SUMMARY
Objective
Epilepsy is one of the most prominent symptoms of the genetic disorder, tuberous sclerosis complex (TSC), and may be related to developmental defects resulting from impaired TSC1 or TSC2 gene function in astrocytes and neurons. Inactivation of the Tsc1 gene driven by a glial-fibrillary acidic protein (GFAP) promoter during embryonic brain development leads to widespread pathological effects on astrocytes and neurons, culminating in severe, progressive epilepsy in mice (Tsc1GFAP-Cre mice). However, the developmental timing and cellular specificity relevant to epileptogenesis in this model has not been well defined. The present study evaluates the effect of postnatal Tsc1 gene inactivation on pathological features of astrocytes and neurons and development of epilepsy.
Methods
An inducible Tsc1 knock-out mouse was created utilizing a tamoxifen-driven GFAP-CreER line (Tsc1GFAP-CreER mice) with TSC1 reduction induced postnatally at 2 and 6 weeks of age, and compared to conventional Tsc1GFAP-Cre mice with prenatal TSC1 reduction. Western blotting, immunohistochemistry, histology, and video-EEG assessed mTOR pathway activation, astrogliosis, neuronal organization, and spontaneous seizures, respectively.
Results
Tsc1 gene inactivation at 2 weeks of age was sufficient to cause astrogliosis and mild epilepsy in Tsc1GFAP-CreER mice, but the phenotype was much less severe than observed with prenatal Tsc1 gene inactivation in Tsc1GFAP-Cre mice. Both astrocytes and neurons were affected by prenatal and postnatal Tsc1 gene activation to a degree similar to the severity of epilepsy, suggesting that both cellular types may contribute to epileptogenesis.
Significance
These findings support a model in which the developmental timing of TSC1 loss dictates the severity of neuronal and glial abnormalities and resulting epilepsy.
Keywords: epilepsy, seizure, tuberous sclerosis, rapamycin, astrocyte, neuron, mice
INTRODUCTION
Tuberous sclerosis complex (TSC) is a genetic disorder, with epilepsy, intellectual disability, and autism representing common neurological manifestations 1; 2. TSC is caused by mutation of either the TSC1 or TSC2 genes, leading to activation of the mechanistic target of rapamycin complex 1 (mTORC1) pathway and associated tumor or hamartoma formation in multiple organs. A leading hypothesis about the pathophysiology of epilepsy in TSC has traditionally focused on the role of cortical tubers, which are focal developmental malformations of the brain, in generating seizures 3. However, the specific cellular and molecular mechanisms of epileptogenesis in TSC are still incompletely understood. Current treatments for epilepsy and other neurological manifestations of TSC are primarily symptomatic, but novel targeted therapies, such as with mTORC1 inhibitors, are being explored as potential mechanistically-based, disease-modifying treatments for TSC 4–6. Further understanding of the cellular and molecular basis of epilepsy in TSC should lead to more effective therapies for this disease and may also have implications for other related disorders involving “mTORopathies”.
Pathological studies of cortical tubers and perituberal regions from TSC patients with intractable epilepsy reveal a variety of cellular and molecular abnormalities 7; 8. In particular, in addition to a loss of normal cortical lamination, a range of normal and abnormal cell types have been described representing a spectrum of neuronal to glial lineages, including dysmorphic neurons, astrogliosis, microglial activation, and the classic giant cells with immature neuronal and glial features. However, the specific roles and relative contribution of these different cell types to epileptogenesis are unknown and difficult to investigate in human studies.
Animal models have provided some insights into the role of specific cells in epileptogenesis in TSC, using cell-targeted conditional knockout of the Tsc1 or Tsc2 genes 9–13. For example, neuron-specific knockout mice, using a synapsin promoter to drive Cre-mediated Tsc1 gene inactivation, have spontaneous seizures, supporting a critical role of neurons in TSC 14. While it is perhaps not surprising that neuronal abnormalities promote seizures in TSC, glial cells have been increasingly recognized in modulating brain excitability and potentially promoting epilepsy in general. Other conditional knockout mouse models have attempted to determine the contribution of astrocytes (glia) to epilepsy in TSC, targeting Tsc gene inactivation with a glial fibrillary acidic protein (GFAP) promoter 15; 16, and identifying a number of abnormal astrocytic mechanisms that promote epileptogenesis in these models 17–20. While GFAP has been largely reported to be “astrocyte-specific”, GFAP is also expressed in embryonic neural progenitor cells and thus prenatal GFAP-driven gene inactivation may have widespread effects on both astrocytes and neurons 21; 22. In this study, we have examined the effect of postnatal Tsc1 gene inactivation, using an inducible-GFAP-Cre mouse model 22; 23, on astrocytic and neuronal abnormalities and seizure generation.
METHODS
Animals and tamoxifen administration
Care and use of all mice were conducted according to an animal protocol approved by the Washington University Animal Studies Committee (IACUC #A-3381-01) and consistent with NIH guidelines on the Care and Use of Laboratory Animals, the ARRIVE guidelines, and the Basel Declaration. In addition, NIH guidelines on Rigor and Reproducibility in Preclinical Research were followed, including use of randomization, blinding, both sexes, and statistical/power analyses. GFAP-CreER mice (obtained from Suzanne Baker’s lab)23 were crossed with Tsc1flox/flox mice to generate the inducible Tsc1 knockout mice (Tsc1GFAP-CreER mice). Littermates (Tsc1 f/+ ;CreER+ or Tsc1 f/f; CreER-) were used as negative controls. For comparison, conventional Tsc1GFAP-Cre mice were also generated by crossing non-inducible GFAP-Cre mice with Tsc1flox/flox mice 15. In separate studies, CgGt(Rosa)26Sortm6(CAG-ZsGreen1)Hze/J (Rosa-Green; Jackson Laboratory) reporter mice were crossed with GFAP-CreER and GFAP-Cre mice to confirm the cellular localization of Cre-mediated recombination induced by tamoxifen.
Mice were randomized into tamoxifen or vehicle treatment groups. Tamoxifen (Sigma, T5648) was dissolved in corn oil (Sigma, C8267) at a concentration of 20 mg/ml. Tamoxifen (9mg/40g body weight; i.p.) was administered to mice once a day for 5 consecutive days at 6-weeks or every other day at 2 weeks of age.
Western blotting
For western blotting, mice were sacrificed 6 weeks after tamoxifen injection. Neocortex and hippocampus were dissected and sonicated in the lysis buffer (0.8M Tris-HCl, 5M NaCl, 0.2M EDTA, 0.1% Triton X-100, 0.2 EDTA). After centrifuging, total proteins were extracted. The concentrations of the total protein extracts were determined by BCA protein assay reagents (Pierce 23223, 23224). Following the normalization of protein concentration, 2X sample buffer (Sigma S3401) was added to the protein extracts. Equal amounts of protein samples were separated by electrophoresis on Bio-rad mini-protean TGXTM gels and transferred to the nitrocellulose membrane. The membrane was then blocked with 5% non-fat milk (BioRad 170-6404) in TBST at room temperature (RT) for 1 hour and incubated in primary antibody (pS6 p240/244, Cell Signaling 2215; S6, Cell Signaling 2217; p-P70S6K, Cell Signaling 9234; P70S6K, Cell Signaling 2708; TSC1, Cell Signaling 4906; TSC2, Cell Signaling 3990; actin, Cell Signaling 4970; gapdh, millipore MAB 374) diluted in TBST overnight (O/N) at 4°C. After washing three times with TBST, the secondary antibody (Goat-anti-rabbit, Cell Signaling 7074 or Goat-anti-mouse, Cell Signaling 7076) was diluted in TBST and the membrane was incubated in the secondary antibody solution at RT for 1 hour. Signals were detected by enzyme chemiluminescence (GE Healthcare Life Science, RPN2235) and quantitatively analyzed with ImageJ software (NIH, Bethesda, MD).
Immunohistochemistry/histology
Mice were anesthetized with isoflurane six weeks after tamoxifen injection and then subjected to cardiac perfusion of phosphate-buffered saline (PBS) followed by 4% of paraformaldehyde (PFA). The brains were dissected out and immersed in 4% PFA O/N at 4°C. After dehydration in 30% sucrose diluted in PBS (~48hrs), the brains were frozen and coronal sections of 40um thickness were cut by a microtome (Microm, HM400).
For antibody staining, the brain slices were blocked in solution with 10% normal goat serum (NGS), 1% BSA and 0.3% triton X-100 in PBS and then incubated in primary antibody (GFAP, Cell Signaling 12389; NeuN, Millipore, MAB 377; S100β, Sigma S2532) diluted in solution of 1% NGS, 1% BSA and 0.3% triton X-100. The brain slices was then incubated in the secondary antibody (Goat anti-rabbit IgG, alexa 488, Life Technologies A11034; Goat anti-rabbit IgG, Cy3 conjugate, Life Technologies A10520; Goat anti-mouse IgG, Cy3 conjugate A10521) solution (PBS with 1% BSA) for 1 hour at RT, avoiding light. Images were acquired with a Zeiss LSM PASCAL confocal microscope (Zeiss Thornwood, NY), or with Nanozoomer HT system (Hamamatsu, Bridgewater, NJ). For quantification, images of comparable regions of interest were taken and the ImageJ software was used for the cell number counting and the fluorescent intensity measurement.
For cresyl violet staining, 0.5% cresyl violet acetate was dissolved in warm water with 0.3% glacial acetic acid. The cresyl violet solution was then cooled and filtered. Brain slices were mounted on the glass slides and passed through the following sequence of washes: 95% EtOH 15 min, 70% EtOH 1min; 50% EtOH 1min, distilled H2O (DH2O) 2min, DH2O 1min, Cresyl Violet 2min, 50% EtOH 1min, 70% acid EtOH 2min, 95% EtOH 2min, 95% EtOH a few dips, 100% EtOH 1min, histoclear 5min. Images were acquired with Nanozoomer HT system.
Video-electroencephalography monitoring
Surgery for implanting epidural electrodes for continuous video-electroencephalography (EEG) recordings were performed at least one week after the mice were injected with tamoxifen. Mice were anesthetized with isoflurane and placed in a stereotaxic frame. Epidural screw electrodes were surgically implanted and secured using dental cement for long-term EEG recordings. Four electrodes were placed on the skull: one right and one left central electrodes (1 mm lateral to midline, 2 mm posterior to bregma), one frontal electrode (0.5 mm anterior and 0.5 mm to the right or left of bregma) and one occipital electrode (0.5 mm posterior and 0.5 mm to the right or left lambda). The typical recording montage involved two EEG channels with the right and left central “active” electrodes being compared to either the frontal or occipital “reference” electrode. Video and EEG data were acquired simultaneously with a Stellate video-EEG system. In a blinded analysis, electrographic seizures were identified by their characteristic pattern of discrete periods of rhythmic spike discharges that evolved in frequency and amplitude lasting at least 10 seconds, typically ending with repetitive burst discharges and voltage suppression. On video analysis, the behavioral correlate to these seizures typically involve head bobbing, rearing with forelimb clonus, and occasional generalized convulsive activity.
Statistical analyses
All statistical analysis was performed using GraphPad Prism 5.0 software (GraphPad Software). Quantitative differences between groups were analyzed by Student’s t test or one-way ANOVA with Tukey’s multiple comparisons post hoc tests when comparing one factor over more than two groups. Quantitative data are expressed as mean ± SEM. Statistical significance was defined as p < 0.05.
RESULTS
Postnatal injection of tamoxifen decreases TSC1 and increases mTORC1 activity in Tsc1GFAP-CreER brains
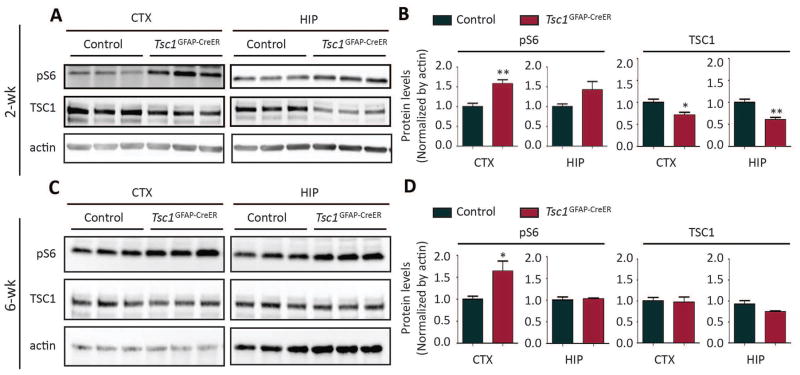
Tsc1GFAP-CreER mice and control littermates were injected with tamoxifen at either 2 weeks or 6 weeks of age. Western blotting was performed six weeks after tamoxifen injection to examine the protein levels of mTORC1 pathway components in the brain. For the injection at 2 weeks of age, the protein levels of TSC1 were reduced significantly in both cortex and hippocampus of Tsc1GFAP-CreER mice compared with control mice (Fig. 1A, 1B), indicating floxed Tsc1 gene recombination mediated by tamoxifen-induced Cre activity. In contrast, TSC2 protein expression was not significantly affected by postnatal TSC1 reduction (Fig. S1). mTORC1 activation, as assessed by expression of phosphorylated S6 (pS6), was increased in both the cortex and hippocampus of Tsc1GFAP-CreER mice six weeks after tamoxifen injection (Fig. 1A, 1B – normalized to actin; Fig. S1 – normalized to total S6). As additional confirmation of mTORC1 signaling involvement, pP70S6K expression was also increased six weeks after tamoxifen injection at 2 weeks of age (Fig. S1). For the tamoxifen injection at 6 weeks of age, although we failed to detect a significant decrease in TSC1 protein in Tsc1GFAP-CreER brains by western blot, there was a significant increase in pS6 levels in the cortex (Fig. 1C, 1D), indicating functional Tsc1 gene inactivation despite an undetectable decrease in protein expression.
Figure 1. Postnatal injection of tamoxifen decreases TSC1 and increases mTORC1 activity in Tsc1GFAP-CreER brains.
Both control (Tsc1f/f;Cre− or Tsc1f/+;Cre+) and Tsc1GFAP-CreER mice were injected with tamoxifen. Western blots were performed 6 weeks after tamoxifen injection. (A–B) Representative blots (A) and quantification (B) show protein levels of TSC1 and pS6 (Ser240/244) in cortex and hippocampus of mice with tamoxifen injection at 2 weeks (*P<0.05; **P<0.01; Student’s t test, n=6 per group,). (C–D) Representative blots (C) and quantification (D) show protein levels of TSC1 and pS6 (Ser240/244) in cortex and hippocampus of mice with tamoxifen injection at 6 weeks (Student’s t test, n=6 per group, *P<0.05)
Tamoxifen injection induces cre-recombinase activity in both astrocytes and neurons
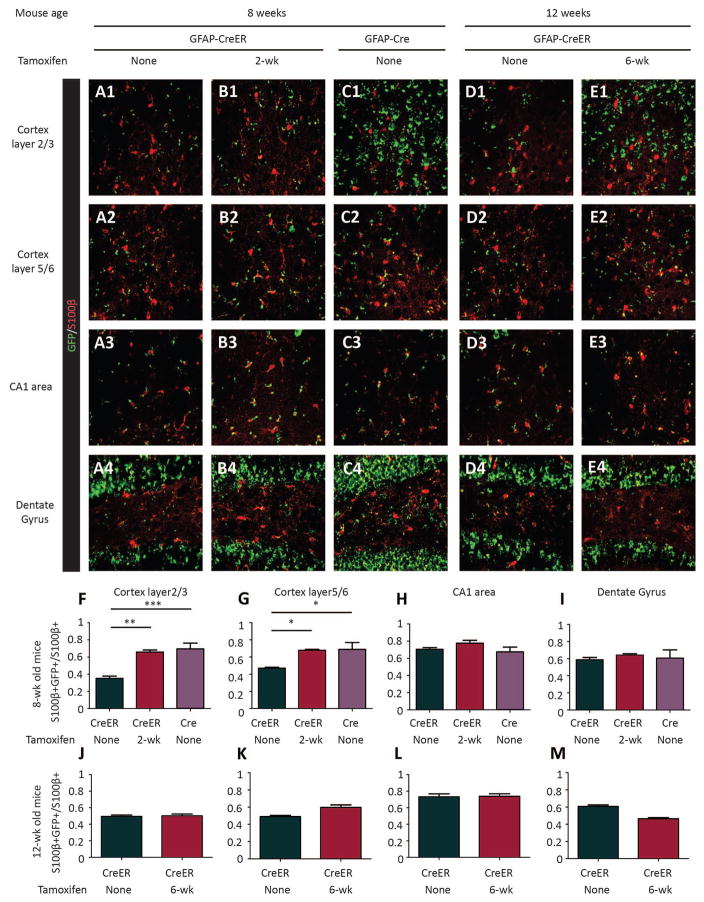
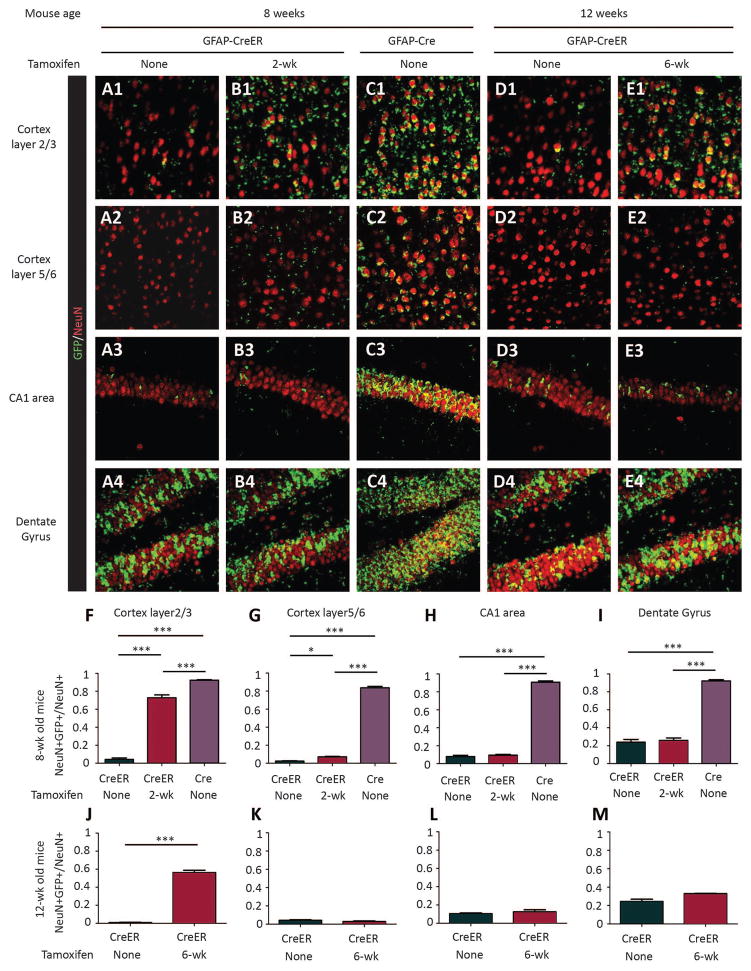
To confirm the identity of cells targeted by Cre-mediated recombination, we crossed Rosa-Green reporter mice with Tsc1GFAP-CreER mice. Expression of the GFP reporter gene was detected in both brain astrocytes and neurons at 8 and 12 weeks of age (six weeks after tamoxifen injections; Fig. 2, 3). Some evidence of Cre activity in Tsc1GFAP-CreER mice was observed even without tamoxifen injection (“leakiness”), especially in astrocytes, but western blot showed comparable protein levels of both TSC1 and pS6 in the cortex and hippocampus of uninjected Tsc1GFAP-CreER mice relative to control littermates (Fig. S2), indicating that the basal Cre expression by itself did not cause any significant functional effect on mTORC1 pathway activation. Furthermore, reporter analysis of uninjected 1 week old Tsc1GFAP-CreER mice found minimal evidence of Cre activity (Fig. S3), indicating that the leakiness occurred postnatally, which would simply reinforce the intent of our studies in targeting postnatal Tsc1 gene inactivation. Compared to non-injected controls, tamoxifen injection increased the number of both astrocytes and neurons with reporter gene expression in Tsc1GFAP-CreER mice, particularly in cortex, although the level of reporter gene expression was not as high as observed in the conventional Tsc1GFAP-Cre knockout mice (Fig. 2, 3).
Figure 2. Tamoxifen injection induces cre-recombinase activity in astrocytes.
(A–E) Confocal images of S100 β staining of astrocytes (red) in the cortex and hippocampus areas of Rosa-Green Cre reporter (green) mice with Tsc1GFAP-CreER (A, B, D, E) or Tsc1GFAP-Cre (C). Tamoxifen was injected in Tsc1+/+ GFAP-CreER or TSC1f/+ GFAP-CreER mice at either 2-wk (B) or 6-wk (E) of age. Mice were sacrificed 6 weeks after tamoxifen was injected for the staining. Controls (A, D, Tsc1+/+ GFAP-CreER or TSC1f/+ GFAP-CreER mice without tamoxifen injection) were sacrificed at 8-wk of age for the tamoxifen injection at 2-wk group and at 12-wk of age for the tamoxifen injection at 6-wk group. The Tsc1GFAP-Cre CKOs were sacrificed at 8 weeks of age only, as they rarely survive to 12 weeks. (F–I) Quantifications of the percentage of GFP+ astrocytes (double-labeled in yellow) in different areas of the brain (*P<0.05; **P<0.01; ***P<0.001; One way ANOVA, n=3 for each group).
Figure 3. Tamoxifen injection induces cre-recombinase activity in neurons.
(A–E) Confocal images of NeuN staining of neurons (red) in the cortex and hippocampus areas of Rosa-Green Cre reporter (green) mice with Tsc1GFAP-CreER (A, B, D, E) or Tsc1GFAP-Cre (C). Tamoxifen was injected in Tsc1+/+ GFAP-CreER or TSC1f/+ GFAP-CreER mice at either 2-wk (B) or 6-wk (E) of age. Mice were sacrificed 6 weeks after tamoxifen was injected for the staining. Controls (A, D, Tsc1+/+ GFAP-CreER or TSC1f/+ GFAP-CreER mice without tamoxifen injection) were sacrificed at 8-wk of age for the tamoxifen injection at 2-wk group and at 12-wk for the tamoxifen injection at 6-wk group. The Tsc1GFAP-Cre CKOs were sacrificed at 8 weeks of age only, as they rarely survive to 12 weeks. (F–I) Quantifications of the percentage of GFP+ neurons (double-labeled in yellow) in different areas of brain (*P<0.05; **P<0.01; ***P<0.001, Student’s t test, n=3 for each group,).
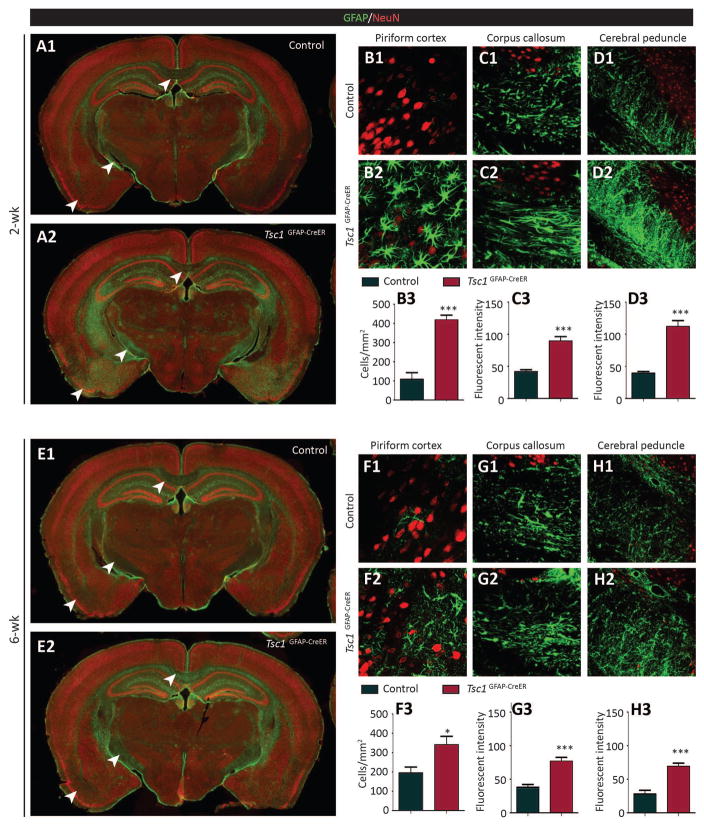
Tamoxifen injection increases GFAP-positive astrocytes in Tsc1GFAP-CreER brains
Conventional Tsc1GFAP-Cre knockout mice show diffuse astrogliosis throughout the brain, manifested by morphological changes of GFAP-positive astrocytes and an increase in astrocyte number 15; 24. To examine whether astrogliosis occurs when TSC1 was reduced postnatally, GFAP staining of Tsc1GFAP-Cre brains was performed six weeks following tamoxifen injection. The brains with tamoxifen injection at either 2 or 6 weeks of age showed an increase in GFAP intensity or GFAP-positive cell number (Fig. 4). However, unlike the widespread astrogliosis found in the conventional Tsc1GFAP-Cre knockout brains, the increase in GFAP staining of Tsc1GFAP-CreER brains was confined to certain brain regions, such as piriform cortex, corpus callosum and cerebral peduncle. We quantified the number of GFAP-positive cells in piriform cortex and the fluorescent intensity in corpus callosum and cerebral peduncle since the boundaries of the individual astrocytes in the latter two areas are difficult to distinguish. The increase in GFAP-staining of the mice injected with tamoxifen at 2 weeks of age was more dramatic than those that received tamoxifen at 6 weeks (Fig. 4B–D, 4F–H).
Figure 4. Tamoxifen injection increases GFAP-positive astrocytes in Tsc1GFAP-CreER brains.
(A) Low power images show the increase of GFAP (green) staining in certain brain regions (indicated by the arrow heads) in Tsc1GFAP-CreER mice compared to control (Tsc1f/f or Tsc1f/+) mice. Both control and Tsc1GFAP-CreER mice were injected with tamoxifen at 2 weeks of age and sacrificed for staining 6 weeks after tamoxifen injection. NeuN (red) was used as counter staining. (B–D) Quantification of GFAP+ cell numbers in piriform cortex (B) and GFAP fluorescent intensity in corpus callosum and cerebral peduncle of the mice injected with tamoxifen at 2 weeks (C, D) (***P<0.001, Student’s t test, n=4 per group). (E) Low power images to show the increase of GFAP (green) staining in certain brain regions (indicated by the arrow heads) in Tsc1GFAP-CreER mice compared to the control (Tsc1f/f or Tsc1f/+) mice. Both control and Tsc1GFAP-CreER mice were injected with tamoxifen at 6 weeks of age and sacrificed for staining 6 weeks after tamoxifen injection. NeuN (red) was used as counter staining. (F–H) Quantification of GFAP+ cell numbers in piriform cortex (F) and GFAP fluorescent intensity in corpus callosum and cerebral peduncle of the mice injected with tamoxifen at 2 weeks (G,H) (***P<0.001, Student’s t test, n=4 per group).
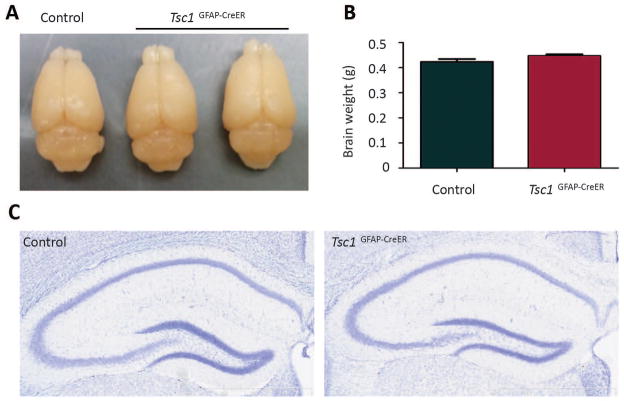
Tsc1GFAP-CreER mice with postnatal tamoxifen injection show normal brain size, weight and hippocampal structure
Conventional Tsc1GFAP-Cre knockout mice have enlarged brain sizes and neuronal disorganization in hippocampus 15; 24. In contrast, postnatal TSC1 reduction did not cause any significant change in brain size and weight or in hippocampal neuronal organization in Tsc1GFAP-CreER mice (Fig. 5). In addition, the conventional Tsc1GFAP-Cre mice all die by 2–3 months of age 15; 24, whereas the tamoxifen-injected Tsc1GFAP-CreER mice lived to at least 6 months of age. The exact life span is not known, since Tsc1GFAP-CreER mice were not followed beyond 6 months of age.
Figure 5. Tsc1GFAP-CreER mice with postnatal tamoxifen injection show normal brain size, weight and hippocampal structure.
Tamoxifen was injected at 2 weeks of age and the brains were dissected 6 weeks after tamoxifen injection for both controls and Tsc1GFAP-CreER mice. (A) Tsc1GFAP-CreER mice showed comparable brain size to the littermate control (Tsc1f/f). (B) The brain weight of Tsc1GFAP-CreER mice were comparable to that of the littermate control (Tsc1f/f or Tsc1f/+) (p>0.05, Student’s t test, n=4 per group). (C) Cresyl violet staining shows that neuronal organization appears normal in Tsc1GFAP-CreER hippocampus.
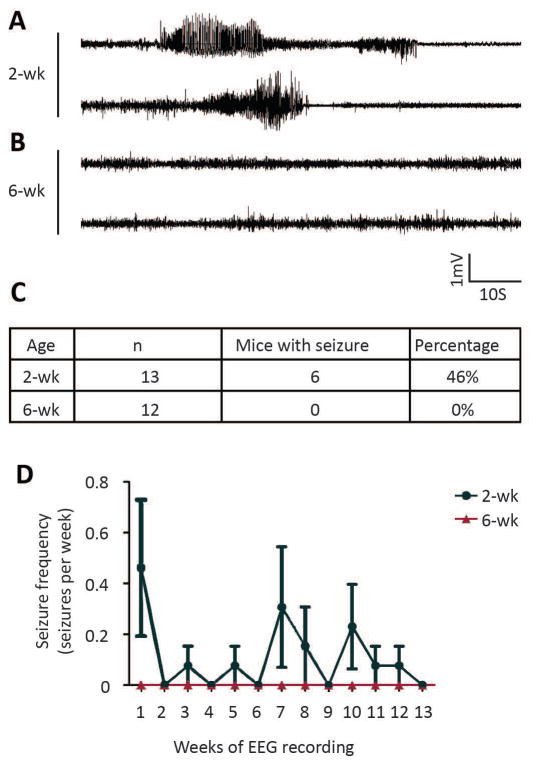
Tamoxifen injection at 2 weeks, but not 6 weeks, induces spontaneous seizures in Tsc1GFAP-CreER mice
Essentially 100% of conventional Tsc1GFAP-Cre knockout mice develop severe, progressive epilepsy, with most mice averaging 10 seizures per day by two months of age 24; 25. In comparison, six out of thirteen monitored Tsc1GFAP-CreER mice with tamoxifen injection at 2 weeks of age exhibited spontaneous seizures (Fig. 6A, 6C), whereas none of the twelve Tsc1GFAP-CreER mice with tamoxifen injection at 6 weeks exhibited any seizures during the 3-month-observation period (Fig. 6B, 6C). Among the mice with spontaneous seizures, seizure onset ranged from 6 weeks to 17 weeks of age (Fig. 6D). One of the mice showed recurrent seizures with the frequency of 1 or 2 seizures a week during the 3-month-observing period. The remaining mice only had rare (1–2 seizures) during the observation period.
Figure 6. Tamoxifen injection at 2 weeks induces spontaneous seizures in Tsc1GFAP-CreER mice.
Representative EEG recordings of seizure from two Tsc1GFAP-CreER mice injected with tamoxifen at 2 weeks. (B) Representative EEG recordings from two Tsc1GFAP-CreER mice injected with tamoxifen at 6 weeks. (C) A table showing the number and percentage of mice exhibiting spontaneous seizures within 3 months after tamoxifen injection at 2-wk and 6-wk. (D) Quantification of the average seizure frequency for mice with spontaneous seizures over time after tamoxifen injection at 2-wk and 6-wk of age.
DISCUSSION
In this study, we investigated the effect of postnatal Tsc1 gene inactivation on epilepsy utilizing an inducible GFAP-Cre line. The primary finding is that postnatal GFAP-driven Tsc1 gene inactivation at 2 weeks of age, but not 6 weeks, is sufficient to cause epilepsy. While postnatal GFAP expression has been reported to be relatively astrocyte specific 22; 23, our reporter data indicates that both astrocytes and neurons can be affected by GFAP-driven Cre expression postnatally, thus making it difficult to determine whether the epilepsy phenotype is primarily related to astrocyte or neuronal TSC1 reduction. In any case, the epilepsy phenotype with postnatal TSC1 reduction in inducible Tsc1GFAP-CreER mice is much milder compared with prenatal TSC1 reduction in Tsc1GFAP-Cre mice, indicating that embryonic effects of Tsc1 gene inactivation on brain development may be more critical for epileptogenesis in TSC and establishing a temporal window for targeting therapies to prevent or modify epilepsy in TSC.
Conventional (non-inducible) GFAP-driven conditional knock-out mice, which exhibit Cre-expression during the embryonic period, were initially thought to be relatively specific for targeting gene inactivation to astrocytes 15; 26. However, the expression of GFAP in embryonic neural progenitor cells results in substantial involvement of derivative neuronal populations, particularly in hippocampus 21; 27. Thus, while conventional Tsc1GFAP-Cre knockout mice have a number of abnormalities in astrocyte function that likely contribute to epileptogenesis, such as impaired astrocytic glutamate transport and potassium buffering 17–20, concurrent involvement of neurons confounds interpretation of the relative contribution of different cell types to epilepsy in these mice. Some studies suggest that postnatal GFAP expression using inducible-CreER systems may be more specific for targeting mature astrocytes22; 23, but our reporter data indicate that there is still a substantial degree of neuronal involvement with postnatal induction, likely due to persistent expression of GFAP in some neural progenitor cells beyond the embryonic stage. Thus, it is still difficult to determine in the Tsc1GFAP-CreER mice whether the seizures are primarily due to astrocyte or neuronal abnormalities or both.
An additional technical issue is that, in contrast to previous reports of the inducible GFAP-CreER line 22; 23, a significant amount of baseline “leakiness” of Cre-recombination was detected in our Rosa-Green reporter mice studies, particularly involving both cortical and hippocampal astrocytes, but also a small percentage of neurons in hippocampus. However, this degree of baseline Cre-recombination did not appear to have any appreciable functional effects, as GFAP-CreER mice not treated with tamoxifen did not exhibits changes in TSC1 protein expression or mTORC1 activation as reflected by pS6 expression, as well as any increase in GFAP expression itself. Furthermore, this leakiness was minimal at one week of age, indicating that it occurred postnatally after one week, so if anything this would help reinforce the effects of the targeted postnatal taxmoxifen injections. Thus, it is reasonable to conclude that postnatal Tsc1 gene inactivation following tamoxifen was primarily responsible for the subsequent seizure generation in the Tsc1GFAP-CreER mice. However, based on the more severe epilepsy and pathological phenotype of conventional Tsc1GFAP-Cre mice, it appears that prenatal effects of Tsc1 gene inactivation on early brain development are more critical for epileptogenesis in TSC than postnatal effects.
Astrogliosis is one of the pathological features of human tubers mimicked in both Tsc1GFAP-Cre and Tsc1GFAP-CreER mice. While the astrogliosis in Tsc1GFAP mice is diffuse, these neuropathological findings in Tsc1GFAP-CreER mice injected with tamoxifen at two weeks was more focal in distribution, although discrete tuber-like abnormalities were not seen. In addition, other cellular features of tubers, such as neuronal disorganization and giant cells, were not seen. Most mouse models of TSC involving embryonic or postnatal Tsc1 or Tsc2 gene inactivation induce relative diffuse neuropathological abnormalities, with the possible exception of the focal in utero electroporation model, which shows some tuber-like abnormalities10. While the lack of tuber-like abnormalities could be considered a limitation of this and other TSC mouse models, these models still have significant utility for investigating cellular and molecular mechanisms of TSC that may occur independently of tubers28. More refined models combining different spatial and temporal properties of Tsc gene inactivation should continue to lead to more accurate models of human TSC.
Other limitations to this study include the possibility that the methods utilized may not have been optimized to exert maximal effects on Cre-expression and corresponding astrocyte or neuronal phenotypes. In particular, differences in Cre-expression driving pre- and postnatal Tsc1 gene inactivation and the resulting TSC1 protein reduction may confound direct comparisons of phenotypic effects. Additional studies varying the timing or dosing of tamoxifen may reveal additional information about the effects of postnatal TSC1 reduction on epilepsy. A previous study with global postnatal inactivation of Tsc1 demonstrated a severe epilepsy phenotype even in the absence of acute pathological changes, but the Cre-driver likely affected most cell types throughout the body, limiting interpretation of the specificity of this effect on epilepsy29. The use of other Cre-driving promoters, such as Glast or Aldh1L1, may increase astrocyte-specificity in attempting to distinguish the relative contribution of astrocytes and neurons to epilepsy. However, the current study illustrates general, practical issues involved in comparing prenatal and postnatal Tsc1 gene inactivation and corresponding effects on brain phenotypes.
Despite these technical caveats and the uncertain contribution of different cell types, a major finding of this study is the developmental differences of the effect of Tsc1 inactivation in the brain on epileptogenesis. Although postnatal Tsc1 gene inactivation at 2 weeks of age was sufficient to cause epilepsy in about 50% of Tsc1GFAP-CreER mice, the epilepsy phenotype was much milder compared with prenatal Tsc1 gene inactivation, which leads to progressive epilepsy in 100% of Tsc1GFAP-Cre mice 15; 24; 25. Furthermore, postnatal Tsc1 gene inactivation at 6 weeks of age did not induce detectable epilepsy. Interestingly, despite the prenatal gene inactivation in Tsc1GFAP-Cre mice, onset of epilepsy in Tsc1GFAP-Cre mice is delayed until approximately 3–4 weeks of age, indicating a several week period of epileptogenesis following Tsc1 gene inactivation. The strong developmental dependence highlights the critical importance of prenatal versus postnatal Tsc1 gene inactivation on epileptogenesis and establishes the temporal window of opportunity for potential disease-modifying interventions. As TSC has been viewed as a feasible model disorder for developing anti-epileptogenic therapies for preventing epilepsy, such as with mTOR inhibitors 5, these findings have direct translational applications for the timing of targeted treatments directed at specific stages of epileptogenesis. Clinical trials of early intervention, such as with vigabatrin, in TSC infants even before the onset of epilepsy are already underway30. As tubers are formed during prenatal brain development, preventative therapies for epilepsy in TSC may actually need to be delivered prenatally to be most effective, as suggested by the more severe phenotype of prenatal TSC1 protein reduction in Tsc1GFAP-Cre mice. However, as epileptogenesis also occurred to a less severe degree with postnatal TSC1 reduction in Tsc1GFAP-CreER mice, this suggests that even later, postnatal treatment has antiepileptogenic potential. Future experiments in the postnatal Tsc1GFAP-CreER mice testing the timing of therapeutic interventions, particularly with rapamycin, should help define more specifically the critical window for preventative antiepileptogenic and direct antiseizure effects at different development stages of TSC.
Supplementary Material
KEY POINTS.
Postnatal Tsc1 inactivation in both astrocytes and neurons is sufficient to cause astrogliosis and mild epilepsy in Tsc1GFAP-CreER mice.
However, the phenotype with postnatal Tsc1 inactivation is much less severe than prenatal Tsc1 inactivation in conventional Tsc1GFAP-Cre mice.
Developmental timing of Tsc1 loss dictates the severity of neuronal and glial abnormalities and resulting epilepsy
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01 NS056872 to MW) and by the Alafi Neuroimaging Lab (S10 RR027552) and the Intellectual and Developmental Disabilities Research Center (U54 HD087011) at Washington University.
Footnotes
DISCLOSURES
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Orlova KA, Crino PB. The tuberous sclerosis complex. Ann N Y Acad Sci. 2010;1184:87–105. doi: 10.1111/j.1749-6632.2009.05117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiMario FJ, Jr, Sahin M, Ebrahimi-Fakhari D. Tuberous Sclerosis Complex. Pediatr Clin North Am. 2015;62:633–648. doi: 10.1016/j.pcl.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Wong M. Mechanisms of epileptogenesis in tuberous sclerosis complex and related malformations of cortical development with abnormal glioneuronal proliferation. Epilepsia. 2008;49:8–21. doi: 10.1111/j.1528-1167.2007.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krueger DA, Wilfong AA, Holland-Bouley K, et al. Everolimus treatment of refractory epilepsy in tuberous sclerosis complex. Ann Neurol. 2013;74:679–687. doi: 10.1002/ana.23960. [DOI] [PubMed] [Google Scholar]
- 5.Wong M. A critical review of mTOR inhibitors and epilepsy: from basic science to clinical trials. Expert Rev Neurother. 2013;13:657–669. doi: 10.1586/ern.13.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.French JA, Lawson JA, Yapici Z, et al. Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): a phase 3, randomised, double-blind, placebo-controlled study. Lancet. 2016;388:2153–2163. doi: 10.1016/S0140-6736(16)31419-2. [DOI] [PubMed] [Google Scholar]
- 7.Ruppe V, Dilsiz P, Reiss CS, et al. Developmental brain abnormalities in tuberous sclerosis complex: a comparative tissue analysis of cortical tubers and perituberal cortex. Epilepsia. 2014;55:539–550. doi: 10.1111/epi.12545. [DOI] [PubMed] [Google Scholar]
- 8.Marcotte L, Aronica E, Baybis M, et al. Cytoarchitectural alterations are widespread in cerebral cortex in tuberous sclerosis complex. Acta Neuropathol. 2012;123:685–693. doi: 10.1007/s00401-012-0950-3. [DOI] [PubMed] [Google Scholar]
- 9.Carson RP, Van Nielen DL, Winzenburger PA, et al. Neuronal and glia abnormalities in Tsc1-deficient forebrain and partial rescue by rapamycin. Neurobiol Dis. 2012;45:369–380. doi: 10.1016/j.nbd.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feliciano DM, Su T, Lopez J, et al. Single-cell Tsc1 knockout during corticogenesis generates tuber-like lesions and reduces seizure threshold in mice. J Clin Invest. 2011;121:1596–1607. doi: 10.1172/JCI44909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goto J, Talos DM, Klein P, et al. Regulable neural progenitor-specific Tsc1 loss yields giant cells with organellar dysfunction in a model of tuberous sclerosis complex. Proc Natl Acad Sci U S A. 2011;108:E1070–1079. doi: 10.1073/pnas.1106454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magri L, Cominelli M, Cambiaghi M, et al. Timing of mTOR activation affects tuberous sclerosis complex neuropathology in mouse models. Dis Model Mech. 2013;6:1185–1197. doi: 10.1242/dmm.012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Way SW, McKenna J, 3rd, Mietzsch U, et al. Loss of Tsc2 in radial glia models the brain pathology of tuberous sclerosis complex in the mouse. Hum Mol Genet. 2009;18:1252–1265. doi: 10.1093/hmg/ddp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meikle L, Talos DM, Onda H, et al. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci. 2007;27:5546–5558. doi: 10.1523/JNEUROSCI.5540-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uhlmann EJ, Wong M, Baldwin RL, et al. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol. 2002;52:285–296. doi: 10.1002/ana.10283. [DOI] [PubMed] [Google Scholar]
- 16.Zeng LH, Rensing NR, Zhang B, et al. Tsc2 gene inactivation causes a more severe epilepsy phenotype than Tsc1 inactivation in a mouse model of tuberous sclerosis complex. Hum Mol Genet. 2011;20:445–454. doi: 10.1093/hmg/ddq491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong M, Ess KC, Uhlmann EJ, et al. Impaired glial glutamate transport in a mouse tuberous sclerosis epilepsy model. Ann Neurol. 2003;54:251–256. doi: 10.1002/ana.10648. [DOI] [PubMed] [Google Scholar]
- 18.Jansen LA, Uhlmann EJ, Crino PB, et al. Epileptogenesis and reduced inward rectifier potassium current in tuberous sclerosis complex-1-deficient astrocytes. Epilepsia. 2005;46:1871–1880. doi: 10.1111/j.1528-1167.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 19.Xu L, Zeng LH, Wong M. Impaired astrocytic gap junction coupling and potassium buffering in a mouse model of tuberous sclerosis complex. Neurobiol Dis. 2009;34:291–299. doi: 10.1016/j.nbd.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang B, Zou J, Rensing NR, et al. Inflammatory mechanisms contribute to the neurological manifestations of tuberous sclerosis complex. Neurobiol Dis. 2015;80:70–79. doi: 10.1016/j.nbd.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fraser MM, Zhu X, Kwon CH, et al. Pten loss causes hypertrophy and increased proliferation of astrocytes in vivo. Cancer Res. 2004;64:7773–7779. doi: 10.1158/0008-5472.CAN-04-2487. [DOI] [PubMed] [Google Scholar]
- 22.Casper KB, Jones K, McCarthy KD. Characterization of astrocyte-specific conditional knockouts. Genesis. 2007;45:292–299. doi: 10.1002/dvg.20287. [DOI] [PubMed] [Google Scholar]
- 23.Chow LM, Zhang J, Baker SJ. Inducible Cre recombinase activity in mouse mature astrocytes and adult neural precursor cells. Transgenic Res. 2008;17:919–928. doi: 10.1007/s11248-008-9185-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng LH, Xu L, Gutmann DH, et al. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erbayat-Altay E, Zeng LH, Xu L, et al. The natural history and treatment of epilepsy in a murine model of tuberous sclerosis. Epilepsia. 2007;48:1470–1476. doi: 10.1111/j.1528-1167.2007.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brenner M, Kisseberth WC, Su Y, et al. GFAP promoter directs astrocyte-specific expression in transgenic mice. J Neurosci. 1994;14:1030–1037. doi: 10.1523/JNEUROSCI.14-03-01030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casper KB, McCarthy KD. GFAP-positive progenitor cells produce neurons and oligodendrocytes throughout the CNS. Mol Cell Neurosci. 2006;31:676–684. doi: 10.1016/j.mcn.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Wong M. The utility of tuberless models of tuberous sclerosis. Epilepsia. 2007;48:1629–1630. doi: 10.1111/j.1528-1167.2007.01178_1.x. author reply 1632–1624. [DOI] [PubMed] [Google Scholar]
- 29.Abs E, Goorden SM, Schreiber J, et al. TORC1-dependent epilepsy caused by acute biallelic Tsc1 deletion in adult mice. Ann Neurol. 2013;74:569–579. doi: 10.1002/ana.23943. [DOI] [PubMed] [Google Scholar]
- 30.Jozwiak S, Kotulska K, Domanska-Pakiela D, et al. Antiepileptic treatment before the onset of seizures reduces epilepsy severity and risk of mental retardation in infants with tuberous sclerosis complex. Eur J Paediatr Neurol. 2011;15:424–431. doi: 10.1016/j.ejpn.2011.03.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.