and NKA in the control of energy balance through a number of pharmacological, behavioral, and genetic studies using the Tac1 knockout mouse as our animal model, including the involvement of Tac1 in the etiology of hepatic steatosis and metabolic syndrome.
RESEARCH DESIGN AND METHODS
Animals
Tac1−/− and ob/ob mice were purchased at The Jackson Laboratories and maintained at Harvard Medical School in a 12:12LD cycle. Time of lights on (7:00am) is considered Zietgeber 0 (ZT0). Animals were housed at 21–22 C. All animal care and procedures were in accordance with guidelines of the Harvard University Institutional Animal Care and Use Committee.
Metabolic Measurements
Adult 12 - 20 week old mice were housed 3–4 mice per cage and fed regular chow (LabDiet #5053) or high fat diet (HFD) (LabDiet #58Y1; 61% fat, 21% carbohydrates, 18% proteins). Insulin and leptin were measured the morning (10:00h) of the final day on HFD (day 16). For the glucose tolerance test (GTT), adult males (n = 6/group) were fasted for 5h before the test and injected intraperitoneally (ip) with 2g/kg glucose (Sigma Aldrich) and glucose levels determined using a glucometer (Accu Check) by tail bleeds at 0, 20, 60 and 120 min.
Whole body composition was determined by MRI following manufacturer’s protocol before and after the Comprehensive Lab Animal Monitoring System (CLAMS) study. For this study, male mice (n = 3/group) were first left to acclimate to the metabolic cage for 24h (START) and then monitored for 4 days fed a HFD (RE-START). Lights and temperature were kept as in their home cages. In the remaining experiments where food intake (FI) was determined, food pellets were placed in small bowls and the amount of food remaining was subtracted from the previous determination.
Neutral lipids accumulation in the liver of double knockouts was determined using Oil Red 0 (ORO) staining and ImageJ (investigator blinded to the group) as described elsewhere (12).
Gene expression determinations
Total RNA was extracted from frozen tissue collected at ZT3 using TRIzol reagent (Invitrogen) followed by chloroform/isopropanol extraction. RNA was quantified using a NanoDrop 2000 spectrophotometer (Thermo Scientific) and 1 µg of RNA was reverse transcribed using Superscript III cDNA synthesis kit (Invitrogen). Quantitative real-time PCR assays were performed on an ABI Prism 7000 sequence detection system, and analyzed using ABI Prism 7000 SDS software (Applied Biosystems). The cycling conditions were the following: 2 min incubation at 95°C (hot start), 45 amplification cycles (95°C for 30 s, 60°C for 30 s, and 45 s at 75°C, with fluorescence detection at the end of each cycle), followed by melting curve of the amplified products obtained by ramped increase of the temperature from 55 to 95°C to confirm the presence of single amplification product per reaction. Information of specific primers in Table S1.
Hormone Determination
Leptin and insulin levels were measured using a Milliplex MAP immunoassay (mouse metabolic panel, Millipore) in the Luminex 200 following the manufacturer’s protocol.
Statistical Tests
Statistical analysis was performed using GraphPad Prism 6. Data are shown as mean ± SEM and the significance level was set as p < 0.05. Differences were determined using a two-tailed unpaired Student's t test or an ANOVA test followed by Newman-Keuls or Tukey post hoc tests.
RESULTS
1. Tac1−/− mice are leaner and resistant to high-fat-diet (HFD) induced obesity
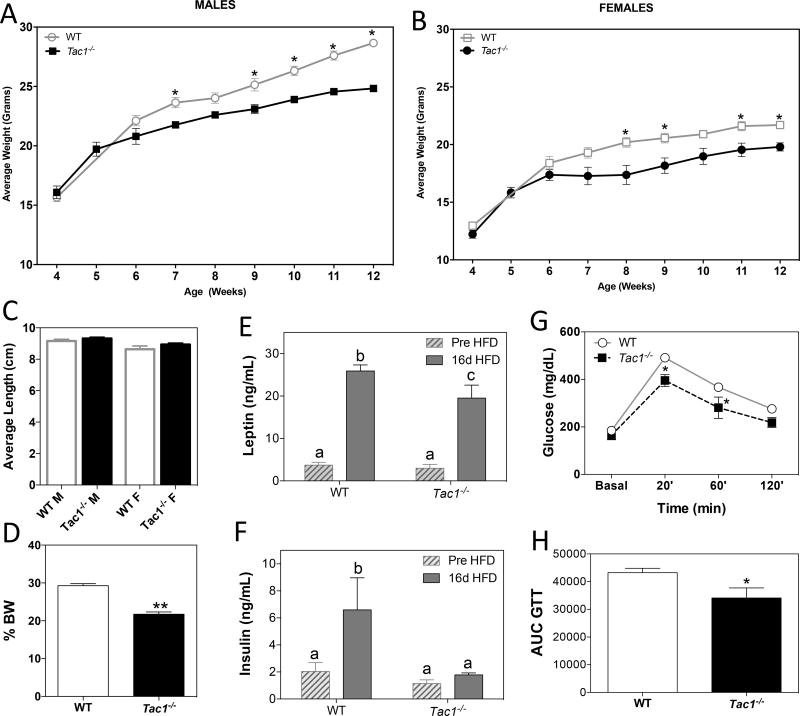
Body weight (BW) of male and female Tac1−/− mice was monitored from 4 to 12 weeks of age. At week 5, BW of Tac1−/− mice of both sexes started to deviate from their control littermates, becoming significantly leaner from 7–8 weeks of age onward (Figure 1 A & B), despite having similar body lengths (Figure 1 C). In order to stress the metabolic phenotype of these animals, adult males were subjected to HFD for 16 days. Proportionally, Tac1−/− mice gained significantly less BW than littermate controls (Figure 1 D). Despite the 20% increase in BW observed in Tac1−/− mice compared with their basal (pre-HFD) state and the significantly higher leptin levels at the end of the treatment (Figure 1 D & E), these animals did not develop the expected insulin resistance associated with HFD (13) at the end of the treatment, as seen in the control group (Figure 1 F). Also, Tac1−/− mice had better glucose tolerance than the WT control mice (Figure 1 G & H), in keeping with previous reports (9, 14), suggesting that SP induces insulin resistance.
Figure 1. Absence of Tac1 results in decreased body weight, resistance to diet-induced obesity and improved glucose tolerance.
(A and B) Weekly determination of BW in male (A) and female (B) WT and Tac1−/− mice (± SEM, *, p < 0.05, n = 3 - 6/group, Repeated measures Two Way ANOVA and Bonferroni post hoc test).
(C) Body length determined by nose to anus distance (39) (n = 3 – 6/group).
(D) Percentage of BW gain during 16 days on HFD (± SEM, n = 4/group, *p<0.05 Two Way ANOVA and Newman-Keuls post hoc test).
(E and F) Leptin (E) and insulin (F) levels (ng/mL) in serum at the end of the HFD treatment (±SEM, n = 4/group). Different letters indicate statistically different values (2 Way ANOVA followed by Newman-Keuls post hoc test, p < 0.05).
(G and H) (G) Glucose tolerance test (GTT) in adult males (n=5–6/group). *p<0.05 Repeated measures Two Way ANOVA and Bonferroni post hoc test. (H) Area under the curve (AUC) for glucose levels over 120 min *p<0.05 Student t-test compared with its respective control.
2. Metabolic characterization of Tac1 null mice in the dark vs. light phase
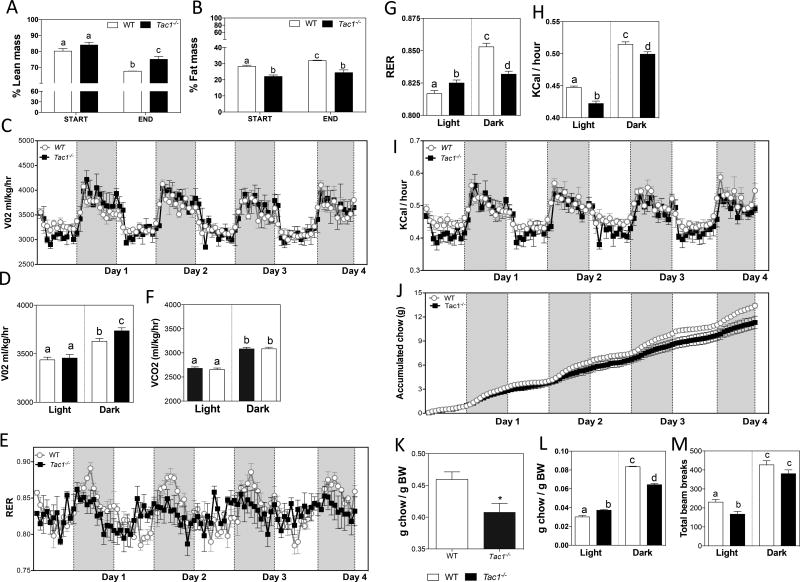
To further explore their metabolic phenotype, adult male mice were subjected to a CLAMS study for four days while fed a HFD. Body composition of these mice was determined by MRI before and after the HFD. Given the baseline BW difference between control and Tac1−/− mice, animals within 2 SD of the average BW of the two genotypes were selected to increase the accuracy of the metabolic comparisons. Lean mass between control and Tac1−/− mice was significantly higher in Tac1−/− mice than controls after HFD for 4 days (Figure 2 A). However, fat mass was lower in Tac1−/− mice than in controls throughout the experiment (Figure 2 B). The metabolic rate of these animals was determined by O2 consumption and CO2 production. Tac1−/− mice displayed higher O2 consumption than controls at night (Figure 2 C & D), while CO2 values were similar between groups (Figure 2 E). Interestingly, the respiratory exchange ratio (RER) was flattened in Tac1−/− mice, indicating decreased circadian rhythm in this otherwise highly oscillatory marker (Figure 2 F). When this ratio was calculated in light vs. dark phases, a robust suppression of the circadian rhythm in Tac1−/− mice compared with controls became evident (Figure 2 G). Energy expenditure, determined as Kcal/hour, was reduced during both phases in knockouts (Figure 2 H & I). RER and Kcal/hour are indicators of the food intake pattern. The blunted oscillations in RER and the overall lower heat production suggested a disruption in the amount and time that food was consumed. Indeed, an overall lower food intake was seen in Tac1−/− mice (Figure 2 J & K). However, they ate more than controls during the day and less during the night (Figure 2 L), which was in keeping with the flattening of diurnal RER values, indicating continued food consumption during the day and consistent with the attenuation of the circadian feeding behavior. Moreover, despite the fact that the light phase is the period of lower activity in the mouse, Tac1−/− mice showed even less activity than controls (Figure 2 M). Whether this is due to a further inhibition of the suprachiasmatic nucleus (SCN) mechanisms that control circadian activity (15) or to the fact that Tac1−/− mice spent more time eating during this period cannot be determined and could contribute to the lower energy expenditure (Figure 2 E & G).
Figure 2. Metabolic characterization of Tac1 null mice under HFD.
(A and B) Percentage of lean (A) and fat (B) mass determined by MRI before (START) and after (END) four days of HFD (± SEM, n = 3/group). Different letters indicate statistically different values (2 Way ANOVA followed by Newman-Keuls post hoc test, p < 0.05).
(C – M) Metabolic rate, indicated by VO2 (ml/kg/hr) (C and D) and VCO2 (ml/kg/hr) (E); respiratory exchange ratio (RER) (F and G); heat produced, indicated by Kcal/hr (H and I); accumulated food intake (J – L); and locomotor activity, indicated by total beam breaks (M) during the dark and light phases (± SEM, n = 3/group). Different letters indicate statistically different values (2 Way ANOVA followed by Newman-Keuls post hoc test, p < 0.05). (H) *p < 0.05 Student t-test
3. Expression of circadian genes in the absence of Tac1
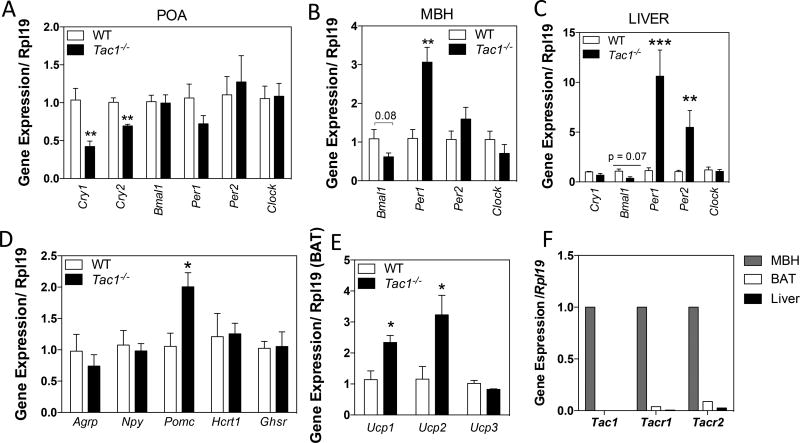
The previous experiment revealed a differential behavioral pattern in Tac1−/− mice compared with their WT controls, suggesting an alteration of the mechanisms that control circadian rhythms in these mice. Thus, we hypothesized that the absence of the Tac1 products would alter the expression of clock genes involved in the control of these rhythmic patterns. Indeed, Tac1−/− mice displayed significantly lower Cry1 and Cry2 levels in the preoptic area (POA, which includes the SCN) at 10:00h (Figure 3 A)—when expression levels are otherwise maximal (16), higher Per1 expression and a trend of lower Bmal1 expression in the mediobasal hypothalamus (MBH, where the main metabolic centers are located) (Figure 3 B), and higher levels of Per1 and Per2 in the liver (Figure 3 C).
Figure 3. Tac1 gene products regulate the circadian clock machinery, hypothalamic metabolic neuropeptides and activate BAT.
(A – C) Expression level of the circadian clock machinery genes in the preotic area (POA) (A), mediobasal hypothalamus (MBH) (B) and liver (C) measured by qRT-PCR and normalized to Rpl19 at ZT3 (10:00am).
*p < 0.05, **p < 0.01, ***p < 0.001. Student t-test, compared with control littermates.
(D) Increased Pomc levels, but not Agrp, Npy, Hcrt1 or Ghsr levels, in the MBH of adult male mice (± SEM, n=5/group). *, p<0.05, Student t-test, compared with control littermates.
(E) Brown adipose tissue (BAT) activation observed by increased Ucp1 and Ucp2 in Tac1−/− male mice (±SEM, n=5/group). *, p<0.05, Student t-test, compared with control littermates.
(F) Expression level of Tac1, Tacr1 and Tacr2 in MBH. BAT and Liver of adult WT male mice measured by qRT-PCR and normalized to Rpl19.
4. The expression of hypothalamic POMC and brown adipose tissue Ucp1 and Ucp2 is altered in the absence of Tac1
Tac1−/− mice display an overall reduction in calorie intake (Figure 2 J-L). Food intake is determined by the interaction of hypothalamic neuropeptides that respond to peripheral indicators of fuel availability, e.g. leptin, ghrelin or insulin (17). Thus, we analyzed the gene expression of the food intake regulators Agouti-related peptide (Agrp), neuropeptide Y (Npy), proopiomelanocortin (Pomc), orexin (Hcrt1) and ghrelin receptor (Ghsr) in the MBH and observed that Tac1−/− mice displayed a significant elevation in Pomc expression (Figure 3 D). Additionally, BAT plays an important role in energy homeostasis by increasing heat production (18). In Tac1−/− mice, BAT is activated as seen by the elevation of gene expression of the uncoupling proteins 1 and 2 (Ucp1 and Ucp2), but not Ucp3 (Figure 3 E), despite the lack of Tac1 (or receptors for the Tac1 products) in the BAT (Figure 3 F), suggesting an indirect activation of the BAT.
5. Tac1 deficiency improves hepatic steatosis of obese (ob/ob) mice
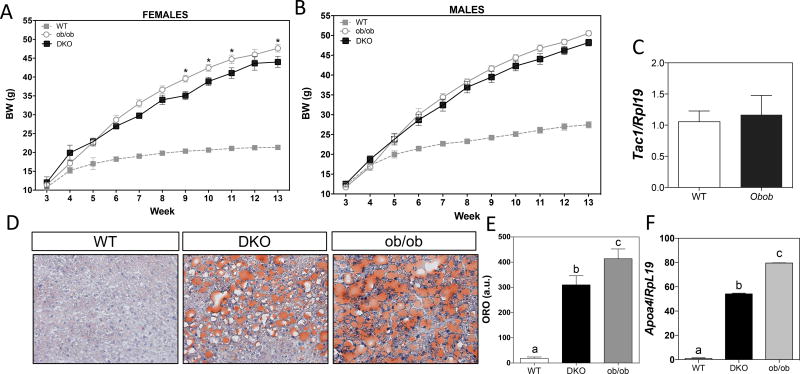
To further explore the role of Tac1 in the control of metabolism, we assessed the effect of Tac1 deletion in severely obese leptin-deficient (ob/ob) mice. Ob/ob:Tac1−/− double knockout, DKO) mice showed a modest, but significant, reduction of BW in females (but not in males) after 9 weeks (Figure 4 A & B). Of note, Tac1 expression (at least in the MBH) is not subject to regulation by leptin (Figure 4 C), suggesting that the action of Tac1 is independent of the action of leptin or limited to post-transcriptional processes. We next aimed to investigate whether the increase in insulin sensitivity and changes in the expression of circadian genes observed in our previous experiments in males lacking a functional Tac1 gene, was enough to improve metabolic syndrome and liver steatosis (present in obese mice) in DKO males. Interestingly, DKO mice had significantly lower lipid content in the liver (Figure 4 D & E). In addition, they displayed significantly lower levels of the plasma triglyceride marker ApoA4 (Figure 4 F). These data indicate an improvement in liver fat accumulation and reduced circulating triglyceride levels compared to age and, importantly, weight matched ob/ob mice, indicating that the reduction of their liver steatosis is not (indirectly) due to changes in BW.
Figure 4. Absence of Tac1 reduces hepatic steatosis in ob/ob mice.
(A – B) BW over 13 weeks of WT, ob/ob and double knockouts (DKO, ob/ob:Tac1−/−) female (A) and male (B) mice. (n=4–7/group).
(C) Tac1 expression in the MBH of WT and ob/ob mice by qRT-PCR (n=3/group). *p < 0.05. 1 Way ANOVA followed by Tukey post hoc test, compared with its respective control.
(D) Representative images of neutral lipid accumulation in the liver by oil red 0 (ORO) staining.
(E) Neutral lipid accumulation in the liver. Different letters indicate statistically different values (1 Way ANOVA followed by Tukey post hoc test, p < 0.05).
(F) Expression level of the triglyceride marker Apoa4 in DKO compared with ob/ob mice (n=4–5/group). *p < 0.05, **p < 0.01, ****p < 0.0001; 1 Way ANOVA followed by Tukey post hoc test.
DISCUSSION
Recent studies have demonstrated a role of SP in the control of metabolism (9–11, 14), but the mechanism of action of the orexigenic role of SP (or NKA) requires further examination. Here we have expanded the characterization of this metabolic component of the Tac1 products and further described the metabolic phenotype of Tac1 null mice that was not documented in previous publications.
SP levels correlate with obesity in humans and rodents (9, 19, 20). While disruption of SP/NK1R signaling had been linked to reduced FI (9), conflicting studies had reported stimulation, no action, or even inhibition of FI by SP (9, 10, 14). Our studies have demonstrated that Tac1−/− mice fed a regular chow diet display significantly lower BW in adulthood and gain significantly lower BW after 60% HFD treatment for 16 days. These latter results are consistent with previous studies by Karagiannides and colleagues in the receptor deficient Tacr1−/− mice (14) but contradict a different report by the same group in Tac1−/− mice, where no changes in BW were detected after 45% HFD (11). This discrepancy between both Tac1−/− models may be derived from the different diet and/or length of the treatment. However, no characterization of the BW in Tac1−/− mice under regular chow diet had been previously reported, so this cannot be compared between both models. Importantly, sex steroids also influence metabolic function through their anorectic action (21). In this context, it is important to mention that while our previous studies have identified a reproductive impairment in male and female Tac1−/− mice, this was limited to the timing of puberty onset (delayed) in both sexes and central induction of ovulation in the female (4, 22). Adult WT and Tac1−/− females displayed the same LH levels in the intact and ovariectomized states (4). Similarly, adult WT and Tac1−/− males displayed the same LH and testosterone levels (22). These data exclude the possibility that different circulating levels of sex steroids could account for the metabolic phenotype describes in Tac1 deficient mice. Moreover, hypogonadism, at least in female rodents, leads to an increase in BW (21), which is opposite to the phenotype observed in Tac1−/− mice.
Interestingly, our studies uncovered a potential role of SP in the control of circadian feeding behavior. Recent studies have provided evidence for the bidirectional interaction between diet and the diurnal pattern of feeding (15, 23). Mice restrict their feeding mostly to the nocturnal phase of the day and restricting food to this phase prevents obesity in animals fed a HFD (24), demonstrating the importance of the circadian feeding behavior. Nonetheless, the mechanisms that link environmental light cues to the regulation of feeding remain unknown. Interestingly, SP is present in the retina, optic nerve and SCN, and is involved in the transmission of photic information from the retina to the SCN (25–30). The SCN is the pacemaker of circadian oscillations. This hypothalamic nucleus holds transcriptional and translational feedback mechanisms that determine the alternation of circadian processes between the light and dark phases by regulating the expression of the clock genes, e.g. Clock, Bmal1, Cry1, Cry2, Per1 and Per2 (23). However, how the SCN integrates the information on light cycles from the retina to synchronize circadian rhythms is unknown. The present studies suggest that Tac1 may be involved in the transmission of this information to the SCN to regulate feeding behavior in a murine model, and open the door for further research to uncover its mechanism of action. The absence of a functional Tac1 gene flattened the circadian RER oscillation as a consequence of higher FI during the light phase and lower during the dark phase compared with controls. The greater inhibition to seek food during the night led to an overall reduction of FI and therefore BW in Tac1−/− animals, consistent with previous pharmacological studies where NK1R antagonists inhibited food intake in mice (9). Of note, HFD is known to disrupt feeding patterns in WT animals (31); however, in our experiment (Figure 2) control WT animals fed a HFD retained their normal feeding behavior (mostly nocturnal), which excludes diet as the source of the disruption in the feeding rhythm in Tac1−/− mice. Importantly, the disruption of the circadian clock machinery, namely Cry1/2, Per1/2, Clock and Bmal1 leads to metabolic diseases in mice (23). We observed that at ZT3, the absence of Tac1 reduced Cry1 and Cry2 expression in the POA, where the SCN is located. In this vein, the resistance to gain BW of Tac1−/− mice under a HFD replicates the phenotype of Cry1−/− mice under the same diet-induced obesity protocol (32). This supports the existence of a SP-Cry pathway in the control of energy balance. Furthermore, despite the unknown role of circadian genes in other tissues, Tac1−/− mice displayed increased Per1 in the MBH, where the central metabolic regulators are located (e.g. AgRP and POMC neurons) and increased Per1 and Per2 expression in the liver, probably as consequence of the altered feeding behavior (33). Future studies will be aimed at determining the magnitude of the changes in the oscillation of these clock genes throughout the day in the absence of Tac1, as well as the neuroanatomical source of SP.
Importantly, these animals showed an increase in the melanocortin pathway, displaying higher Pomc expression, which encodes the anorexigenic peptide α-MSH, and a trend to lower AgRP levels. A recent characterization of the transcriptome of ARC neurons documented the presence, albeit at low levels, of Tac1r, but not Tacr2, in POMC and AgRP neurons (34). However, whether SP can modulate the action of POMC and/or AgRP neurons directly remains to be deciphered. Nonetheless, these data suggested that the overall reduction in food intake observed in these mice may be due to the activation of the melanocortin pathway. Importantly, whether this reduction in food intake leads, in turn, to further alteration in the expression of the circadian genes, remains to be deciphered.
Furthermore, in order to address whether the leaner phenotype of Tac1KO mice could also involve the activation of mechanisms of heat dissipation, we investigated the effect of SP absence on the activation of the thermogenic markers Ucp1, Ucp2 and Ucp3 in BAT. Indeed, we observed that Ucp1 and Ucp2 were significantly upregulated. The activation of BAT is a critical component in energy homeostasis and, therefore, a likely contributor to the leaner phenotype seen in Tac1−/− mice. It is also possible that Tac1−/− mice are more sensitive to a moderate cold stress (21–22 C) that leads to further activation of BAT in null animals. Paradoxically, these mice showed lower energy expenditure under HFD (Figure 2H), probably due to the fact that a larger BW and higher leptin levels in WT mice under HFD (Figure 1E) increased their energy expenditure (35) above the level of Tac1−/− mice. However, neither Tac1 nor Tacr1 mRNA were detected in BAT (Figure 3F). Interestingly, this may suggest a central action through the sympathetic nervous system, based on a previous study (36) that showed activation of BAT through an E2-dependent decrease in AMP-activated protein kinase (AMPK) at the central level. In this context, it has been demonstrated that SP can stimulate AMPK (37), therefore, we can speculate that the lack of SP may lead to a decrease in AMPK and, therefore, to the activation of BAT (mimicking the E2 effect).
Finally, we hypothesize that if the absence of SP activates the melanocortin pathway and heat dissipation, removing this gene from a model of severe obesity, e.g. leptin deficiency (ob/ob mice), would lead to an improvement of their metabolic phenotype. Previous studies had reported a robust decrease in BW in ob/ob mice after NK1R antagonist treatment (9). Thus, we aimed to use a model of double Tac1−/− and ob/ob knockouts (DKO) to address our hypothesis. We observed that female, but not male, DKO mice showed a modest but significant reduction of BW in comparison to ob/ob mice at the end of the study, suggesting that a portion of the obese phenotype, at least in obese female mice, may come from the effect of SP. Interestingly, Tac1 is highly enriched in LepRb neurons (38), suggesting that leptin may inhibit the release of SP from leptin responsive neurons as part of its anorexic role. If this is true, the ablation of SP release in ob/ob mice may account for the decrease in BW observed (at least in females). Still, in males, despite the absence of a BW difference between DKO and ob/ob mice, DKO mice displayed a significant improvement in the triglyceride marker Apoa4 in the liver, as well as significantly reduced hepatic steatosis compared with ob/ob mice as seen by decreased lipid content.
In summary, the present results add further evidence for the role of SP in the control of food intake, which may involve a role in the control of circadian feeding behavior and its interactions with the melanocortin system and the peripheral stimulation of energy expenditure through BAT activation. These data placed SP as a critical player in the development of metabolic diseases (e.g., obesity, type 2 diabetes, hypertriglyceridemia, NASH/NAFDL), suggesting that SP blockade may serve as a novel therapeutic target for their treatment.
Supplementary Material
Acknowledgments
We thank Dr. Alex Banks and Katherine Leclair for their help with the CLAMS studies. This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development through cooperative agreement U54 HD028138, R01 HD019938 and R01 HD082314 to U.B.K.; R00 HD071970, Charles H. Hood Foundation for Child Health Research Program to V.M.N.
Footnotes
Disclosure statement: The authors have nothing to disclose.
References
- 1.Onaga T. Tachykinin: recent developments and novel roles in health and disease. Biomol Concepts. 2014;5(3):225–43. doi: 10.1515/bmc-2014-0008. [DOI] [PubMed] [Google Scholar]
- 2.Navarro VM, Bosch MA, Leon S, Simavli S, True C, Pinilla L, et al. The integrated hypothalamic tachykinin-kisspeptin system as a central coordinator for reproduction. Endocrinology. 2015;156(2):627–37. doi: 10.1210/en.2014-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruiz-Pino F, Garcia-Galiano D, Manfredi-Lozano M, Leon S, Sanchez-Garrido MA, Roa J, et al. Effects and interactions of tachykinins and dynorphin on FSH and LH secretion in developing and adult rats. Endocrinology. 2015;156(2):576–88. doi: 10.1210/en.2014-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simavli S, Thompson IR, Maguire CA, Gill JC, Carroll RS, Wolfe A, et al. Substance p regulates puberty onset and fertility in the female mouse. Endocrinology. 2015;156(6):2313–22. doi: 10.1210/en.2014-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navarro VM. New insights into the control of pulsatile GnRH release: the role of Kiss1/neurokinin B neurons. Frontiers in endocrinology. 2012;3:48. doi: 10.3389/fendo.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruiz-Pino F, Navarro VM, Bentsen AH, Garcia-Galiano D, Sanchez-Garrido MA, Ciofi P, et al. Neurokinin B and the control of the gonadotropic axis in the rat: developmental changes, sexual dimorphism, and regulation by gonadal steroids. Endocrinology. 2012;153(10):4818–29. doi: 10.1210/en.2012-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodman RL, Coolen LM, Lehman MN. A role for neurokinin B in pulsatile GnRH secretion in the ewe. Neuroendocrinology. 2014;99(1):18–32. doi: 10.1159/000355285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navarro VM, Kaiser UB. Metabolic influences on neuroendocrine regulation of reproduction. Current opinion in endocrinology, diabetes, and obesity. 2013;20(4):335–41. doi: 10.1097/MED.0b013e32836318ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karagiannides I, Torres D, Tseng YH, Bowe C, Carvalho E, Espinoza D, et al. Substance P as a novel anti-obesity target. Gastroenterology. 2008;134(3):747–55. doi: 10.1053/j.gastro.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trivedi C, Shan X, Tung YC, Kabra D, Holland J, Amburgy S, et al. Tachykinin-1 in the central nervous system regulates adiposity in rodents. Endocrinology. 2015;156(5):1714–23. doi: 10.1210/en.2014-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karagiannides I, Bakirtzi K, Kokkotou E, Stavrakis D, Margolis KG, Thomou T, et al. Role of substance P in the regulation of glucose metabolism via insulin signaling-associated pathways. Endocrinology. 2011;152(12):4571–80. doi: 10.1210/en.2011-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehlem A, Hagberg CE, Muhl L, Eriksson U, Falkevall A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat Protoc. 2013;8(6):1149–54. doi: 10.1038/nprot.2013.055. [DOI] [PubMed] [Google Scholar]
- 13.Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR) J Clin Invest. 1997;100(5):1166–73. doi: 10.1172/JCI119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karagiannides I, Stavrakis D, Bakirtzi K, Kokkotou E, Pirtskhalava T, Nayeb-Hashemi H, et al. Substance P (SP)-neurokinin-1 receptor (NK-1R) alters adipose tissue responses to high-fat diet and insulin action. Endocrinology. 2011;152(6):2197–205. doi: 10.1210/en.2010-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bechtold DA, Loudon AS. Hypothalamic clocks and rhythms in feeding behaviour. Trends Neurosci. 2013;36(2):74–82. doi: 10.1016/j.tins.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Morse D, Sassone-Corsi P. Time after time: inputs to and outputs from the mammalian circadian oscillators. Trends Neurosci. 2002;25(12):632–7. doi: 10.1016/s0166-2236(02)02274-9. [DOI] [PubMed] [Google Scholar]
- 17.Waterson MJ, Horvath TL. Neuronal Regulation of Energy Homeostasis: Beyond the Hypothalamus and Feeding. Cell Metab. 2015 doi: 10.1016/j.cmet.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 18.Argyropoulos G, Harper ME. Uncoupling proteins and thermoregulation. J Appl Physiol (1985) 2002;92(5):2187–98. doi: 10.1152/japplphysiol.00994.2001. [DOI] [PubMed] [Google Scholar]
- 19.Baroncelli GI, Bertelloni S, Buggiani B, Papini A, Gualtieri M, Saggese G. Evidence of increased levels of substance P in obese children. Funct Neurol. 1989;4(2):183–4. [PubMed] [Google Scholar]
- 20.Fu J, Liu B, Liu P, Liu L, Li G, Wu B, et al. Substance P is associated with the development of obesity, chronic inflammation and type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2011;119(3):177–81. doi: 10.1055/s-0030-1261965. [DOI] [PubMed] [Google Scholar]
- 21.Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, Lai J, Ciofi P, McMullen NT, et al. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153(6):2800–12. doi: 10.1210/en.2012-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maguire CA, Song YB, Wu M, Leon S, Carroll RS, Alreja M, et al. Tac1 Signaling is Required for Sexual Maturation and Responsiveness of GnRH Neurons to Kisspeptin in the Male Mouse. Endocrinology. 2017 doi: 10.1210/en.2016-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol Rev. 2013;93(1):107–35. doi: 10.1152/physrev.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15(6):848–60. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abe H, Honma S, Shinohara K, Honma K. Substance P receptor regulates the photic induction of Fos-like protein in the suprachiasmatic nucleus of Syrian hamsters. Brain Res. 1996;708(1–2):135–42. doi: 10.1016/0006-8993(95)01298-2. [DOI] [PubMed] [Google Scholar]
- 26.Gannon RL, Millan MJ. The selective tachykinin neurokinin 1 (NK1) receptor antagonist, GR 205,171, stereospecifically inhibits light-induced phase advances of hamster circadian activity rhythms. Eur J Pharmacol. 2005;527(1–3):86–93. doi: 10.1016/j.ejphar.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Kim DY, Kang HC, Shin HC, Lee KJ, Yoon YW, Han HC, et al. Substance p plays a critical role in photic resetting of the circadian pacemaker in the rat hypothalamus. J Neurosci. 2001;21(11):4026–31. doi: 10.1523/JNEUROSCI.21-11-04026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim YI, Kim SH, Kim DY, Lee HW, Shin HC, Chung JM, et al. Electrophysiological evidence for the role of substance P in retinohypothalamic transmission in the rat. Neurosci Lett. 1999;274(2):99–102. doi: 10.1016/s0304-3940(99)00681-3. [DOI] [PubMed] [Google Scholar]
- 29.Mick G, Shigemoto R, Kitahama K. Localization of substance P receptors in central neural structures controlling daily rhythms in nocturnal rodents. C R Acad Sci III. 1995;318(2):209–17. [PubMed] [Google Scholar]
- 30.Takatsuji K, Oyamada H, Tohyama M. Postnatal development of the substance P-, neuropeptide Y- and serotonin-containing fibers in the rat suprachiasmatic nucleus in relation to development of the retino-hypothalamic projection. Brain Res Dev Brain Res. 1995;84(2):261–70. doi: 10.1016/0165-3806(94)00209-i. [DOI] [PubMed] [Google Scholar]
- 31.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6(5):414–21. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Griebel G, Ravinet-Trillou C, Beeske S, Avenet P, Pichat P. Mice deficient in cryptochrome 1 (cry1 (−/−)) exhibit resistance to obesity induced by a high-fat diet. Front Endocrinol (Lausanne) 2014;5:49. doi: 10.3389/fendo.2014.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci U S A. 2009;106(50):21453–8. doi: 10.1073/pnas.0909591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell JN, Macosko EZ, Fenselau H, Pers TH, Lyubetskaya A, Tenen D, et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat Neurosci. 2017;20(3):484–96. doi: 10.1038/nn.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scarpace PJ, Matheny M, Pollock BH, Tumer N. Leptin increases uncoupling protein expression and energy expenditure. Am J Physiol. 1997;273(1 Pt 1):E226–30. doi: 10.1152/ajpendo.1997.273.1.E226. [DOI] [PubMed] [Google Scholar]
- 36.Martinez de Morentin PB, Gonzalez-Garcia I, Martins L, Lage R, Fernandez-Mallo D, Martinez-Sanchez N, et al. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 2014;20(1):41–53. doi: 10.1016/j.cmet.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dubon MJ, Byeon Y, Park KS. Substance P enhances the activation of AMPK and cellular lipid accumulation in 3T3L1 cells in response to high levels of glucose. Mol Med Rep. 2015;12(6):8048–54. doi: 10.3892/mmr.2015.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allison MB, Patterson CM, Krashes MJ, Lowell BB, Myers MG, Jr, Olson DP. TRAP-seq defines markers for novel populations of hypothalamic and brainstem LepRb neurons. Mol Metab. 2015;4(4):299–309. doi: 10.1016/j.molmet.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gargiulo S, Gramanzini M, Megna R, Greco A, Albanese S, Manfredi C, et al. Evaluation of growth patterns and body composition in C57Bl/6J mice using dual energy X-ray absorptiometry. Biomed Res Int. 2014;2014:253067. doi: 10.1155/2014/253067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.