Abstract
Objective
To evaluate risk factors for mortality, including healthcare insurance status, among patients with the AIDS in the era of modern combination antiretroviral therapy (cART).
Design
Prospective, multicenter, observational study, the Longitudinal Study of the Ocular Complications of AIDS (LSOCA)
Methods
Patients were classified as having private healthcare insurance, Medicare, Medicaid, or no insurance. Hazard ratios for death were calculated using proportional hazards regression models and staggered entries, anchored to the AIDS diagnosis date.
Results
Among 2363 participants with AIDS, 97% were treated with cART. At enrollment, 31% of participants had private insurance, 29% Medicare, 24% Medicaid, and 16% were uninsured. Non-infectious, age-related diseases, such as hypertension, diabetes, and renal disease were more frequent among persons with Medicare than among those with private insurance. Mortality vs. privately-insured was greater among participants with Medicare (adjusted hazard ratio [HRadj] = 1.35; 95% confidence interval [CI] 1.09–1.67; P=0.008). Among participants with suppressed HIV viral load, HRsadj for mortality vs. privately-insured were: Medicare, 1.93 (95% CI 1.08–3.44; P=0.02) and Medicaid 2.09 (95% CI 1.02–4.27; P=0.04). Mortality among initially uninsured participants was not significantly different than for privately-insured, but these participants typically obtained antiretroviral therapy and insurance during follow-up. Time-updated HRsadj for mortality vs. privately-insured were: Medicare 1.34 (95% CI 1.05–1.70; P=0.02), Medicaid 1.34 (95%CI 1.01–1.80; P=0.05), and uninsured 1.35 (95% CI 0.97–1.88; P=0.05).
Conclusions
Compared to persons with AIDS and private insurance, persons with public Insurance have increased mortality, possibly due to a greater burden of non-Infectious, age-related diseases.
Keywords: Acquired immune deficiency syndrome, Mortality, Health insurance
Mortality among human immunodeficiency virus (HIV)-infected persons decreased dramatically with the introduction of modern combination antiretroviral therapy (cART).1–6 Although lower than in the era before cART, mortality remains higher than that among comparably aged HIV-uninfected persons, largely due to an increase in common age-related, non-infectious chronic diseases that occur among HIV-infected persons.7–11 Although traditional HIV-related risk factors for mortality, such as the level of plasma HIV RNA (HIV viral load), and CD4+ T-lymphocyte cell counts, are well established, other socio-demographic factors are less so. The HIV Outpatient Study (HOPS) suggested that mortality was greater among cART-treated, virally-suppressed, HIV-infected persons with public health insurance compared to persons with private insurance, and that public insurance may be a marker for chronic non-infectious, age-related co-morbidities.12 We evaluated risk factors for mortality in the Longitudinal Study of the Ocular Complications of AIDS (LSOCA).
Patients and Methods
The LSOCA is a prospective, multicenter, observational study of patients with AIDS conducted in the era of modern cART.13,14 Eligible participants were age 13 years or older and had a diagnosis of the acquired immunodeficiency syndrome (AIDS) according to the Centers for Disease Control and Prevention 1993 revised surveillance case definition.15 Enrollment occurred between 1 September 1998 and 31 July 2011; follow-up continued through 31 July 2013. The study and its procedures were approved by the institutional review boards of the clinical and resource centers and adhered to the principles of the Declaration of Helsinki. All participants gave written, informed consent.
Enrollment data collection included demographic information, medical history (including a complete medication history), nadir CD4+ T cell counts prior to enrollment, maximum HIV viral load prior to enrollment, estimate of Karnofsky performance score, and a complete ophthalmologic examination for the presence of cytomegalovirus (CMV) retinitis.13,14,16 Standard 60° retinal photographs were taken and graded at a Reading Center.13,14,17 Laboratory measurements at enrollment included a complete blood count, CD4+ T cell counts, plasma HIV viral load, and hepatitis C virus (HCV) antibody status.13,14,18 Elements of the history were verified from medical records. Insurance status was classified as private insurance, Medicare, Medicaid, CHAMPUS or Veterans’ Hospital coverage, and uninsured. In the United States, Medicare is a federally-funded program for the elderly and disabled, Medicaid is a federally- and state-funded program for low-income individuals, administered by the individual states, and private insurance is provided by commercial companies and purchased most often by employers (as a benefit) or by individuals. Because of the small number of participants with CHAMPUS (n=10) or Veterans’ Administration (n=15) coverage, they were excluded from the analyses; four participants had no insurance data collected. Patients with private insurance and Medicare or Medicaid (N=153) were classified as having private insurance, and patients with both Medicare and Medicaid (N=358) were classified as having Medicare. Sensitivity analyses classifying these participants with more than one type of insurance as separate groups gave qualitatively similar results (data not shown). Combination antiretroviral therapy was defined as: any 3-drug regimen including a protease inhibitor, a non-nucleoside reverse transcriptase inhibitor, or a fusion, integrase, or entry inhibitor or regimens with similar efficacy, such as triple nucleoside reverse transcriptase inhibitor therapy, or dual boosted protease inhibitor therapy.6,19 Non-AIDS co-morbidities were ascertained from the medical history and current medication log. The Karnofsky performance score was estimated by the enrolling physician and was graded between 100 (perfect health) and 0 (death) in 10-point decrements. A score of 80 points corresponds to a health status of “normal activity with effort; some signs and symptoms of disease”.16 Measurements of CD4+ T cells and HIV viral load were performed at the individual clinical centers using standard assays, and HCV antibody measurements were performed at a central laboratory on cryopreserved plasma specimens.18
Characteristics of CMV retinitis lesions were determined from retinal photographs graded at the Reading Center by trained graders masked to other participant clinical features and outcomes, as previously described.6,13,14,17,20–24 Best corrected visual acuity was measured after standardized refraction using logarithmic visual acuity charts.13,14,17,24 Visual impairment was defined as a best corrected acuity of worse than 20/40; and bilateral visual impairment was defined as best corrected acuity worse than 20/40 in both eyes.25,26
Follow-up visits occurred every three months for patients with an ocular opportunistic infection (e.g. CMV retinitis) and every six months for all others until 2008 when follow-up became every six months for all participants. Mortality data were collected on an ongoing basis with confirmation of death obtained from death certificates or the National Death Index. Use of the National Death Index permitted obtaining mortality data on participants lost to follow-up.
Statistical analyses
Variables were categorized using biologically meaningful cutoffs, or by medians and quartiles. Comparisons of insurance status with enrollment characteristics were made using the Pearson Χ2 test for categorical variables and the Kruskal-Wallis test for continuous variables.27 Follow-up time was calculated as time from study enrollment until death, loss to follow-up, or administrative censoring. In order to anchor data to a biologically meaningful date, and to account for the duration of AIDS prior to enrollment, survival analyses were performed using staggered entries anchored at the AIDS diagnosis date.28,29 Analyses using only study follow-up time and adjusting for duration of AIDS, and not using staggered entries, produced similar results (data not shown). Cox proportional hazard regression was used to estimate hazard ratios for death.30 Models were both unadjusted and adjusted for demographic data and known risk factors for mortality in patients with AIDS (e.g. cART use, CD4+ T cells, plasma HIV viral load, and CMV disease). Because weight at enrollment was a predictor of mortality and had an 11% missing rate, multiple imputation was used for the time-independent regression analyses adjusting for weight. Observations with missing weight at enrollment were imputed using 5 datasets. The imputation model included all baseline covariates. Statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC) and Stata release 13 (StataCorp, College Station, TX).
Results
Characteristics of the study population
There were 2392 participants enrolled in LSOCA, of which 2363 are included in these analyses. Enrollment characteristics of the study population by insurance status are presented as Table 1. There were evident differences by insurance status. Participants with private insurance had the highest proportion of men (90.2%) and White, non-Hispanic persons (67.3%); participants with Medicaid had the highest proportion of women (38.0%) and African American persons (53.2%). Privately-insured participants were most likely to have male to male sexual contact as their HIV transmission risk (76.8%) and least likely to have a history of injection drug use (4.1%); participants with Medicaid were most likely to have injection drug use (19.8%) and heterosexual contact (40.4%) as their HIV transmission risk. Privately-insured patients were most likely to be employed at enrollment (52.2%); those with Medicare (7.1%) and Medicaid (6.4%) were less likely. Patients with Medicare (78.9%) and Medicaid (69.1%) were most likely to be considered disabled. Although uninsured participants had a significantly lower enrollment cART use (77.2%, P=0.0009), cART use at any time prior to or at enrollment or during follow-up ranged from 96.5 to 97.8% of participants for all insurance groups and was not significantly different (P=0.12) across groups. Uninsured participants had lower enrollment CD4+ T cell counts (median 151 cells/μL) than the other groups (medians 171 to 200 cells/μL, P=0.002). There were no significant differences in nadir CD4+ T cell counts or enrollment HIV viral loads among the four insurance groups. Maximum HIV viral load prior to study enrollment was lower among participants with private insurance (5.3 log10[copies/mL]) than the other three groups (5.4 log10[copies/mL], P=0.02). Cytomegalovirus retinitis was present more often among privately-insured participants (24.9%) or those with Medicare (22.4%) than among those with Medicaid (17.3%) or who were uninsured (17.5%). Privately-insured participants were most likely to have an enrollment Karnofsky performance score ≥80 (87.1%), whereas Medicaid recipients had the lowest proportion with a Karnofsky score ≥80 (70.9%). Some non-AIDS co-morbidities, including hypertension, diabetes, and renal disease, appeared to be most frequent among participants with Medicare and least frequent among uninsured participants (Table 1).
Table 1.
Characteristics at Enrollment by Insurance Type of Patients with AIDS in the Longitudinal Study of the Ocular Complications of AIDS
| Characteristic | Total | Insurance Status* | P-value | |||
|---|---|---|---|---|---|---|
| Private | Medicare | Medicaid | Uninsured | |||
| Number participants | 2363 | 743 | 688 | 560 | 372 | |
| Age (years) | <0.0001 | |||||
| Median | 43 | 44 | 44 | 41 | 40 | |
| 25th, 75th percentile | 37, 49 | 39, 50 | 39, 49 | 35, 47 | 34, 46 | |
| Gender (%) | <0.0001 | |||||
| Men | 80.4 | 90.2 | 84.3 | 62.0 | 81.2 | |
| Women | 19.6 | 9.8 | 15.7 | 38.0 | 18.8 | |
| Race/Ethnicity (%) | <0.0001 | |||||
| White, non-Hispanic | 45.4 | 67.3 | 45.1 | 24.8 | 33.1 | |
| Black, non-Hispanic | 36.5 | 18.7 | 39.1 | 53.2 | 42.2 | |
| Hispanic | 14.2 | 10.3 | 11.8 | 17.5 | 21.5 | |
| Other | 3.9 | 3.6 | 4.1 | 4.5 | 3.2 | |
| Education (%) | <0.0001 | |||||
| Less than high school grad | 16.4 | 2.4 | 15.0 | 32.4 | 23.2 | |
| Secondary- high school grad | 23.2 | 10.4 | 25.7 | 30.6 | 32.7 | |
| Some college or college grad | 48.0 | 59.5 | 51.4 | 33.3 | 40.8 | |
| Post-college | 12.4 | 27.7 | 7.8 | 3.6 | 3.2 | |
| Employment status at enrollment (%) | <0.0001 | |||||
| Disabled | 57.2 | 34.7 | 78.9 | 69.1 | 43.8 | |
| Employed | 24.1 | 52.2 | 7.1 | 6.4 | 26.1 | |
| Unemployed | 12.8 | 6.6 | 8.2 | 18.2 | 25.6 | |
| Other (student, homemaker, retired) | 5.9 | 6.5 | 5.8 | 6.2 | 4.6 | |
| HIV transmission category (%) | <0.0001 | |||||
| Male to male sexual contact only | 55.0 | 76.8 | 54.6 | 30.9 | 48.4 | |
| Injection drug use only | 8.6 | 2.2 | 8.7 | 15.7 | 10.8 | |
| Male to male sexual contact & injection drug use | 4.1 | 1.9 | 6.0 | 4.1 | 4.8 | |
| Heterosexual contact | 26.0 | 14.9 | 24.6 | 40.4 | 29.0 | |
| Other/not reported | 6.3 | 4.2 | 6.1 | 8.9 | 7.0 | |
| AIDS defining illness (%) | 0.25 | |||||
| Opportunistic infection | 38.0 | 38.0 | 37.5 | 40.9 | 34.4 | |
| T cell lymphocytopenia | 62.0 | 62.0 | 62.5 | 59.1 | 65.6 | |
| Cigarette smoking (%) | <0.0001 | |||||
| Current | 26.9 | 15.8 | 29.5 | 35.7 | 30.9 | |
| Former | 20.0 | 22.8 | 22.7 | 15.5 | 16.1 | |
| Never | 32.8 | 41.3 | 29.4 | 28.0 | 29.0 | |
| Missing | 20.4 | 20.2 | 18.5 | 20.7 | 23.9 | |
| Duration of AIDS (years) | ||||||
| Median | 4.3 | 4.5 | 5.7 | 3.4 | 1.6 | <0.0001 |
| 25th, 75th percentile | 1.6, 7.2 | 1.7, 7.2 | 3.6, 8.2 | 1.1, 6.3 | 0.5, 4.1 | |
| Combination antiretroviral treatment (%) | ||||||
| Prior to enrollment† | 91.0 | 94.1 | 92.2 | 88.9 | 85.4 | <0.0001 |
| At enrollment | 82.4 | 85.6 | 83.1 | 81.4 | 76.1 | 0.001 |
| Ever prior to or at enrollment or during follow-up | 97.8 | 97.8 | 98.7 | 97.3 | 96.5 | 0.12 |
| Enrollment CD4+ T lymphocyte cell count | ||||||
| Median (cells/μL) | 179 | 185 | 200 | 171 | 151 | 0.002 |
| 25th, 75th percentile (cells/μL) | 66, 344 | 68, 362 | 69, 398 | 53, 310 | 62, 299 | |
| <50 cells/μL (%) | 21.7 | 20.6 | 20.6 | 24.1 | 22.4 | 0.004 |
| 50–99 cells/μL (%) | 11.6 | 13.0 | 9.6 | 9.9 | 15.4 | |
| 100–199 cells/μL (%) | 20.3 | 18.1 | 19.5 | 21.9 | 23.5 | |
| ≥200 cells/μL (%) | 46.4 | 48.3 | 50.3 | 44.2 | 38.6 | |
| Nadir CD4+ T lymphocyte cell count prior to enrollment | ||||||
| Median (cells/μL) | 30 | 30 | 30 | 24 | 34 | 0.12 |
| 25th, 75th percentile (cells/μL) | 9, 90 | 10, 87 | 8, 92 | 8, 85 | 11, 103 | |
| <50 cells/μL (%) | 61.4 | 62.1 | 60.9 | 62.5 | 59.0 | 0.87 |
| 50–99 cells/μL (%) | 16.1 | 16.0 | 16.7 | 15.9 | 15.6 | |
| ≥100 cells/μL (%) | 22.5 | 21.8 | 22.4 | 21.6 | 25.3 | |
| Rate of increase in CD4+ T cells from nadir after initiation of cART (cells/μL/month) | <0.0001 | |||||
| Number. evaluable patients | 1503 | 502 | 432 | 341 | 228 | |
| Median | 4.8 | 5.1 | 3.6 | 4.7 | 8.9 | |
| 25th, 75th percentile | 1.1, 12.6 | 1.4, 13.1 | 0.9, 8.6 | 0.7, 13.2 | 2.0, 22.4 | |
| Enrollment plasma HIV viral load | ||||||
| Median (log10(copies/mL)) | 2.8 | 2.7 | 2.8 | 2.9 | 2.7 | 0.21 |
| 25th, 75th percentile (log10(copies/mL)) | 1.9, 4.7 | 1.9, 4.5 | 1.9, 4.7 | 1.9, 4.7 | 2.3, 4.9 | |
| <2.6 (%) | 35.6 | 38.0 | 37.8 | 34.9 | 27.9 | 0.01 |
| 2.6–5.0 (%) | 45.9 | 44.5 | 42.0 | 48.1 | 52.6 | |
| >5.0 (%) | 18.5 | 17.5 | 20.2 | 17.0 | 19.5 | |
| Maximum plasma HIV viral load prior to enrollment | ||||||
| Median (log10(copies/mL)) | 5.3 | 5.3 | 5.4 | 5.4 | 5.4 | 0.02 |
| 25th, 75th percentile (log10(copies/mL)) | 4.7, 5.8 | 4.7, 5.7 | 4.7, 5.8 | 4.9, 5.8 | 4.8, 5.8 | |
| <2.6 (%) | 2.9 | 2.7 | 4.9 | 1.9 | 0.8 | 0.001 |
| 2.6–5.0 (%) | 31.3 | 34.0 | 30.4 | 28.6 | 32.0 | |
| >5.0 (%) | 65.8 | 63.3 | 64.7 | 69.5 | 67.1 | |
| Karnofsky score (%) | ||||||
| ≤80 | 20.2 | 12.9 | 21.0 | 29.1 | 20.2 | <0.0001 |
| >80 | 79.8 | 87.1 | 79.0 | 70.9 | 79.8 | |
| Weight (kg)‡ | <0.0001 | |||||
| Median | 73.9 | 75.0 | 75.0 | 71.8 | 73.3 | |
| 25th, 75th percentile | 65.8, 83.4 | 68.0, 84.1 | 66.8, 84.2 | 62.7, 81.0 | 64.5, 82.1 | |
| Hepatitis C virus antibody status (%) | ||||||
| Positive | 20.2 | 8.2 | 22.8 | 31.0 | 23.8 | <0.0001 |
| Negative | 79.8 | 91.8 | 77.2 | 69.0 | 76.2 | |
| Non-AIDS co-morbidities (%) | ||||||
| Hypertension | 20.2 | 19.0 | 26.9 | 18.1 | 13.2 | <0.0001 |
| Diabetes | 9.0 | 8.4 | 11.8 | 8.8 | 5.4 | 0.005 |
| Hyperlipidemia | 19.9 | 28.4 | 22.5 | 13.8 | 7.5 | <0.0001 |
| Renal disease | 7.1 | 6.2 | 9.4 | 5.9 | 6.4 | 0.04 |
| Cardiovascular disease | 12.8 | 14.0 | 12.7 | 14.5 | 7.9 | 0.04 |
| Anemia (hemoglobin <10 gm/dL) | 4.8 | 3.8 | 3.9 | 7.3 | 4.6 | 0.01 |
| Cytomegalovirus retinitis (% of cohort) | 21.2 | 24.9 | 22.4 | 17.3 | 17.5 | 0.002 |
| Cytomegalovirus retinitis characteristics | ||||||
| Number patients | 500 | 185 | 153 | 97 | 65 | |
| Bilateral disease (% patients with CMV retinitis) | 41.0 | 43.8 | 38.6 | 43.3 | 35.4 | 0.57 |
| Zone 1† disease in either eye (% patients) | 46.2 | 43.2 | 51.0 | 37.1 | 56.9 | 0.04 |
| Area retinitis ≥25% retina in either eye (% patients) | 42.2 | 38.4 | 51.6 | 36.1 | 40.0 | 0.04 |
| Active retinitis in either eye (% patients) | 11.6 | 9.7 | 13.7 | 11.3 | 12.3 | 0.72 |
Participants classified as private insurance if they had any private insurance and Medicare if they had either Medicare only or Medicare and Medicaid.
Excluding 272 participants with unknown prior history of cART
Excluding 270 participants with unknown weight
Mortality
Mean follow-up was 7.75 years during which there were 733 deaths, resulting in a mortality rate of 4.0/100 person-years (PY). Mortality was greatest among publicly-insured participants, both Medicare and Medicaid (Figure 1 and Table 2) compared to privately-insured persons. Unadjusted hazard ratios (HR) for mortality were 1.54 (95% confidence interval [CI] 1.28–1.86; P<0.001) for Medicare and 1.36 (95% CI 1.11–1.66; P=0.003) for Medicaid. Uninsured participants’ unadjusted HR for mortality was 1.00 (95% CI 0.79–1.28; P=0.97) compared to the privately-insured. Adjustment for enrollment characteristics attenuated these differences (Table 3). Adjusted HRs (HRadj) for mortality were: 1.35 (95% CI 1.08–1.67; P=0.008) for participants with Medicare and 1.21 (95% CI 0.94–1.55; P=0.14) for Medicaid vs. privately-insured participants. Participants uninsured at enrollment were not significantly different from privately-insured (HRadj 0.89; 95% CI 0.68–1.18; P=0.42).
Figure 1.
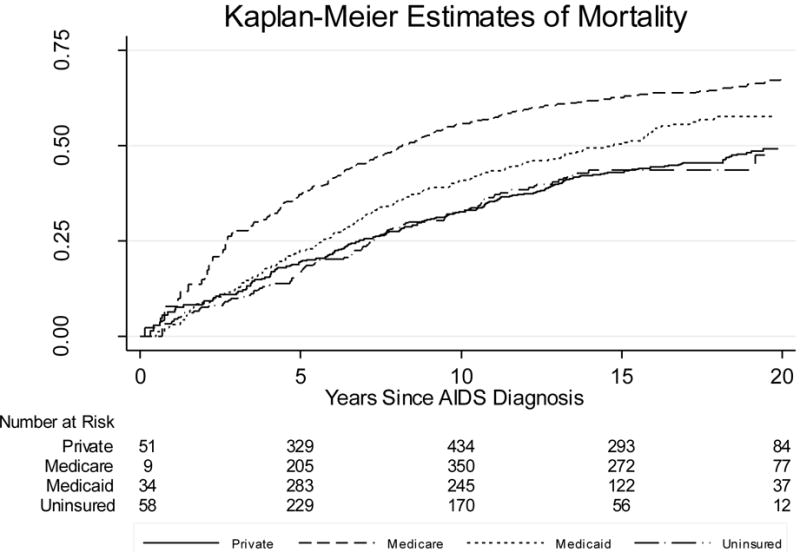
Table 2.
Association of Insurance Status with Mortality among Patients with AIDS in the Longitudinal Study of the Ocular Complications of AIDS Cohort
| Insurance status* | Entire Cohort (N=2363) | Patients with Suppressed HIV Load (N=794) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Deaths/N† | Rate (/100 PY)‡ | HR§ | 95% CI¶ | P-value | Deaths/N | Rate (/100 PY) | HR | 95% CI | P-value | ||
| Private | 205/743 | 3.3 | 1.00 | 27/271 | 1.1 | 1.00 | |||||
| Medicare | 235/688 | 4.9 | 1.54 | 1.28–1.86 | <0.0001 | 49/247 | 2.7 | 2.53 | 1.57–4.09 | 0.0001 | |
| Medicaid | 188/560 | 5.0 | 1.36 | 1.11–1.66 | 0.003 | 24/189 | 1.8 | 1.60 | 0.91–2.81 | 0.10 | |
| Uninsured | 105/372 | 3.8 | 1.00 | 0.79–1.28 | 0.97 | 6/97 | 0.9 | 0.77 | 0.31–1.90 | 0.57 | |
Patients with private insurance and a second insurance classified as private; patients with Medicare and Medicaid classified as Medicare.
N = number.
PY = person-years.
HR = hazard ratio.
CI = confidence interval.
Table 3.
Adjusted Associations of Risk Factors with Mortality in the Longitudinal Study of the Ocular Complications of AIDS Cohort
| Risk Factor | Entire Cohort (N=1913) |
Patients with Suppressed HIV Load (N=630) |
||||
|---|---|---|---|---|---|---|
| HRadj* | 95% CI† | P-value | HRadj | 95% CI | P-value | |
| Enrollment insurance status‡ | ||||||
| Private | 1.00 | 1.00 | ||||
| Medicare | 1.35 | 1.08–1.67 | 0.008 | 1.93 | 1.08–3.44 | 0.02 |
| Medicaid | 1.21 | 0.94–1.55 | 0.14 | 2.09 | 1.02–4.27 | 0.04 |
| Uninsured | 0.89 | 0.68–1.18 | 0.42 | 0.72 | 0.25–2.05 | 0.54 |
| Gender | ||||||
| Female | 1.00 | 1.00 | ||||
| Male | 1.12 | 0.87–1.45 | 0.37 | 1.25 | 0.62–2.55 | 0.54 |
| Race/ethnicity | ||||||
| White, non-Hispanic | 1.00 | 1.00 | ||||
| African American | 1.07 | 0.88–1.31 | 0.50 | 0.70 | 0.40–1.23 | 0.22 |
| Hispanic | 0.70 | 0.53–0.94 | 0.02 | 0.54 | 0.24–1.23 | 0.14 |
| Other | 0.71 | 0.42–1.20 | 0.20 | 0.74 | 0.24–2.25 | 0.59 |
| Age (per year) | 1.02 | 1.01–1.03 | <0.001 | 1.04 | 1.01–1.07 | 0.005 |
| Education | ||||||
| College graduate | 1.00 | 1.00 | ||||
| Less than high school | 1.43 | 1.00–2.06 | 0.05 | 1.36 | 0.52–3.59 | 0.53 |
| High school graduate | 1.41 | 1.01–1.96 | 0.04 | 2.21 | 0.95–5.16 | 0.07 |
| Some college, not graduate | 1.21 | 0.91–1.61 | 0.19 | 1.55 | 0.74–3.26 | 0.24 |
| HIV transmission category | ||||||
| Injection drug use only | 1.00 | 1.00 | ||||
| Male to male sexual contact only | 1.26 | 0.90–1.77 | 0.18 | 0.83 | 0.34–2.02 | 0.69 |
| MSM & IDU§ | 1.38 | 0.86–2.20 | 0.18 | 1.22 | 0.34–4.34 | 0.76 |
| Heterosexual contact | 1.23 | 0.86–1.74 | 0.26 | 0.85 | 0.35–2.02 | 0.71 |
| Other | 1.21 | 0.79–1.85 | 0.37 | 1.23 | 0.43–3.49 | 0.70 |
| Antiretroviral treatment | ||||||
| No cART at enrollment¶ | 1.00 | 1.00 | ||||
| cART at enrollment | 0.86 | 0.71–1.04 | 0.12 | 0.94 | 0.44–2.04 | 0.88 |
| Immunology and Virology | ||||||
| Baseline CD4+ T lymphocyte cells (per 100 cell/μL) | 0.86 | 0.80–0.92 | <0.001 | 0.97 | 0.86–1.08 | 0.55 |
| Nadir CD4+ T lymphocyte cells (per 100 cell/μL) | 1.04 | 0.88–1.23 | 0.63 | 0.86 | 0.59–1.25 | 0.43 |
| Baseline plasma HIV viral load (per log10(copies/mL)) | 1.44 | 1.34–1.54 | <0.001 | 1.34 | 0.72–2.51 | 0.35 |
| Maximum pre-enrollment plasma HIV viral load (per log10(copies/mL)) | 1.09 | 0.98–1.22 | 0.12 | 1.16 | 0.94–1.43 | 0.16 |
| Karnofsky Score | ||||||
| <80 | 1.00 | 1.00 | ||||
| ≥80 | 0.81 | 0.67–0.98 | 0.03 | 0.88 | 0.50–1.57 | 0.67 |
| Weight (/10 kg) | 0.88 | 0.82–0.95 | <0.001 | 0.80 | 0.66–0.96 | 0.02 |
| Comorbidities | ||||||
| Hepatitis C virus infection vs none | 1.33 | 1.06–1.65 | 0.01 | 1.24 | 0.69–2.24 | 0.47 |
| Cytomegalovirus retinitis vs none | 1.69 | 1.40–2.04 | <0.001 | 0.88 | 0.48–1.61 | 0.68 |
| Hypertension vs none | 1.31 | 1.07–1.62 | 0.01 | 1.80 | 1.07–3.03 | 0.03 |
| Diabetes vs none | 0.96 | 0.71–1.28 | 0.76 | 1.01 | 0.52–1.99 | 0.97 |
| Hyperlipidemia vs none | 1.04 | 0.82–1.31 | 0.76 | 0.94 | 0.55–1.60 | 0.82 |
| Renal disease vs none | 1.43 | 1.08–1.91 | 0.01 | 2.03 | 1.09–3.77 | 0.02 |
| Anemia (Hgb<10 g/dL) vs none | 1.09 | 0.80–1.50 | 0.58 | 1.32 | 0.36–4.85 | 0.67 |
| Other | ||||||
| Enrollment year (/year) | 0.96 | 0.92–0.99 | 0.02 | 0.90 | 0.82–0.98 | 0.02 |
HRadj = adjusted hazard ratio. Adjusted for age, race/ethnicity, gender, education, HIV transmission category, insurance status, combination antiretroviral therapy use, CD4+ T cell count, nadir CD4+ T cell count prior to enrollment, HIV viral load, maximum HIV viral load prior to enrollment, Karnofsky performance score, cytomegalovirus retinitis, hepatitis C virus sero-status, hypertension, diabetes, hyperlipidemia, renal disease, anemia, year of enrollment and weight.
CI = confidence interval.
Insurance status = private insurance with or without other insurance and Medicare for Medicare with or without Medicaid.
MSM = male to male sexual contact. IDU = injection drug use.
cART = combination antiretroviral therapy.
Other risk factors associated with mortality included race/ethnicity (Hispanic HRadj vs. non-Hispanic whites 0.70; 95% CI 0.53–0.94; P=0.02), being older (HRadj1.02 per year of age; 95% CI 1.01–1.03; P<0.001), having less education, having a lower enrollment CD4+ T cell count (HRadj 0.86 per 100 CD4+ T cells/μL; 95% CI 0.80–0.92; P<0.001), having a higher enrollment HIV viral load (HRadj 1.44 per log10(copies/mL); 95% CI 1.36–1.54; P<0.001), having a lower enrollment Karnofsky performance score (HRadj for Karnofsky ≥80 = 0.81; 95% CI 0.67–0.98; P=0.04), weighing less (HRadj per 10 kg body weight 0.88; 95% CI 0.82–0.95; P<0.001), being serum HCV antibody positive at enrollment (HRadj 1.33; 95 % CI 1.06–1.63; P=0.01); having CMV retinitis at enrollment (HRadj 1.69; 95% CI 1.40–2.04; P<0.001), having hypertension (HRadj 1.31; 95% CI 1.07–1.62; P=0.01), having renal disease (HRadj 1.43; 95% CI 1.08–1.91; P=0.01), and being enrolled earlier in the study (HRadj 0.96 per calendar year of enrollment; 95% CI 0.92–0.99; P=0.02).
Seven hundred ninety-four participants had enrollment plasma HIV viral loads <2.6 log10(copies/mL) and were considered to be “virologically suppressed” for HIV. Risk factors for mortality among these participants (Tables 2 and 3) included public insurance (HRadj for Medicare 1.93; 95% CI 1.08–3.44; P=0.02 and HRadj for Medicaid 2.09; 95% CI 1.02–4.27; P=0.04) vs. private insurance, older age (HRadj = 1.05 per year of age; 95% CI 1.01–1.07; P=0.005), hypertension (HRadj 1.80; 95% CI 1.07–3.03; P=0.03), renal disease (HR adj 2.03; 95% CI 1.09–3.77; P=0.02), and being enrolled earlier in the study (HRadj 0.90 per calendar year of enrollment; 95% CI 0.82–0.98; P=0.02).
Participants with healthcare insurance tended to remain insured with the same insurance category throughout the study. Among privately-insured persons at enrollment who were alive at the end of the study, 73% had private insurance at the end of the study, and 14% Medicare; among privately-insured persons at enrollment who died during the study, 65% had private insurance at the visit just prior to death, and 19% Medicare. Among persons with Medicare at enrollment who were alive at the end of the study, 75% had Medicare at the end of the study, and 9% private insurance; among persons with Medicare at enrollment who died, 77% had Medicare, and 8% Medicaid at the visit prior to death. Among persons with Medicaid at enrollment who were alive at the end of the study, 50% had Medicaid and 30% Medicare at the end of the study; among persons with Medicaid at enrollment who died during the study, 60% had Medicaid and 24% Medicare at the visit just prior to death. Among persons who were uninsured at enrollment and were alive at the end of the study, only 31% were uninsured at the end of the study, and among those who died, only 36% were uninsured at the visit prior to death. The proportions of uninsured at enrollment with insurances were private 12%, Medicare 29%, and Medicaid 20% at the end of the study for those alive at the end of the study and private 8%, Medicare 25%, and Medicaid 21% at the visit prior to death for those who died during the study. Because of changes in insurance status during the study a time-updated analysis of insurance vs. mortality was performed (Table 4). Compared to persons with private insurance, the time-updated HRsadj for mortality were 1.34 (95% CI 1.05–1.67; P=0.02) for Medicare, 1.34 (95%CI 1.01–1.80; P=0.05) for Medicaid; and 1.35 (95% CI 0.97–1.88; P=0.05) for remaining uninsured.
Table 4.
Time-updated Association of Insurance with Mortality in the Longitudinal Study of the Ocular Complications of AIDS Cohort
| Time-updated Insurance status* | Unadjusted | Adjusted† | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Private | 1.00 | |||||
| Medicare | 1.63 | 1.35–1.97 | <0.0001 | 1.34 | 1.05–1.70 | 0.02 |
| Medicaid | 1.65 | 1.32–2.05 | <0.0001 | 1.34 | 1.01–1.80 | 0.05 |
| Uninsured | 1.44 | 1.09–1.89 | 0.009 | 1.35 | 0.97–1.88 | 0.05 |
Participants classified as having private insurance if they had any private insurance and Medicare if they had Medicare with or without Medicaid. Insurance status was updated at every visit.
Cox proportional hazard model with staggered entry adjusted for age, race/ethnicity, gender, education, HIV transmission category, insurance status, combination antiretroviral therapy use, CD4+ T cell count, nadir CD4+ T cell count prior to enrollment, HIV viral load, maximum HIV viral load prior to enrollment, Karnofsky performance score, cytomegalovirus retinitis, hepatitis C virus sero-status, hypertension, diabetes, hyperlipidemia, renal disease, anemia, year of enrollment and weight.
Patients with CMV retinitis and Medicare appeared to have worse visual outcomes in the unadjusted analysis (HR for bilateral visual impairment 1.91; 95% CI 1.05–3.46; P=0.03) vs privately-insured, but this outcome was confounded by the worse CMV retinitis at presentation (Table 1), as evidenced by larger lesions and more posterior located lesions.26 In the adjusted analysis, the results were not significantly different among the four insurance groups (Table 5).
Table 5.
Incidence of Bilateral Visual Impairment among Patients with Cytomegalovirus Retinitis in the Longitudinal Study of the Ocular Complications of AIDS Cohort
| Enrollment insurance status | Events/N† | Rate (/PY)‡ | Unadjusted | Adjusted* | ||||
|---|---|---|---|---|---|---|---|---|
| HR§ | 95% CI¶ | P-value | HR | 95% CI | P-value | |||
| Private | 19/162 | 2.1 | 1.00 | 1.00 | ||||
| Medicare | 25/129 | 4.2 | 1.91 | 1.05–3.46 | 0.03 | 1.68 | 0.91–3.12 | 0.10 |
| Medicaid | 11/78 | 2.6 | 1.20 | 0.57–2.52 | 0.63 | 0.70 | 0.32–1.53 | 0.37 |
| Uninsured | 7/54 | 2.5 | 1.08 | 0.46–2.58 | 0.86 | 1.33 | 0.56–3.19 | 0.52 |
Adjusted for characteristics of cytomegalovirus retinitis at enrollment: bilateral disease, location of retinitis in retina, border activity, lesion size, and visual acuity in the better eye.
N = number.
PY = person-years.
HR = hazard ratio.
Discussion
The LSOCA cohort is unique among cohort studies of HIV-infected persons in the United States in that it enrolled only participants with late-stage HIV disease (i.e. AIDS) and from all HIV risk groups (as opposed to single risk group or single gender studies).13 Hence, the LSOCA cohort provides important information on patients with late-stage HIV disease and on those who present with AIDS.
Our data suggest that publicly-insured persons with AIDS have a greater risk for mortality than privately-insured persons. In the entire cohort there was a significantly greater mortality in the unadjusted and adjusted analyses among Medicare recipients and in the unadjusted analysis for Medicaid recipients when compared to privately-insured persons. Among participants with a suppressed plasma HIV viral load at enrollment, mortality in both the unadjusted and adjusted analyses was greater among both Medicare and Medicaid recipients compared to privately-insured persons, and in the time-updated analysis there was a greater mortality associated with these same insurance types. The seemingly paradoxical result that the uninsured had a similar mortality risk compared to privately-insured persons appears to be explained by uninsured persons obtaining insurance during the study, their similar access to antiretroviral therapy, and their lower burden of co-morbidities at enrollment. In the United States, uninsured HIV-infected patients often obtain antiretroviral therapy through the Ryan White HIV/AIDS Program, which provides primary medical care and treatment for HIV-infected persons who are uninsured or underinsured.31 In the time-updated analysis, being uninsured was associated with a greater mortality than having private insurance. During the period covered by LSOCA, estimates of the uninsured in the United States increased from ~16% to ~19% of the population,32,33 so that LSOCA’s 16% uninsured was similar to that of the United States as a whole.
Other risk factors for mortality included co-infection with HCV, CMV retinitis, lower CD4+ T cells, and higher plasma HIV viral load, older age, lower body weight, and the age-related co-morbidities of hypertension and renal disease. Mortality decreased throughout the calendar period during which the study was conducted, as evidenced by the decline in mortality with later year of study entry, consistent with the advances in and increased options for antiretroviral therapy.
Cytomegalovirus retinitis management requires close follow-up, management of potentially toxic drugs, and coordinated care between ophthalmology and infectious disease physicians.20–23,34 As such, we evaluated bilateral visual impairment among patients with CMV retinitis as a marker of quality of care, and after adjustment for baseline imbalances in the severity of CMV retinitis,26 there were no significant differences among the four insurance groups. Because there were no differences in the outcome of the complicated management of CMV retinitis, insurance-related differences in mortality may not reflect differences in access to or process of care, but may reflect “burden of disease-related” factors. In this regard, patients with Medicare and Medicaid had lower enrollment Karnofsky performance scores, greater percentages of disabled persons, and greater proportions with HCV infection. Patients with Medicare had more non-AIDS co-morbidities, including hypertension, diabetes, and renal disease. Finally, participants with Medicare and, to a lesser extent, Medicaid appeared to have a less robust response to antiretroviral therapy as evidenced by a slower rate of rise of CD4+ T cell counts after initiation of antiretroviral therapy.
All participants in LSOCA had AIDS, not earlier stages of HIV, so the results may not be generalizable to the entire HIV epidemic. Because of an interest in ocular outcomes, the cohort was enriched for CMV retinitis;6,13,20–22,35 hence LSOCA may not represent the AIDS epidemic as it exists today with a reduced incidence of CMV disease.36 However, the LSOCA cohort is the only prospective cohort of patients with AIDS (as opposed to earlier stages of HIV infection) and with all HIV transmission risk groups in the United States,13 and therefore provides important information on outcomes in late-stage HIV disease (i.e. AIDS). Furthermore, the LSOCA cohort’s demographic characteristics are similar to that of the AIDS epidemic, except for a slightly lower proportion of participants with a history of injection drug use, suggesting overall good generalizability to persons with AIDS.13 Although one goal of antiretroviral therapy is to prevent the progression of HIV infection to AIDS, late diagnosis of HIV infection and difficulties in achieving viral suppression for all patients, even among patients retained in care, suggest that many HIV-infected persons will progress to AIDS.37,38 Furthermore, among HIV-infected persons, mortality is highest among persons who initiate cART late in the course of HIV infection compared to those who initiate cART at higher CD+ T cells counts. Additionally, among persons who initiate cART with advanced HIV disease, a subset may not achieve immune reconstitution, as evidence by less robust increases in CD4+ T cell counts, resulting in a greater risk for mortality.39,40 One study suggested that cART may be initiated at lower CD4+ T cells among persons with Medicare or Medicaid than among privately-insured,41 which could contribute to differences in mortality.
Our definition of suppressed plasma HIV viral load was based upon the detection threshold of the assay used at the start of LSOCA. Newer assays detect much lower levels of HIV replication and provide more precision regarding HIV replicative activity. Nevertheless, our findings regarding mortality rates by insurance status among participants with “suppressed” HIV viral loads mirror the findings of the entire cohort, suggesting that use of more recent HIV viral load assays and definitions of “virologic suppression” would not alter the conclusions. Although we collected information on non-AIDS co-morbidities, data regarding cardiovascular disease were incomplete, as collecting detailed cardiovascular disease data began only after 80% of the cohort had been enrolled. Because there was no opportunity to collect the data for participants who died early in the study, having missing cardiovascular disease data was a risk factor for mortality, and cardiovascular disease could not be included in the adjusted model. Nevertheless we did have data regarding risk factors for cardiovascular disease, including hypertension, hyperlipidemia, and diabetes, and included all of these in the adjusted models. Finally we did not have detailed data on potential insurance-related barriers to accessing care, such as co-pays and deductibles.
Our data complement and extend the observations in the HIV Outpatient Study (HOPS) regarding the relationship between health insurance and mortality, as we have reported increased mortality among patients with AIDS and among patients with and without HIV virologic suppression. The HOPS analysis evaluated only persons with suppressed HIV load. We evaluated CMV retinitis outcomes as a marker for process of care and, after adjustment for baseline differences, could not detect a difference among the four groups.
Healthcare insurance status has been associated with mortality and worse outcomes among persons with lung cancer, Hodgkin disease, vascular disease, and renal disease; in these studies, patients with public insurance (particularly Medicaid) or are uninsured have a worse outcomes than those with private insurance.42–47 Many studies demonstrate more advanced disease and greater co-morbidities among persons with public or no insurance, accounting at least in part for the differences in outcomes,42–47 but some studies demonstrate a persistently worse outcomes for those with public or no insurance even after adjustment, suggesting access to care or process of care issues.42, 46,47 In LSOCA non-infectious, age-related co-morbidities were greater among those with public insurance, accounting at least in part for the difference in mortality. Although participants in LSOCA uninsured at enrollment had a similar mortality to privately-insured participants, our data suggest that these patients accessed care (e.g. antiretroviral therapy) and often obtained insurance. These facts plus their lower frequency of non-infectious, age-related co-morbidities may account for any differences between our results and those in other diseases. Consistent with this interpretation is the greater mortality among those in LSOCA who remained uninsured in the time-updated analysis.
In conclusion, our data suggest a greater mortality among persons with AIDS in the United States who have publicly-funded healthcare insurance, both Medicare and Medicaid, compared to private healthcare insurance. These differences may reflect differences in other-than-HIV co-morbid disease burden, particularly age-related diseases, the diagnosis and prevention of which should be undertaken routinely and the management of which pursued aggressively. Although we did not detect differences in one outcome related to the process of care, we cannot exclude insurance status as an independent risk factor. Future research should evaluate insurance status as a potential marker for access-to-care barriers.
Acknowledgments
Grant Support: Supported by cooperative agreements from the National Eye Institute, the National Institutes of Health, Bethesda, MD, to the Icahn School of Medicine at Mount Sinai, New York, NY (U10 EY 08052); The Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD (U10 EY 08057); and the University of Wisconsin, Madison School of Medicine and Public Health, Madison, WI (U10 EY 08067).
Footnotes
Conflict of Interest: Alexandra W. Jabs: none; Douglas A. Jabs: none; Mark L. Van Natta: none; Frank J. Palella: consultant and/or on the speakers’ bureau for Gilead Sciences, Janssen Pharmaceutical, Merck and Co, and Bristol Myers Squibb; Curtis L. Meinert: none.
References
- 1.Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338(13):853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Murphy EL, Collier AC, Kalish LA, et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001;135(1):17–26. doi: 10.7326/0003-4819-135-1-200107030-00005. [DOI] [PubMed] [Google Scholar]
- 3.Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients stating highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360(9327):119–29. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 4.Hessol NA, Kalinowski A, Benning L, et al. Mortality among participants in the Multicenter AIDS Cohort Study and the Women’s Interagency HIV Study. Clin Infect Dis. 2007;44(2):287–94. doi: 10.1086/510488. [DOI] [PubMed] [Google Scholar]
- 5.Braithwaite RS, Roberts MS, Chang CC, et al. Influence of alternative thresholds for initiating HIV treatment on quality-adjusted life expectancy. Ann Intern Med. 2008;148(3):178–85. doi: 10.7326/0003-4819-148-3-200802050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jabs DA, Ahuja A, Van Natta ML, Lyon A, Yeh S, Danis R, Studies of the Ocular Complications of AIDS Research Group Long-term outcomes of cytomegalovirus retinitis in the era of modern antiretroviral therapy: results from a United States cohort. Ophthalmology. 2015;122(7):1452–63. doi: 10.1016/j.ophtha.2015.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lohse N, Hansen AB, Pedersen G, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med. 2007;146(2):87–95. doi: 10.7326/0003-4819-146-2-200701160-00003. [DOI] [PubMed] [Google Scholar]
- 8.Hogg R, Lima V, Sterne JA, et al. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372(9635):293–9. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan RC, Kingsley LA, Sarrett AR, et al. Ten-year predicted coronary heart disease risk in HIV-infected men and women. Clin Infect Dis. 2007;45(8):1074–81. doi: 10.1086/521935. [DOI] [PubMed] [Google Scholar]
- 10.Deeks SG. HIV Infection, inflammation, immunosenescence, and aging. Annual Rev Med. 2011;62:141–55. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pathai S, Bajillan H, Landay AL, High KP. Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci. 2014;69:833–42. doi: 10.1093/gerona/glt168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pallela FJ, Jr, Baker RK, Buchacz K, et al. Increased mortality among publicluy insured participants in the HIV Outpatient Study despite HAART treatment. AIDS. 2011;25(15):1865–76. doi: 10.1097/QAD.0b013e32834b3537. [DOI] [PubMed] [Google Scholar]
- 13.Jabs DA, Van Natta ML, Holbrook JT, et al. The Longitudinal Study of the Ocular Complications of AIDS: 1. Ocular diagnoses at enrollment. Ophthalmology. 2007;114(4):780–86. doi: 10.1016/j.ophtha.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Jabs DA, Van Natta ML, Holbrook JT, et al. The Longitudinal Study of the Ocular Complications of AIDS: 2. Ocular examination results at enrollment. Ophthalmology. 2007;114(4):787–93. doi: 10.1016/j.ophtha.2006.07.065. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morb Mortal Wkly Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 16.Karnofsky DA, Abelmann WH, Craver LF, Burchenal JH. The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948;1:634–56. [Google Scholar]
- 17.Studies of the Ocular Complications of AIDS Research Group. The foscarnet gancicolvir cytomegalovirus retinitis trial: 1. Rationale, design, and methods. Control Clin Trials. 1992;13(1):22–39. doi: 10.1016/0197-2456(92)90027-w. [DOI] [PubMed] [Google Scholar]
- 18.Branch AD, Van Natta ML, Vachon ML, et al. Mortality in hepatitis C virus-infected patients with a diagnosis of AIDS in era of combination antiretroviral therapy. Clin Infect Dis. 2012;155(1):137–44. doi: 10.1093/cid/cis404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jabs DA, Van Natta ML, Sezgin E, et al. Prevalence of intermediate-stage age-related macular degeneration in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 2015;159(6):1115–22. doi: 10.1016/j.ajo.2015.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jabs DA, Van Natta ML, Thorne JE, et al. Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: 1. Retinitis progression. Ophthalmology. 2004;111:2224–31. doi: 10.1016/j.ophtha.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 21.Jabs DA, Ahuja A, Van Natta ML, et al. Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: five-year outcomes. Ophthalmology. 2010;117:2152–61. doi: 10.1016/j.ophtha.2010.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jabs DA, Van Natta M, Dunn JP, et al. Comparison of treatment regimens for cytomegalovirus retinitis in patients with AIDS in the era of highly active antiretroviral therapy. Ophthalmology. 2013;120:1262–70. doi: 10.1016/j.ophtha.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jabs DA. Cytomegalovirus retinitis and the acquired immune deficiency syndrome: bench to bedside: LXVII Edward Jackson Memorial Lecture. Am J Ophthalmol. 2011;151(2):198–216. doi: 10.1016/j.ajo.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferris FL, III, Kassof A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94:91–6. [PubMed] [Google Scholar]
- 25.Jabs DA, Rosenbaum JT, Nussenblatt RB. The Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005;140:509–16. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thorne JE, Jabs DA, Kempen JH, et al. Incidence of and risk factors for visual acuity loss among patient with AIDS and cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Ophthalmology. 2006;113:1432–40. doi: 10.1016/j.ophtha.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 27.Altman DG. Practical Statistics for Medical Research. London: Chapman and Hall; 1991. [Google Scholar]
- 28.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 29.Tarwater PM, Mellors J, Gore ME, et al. Methods to assess population effectiveness of therapies in human immunodeficiency virus incident and prevalent cohorts. Am J Epidemiol. 2001;154:675–81. doi: 10.1093/aje/154.7.675. [DOI] [PubMed] [Google Scholar]
- 30.Lin DY. Cox regression analysis of multivariate failure time data: the marginal approach. Stat Med. 1994;13:2233–47. doi: 10.1002/sim.4780132105. [DOI] [PubMed] [Google Scholar]
- 31.https://hab.hrsa.gov/about-ryan-white-hivaids-program/about-ryan-white-hivaids-program. Accessed 20 march 2017
- 32.National Center for Health Statistics. Health, United States 2010: with Special Feature on Death and Dying. Hyattsville, MD: 2011. p. 397. [PubMed] [Google Scholar]
- 33.Holohan J. The 2007–2009 recession and health insurance coverage. Health Affairs. 2011;30:145–52. doi: 10.1377/hlthaff.2010.1003. [DOI] [PubMed] [Google Scholar]
- 34.Jabs DA, Holbrook JT, Van Natta ML, et al. Risk factors for mortality in patients with AIDS in the era of highly active antiretroviral therapy. Ophthalmology. 2005;112(5):771–9. doi: 10.1016/j.ophtha.2004.10.049. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. Group guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. Morb Mortal Wkly Rep. 2009;58:55–60. [Google Scholar]
- 36.Sugar EA, Jabs DA, Ahuja A, et al. Incidence of cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Am J Ophthalmol. 2012;153(6):1016–24. doi: 10.1016/j.ajo.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. Late HIV testing – 34 states, 1996–2005. Morb Mortal Wkly Rep. 2009;58:661–5. [PubMed] [Google Scholar]
- 38.Edison L, Hughes D, Drenzek C, Kelly J. Prevalence and indicators of viral suppression among persons diagnosed with HIV infection retained in care – Georgia, 2010. Morb Mortal Wkly Rep. 2014;63:55–8. [PMC free article] [PubMed] [Google Scholar]
- 39.Palella FJ, Deloria-Knoll M, Chmiel JS, Moorman AC, Wood KC, Greenberg AE, Holmberg SC. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med. 2003;138:620–6. doi: 10.7326/0003-4819-138-8-200304150-00007. [DOI] [PubMed] [Google Scholar]
- 40.Taiwo B, Li X, Palella F, Jacobson L, Margolick J, Detels R, Rinaldo C, Phair J. Higher risk of death in patients with lower CD4 cell counts after virally suppressive HAART. HIV Medicine. 2009;10:657–60. doi: 10.1111/j.1468-1293.2009.00739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneider G, Juday T, Wentworth C, III, Lanes S, Hebden T, Seekins D. Impact of health care payer type on HIV stage illness at time of initiation of antiretroviral therapy in the USA. AIDS Care. 2013;25(11):1470–6. doi: 10.1080/09540121.2013.774316. [DOI] [PubMed] [Google Scholar]
- 42.Shi R, Diaz R, Zhenzhen S, Duvall E, Mills G. The effect of payer status on survival of patients with stage I/II non-small cell lung cancer: NCDB 1998–2011. Anticancer Research. 2016;36:319–26. [PubMed] [Google Scholar]
- 43.Slatore CG, Au DH, Gould MK. An official American Thoracic Society systematic review: insurance status and disparities in lung cancer practices and outcomes. Am J Respir Crti Care Med. 2010;182(9):1195–1205. doi: 10.1164/rccm.2009-038ST. [DOI] [PubMed] [Google Scholar]
- 44.Parikh RR, Grossbard ML, Green BL, Harrison LB, Yahalom J. Disparities in survival by insurance status in patients Hodgkin lymphoma. Cancer. 2015;121:3515–24. doi: 10.1002/cncr.29518. [DOI] [PubMed] [Google Scholar]
- 45.Boxer LK, Dimick JB, Wainess RM, et al. Payer status is related to differences in access and outcomes of abdominal aortic aneurysm repair in the United States. Surgery. 2003;134(15):1865–76. doi: 10.1067/msy.2003.214. [DOI] [PubMed] [Google Scholar]
- 46.Giacovelli JK, Egorova N, Nowygrod R, Gelijns A, Kent KC, Morrissey NJ. Insurance status predicts access to care and outcomes of vascular disease. J Vasc Surg. 2008;48(4):905–11. doi: 10.1016/j.jvs.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saunders M, Ricardo AC, Chen J, Chin MH, Lash JP. Association between insurance status and mortality in individuals with albuminuria: an observational cohort study. BMC Nephrology. 2016;17:27–33. doi: 10.1186/s12882-016-0239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


