Summary
Objective
Medically refractory epilepsy is a debilitating disorder that is particularly challenging to treat in patients who have already failed a surgical resection. Evidence regarding outcomes of further epilepsy surgery is limited to small case series and reviews. Therefore, our group performed the first quantitative meta-analysis of the literature from the past 30 years to assess for rates and predictors of successful reoperations.
Methods
A PubMed search was conducted for studies reporting outcomes of repeat epilepsy surgery. Studies were excluded if they reported fewer than 5 eligible patients or had average follow ups < 1 year, and patients were excluded from analysis if they received a non-resective intervention. Outcomes were stratified by each variable of interest and quantitative meta-analysis was performed to generate odds ratios (OR) and 95% confidence intervals (CI).
Results
782 patients who received repeat resective epilepsy surgery from 36 studies were included. Engel I outcome was observed in 47% (N=369) of patients. Significant predictors of seizure freedom in included congruent over non-congruent electrophysiology data (OR 3.6, 95% CI 1.6–8.2), lesional over non-lesional epilepsy (OR 3.2, 95% CI 1.9–5.3), and surgical limitations over disease-related factors associated with failure of the first surgery (OR 2.6, 95% CI 1.3–5.3). Among patients with at least one of these predictors, seizure freedom was achieved in 58%. Conversely, the use of invasive monitoring was associated with worse outcome (OR 0.4, 95% CI 0.2–0.9). Temporal lobe over extratemporal/multilobe resection (OR 1.5, 95% CI 0.8–3.0) and abnormal over normal pre-operative MRI (OR 1.9, 95% CI 0.6–5.4) showed non-significant trends toward seizure freedom.
Significance
This analysis supports considering further resection in patients with intractable epilepsy who continue to have debilitating seizures after an initial surgery, especially in the context of factors predictive of a favorable outcome.
Keywords: Seizure, reresection, reoperation, secondary, redo
Introduction
Medically refractory epilepsy is a debilitating disorder that is challenging to treat. Surgical resection has been shown to provide long term seizure control in about 40–80%1 of operative candidates (60–80% in temporal lobe epilepsy2–5 and 40–60% in extratemporal lobe epilepsy6). However, in patients who continue to have seizures after surgery, treatment becomes even more difficult7 and can frustrate both patients and clinicians. Further therapeutic options include continued antiepileptic drug (AED) modifications8 while awaiting seizure “run down”9–11, vagus nerve stimulation (VNS)12,13, responsive neurostimulation (RNS)14, or a second surgery. While some of these options are palliative, repeat surgical intervention may still provide an opportunity for seizure freedom. Since seizure freedom is the strongest predictor of quality of life15,16, and continued seizures are known to increase morbidity, mortality, neurocognitive decline, and psychosocial problems16–18, it is important to explore which patients might have the best chance of success with further resection.
Current evidence regarding outcomes from repeat surgery is limited to a number of small case series and reviews19–22, and results are often inconclusive. As such, drawing clear conclusions regarding predictors of success can be challenging. Furthermore, our group is unaware of any plans for large, prospective, randomized trials in this patient population. As such, here we provide a systematic review and the first quantitative meta-analysis of outcomes following repeat epilepsy surgery. We analyze published literature on this topic from the past 30 years and examine patient demographics, diagnostic data, epilepsy characteristics, and surgical factors as possible predictors of outcome. Our results suggest that repeat surgery for intractable epilepsy can be efficacious for properly selected patients.
Methods
Literature Search
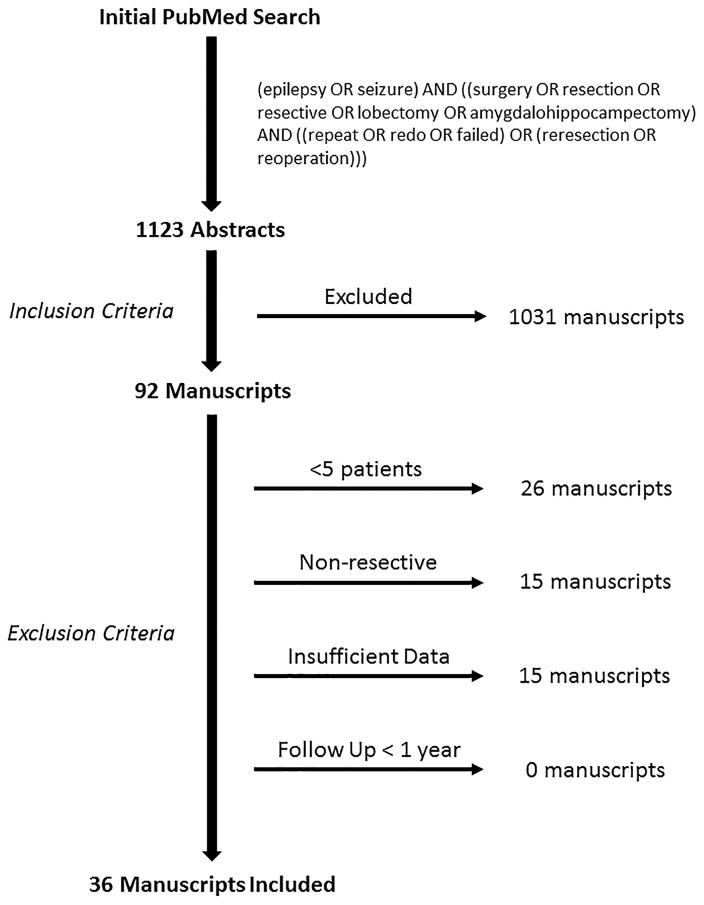
A PubMed search for peer-reviewed articles containing outcomes of repeat epilepsy surgeries was conducted using the following query guidelines: (epilepsy OR seizure) AND ((surgery OR resection OR resective OR lobectomy OR amygdalohippocampectomy) AND ((repeat OR redo OR failed) OR (reresection OR reoperation))). This resulted in 1123 titles and abstracts that were examined for the following inclusion criteria: reported primary outcomes of repeat epilepsy surgery, published between 1986 and April 2017, and available in the English language. 92 manuscripts met such criteria and were examined thoroughly for the following exclusion criteria: mean or median post-operative follow up less than 1 year, insufficiently disaggregated outcomes, or fewer than 5 eligible patients reported. Ultimately, 36 eligible studies were identified and included in our analysis (see Figure 1 for a summary of this process). Individual patients were disqualified from analysis if they received a non-resective intervention prior to or instead of a resection (e.g. VNS, corpus callosotomy, or radiation). This literature search and study design were guided by PRISMA recommendations23.
Figure 1.
Literature search guided by PRISMA23.
Data Collection
Outcomes following repeat surgery were classified as free of disabling seizure (Engel class IA–D) or not seizure-free (Engel class II–IV). To assess for possible relationships with outcome, data for the following variables were collected when available: age of seizure onset, epilepsy duration prior to 1st surgery, age at 1st surgery, age at last surgery, time between surgeries, gender, laterality of surgeries, imaging abnormalities, use of invasive monitoring, congruency of electrophysiological data, predominant seizure type, seizure generalization, anatomical site of surgeries, underlying pathology, and cited reason for failure of the initial surgery. If more than 2 surgeries were performed on a patient, only outcome data relating to the final surgery were included. Any cited complications (or lack thereof) from the repeat surgery were also noted.
Anatomical site of surgery was categorized as temporal or extratemporal (including multilobar), pathology as lesional (tumor, cyst, or vascular malformation) or non-lesional (all other pathologies), and electrophysiology results as congruent (focal, localizing) or incongruent (bilateral, widespread, or non-localizing patterns). Studies varied in terms of which electrophysiological data was obtained and/or presented for each patient (e.g. ictal, interictal, scalp electroencephalography [EEG], subdural electrocorticography [ECoG], or depth electrode data). Therefore, if any data suggested a non-localizing, bilateral, widespread, or incongruent pattern, the patient’s data was categorized as incongruent.
Additionally, authors often cited a known or suspected reason that the primary epilepsy surgery failed. Such explanations were dichotomized into “surgery-related” (e.g. extension of epileptogenic zones into functional areas, missed lesions, sub-total resections, lesional recurrence, improperly categorized epileptogenic areas, or residual mesiotemporal structures) or “disease-related” (e.g. emergence of new epileptiform areas postoperatively, known widespread/bilateral disease, or palliative cases) factors, as explained more fully in the Discussion.
Statistical Analysis
For preliminary analysis, outcomes from repeat surgeries were stratified by each variable of interest. For summary purposes and to help identify factors for meta-analysis, potential outcome predictors were compared using unpaired, two-way Student’s t-tests for continuous variables and Pearson’s chi-squared (X2) tests for categorical variables. Variables potentially associated with seizure outcome (p-value ≤ 0.05) were subjected to formal meta-analysis. Heterogeneity across studies was examined using both Cochran’s Q and I2 tests, which identified a fixed effects model as appropriate in all cases. Cochran–Mantel–Haenszel (CMH) testing was used to calculate odds ratios (OR) and 95% confidence intervals (CI). All statistical analyses were computed using Wizard Pro 1.8.28 (207), MATLAB® R2016b, and Review Manager v5.3 (The Nordic Cochrane Centre, Rigshospitalet 2008).
Results
Data from 782 patients across 36 case series were analyzed (Table 1). No randomized or controlled trials were identified. The overall seizure freedom rate after repeat epilepsy surgery was 47% and did not change significantly over time (r = 0.14, p = 0.33) (Figure 2). Table 2 displays all summary data. Patient demographics displayed in Table 2a were not significantly associated with outcome. Factors found to be potentially associated with positive outcome after repeat surgery during preliminary analysis included abnormal imaging (p=0.03), congruent electrophysiology data (p<0.01), predominantly focal onset seizures with impaired awareness (i.e. complex-partial seizure) semiology (p=0.01), surgery-related limitation as the cited reason for failure of initial surgery (p<0.01), lesional pathology (p<0.01), temporal lobe resection in the initial surgery (p=0.04), and temporal lobe resection in the final surgery (p<0.01) (Table 2). The use of invasive monitoring correlated with a worse outcome (p<0.01). The presence of generalized seizures showed a trend toward worse outcomes, but this association did not reach statistical significance (p=0.08).
Table 1.
Included Studies
| First Author | Year | Patients |
|---|---|---|
| Abosch24 | 2002 | 13 |
| Awad25 | 1991 | 15 |
| Benifla26 | 2006 | 12 |
| Bauman27 | 2008 | 13 |
| Bower28 | 2015 | 10 |
| Englot6 | 2014 | 16 |
| Fauser29 | 2015 | 19 |
| Germano30 | 1994 | 40 |
| Goellner31 | 2013 | 11 |
| Gonzalez-Martinez32 | 2007 | 57 |
| Greiner33 | 2016 | 30 |
| Grote34 | 2016 | 66 |
| Hallbook35 | 2013 | 21 |
| Holmes36 | 1999 | 21 |
| Jehi37 | 2010 | 15 |
| Jooma38 | 1995 | 8 |
| Juhasz39 | 2004 | 7 |
| Jung40 | 2013 | 17 |
| Kirchberger41 | 1998 | 5 |
| Koh42 | 2004 | 14 |
| Munari43 | 2000 | 42 |
| Otsuki44 | 2013 | 9 |
| Pati45 | 2011 | 14 |
| Ramantani46 | 2013 | 23 |
| Ramos47 | 2009 | 5 |
| Ryzi7 | 2015 | 6 |
| Sacino48 | 2017 | 22 |
| Salanova49 | 1994 | 35 |
| Salanova50 | 2005 | 21 |
| Schulz51 | 2011 | 22 |
| Schwartz52 | 2001 | 17 |
| Shaver53 | 1997 | 20 |
| Siegel54 | 2004 | 64 |
| Tian55 | 2011 | 9 |
| Vadera56 | 2012 | 36 |
| Wyler57 | 1989 | 27 |
|
| ||
| Total | 782 | |
Figure 2.
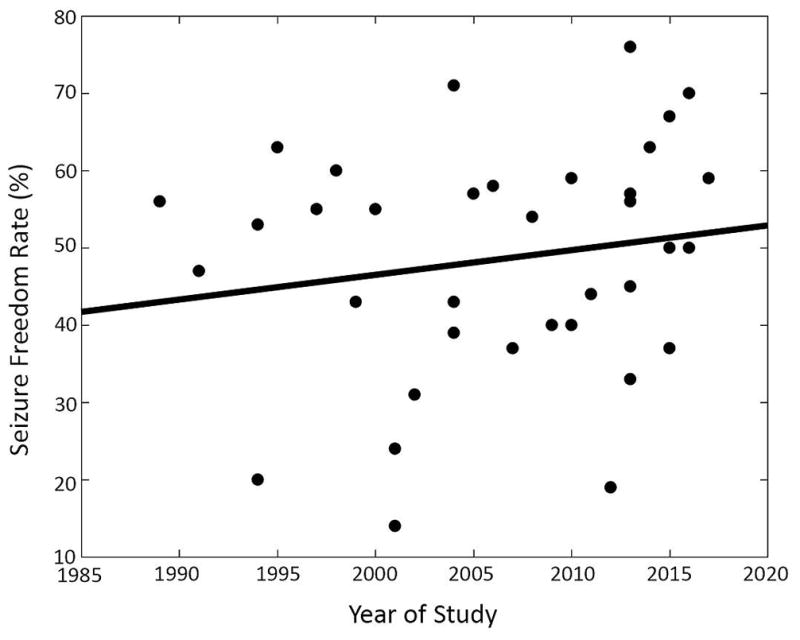
Reported seizure free rates of repeat epilepsy surgeries over time. Linear regression revealed no significant trend (r = 0.14, p = 0.33).
Table 2.
Postoperative seizure outcomes stratified by patient characteristics
| Engel I | Engel II–IV | P value | |||
|---|---|---|---|---|---|
|
| |||||
| Total | 369 (47%) | 413 (53%) | |||
| A) Demographics | Gender | Female | 31 (56%) | 24 (44%) | 0.56 |
| Male | 44 (52%) | 41 (46%) | |||
| Age of seizure onset, years (mean ± SEM) | 7.1 ± 0.9 | 6.6 ± 0.9 | 0.64 | ||
| N = 78 | N = 67 | ||||
| Age at initial surgery, years (mean ± SEM) | 15.9 ± 1.3 | 14.9 ± 1.3 | 0.57 | ||
| N = 105 | N = 89 | ||||
| Age at last surgery, years (mean ± SEM) | 16.8 ± 1.4 | 15.4 ± 1.3 | 0.48 | ||
| N = 87 | N = 77 | ||||
| B) Epilepsy characteristics | Pathology | Lesional | 95 (61%) | 62 (39%) | < 0.01* |
| Non-Lesional | 145 (41%) | 205 (59%) | |||
| Secondary seizure generalization | Yes | 22 (38%) | 36 (62%) | < 0.08 | |
| No | 46 (53%) | 41 (47%) | |||
| Predominant seizure type | FOIA | 54 (51%) | 51 (49%) | 0.01* | |
| FOA | 38 (45%) | 46 (55%) | |||
| Other | 2 (12%) | 15 (88%) | |||
| Duration of epilepsy, years (mean ± SEM) | 12.3 ± 1.9 | 8.5 ± 1.9 | 0.18 | ||
| N = 36 | N = 24 | ||||
| C) Diagnostics | MRI findings prior to 1st surgery | Normal | 18 (35%) | 34 (65%) | 0.03* |
| Abnormal | 76 (53%) | 68 (47%) | |||
| Electrophysiology | Congruent | 87 (69%) | 39 (31%) | < 0.01* | |
| Incongruent | 31 (47%) | 35 (53%) | |||
| Invasive Monitoring | Used | 67 (52%) | 61 (48%) | < 0.01* | |
| Not used | 62 (76%) | 20 (24%) | |||
| D) Surgical factors | Time between resections, years (mean ± SEM) | 3.1 ± 0.3 | 3.0 ± 0.3 | 0.83 | |
| N = 96 | N = 92 | ||||
| Resection location – initial surgery | Temporal | 94 (49%) | 99 (51%) | 0.04* | |
| Extratemporal | 99 (39%) | 155 (61%) | |||
| Resection location – last surgery | Temporal | 129 (54%) | 112 (47%) | < 0.01* | |
| Extratemporal | 95 (37%) | 160 (63%) | |||
| Side of initial surgery | Left | 47 (48%) | 50 (52%) | 0.26 | |
| Right | 68 (56%) | 53 (48%) | |||
| Side of last surgery | Left | 46 (49%) | 47 (51%) | 0.34 | |
| Right | 65 (55%) | 51 (45%) | |||
| Reason for failure of initial surgery | Surgical limitation | 100 (50%) | 102 (50%) | < 0.01* | |
| Disease-related | 23 (32%) | 48 (68%) | |||
Statistically significant value (p ≤ 0.05).
Data are reported as N values (with corresponding percent) unless otherwise indicated. SEM = standard error of the mean; FOIA = focal onset with impaired awareness (i.e. complex partial); FOA = focal onset aware (i.e. simple partial); MRI = magnetic resonance imaging.
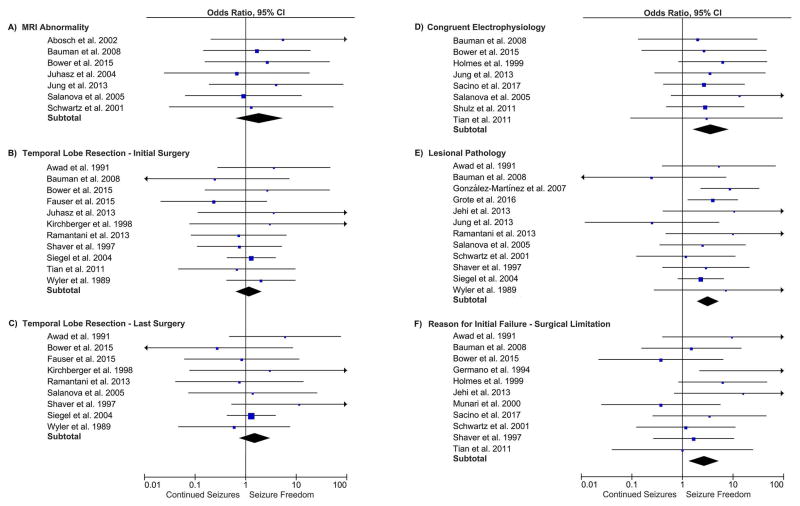
Formal meta-analysis was conducted on factors identified as potentially predictive of outcome (Figure 3). Higher odds of seizure freedom after a repeat surgery were identified with congruent over non-congruent electrophysiological data (OR 3.6, 95% CI 1.6–8.2), lesional over non-lesional epilepsy (OR 3.2, 95% CI 1.9–5.3), and surgical limitations over disease-related factors as the cited reason for failure of the initial surgery (OR 2.6, 95% CI 1.3–5.3). For patients with at least one of these positive factors, the rate of seizure freedom increased to 58% (N = 282 out of 485). The use of invasive monitoring was associated with worse outcome (OR 0.4, 95% CI 0.2–0.9). Temporal lobe over extratemporal/multilobe (OR 1.5, 95% CI 0.8–3.0) and abnormal over normal pre-operative MRI (OR 1.9, 95% CI 0.6–5.4) showed non-significant trends toward better outcomes. Meta-analysis was unable to be conducted for seizure semiology due to a paucity (<4) eligible studies.
Figure 3.
Forest plots showing meta-analysis results.
Reported complications across all studies included neurological deficits (e.g. weakness, visual field cut, aphasia), vascular injury (e.g. hemorrhage, stroke), infection, and cerebrospinal fluid (CSF) disturbances (e.g. CSF leak, pseudomeningocele, or hydrocephalus). There were no short term surgical mortalities reported from repeat surgeries. When reported, complication rates varied widely across studies, as shown in Table 3.
Table 3.
Complication Rates in Repeat Epilepsy Surgery
| First author | Year | No. Patients | Overall Complication Rate | Neurological Complication Rate | Non-neurological Complication Rate |
|---|---|---|---|---|---|
| Awad | 1991 | 15 | 0% | 0% | 0% |
| Bower | 2015 | 10 | 50% | 10% | 40% |
| Germano | 1994 | 40 | 8% | 3% | 5% |
| Gonzalez-Martinez | 2007 | 57 | 11% | 9% | 2% |
| Grote | 2016 | 66 | 29% | 20% | 9% |
| Holmes | 1999 | 21 | 0% | 0% | 0% |
| Ramantani | 2013 | 23 | 57% | 44% | 13% |
| Sacino | 2017 | 22 | 36% | 32% | 5% |
| Salanova | 1994 | 35 | 36% | 36% | 0% |
| Schulz | 2011 | 22 | 9% | 9% | NR |
| Shaver | 1997 | 20 | 20% | 10% | 10% |
| Siegel | 2004 | 64 | 20% | 16% | 5% |
| Tian | 2011 | 9 | 22% | 0% | 22% |
| Vadera | 2012 | 36 | 14% | 0% | 14% |
| Wyler | 1989 | 27 | 11% | 11% | 0% |
NR = not reported
Discussion
Medically refractory epilepsy is a debilitating disease and can be particularly challenging to treat after an initial surgical intervention has failed. Existing data to help select candidates for repeat surgery have been limited to small case series and reviews19–22. Here, our group analyzed outcomes from 782 patients across 36 case series to provide the first quantitative meta-analysis of seizure outcomes following repeat epilepsy surgery. We found that 47% of patients across the literature achieved seizure freedom after further resection – a percentage higher than appreciated in a recent review19. We also found these outcomes to be positively correlated with electrophysiological congruency, lesional epilepsy, and surgical limitations as the cause of initial surgical failure. For patients who have at least one of these predictors, the seizure freedom rate after repeat surgery increased to 58%. It should be noted that this percentage does not include Engel II outcomes, which some consider a good result58. While temporal lobe surgery and MRI abnormalities were positively associated with seizure freedom in summary analysis, these traits did not reach statistical significance in quantitative meta-analysis. Similarly, predominantly focal onset seizures with impaired awareness showed a favorable association but was unable to undergo formal meta-analysis due to a paucity of studies with patients in both categories (seizure free and not seizure free, with and without predominantly focal onset seizures with impaired awareness). Perhaps intuitively, the amalgam of the attributes that predict seizure freedom here resemble those that predict good outcomes for an initial surgical resection59. Poorer outcomes were seen in patients who required invasive monitoring, possibly because this intervention is pursued in the more challenging cases. Ultimately, our findings demonstrate a previously underappreciated efficacy of repeat surgery in properly selected patients and supports evaluation for further surgical resection in patients who have failed an initial operation.
Predictors of Successful Repeat Surgery
Our group’s analysis demonstrated higher odds of seizure freedom in patients with lesional pathology, electrophysiological congruence, and surgical limitations as the cited reason for failure of the initial surgery. While the predictive value of the first two factors seems intuitive, not all studies have found them to be associated with favorable outcome36,40. The third factor, cited reasons for failure of the primary surgery, was divided into “surgical limitations” and “disease-related factors” for our analysis. Surgical limitations included reasons that implied an incomplete initial resection, such as errors in preoperative planning (e.g. wrong diagnosis or incomplete characterization of epileptogenic focus), overlap of functional and epileptogenic zones preventing complete resection, sub-optimal execution of surgical plan (e.g. sub-total resection), and regrowth or recurrence of a lesion (which also implies a sub-total resection). Disease-related factors suggested failure due to disease processes that maybe too widespread or evolving to cure, including emergence of new epileptiform areas postoperatively and widespread, multifocal, progressive, or bilateral disease (including palliative surgeries). The categorization of each result into the two groups relied completely on the description provided by the authors of the cited studies. While these two categories admittedly contain some overlap, the overall purpose of the dichotomy was to divide patients with foci that may have been amenable to complete resection were it not limited by surgical/diagnostic factors from those who have disease processes that may never be amenable to complete resection. The finding that surgical limitations portend higher odds of success with a repeat resection than disease-related factors is consistent with findings from Ramantani et al. and Sacino et al. demonstrating that when functional limitations prevented a complete primary resection, patients had better outcomes after further surgery46,48. Munari et al. have suggested that persistent seizures after resection of eloquent epileptogenic regions may not always represent a true failure, if the intent is a palliative operation aimed at reducing seizure frequency or severity43. Ultimately, our result illustrates the principle that those with epileptogic foci amenable to complete resection have a greater chance of success from a second surgery than those with progressive disease that may never be completely resected.
Factors Not Predictive of Outcome
Gender, epilepsy duration, age at either surgery, time between resections, laterality of resections, and seizure generalization showed no statistically significant association with outcome (although seizure generalizations did show a non-significant trend toward worse outcomes). While summary data suggested a significant association between temporal lobe resection and MRI abnormalities with better outcomes, formal meta-analysis did not demonstrate significant relationships for either of these variables. The lack of a clear distinction between temporal and extratemporal outcomes may be due to underappreciated heterogeneity in the temporal group, as it has been reported in multiple studies that the discovery of extratemporal epileptogenic foci (i.e. “temporal-plus” epilepsy) is correlated with initial surgical failure24,60–62. Therefore, failed temporal lobe-only resections may be due an inaccurate classification of temporal lobe epilepsy in these patients. Furthermore, after a temporal lobe resection, whether residual mesiotemporal structures cause continued seizures is not clear, as there is evidence for63–65 and against24,47,66 this finding. This makes it difficult to focus the target of re-resection after a failed temporal lobe resection. Ramos et al. argue that failure after temporal lobe surgery is most likely due to dual pathological entities, scar tissue, or contralateral temporal epileptogenesis, and that incomplete resection of the epileptogenic zones in the form of residual hippocampus is not the most likely cause for surgical failures47. Similarly, Hennessey et al. suggest that extrahippocampal or extratemporal epileptogenesis accounts for the majority of temporal surgical failures, regardless of the underlying pathology60. Abosch et al. argue that failure of selective amygdalohippocampectomy is usually because patients were improperly identified as having unilateral temporal lobe epilepsy24. Even so, the latter authors still include inadequate resection of mesial structures in their differential diagnosis of a failed resection. Furthermore, epileptogenic structures found surrounding the resection may have arisen postoperatively47 or were always present but not properly resected24,63 – a topic that remains in debate67.
Unfortunately, there was not enough disaggregated data to formally address the question of whether residual temporal structures on a postoperative MRI predicts success of a secondary surgery. Similarly, while abnormal MRIs showed an association with seizure freedom in our summary data, meta-analysis did not find a significant difference. This, too, is likely due to the heterogeneity of the abnormal MRI group. The fact that lesional versus non-lesional epilepsy did show significance whereas abnormal versus normal MRI did not suggests that the underlying pathology is more prognostic. In our analysis, abnormal MRIs included some cases that were considered non-lesional (e.g. mesiotemporal sclerosis [MTS] and cortical dysplasia [CD]), thus creating enough of an overlap to diminish its significance (see above discussion regarding temporal lobe epilepsy). González-Martínez et al. suggest that MTS and CD are the most likely pathologies to be associated with dual pathology, hence leading to worse outcomes after surgery32. There is also evidence that CD is associated with worse outcomes in temporal lobe epilepsy4. This is consistent with our finding that non-lesional epilepsy (which included these pathologic diagnoses in our analysis) portends lower odds for success than its counterpart. Although some studies classify focal CD and nodular heterotopias as lesional34,54, our group did not because it was often difficult to tell which CDs were focal versus widespread, and CD was often part of a dual pathology or presented at distant locations. Ultimately, the lack of significant association with outcome for abnormal MRIs and temporal resections may be due to the confounders explored in this section, or simply a result of underpowering.
Predictors of Failed Repeat Surgery
Previously cited predictors of poor outcome after repeat epilepsy surgery have included: distant or multifocal epileptiform discharges (i.e. widespread disease)30,46,51,52, history of encephalitis after 4 years of age36, inadequate second surgery (e.g. lesional excision or volume of temporal lobe taken)36,51, pathology consistent with CD or MTS32, recurrent or new epileptiform areas despite adequate achievement of primary surgical targets52, history of central nervous system (CNS) infection36, history of traumatic brain injury (TBI)40, and palliative procedures52. Most of these variables were not reported at a high enough rate to be quantitatively analyzed in our study. However, odds of negative outcomes were higher with the use of invasive monitoring and a disease-related failure in the initial surgery. The former association is controversial in the literature. While some studies suggest that invasive recording is associated with a higher rate of primary surgical seizure failure50,68, other studies advocate for its necessity34,52,57,69–71. The most likely explanation for invasive monitoring’s association with worse outcomes is its use in more complex cases. In some cases, invasive monitoring may enable surgery to be performed on patients who would otherwise not be considered surgical candidates. Some authors argue that lack of invasive monitoring during an initial surgery leaves room for improvement on the repeat surgery, thus predicting a successful reoperation52. Therefore, our data suggest that if the first surgery failed despite the use of invasive monitoring and due to disease-related factors, caution should be taken when considering a second intervention.
Morbidity and Complications
The overall complication rate of repeat epilepsy surgery was most recently reported as 13.5%19, but as we have shown, it varies greatly across studies (Table 3). For example, Germano et al. published a series where 39 of 40 patients had no neurological complications, and one patient experienced only temporary worsening of preoperative visual deficit30. Awad et al. and Holmes et al. report 0% complication rates25,36. However, Bower et al. cite a complication rate as high as 50% including cases of hydrocephalus causing pseudomeningoceles requiring shunt placement (mostly after functional hemispherectomies)28, and Ramantani et al. report that 57% of their patients had some form of adverse event46. The large discrepancy in complication rate is most likely related to the breadth of surgical interventions offered. If primary surgeries failed due to an overly conservative resection, a higher risk of neurological deficits such as visual field cuts, motor weakness, or aphasias might be expected from repeat, larger resections that extend into eloquent areas to control exceptionally difficult seizures46. Neurocognitive decline after secondary surgery has been reported as well, but it is also often followed by late neurocognitive improvements in patients who ultimately achieve seizure freedom34,40,45. Of note, there was no acute surgical mortality reported in any of the studies reviewed.
Reasons for Failure of Initial Surgery
Defining failure for epilepsy surgery can be challenging and should be analyzed on an individual basis and in the context of the patient and family’s expectations and goals. A broad definition of surgical failure might be the return to a state of medically intractable seizures, or a non-achievement of preoperative goals. While not all postoperative seizures predict true failure9, there is some evidence that latency to first seizure is related to long-term outcome in temporal lobe epilepsy72. Current evidence suggests that having 2 unprovoked seizures within 6 months of surgery and recurrence of ipsilateral epileptiform discharges on a 6 month postoperative evaluation constitutes a very high probability (>95%) of continuing into persistent medically refractory seizures73. Therefore, it is reasonable to consider a second surgery on this subset of patients.
Study Limitations
The use of retrospective studies in meta-analyses contains many inherent limitations.74 In our case, although data from 782 patients were utilized, not all variables were reported for each patient. Therefore, each analysis comprised only a subset of the total study population. For example, data regarding secondary seizure generalization were available in 145 (18.5%) patients, predominant seizure type in 206 (26.3%) patients, MRI findings in 196 (25.1%) patients, electrophysiological congruency in 192 (24.6%) patients, invasive monitoring in 210 (26.9%) patients, pathology in 507 (65%) patients, initial surgical location in 447 (57.2%) patients, final surgical location in 496 (63.4%) patients, and reason for failure of initial surgery in 283 (36.2%) patients (see Table 2). The inconsistent availability of variables of interest across reports is a limitation inherent to any meta-analysis of previously published studies, and it is important to consider when interpreting our results. Furthermore, drawing conclusions from data across multiple case series is always subject to a positive skew from publication bias, as positive results may be more likely to be published than negative ones. However, in the absence of a prospective, randomized, controlled trial, these methods allow us to capture the largest amount of data possible to quantitatively analyze potentially prognostic relationships between variables of interest and patient outcomes after repeat epilepsy surgery. Unfortunately, only two randomized, controlled studies of epilepsy resection exist75,76, and no controlled studies of repeat epilepsy surgery have ever been performed. Pursuing further randomized, controlled trials in epilepsy surgery remains a valuable future goal.
Additionally, this analysis contains patients, epileptologists, and surgeons from different countries and training backgrounds who utilize different diagnostic modalities and techniques. The included studies span over 30 years, an epoch that has seen advances in MRI techniques, positron-emission tomography (PET) scans62, AEDs, surgical practices, and knowledge of disease entities77,78, all of which can affect outcomes. However, our analysis found no significant trend in outcomes over time (Figure 2). To further ensure that the reported results were not skewed by older data, we repeated our analysis using only studies from the past 20 years and found no notable differences. It should also be noted that, while this study analyzes repeat surgery for epilepsy, it does not directly compare these results to other treatment modalities, such as medication adjustments or VNS.
Furthermore, there was a great deal of heterogeneity in the electrophysiological work-ups reported across studies. Unfortunately, available data were insufficient to separately analyze ictal versus non-ictal, invasive versus non-invasive, and subdural versus stereo-EEG data in a meaningful way. Thus, combining information allowed us to assess for any evidence of localized or non-localized findings across electrophysiological studies reported, which may provide some insight into the importance of overall electrophysiology congruency in predicting outcome. Therefore, conclusions related to individual electrophysiological exams cannot be made from our data, making it an important focus for future studies.
Conclusion
Here we provide the first quantitative meta-analysis of rates and predictors of seizure freedom following repeat epilepsy surgery. Freedom from disabling seizures (Engel I outcome) was observed in 47% of patients after repeat surgery, a rate higher than the previously reported 37%19. Additionally, this rate increased to 58% in patients who had a least one factor associated with a favorable outcome. The higher rate of success found in our analysis compared to historically reported values19 may be due to the inclusion of more patients and newer studies, or the exclusion of patients undergoing non-resective procedures like VNS or callosotomies. Significant predictors of seizure freedom in these patients resembled those for initial epilepsy surgery59, and included lesional over non-lesional epilepsy, congruent over non-congruent EEG data, and surgical over disease-related factors as the cited reason for failure of the first surgery. Invasive monitoring was associated with worse outcomes, and temporal lobe resections and abnormal MRIs trended toward better outcomes but did not reach statistical significance. Ultimately, these data support considering a repeat resection in patients who fail an initial intervention, especially in the setting of predictors of a favorable response.
Key Points.
Medically refractory epilepsy is particularly challenging to treat after an initial surgical resection has failed
Here we present the first quantitative meta-analysis of 30 years of literature to assess for rates and predictors of seizure freedom after reoperation
Predictors of seizure freedom include lesional over non-lesional pathology and all congruent over any non-congruent electrophysiological data
If the initial surgery failed due to surgical limitations versus disease-related factors, there are better odds of seizure freedom after reresection
Invasive monitoring portends a worse outcome; abnormal MRIs and temporal lobe-only resections show non-significant trends toward seizure freedom
Acknowledgments
Max O. Krucoff, MD, is supported by a grant from the National Institute of Neurological Disorders and Stroke (NINDS)/National Institute of Health (NIH) (R25, 5R25NS065731-08). Dario J. Englot, MD, PhD, is also supported by a grant from the NINDS/NIH (K99, NS097618).
Footnotes
Conflicts of Interest
The authors declare we have no conflicts of interest
Ethics Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure
None of the authors has any conflict of interest to disclose
References
- 1.Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7:525–37. doi: 10.1016/S1474-4422(08)70109-1. [DOI] [PubMed] [Google Scholar]
- 2.Englot DJ, Lee AT, Tsai C, et al. Seizure types and frequency in patients who “fail” temporal lobectomy for intractable epilepsy. Neurosurgery. 2013;73:838–44. doi: 10.1227/NEU.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 3.Yasuda CL, Tedeschi H, Oliveira ELP, et al. Comparison of short-term outcome between surgical and clinical treatment in temporal lobe epilepsy: A prospective study. Seizure. 2006;15(1):35–40. doi: 10.1016/j.seizure.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Xu R, Liu Z, Zhang X, et al. Temporal lobe epilepsy surgery and preoperative factors predictive of postoperative outcome: retrospective analysis of 143 cases. Academic journal of the first medical college of PLA. 2003;23:663–7. [PubMed] [Google Scholar]
- 5.Sinclair DB, Aronyk K, Snyder T, et al. Pediatric temporal lobectomy for epilepsy. Pediatr Neurosurg. 2003;38:195–205. doi: 10.1159/000069099. [DOI] [PubMed] [Google Scholar]
- 6.Englot DJ, Raygor KP, Molinaro AM, et al. Factors associated with failed focal neocortical epilepsy surgery. Neurosurgery. 2014;75:648–55. doi: 10.1227/NEU.0000000000000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryzí M, Brázdil M, Novák Z, et al. Long-term outcomes in patients after epilepsy surgery failure. Epilepsy Res. 2015;110:71–7. doi: 10.1016/j.eplepsyres.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Wolf P. Treatment of epilepsy following rejection of epilepsy surgery. Seizure. 1998;7:25–9. doi: 10.1016/s1059-1311(98)90004-8. [DOI] [PubMed] [Google Scholar]
- 9.Salanova V, Andermann F, Rasmussen T, et al. The running down phenomenon in temporal lobe epilepsy. Brain. 1996;119:989–96. doi: 10.1093/brain/119.3.989. [DOI] [PubMed] [Google Scholar]
- 10.Ryzi M, Oslejskova H, Rektor I, et al. Long-term approach to patients with postsurgical seizures. Epilepsia. 2016;57:597–604. doi: 10.1111/epi.13343. [DOI] [PubMed] [Google Scholar]
- 11.Rasmussen T. Modern problems of pharmacopsychiatry. In: Niedermeyer E, editor. The Neurosurgical Treatment of Focal Epilepsy First. New York: Krager; 1970. pp. 306–25. [Google Scholar]
- 12.Vale FL, Ahmadian A, Youssef AS, et al. Long-term outcome of vagus nerve stimulation therapy after failed epilepsy surgery. Seizure. 2011;20:244–8. doi: 10.1016/j.seizure.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Elliott RE, Morsi A, Geller EB, et al. Impact of failed intracranial epilepsy surgery on the effectiveness of subsequent vagus nerve stimulation. Neurosurgery. 2011;69:1210–7. doi: 10.1227/NEU.0b013e3182230ae3. [DOI] [PubMed] [Google Scholar]
- 14.Morrell MJ. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 15.McLachlan RS, Rose KJ, Derry PA, et al. Health-related quality of life and seizure control in temporal lobe epilepsy. Ann Neurol. 1997;41:482–9. doi: 10.1002/ana.410410411. [DOI] [PubMed] [Google Scholar]
- 16.Keene DL, Higgins MJ, Ventureyra ECG. Outcome and life prospects after surgical management of medically intractable epilepsy in patients under 18 years of age. Child’s Nerv Syst. 1997;13:530–5. doi: 10.1007/s003810050132. [DOI] [PubMed] [Google Scholar]
- 17.Derry PA, Wiebe S. Psychological adjustment to success and to failure following epilepsy surgery. Can J Neurol Sci. 2000;27(Suppl 1):S116-20-5. doi: 10.1017/s0317167100000779. [DOI] [PubMed] [Google Scholar]
- 18.Bourgeois M, Sainte-Rose C, Lellouch-Tubiana A, et al. Surgery of epilepsy associated with focal lesions in childhood. J Neurosurg. 1999;90:833–42. doi: 10.3171/jns.1999.90.5.0833. [DOI] [PubMed] [Google Scholar]
- 19.Surges R, Elger CE. Reoperation after failed resective epilepsy surgery. Seizure. 2013;22:493–501. doi: 10.1016/j.seizure.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 20.El Tahry R, Wang IZ. Failed epilepsy surgery: is this the end? Acta Neurol Belg. 2017 doi: 10.1007/s13760-017-0769-8. [DOI] [PubMed] [Google Scholar]
- 21.Goodman RR. AES 2009 Annual Course: Reoperation for medically refractory epilepsy. Epilepsy Behav. 2011;20:241–6. doi: 10.1016/j.yebeh.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 22.Téllez-Zenteno JF, Moien-Afshari F, Hernández-Ronquillo L, et al. Reasons for reoperation after epilepsy surgery: a review based on a complex clinical case with three operations. Neuropsychiatr Dis Treat. 2010;6:409–15. doi: 10.2147/ndt.s10384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abosch AA, Bernasconi NN, Boling WW, et al. Factors predictive of suboptimal seizure control following selective amygdalohippocampectomy. J Neurosurg. 2002;97:1142–51. doi: 10.3171/jns.2002.97.5.1142. [DOI] [PubMed] [Google Scholar]
- 25.Awad IA, Nayel MH, Ch MBB, et al. Second Operation after the Failure of Previous Resection for Epilepsy. Neurosurgery. 1991;28:510–8. doi: 10.1097/00006123-199104000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Benifla M, Otsubo H, Ochi A, et al. Temporal lobe surgery for intractable epilepsy in children: An analysis of outcomes in 126 children. Neurosurgery. 2006;59:1203–13. doi: 10.1227/01.NEU.0000245615.32226.83. [DOI] [PubMed] [Google Scholar]
- 27.Bauman JA, Feoli E, Romanelli P, et al. Multistage epilepsy surgery: safety, efficacy, and utility of a novel approach in pediatric extratemporal epilepsy. Neurosurgery. 2008;62(Suppl 2):489–505. doi: 10.1227/01.neu.0000316252.47028.2d. [DOI] [PubMed] [Google Scholar]
- 28.Bower RS, Wirrell EC, Eckel LJ, et al. Repeat resective surgery in complex pediatric refractory epilepsy: lessons learned. J Neurosurg Pediatr. 2015;16:94–100. doi: 10.3171/2014.12.PEDS14150. [DOI] [PubMed] [Google Scholar]
- 29.Fauser S, Essang C, Altenmüller D-M, et al. Long-term seizure outcome in 211 patients with focal cortical dysplasia. Epilepsia. 2015;56(1):66–76. doi: 10.1111/epi.12876. [DOI] [PubMed] [Google Scholar]
- 30.Germano IM, Poulin N, Ed M, et al. Reoperation for Recurrent Temporal Lobe Epilepsy. J Neurosurg. 1994;81:31–6. doi: 10.3171/jns.1994.81.1.0031. [DOI] [PubMed] [Google Scholar]
- 31.Goellner E, Bianchin MM, Burneo JG, et al. Timing of early and late seizure recurrence after temporal lobe epilepsy surgery. Epilepsia. 2013;54:1933–41. doi: 10.1111/epi.12389. [DOI] [PubMed] [Google Scholar]
- 32.González-Martínez JA, Srikijvilaikul T, Nair D, et al. Long-term seizure outcome in reoperation after failure of epilepsy surgery. Neurosurgery. 2007;60:873–9. doi: 10.1227/01.NEU.0000255438.13871.FA. [DOI] [PubMed] [Google Scholar]
- 33.Greiner HM, Horn PS, Tenney JR, et al. Should spikes on post-resection ECoG guide pediatric epilepsy surgery? Epilepsy Res. 2016;122:73–8. doi: 10.1016/j.eplepsyres.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Grote A, Witt JA, Surges R, et al. A second chance-reoperation in patients with failed surgery for intractable epilepsy: long-term outcome, neuropsychology and complications. J Neurol Neurosurg Psychiatry. 2016;87:379–85. doi: 10.1136/jnnp-2015-310322. [DOI] [PubMed] [Google Scholar]
- 35.Hallbook T, Tideman P, Rosen I, et al. Epilepsy surgery in children with drug-resistant epilepsy, a long-term follow-up. Acta Neurol Scand. 2013;128:414–21. doi: 10.1111/ane.12154. [DOI] [PubMed] [Google Scholar]
- 36.Holmes MD, Wilensky AJ, Ojemann LM, et al. Predicting outcome following reoperation for medically intractable epilepsy. Seizure. 1999;8:103–6. doi: 10.1053/seiz.1998.0256. [DOI] [PubMed] [Google Scholar]
- 37.Jehi LE, Silveira DC, Bingaman W, et al. Temporal lobe epilepsy surgery failures: predictors of seizure recurrence, yield of reevaluation, and outcome following reoperation. J Neurosurg. 2010;113:1186–94. doi: 10.3171/2010.8.JNS10180. [DOI] [PubMed] [Google Scholar]
- 38.Jooma R, Yeh HS, Privitera MD, et al. Lesionectomy versus electrophysiologically guided resection for temporal lobe tumors manifesting with complex partial seizures. J Neurosurg. 1995;83:231–6. doi: 10.3171/jns.1995.83.2.0231. [DOI] [PubMed] [Google Scholar]
- 39.Juhasz C, Chugani DC, Padhye UN, et al. Evaluation with alpha-[11C]methyl-L-tryptophan positron emission tomography for reoperation after failed epilepsy surgery. Epilepsia. 2004;45:124–30. doi: 10.1111/j.0013-9580.2004.30303.x. [DOI] [PubMed] [Google Scholar]
- 40.Jung R, Aull-Watschinger S, Moser D, et al. Is reoperation an option for patients with temporal lobe epilepsy after failure of surgery? Seizure. 2013;22:502–6. doi: 10.1016/j.seizure.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 41.Kirchberger K, Hummel C, Stefan H. Postoperative multichannel magnetoencephalography in patients with recurrent seizures after epilepsy surgery. Acta Neurol Scand. 1998;98:1–7. doi: 10.1111/j.1600-0404.1998.tb07370.x. [DOI] [PubMed] [Google Scholar]
- 42.Koh S, Nguyen S, Asarnow RF, et al. Five or More Acute Postoperative Seizures Predict Hospital Course and Long-term Seizure Control after Hemispherectomy. Epilepsia. 2004;45:527–33. doi: 10.1111/j.0013-9580.2004.50203.x. [DOI] [PubMed] [Google Scholar]
- 43.Munari C, Berta E, Tassi L, et al. Analysis of failures and reoperations in resective epilepsy surgery. Adv Neurol. 2000;84:605–14. [PubMed] [Google Scholar]
- 44.Otsuki T, Honda R, Takahashi A, et al. Surgical management of cortical dysplasia in infancy and early childhood. Brain Dev. 2013;35:802–9. doi: 10.1016/j.braindev.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Pati S, Abla AA, Rekate HL, et al. Repeat surgery for hypothalamic hamartoma in refractory epilepsy. Neurosurg Focus. 2011;30:E3. doi: 10.3171/2010.11.FOCUS10248. [DOI] [PubMed] [Google Scholar]
- 46.Ramantani G, Strobl K, Stathi A, et al. Reoperation for refractory epilepsy in childhood: A second chance for selected patients. Neurosurgery. 2013;73:695–704. doi: 10.1227/NEU.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 47.Ramos E, Benbadis S, Vale FL. Failure of temporal lobe resection for epilepsy in patients with mesial temporal sclerosis: results and treatment options. J Neurosurg. 2009;110:1127–34. doi: 10.3171/2009.1.JNS08638. [DOI] [PubMed] [Google Scholar]
- 48.Sacino MF, Ho C, Whitehead MT, et al. Repeat surgery for focal cortical dysplasias in children: indications and outcomes. J Neurosurg Pediatr. 2017;19:174–81. doi: 10.3171/2016.8.PEDS16149. [DOI] [PubMed] [Google Scholar]
- 49.Salanova V, Quesney LF, Rasmussen T, et al. Reevaluation of Surgical Failures and the Role of Reoperation in 39 Patients with Frontal Lobe Epilepsy. Epilepsia. 1994;35:70–80. doi: 10.1111/j.1528-1157.1994.tb02914.x. [DOI] [PubMed] [Google Scholar]
- 50.Salanova V, Markand O, Worth R. Temporal lobe epilepsy: Analysis of failures and the role of reoperation. Acta Neurol Scand. 2005;111:126–33. doi: 10.1111/j.1600-0404.2005.00371.x. [DOI] [PubMed] [Google Scholar]
- 51.Schulz R, Hoppe M, Boesebeck F, et al. Analysis of reoperation in mesial temporal lobe epilepsy with hippocampal sclerosis. Neurosurgery. 2011;68:89–97. doi: 10.1227/NEU.0b013e3181fdf8f8. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz T, Spencer D. Strategies for reoperation after comprehensive epilepsy surgery. J Neurosurg. 2001;95:615–23. doi: 10.3171/jns.2001.95.4.0615. [DOI] [PubMed] [Google Scholar]
- 53.Shaver EG, Harvey AS, Morrison G, et al. Results and Complications After Reoperation for Failed Epilepsy Surgery in Children. Pediatr Neurosurg. 1997;27:194–202. doi: 10.1159/000121251. [DOI] [PubMed] [Google Scholar]
- 54.Siegel aM, Cascino GD, Meyer FB, et al. Resective reoperation for failed epilepsy surgery: seizure outcome in 64 patients. Neurology. 2004;63:2298–302. doi: 10.1212/01.wnl.0000147476.86575.a7. [DOI] [PubMed] [Google Scholar]
- 55.Tian AG, Edwards MSB, Williams NJ. Epilepsy surgery following brain tumor resection in children. J Neurosurg Pediatr. 2011;7:229–34. doi: 10.3171/2010.12.PEDS10293. [DOI] [PubMed] [Google Scholar]
- 56.Vadera S, Moosa ANV, Jehi L, et al. Reoperative hemispherectomy for intractable epilepsy: A report of 36 patients. Neurosurgery. 2012;71:388–92. doi: 10.1227/NEU.0b013e31825979bb. [DOI] [PubMed] [Google Scholar]
- 57.Wyler aR, Hermann BP, Richey ET. Results of reoperation for failed epilepsy surgery. J Neurosurg. 1989;71:815–9. doi: 10.3171/jns.1989.71.6.0815. [DOI] [PubMed] [Google Scholar]
- 58.Wetjen NM, Cascino GD, Fessler aJ, et al. Subtraction ictal single-photon emission computed tomography coregistered to magnetic resonance imaging in evaluating the need for repeated epilepsy surgery. J Neurosurg. 2005;105:71–6. doi: 10.3171/jns.2006.105.1.71. [DOI] [PubMed] [Google Scholar]
- 59.Englot DJ, Han SJ, Rolston JD, et al. Epilepsy surgery failure in children: a quantitative and qualitative analysis. J Neurosurg Pediatr. 2014;14:386–95. doi: 10.3171/2014.7.PEDS13658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hennessy MJ, Elwes RD, Binnie CD, et al. Failed surgery for epilepsy. A study of persistence and recurrence of seizures following temporal resection. Brain. 2000;123(Pt 12):2445–66. doi: 10.1093/brain/123.12.2445. [DOI] [PubMed] [Google Scholar]
- 61.Barba C, Rheims S, Minotti L, et al. Temporal plus epilepsy is a major determinant of temporal lobe surgery failures. Brain. 2016;139:444–51. doi: 10.1093/brain/awv372. [DOI] [PubMed] [Google Scholar]
- 62.Chassoux F, Laurent A, Landré E. F-FDG-PET patterns of surgical success and failure in mesial temporal lobe epilepsy. Neurology. 2017;16:1–10. doi: 10.1212/WNL.0000000000003714. [DOI] [PubMed] [Google Scholar]
- 63.Zachenhofer I, Novak K, Baumgartner C, et al. Reoperation after selective amygdalohippocampectomy: An MRI analysis of the extent of temporomesial resection in ten cases. Acta Neurochir (Wien) 2011;153:239–48. doi: 10.1007/s00701-010-0802-7. [DOI] [PubMed] [Google Scholar]
- 64.Erdem A, Kahilogullari G, Erbas YC, et al. Reoperation of a recurrent temporal lobe epilepsy: a technical case report. Surg Neurol. 2005;64(Suppl 2):S102–5. doi: 10.1016/j.surneu.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 65.Abou-Khalil B, Andermann E, Andermann F, Olivier a, Quesney LF. Temporal lobe epilepsy after prolonged febrile convulsions: excellent outcome after surgical treatment. Epilepsia. 1993;34:878–83. doi: 10.1111/j.1528-1157.1993.tb02105.x. [DOI] [PubMed] [Google Scholar]
- 66.Malikova H, Liscak R, Vojtech Z, et al. Stereotactic radiofrequency amygdalohippocampectomy: Does reduction of entorhinal and perirhinal cortices influence good clinical seizure outcome? Epilepsia. 2011;52:932–40. doi: 10.1111/j.1528-1167.2011.03048.x. [DOI] [PubMed] [Google Scholar]
- 67.Wieser HG. Mesial temporal lobe epilepsy versus amygdalar epilepsy: late seizure recurrence after initially successful amygdalotomy and regained seizure control following hippocampectomy. Epileptic Disord. 2000;2:141–52. [PubMed] [Google Scholar]
- 68.Blume WT, Ganapathy GR, Munoz D, et al. Indices of Resective Surgery Effectiveness for Intractable Nonlesional Focal Epilepsy. Epilepsia. 2004;45:46–53. doi: 10.1111/j.0013-9580.2004.11203.x. [DOI] [PubMed] [Google Scholar]
- 69.Wellmer J, Von Der Groeben F, Klarmann U, et al. Risks and benefits of invasive epilepsy surgery workup with implanted subdural and depth electrodes. Epilepsia. 2012;53:1322–32. doi: 10.1111/j.1528-1167.2012.03545.x. [DOI] [PubMed] [Google Scholar]
- 70.Gelinas JN, Battison AW, Smith S, et al. Electrocorticography and seizure outcomes in children with lesional epilepsy. Child’s Nerv Syst. 2011;27:381–90. doi: 10.1007/s00381-010-1279-7. [DOI] [PubMed] [Google Scholar]
- 71.Siegel AM, Roberts DW, Thadani VM, et al. The Role of Intracranial Electrode Reevaluation in Epilepsy Patients After Failed Initial Invasive Monitoring. Epilepsia. 2000;41:571–80. doi: 10.1111/j.1528-1157.2000.tb00211.x. [DOI] [PubMed] [Google Scholar]
- 72.Buckingham SE, Chervoneva I, Sharan A, et al. Latency to first seizure after temporal lobectomy predicts long-term outcome. Epilepsia. 2010;51:1987–93. doi: 10.1111/j.1528-1167.2010.02721.x. [DOI] [PubMed] [Google Scholar]
- 73.Jehi L, Sarkis R, Bingaman W, et al. When is a postoperative seizure equivalent to “epilepsy recurrence” after epilepsy surgery? Epilepsia. 2010;51:994–1003. doi: 10.1111/j.1528-1167.2010.02556.x. [DOI] [PubMed] [Google Scholar]
- 74.Sampson JH, Barker FG. Methodology and Reporting of Meta-Analyses in the Neurosurgical Literature. J Neurosurg. 2014;120:791–5. doi: 10.3171/2013.10.JNS13724. [DOI] [PubMed] [Google Scholar]
- 75.Wiebe S, Blume WT, Girvin JP, et al. A randomized, controlled trial of surgery for tempral-lobe epilepsy. N Engl J Med. 2001;345:311–8. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 76.Engel J, Jr, Mcdermott MP, Wiebe S, et al. Early Surgical Therapy for Drug-Resistant Temporal Lobe Epilepsy. JAMA. 2012:307. doi: 10.1001/jama.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Colosimo E, Gambardella A, Mantegazza M, et al. Electroclinical features of a family with simple febrile seizures and temporal lobe epilepsy associated with SCN1A loss-of-function mutation. Epilepsia. 2007;48:1691–6. doi: 10.1111/j.1528-1167.2007.01153.x. [DOI] [PubMed] [Google Scholar]
- 78.Bien CG, Urbach H, Schramm J, et al. Limbic encephalitis as a precipitating event in adult-onset temporal lobe epilepsy. Neurology. 2007;69:1236–44. doi: 10.1212/01.wnl.0000276946.08412.ef. [DOI] [PubMed] [Google Scholar]