Abstract
Spontaneous physical activity (SPA) is physical activity not motivated by a rewarding goal, such as that associated with food-seeking or wheel running behavior. SPA is often thought of as only “fidgeting”, but that is a mischaracterization, since fidgety behavior can be linked to stereotypies in neurodegenerative disease and other movement disorders. Instead, SPA should be thought of as all physical activity behavior that emanates from an unconscious drive for movement. An example of this may be restless behavior, which can include fidgeting and gesticulating, frequent sit-to-stand movement, and more time spent standing and moving. All physical activity burns calories, and as such, SPA could be manipulated as a means to burn calories, defend against weight gain and reduce excess adiposity. In this review, we discuss human and animal literature on the use of SPA in reducing weight gain, the neuromodulators that could be targeted to this end, and future directions in this field.
Keywords: Spontaneous physical activity, non-exercise energy expenditure, locomotion, exercise, obesity, food intake, eating behavior, brain, central nervous system, orexin, dynorphin, DREADD, optogenetics, human, animal
INTRODUCTION
Susceptibility to obesity in humans depends on biological mechanisms and environmental effects [1–3]. There is extensive inter-individual variability in diet-induced obesity (DIO) susceptibility [4, 5] and co-morbidity of obesity with other pathologies, such as metabolic disorder and cardiovascular disease [6]. Spontaneous physical activity (SPA) and its associated thermogenesis (NEAT, non-exercise induced thermogenesis) are major contributors to the variability between humans in diet-induced obesity [7, 8, 5]. In humans, SPA includes fidgeting, time spent standing and ambulating [9, 10], and thus it is thought to be a reflection of activity that is not goal oriented, but an expression of an inherent drive for activity [11, 12]. Spontaneous physical activity and NEAT can account for up to 30 percent of daily energy expenditure in humans [8, 13]. In response to overfeeding, some humans increase their SPA and resist obesity [5], whereas others do not. Individual variation in SPA and NEAT has important implications for energy balance. Mechanisms controlling SPA and NEAT are not well defined, but have been shown to involve several neuropeptide systems, including orexins [14, 10, 15]. We propose that the orexins and their receptors represent a neurobiological system that is central to the control of SPA and NEAT.
HUMAN SPA STUDIES
Recent cross-sectional and longitudinal studies show that individuals with higher SPA levels weigh less [16–18] and gain less weight over time [16, 19, 20]. It has also been shown that physical activity [21], including habitual or spontaneous levels of physical activity [22] can moderate or eliminate increased weight among those carrying a risk gene at the FTO genetic locus, the most common risk gene for obesity. Blood levels of the activity related neuropeptide orexin (discussed below) are higher among people with higher amounts of physical activity [23]. Few intervention studies have addressed the clinical significance of elevated SPA to human obesity or addressed whether an increase in SPA mitigates weight gain in the predicted manner by increasing total energy expenditure in adults [24, 25]. Despite the limitations that were acknowledged in those studies [24, 25], the difficulty of designing SPA interventions may be related to the inherent difficulty of intervening to increase SPA levels based on its operational definition as low intensity physical activity that distinguishes this “non-exercise physical activity” from formal exercise. Hence, aside from modifying the obesogenic environment to promote SPA (e.g. remove labor-saving devices such as escalators or elevators, move parking lots further from entrances, abolish the drive-through window at fast-food restaurants, use monetary incentives for public transportation and biking to work, or stand up during meetings at work), is it feasible to intervene with the intention of increasing SPA levels without imposing a structured intervention to increase low intensity physical activity such as walking? If we adhere to the strict definition of SPA, then we would theorize that those with higher SPA levels would weigh less and gain less weight over time similar to pre-clinical models with high and low SPA levels. Thus, SPA levels should distinguish individuals classified as being more prone or resistant to obesity. Based on this idea, Schmidt and colleagues determined SPA levels before and after 3-d of overfeeding among adults that were classified as being prone or resistant to obesity based on family weight history, BMI and self-identification [26]. Despite that SPA indicated by walking was similar between the obesity prone and resistant individuals before overfeeding, obesity prone adults responded to overfeeding with a reduction in walking while obesity resistant adults maintained their level of walking. It’s difficult to reconcile why SPA levels were similar between obesity prone and resistant adults before overfeeding based on their different BMI and the inverse relationship between SPA levels and bodyweight [16–18], but it is unknown whether weight gain trajectories would have tracked as predicted based on SPA levels prior to overfeeding.
Consistent with the strict definition of SPA, Villablanca and colleagues reviewed literature that targeted sedentary work environments and proposed interventions that modified occupational settings and leisure-time activities as feasible approaches to increasing NEAT (reviewed in [27]). Several approaches have targeted the traditional office environment by replacing chair-based desks with upright/standing desks, treadmill desks, “fidget” chairs or chairs designed to increase leg movement. Koepp and colleges reported that the addition of an apparatus under a desk designed to promote leg movement or increase fidgeting increased total EE by approximately 13–22kcal/h more than a traditional seated chair [28, 29]. Dutta and colleagues reported that installation of a sit-to-stand desk for 4-weeks reduced time spent seated by 20% with no change in activity during non-work hours and the authors projected a 3.2-h reduction in sedentary time during a 40-h week [30]. Likewise, Thompson and colleagues reported that a 24-week intervention among physicians that used a treadmill desk increased daily physical activity by 197 kcal per day, which was paralleled by significant reduction in bodyweight and percent body fat [31]. While results from physicians may not apply to the general population, Koepp and colleagues reported that the addition of a treadmill desk to employees for one year increased daily physical activity, reduced sedentary time defined as time spent with zero activity, and despite that the whole group lost an average of 1.4 ± 3.3 kg, those with obesity lost on average 2.3 ± 3.5 kg [32]. Finally, McCrady-Spitzer and colleagues have intervened within children’s work environments by increasing NEAT within classrooms. They reported that the addition of “Active Class Equipment” resulted in a sequential increase in activity units per minute (measured with Triaxial accelerometers) with each successive quarter during the 9-month school year among first grade students that used the equipment for 30-minutes per day [33]. Thus, re-designing office and classroom environments by removing or modifying chairs promotes SPA and would be expected to mitigate weight gain.
Another question that arises when considering challenges to designing SPA interventions is related to the principles of physical activity or exercise prescription (e.g. mode, duration and intensity of activity). Since SPA is often referred to as low intensity physical activity, should all interventions aimed at increasing walking be classified as SPA intervention studies? While this seems to be an oversimplification, free-living SPA is often indicated by walking and the intensity of walking or the imposition of a structured walking program is often used to address whether structured exercise or the intensity of exercise leads to a compensatory reduction in SPA and its associated energy expenditure (non-exercise activity thermogenesis, NEAT). This literature has already been extensively reviewed [34–36]. Briefly, Washburn and colleagues performed a systematic review of the literature to access the influence of exercise on SPA and energy expenditure [35]. They reported that there was minimal evidence in support of the hypothesis that structured exercise decreases SPA and energy expenditure in healthy adults [35]. The authors’ highlighted that the heterogeneity across studies, which when combined with other factors that influence SPA (e.g. age, gender, body mass and training status) [34–36], underscores the unique opportunity to resolve whether imposed exercise causes a compensatory change in SPA or the associated NEAT.
Although it is unclear how much exercise modifies SPA, total energy expenditure, and its components, several models have been proposed to explain the relationship between SPA and energy expenditure as they relate to weight gain [37–39, 34] and recently reviewed by [34]. Based on the definition of energy balance, whereby weight maintenance persists when energy intake equals energy expenditure, one would expect that increasing SPA would in turn increase overall energy expenditure [38] and mitigate weight gain if daily calorie intake remained constant. Yet the paradox of lower than predicted energy expenditure has been linked to weight regain after weight loss or exercise, and the latter has been attributed in part to a compensatory reduction in resting energy expenditure, SPA or improved muscle efficiency during physical activity (e.g. reduced energetic cost of physical activity) [40]. The ‘ActivityStat’ hypothesis [37, 41] and several models have been proposed (e.g. allocation, independent/additive, performance, constrained energy expenditure) [38, 39] including an alternative model which proposes that exercise modifies an additional and unidentified component of total EE alone or in parallel to the EE due to SPA, the thermic effect of food or resting metabolism [34]. The efficiency of performing SPA may not be constant and thus an increase in SPA may not translate into the expected increase in NEAT. In addition to the methodological considerations reviewed previously [34, 35] (e.g. more accurate and precise methods to quantify SPA, components of total energy expenditure and sedentary behavior), addressing the clinical significance of SPA and NEAT to energy balance will require additional studies in free-living environments versus whole-room calorimeters. The latter is highlighted by an early study reporting that despite that positive association between measured level of SPA in a whole-room calorimeter with free-living SPA [42], physical activity was lower in the room calorimeter compared to free-living conditions, which translated into a 47% reduction in activity-related energy expenditure in the calorimeter compared to that during free-living conditions. Thus, disentangling the relationship between SPA, NEAT and total energy expenditure to overall energy balance will require additional studies in free-living environments to avoid artificially reducing SPA when measured in whole room calorimeters.
ANIMAL MODELS OF SPA AND OBESITY
Most animal evidence supports the concept that SPA and its associated energy expenditure (NEAT) can protect against obesity. In animal models, SPA is usually understood to describe home-cage activity or overall activity and different from motivated behaviors, such as running wheel activity [10]. Yet, SPA is loosely applied to describe the outcome of different methods to quantify animal behavior, including telemetry, video analysis or IR sensors [43]. We propose that SPA should include all movement (locomotor, rearing and grooming) performed by the animal in the absence of an immediate goal and external influences that disrupt normal behavior (i.e. novelty) over an extended period of time (i.e. days) [43]. However, this is a difficult definition of SPA to fulfill, which is further complicated by the fact that most methods used to quantify SPA require isolation, known to alter animal behavior [44]. Whether the isolation has a significant effect on SPA requires the ability to accurately measure SPA in isolated and group-housed animals, which is currently an unsolved technical challenge. Furthermore, even in isolated animals, the different methods used to quantify SPA measure unique aspects of this behavior, which do not necessarily provide equivalent information [43]. Therefore, the analysis of SPA in rodents can be deceiving and studies should include detailed information regarding the method of SPA to determine exactly what is being measured under the specific experimental conditions.
When considering the influence of SPA on obesity, measuring NEAT and not SPA should be the ultimate goal, yet only recently technological advances have been able to provide accurate quantification of NEAT in rodents using specialized equipment [45, 46], which helps explain why SPA is more commonly measured as compared to NEAT. Currently, NEAT is determined by analyzing overall energy expenditure (i.e. by indirect calorimetry) together with some measurement of activity, which allows linking energy expenditure to specific behavioral states [43]. Yet this method of estimating NEAT is further complicated by the influence of isometric skeletal muscle contraction to energy expenditure [47] which cannot be estimated using the type of analysis outlined. Here, we discuss animal studies that support the concept that SPA and NEAT can protect against obesity, yet we make the distinction where SPA and NEAT have been measured and the components of SPA analyzed in the study.
The available evidence suggests that in rats bred selectively for high (obesity-prone, OP) or low (obesity resistant, OR) weight gain when fed a high-fat diet [48], OR rats show higher SPA (time spent in locomotor and rearing activity) compared to non-selectively bred and OP rats [49]. Moreover, the respective phenotypes were maintained across the lifespan [50]. In studies using a different approach, non-selectively bred rats were screened for their SPA (time spent in locomotor and rearing activity) and classified as high activity (HA) or low activity (LA) rats. This model showed that HA rats were more resistant to obesity induced by HF diet compared to LA rats [51], which is direct support for the hypothesis that individual variation in SPA has a noticeable effect on diet-induced obesity. The HA/LA rats illustrate the inter-individual variability in SPA within a particular rodent strain, which has been observed in other rodent strains and related to variability in response to metabolic challenges [52, 53]. For example, recently, work in Balb/c mice fed cafeteria diet showed that obesity-resistant mice increase their SPA (locomotor activity) in response to CAF diet, yet there were no differences in their SPA prior to CAF diet feeding [54]. In C57 mice, HFD feeding decreased SPA (locomotor activity) and NEAT [55], highlighting the bidirectional nature of the relationship between SPA and NEAT. Together, these data support the concept that SPA and NEAT contribute to obesity resistance, but there is extensive individual variation in the magnitude of effect, and the mechanisms by which SPA protects against obesity (e.g. increasing on a high-energy diet and/or maintaining high and low activity regardless of diet). Recent evidence has explored whether other traits related to energy balance and physical activity are correlated with or influence SPA. For example, aerobic capacity does not correlate with SPA [56] while basal metabolic rate does [57], and the evidence is still mixed as to the effects of voluntary exercise on SPA [58–61]. Future challenges include the accurate measurement of SPA, its different components and NEAT, the possibility of measuring SPA and NEAT in group housed animals, and accurate comparison of the physiological effects and mechanisms of SPA between males and females.
Neural Regulators of SPA
There are several neuroregulators of SPA as has been previously reviewed by this group [14]. This section will not cover the data supporting all of those neuroregulators as having roles in the regulation of SPA, but these include dynorphin, neuromedin, oxyntmodulin, leptin, melanocortins and others. The best characterized in terms of its role in SPA and the defense against obesity, is orexin, also known as hypocretin. There are two orexin peptides (orexin A and orexin B) and two receptors (orexin receptor 1, OX1R and orexin receptor 2, OX2R) [62, 63]. The orexin neurons send efferent projections to multiple brain sites important for physical activity, including but not limited to the substantia nigra, dorsal raphe nucleus, and the locus coeruleus [64]. Expression of orexin receptors (OXR) is differential, varying widely among brain sites [65, 66]. The functional significance of the two orexin receptors (OXR1 and OXR2) is unclear.
In accordance with a role in daily energy balance regulation, orexin neurons display rhythmicity that is entrained to environmental cues and physiological signals. Orexin neuron activity increases during the waking phase, fasting or caloric restriction [67]. The activity of orexin neurons is modulated by multiple metabolic indicators (i.e. glucose, leptin and amino acids) [68–70] and intra- and extra-hypothalamic synaptic inputs [71–73]. Orexin peptides modulate energy metabolism and arousal [74–80]. Our lab and others have repeatedly demonstrated that orexins protect against obesity. An animal model with neurodegeneration of orexin neurons develops obesity despite reduced food intake [75]. Mice over-expressing orexin peptides show resistance to obesity [81]. Current data support that resistance to obesity correlates with greater behavioral effects of orexin A [49], higher expression of prepro-orexin in the LH, and higher sensitivity to orexin A in rostral LH on SPA [82]. Orexin neurons send collateral projections within the CNS [83, 84], but data describing the organization of the orexin field is lacking. It has been proposed that orexin neurons located in LH mediate reward behaviors and those located in the PFA/DMH area are involved in arousal and stress [85, 86]. The pattern of circadian Fos expression in orexin neurons supports this idea [76], but it is not clear to what extent there is overlapping versus specialized function of the orexin neurons, and which set of orexin neurons are important to SPA.
Orexin-dependent modulation of SPA and NEAT involves OXR subtypes in several brain areas [87–89], including the rostral LH [90, 88], dorsal raphe nucleus, substantia nigra, ventral lateral preoptic area and locus coeruleus [91]. Orexin A in rostral LH increases NEAT [90, 88, 92], and repeated injection reduces adiposity [93]. These data suggest the orexin receptors located in this area of the LH may be located on NEAT-inducing neurons [94], though the phenotype of these neurons is yet unknown. A recent study however, indicates that orexin may be acting through glutamic acid decarboxylase 65 (GAD65) neurons. In this study, Kosse et al [95] demonstrated that a network of neurons expressing glutamic acid decarboxylase 65 are located within the LH, separate from MCH and orexin neurons, and may be responsible for facilitating changes in locomotor activity. Specifically, they showed that GAD65 neurons are necessary for carrying out orexin-mediated changes in locomotor activity [95]. Therefore, it is possible that the changes in SPA observed in our studies are facilitated by changes in GAD-65 produced by orexin neuron activation.
Recent studies from our group show that optogenetic stimulation of orexin neurons increases SPA, suggesting that orexin neurons regulate SPA and could be an important therapeutic target (Fig. 1). To test this idea, we then used another approach, the Designer Receptors Exclusively Activated by Designer Drugs (DREADD) technique, which exploits viral delivery of a G-protein coupled receptor that has been modified to respond only to an otherwise biologically inert drug. Expression of DREADDs can be genetically restricted to orexin neurons using a Cre-lox system, in which the DNA recombinase, Cre, is excised and re-orients the genetic material encoding DREADDs so that transcription can proceed. In a recent publication by our group [96] we used transgenic (Orexin-Cre) mice in which expression of Cre is under the control of the orexin promoter. We further restricted the area of stimulation by use of orexin-Cre mice in combination with a stereotaxically controlled viral DREADD delivery to express specific regulation only in orexin neurons within the lateral portion of the lateral hypothalamus. In this way, we are able to activate only these specific orexin neurons with the designer drug. Using this method, we showed increases in SPA and NEAT by once daily administration of CNO, and prevention of adiposity gain in mice given a high fat diet [96]. Importantly, these data demonstrate strong proof-of-concept that stimulation of SPA and NEAT through orexin neurons can be used therapeutically to prevent adiposity and obesity. It is very likely that other neuromodulators of SPA and NEAT could be exploited to this end.
Figure 1.
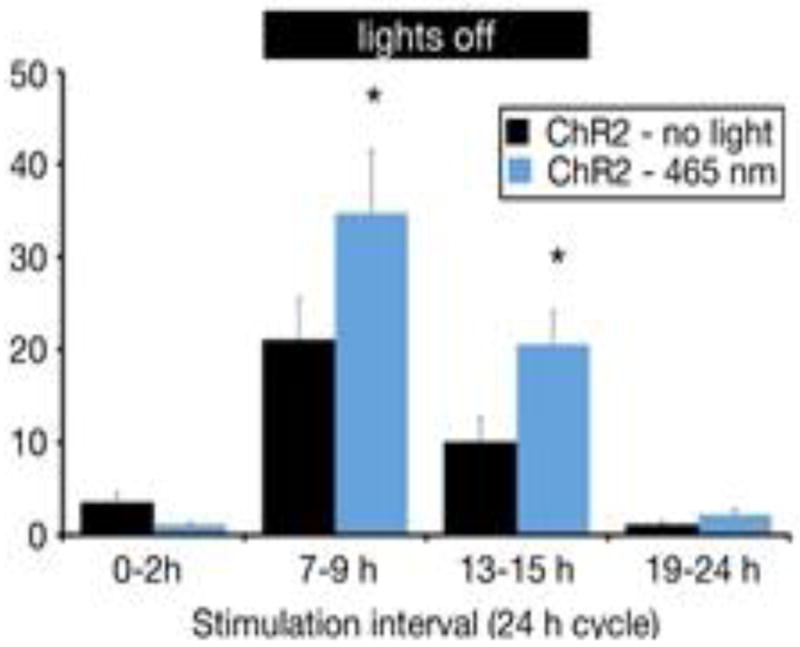
Optogenetic control of orexin neurons and SPA. Orexin-cre::ChR2 mice were stimulated for 2 h (10 sec every 15 sec at 10 Hz) every 6 h across the circadian cycle. Black header bar, lights-off period. Optogenetic stimulation is indicated by the blue bars. * P≤0.05 for pairwise comparison between groups. Y-axis is SPA in minutes, mean ± s.e.m.
Aging is associated with a decrease in SPA, but does not appear to have a uniform effect on orexin neuron survival. Rather, reductions in orexin neuron cell bodies are most prominent in medial portions of the hypothalamus, whereas orexin neurons in the LH are relatively spared. Beyond overt cell loss, there is also age-related reduction in prepro-orexin mRNA in the hypothalamus [97]. Importantly, it is still unknown if age-dependent changes in orexin neuron function contribute to decreased levels of physical activity and weight gain [97], but NEAT is an attractive target for reducing age-associated weight gain and comorbid health disorders.
Susceptibility to obesity development differs between sexes. When fed a high fat diet females gain a greater percentage body fat than males [98]. Moreover, in two types of mouse models with reduced orexin signaling, females consistently gain more weight [99]. Sexual dimorphism in the brain may be responsible for some of the phenotypic differences observed between genders, as is the case with leptin [99]. Although no gender- or sex hormone-dependent differences are present in prepro-orexin mRNA, healthy female mice show higher OXR1 mRNA expression in the hypothalamus than males [100]. Human females also have higher circulating orexin levels in their cerebrospinal fluid [101]. Diet and energy state affect orexin neurons in the LH differently in males and females. Orexin neurons in females appear to be more sensitive to environmental manipulations. Both fasting and exposure to a high fat diet increase markers of neuronal activation in orexin cells more in females than males [98, 102]. Even on a standard diet, basal levels of orexin neuron activity differ between sexes, with females displaying relatively low numbers of orexin neurons expressing cFos compared to males [98].
Future Directions
The long-term goal of SPA studies is identification of obesity treatment strategies that exploit the knowledge mechanism and impact reviewed above. Greater definition of brain networks regulating SPA is needed, including the functional anatomy, identification of neuroregulators such as orexin, and how and when these networks are active. With this knowledge, pharmaceutical interventions aimed at defending against obesity by stimulating unconscious movement will be possible. It may also become possible to directly stimulate key brain sites and pathways to more precisely regulate spontaneous activity. The impact of such manipulations will require clinical trial experience.
Whether it is possible to use conscious or behavioral modification strategies to increase SPA is an equally important question. Such increases could defend against weight gain or contribute to treatment of existing obesity. The degree to which such strategies, aimed at teaching people to move more throughout the day, can overcome the biological predisposition defined by genes, development and brain pathways is yet to be determined. Again, better knowledge of the underlying mechanisms should shed light on this question. In the meantime, clinical studies aimed at teaching or modifying behaviors are a sensible approach.
Conclusions
In conclusion, both human and animal literature point to an important role of SPA in predicting obesity, and in the potential for therapeutic manipulation of SPA. Animal work shows that orexin neurons can be stimulated to increase SPA and NEAT, such that adiposity gains are mitigated in the face of an obesogenic diet. Future studies should focus on better quantification and standardization of SPA measurement in humans and on therapeutic neuromodulation of SPA as a defense against obesity.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Catherine M. Kotz declares that she has no conflict of interest.
Claudio E. Perez-Leighton declares that he has no conflict of interest.
Jennifer A. Teske declares that she has no conflict of interest.
Charles J. Billington has received compensation from Novo Nordisk, EnteroMedics, and Optum Health for service as a consultant.
References
- 1.Bouchard C. Gene-environment interactions in the etiology of obesity: defining the fundamentals. Obesity. 2008;16(Suppl 3):S5–S10. doi: 10.1038/oby.2008.528. [DOI] [PubMed] [Google Scholar]
- 2.Lindgren CM, McCarthy MI. Mechanisms of disease: genetic insights into the etiology of type 2 diabetes and obesity. Nature clinical practice Endocrinology & metabolism. 2008;4(3):156–63. doi: 10.1038/ncpendmet0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crocker MK, Yanovski JA. Pediatric obesity: etiology and treatment. Endocrinology and metabolism clinics of North America. 2009;38(3):525–48. doi: 10.1016/j.ecl.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blundell JE, Stubbs RJ, Golding C, Croden F, Alam R, Whybrow S, et al. Resistance and susceptibility to weight gain: individual variability in response to a high-fat diet. Physiology & behavior. 2005;86(5):614–22. doi: 10.1016/j.physbeh.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 5.Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283(5399):212–4. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- 6.Jordan J, Yumuk V, Schlaich M, Nilsson PM, Zahorska-Markiewicz B, Grassi G, et al. Joint statement of the European Association for the Study of Obesity and the European Society of Hypertension: obesity and difficult to treat arterial hypertension. Journal of hypertension. 2012;30(6):1047–55. doi: 10.1097/HJH.0b013e3283537347. [DOI] [PubMed] [Google Scholar]
- 7.Zurlo F, Ferraro RT, Fontvielle AM, Rising R, Bogardus C, Ravussin E. Spontaneous physical activity and obesity: cross-sectional and longitudinal studies in Pima Indians. The American journal of physiology. 1992;263(2 Pt 1):E296–300. doi: 10.1152/ajpendo.1992.263.2.E296. [DOI] [PubMed] [Google Scholar]
- 8.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. The Journal of clinical investigation. 1986;78(6):1568–78. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanltallie TB. Resistance to weight gain during overfeeding: a NEAT explanation. Nutrition reviews. 2001;59(2):48–51. doi: 10.1111/j.1753-4887.2001.tb06975.x. [DOI] [PubMed] [Google Scholar]
- 10.Garland T, Jr, Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE, et al. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. The Journal of experimental biology. 2011;214(Pt 2):206–29. doi: 10.1242/jeb.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotz CM, Levine JA. Role of nonexercise activity thermogenesis (NEAT) in obesity. Minnesota medicine. 2005;88(9):54–7. [PubMed] [Google Scholar]
- 12.Levine JA, Vander Weg MW, Hill JO, Klesges RC. Non-exercise activity thermogenesis: the crouching tiger hidden dragon of societal weight gain. Arteriosclerosis, thrombosis, and vascular biology. 2006;26(4):729–36. doi: 10.1161/01.ATV.0000205848.83210.73. [DOI] [PubMed] [Google Scholar]
- 13.Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, et al. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307(5709):584–6. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]
- 14.Teske JA, Billington CJ, Kotz CM. Neuropeptidergic mediators of spontaneous physical activity and non-exercise activity thermogenesis. Neuroendocrinology. 2008;87(2):71–90. doi: 10.1159/000110802. [DOI] [PubMed] [Google Scholar]
- 15.Nixon JP, Kotz CM, Novak CM, Billington CJ, Teske JA. Neuropeptides controlling energy balance: orexins and neuromedins. Handbook of experimental pharmacology. 2012;(209):77–109. doi: 10.1007/978-3-642-24716-3_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shook RP, Hand GA, Drenowatz C, Hebert JR, Paluch AE, Blundell JE, et al. Low levels of physical activity are associated with dysregulation of energy intake and fat mass gain over 1 year. Am J Clin Nutr. 2015;102(6):1332–8. doi: 10.3945/ajcn.115.115360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uemura H, Katsuura-Kamano S, Yamaguchi M, Nakamoto M, Hiyoshi M, Arisawa K. Abundant daily non-sedentary activity is associated with reduced prevalence of metabolic syndrome and insulin resistance. J Endocrinol Invest. 2013;36(11):1069–75. doi: 10.3275/9066. [DOI] [PubMed] [Google Scholar]
- 18.Piaggi P, Thearle MS, Krakoff J, Votruba SB. Higher Daily Energy Expenditure and Respiratory Quotient, Rather Than Fat-Free Mass, Independently Determine Greater ad Libitum Overeating. J Clin Endocrinol Metab. 2015;100(8):3011–20. doi: 10.1210/jc.2015-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drenowatz C, Hill JO, Peters JC, Soriano-Maldonado A, Blair SN. The association of change in physical activity and body weight in the regulation of total energy expenditure. Eur J Clin Nutr. 2017;71(3):377–82. doi: 10.1038/ejcn.2016.228. [DOI] [PubMed] [Google Scholar]
- 20.Piaggi P, Thearle MS, Bogardus C, Krakoff J. Lower energy expenditure predicts long-term increases in weight and fat mass. J Clin Endocrinol Metab. 2013;98(4):E703–7. doi: 10.1210/jc.2012-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmad S, Rukh G, Varga TV, Ali A, Kurbasic A, Shungin D, et al. Gene x physical activity interactions in obesity: combined analysis of 111,421 individuals of European ancestry. PLoS Genet. 2013;9(7):e1003607. doi: 10.1371/journal.pgen.1003607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rampersaud E, Mitchell BD, Pollin TI, Fu M, Shen H, O'Connell JR, et al. Physical activity and the association of common FTO gene variants with body mass index and obesity. Arch Intern Med. 2008;168(16):1791–7. doi: 10.1001/archinte.168.16.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hao YY, Yuan HW, Fang PH, Zhang Y, Liao YX, Shen C, et al. Plasma orexin-A level associated with physical activity in obese people. Eat Weight Disord. 2017;22(1):69–77. doi: 10.1007/s40519-016-0271-y. [DOI] [PubMed] [Google Scholar]
- 24.Nicklas BJ, Gaukstern JE, Beavers KM, Newman JC, Leng X, Rejeski WJ. Self-monitoring of spontaneous physical activity and sedentary behavior to prevent weight regain in older adults. Obesity (Silver Spring) 2014;22(6):1406–12. doi: 10.1002/oby.20732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elbelt U, Schuetz T, Knoll N, Burkert S. Self-Directed Weight Loss Strategies: Energy Expenditure Due to Physical Activity Is Not Increased to Achieve Intended Weight Loss. Nutrients. 2015;7(7):5868–88. doi: 10.3390/nu7075256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt SL, Harmon KA, Sharp TA, Kealey EH, Bessesen DH. The effects of overfeeding on spontaneous physical activity in obesity prone and obesity resistant humans. Obesity (Silver Spring) 2012;20(11):2186–93. doi: 10.1038/oby.2012.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•*.Villablanca PA, Alegria JR, Mookadam F, Holmes DR, Jr, Wright RS, Levine JA. Nonexercise activity thermogenesis in obesity management. Mayo Clin Proc. 2015;90(4):509–19. doi: 10.1016/j.mayocp.2015.02.001. This paper reviews the available methodologies for changing spontaneous physical activity and the associated nonexercise thermogenesis as part of weight management strategies. [DOI] [PubMed] [Google Scholar]
- 28.Koepp GA, Moore G, Levine JA. An Under-the-Table Leg-Movement Apparatus and Changes in Energy Expenditure. Front Physiol. 2017;8:318. doi: 10.3389/fphys.2017.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koepp GA, Moore GK, Levine JA. Chair-based fidgeting and energy expenditure. BMJ Open Sport Exerc Med. 2016;2(1):e000152. doi: 10.1136/bmjsem-2016-000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dutta N, Koepp GA, Stovitz SD, Levine JA, Pereira MA. Using sit-stand workstations to decrease sedentary time in office workers: a randomized crossover trial. Int J Environ Res Public Health. 2014;11(7):6653–65. doi: 10.3390/ijerph110706653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson WG, Koepp GA, Levine JA. Increasing physician activity with treadmill desks. Work. 2014;48(1):47–51. doi: 10.3233/WOR-131708. [DOI] [PubMed] [Google Scholar]
- 32.Koepp GA, Manohar CU, McCrady-Spitzer SK, Ben-Ner A, Hamann DJ, Runge CF, et al. Treadmill desks: A 1-year prospective trial. Obesity. 2013;21(4):705–11. doi: 10.1002/oby.20121. [DOI] [PubMed] [Google Scholar]
- 33.McCrady-Spitzer SK, Manohar CU, Koepp GA, Levine JA. Low-cost and Scalable Classroom Equipment to Promote Physical Activity and Improve Education. J Phys Act Health. 2015;12(9):1259–63. doi: 10.1123/jpah.2014-0159. [DOI] [PubMed] [Google Scholar]
- 34.Melanson EL. The effect of exercise on non-exercise physical activity and sedentary behavior in adults. Obes Rev. 2017;18(Suppl 1):40–9. doi: 10.1111/obr.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Washburn RA, Lambourne K, Szabo AN, Herrmann SD, Honas JJ, Donnelly JE. Does increased prescribed exercise alter non-exercise physical activity/energy expenditure in healthy adults? A systematic review. Clin Obes. 2014;4(1):1–20. doi: 10.1111/cob.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Westerterp KR. Control of energy expenditure in humans. Eur J Clin Nutr. 2017;71(3):340–4. doi: 10.1038/ejcn.2016.237. [DOI] [PubMed] [Google Scholar]
- 37.Gomersall SR, Rowlands AV, English C, Maher C, Olds TS. The ActivityStat hypothesis: the concept, the evidence and the methodologies. Sports Med. 2013;43(2):135–49. doi: 10.1007/s40279-012-0008-7. [DOI] [PubMed] [Google Scholar]
- 38.Mathot KJ, Dingemanse NJ. Energetics and behavior: unrequited needs and new directions. Trends Ecol Evol. 2015;30(4):199–206. doi: 10.1016/j.tree.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 39.Pontzer H. Constrained Total Energy Expenditure and the Evolutionary Biology of Energy Balance. Exerc Sport Sci Rev. 2015;43(3):110–6. doi: 10.1249/JES.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 40.Ravussin E, Peterson CM. Physical Activity and the Missing Calories. Exerc Sport Sci Rev. 2015;43(3):107–8. doi: 10.1249/JES.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomersall SR, Maher C, English C, Rowlands AV, Dollman J, Norton K, et al. Testing the activitystat hypothesis: a randomised controlled trial. BMC Public Health. 2016;16:900. doi: 10.1186/s12889-016-3568-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snitker S, Tataranni PA, Ravussin E. Spontaneous physical activity in a respiratory chamber is correlated to habitual physical activity. Int J Obes Relat Metab Disord. 2001;25(10):1481–6. doi: 10.1038/sj.ijo.0801746. [DOI] [PubMed] [Google Scholar]
- 43••.Teske JA, Perez-Leighton CE, Billington CJ, Kotz CM. Methodological considerations for measuring spontaneous physical activity in rodents. American journal of physiology Regulatory, integrative and comparative physiology. 2014;306(10):R714–21. doi: 10.1152/ajpregu.00479.2013. This paper shows that different methods of measuring SPA (e.g. timing of measurement, housing etc.) can yield different absolute numbers and may alter data interpretation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cacioppo JT, Cacioppo S, Capitanio JP, Cole SW. The neuroendocrinology of social isolation. Annu Rev Psychol. 2015;66:733–67. doi: 10.1146/annurev-psych-010814-015240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bunney PE, Zink AN, Holm AA, Billington CJ, Kotz CM. Orexin activation counteracts decreases in nonexercise activity thermogenesis (NEAT) caused by high-fat diet. Physiology & behavior. 2017;176:139–48. doi: 10.1016/j.physbeh.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coborn JE, DePorter DP, Mavanji V, Sinton CM, Kotz CM, Billington CJ, et al. Role of orexin-A in the ventrolateral preoptic area on components of total energy expenditure. International journal of obesity. 2017;41(8):1256–62. doi: 10.1038/ijo.2017.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dulloo AG, Miles-Chan JL, Montani JP, Schutz Y. Isometric thermogenesis at rest and during movement: a neglected variable in energy expenditure and obesity predisposition. Obes Rev. 2017;18(Suppl 1):56–64. doi: 10.1111/obr.12505. [DOI] [PubMed] [Google Scholar]
- 48.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. The American journal of physiology. 1997;273(2 Pt 2):R725–30. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- 49.Teske JA, Levine AS, Kuskowski M, Levine JA, Kotz CM. Elevated hypothalamic orexin signaling, sensitivity to orexin A, and spontaneous physical activity in obesity-resistant rats. American journal of physiology Regulatory, integrative and comparative physiology. 2006;291(4):R889–99. doi: 10.1152/ajpregu.00536.2005. [DOI] [PubMed] [Google Scholar]
- 50.Teske JA, Billington CJ, Kuskowski MA, Kotz CM. Spontaneous physical activity protects against fat mass gain. International journal of obesity. 2012;36(4):603–13. doi: 10.1038/ijo.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perez-Leighton CE, Boland K, Billington C, Kotz CM. High and low activity rats: Elevated intrinsic physical activity drives resistance to diet induced obesity in non-bred rats. Obesity. 2012 doi: 10.1002/oby.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perez-Leighton CE, Grace M, Billington CJ, Kotz CM. Role of spontaneous physical activity in prediction of susceptibility to activity based anorexia in male and female rats. Physiology & behavior. 2014;135:104–11. doi: 10.1016/j.physbeh.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smyers ME, Bachir KZ, Britton SL, Koch LG, Novak CM. Physically active rats lose more weight during calorie restriction. Physiology & behavior. 2015;139:303–13. doi: 10.1016/j.physbeh.2014.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gac L, Kanaly V, Ramirez V, Teske JA, Pinto MP, Perez-Leighton CE. Behavioral characterization of a model of differential susceptibility to obesity induced by standard and personalized cafeteria diet feeding. Physiology & behavior. 2015;152(Pt A):315–22. doi: 10.1016/j.physbeh.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Moretto TL, Benfato ID, de Carvalho FP, Barthichoto M, Le Sueur-Maluf L, de Oliveira CAM. The effects of calorie-matched high-fat diet consumption on spontaneous physical activity and development of obesity. Life Sci. 2017;179:30–6. doi: 10.1016/j.lfs.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 56.Sadowska J, Gebczynski AK, Konarzewski M. Selection for high aerobic capacity has no protective effect against obesity in laboratory mice. Physiology & behavior. 2017;175:130–6. doi: 10.1016/j.physbeh.2017.03.034. [DOI] [PubMed] [Google Scholar]
- 57.Sadowska J, Gebczynski AK, Konarzewski M. Metabolic risk factors in mice divergently selected for BMR fed high fat and high carb diets. PLoS One. 2017;12(2):e0172892. doi: 10.1371/journal.pone.0172892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Acosta W, Meek TH, Schutz H, Dlugosz EM, Vu KT, Garland T., Jr Effects of early-onset voluntary exercise on adult physical activity and associated phenotypes in mice. Physiology & behavior. 2015;149:279–86. doi: 10.1016/j.physbeh.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 59.Copes LE, Schutz H, Dlugosz EM, Acosta W, Chappell MA, Garland T., Jr Effects of voluntary exercise on spontaneous physical activity and food consumption in mice: Results from an artificial selection experiment. Physiology & behavior. 2015;149:86–94. doi: 10.1016/j.physbeh.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 60.de Carvalho FP, Benfato ID, Moretto TL, Barthichoto M, de Oliveira CA. Voluntary running decreases nonexercise activity in lean and diet-induced obese mice. Physiology & behavior. 2016;165:249–56. doi: 10.1016/j.physbeh.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 61.Scariot PP, de Manchado-Gobatto FB, Torsoni AS, Dos Reis IG, Beck WR, Gobatto CA. Continuous Aerobic Training in Individualized Intensity Avoids Spontaneous Physical Activity Decline and Improves MCT1 Expression in Oxidative Muscle of Swimming Rats. Front Physiol. 2016;7:132. doi: 10.3389/fphys.2016.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(1):322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–85. doi: 10.1016/s0092-8674(00)80949-6. S0092-8674(00)80949-6 [pii] [DOI] [PubMed] [Google Scholar]
- 64.Kotz C, Nixon J, Butterick T, Perez-Leighton C, Teske J, Billington C. Brain orexin promotes obesity resistance. Ann N Y Acad Sci. 2012;1264:72–86. doi: 10.1111/j.1749-6632.2012.06585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. The Journal of comparative neurology. 2001;435(1):6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 66.Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS letters. 1998;438(1–2):71–5. doi: 10.1016/s0014-5793(98)01266-6. S0014-5793(98)01266-6 [pii] [DOI] [PubMed] [Google Scholar]
- 67.Alam MN, Kumar S, Bashir T, Suntsova N, Methippara MM, Szymusiak R, et al. GABA-mediated control of hypocretin- but not melanin-concentrating hormone-immunoreactive neurones during sleep in rats. The Journal of physiology. 2005;563(Pt 2):569–82. doi: 10.1113/jphysiol.2004.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karnani MM, Apergis-Schoute J, Adamantidis A, Jensen LT, de Lecea L, Fugger L, et al. Activation of central orexin/hypocretin neurons by dietary amino acids. Neuron. 2011;72(4):616–29. doi: 10.1016/j.neuron.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 69.Williams RH, Alexopoulos H, Jensen LT, Fugger L, Burdakov D. Adaptive sugar sensors in hypothalamic feeding circuits. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(33):11975–80. doi: 10.1073/pnas.0802687105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Edwards CM, Abusnana S, Sunter D, Murphy KG, Ghatei MA, Bloom SR. The effect of the orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin. The Journal of endocrinology. 1999;160(3):R7–12. doi: 10.1677/joe.0.160r007. [DOI] [PubMed] [Google Scholar]
- 71.Shirasaka T, Miyahara S, Kunitake T, Jin QH, Kato K, Takasaki M, et al. Orexin depolarizes rat hypothalamic paraventricular nucleus neurons. American journal of physiology Regulatory, integrative and comparative physiology. 2001;281(4):R1114–8. doi: 10.1152/ajpregu.2001.281.4.R1114. [DOI] [PubMed] [Google Scholar]
- 72.Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell metabolism. 2009;10(2):89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, et al. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46(2):297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 74.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98(4):437–51. doi: 10.1016/s0092-8674(00)81973-x. S0092-8674(00)81973-X [pii] [DOI] [PubMed] [Google Scholar]
- 75.Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30(2):345–54. doi: 10.1016/s0896-6273(01)00293-8. S0896-6273(01)00293-8 [pii] [DOI] [PubMed] [Google Scholar]
- 76.Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, et al. Fos expression in orexin neurons varies with behavioral state. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21(5):1656–62. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46(5):787–98. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takahashi K, Lin JS, Sakai K. Neuronal activity of orexin and non-orexin waking-active neurons during wake-sleep states in the mouse. Neuroscience. 2008;153(3):860–70. doi: 10.1016/j.neuroscience.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 79.Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J, et al. Release of hypocretin (orexin) during waking and sleep states. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22(13):5282–6. doi: 10.1523/JNEUROSCI.22-13-05282.2002. 20026541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450(7168):420–4. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Funato H, Tsai AL, Willie JT, Kisanuki Y, Williams SC, Sakurai T, et al. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell metabolism. 2009;9(1):64–76. doi: 10.1016/j.cmet.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perez-Leighton CE, Boland K, Teske JA, Billington C, Kotz CM. Behavioral responses to orexin, orexin receptor gene expression, and spontaneous physical activity contribute to individual sensitivity to obesity. American journal of physiology Endocrinology and metabolism. 2012;303(7):E865–74. doi: 10.1152/ajpendo.00119.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Espana RA, Reis KM, Valentino RJ, Berridge CW. Organization of hypocretin/orexin efferents to locus coeruleus and basal forebrain arousal-related structures. The Journal of comparative neurology. 2005;481(2):160–78. doi: 10.1002/cne.20369. [DOI] [PubMed] [Google Scholar]
- 84.Oldfield BJ, Allen AM, Davern P, Giles ME, Owens NC. Lateral hypothalamic 'command neurons' with axonal projections to regions involved in both feeding and thermogenesis. The European journal of neuroscience. 2007;25(8):2404–12. doi: 10.1111/j.1460-9568.2007.05429.x. [DOI] [PubMed] [Google Scholar]
- 85.Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behavioural brain research. 2007;183(1):43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends in neurosciences. 2006;29(10):571–7. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 87.Kiwaki K, Kotz CM, Wang C, Lanningham-Foster L, Levine JA. Orexin A (hypocretin 1) injected into hypothalamic paraventricular nucleus and spontaneous physical activity in rats. American journal of physiology Endocrinology and metabolism. 2004;286(4):E551–9. doi: 10.1152/ajpendo.00126.2003. [DOI] [PubMed] [Google Scholar]
- 88.Kotz CM, Wang C, Teske JA, Thorpe AJ, Novak CM, Kiwaki K, et al. Orexin A mediation of time spent moving in rats:neural mechanisms. Neuroscience. 2006;142(1):29–36. doi: 10.1016/j.neuroscience.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 89.Thorpe AJ, Kotz CM. Orexin A in the nucleus accumbens stimulates feeding and locomotor activity. Brain research. 2005;1050(1–2):156–62. doi: 10.1016/j.brainres.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 90.Kotz CM, Teske JA, Levine JA, Wang C. Feeding and activity induced by orexin A in the lateral hypothalamus in rats. Regulatory peptides. 2002;104(1–3):27–32. doi: 10.1016/s0167-0115(01)00346-9. S0167011501003469 [pii] [DOI] [PubMed] [Google Scholar]
- 91.Teske JA, Perez-Leighton CE, Billington CJ, Kotz CM. Role of the locus coeruleus in enhanced orexin A-induced spontaneous physical activity in obesity-resistant rats. American journal of physiology Regulatory, integrative and comparative physiology. 2013;305(11):R1337–45. doi: 10.1152/ajpregu.00229.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Teske JA, Billington CJ, Kotz CM. Hypocretin/orexin and energy expenditure. Acta physiologica. 2010;198(3):303–12. doi: 10.1111/j.1748-1716.2010.02075.x. [DOI] [PubMed] [Google Scholar]
- 93.Perez-Leighton CE, Butterick-Peterson TA, Billington CJ, Kotz CM. Role of orexin receptors in obesity: from cellular to behavioral evidence. International journal of obesity. 2013;37(2):167–74. doi: 10.1038/ijo.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94•.Perez-Leighton C, Little MR, Grace M, Billington C, Kotz CM. Orexin signaling in rostral lateral hypothalamus and nucleus accumbens shell in the control of spontaneous physical activity in high- and low-activity rats. American journal of physiology Regulatory, integrative and comparative physiology. 2017;312(3):R338–R46. doi: 10.1152/ajpregu.00339.2016. This paper shows the differential interplay of brain regions in the control of SPA, in high vs. low activity rats. Put more simply, the brains of high activity rats may be functionally organized differently than those of low physical activity rats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95••.Kosse C, Schone C, Bracey E, Burdakov D. Orexin-driven GAD65 network of the lateral hypothalamus sets physical activity in mice. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(17):4525–30. doi: 10.1073/pnas.1619700114. This paper shows a link between orexin neurons and another set of neurons that may be important in understanding additional mechanisms by which orexin induces SPA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96•.Zink AN, Bunney PE, Holm AA, Billington CJ, Kotz CM. Neuromodulation of Orexin Neurons Reduces Diet-Induced Adiposity. International journal of obesity. 2017 doi: 10.1038/ijo.2017.276. in press. These studies provide direct evidence that activation of induces SPA and protects against weight gain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kessler BA, Stanley EM, Frederick-Duus D, Fadel J. Age-related loss of orexin/hypocretin neurons. Neuroscience. 2011;178:82–8. doi: 10.1016/j.neuroscience.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pirnik Z, Bundzikova J, Mikkelsen JD, Zelezna B, Maletinska L, Kiss A. Fos expression in hypocretinergic neurons in C57B1/6 male and female mice after long-term consumption of high fat diet. Endocrine regulations. 2008;42(4):137–46. [PubMed] [Google Scholar]
- 99.Fujiki N, Yoshida Y, Zhang S, Sakurai T, Yanagisawa M, Nishino S. Sex difference in body weight gain and leptin signaling in hypocretin/orexin deficient mouse models. Peptides. 2006;27(9):2326–31. doi: 10.1016/j.peptides.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Johren O, Bruggemann N, Dendorfer A, Dominiak P. Gonadal steroids differentially regulate the messenger ribonucleic acid expression of pituitary orexin type 1 receptors and adrenal orexin type 2 receptors. Endocrinology. 2003;144(4):1219–25. doi: 10.1210/en.2002-0030. [DOI] [PubMed] [Google Scholar]
- 101.Schmidt FM, Kratzsch J, Gertz HJ, Tittmann M, Jahn I, Pietsch UC, et al. Cerebrospinal fluid melanin-concentrating hormone (MCH) and hypocretin-1 (HCRT-1, orexin-A) in Alzheimer's disease. PLoS One. 2013;8(5):e63136. doi: 10.1371/journal.pone.0063136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Funabashi T, Hagiwara H, Mogi K, Mitsushima D, Shinohara K, Kimura F. Sex differences in the responses of orexin neurons in the lateral hypothalamic area and feeding behavior to fasting. Neuroscience letters. 2009;463(1):31–4. doi: 10.1016/j.neulet.2009.07.035. [DOI] [PubMed] [Google Scholar]


