Summary
Background
Increased expression of vitronectin (VN) by smooth muscle cells (SMCs) promotes neointima formation after vascular injury and may contribute to chronic vascular diseases, such as atherosclerosis. However, the molecular regulation of vascular VN expression is poorly defined. Given the overlapping expression profiles and functions of VN and plasminogen activator inhibitor-1 (PAI-1), we hypothesized that PAI-1 regulates vascular VN expression.
Objectives
Determine whether PAI-1 regulates VN expression in SMCs and in vivo.
Methods
Effects of genetic alterations in PAI-1 expression, pharmacologic PAI-1 inhibition, and recombinant PAI-1 on SMC VN expression were studied and vascular VN expression in wild-type (WT) and PAI-1-deficient mice was assessed.
Results
VN expression was significantly lower in PAI-1-deficient SMCs and significantly increased in PAI-1-over-expressing SMCs. PAI-1 siRNA and pharmacological PAI-1 inhibition significantly decreased SMC VN expression. Recombinant PAI-1 stimulated VN expression by binding LDL receptor-related protein-1 (LRP1), but another LRP1 ligand, α2-macroglobulin, did not. Compared to WT controls, carotid artery VN expression was significantly lower in PAI-1-deficient mice and significantly higher in PAI-1-transgenic mice. In a vein graft (VG) model of intimal hyperplasia, VN expression was significantly attenuated in PAI-1-deficient VGs compared to WT controls. Plasma VN concentration was significantly decreased in PAI-1-deficient mice vs. WT controls at 4 weeks, but not at 5 days or 8 weeks, after surgery.
Conclusions
PAI-1 stimulates SMC VN expression by binding LRP1 and controls vascular VN expression in vivo. Autocrine regulation of vascular VN expression by PAI-1 may play important roles in vascular homeostasis and pathological vascular remodeling.
Keywords: muscle, smooth, vascular; plasminogen inactivators; serine proteinase inhibitors; vascular remodeling; vitronectin
Vitronectin (VN) is an approximately 75 kDa acute-phase-reactant plasma protein that is synthesized predominantly in the liver [1]. VN binds plasminogen activator inhibitor-1 (PAI-1), a serpin superfamily member that is the primary inhibitor of tissue-type plasminogen activator (tPA) and urinary-type PA (uPA) [2], which stabilizes PAI-1 in its active conformation and inhibits fibrinolysis [3, 4]. In addition to the plasma, VN is present in the extracellular matrix (ECM) of blood vessel walls and many other tissues, where it regulates cell adhesion and proteolysis through binding interactions with cell surface receptors and protease inhibitors, respectively [1]. VN also binds to structural proteins in the ECM, including collagen and elastin [5, 6]. Vascular smooth muscle cells (SMCs) express VN [7]. Under pathological conditions, vascular wall expression of VN increases significantly, which promotes intimal hyperplasia and vascular inflammation [8]. Vascular VN expression has also been implicated in the pathogenesis of atherosclerosis [9–11]. Similar to VN, PAI-1 expression in the blood vessel wall increases in diseases characterized by vascular intimal hyperplasia, including diabetes mellitus and atherosclerosis [12, 13]. The binding interaction between VN and PAI-1 not only stabilizes PAI-1, but also regulates VN function, as PAI-1 competitively blocks binding of VN to αVβ3 integrin and the uPA receptor (uPAR), which are expressed by SMCs, producing an anti-migratory effect [14–16]. PAI-1 also induces VN multimerization [17].
Given VN’s important vascular functions, regulation of VN expression in the wall of blood vessels is likely to have pathophysiological significance. Inflammatory signaling pathways have been shown to increase VN expression [18]. However, the control of vascular VN expression remains poorly understood. In a previous study we demonstrated that the stoichiometric relationship between VN and PAI-1 in the ECM plays a key role in determining the effects of PAI-1 on SMC migration [19]. Given that PAI-1 and VN regulate each other’s functions, SMCs express both VN and PAI-1 [7, 20], and PAI-1/VN stoichiometry has physiological significance, we hypothesized that PAI-1 regulates VN expression by SMCs and controls vascular VN expression in vivo. We tested our hypothesis by analyzing the effects of genetic alterations in PAI-1 expression, recombinant PAI-1 proteins, and a pharmacological PAI-1 inhibitor on expression of VN by SMCs grown in culture, and by analysis of vascular wall and plasma expression of VN in mice. Our results suggest that PAI-1 has a previously unrecognized role in regulating the expression and distribution of VN in the vasculature.
Materials and Methods
Animals
C57BL/6J mice were from Jackson Labs. C57BL/6J-congenic PAI-1-deficient (pai1−/−) mice were a gift from Dr. Peter Carmeliet, University of Leuven, Leuven, Belgium [21]. C57BL/6J-congenic VN-deficient (vn−/−) mice and PAI-1-transgenic (Tg) mice that over-express PAI-1 under the control of the CMV promoter were from Dr. David Ginsburg, University of Michigan [22, 23]. Mice received standard chow. All animal care and experimental procedures were approved by the University of Missouri Animal Care and Use Committee.
Recombinant PAI-1s and PAI-1 inhibitor
Recombinant, active murine PAI-1 (MPAI) was from Molecular Innovations. Recombinant human PAI-1 mutants were a gift from Dan Lawrence, PhD, University of Michigan and were expressed and purified as described previously [24]. PAI-1 mutants were 1) PAI-1-14-1B (PAI-1 N150H, K154T, Q319L, and M354I), an active, stable mutant (half-life of >140 hours under physiological conditions) which binds VN with normal affinity [25], 2) PAI-1-AK (PAI-1 N150H, K154T, Q319L, M354I, R101A, Q123K), an active, stable mutant with no detectable VN binding [26], and 3) PAI-1-E (PAI-1 N150H, K154T, Q319L, M354I, R76E), an active stable mutant with markedly reduced binding affinity for low-density-lipoprotein receptor-related protein 1 (LRP1), but normal VN binding affinity [19]. PAI-039, a pharmacological inhibitor of PAI-1, was from Pfizer [27].
Cell culture
SMCs were isolated from mouse aortas and cell culture lines were established as described previously [28]. SMCs (2×105) were seeded in 6-well cell culture plates in DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS) and grown to about 80% confluency, then medium was removed and serum-free DMEM/F12 was added to wells. Cells were treated with recombinant PAI-1, PAI-039, or α2-macroglobulin-trypsin complex for 4–24 hrs, after which cells were washed and harvested for analysis. Complexes of human α2-macroglobulin (Sigma-Aldrich, Cat. No. 63013, St. Louis, MO, USA) and trypsin (Sangon Biotech, Shanghai, China) were prepared and treated with soybean trypsin inhibitor (Sangon Biotech) to inactive free trypsin, as described [29, 30]. In some experiments, SMCs were pre-incubated with a rabbit anti-LRP1 antibody (R2629, 100 µg/mL, kindly provided by Dudley Strickland, PhD, U. Maryland) prior to addition of PAI-1. R2629 does not cross react with any other known LDL receptor family member [31].
Quantitative reverse-transcriptase PCR (qRT-PCR)
Total RNA was isolated from cells and tissues using TRIzol (Thermo Fisher Scientific, Waltham, MA, USA), according to manufacturer’s instructions. cDNA was synthesized with Prime Script RT reagent kit (Takara Bio, Mountain View, CA, USA), subjected to qRT-PCR using the SYBR Green method, and quantified by the 2−ΔΔCT method, as described previously [32]. For amplification of murine PAI-1, the sequences 5'- GCTGCAGATGACCACAGCGGG -3' and 5'- CCGCAGTACTGATCTCATTC -3' were used as forward and reverse primers, respectively. For amplification of murine VN, the sequences 5'-CCCCTGAGGCCCTTTTTCATA -3' and 5'-CAAAGCTCGTCACACTGACA -3' were used. For amplification of β-actin, the sequences 5'-AAGAGCTATGAGCTGCCTGA-3' and 5'- TACGGATGTCAACGTCACAC -3' were used.
Western blotting
SMCs were lysed by suspending them in RIPA buffer (Thermo Fisher Scientific). Lysates were centrifuged and supernatants were collected. Samples (25 µg total protein, determined by BCA reagent [Thermo Fisher Scientific]) were subjected to SDS-PAGE (using 4–20% gradient acrylamide gels) and transferred to PVDF membrane (BioRad, CA, USA). After blocking, membranes were incubated with rabbit antibodies raised against murine PAI-1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), VN (Cell Signaling Technology, Danvers, MA), and β-actin (Cell Signaling Technology). Secondary antibody was horseradish-peroxidase (HRP)-conjugated goat IgG raised against rabbit or mouse IgG (Santa Cruz Biotechnology). Blots were developed with ECL substrate (Thermo Fisher Scientific). Image J software was used to quantify band intensity.
ELISA
Concentration of VN in plasma was measured with Mouse Vitronectin Elisa Kit (Molecular Innovations, Novi, MI, USA) according to manufacturer’s instructions.
Silencing of PAI-1 gene expression
Murine SMCs were transfected with siRNA duplexes (5nM) directed against PAI-1 or negative control siRNAs (obtained from Qiagen, Hilden, Germany) using HiPerFect reagent (Qiagen), according to the manufacturer’s instructions. Cells were harvested 24 h after transfection.
In vivo analyses of VN expression
Adult male mice were anesthetized. Blood was collected by cardiac puncture into citrate anticoagulant and platelet-poor plasma was prepared and frozen. The vasculature was perfused with saline. Common carotid arteries were excised, and total cellular RNA was extracted from one artery using TRIzol, according to the manufacturer’s instructions. Proteins were extracted from the other carotid artery by cutting it into small pieces, placing them in RIPA buffer, and grinding them with a hand-held tissue homogenizer. Homogenates were centrifuged and supernatants were prepared by centrifugation and saved for Western blot analysis. To study VN expression under pathological conditions, a vein graft model of intimal hyperplasia was utilized, which involves transplant of a segment of inferior vena cava (IVC) from a donor mouse into the carotid artery of a recipient mouse [33]. Blood samples were collected from mice at 5 days, 4 weeks, and 8 weeks after surgery and citrated plasma was prepared and frozen. Vein grafts were harvested 4 weeks after surgery. Cross-sections (5 µm thickness) were mounted on glass slides, de-paraffinized, hydrated, incubated 10 minutes in methanol containing 3% H2O2, and rinsed. VN immunostaining in vein grafts was performed using a rat anti-mouse VN monoclonal antibody (MAB3875, R&D Systems, Inc.) and horseradish-peroxidase-conjugated broad spectrum secondary antibody (Histomouse-MAX Kit, Invitrogen). As a negative control, immunostaining was also performed by substituting non-immune rat IgG for anti-VN IgG. Identical, simultaneously performed immunostaining techniques (i.e. antibody dilutions, incubation and wash times) were used for all samples.
Statistical analyses
All experiments were performed at least in triplicate, with results reported as mean ± standard error of mean. Student’s t-test or one-way analysis of variance (ANOVA) was used to compare experimental groups, as appropriate.
Results
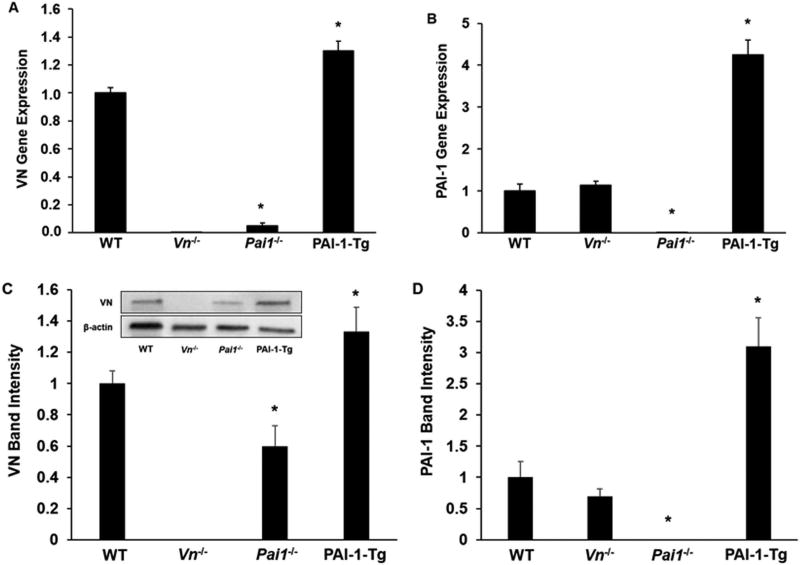
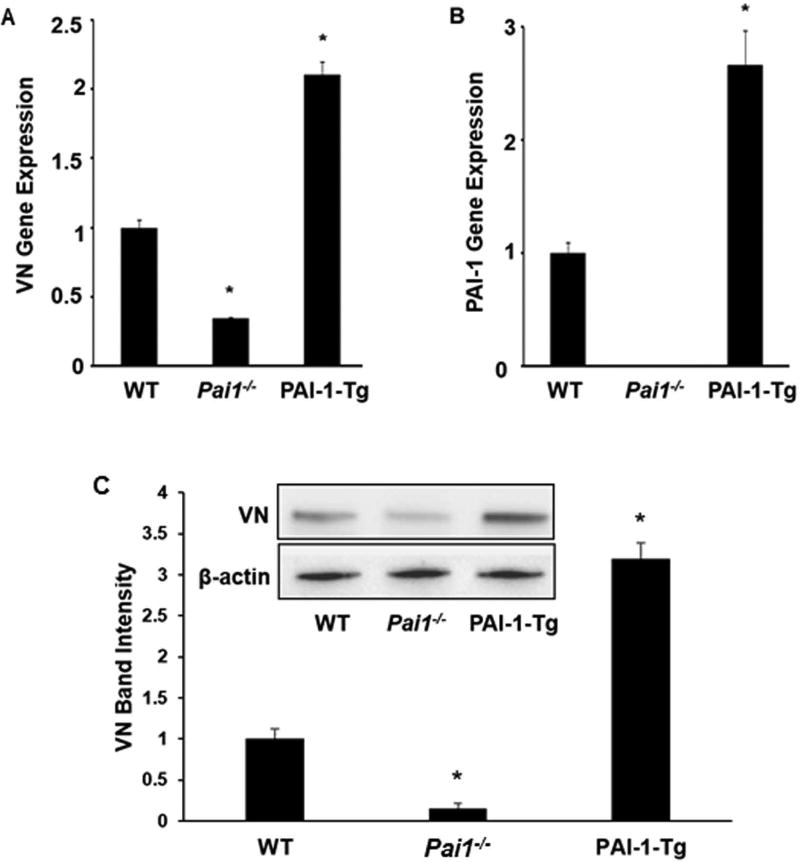
VN gene expression and protein concentration are decreased in PAI-1-deficient SMCs. We used qRT-PCR to study VN gene expression in murine SMCs grown in culture. VN gene expression was markedly decreased in PAI-1-deficient SMCs compared to wild-type (WT) controls (Fig. 1A). Conversely, PAI-1 gene expression was not significantly altered in VN-deficient SMCs compared to WT controls (Fig. 1B). VN gene expression was significantly increased in PAI-1-Tg SMCs compared to WT controls (Fig. 1A). Western blot analysis of SMC lysates confirmed that VN protein concentration was significantly reduced in PAI-1 deficient SMCs compared to WT controls, while the difference in VN protein concentration in lysates prepared from PAI-1-Tg vs. WT SMCs did not achieve statistical significance (Fig. 1C). Consistent with the gene expression data, PAI-1 protein content did not differ significantly between WT and VN-deficient SMCs (Fig. 1D). Overall, these results suggested that genetic alterations in PAI-1 expression significantly alter VN expression in vascular SMCs.
Figure 1.
PAI-1 regulates vitronectin (VN) expression in vascular SMCs. VN and PAI-1 gene expression and protein content in cultured SMCs isolated from mice with the indicated genotypes were assessed by qRT-PCR and Western blotting, respectively. (A) VN gene expression. (B) PAI-1 gene expression. (C) VN protein content, assessed by densitometric analysis of band densities. Inset shows results of a representative Western blot. (D) PAI-1 protein content. Data are expressed as fold-control (WT SMCs) and are from 3 independent experiments. *P<0.05 vs. WT SMCs. PAI-1-Tg = PAI-1-transgenic.
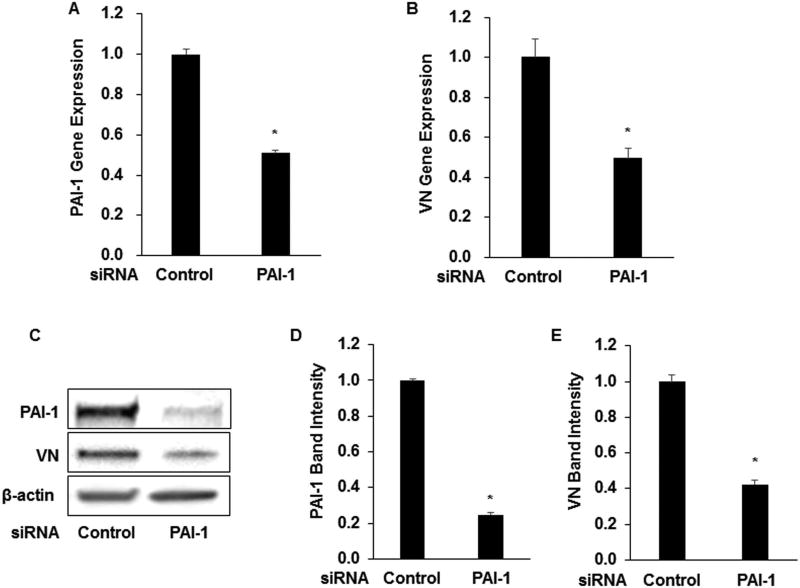
Silencing of PAI-1 gene expression decreases VN gene expression in SMCs. We treated murine SMCs grown in culture with PAI-1 siRNA. This intervention, which resulted in an approximately 50% reduction in PAI-1 gene expression and an approximately 70% reduction in PAI-1 protein concentration compared to negative control siRNA (Fig. 2A, C–D), was accompanied by a similar reduction in VN gene expression and protein concentration (Fig. 2B–C, E). These results suggested that a short-term reduction in PAI-1 expression decreases VN expression by SMCs.
Figure 2.
Silencing of PAI-1 gene expression reduces SMC VN gene expression and protein content. Murine wild-type SMCs were transfected with PAI-1 siRNA expression plasmid or negative control plasmid. Gene expression was assessed by qRT-PCR and protein content was assessed by Western blotting. (A) PAI-1 gene expression. (B) VN gene expression. (C) Representative Western blot. (D–E) Densitometric analysis of PAI-1 and VN band density, respectively. Data are mean of 3 independent experiments and are expressed as fold-control. *P<0.05 vs. control.
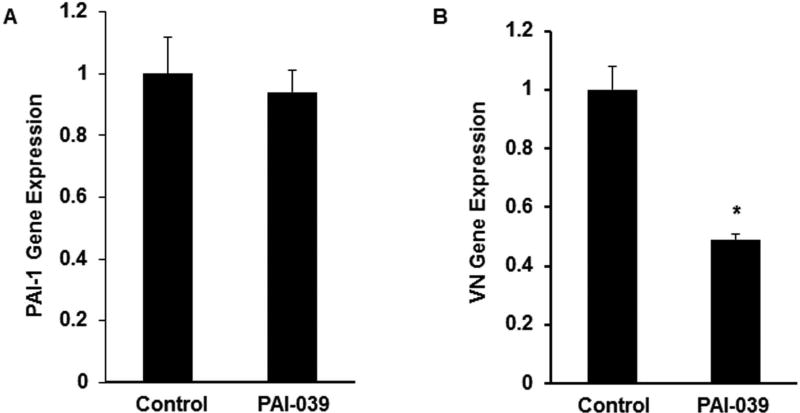
Pharmacological inhibition of PAI-1 decreases VN expression. Murine SMCs were grown in culture and allowed to achieve approximately 80% confluency. PAI-039 (25 µM), a highly specific PAI-1 inhibitor, or vehicle control was added and cells were incubated for an additional 24 hrs, after which total cellular RNA was isolated and analyzed by RT-PCR. PAI-039 significantly decreased VN gene expression without inhibiting PAI-1 gene expression (Fig. 3), suggesting that PAI-1 anti-protease activity was required for stimulation of VN gene expression.
Figure 3.
Pharmacological inhibition of PAI-1 with PAI-039 decreases VN gene expression by SMCs. Wild-type SMCs were incubated in the absence (control) or presence of PAI-039 (25 µM) for 24 hrs, after which (A) PAI-1 and (B) VN gene expression were measured by qRT-PCR. Results are mean of 3 experiments and are expressed as fold-control. *P<0.05 vs. control.
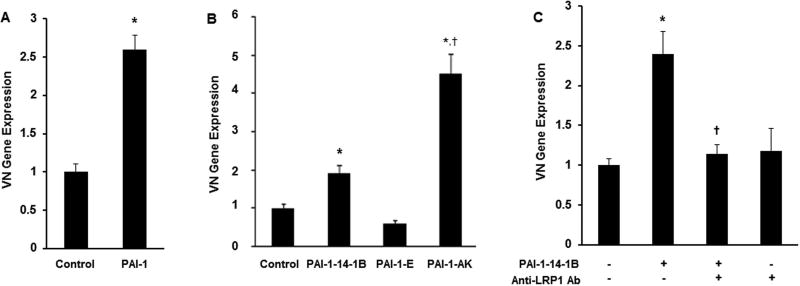
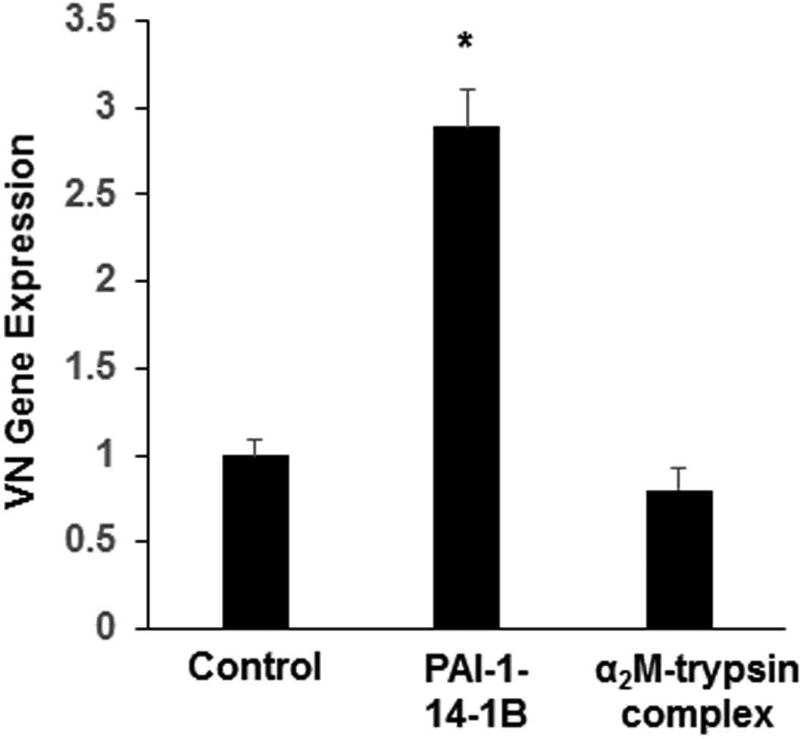
Recombinant PAI-1 stimulates SMC VN expression by binding to LRP1. Addition of recombinant PAI-1 to cell culture medium stimulated VN gene expression in wild-type mouse SMCs (Fig. 4A). To study the mechanism underlying this effect, we treated PAI-1-deficient murine SMCs with recombinant human PAI-1 mutants. PAI-1-14-1B, a stable mutant with intact anti-protease and VN binding functions, significantly stimulated VN expression (Fig. 4B). PAI-1-AK, an active mutant deficient in VN binding function, also significantly increased VN expression, producing an effect that was significantly greater than that of PAI-1-14-1B. However, PAI-1-E, an active mutant defective in binding to LRP1 and other LDL receptor family members, did not stimulate SMC VN expression. R2629, a specific anti-LRP1 antibody that does not recognize other LDL receptor family members [31], completely blocked the capacity of PAI-1-14-1B to stimulate VN expression (Fig. 4C). The capacity of another LRP1 ligand, α2-macroglobulin, to stimulate VN expression was studied by incubating SMCs with complexes composed of α2-macroglobulin and trypsin, which bind LRP1 and induce intracellular signaling [34, 35]. Unlike PAI-1, α2-macroglobulin-trypsin complex had no significant effect on VN expression by SMCs (Fig. 5). Together, these results suggested that 1) the stimulatory effect of extracellular PAI-1 on SMC VN expression is mediated by binding to LRP1, 2) endogenous expression of PAI-1 is not required to stimulate SMC VN expression, and 3) not all LRP1 ligands enhance VN expression by SMCs.
Figure 4.
PAI-1 increases SMC VN expression by binding to LRP1. (A) Wild-type PAI-1 stimulates SMC VN expression. Murine wild-type SMCs were incubated with recombinant murine PAI-1 for 4 hrs, after which cells were washed, harvested, and VN gene expression was determined by qRT-PCR. (B) PAI-1 mutant defective in LRP1 binding does not stimulate VN expression. PAI-1-deficient SMCS were incubated with PAI-1-14-1B (stable mutant), PAI-1-E (defective in LRP1 binding), or PAI-1-AK (defective in VN binding) for 4 hrs (concentration of each mutant was 1 µg/mL; control SMCs were incubated in absence of PAI-1), after which VN gene expression was measured by qRT-PCR. (C) Anti-LRP1 antibody (Ab) blocks enhancement of VN expression by PAI-1-14-1B. Murine wild-type SMCs were incubated in the presence (+) or absence (−) of PAI-1-14-1B (1 µg/mL) or anti-LRP1 antibody (100 µg/mL) for 4 hrs, after which cells were washed, harvested, and VN gene expression was determined by qRT-PCR. All data shown are mean of 3 experiments and are expressed as fold-control (left-most bar in each figure). *P < 0.05 vs. control; †P<0.05 vs. PAI-1-14-1B.
Figure 5.
α2-Macroglobulin (α2M) does not stimulate SMC VN expression. Cultured murine SMCs were incubated with PAI-1-14-1B (21.3 nM), α2-macroglobulin-trypsin complex (100 nM), or vehicle control for 4 hrs, after which cells were washed and harvested and VN gene expression was determined by qRT-PCR. Data are mean of triplicate experiments. *P<0.05 vs. control.
PAI-1 regulates VN expression in vivo. To examine the in vivo significance of our findings, we compared VN expression in blood vessels from wild-type mice, PAI-1-deficient mice, and PAI-1-Tg mice. There were significantly lower levels of VN mRNA and protein in carotid arteries of PAI-1-deficient mice compared to those of wild-type controls, and significantly higher levels of VN mRNA and protein in carotid arteries of PAI-1-Tg mice compared to wild-type controls (Fig. 6A, C). Quantitative RT-PCR analysis confirmed that PAI-1 gene expression was increased in carotid arteries of PAI-1-Tg mice compared to wild-type controls and absent in pai1−/− mice (Fig. 6B).
Figure 6.
PAI-1 regulates VN gene expression in the carotid artery. Total RNA and protein were extracted from murine common carotid arteries. VN and PAI-1 gene expression and protein content were assessed by qRT-PCR and Western blotting, respectively. (A) VN gene expression. (B) PAI-1 gene expression. (C) VN protein content, assessed by band densitometry. Inset is a representative Western blot of carotid artery extracts prepared from one mouse of each genotype. Data in all graphs are derived from 3 mice/group and are expressed as fold-control (wild-type mice = WT). PAI-1-transgenic mice = PAI-1-Tg. *P<0.05 vs. WT group.
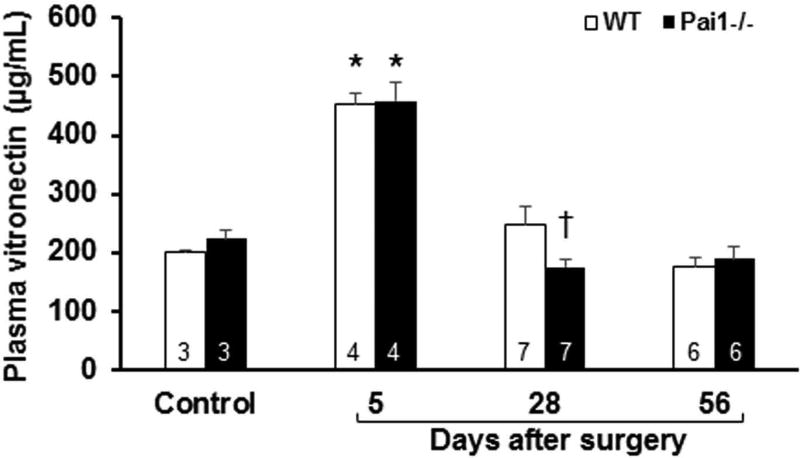
To examine the effects of PAI-1 on VN expression under a clinically relevant form of acute stress, we subjected mice to vein graft surgery, which involves interposition of a segment of vena cava harvested from a donor mouse into the transected carotid artery of a recipient mouse [33, 36] Plasma VN in recipient wild-type mice 5 days after surgery was significantly higher than in non-operated wild-type controls (Fig. 7), consistent with the acute-phase regulation of VN expression [18]. At this early time point after surgery there was no significant difference in plasma VN concentration between WT mice and PAI-1-deficient mice. However, at 4 weeks after surgery, plasma VN was significantly higher in WT mice compared to PAI-1-deficient mice. At 8 weeks after surgery, plasma VN was similar to that found in non-operated controls and did not differ significantly between WT mice and PAI-1-deficient mice.
Figure 7.
Effect of PAI-1 genotype on plasma VN concentration after surgery. VN concentration in plasma was measured by ELISA in non-operated mice (control) and in mice after vein graft surgery, at the indicated time points. Number of mice in each group are shown. All data shown are from mice that received vein grafts from donor mice with the same genotype (i.e. wild-type [WT] vein segments were transplanted into WT mice and PAI-1-deficient vein segments were transplanted into pai1−/− mice). *P<0.05 vs. control mice of same genotype. †P<0.05 vs. WT mice at same time point.
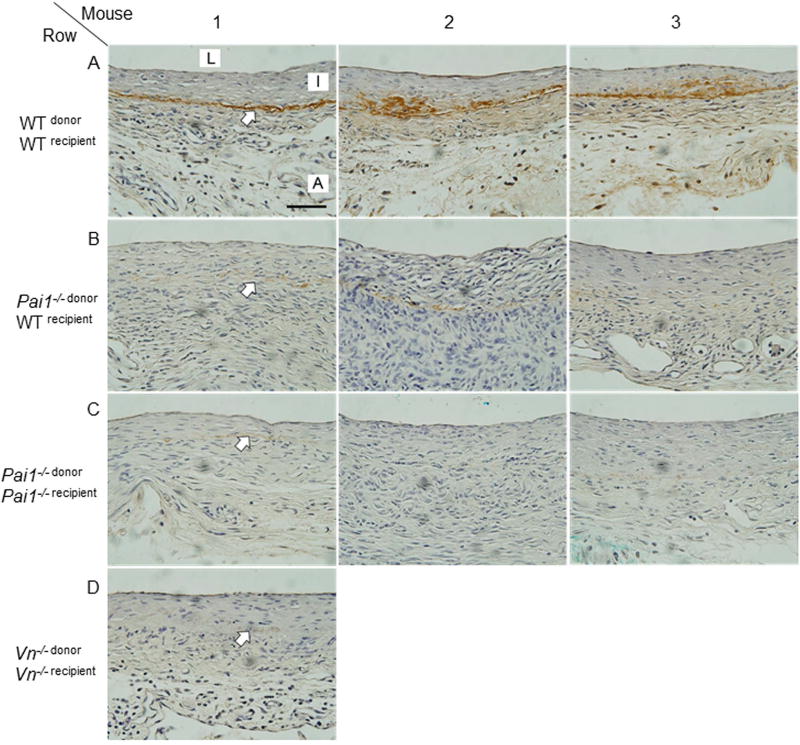
Immunohistochemistry was used to assess VN expression and localization in vein grafts retrieved 4 weeks after surgery. In WT mice VN was localized in the interface of the intimal and medial vascular layers (Fig. 8, row A). In contrast, VN protein concentration in neointimal lesions was significantly less in PAI-1-deficient mice (Fig. 8, row C). To further explore the role of PAI-1 in regulating VN expression in vein grafts, we performed surgeries involving interposition of PAI-1-deficient vein segments into the carotid arteries of WT mice to generate Pai1−/− donor/WTrecipient mice. VN expression in vein grafts harvested 4 weeks after surgery was significantly reduced in Pai1−/− donor/WTrecipient mice (Fig. 8, row B) compared to WTdonor/WTrecipient mice and similar to that observed in PAI-1-deficient (i.e. Pai1−/− donor/Pai1−/− recipient) mice. As a whole, these in vivo results support an important role of PAI-1 in regulating VN expression in plasma and the vascular wall.
Figure 8.
PAI-1 deficiency decreases VN content of murine vein grafts. Vein grafts were harvested 4 weeks after surgery and subjected to VN immunostaining. Vein grafts harvested from 3 mice (numbered 1, 2, and 3) from each group are shown in each of rows A–C. Genotypes of vein segment donor and recipient mice are indicated for each group. Row D shows results of a single Vn−/− donor/Vn−/− recipient mouse (negative control). L, lumen; I, intima, A, adventitia. Arrows denote interface of intima and media, where VN immunostaining (brick red color) localizes. Scale bar = 50 µm.
Discussion
VN expression increases in blood vessels after injury, which supports neointimal growth [8]. However, the factors that regulate vascular VN expression are poorly defined. In this study we performed a series of in vitro and in vivo experiments to study the effects of PAI-1 on the regulation of VN expression in the vasculature. Our in vitro experiments focused on SMCs, which express PAI-1 and VN and play key roles in normal vascular function and pathological vascular remodeling. Our results demonstrate that PAI-1 regulates VN gene expression. To our knowledge, a role of PAI-1 in controlling gene expression has not been reported previously. However, there is a precedent for regulation of gene expression by a serpin family member, as antithrombin III has been shown to significantly down-regulate expression of host cell signal transduction factors, most notably bone morphogenetic protein 2 (BMP2) [37].
PAI-1 and VN are mutually dependent on each other for their function. VN stabilizes PAI-1 in an active conformation [3, 4]. PAI-1 controls the cell adhesion function of VN by competitively blocking its binding to αVβ3 integrin and uPAR [16]. Furthermore, the stoichiometric relationship of PAI-1 and VN plays an important role in controlling SMC migration [19]. Hence, the existence of systems for coordinated expression of VN and PAI-1, including an autocrine loop by which PAI-1 released by SMCs regulates their production of VN, would not be unexpected. The regulation of VN expression by PAI-1 has the potential to have considerable significance in vascular disorders, including chronic diseases characterized by over-expression of PAI-1, such as diabetes mellitus and atherosclerosis [12, 13]. In addition, short-term increases in PAI-1 concentration in the vasculature, as occurs with thrombosis and balloon angioplasty [38, 39], may contribute to the surge in VN expression after vascular injury that contributes to neointima formation [8].
Our data with recombinant PAI-1 mutants and anti-LRP1 antibody suggest that binding of PAI-1 to LRP1 is required to enhance VN expression. Binding of PAI-1 to LRP1 activates intracellular signaling pathways that regulate gene expression, including JAK/STAT1 signaling [32, 40]. Of note, we observed significantly greater stimulation of VN gene expression by PAI-1-AK, which does not bind VN, as compared to PAI-1-14-1B, which is most likely explained by the recognized capacity of VN to down-regulate binding of PAI-1 to LRP1 [41]. PAI-1’s capacity to signal via LRP1 is complex and can involve binding of PAI-1 to uPA, the latter of which may be bound to its receptor, uPAR [42]. Binding of PAI-1 to uPA induces a conformational change in PAI-1 that exposes its binding site for LRP1 [43]. uPAR can bind directly to LRP1 and to integrins, which can trigger intracellular signaling [44, 45]. The macro-complex of LRP1, PAI-1, uPA, uPAR, and integrin undergoes endocytosis, with targeting of PAI-1 and u-PA to endosomes for degradation and recycling of LRP1, uPAR, and integrin to the cell membrane. In addition to controlling cell adhesion and migration, this LRP1-mediated endocytic process is accompanied by activation of intracellular signaling pathways that regulate cell migration and other functions [42]. While we did not measure uPA in our experiments, uPA is expressed by SMCs [46] and likely was present in our cell culture studies. Our experiments did not resolve whether PAI-1 must be internalized to stimulate VN expression, nor did they determine if PAI-1 acts via an intracellular mechanism in up-regulating VN expression. However, our experiments involving addition of recombinant PAI-1 to PAI-1-deficient SMCs demonstrate that cell synthesis of PAI-1 is not required for PAI-1 to stimulate VN expression and support an autocrine mechanism by which secreted PAI-1 binds LRP1 and activates an outside-in signaling pathway that up-regulates VN gene expression. Additional studies will be needed to clarify the precise molecular signaling events that mediate PAI-1’s effects on VN expression. It also will be of interest to study the effects of LRP1’s many other ligands on VN expression. Our finding that α2-macroglobulin, which binds and is endocytosed by LRP1 [34], does not stimulate SMC VN expression suggests that there are significant differences in VN expression in response to different LRP1 ligands.
There is growing interest in the potential of pharmacologic PAI-1 inhibitors to treat and prevent vascular diseases [47, 48]. We have shown that pharmacologic targeting of PAI-1 with PAI-039, a highly specific PAI-1 inhibitor, decreases VN expression, suggesting that down-regulation of VN expression may be an additional mechanism by which pharmacologic inhibition of PAI-1 decreases SMC migration and adverse vascular remodeling [32].
Our studies involving assessment of VN expression in arteries and vein grafts confirm that increases and decreases in PAI-1 expression produce commensurate changes in vascular VN expression in vivo. Furthermore, our experiments involving a vein graft model provide important insights into the source of PAI-1 that regulates neointimal VN expression. In a previous study we showed that plasma PAI-1 concentration does not differ significantly in mice that receive WT vs. PAI-1-deficient vein grafts [36]. Therefore, the significant reduction in VN content in vein grafts from Pai1−/− donor/WTrecipient mice compared to WTdonor/WTrecipient mice suggests that local expression of PAI-1 is a major determinant of VN expression in vein graft neointimal lesions, further supporting an autocrine loop mechanism of regulation of VN expression by PAI-1.
VN is an acute-phase reactant plasma protein whose concentration increases significantly in response to stress [18]. In our murine surgical model, we found that plasma VN increased significantly on the fifth post-operative day. However, at this early time point, when multiple pathways that stimulate expression of VN and other acute-phase reactant proteins would be expected to be strongly activated, there was no discernable effect of PAI-1 genotype on plasma VN concentration. At 4 weeks after surgery, plasma VN was significantly lower in PAI-1-deficient mice compared to WT controls, though this difference between genotypes dissipated by 8 weeks after surgery, at which time animals would be anticipated to be completely recovered from surgery. Consistent with our late post-operative data, there was no significant difference in plasma VN between WT and PAI-1-deficient mice under basal conditions. Overall, these results suggest that PAI-1 can modulate plasma VN concentration under stress conditions, but does not exert a major effect in comparison to other inflammatory mediators, such as interleukin-6 [49]. Interestingly, Ekmekci et al. found a positive correlation between plasma PAI-1 activity and VN concentration in patients with symptomatic carotid artery disease [50], consistent with our cell culture and in vivo data and supporting the hypothesis that PAI-1 regulates VN expression.
In summary, we have shown that PAI-1 plays a previously unrecognized role in regulating expression of VN by SMCs and controlling vascular VN expression in vivo. Therefore, downstream effects of PAI-1 on vascular expression of VN may represent another important mechanism by which PAI-1 regulates SMC migration and vascular remodeling. The stimulatory effect of PAI-1 on SMC VN expression is LRP1-dependent. As a whole, these findings demonstrate that VN depends on PAI-1 for its function, not only through direct binding interactions, but also via regulation of VN gene expression by PAI-1.
Essentials.
Vitronectin (VN) is produced by smooth muscle cells (SMCs) and promotes neointima formation.
We studied the regulation of vascular VN expression by plasminogen activator inhibitor-1 (PAI-1).
PAI-1 stimulates VN gene expression in SMCs by binding LDL receptor-related protein 1 (LRP1).
Stimulation of VN gene expression may be a mechanism by which PAI-1 controls vascular remodeling.
Acknowledgments
None.
Sources of Funding
This work was supported by grants from the National Natural Science Foundation of China (81172050, 81570263; JW), NIH grant HL095951 (WPF) and a Department of Veterans Affairs Merit Review Award (CARA-007-12S; WPF).
Footnotes
Addendum
M. Luo, Y. Luo, and R. Li performed experiments involving cultured smooth muscle cells and carotid arteries, analyzed data, and prepared figures. Y. Ji performed vein graft and plasma experiments, analyzed data, and prepared figures. W. P. Fay designed experiments performed by Y. Ji, discussed and analyzed data, and assisted with figure preparation and manuscript writing. J. Wu designed experiments performed by M. Luo, Y. Luo, and R. Li, analyzed and discussed data, prepared figures, and assisted with figure preparation and manuscript writing.
Disclosures
The authors declare no competing interests.
References
- 1.Preissner KT, Reuning U. Vitronectin in vascular context: facets of a multitalented matricellular protein. Semin Thromb Hemost. 2011;37:408–24. doi: 10.1055/s-0031-1276590. [DOI] [PubMed] [Google Scholar]
- 2.Declerck PJ, Gils A. Three decades of research on plasminogen activator inhibitor-1: a multifaceted serpin. Semin Thromb Hemost. 2013;39:356–64. doi: 10.1055/s-0033-1334487. [DOI] [PubMed] [Google Scholar]
- 3.Mimuro J, Loskutoff DJ. Purification of a protein from bovine plasma that binds to type 1 plasminogen activator inhibitor and prevents its interaction with extracellular matrix. Evidence that the protein is vitronectin. J Biol Chem. 1989;264:936–9. [PubMed] [Google Scholar]
- 4.Mimuro J, Loskutoff DJ. Binding of type 1 plasminogen activator inhibitor to the extracellular matrix of cultured bovine endothelial cells. J Biol Chem. 1989;264:5058–63. [PubMed] [Google Scholar]
- 5.Gebb C, Hayman EG, Engvall E, Ruoslahti E. Interaction of vitronectin with collagen. J Biol Chem. 1986;261:16698–703. [PubMed] [Google Scholar]
- 6.Reilly JT, Nash JR. Vitronectin (serum spreading factor): its localisation in normal and fibrotic tissue. J Clin Pathol. 1988;41:1269–72. doi: 10.1136/jcp.41.12.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dufourcq P, Louis H, Moreau C, Daret D, Boisseau MR, Lamazière JMD, Bonnet J. Vitronectin Expression and Interaction With Receptors in Smooth Muscle Cells From Human Atheromatous Plaque. Arterioscler Thromb Vasc Biol. 1998;18:168–76. doi: 10.1161/01.atv.18.2.168. [DOI] [PubMed] [Google Scholar]
- 8.Dufourcq P, Couffinhal T, Alzieu P, Daret D, Moreau C, Duplàa C, Bonnet J. Vitronectin is up-regulated after vascular injury and vitronectin blockade prevents neointima formation. Cardiovasc Res. 2002;53:952–62. doi: 10.1016/s0008-6363(01)00547-8. [DOI] [PubMed] [Google Scholar]
- 9.Ekmekci OB, Ekmekci H. Vitronectin in atherosclerotic disease. Clin Chim Acta. 2006;368:77–83. doi: 10.1016/j.cca.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 10.van Aken EB, Seiffert D, Thinnes T, Loskutoff JD. Localization of vitronectin in the normal and atherosclerotic human vessel wall. Histochem Cell Biol. 1997;107:313–20. doi: 10.1007/s004180050116. [DOI] [PubMed] [Google Scholar]
- 11.Niculescu F, Rus HG, Poruţiu D, Ghiurca V, Vlaicu R. Immunoelectron-microscopic localization of S-protein/vitronectin in human atherosclerotic wall. Atherosclerosis. 1989;78:197–203. doi: 10.1016/0021-9150(89)90223-2. [DOI] [PubMed] [Google Scholar]
- 12.Pandolfi A, Cetrullo D, Polishuck R, Alberta MM, Calafiore A, Pellegrini G, Vitacolonna E, Capani F, Consoli A. Plasminogen Activator Inhibitor Type 1 Is Increased in the Arterial Wall of Type II Diabetic Subjects. Arterioscler Thromb Vasc Biol. 2001;21:1378–82. doi: 10.1161/hq0801.093667. [DOI] [PubMed] [Google Scholar]
- 13.Schneiderman J, Sawdey MS, Keeton MR, Bordin GM, Bernstein EF, Dilley RB, Loskutoff DJ. Increased type 1 plasminogen activator inhibitor gene expression in atherosclerotic human arteries. Proc Natl Acad Sci USA. 1992;89:6998–7002. doi: 10.1073/pnas.89.15.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Germer M, Kanse SM, Kirkegaard T, Kjøller L, Felding-Habermann B, Goodman S, Preissner KT. Kinetic analysis of integrin-dependent cell adhesion on vitronectin. Eur J Biochem. 1998;253:669–74. doi: 10.1046/j.1432-1327.1998.2530669.x. [DOI] [PubMed] [Google Scholar]
- 15.Deng G, Curriden SA, Wang S, Rosenberg S, Loskutoff DJ. Is plasminogen activator inhibitor-1 the molecular switch that governs urokinase receptor-mediated cell adhesion and release? J Cell Biol. 1996;134:1563–71. doi: 10.1083/jcb.134.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stefansson S, Lawrence DA. The serpin PAI-1 inhibits cell migration by blocking integrin alpha V beta 3 binding to vitronectin. Nature. 1996;383:441–3. doi: 10.1038/383441a0. [DOI] [PubMed] [Google Scholar]
- 17.Minor KH, Peterson CB. Plasminogen Activator Inhibitor Type 1 Promotes the Self-association of Vitronectin into Complexes Exhibiting Altered Incorporation into the Extracellular Matrix. J Biol Chem. 2002;277:10337–45. doi: 10.1074/jbc.M109564200. [DOI] [PubMed] [Google Scholar]
- 18.Seiffert D, Crain K, Wagner NV, Loskutoff DJ. Vitronectin gene expression in vivo. Evidence for extrahepatic synthesis and acute phase regulation. J Biol Chem. 1994;269:19836–42. [PubMed] [Google Scholar]
- 19.Garg N, Goyal N, Strawn TL, Wu J, Mann KM, Lawrence DA, Fay WP. Plasminogen activator inhibitor-1 and vitronectin expression level and stoichiometry regulate vascular smooth muscle cell migration through physiological collagen matrices. J Thromb Haemost. 2010;8:1847–54. doi: 10.1111/j.1538-7836.2010.03907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noda-Heiny H, Fujii S, Sobel BE. Induction of vascular smooth muscle cell expression of plasminogen activator inhibitor-1 by thrombin. Circ Res. 1993;72:36–43. doi: 10.1161/01.res.72.1.36. [DOI] [PubMed] [Google Scholar]
- 21.Carmeliet P, Kieckens L, Schoonjans L, Ream B, van Nuffelen A, Prendergast G, Cole M, Bronson R, Collen D, Mulligan RC. Plasminogen activator inhibitor-1 gene-deficient mice. I. Generation by homologous recombination and characterization. J Clin Invest. 1993;92:2746–55. doi: 10.1172/JCI116892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng X, Saunders TL, Camper SA, Samuelson LC, Ginsburg D. Vitronectin is not essential for normal mammalian development and fertility. Proc Natl Acad Sci USA. 1995;92:12426–30. doi: 10.1073/pnas.92.26.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eitzman DT, McCoy RD, Zheng X, Fay WP, Shen T, Ginsburg D, Simon RH. Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene. J Clin Invest. 1996;97:232–7. doi: 10.1172/JCI118396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stefansson S, Petitclerc E, Wong MKK, McMahon GA, Brooks PC, Lawrence DA. Inhibition of Angiogenesis in Vivo by Plasminogen Activator Inhibitor-1. J Biol Chem. 2001;276:8135–41. doi: 10.1074/jbc.M007609200. [DOI] [PubMed] [Google Scholar]
- 25.Berkenpas MB, Lawrence DA, Ginsburg D. Molecular evolution of plasminogen activator inhibitor-1 functional stability. EMBO J. 1995;14:2969–77. doi: 10.1002/j.1460-2075.1995.tb07299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Courey AJ, Horowitz JC, Kim KK, Koh TJ, Novak ML, Subbotina N, Warnock M, Xue B, Cunningham AK, Lin Y, Goldklang MP, Simon RH, Lawrence DA, Sisson TH. The vitronectin-binding function of PAI-1 exacerbates lung fibrosis in mice. Blood. 2011;118:2313–21. doi: 10.1182/blood-2010-12-324574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elokdah H, Abou-Gharbia M, Hennan JK, McFarlane G, Mugford CP, Krishnamurthy G, Crandall DL. Tiplaxtinin, a novel, orally efficacious inhibitor of plasminogen activator inhibitor-1: design, synthesis, and preclinical characterization. J Med Chem. 2004;47:3491–4. doi: 10.1021/jm049766q. [DOI] [PubMed] [Google Scholar]
- 28.Wu J, Stevenson MJ, Brown JM, Grunz EA, Strawn TL, Fay WP. C-reactive protein enhances tissue factor expression by vascular smooth muscle cells: mechanisms and in vivo significance. Arterioscler Thromb Vasc Biol. 2008;28:698–704. doi: 10.1161/ATVBAHA.107.160903. [DOI] [PubMed] [Google Scholar]
- 29.Mikhailenko I, Battey FD, Migliorini M, Ruiz JF, Argraves K, Moayeri M, Strickland DK. Recognition of α2-Macroglobulin by the Low Density Lipoprotein Receptor-related Protein Requires the Cooperation of Two Ligand Binding Cluster Regions. J Biol Chem. 2001;276:39484–91. doi: 10.1074/jbc.M104382200. [DOI] [PubMed] [Google Scholar]
- 30.Ashcom JD, Tiller SE, Dickerson K, Cravens JL, Argraves WS, Strickland DK. The human alpha 2-macroglobulin receptor: identification of a 420-kD cell surface glycoprotein specific for the activated conformation of alpha 2-macroglobulin. J Cell Biol. 1990;110:1041–8. doi: 10.1083/jcb.110.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao C, Lawrence DA, Li Y, Von Arnim CA, Herz J, Su EJ, Makarova A, Hyman BT, Strickland DK, Zhang L. Endocytic receptor LRP together with tPA and PAI-1 coordinates Mac-1-dependent macrophage migration. EMBO J. 2006;25:1860–70. doi: 10.1038/sj.emboj.7601082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji Y, Weng Z, Fish P, Goyal N, Luo M, Myears SP, Strawn TL, Chandrasekar B, Wu J, Fay WP. Pharmacological Targeting of Plasminogen Activator Inhibitor-1 Decreases Vascular Smooth Muscle Cell Migration and Neointima Formation. Arterioscler Thromb Vasc Biol. 2016;36:2167–75. doi: 10.1161/ATVBAHA.116.308344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zou Y, Dietrich H, Hu Y, Metzler B, Wick G, Xu Q. Mouse model of venous bypass graft arteriosclerosis. Am J Pathol. 1998;153:1301–10. doi: 10.1016/S0002-9440(10)65675-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strickland DK, Ashcom JD, Williams S, Burgess WH, Migliorini M, Argraves WS. Sequence identity between the alpha 2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J Biol Chem. 1990;265:17401–4. [PubMed] [Google Scholar]
- 35.Misra UK, Pizzo SV. Binding of Receptor-recognized Forms of α2-Macroglobulin to the α2-Macroglobulin Signaling Receptor Activates Phosphatidylinositol 3-Kinase. J Biol Chem. 1998;273:13399–402. doi: 10.1074/jbc.273.22.13399. [DOI] [PubMed] [Google Scholar]
- 36.Ji Y, Strawn TL, Grunz EA, Stevenson MJ, Lohman AW, Lawrence DA, Fay WP. Multifaceted Role of Plasminogen Activator Inhibitor-1 in Regulating Early Remodeling of Vein Bypass Grafts. Arterioscler Thromb Vasc Biol. 2011;31:1781–7. doi: 10.1161/ATVBAHA.111.228767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asmal M, Seaman M, Lin W, Chung RT, Letvin NL, Geiben-Lynn R. Inhibition of HCV by the serpin antithrombin III. Virology Journal. 2012;9:226-. doi: 10.1186/1743-422X-9-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sawa H, Fujii S, Sobel BE. Augmented arterial wall expression of type-1 plasminogen activator inhibitor induced by thrombosis. Arterioscler Thromb Vasc Biol. 1992;12:1507–15. doi: 10.1161/01.atv.12.12.1507. [DOI] [PubMed] [Google Scholar]
- 39.Sawa H, Lundgren C, Sobel BE, Fujii S. Increased intramural expression of plasminogen activator inhibitor type 1 after balloon injury: A potential progenitor of restenosis. J Am Coll Cardiol. 1994;24:1742–8. doi: 10.1016/0735-1097(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 40.Degryse B, Neels JG, Czekay RP, Aertgeerts K, Kamikubo Y, Loskutoff DJ. The low density lipoprotein receptor-related protein is a motogenic receptor for plasminogen activator inhibitor-1. J Biol Chem. 2004;279:22595–604. doi: 10.1074/jbc.M313004200. [DOI] [PubMed] [Google Scholar]
- 41.Kamikubo Y, Neels JG, Degryse B. Vitronectin inhibits plasminogen activator inhibitor-1-induced signalling and chemotaxis by blocking plasminogen activator inhibitor-1 binding to the low-density lipoprotein receptor-related protein. Int J Biochem Cell Biol. 2009;41:578–85. doi: 10.1016/j.biocel.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Stefansson S, Lawrence DA. Old Dogs and New Tricks, Proteases, Inhibitors, and Cell Migration. Science Signaling. 2003;2003:e24. doi: 10.1126/stke.2003.189.pe24. [DOI] [PubMed] [Google Scholar]
- 43.Stefansson S, Muhammad S, Cheng X-F, Battey FD, Strickland DK, Lawrence DA. Plasminogen Activator Inhibitor-1 Contains a Cryptic High Affinity Binding Site for the Low Density Lipoprotein Receptor-related Protein. J Biol Chem. 1998;273:6358–66. doi: 10.1074/jbc.273.11.6358. [DOI] [PubMed] [Google Scholar]
- 44.Czekay R-P, Kuemmel TA, Orlando RA, Farquhar MG. Direct Binding of Occupied Urokinase Receptor (uPAR) to LDL Receptor-related Protein Is Required for Endocytosis of uPAR and Regulation of Cell Surface Urokinase Activity. Mol Biol of the Cell. 2001;12:1467–79. doi: 10.1091/mbc.12.5.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Czekay RP, Aertgeerts K, Curriden SA, Loskutoff DJ. Plasminogen activator inhibitor-1 detaches cells from extracellular matrices by inactivating integrins. J Cell Biol. 2003;160:781–91. doi: 10.1083/jcb.200208117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lau HKF. Regulation of proteolytic enzymes and inhibitors in two smooth muscle cell phenotypes. Cardiovasc Res. 1999;43:1049–59. doi: 10.1016/s0008-6363(99)00136-4. [DOI] [PubMed] [Google Scholar]
- 47.Fortenberry YM. Plasminogen activator inhibitor-1 inhibitors: a patent review (2006-present) Expert Opin Ther Patents. 2013;23:801–15. doi: 10.1517/13543776.2013.782393. [DOI] [PubMed] [Google Scholar]
- 48.Vaughan DE, De Taeye BM, Eren M. PAI-1 antagonists: predictable indications and unconventional applications. Curr Drug Targets. 2007;8:962–70. doi: 10.2174/138945007781662364. [DOI] [PubMed] [Google Scholar]
- 49.Seiffert D, Geisterfer M, Gauldie J, Young E, Podor TJ. IL-6 stimulates vitronectin gene expression in vivo. J Immunol. 1995;155:3180–5. [PubMed] [Google Scholar]
- 50.Ekmekci HGO Z, Ekmekci OB, Isler Butun I, Besirli K, Gode S, Atukeren P, Sonmez H. Significance of vitronectin and PAI-1 activity levels in carotid artery disease: comparison of symptomatic and asymptomatic patients. Minerva Med. 2013;104:215–23. [PubMed] [Google Scholar]