Abstract
Understanding functions of Foxp3+ regulatory T (Treg) cells during allergic airway inflammation remains incomplete. In this study, we report that during cockroach antigen (CA)-induced allergic airway inflammation Foxp3+ Treg cells are rapidly mobilized into the inflamed lung tissues. However, the level of Treg cell accumulation in the lung was different depending on the type of inflammation. During eosinophilic airway inflammation, ~30% of lung infiltrating CD4 T cells express Foxp3, an indicative of Treg cells. On the contrary, only ~10% of infiltrating CD4 T cells express Foxp3 during neutrophilic airway inflammation. Despite the different accumulation, both the lung inflammation and inflammatory T cell responses were aggravated following Treg cell depletion regardless of the type of inflammation, suggesting regulatory roles of Treg cells. Interestingly, however, the extent to which inflammatory responses are aggravated by Treg cell depletion was significantly greater during eosinophilic airway inflammation. Indeed, lung infiltrating Treg cells exhibit phenotypic and functional features associated with potent suppression. Our results demonstrate that Treg cells are essential regulators of inflammation regardless of the type of inflammation, although the mechanisms employed by Treg cells to control inflammation may be shaped by environmental cues available to those Treg cells.
Introduction
The immune system of the lung mucosal tissues is continuously exposed to inhaled antigens, requiring regulatory mechanisms to prevent uncontrolled immune activation against otherwise innocuous antigens, yet to mount protective immunity against invading pathogens. Dysregulated immune responses to the harmless environmental antigens often result in asthma, a chronic inflammatory disease of the airway (1). Allergen-specific effector CD4 T cells producing Th2 type cytokines, namely IL-4, IL-5, and IL-13, mediate the disease processes, inducing eosinophil infiltration, IgE isotype switching, airway hyperresponsiveness and airway remodeling (2, 3). In addition to Th2 type effector T cells, Th17 type CD4 T cells producing the signature cytokine IL-17, also induce airway inflammation in which neutrophils, instead of eosinophils, are the dominant inflammatory leukocytes infiltrating the lung tissues (4, 5), and Th17-mediated neutrophilic asthma is associated with a severe persistent form (6, 7). The mechanisms underlying these distinct forms of airway inflammation remain elusive.
Foxp3+ regulatory CD4 T (Treg) cells are central regulators of immunity and tolerance (8). Defects in Treg cell generation and/or function are coupled with uncontrolled lymphoproliferative diseases both in human and mouse (8). In particular, patients with Foxp3 mutation exhibit pathologies at the mucosal tissues associated with allergic inflammation (9, 10), suggesting that Treg cells are key regulators of allergic inflammation. Treg cells are recruited to the inflammatory sites, where they exert regulatory functions to dampen the inflammation (11). Indeed, the proportions of Treg cells are significantly elevated in bronchoalveolar lavage (BAL) fluid from asthmatic patients compared to that from healthy subjects (12). However, others reported that Treg cell proportions are comparable between patients and healthy controls, although lower level of Foxp3 mRNA is found in peripheral blood from asthmatics (13, 14). These conflicting results warrant further investigation with regard to regulatory roles of Treg cells during airway inflammation. Moreover, the role of lung infiltrating Treg cells during Th2 type eosinophilic and Th17 type neutrophilic airway inflammation has not formally been tested.
In this study, we examined the role of Treg cells utilizing murine models of eosinophilic and neutrophilic allergic inflammation induced via different adjuvants. We found that Treg cell accumulation in the inflamed lung tissues is dramatically different between the models. In eosinophilic inflammation, substantial proportions of infiltrating CD4 T cells were Foxp3+ Treg cells, while the proportion was significantly lower during neutrophilic inflammation. Nonetheless, Treg cells play a role in controlling both types of inflammation as depleting Treg cells during allergen challenge exacerbated the overall inflammation and inflammatory T cell responses, although the extent to which inflammatory responses are aggravated by Treg cell depletion was greater during eosinophilic inflammation. Phenotypic analysis of lung infiltrating Treg cells further uncovered that those Treg cells from mice induced for eosinophilic inflammation display phenotypic and functional features associated with more potent suppression. Our results demonstrate that the suppressive mechanisms expressed by infiltrating Treg cells may be shaped by environmental cues available to those Treg cells infiltrating the inflamed tissues.
Materials and Methods
Animals
C57BL/6 and C57BL/6 Foxp3.DTR mice were purchased from the Jackson Laboratory (Bar Harbor, ME). C57BL/6 Foxp3.GFP KI mice were previously reported (15). All the mice were maintained under specific pathogen free facility located in the Lerner Research Institute. All animal experiments were performed in accordance with approved protocols for the Institutional Animal Care and Usage Committee.
Airway inflammation
For eosinophilic airway inflammation, mice were intraperitoneally injected with 5µg cockroach antigen (CA, Greer laboratory, Lenoir, NC) mixed in 100µl alum adjuvant (aluminum hydroxide, Sigma, St. Louis, MO). Another injection was made seven days later. Starting day 14, the mice were daily challenged with intranasal cockroach antigen injection (5µg in 50µl) for 4 days. Mice were sacrificed 24 hours after the last antigen challenge. For neutrophilic airway inflammation, mice were subcutaneously immunized with 5µg cockroach antigen emulsified in 100µl complete Fruend’s adjuvant containing 5mg/ml H37Ra (BD Difco, Franklin Lakes, NJ). Starting day 14, the mice were challenged with cockroach antigen and sacrificed similar to eosinophilic inflammation model. In some experiments, Treg cells were depleted by intraperitoneally injecting 1µg diphtheria toxin (DTX, Sigma). Lung tissues were prepared from paraffin embedded blocks and stained with haematoxylin-eosin. Inflammation was scored as previously reported (16): 0, no or occational cells; 1: one or two concentric rows of perivascular and peribronchial cell accumulation; 2: three or more concentric rows of perivascular and peribronchial cell accumulation; 3: continuous perivascular and peribronchial cell accumulation.
Flow Cytometry
At sacrifice, the bronchoalveolar lavage (BAL) cells were collected. Lung tissue was minced with scissors, and single cell suspension was obtained. Mononuclear cells were isolated from the 30/70% percoll interface. Red blood cells were lysed with ACK lysis buffer. Cells were stained with anti-CD4 (RM4-5), anti-Ly6G (1A8), anti-Siglec-F (1RNM44N), anti-ICOS (C398.4A), anti-CD39 (24DMS1), anti-GITR (DTA-1), anti-ICOSL (HK5.3), anti-CD25 (PC61), anti-CD31 (MEC13.3), anti-Vcam1 (429), anti-Nrp1 (3DS304M), anti-CD11c (N418), anti-CD11b (M1/70), anti-CD80 (16-10A1), anti-CD86 (GL1), anti-BrdU (3D4), and anti-CD49d (R1–2). For intracellular staining, cells were stained with anti-Ki-67 (B56), anti-IL-4 (11B11), anti-IL-10 (JES5-16E3), anti-IL-13 (eBio13A), anti-IL-17 (TC11-18H10), and anti-IFNγ (XMG1.2). All the antibodies used in this study were purchased from eBioscience (San Diego, CA), BD PharMingen (San Jose, CA), and Biolegend (San Diego, CA). Cells were acquired using a LSR II (BD Biosciences, San Jose, CA) and analyzed using a FlowJo software (Treestar, Ashland, OR). For Intracellular staining, cells were separately harvested and ex vivo stimulated with PMA (10 ng/ml) and Ionomycin (1µM) for 4 hrs in the presence of 2µM monensin (Calbiochem, San Diego, CA) during the last 2 hrs of stimulation. Cells were immediately fixed with 4% paraformaldehyde, permeabilized, and stained with fluorescence conjugated antibodies. In some experiments, mice were injected with 1mg BrdU (Sigma) 24 hours prior to sacrifice. In vivo BrdU incorporation was measured using a BrdU kit (PharMingen) according to the manufacturer.
Suppression assay
Foxp3+ Treg cells were FACS sorted from inflamed lung tissues using a FACSAria sorter (BD Biosciences). Naïve responder CD4 T cells were sorted from B6 mice, CFSE labeled, and cultured with anti-CD3 mAb plus irradiated T cell-depleted splenocytes for 3 days. Different numbers of Treg cells were added in the culture. CFSE dilution was determined by FACS analysis. Relative proliferation was calculated based on CFSE dilution without Treg cells.
ELISA
Draining mediastinal LN cells isolated from animals induced for eosinophilic- and neutrophilic-airway inflammation were in vitro stimulated with 10µg/ml CA or OVA protein for 3 days. Culture supernatant was collected and IL-4/IL-17 secretion was determined by ELISA. In some experiments, bronchoalveolar lavage (BAL) fluids were collected from mice induced for eosinophilic- and neutrophilic-inflammation and treated with PBS or DTX. IL-4 and IL-17 secretion was determined by ELISA.
Inflammation Antibody array
Assay for Mouse Inflammation Antibody Array C1 (RayBiotech®, Inc., Norcross, GA, #AAM-INF-1-4) was carried out according to manufacturer’s instructions. Briefly, antibody-coated array membranes were first incubated for 30 min with 1 ml of blocking buffer. After 30 min, blocking buffer was decanted and replaced with 1 ml of the samples at 4°C overnight. The samples were decanted from each container and washed 3 times with wash buffer at room temperature with shaking for 10 min. After extensive washing to remove unbound materials, membranes were then incubated with diluted biotin-conjugated antibodies at 4°C overnight. Membranes were washed and then incubated with HRP-conjugated streptavidin at room temperature for 2 h. Membranes were then washed thoroughly and exposed to detection buffer in the dark before being exposed to X-ray film, and images were developed using a film scanner. Intensities of signals were quantified using ImageJ software. Positive controls were used to normalize results from different membranes being compared. Fold changes in protein expression were calculated.
Gene expression analysis
RNA was isolated from inflamed lung tissues or FACS sorted cells using a GeneJet RNA isolation kit (ThermoFisher, Waltham, MA cDNA was synthesized using M-MLV reverse transcriptase (Promega, Madison, WI). Real time PCR analysis was performed using an ABI7500 real time PCR system (Applied Biosystem, Waltham, MA), a SYBR master mix (Applied Biosystem), and gene specific primers.
Statistics
Hierarchical clustering and heat map generation were performed in nSolver software. Statistical significance was determined by t test (two-tailed) using the Prism 5 software (GraphPad, La Jolla, CA). A p value of <0.05 was considered statistically significant.
Results
Model system
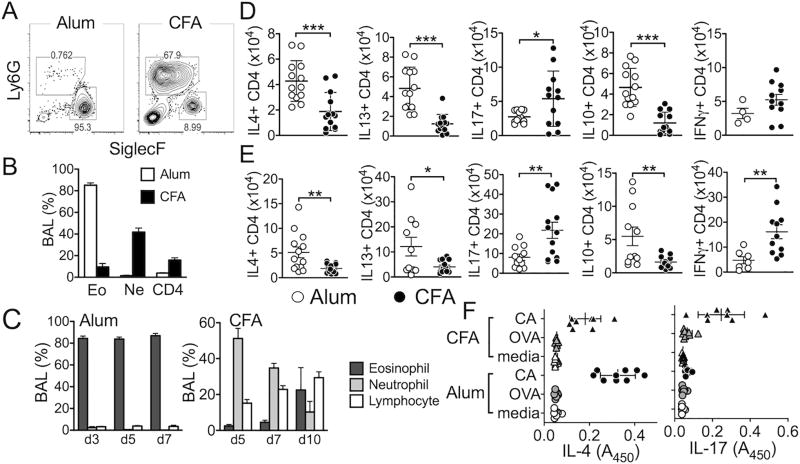
Using the cockroach antigen (CA) as a model allergen, we employed Alum- and CFA-based sensitization protocols known to induce eosinophilic and neutrophilic airway inflammation, respectively (17–20). Mice were sensitized with CA in Alum and intranasally challenged with the CA as illustrated in Supplementary Fig S1. When measured 24 hours after the last challenge (day 5), they developed eosinophilic inflammation in the airway, characterized by predominant SiglecF+ (Ly6G-) eosinophil infiltration in the bronchoalveolar lavage (BAL) (Fig 1A). ~90% of infiltrating cells were eosinophils, <1% were neutrophils, and CD4 T cells constituted <5% (Fig 1B). On the other hand, mice sensitized with CA in CFA followed by intranasal allergen challenge developed airway inflammation with predominantly neutrophils (Ly6G+ SiglecF-) in the BAL (Supplementary Fig S1, Fig 1A). ~50% of the infiltrating cells were neutrophils, while eosinophils were <10% (Fig 1B). Eosinophil-dominant BAL cell profiles remained consistent before and after the peak responses (Fig 1C, Supplementary Fig S2). In CFA-induced inflammation, neutrophils were dominant at days 5 and 7, although their proportions and numbers gradually decreased after day 7 (Fig 1C, Supplementary Fig S2). CD4 T cells expressing Th2 and Th17 signature cytokines are the mediators of eosinophilic and neutrophilic inflammation, respectively (5, 7, 21). Indeed, Alum-induced inflammation was strongly associated with Th2 type effector CD4 T cells in the draining LN and lung tissues. T cells expressing Th2 type cytokines, namely IL-4, IL-13, and IL-10 were more abundant compared to T cells expressing IFNγ or IL-17 (Fig 1D and 1E). Likewise, IFNγ- and IL-17-producing effector CD4 T cells were significantly elevated in mice induced for neutrophilic inflammation, while T cells expressing Th2 type cytokines were substantially lower compared to those from Alum-induced inflammation (Fig 1D and 1E). Th2 and Th17 type responses were antigen specific, as draining LN CD4 T cells from Alum- and CFA-sensitized mice primarily produced IL-4 and IL-17, respectively, upon restimulation with CA antigens but not with irrelevant antigens (Fig 1F). Taken together, these results demonstrate two distinct inflammatory responses in the airway characterized by Th2/eosinophils or by Th1/Th17/neutrophils.
Figure 1. The model system of eosinophilic and neutrophilic airway inflammation.
B6 mice were either injected i.p. with CA/Alum or s.c. with CA/CFA as described in Supplementary Fig S1. Two weeks later mice were intranasally challenged for 4 consecutive days with CA in PBS. Mice were sacrificed 24 hours after the last challenge. (A) Cells from BAL were stained for Ly6G and Siglec F expression. (B) Differential cell count of BAL cells was performed using FACS. (C) Mice were sacrificed at different time points as shown in Supplementary Fig S1. BAL cells were then stained for eosinophils, neutrophils, and CD4 T cells. (D and E) Draining mediastinal LN (D) and Lung (E) cells were collected and ex vivo stimulated to measure intracellular cytokine expression. (F) Draining LN cells were ex vivo stimulated with CA or OVA protein for 3 days. Cytokine secretion was determined by ELISA. Each symbol represents individually tested mice. The data shown are the mean ± s.d. of three independent experiments. *, p<0.05; **, p<0.01; ***, p<0.001.
Foxp3+ Treg cell accumulation in the inflamed tissues is different depending on the types of inflammation
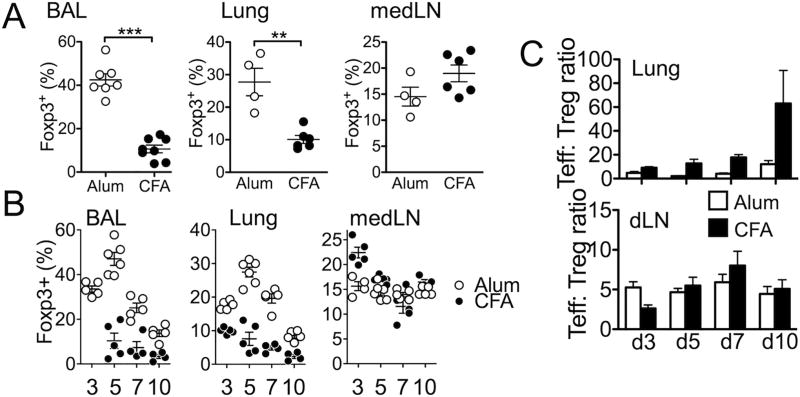
Foxp3+ Treg cells accumulate in inflamed tissues, where they are thought to exert regulatory functions (22, 23). There is increasing evidence that Treg cell functions can be compromised during allergic inflammation and that such defective functions contribute to the disease aggravation (13, 24). To investigate tissue infiltrating Treg cells in eosinophilic and neutrophilic inflammation Foxp3GFP reporter animals were induced for the inflammation and Treg cell accumulation in the inflamed lung tissues at peak of the responses (24 hours after the last challenge, day 5) was compared. We found that ~30% of CD4 T cells infiltrating the BAL and lung tissues of mice induced for eosinophilic inflammation expressed GFP, whereas Treg cell infiltration to the inflamed tissues during neutrophilic inflammation were only ~10% among the entire CD4 T cells (Fig 2A). The differential Treg cell accumulation was only found in the inflamed tissues, because Treg cell levels in the draining medLN and other lymphoid tissues were comparable between the groups (Fig 2A and data not shown).
Figure 2. Foxp3+ Treg cell accumulation during eosinophilic and neutrophlic airway inflammation.
Foxp3.GFP mice were induced for eosinophilic and neutrophilic inflammation as described in Figure 1. (A) After 24 hours of the last antigen challenge mice were sacrificed. Foxp3 expression of the total CD4 T cells in the indicated tissues was determined by flow analysis. (B) Mice were sacrificed at different time points. Indicated tissues were collected and examined for Foxp3 expression by flow analysis. (C) The ratio of Foxp3− and Foxp3+ cells was calculated from the indicated tissues. The data shown represent the mean ± s.d. of two independent experiments. Each symbol represents individually tested mouse. *, p<0.05; **, p<0.01; ***, p<0.001.
To ensure that differential Treg cell accumulation in the inflamed tissues of aforementioned experiments is not due to different kinetics of Treg cell trafficking, we compared Treg cell infiltration during and after Ag challenge. Four time points were chosen as described in Supplementary Fig S1: 1 day after the second CA challenge (d3), and 1 day (d5), 3 days (d7), and 6 days (d10) after the last CA challenge. In case of Alum-induced inflammation, Treg cell accumulation in the target tissues (BAL and lung) was already elevated at day 3, peaked around day 5, and gradually diminished thereafter (Fig 2B). On the other hand, the levels of Treg cells remained at low levels throughout the experiments in CFA-induced inflammation (Fig 2B). Again, Treg cell levels in the draining LN were comparable at all times examined in both groups (Fig 2B). Interestingly, the ratio between Treg cells and effector CD4 T cells was drastically different between the two groups. As shown in Fig 2C, effector T cells significantly outnumbered Treg cells in the lung of CFA-sensitized mice. On the other hand, the ratio of effector to Treg cells remained relatively low in Alum-sensitized mice (Fig 2C). The ratio remained constant in the draining LN regardless of the mode of sensitization (Fig 2C).
The roles of Treg cells during eosinophilic airway inflammation
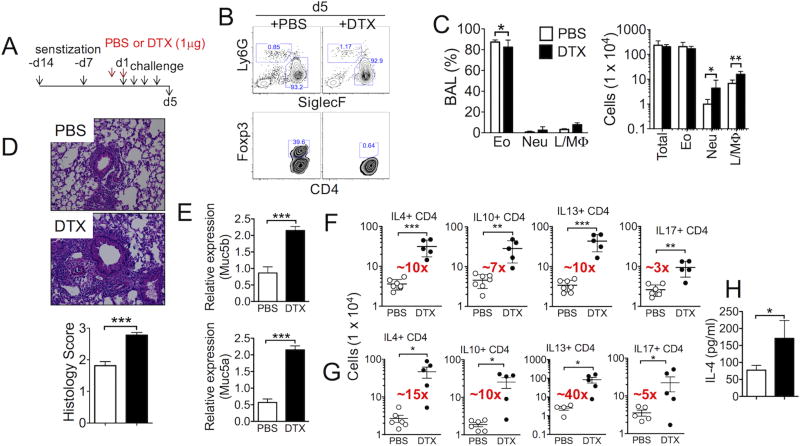
Our results thus far demonstrate that Treg cell accumulation in the inflamed lung tissues is differentially controlled by the nature of airway inflammation, that is, eosinophilic vs. neutrophilic inflammation. The role of Treg cells during allergen-induced airway inflammation has previously been examined. Utilizing BAC-transgenic Foxp3.DTR (DEREG) mice, Baru et al. reported that the lack of Treg cells during ovalbumin antigen challenge does not aggravate allergic airway inflammation (25). We tested the roles of Treg cells during CA-induced eosinophilic inflammation using Foxp3.DTR knockin mice (26). Following CA sensitization in Alum, the diphtheria toxin (DTX) was injected one day prior to and on the first day of CA challenge (Fig 3A). Treg cells were efficiently ablated when analyzed 24 hours after the last CA challenge (Fig 3B). Eosinophils were still the dominant population in the BAL after Treg cell depletion, although the absolute numbers of lymphocytes/macrophages and neutrophils were significantly elevated (Fig 3C). Unlike the previous report (25), however, we found that Treg cell depletion during allergen challenge dramatically exacerbated the lung inflammation as determined by H&E (Fig 3D) and PAS staining (data not shown). In support of PAS staining, Muc5a and Muc5b mRNA expression was significantly elevated in the lung of Treg cell depleted animals (Fig 3E). The levels of lung infiltrating CD4 T cells producing Th2 type cytokines were similarly elevated upon Treg cell depletion (Fig 3F). We estimated ~10-fold increase in effector T cells expressing Th2 type cytokines in the lung (Fig 3F). IL-17-producing CD4 T cells were also increased ~3-fold following depletion (Fig 3F), which may account for increased neutrophil infiltration (Fig 3C). The magnitude of increase in cytokine producing cells was even greater in the draining medLN (Fig 3G). Consistent with intracellular cytokine expression, we detected increased IL-4 secretion in the BAL fluid after Treg cell depletion (Fig 3H). Altered inflammatory responses following DTX-mediated Treg cell depletion was not observed in B6 non-DTR mice (Supplementary Fig S3), suggesting that the enhanced inflammatory responses are not ‘off-target’ effects caused by potential adjuvant effect of DTX injected. Taken together, these results strongly suggest that Foxp3+ Treg cells play an essential role in controlling eosinophilic airway inflammation during allergen challenge.
Figure 3. Depletion of Treg cells during allergen challenge exacerbates Alum-induced eosinophilic airway inflammation.
(A) Experimental scheme. Mice were sensitized with CA antigens in Alum and intranasally challenged. PBS or diphtheria toxin (DTX) was injected one day before and on the day of first antigen challenge. (B) BAL cells were examined for eosinophils, neutrophils, and Foxp3 expression in CD4 T cells. (C) The proportion and absolute numbers of each inflammatory cell type were determined. (D) H&E staining of the lung tissues. Pathology score was determined as described in the Methods. (E) Muc5a and Muc5b mRNA expression in the lung tissue was determined by qPCR. (F and G) Lung and draining LN cells were ex vivo stimulated to measure intracellular cytokine expression. The total numbers of cytokine producing CD4 T cells in the lung (F) and draining LN (G) were enumerated by flow analysis. (H) IL-4 secretion in the BAL fluid was determined by ELISA. The data shown represent the mean ± s.d. of more than two independent experiments. Each symbol represents individually tested mouse. The experiments were repeated twice and similar results were observed. *, p<0.05; **, p<0.01; ***, p<0.001.
The role of Treg cells during neutrophilic airway inflammation
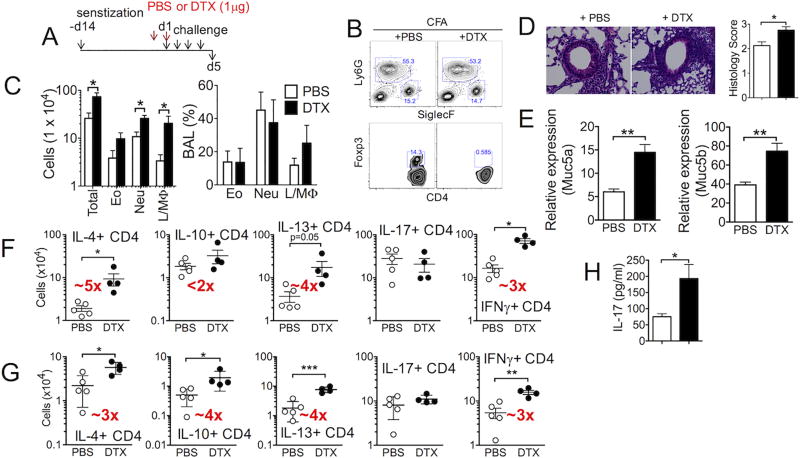
Treg cell accumulation in the lung tissues during CFA-induced neutrophilic airway inflammation was substantially lower than that during eosinophilic inflammation (Fig 2A). Whether these Treg cells still play a role in controlling inflammatory responses in this condition was next tested. Analogous to Alum-induced model (Fig 3), diphtheria toxin (DTX) was injected into Foxp3.DTR mice sensitized with CA in CFA adjuvant one day prior to and on the first day of CA challenge (Fig 4A). Complete depletion of Treg cells was confirmed at sacrifice (Fig 4B). Without Treg cells, neutrophils remained the dominant population in the BAL cells; however, the overall cellularity of inflammatory cells in the BAL was significantly elevated (Fig 4C). Lung inflammation was aggravated following Treg cell depletion (Fig 4D), suggesting that Treg cells, albeit lower in proportion, still play an important regulatory role. Likewise, Muc5a and Muc5b mRNA expression was elevated (Fig 4E). Notably, Treg cell depletion during neutrophilic inflammation increased the numbers of T cells expressing Th2 type cytokines (IL-4 and IL-13), although the magnitude of the increase was considerably lower than that seen during Alum-induced inflammation (Fig 4F and 4G). The accumulation of IL-17-expressing CD4 T cells remained unchanged when measured by intracellular cytokine staining; however, IL-17 secretion in the BAL fluid was significantly increased following Treg cell depletion (Fig 4F, 4G and 4H). IFNγ+ CD4 T cell accumulation was significantly increased by Treg cell depletion. Therefore, these results suggest that Treg cells still play a key regulatory role during CFA-induced neutrophilic airway inflammation, although their lung accumulation is less evident. It is important to point out that the magnitude of enhanced inflammation following Treg cell depletion is greater during eosinophilic (Fig 3F and 3G) than during neutrophilic inflammation (Fig 4F and 4G), suggesting that the extent to which Treg cells control inflammatory responses may be different depending on the inflammatory environments.
Figure 4. Depletion of Treg cells during allergen challenge exacerbates CFA-induced neutrophilic airway inflammation.
(A) Experimental scheme. Mice were sensitized and challenged as described in Supplementary Fig S1. PBS or diphtheria toxin was injected one day before and on the day of first antigen challenge. (B) BAL cell profiles at sacrifice. (C) Total BAL cell numbers were analyzed. (D) H&E staining of the lung tissues. Pathology score was determined as described in the Methods. (E) Muc5a and Muc5b mRNA expression in the lung tissue was determined by qPCR. (F and G) Lung (F) and draining LN (G) cells were ex vivo stimulated to measure intracellular cytokine expression. The total numbers of cytokine producing CD4 T cells were enumerated by flow analysis. (H) IL-17 secretion in the BAL fluid was determined by ELISA. The data shown represent the mean ± s.d. of more than two independent experiments. Each symbol represents individually tested mouse. The experiments were repeated twice and similar results were observed. *, p<0.05; **, p<0.01; ***, p<0.001.
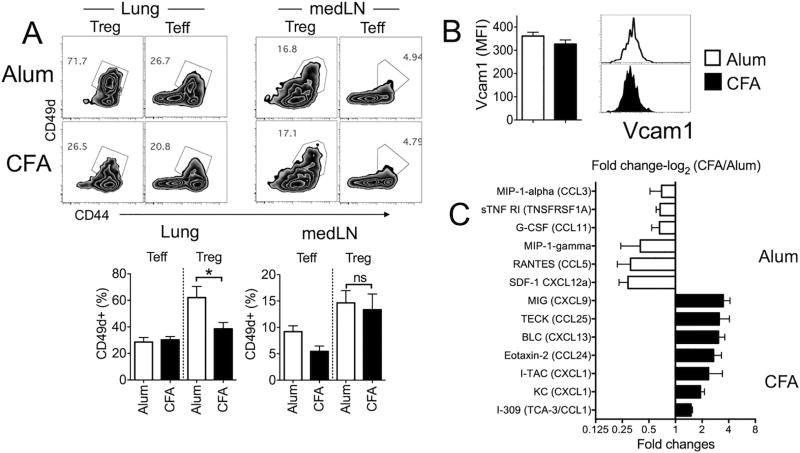
Elevated CD49d expression in Treg cells during eosinophilic inflammation
Allergen specific CD4 T cells upregulate the α4 integrin (CD49d) that facilitates T cell migration to the sites of inflammation (27, 28). CD49d upregulated on activated T cells forms the α4β1 integrin complexes (VLA-4), which then binds to the fibronectin and VCAM-1 for lung infiltration during airway inflammation (29). Proper control of Treg cell migration is an essential feature for successful Treg cell functions in vivo (30). We thus examined whether CD49d is differentially expressed by lung infiltrating Treg cells during Alum- and CFA-induced inflammation. CD49d+ Foxp3+ Treg cells within the inflamed tissues was significantly greater in Alum-induced than in CFA-induced inflammation, while LN resident Treg cells expressed comparable levels of CD49d (Fig 5A). Interestingly, CD49d expression on effector CD4 T cells in the lung was comparable (Fig 5A). The ligand for CD49d, VCAM-1 expression on lung endothelial cells was comparable between the groups (Fig 5B). Besides tissue-trafficking adhesion molecules, inflammatory chemokine expression may play an additional role in differentially attracting Treg cells during Alum- and CFA-induced inflammation. Measuring chemokine expression in the lungs by protein array analysis revealed distinct chemokine expression pattern in Alum- and CFA-induced lung inflammation (Fig 5C). In Alum-induced inflammation, higher level expression of CXCL12 and CCL5 was observed, while CXCL9, CCL25, CXCL13, CCL24, and CXCL1 expression was significantly increased in the lungs with CFA-induced inflammation (Fig 5C). Treg cells migrate to the airways via CCR4 and attenuate allergic inflammation (31), and the CCR4 ligand, CCL17 and CCL22, expression is essential in recruiting Treg cells to the inflamed lung during allergen challenge (32). However, we found that CCR4 expression in infiltrating Treg cells and the CCR4 ligand expression in the lung tissues in both models remained comparable (data not shown), suggesting that the CCL17/CCL22-CCR4 axis plays little role during elevated Treg cell recruitment in Alum-induced inflammation.
Figure 5. Treg cell expression of adhesion molecule and chemokine expression in the lung.
(A) CD49d expression of Treg cells and conventional CD4 T cells in the listed tissues was determined by flow analysis. (B) Vcam1 expression of CD45− CD31+ lung endothelial cells was measured. The data shown represent the mean ± s.d. of three independent experiments. (C) Lung tissues were collected and tissue homogenates were subjected to protein array analysis as described in Methods section. The data shown are the mean ± s.d. of two independent experiments.
Phenotypes of Treg cells within the inflamed tissues
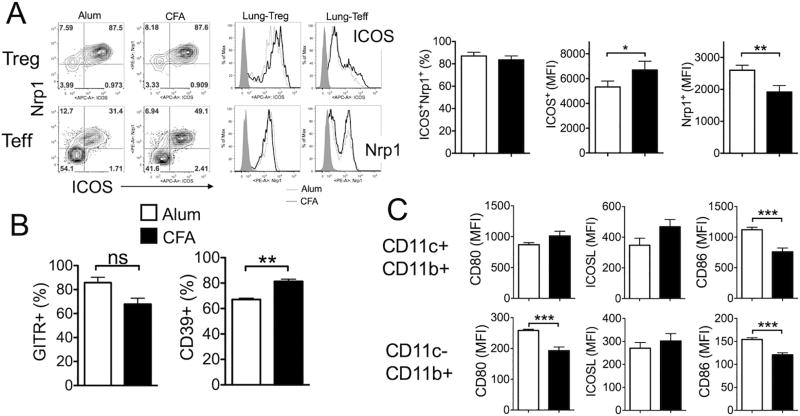
Although Treg cell depletion in both Alum- and CFA-induced models resulted in aggravation of lung inflammation (Fig 3 and 4), we noted that the extent to which inflammatory T cell responses are enhanced by Treg cell depletion was especially greater in Alum-induced model, raising a possibility that the mechanism by which Treg cells control the inflammation may be different. The expression of surface molecules associated with suppressive functions on the lung-infiltrating Treg cells was measured. Inducible T cell costimulatory (ICOS) is a marker for highly suppressive antigen specific Treg cells in part by controlling Treg cell expansion during helminth infections (33, 34). Indeed, lung infiltrating Treg cells expressed high levels of surface ICOS (Fig 6A). The proportion of ICOS+ Treg cells was comparable between the groups; however, the mean fluorescence intensity (MFI) of ICOS expression was reproducibly higher in Treg cells from CFA-sensitized mice (Fig 6A). ICOS+ Treg cells also expressed Nrp1, a thymus-derived tTreg cell specific marker implicated in Treg cell stability (35, 36). Unlike ICOS, however, the MFI of Nrp1 expression was greater in Treg cells from Alum-sensitized mice (Fig 6A). Unexpectedly, we noticed that substantial proportion of Foxp3− lung infiltrating effector CD4 T cells also expressed Nrp1, which also expressed ICOS (Fig 6A). It appears that these Nrp1+ Foxp3− CD4 T cells are highly activated effector but not a subset of ‘regulatory’ T cells, because they were highly proliferating BrdU+ cells and dramatically increased following Treg depletion (our unpublished results). The proportions of Treg cells expressing the Glucocorticoid-induced TNF receptor (GITR) and CTLA4 were comparable between the groups (Fig 6B and data not shown). CD39, an ectoenzyme that hydrolyzes ATP, is highly expressed in Treg cells at the site of inflammation (37, 38). We found that Treg cells expressing CD39 were more abundant in the lung tissues than in the lymphoid tissues (data not shown) and that the level was higher in CFA-sensitized mice (Fig 6B). Therefore, Treg cells infiltrating the lung tissues may mediate regulatory functions via different mechanisms depending on the types of inflammation.
Figure 6. Surface phenotypes of infiltrating Foxp3+ Treg cells during eosinophilic and neutrophilic airway inflammation.
(A) Lung infiltrating Treg cells from Alum- and CFA-sensitized animals were analyzed for the surface expression of ICOS and Nrp1. (B) CD39 and GITR expression on Foxp3+ Treg cells in the lung were also examined. (C) Lung cells from Alum- and CFA-sensitized mice were stained for CD11c, CD11b, CD80, ICOSL, and CD86. The data shown represent the mean fluorescence intensity (MFI) of the indicated surface molecules in CD11c+ CD11b+ or CD11c− CD11b+ cells. The data shown represent the mean ± s.d. of more than two independent experiments. *, p<0.05; **, p<0.01; ***, p<0.001; ns, not-significant.
Costimulatory signals are critical to control Treg cell functions (39). We next compared antigen presenting cell (APC) expression of costimulatory ligands implicated in controlling Treg cell functions. We found that CD80 and CD86 expression was also higher during Alum-induced inflammation models (Fig 6C). ICOS ligand (ICOSL) expression remained comparable (Fig 6C).
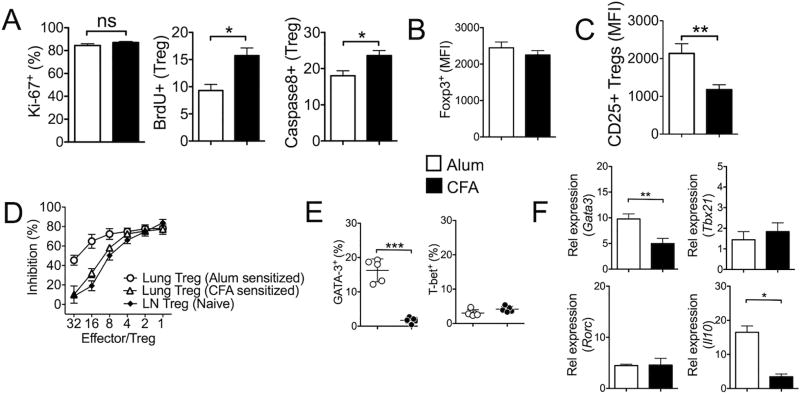
Treg cells are a highly proliferative population in vivo, and ~20% of LN Treg cells are shown in active cell cycle (40, 41). Treg cells in the lung tissues expressed high levels (~80%) of Ki-67, which is in stark contrast with the proportion of cycling Treg cells in the medLN (30~40% Ki-67+) (Fig 7A). The proportions of cells in active cell cycle was similar regardless of the types of inflammation (Fig 7A). However, when the rate of cell proliferation was determined by in vivo BrdU incorporation that only detects the S phase entry of the cell cycle over 24 hour period, BrdU incorporation was significantly higher in Treg cells from CFA-sensitized mice, suggesting that more Treg cells divide in CFA-induced inflammation (Fig 7A). These Treg cells, however, expressed higher active caspase 8 (Fig 7A). Therefore, Treg cells differentially infiltrating the inflamed lung tissues during eosinophilic and neutrophilic airway inflammation display distinct homeostatic behaviors. Of note, Foxp3 expression level was comparable (Fig 7B).
Figure 7. Treg cells from Alum-sensitized mice display more suppressive phenotypes.
(A) Lung infiltrating Foxp3+ Treg cells from Alum- and CFA-sensitized mice were examined for Ki67 expression. Mice were injected with BrdU 24 hours prior to sacrifice. BrdU incorporation of Treg cells was determined by FACS. Active caspase 8 expression was also measured. (B) The level of Foxp3 expression was measured by GFP expression between the groups. (C) Treg cells were stained for surface CD25 expression. (D) Foxp3+ Treg cells were FACS sorted from the lung tissues of Alum- and CFA-sensitized animals. Treg cell suppression assay was performed using CFSE labeled naïve CD4 T cells as described in the Methods. Foxp3+ Treg cells isolated from lymph nodes of naïve animals were used as controls. % inhibition was calculated based on the CFSE dilution of responder CD4 T cells without Treg cells in the culture. (E) Gata3 and T-bet expression was determined by intracellular FACS analysis. (F) Treg cells were FACS sorted and the expression of the indicated genes was determined by real time PCR analysis. The data shown represent the mean ± s.d. of more than two independent experiments. *, p<0.05; **, p<0.01; ns, not-significant.
It was previously reported that Treg cell accumulation pattern in the inflamed skin is linked to CD25 expression (42). Consistent with this study, we found that Treg cells infiltrating the lung tissues during Alum-induced inflammation expressed significantly higher surface CD25 (Fig 7C). Furthermore, those Treg cells expressed more potent suppressive activity compared to that from CFA-sensitized groups (Fig 7D). Treg cell mediated control of tissue inflammation can dynamically be changed by inflammatory signals (43). In particular, Gata3, a Th2 lineage transcription factor, plays a crucial role in regulating Treg cell accumulation in the inflamed sites and to maintain Foxp3 expression (44). Indeed, Treg cell expression of Gata3 was dramatically higher in Treg cells during Alum-induced inflammation (Fig 7E and 7F), while the expression of other lineage-specific transcription factors such as T-bet or RORγt remained comparable (Fig 7F). Furthermore, these Treg cells also expressed higher IL-10 (Fig 7F), further supporting more potent suppressive capacity as well as increased accumulation in the inflamed lung tissues. Taken together, those Treg cells recruited into the inflammatory sites during eosinophilic airway inflammation appear to express key molecules that promote the suppressive functions possibly in response to inflammatory signals available to them.
Discussion
Accumulation of Foxp3+ Treg cells in inflamed tissues is a critical regulatory mechanism by which the inflammatory responses are resolved (11). Here, we report that Treg cell accumulation in the lung tissues during allergic airway inflammation is differentially controlled by the types of inflammation (i.e., eosinophilic vs. neutrophilic). In Alum adjuvant-induced inflammation model where eosinophils and Th2 type effector CD4 T cells are the dominant inflammatory cell types, substantial (20~30%) proportions of lung infiltrating CD4 T cells are Treg cells. On the contrary, the proportion of lung infiltrating Treg cells is merely ~10% during CFA adjuvant-induced airway inflammation where neutrophils and Th1/Th17 type effector CD4 T cells are predominantly found. The proportions of Treg cells in the draining LN remain comparable between the two model systems, suggesting that different levels of Treg cell accumulation within the inflamed tissues are likely due to differential recruitment, expansion, survival, or any combination thereof, resulting in different regulatory mechanisms to control inflammation.
Faustino et al. reported that Treg cells expressing effector/memory phenotypes accumulate in the lung of allergic animals and that these Treg cells efficiently suppress pulmonary T cell proliferation but not Th2 cytokine production (45). Baru et al. reported that the absence of Treg cells during ovalbumin allergen challenge does not exacerbate allergic airway inflammation (25). Our results clearly demonstrate that accumulation of effector CD4 T cells expressing inflammatory cytokines in the lung tissues are dramatically elevated upon Treg cell depletion during allergen challenge in both models of lung inflammation, suggesting that Treg cells play a regulatory role in controlling both Th2 and Th1/17 type airway inflammation during allergen challenge. The difference behind the discrepancy is unclear. Unlike ovalbumin typically used, our study utilized cockroach antigen capable of activating protease-activated receptors (46). Protease-activated receptors enhance production of inflammatory cytokines and potentiate Th2 type responses (47). The model allergen may affect the role of Treg cells during allergic inflammation.
Fowell and colleagues previously reported that the magnitude of Treg cell accumulation in the inflamed skin varies depending on the adjuvants used to immunize animals (42). They similarly found that Treg cell accumulation after Alum-induced skin inflammation is greater than that induced from CFA immunization (42). Treg cell accumulation pattern was found to be linked to CD25 expression in Treg cells (42). Consistently, we found that Treg cells infiltrating the lung tissues from Alum-induced inflammation expressed higher CD25. One potential modulators that control infiltrating Treg cell functions are APC. It was noted that APC expression of costimulatory molecules such as CD80/CD86 is directly linked to Treg cell expansion/accumulation (42). We also found that the expression of CD80 and CD86 was significantly upregulated especially in CD11c− CD11b+ macrophages in the lung during eosinophilic airway inflammation, possibly supporting the importance of the CD28/B7 pathway for the homeostasis of Treg cells to control inflammatory responses and to maintain CD25 expression on Treg cells (48, 49). During CFA and Alum adjuvant-induced skin inflammation the expression of inflammatory signature genes remained unchanged in Treg cells (42). However, we found that Gata3 expression is considerably elevated in Treg cells following Alum-induced airway inflammation. Supporting these findings is that Gata3 expression in Foxp3+ Treg cells critically control Treg cell accumulation at inflamed barrier sites and that Gata3 expression by Treg cells is positively regulated by IL-2 (44).
The findings that the magnitude of aggravation of inflammatory responses following Treg cell depletion is different between eosinophilic and neutrophilic inflammation raises an interesting possibility that the regulatory functions of Treg cells may be controlled at sites of inflammation. As previously proposed (43), inflammatory factors produced by effector cells may be capable of altering transcription factor expression (such as Stat3, IRF4, Gata3, etc) in Treg cells, from which Treg cells may acquire distinct regulatory mechanisms to control inflammatory responses. Particularly interesting is that Treg cell depletion during eosinophilic inflammation enhances effector CD4 T cells producing Th2 as well as Th17 type cytokines. On the contrary, IL-17 production remains unchanged following Treg cell depletion during neutrophilic inflammation. Instead, significant increases in T cells producing Th2 type cytokines and IFNγ are observed in this condition, although the levels are still significantly lower than those found in eosinophilic inflammation. Understanding the precise nature of crosstalk between infiltrating Treg cells and inflammatory factors, and the specific regulatory functions that Treg cells carry out under such conditions are the subjects of future investigation.
In conclusion, the current results demonstrate that the magnitude of Treg cell accumulation in the inflamed lung tissues may still be an important factor that mediates Treg cell functions to control inflammation. However, Treg cell extrinsic factors from inflammatory milieu and APCs may be equally important to shape Treg cell functions.
Supplementary Material
Acknowledgments
The authors thank Jennifer Powers for cell sorting.
Supported by the American Asthma Foundation and NIH grants AI121524 and AI125247 (to B.M.)
References
- 1.Tournoy KG, Provoost S, Van Hove C, Joos G. The role of immune tolerance in asthma pathogenesis. Curr Allergy Asthma Rep. 2006;6:437–443. doi: 10.1007/s11882-996-0018-3. [DOI] [PubMed] [Google Scholar]
- 2.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012;18:673–683. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 3.Holtzman MJ. Asthma as a chronic disease of the innate and adaptive immune systems responding to viruses and allergens. J Clin Invest. 2012;122:2741–2748. doi: 10.1172/JCI60325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Y, Yang J, Gao YD, Guo W. Th17 immunity in patients with allergic asthma. Int Arch Allergy Immunol. 2010;151:297–307. doi: 10.1159/000250438. [DOI] [PubMed] [Google Scholar]
- 5.Cosmi L, Liotta F, Maggi E, Romagnani S, Annunziato F. Th17 cells: new players in asthma pathogenesis. Allergy. 2011;66:989–998. doi: 10.1111/j.1398-9995.2011.02576.x. [DOI] [PubMed] [Google Scholar]
- 6.Wang YH, Wills-Karp M. The potential role of interleukin-17 in severe asthma. Curr Allergy Asthma Rep. 2011;11:388–394. doi: 10.1007/s11882-011-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choy DF, Hart KM, Borthwick LA, Shikotra A, Nagarkar DR, Siddiqui S, Jia G, Ohri CM, Doran E, Vannella KM, Butler CA, Hargadon B, Sciurba JC, Gieseck RL, Thompson RW, White S, Abbas AR, Jackman J, Wu LC, Egen JG, Heaney LG, Ramalingam TR, Arron JR, Wynn TA, Bradding P. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med. 2015;7:301ra129. doi: 10.1126/scitranslmed.aab3142. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Verbsky JW, Chatila TA. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) and IPEX-related disorders: an evolving web of heritable autoimmune diseases. Curr Opin Pediatr. 2013;25:708–714. doi: 10.1097/MOP.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ray A, Khare A, Krishnamoorthy N, Qi Z, Ray P. Regulatory T cells in many flavors control asthma. Mucosal Immunol. 2010;3:216–229. doi: 10.1038/mi.2010.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow Z, Banerjee A, Hickey MJ. Controlling the fire--tissue-specific mechanisms of effector regulatory T-cell homing. Immunol Cell Biol. 2015;93:355–363. doi: 10.1038/icb.2014.117. [DOI] [PubMed] [Google Scholar]
- 12.Smyth LJ, Eustace A, Kolsum U, Blaikely J, Singh D. Increased airway T regulatory cells in asthmatic subjects. Chest. 2010;138:905–912. doi: 10.1378/chest.09-3079. [DOI] [PubMed] [Google Scholar]
- 13.Hartl D, Koller B, Mehlhorn AT, Reinhardt D, Nicolai T, Schendel DJ, Griese M, Krauss-Etschmann S. Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. J Allergy Clin Immunol. 2007;119:1258–1266. doi: 10.1016/j.jaci.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Yu HH, Wang LC, Yang YH, Lin YT, Chiang BL. The levels of CD4+CD25+ regulatory T cells in paediatric patients with allergic rhinitis and bronchial asthma. Clin Exp Immunol. 2007;148:53–63. doi: 10.1111/j.1365-2249.2007.03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 16.Singh B, Shinagawa K, Taube C, Gelfand EW, Pabst R. Strain-specific differences in perivascular inflammation in lungs in two murine models of allergic airway inflammation. Clin Exp Immunol. 2005;141:223–229. doi: 10.1111/j.1365-2249.2005.02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogaert P, Naessens T, De Koker S, Hennuy B, Hacha J, Smet M, Cataldo D, Di Valentin E, Piette J, Tournoy KG, Grooten J. Inflammatory signatures for eosinophilic vs. neutrophilic allergic pulmonary inflammation reveal critical regulatory checkpoints. Am J Physiol Lung Cell Mol Physiol. 2011;300:L679–690. doi: 10.1152/ajplung.00202.2010. [DOI] [PubMed] [Google Scholar]
- 18.Ito K, Herbert C, Siegle JS, Vuppusetty C, Hansbro N, Thomas PS, Foster PS, Barnes PJ, Kumar RK. Steroid-resistant neutrophilic inflammation in a mouse model of an acute exacerbation of asthma. Am J Respir Cell Mol Biol. 2008;39:543–550. doi: 10.1165/rcmb.2008-0028OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao J, Lloyd CM, Noble A. Th17 responses in chronic allergic airway inflammation abrogate regulatory T-cell-mediated tolerance and contribute to airway remodeling. Mucosal Immunol. 2013;6:335–346. doi: 10.1038/mi.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A, Kolls JK. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Possa SS, Leick EA, Prado CM, Martins MA, Tiberio IF. Eosinophilic inflammation in allergic asthma. Front Pharmacol. 2013;4:46. doi: 10.3389/fphar.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 23.Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005;201:1037–1044. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai YG, Niu DM, Yang KD, Hung CH, Yeh YJ, Lee CY, Lin CY. Functional defects of CD46-induced regulatory T cells to suppress airway inflammation in mite allergic asthma. Lab Invest. 2012;92:1260–1269. doi: 10.1038/labinvest.2012.86. [DOI] [PubMed] [Google Scholar]
- 25.Baru AM, Ganesh V, Krishnaswamy JK, Hesse C, Untucht C, Glage S, Behrens G, Mayer CT, Puttur F, Sparwasser T. Absence of Foxp3+ regulatory T cells during allergen provocation does not exacerbate murine allergic airway inflammation. PLoS One. 2012;7:e47102. doi: 10.1371/journal.pone.0047102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 27.Henderson WR, Jr, Chi EY, Albert RK, Chu SJ, Lamm WJ, Rochon Y, Jonas M, Christie PE, Harlan JM. Blockade of CD49d (alpha4 integrin) on intrapulmonary but not circulating leukocytes inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. J Clin Invest. 1997;100:3083–3092. doi: 10.1172/JCI119863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pacheco KA, Tarkowski M, Klemm J, Rosenwasser LJ. CD49d expression and function on allergen-stimulated T cells from blood and airway. Am J Respir Cell Mol Biol. 1998;18:286–293. doi: 10.1165/ajrcmb.18.2.2687. [DOI] [PubMed] [Google Scholar]
- 29.Nakajima H, Sano H, Nishimura T, Yoshida S, Iwamoto I. Role of vascular cell adhesion molecule 1/very late activation antigen 4 and intercellular adhesion molecule 1/lymphocyte function-associated antigen 1 interactions in antigen-induced eosinophil and T cell recruitment into the tissue. J Exp Med. 1994;179:1145–1154. doi: 10.1084/jem.179.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Afshar R, Strassner JP, Seung E, Causton B, Cho JL, Harris RS, Hamilos DL, Medoff BD, Luster AD. Compartmentalized chemokine-dependent regulatory T-cell inhibition of allergic pulmonary inflammation. J Allergy Clin Immunol. 2013;131:1644–1652. doi: 10.1016/j.jaci.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faustino L, da Fonseca DM, Takenaka MC, Mirotti L, Florsheim EB, Guereschi MG, Silva JS, Basso AS, Russo M. Regulatory T cells migrate to airways via CCR4 and attenuate the severity of airway allergic inflammation. J Immunol. 2013;190:2614–2621. doi: 10.4049/jimmunol.1202354. [DOI] [PubMed] [Google Scholar]
- 32.Kearley J, Robinson DS, Lloyd CM. CD4+CD25+ regulatory T cells reverse established allergic airway inflammation and prevent airway remodeling. J Allergy Clin Immunol. 2008;122:617–624. e616. doi: 10.1016/j.jaci.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Redpath SA, van der Werf N, Cervera AM, MacDonald AS, Gray D, Maizels RM, Taylor MD. ICOS controls Foxp3(+) regulatory T-cell expansion, maintenance and IL-10 production during helminth infection. Eur J Immunol. 2013;43:705–715. doi: 10.1002/eji.201242794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vocanson M, Rozieres A, Hennino A, Poyet G, Gaillard V, Renaudineau S, Achachi A, Benetiere J, Kaiserlian D, Dubois B, Nicolas JF. Inducible costimulator (ICOS) is a marker for highly suppressive antigen-specific T cells sharing features of TH17/TH1 and regulatory T cells. J Allergy Clin Immunol. 2010;126:280–289. 289, e281–287. doi: 10.1016/j.jaci.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 35.Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, Anthony BA, Sverdrup FM, Head R, Kuster DJ, Ruminski P, Weiss D, Von Schack D, Bluestone JA. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209:1713–1722. S1711–1719. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J, Workman CJ, Vignali DA. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature. 2013;501:252–256. doi: 10.1038/nature12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herrath J, Chemin K, Albrecht I, Catrina AI, Malmstrom V. Surface expression of CD39 identifies an enriched Treg-cell subset in the rheumatic joint, which does not suppress IL-17A secretion. Eur J Immunol. 2014;44:2979–2989. doi: 10.1002/eji.201344140. [DOI] [PubMed] [Google Scholar]
- 38.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, Centonze D, Bernardi G, Dell'Acqua ML, Rossini PM, Battistini L, Rotzschke O, Falk K. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 39.Bour-Jordan H, Bluestone JA. Regulating the regulators: costimulatory signals control the homeostasis and function of regulatory T cells. Immunol Rev. 2009;229:41–66. doi: 10.1111/j.1600-065X.2009.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fisson S, Darrasse-Jeze G, Litvinova E, Septier F, Klatzmann D, Liblau R, Salomon BL. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J Exp Med. 2003;198:737–746. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Attridge K, Walker LS. Homeostasis and function of regulatory T cells (Tregs) in vivo: lessons from TCR-transgenic Tregs. Immunol Rev. 2014;259:23–39. doi: 10.1111/imr.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Billroth-MacLurg AC, Ford J, Rosenberg A, Miller J, Fowell DJ. Regulatory T Cell Numbers in Inflamed Skin Are Controlled by Local Inflammatory Cues That Upregulate CD25 and Facilitate Antigen-Driven Local Proliferation. J Immunol. 2016;197:2208–2218. doi: 10.4049/jimmunol.1502575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaudhry A, Rudensky AY. Control of inflammation by integration of environmental cues by regulatory T cells. J Clin Invest. 2013;123:939–944. doi: 10.1172/JCI57175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wohlfert EA, Grainger JR, Bouladoux N, Konkel JE, Oldenhove G, Ribeiro CH, Hall JA, Yagi R, Naik S, Bhairavabhotla R, Paul WE, Bosselut R, Wei G, Zhao K, Oukka M, Zhu J, Belkaid Y. GATA3 controls Foxp3(+) regulatory T cell fate during inflammation in mice. J Clin Invest. 2011;121:4503–4515. doi: 10.1172/JCI57456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faustino L, Mucida D, Keller AC, Demengeot J, Bortoluci K, Sardinha LR, Carla Takenaka M, Basso AS, Faria AM, Russo M. Regulatory T cells accumulate in the lung allergic inflammation and efficiently suppress T-cell proliferation but not Th2 cytokine production. Clin Dev Immunol. 2012;2012:721817. doi: 10.1155/2012/721817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hong JH, Lee SI, Kim KE, Yong TS, Seo JT, Sohn MH, Shin DM. German cockroach extract activates protease-activated receptor 2 in human airway epithelial cells. J Allergy Clin Immunol. 2004;113:315–319. doi: 10.1016/j.jaci.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 47.Ebert CS, Jr, McKinney KA, Urrutia G, Wu M, Rose AS, Fleischman GM, Thorp B, Senior BA, Zanation AM. Expression of protease-activated receptors in allergic fungal rhinosinusitis. Int Forum Allergy Rhinol. 2014;4:266–271. doi: 10.1002/alr.21295. [DOI] [PubMed] [Google Scholar]
- 48.Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, Zheng XX, Strom TB, Bluestone JA. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 49.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.