Abstract
Sustained, localized protein delivery can enhance the safety and activity of protein drugs in diverse disease settings. While hydrogel systems are widely studied as vehicles for protein delivery, they often suffer from rapid release of encapsulated cargo, leading to a narrow duration of therapy, and protein cargo can be denatured by incompatibility with the hydrogel crosslinking chemistry. In this work, we describe injectable nanocomposite hydrogels that are capable of sustained, bioactive, release of a variety of encapsulated proteins. Injectable and porous cryogels were formed by bio-orthogonal crosslinking of alginate using tetrazine-norbornene coupling. To provide sustained release from these hydrogels, protein cargo was pre-adsorbed to charged Laponite nanoparticles that were incorporated within the walls of the cryogels. The presence of Laponite particles substantially hindered the release of a number of proteins that otherwise showed burst release from these hydrogels. By modifying the Laponite content within the hydrogels, the kinetics of protein release could be precisely tuned. This versatile strategy to control protein release simplifies the design of hydrogel drug delivery systems.
Keywords: drug delivery, injectable hydrogel, protein, alginate, Laponite
Graphical Abstract
1.1 Introduction
Macroscale protein drug delivery systems offer a means to control the spatiotemporal delivery of drugs to specific tissues, possibly reducing side effects and improving efficacy at the target site compared to systemic delivery.[1] The creation of an effective polymer drug delivery system for a given protein often requires careful material selection and optimization of protein loading to achieve the desired protein release profile and maintain bioactivity.[2] Platform drug delivery systems capable of controlled release of a variety of proteins may accelerate the pace of therapeutic protein delivery research and translation.
Hydrogels are water-swollen crosslinked hydrophilic polymer networks that have been used successfully as matrices to protect and facilitate controlled release of proteins at defined tissue sites. Hydrogel crosslinking typically occurs in aqueous conditions, often allowing protein encapsulation without denaturation. Physicochemical properties of hydrogel matrices such as crosslink density, polymer concentration, degradation, and affinity for a given protein govern the rate of protein release.[2] However, many hydrogels have mesh sizes larger than the size of typical proteins, which may not interact strongly with the hydrogel polymer backbone, leading to rapid release from these matrices by diffusion.[3] For example, alginate, a polysaccharide commonly used for hydrogel drug delivery, can be used to encapsulate proteins under mild conditions when it is crosslinked with calcium ions. The affinity of heparin-binding proteins for alginate enables alginate hydrogels to encapsulate and release such proteins in a controlled manner due to ionic interactions between the protein and the alginate backbone. However, many proteins do not strongly interact with alginate polymers and demonstrate burst release kinetics from alginate hydrogels. This issue with many hydrogels has been overcome by various means, for example, first loading the protein in another phase (e.g. poly(lactide-co-glycolide) microspheres[4]) that offers controlled protein release and then encapsulating this phase within a hydrogel. However, entrapment of proteins in these carriers prior to incorporation into a hydrogel often requires laborious optimization, harsh conditions involving organic solvents, and frequently results in poor encapsulation efficiencies.[5] Alternative strategies for protein entrapment and controlled release from hydrogels are needed.
Controlled protein release from hydrogels may be achieved by inclusion of particles that have substantial interactions with proteins within the hydrogel matrix.[2] One potential particle is Laponite XLG, which is a synthetic smectite clay in the form of ~25 nm diameter and ~1 nm thickness nanoplatelets with a surface area of 350 m2/g. Laponite nanoplatelets bear a high negative surface charge on their faces and a pH-dependent charge on their rim (positive at pH<9).[6] Laponite has been explored extensively as a drug carrier via loading of small molecule drugs into the interlayer space of the discs.[7–10] Physical gels formed from the interaction of Laponite with electrolytes have been shown to adsorb and release bioactive proteins.[11,12] However, gels formed from pure Laponite offer little control over protein release kinetics and lack control over gel properties such as cell adhesion and macroscale porosity. Laponite has been included in many hydrogel systems to form nanocomposite hydrogels primarily to alter their mechanical properties.[13–15] Although some evidence for the utility of Laponite for retarding protein release from hydrogels has been reported,[16] a systematic study of this behavior has not been undertaken.
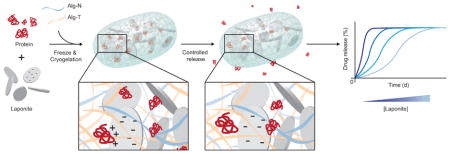
In this study, it was hypothesized that pre-adsorbing proteins onto charged Laponite nanoplatelets and incorporation into injectable hydrogels would allow sustained bioactive release of an array of therapeutically relevant proteins (Figure 1). Cryogels were used to explore this possibility, in place of the typical in situ gelling or shear-thinning hydrogels often used for drug delivery.[17,18] The latter are mechanically weak, allow little control of hydrogel geometry and microstructure when injected into tissues, and it is difficult to control their precise location and dose at a particular injection site. In contrast, sponge-like injectable preformed scaffolds created from cryopolymerization can be injected into the body with a conventional needle. These gels demonstrate shape memory, allowing discrete gels of a predefined volume, microarchitecture and shape to be delivered at a tissue site in a minimally invasive manner. Additionally, the tunable macroscale porosity of these gels, that can be achieved by altering the freezing conditions, [19] affords cell recruitment and trafficking for cell delivery and vaccine applications. Proteins encapsulated in cryogels typically demonstrate rapid diffusion-mediated burst release, as in other hydrogel systems. However, the crosslinking chemistries used in hydrogel formation can crossreact with encapsulated proteins, potentially resulting in loss of bioactivity.[20,21] Recently, a bio-orthogonal click chemistry strategy using tetrazine-norbornene conjugation was introduced that enabled the formation of covalently crosslinked hydrogels without damaging encapsulated biological cargo.[22–24] Creation of cryogels using this chemistry, with incorporation of Laponite nanoparticles, may allow mechanically robust injectable hydrogels to be formed with improved protein delivery capabilities. Here we explore this possibility, and also the ability to control and tune the release of bioactive proteins from these nanocomposite materials.
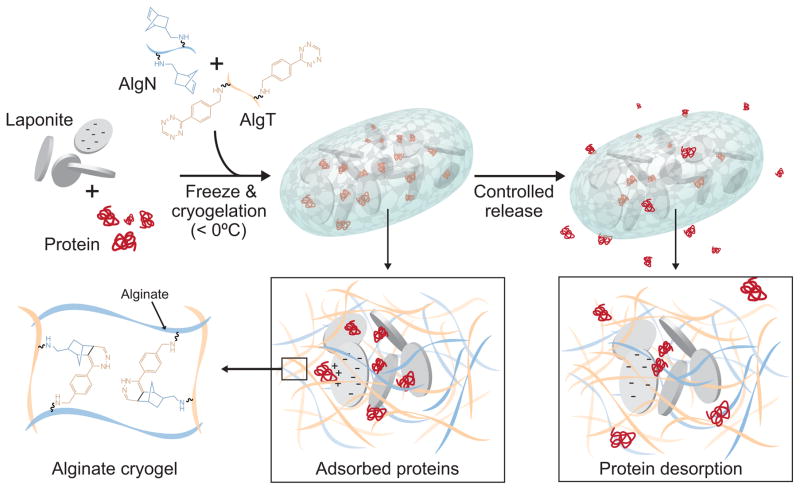
Figure 1.
Schematic of drug loading and release in Laponite-containing click alginate cryogels. Laponite and recombinant proteins are first mixed together to allow adsorption. Alginates coupled to norbornene (AlgN) or tetrazine (AlgT) are added to the Laponite/protein, and this mixture is partially frozen. AlgN and AlgT covalently crosslink to form a porous cryogel with walls containing protein that is bound to Laponite. The thawed cryogels release the bound protein over time, facilitating controlled release into the surroundings.
1.2 Materials and Methods
1.2.1 Click alginate synthesis
Click alginate was synthesized similarly to a previously described method with some modifications.[23] Individual batches of PRONOVA UP MVG alginate (NovaMatrix) were coupled with (4-(1,2,4,5-tetrazin-3-yl)phenyl methanamine hydrochloride (tetrazine; Karebay Biochem) and 5-norbornene-2-methylamine (norbornene; TCI) using carbodiimide chemistry. Alginate was dissolved in 0.1 M 2-(N-morpholino)ethanesulfonic acid (MES), 0.3 M NaCl, pH 6.5 at 5 mg/ml. 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (ThermoFisher) and N-hydroxysuccinimide (ThermoFisher) were added at 5-molar excess of the carboxylic acid groups present on alginate. Tetrazine or norbornene were added at 1 mM per gram of alginate and allowed to react for 24 hours at room temperature. Modified alginate was purified using tangential flow filtration (KrosFlow KR2i; Spectrum Labs) with a 5 kDa MWCO hollow fiber membrane (mPES; Spectrum Labs) through a decreasing salt gradient from 150 mM to 0 mM sodium chloride in Milli-Q (Millipore) distilled water (dH20), followed by treatment with activated charcoal, and filtration through a 0.22 μm vacuum filter. The polymers were lyophilized and stored at −20 °C until use. The degree of substitution was 5% tetrazine or norbornene per alginate chain, as determined by 1H-NMR as described previously.[23]
1.2.2 Click alginate cryogel formation
To form cryogels, alginate-norbornene (AlgN) and alginate-tetrazine (AlgT) were separately dissolved in dH20 at 15 mg/ml, mixed at a 1:1 ratio, and diluted to a final concentration of 10 mg/ml alginate. For each gel, 50 μl of this mixture was immediately pipetted into polystyrene molds and placed in a freezer set to −12 °C overnight for cryopolymerization. Samples were thawed by placement into room temperature buffer, such as Dulbecco’s phosphate buffered saline (DPBS; Gibco).
To incorporate Laponite XLG (BYK Additives), it was allowed to disperse in dH20 at 5 mg/ml for 30 minutes with occasional vortexing. Various amounts of this suspension were diluted with AlgN and AlgT prior to cryogelation (0.1–0.5 mg/ml final Laponite concentration), with or without pre-mixing with protein, as described below.
1.2.3 Scanning electron microscopy
For scanning electron microscopy (SEM), click alginate cryogels were transitioned through a series of ethanol solutions in dH20 (0%, 30%, 50%, 70%, 90%, 95%, 100% ethanol) for 20 minutes each. Samples were then placed in a 1:1 solution of ethanol and hexamethyldisilazane (HMDS; Electron Microscopy Sciences) for 10 minutes, followed by a 5 minute incubation in 100% HMDS. Samples were desiccated overnight, mounted on SEM sample stubs using carbon tape, and sputter coated with a 5 nm layer of platinum-palladium. SEM imaging was performed using a Supra 55 VP field emission SEM (Carl Zeiss) using secondary electron detection.
1.2.4 Nanoindentation testing
All nanoindentation (Agilent G200, Agilent Technologies, Santa Clara, USA) tests were conducted with a conical diamond probe 400 μm in radius (120°±5°, Agilent XP/CON120/400/020). The probe was calibrated on fused silica prior to measurements. Four separate hydrogels for each Laponite concentration were measured, and 16 indents were performed in an array of 4x4, 100 μm apart at the resonant frequency of 110 Hz. Moduli were obtained from continuous stiffness measurement (CSM) indentation at an amplitude of 500 nm, with a preload of 2 μm, and an assumed Poisson ratio of 0.5.
1.2.5 Inductively coupled plasma atomic emission spectrometry
Cryogels containing various amounts of Laponite were formed, washed for 1 hour in dH20, and lyophilized. Inductively coupled plasma atomic emission spectrometry (ICP-AES) was performed by Galbraith Laboratories to analyze silicon content within the gels. Percent retention of the Laponite within the gels was calculated comparing to silicon content in a known mass of pure Laponite sample.
1.2.6 Protein loading into cryogels
For protein release studies, recombinant murine granulocyte-macrophage colony-stimulating factor (GM-CSF), FMS-like tyrosine kinase 3 ligand (FlT3L), interleukin 15 (IL-15), and chemokine (C-C motif) ligand 20 (CCL20) were purchased from Peprotech, and recombinant murine interleukin 2 (IL-2) was acquired from Biolegend. The electrostatic properties of the proteins were calculated by inputting the amino acid sequence supplied by the manufacturer into an online protein isoelectric point calculator (http://isoelectric.ovh.org).[25]
In most studies, Laponite (0–25 μg per gel) and recombinant protein (1 μg per gel) or fluorescent molecules (Alexa Fluor 488-azide, Alexa Fluor 488-IgG; Life Technologies) were premixed for 1 hour at room temperature in dH20. This solution was diluted with 1.5 mg/ml AlgN and AlgT to achieve a 1 mg/ml final alginate concentration before cryopolymerization to form 50 μl disc-shaped cryogels as described above.
1.2.7 Protein release studies
Frozen cryogels were removed from molds and placed into a solution of 1% bovine serum albumin in DPBS (release buffer) within a low protein-binding 1.5 ml centrifuge tube to thaw for one hour at room temperature. Gels were moved into a fresh release buffer and placed at 37 °C with complete exchange of release buffer at the indicated timepoints. Protein release supernatants were frozen at −20 °C immediately upon retrieval until further use.
Needle injection tests were performed after initially thawing the gel for one hour at room temperature. Thawed gels were placed into a syringe containing 200 μl of release buffer and equipped with a 16G needle. Gels were injected into a centrifuge tube containing 800 μl of release buffer and immediately removed. The release buffer was then assayed for protein release by enzyme-linked immunosorbent assay (ELISA).
ELISA kits for GM-CSF (R&D Systems), CCL20 (R&D Systems), Flt3L (R&D Systems), IL-15 (Peprotech), and IL-2 (Biolegend) were used to assay protein release from cryogels according to the manufacturer’s instructions. For standard curves, a dilution series was prepared from the recombinant protein solution used for initial protein loading into drug-releasing cryogels.
1.2.8 GM-CSF in vitro bioactivity assay
The in vitro bioactivity of GM-CSF released from cryogels was assessed using a previously published method.[17] Briefly, femurs were harvested from euthanized C57Bl/6J mice (female, 6–12 weeks) and the bone marrow was flushed into Hanks Balanced Salt Solution (HBSS). Isolated bone marrow cells were then suspended in 100 μl of Roswell Park Memorial Institute medium (RPMI 1640; Lonza) containing 10% v/v heat-inactivated fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin, and 50 μM β-mercaptoethanol in flat-bottom 96 well plates at 4x104 cells/well. 100 μl of known standards (final concentration 10 ng/ml–19 pg/ml GM-CSF) or diluted release samples were added to the wells in triplicate. Samples were incubated at 37 °C in a 5% CO2 atmosphere for five days. PrestoBlue Cell Viability Reagent (Thermo) was used to assess metabolic activity in the wells according to the manufacturer’s instructions. The equivalent amount of bioactive GM-CSF in the unknown samples was back-calculated by comparing to the standard curve.
1.2.9 IL-2 bioactivity assay
CTLL-2 cells (American Type Culture Collection #TIB-214) were maintained in RPMI 1640 with 10% FCS, 2 mM L-glutamine, 1 mM sodium pyruvate, 100 U/ml penicillin, and 100 μg/ml streptomycin with 16 ng/ml recombinant murine IL-2 (Biolegend). For assessing IL-2 bioactivity, cells were placed in flat-bottom 96 well plates at 1x105 cells/well in 100 μl of medium. Diluted samples and standards were added in 100 μl to the wells and the plates were incubated at 37 °C in a 5% C02 atmosphere for 24 hours. PrestoBlue Cell Viability Reagent (Thermo) was used to measure metabolic activity in the wells according to the manufacturer’s instructions. The equivalent amount of bioactive IL-2 in the unknown samples was back-calculated by comparing to the standard curve.
1.2.10 Statistical methods
All data are shown as mean and standard deviation and statistical tests were calculated with GraphPad Prism 6.0. Release curves in Figures 3 and 4 were compared with an unpaired two-tailed t-test with Welch’s correction at the final timepoint, and in Figure 5 the curves were compared with ordinary one-way ANOVA with Tukey’s multiple comparison’s test at the final timepoint. The rheological data of Figure S3 were analyzed by comparing the mean rank of each group to the 0 mg/ml Laponite condition by Kruskal-Wallis multiple comparison test. p values are indicated in the figure legends.
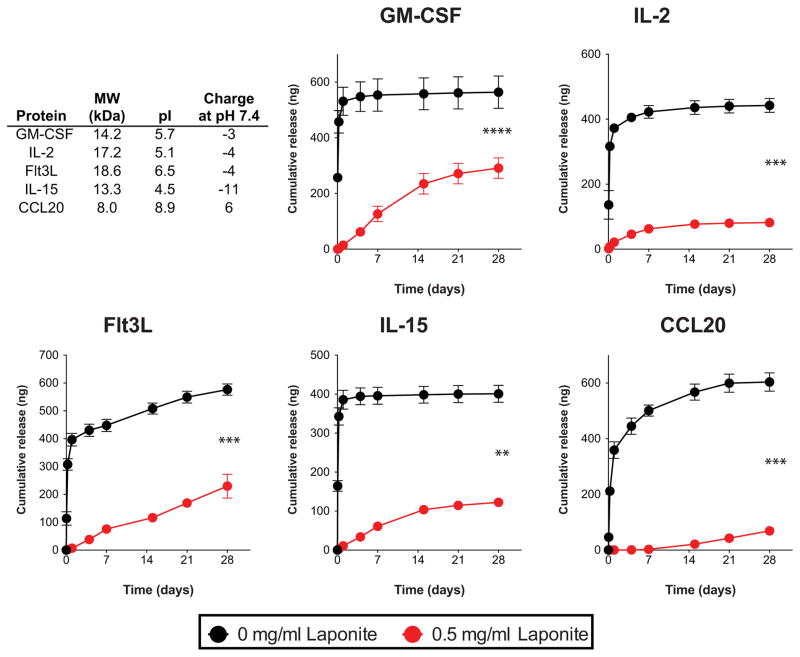
Figure 3.
Effect of Laponite pre-adsorption on protein release from click alginate cryogels. 1 μg of recombinant protein either in free form, or pre-incubated with 25 μg of Laponite was incorporated in each 50 μl gel. Supernatants were collected from protein-loaded gels incubated in 1% BSA in DPBS, and the recombinant protein release was assessed by ELISA (n=3 gels/condition, unpaired two-tailed t-test with Welch’s correction, ****p<0.0001, ***p<0.001, **p<0.01). Data are shown as mean and standard deviation.
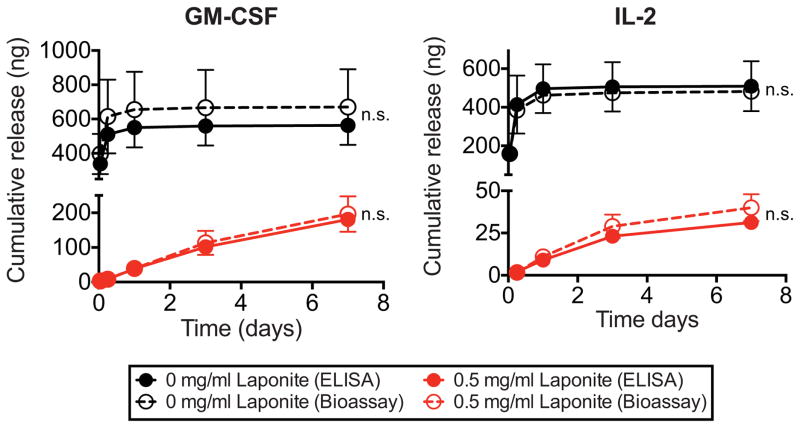
Figure 4.
Comparison of ELISA and bioassay-detected IL-2 and GM-CSF release from click alginate cryogels with and without Laponite. 1 μg of recombinant protein either in free form, or pre-incubated with 25 μg of Laponite was incorporated in each 50 μl gel. Supernatants were collected with from gels incubated in 1% BSA in DPBS, and the recombinant protein release was assessed by ELISA. In parallel, samples were used in cell based proliferation assays with mouse bone marrow cells or CTLL-2 cells for GM-CSF and IL-2, respectively, to determine bioactivity (n=3 gels/condition, unpaired two-tailed t-test with Welch’s correction, n.s. – not significant). Data are shown as mean and standard deviation.
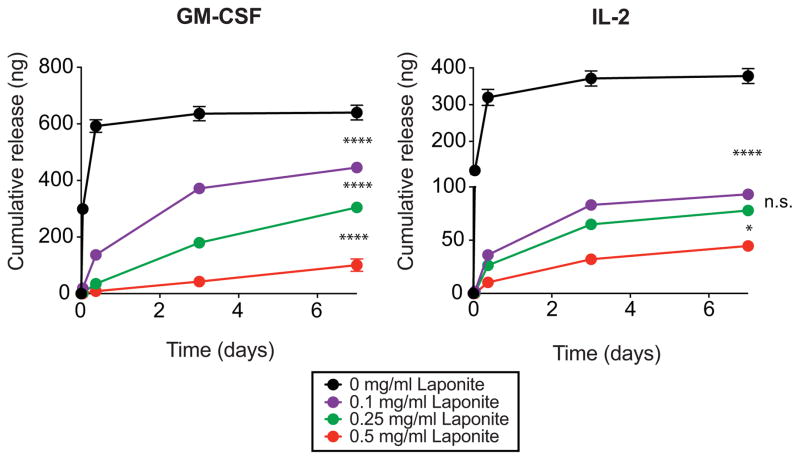
Figure 5.
Effect of initia Laponite concentration on GM-CSF release from click alginate cryogels. Recombinant GM-CSF (1 μg) was incubated with various initial amounts of Laponite (0-25 μg) and incorporated into 50 μl click alginate cryogels (n=3 gels/condition, one-way ANOVA with Tukey’s multiple comparison’s test, ****p<0.0001, *p<0.05). Data are shown as mean and standard deviation.
1.3 Results
1.3.1 Formation of click alginate cryogels incorporating Laponite
The ability to form cryogels using click alginate was first tested (Figure S1). Click alginate prepolymer solutions at 1% (w/v) were cryopolymerized to form porous cryogels. The cryogels exhibited an interconnected porous structure, high deformability, and were injectable through a 16 gauge needle (Figure S2) as has been previously reported with other cryogel systems.[18,26]
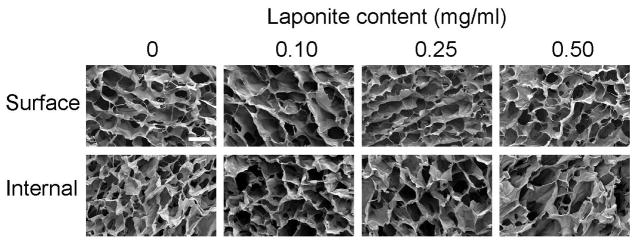
Next, the ability to incorporate Laponite particles into click alginate cryogels was tested. Addition of 0–0.5 mg/ml Laponite prior to cryogelation again resulted in hydrogels with surface and internal macroporosity (Figure 2). The pore size range was between 50–300 μm, and no obvious impact of Laponite addition on pore architecture was seen over this range. These cryogels were highly deformable and could be injected through a 16 gauge needle. Higher initial concentrations of Laponite resulted in gels that were not reversibly deformable and fragmented when injected. This phenomenon was evident in the apparent decrease in loss modulus with respect to increasing concentrations of Laponite (Figure S3). As the Laponite content is increased, the cryogels lose their viscous properties as the tan(δ) decreases, resulting in brittle gels that cannot be injected without damage. An input Laponite concentration of 0.5 mg/ml was considered the upper limit for further studies.
Figure 2.
SEM images of cryogels with various input Laponite concentrations. A surface view (top) and internal cross section after cutting the gel (bottom) are shown for 50 μl cryogels formed with the indicated initial Laponite concentration. Scale bar is 100 μm. Images are representative of n=3 gels/condition.
Elemental analysis by ICP-AES was used to assess Laponite retention within the gels after cryopolymerization and thawing into dH20 for one hour. Using the silicon content of the pure Laponite used in these studies as a basis, the amount of input Laponite present in the cryogels was determined (Figure S4). A large fraction (>70%) of the input Laponite was retained at all of the initial Laponite concentrations tested.
Finally, the distribution of Laponite within the walls of cryogel was visualized using confocal microscopy. A uniform distribution of fluorescence signal from a dye bound to the Laponite particles was observed (Figure S5), indicating no aggregation of Laponite particles when suspended in alginate and after cryogelation.
1.3.2 Effect of Laponite on protein release from click alginate cryogels
The ability of Laponite to impact protein release from click alginate cryogels was next tested. Five immune-related proteins of varying size, net charge, and immune function were chosen, as representative candidates for biomaterial-based immunotherapies (Figure 3A).[27] Proteins were incubated with Laponite before incorporation into click alginate cryogels, and their release over time was assessed. All five tested proteins showed burst release from cryogels made solely from click alginate (Figure 3B–F), as is typical with many diffusion-dominated drug release systems.[2] Incorporation of Laponite eliminated burst release of all of the proteins tested and maintained their controlled release over the course of four weeks. Injection of newly formed Laponite-containing cryogels loaded with 1 μg of GM-CSF through a needle resulted in the loss of only 3.6 ± 0.9 ng of protein, which is <0.5% of the encapsulated protein and ~2% of the amount released over the four week period. Additionally, soaking pre-formed Laponite-containing click alginate cryogels in solutions of recombinant protein resulted in greater uptake of proteins from solution into the gel and slower release over time compared to pristine click alginate cryogels (Figure S6). Importantly, the binding of encapsulated proteins to the Laponite particles still allowed uniform distribution of the particles within the cryogel (Figure S7). These results show that incorporated Laponite can bind and slow the release of encapsulated proteins from click alginate cryogels.
1.3.3 Protein bioactivity after release from cryogels
Next, the bioactivity of proteins released from click alginate cryogels containing Laponite was studied. GM-CSF and IL-2 were chosen for testing in these studies since the human versions of these two molecules are used clinically for immunotherapy. When cell-based assays were performed to assess released protein bioactivity, the results showed no significant difference from the values measured by ELISA for cryogels with and without Laponite (Figure 4). These data suggest that all of the detectable GM-CSF and IL-2 released from the cryogels is bioactive.
1.3.4 Tuning protein release from cryogels by varying Laponite content
Finally, the ability to tune protein release by varying the Laponite content was explored. Murine GM-CSF and IL-2 were incubated with various amounts of Laponite prior to encapsulation into and release from click alginate cryogels (Figure 5). Release studies revealed that increasing the amount of Laponite used for pre-adsorption of proteins reduced burst release and slowed the elution of proteins over time from the gels, enabling tuning of the release rate of protein from the gels.
1.4 Discussion
This work explored the effect of Laponite XLG incorporation into hydrogels on protein release. An injectable, macroporous hydrogel system was developed that is capable of releasing bioactive protein drugs in a controlled manner. This nanocomposite system is simple to fabricate, and is functionally versatile, allowing the sustained release of a number of proteins with diverse properties. These features address many shortcomings of current, widely used hydrogel systems for drug delivery.
The results show that injectable cryogels can be formed with high retention of encapsulated Laponite. Porous cryogels were formed by partially freezing a prepolymer solution containing AlgN, AlgT, and Laponite nanoplatelets. The freezing process results in phase separation into a polymer-rich and water-rich phase. Crosslinking via spontaneous tetrazine-norbornene ligation occurs in this partially frozen phase. When the gel is thawed, a thin-walled hydrogel with high porosity and interconnectivity is formed. The high retention of Laponite within the cryogels after thawing suggests efficient partitioning of the Laponite into the polymer-rich phase during freezing. A limit to the Laponite content, in terms of embrittlement of the cryogels, was observed at Laponite concentrations higher than 0.5 mg/ml. Alginate contains anionic carboxyl groups in its polymer backbone that are thought to electrostatically interact with the cationic rims of the Laponite nanoplatelets.[28] This interaction may alter the crosslinking and swelling behaviors of these hydrogels at high Laponite concentrations, resulting in brittle gels that cannot be injected without breaking. The use of injectable cryogels in this work allows one to create preformed, porous scaffolds that can be injected precisely into the body in a minimally invasive manner. The macroporosity of these gels may allow desirable cell recruitment into the material, as in the case of a recently reported cryogel-based cancer vaccine.[17] Cell infiltration may alter the scaffold microenvironmental conditions (e.g. pH) leading to alterations of the release profile of bound-proteins from the gels. Alginate is used medically in wound-healing applications, as a carrier for therapeutic proteins (e.g. Emdogain, Progenix Putty) and has demonstrated safety in extensive preclinical tests for regenerative medicine and drug delivery applications.[29] Laponite has been used in the cosmetic and pharmaceutical industry and degrades to release non-toxic components (silicic acid, and ions of lithium, magnesium, and sodium[30]). These favorable properties of the base materials position the nanocomposite hydrogels reported here for further development for clinical applications.
Pre-adsorption with Laponite can prevent burst release and provides sustained elution of proteins of various size and charge from hydrogels. Five immune-related proteins encapsulated in click alginate cryogels showed substantial initial burst release, which was hindered by addition of Laponite. Laponite-containing hydrogels showed sustained release of the encapsulated protein over time. Additionally, this approach enables all of the input protein to be encapsulated within the hydrogel, unlike approaches using pre-encapsulation in particles where significant protein loss occurs prior to addition of the carrier into hydrogels.[4] Since the interaction of encapsulated proteins with Laponite is non-covalent, it is expected that all of the protein is released over time. Several potential mechanisms of hindered release using clay particles in hydrogels have been described including (1) reduced polymer swelling, (2) formation of barriers to diffusion, (3) slowing degradation of the hydrogel, and (4) interaction with the clay particles.[11] A reduction in polymer swelling or the formation of a barrier are unlikely to dominate the mechanism since Laponite-containing cryogels were able to rapidly (1 hour) take up proteins from solution and facilitate their slow release over several days. Additionally, CCL20, which was the lowest mass protein tested, showed the highest retention within the gels, suggesting that protein size was not the key property determining release, as would be the case if changes in the mesh size or tortuosity of the gel were the limiting mechanism for protein release. A reduction in cryogel degradation is unlikely to facilitate hindered protein release since click alginate cryogels are not expected to degrade significantly over this timescale.[23] A number of interactions between proteins and the Laponite particles are possible including: hydrogen bonding, Van der Waals forces, and electrostatic interactions, among others.[6] The negatively-charged face of Laponite nanoplatelets should be capable of binding positively-charged domains found on proteins, and this interaction is likely to be responsible for slow release of proteins from the cryogels. This hypothesis is supported by the release curve observed for CCL20, the only net positively charged protein tested, which showed the lowest release rate from the gel. Electrostatically driven release of net positively charged proteins has recently been reported using adsorption to PLGA particles within hydrogels.[31] However, this method was unable to sustain the release of a net negatively charged protein (erythropoietin). In this work, interactions with Laponite allowed the release of several net negatively charged proteins, suggesting that the presence of positively charged domains on the protein may be sufficient for interaction with Laponite. This robust electrostatic interaction with proteins is simple to introduce into the cryogels and may be more generalizable to protein release than strategies based on introducing covalent coupling, other non-covalent interactions, mesh size, or degradation.[2] Previous work with Laponite ceramics have shown that this material may have some impact on bone formation.[32]. The possible calcification of Laponite cryogels could be a barrier to release of protein from the Laponite-laden cryogels in vivo. Laponite-containing click alginate hydrogels may be able to facilitate the controlled release of a number of therapeutic proteins that was previously unachievable.
Cell-based proliferation assays using GM-CSF and IL-2 showed maintenance of protein bioactivity after release from the alginate-based cryogels, indicating neither the crosslinking method nor interaction with Laponite induced irreversible protein damage. Encapsulation of proteins in hydrogels crosslinked using chain growth polymerization of acrylates is a common strategy, but can lead to the damage of proteins by radical-mediated oxidation, denaturation, protein cleavage, or conjugation to the hydrogel.[21] In contrast, the click reaction between tetrazine and norbornene used for crosslinking the cryogels of this report does not react with or damage biological species.[22–24,33] Encapsulation within PLGA particles is a widely used technique for protein release, but can cause protein inactivation through various mechanisms.[5] The encapsulation-free approach used here, via interaction with Laponite, appears to avoid protein denaturation, although further validation with other proteins will be required.
Protein release profiles can be tuned by adjusting the Laponite content within hydrogels. Increasing the Laponite amount pre-mixed with proteins increases the potential surface area available for protein binding. Furthermore, an increased amount of Laponite likely hinders release by allowing an increased concentration of sites available for rebinding of proteins prior to exiting the gel. This effect was likely enhanced in this specific system due to the concentration of the Laponite in the gel walls, which comprise only a small fraction of the overall hydrogel volume. Facile modification of protein release kinetics may allow these drug delivery systems to be used to study the impact of protein release kinetics on biological responses to protein drugs.
1.5 Conclusions
In this work, the incorporation of Laponite XLG into hydrogels containing encapsulated proteins was demonstrated to slow protein release. Laponite could be retained in preformed click alginate cryogels while maintaining gel injectability. Bioactive proteins of varying size and charge could be delivered from the nanocomposite hydrogels in a sustained manner without burst release. The release kinetics of proteins from the gels could be tuned by varying the Laponite content within the gels. These results demonstrate that click alginate-Laponite nanocomposite hydrogels are a platform for controlled release of recombinant proteins. This strategy is likely to also be useful with a variety of other hydrogels used for sustained drug delivery.
Supplementary Material
Statement of Significance.
Here we present an injectable nanocomposite hydrogel for simple and versatile controlled release of therapeutic proteins. Protein release from hydrogels often requires first entrapping the protein in particles and embedding these particles within the hydrogel to allow controlled protein release. This pre-encapsulation process can be cumbersome, can damage the protein’s activity, and must be optimized for each protein of interest. The strategy presented in this work simply premixes the protein with charged nanoparticles that bind strongly with the protein. These protein-laden particles are then placed within a hydrogel and slowly release the protein into the surrounding environment. Using this method, tunable release from an injectable hydrogel can be achieved for a variety of proteins. This strategy greatly simplifies the design of hydrogel systems for therapeutic protein release applications.
Acknowledgments
We thank Akhilesh Gaharwar, Jianyu Li, Catia Verbeke, Luo Gu, and Ryland Bates for useful scientific discussions and helpful advice. Sandeep T. Koshy is grateful for support from an HHMI ISRF award. David K.Y. Zhang acknowledges support from the Canadian Institutes of Health Research (CIHR-DFSA). This work was supported by the National Institutes of Health (R01 EB015498; R01 HL021796) and the Wyss Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
1.7 References
- 1.Kearney CJ, Mooney DJ. Macroscale delivery systems for molecular and cellular payloads. Nat Mat. 2013;12:1004–1017. doi: 10.1038/nmat3758. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1:1–18. doi: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boontheekul T, Kong HJ, Mooney DJ. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials. 2005;26:2455–2465. doi: 10.1016/j.biomaterials.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 4.Lee J, Lee KY. Injectable Microsphere/Hydrogel Combination Systems for Localized Protein Delivery. Macromol Biosci. 2009;9:671–676. doi: 10.1002/mabi.200800317. [DOI] [PubMed] [Google Scholar]
- 5.van de Weert M, Hennink WE, Jiskoot W. Protein Instability in Poly(Lactic-co-Glycolic Acid) Microparticles. Pharm Res. 2000;17:1159–1167. doi: 10.1023/a:1026498209874. [DOI] [PubMed] [Google Scholar]
- 6.Dawson JI, Oreffo ROC. Clay: New Opportunities for Tissue Regeneration and Biomaterial Design. Adv Mater. 2013;25:4069–4086. doi: 10.1002/adma.201301034. [DOI] [PubMed] [Google Scholar]
- 7.Li K, Wang S, Wen S, Tang Y, Li J, Shi X, Zhao Q. Enhanced In Vivo Antitumor Efficacy of Doxorubicin Encapsulated within Laponite Nanodisks. ACS Appl Mater Interfaces. 2014;6:12328–12334. doi: 10.1021/am502094a. [DOI] [PubMed] [Google Scholar]
- 8.Wang S, Wu Y, Guo R, Huang Y, Wen S, Shen M, Wang J, Shi X. Laponite Nanodisks as an Efficient Platform for Doxorubicin Delivery to Cancer Cells. Langmuir. 2013;29:5030–5036. doi: 10.1021/la4001363. [DOI] [PubMed] [Google Scholar]
- 9.Ghadiri M, Hau H, Chrzanowski W, Agus H, Rohanizadeh R. Laponite clay as a carrier for in situ delivery of tetracycline. RSC Adv. 2013;3:20193–9. [Google Scholar]
- 10.Ghadiri M, Chrzanowski W, Rohanizadeh R. Antibiotic eluting clay mineral (Laponite®) for wound healing application: an in vitro study. J Mater Sci: Mater Med. 2014;25:2513–2526. doi: 10.1007/s10856-014-5272-7. [DOI] [PubMed] [Google Scholar]
- 11.Dawson JI, Kanczler JM, Yang XB, Attard GS, Oreffo ROC. Clay Gels For the Delivery of Regenerative Microenvironments. Adv Mater. 2011;23:3304–3308. doi: 10.1002/adma.201100968. [DOI] [PubMed] [Google Scholar]
- 12.Gibbs DMR, Black CRM, Hulsart-Billstrom G, Shi P, Scarpa E, Oreffo ROC, Dawson JI. Bone induction at physiological doses of BMP through localization by clay nanoparticle gels. Biomaterials. 2016;99:16–23. doi: 10.1016/j.biomaterials.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Chen P, Xu S, Wu R, Wang J, Gu R, Du J. A transparent Laponite polymer nanocomposite hydrogel synthesis via in-situ copolymerization of two ionic monomers. Appl Clay Sci. 2013;72:196–200. [Google Scholar]
- 14.Wu CJ, Gaharwar AK, Chan BK, Schmidt G. Mechanically Tough Pluronic F127/Laponite Nanocomposite Hydrogels from Covalently and Physically Cross-Linked Networks. Macromolecules. 2011;44:8215–8224. [Google Scholar]
- 15.Chang CW, van Spreeuwel A, Zhang C, Varghese S. PEG/clay nanocomposite hydrogel: a mechanically robust tissue engineering scaffold. Soft Matter. 2010;6:5157–8. [Google Scholar]
- 16.Gaharwar AK, Schexnailder PJ, Kline BP, Schmidt G. Assessment of using Laponite® cross-linked poly(ethylene oxide) for controlled cell adhesion and mineralization. Acta Biomater. 2011;7:568–577. doi: 10.1016/j.actbio.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Bencherif SA, Sands RW, Ali OA, Li WA, Lewin SA, Braschler TM, Shih TY, Verbeke CS, Bhatta D, Dranoff G, Mooney DJ. Injectable cryogel-based whole-cell cancer vaccines. Nat Commun. 1AD;6:1–13. doi: 10.1038/ncomms8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koshy ST, Ferrante TC, Lewin SA, Mooney DJ. Injectable, porous, and cell-responsive gelatin cryogels. Biomaterials. 2014;35:2477–2487. doi: 10.1016/j.biomaterials.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutiérrez MC, Ferrer ML, del Monte F. Ice-Templated Materials: Sophisticated Structures Exhibiting Enhanced Functionalities Obtained after Unidirectional Freezing and Ice-Segregation-Induced Self-Assembly. Chem Mater. 2008;20:634–648. [Google Scholar]
- 20.Lin CC, Sawicki SM, Metters AT. Free-Radical-Mediated Protein Inactivation and Recovery during Protein Photoencapsulation. Biomacromolecules. 2008;9:75–83. doi: 10.1021/bm700782c. [DOI] [PubMed] [Google Scholar]
- 21.McCall JD, Anseth KS. Thiol–Ene Photopolymerizations Provide a Facile Method To Encapsulate Proteins and Maintain Their Bioactivity. Biomacromolecules. 2012;13:2410–2417. doi: 10.1021/bm300671s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alge DL, Azagarsamy MA, Donohue DF, Anseth KS. Synthetically Tractable Click Hydrogels for Three-Dimensional Cell Culture Formed Using Tetrazine–Norbornene Chemistry. Biomacromolecules. 2013;14:949–953. doi: 10.1021/bm4000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desai RM, Koshy ST, Hilderbrand SA, Mooney DJ, Joshi NS. Versatile click alginate hydrogels crosslinked via tetrazine–norbornene chemistry. Biomaterials. 2015 doi: 10.1016/j.biomaterials.2015.01.048. [DOI] [PubMed] [Google Scholar]
- 24.Koshy ST, Desai RM, Joly P, Li J, Bagrodia RK, Lewin SA, Joshi NS, Mooney DJ. Click-Crosslinked Injectable Gelatin Hydrogels. Adv Healthcare Mater. 2016;5:541–547. doi: 10.1002/adhm.201500757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozlowski LP. IPC – Isoelectric Point Calculator. Biol Direct. 2016:1–16. doi: 10.1186/s13062-016-0159-9. [DOI] [PMC free article] [PubMed]
- 26.Bencherif SA, Sands RW, Bhatta D, Arany P, Verbeke CS, Edwards DA, et al. Injectable preformed scaffolds with shape-memory properties. Proc Natl Acad Sci USA. 2012;109:19590–19595. doi: 10.1073/pnas.1211516109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koshy ST, Mooney DJ. Biomaterials for enhancing anti-cancer immunity. Curr Opin Biotechnol. 2016;40:1–8. doi: 10.1016/j.copbio.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dávila JL, d’Ávila MA. Laponite as a rheology modifier of alginate solutions: Physical gelation and aging evolution. Carbohydr Polym. 2017;157:1–8. doi: 10.1016/j.carbpol.2016.09.057. [DOI] [PubMed] [Google Scholar]
- 29.Lee KY, Mooney DJ. Alginate: Properties and biomedical applications. Prog Polym Sci. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson DW, Butterworth JT. The nature of laponite and its aqueous dispersions. J Colloid and Interface Sci. 1992;151:236–243. [Google Scholar]
- 31.Pakulska MM, Elliott Donaghue I, Obermeyer JM, Tuladhar A, McLaughlin CK, Shendruk TN, et al. Encapsulation-free controlled release: Electrostatic adsorption eliminates the need for protein encapsulation in PLGA nanoparticles. Sci Adv. 2016;2:e1600519–e1600519. doi: 10.1126/sciadv.1600519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang C, Wang S, Li K, Ju Y, Li J, Zhang Y, et al. Preparation of Laponite Bioceramics for Potential Bone Tissue Engineering Applications. PLoS ONE. 2014;9:e99585–11. doi: 10.1371/journal.pone.0099585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devaraj NK, Weissleder R, Hilderbrand SA. Tetrazine-Based Cycloadditions: Application to Pretargeted Live Cell Imaging. Bioconjugate Chem. 2008;19:2297–2299. doi: 10.1021/bc8004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.