Abstract
Oral squamous cell carcinoma (OSCC) is preceded by progressive oral premalignant lesions (OPLs). Therefore, therapeutic strategies that prevent malignant progression of OPLs are expected to reduce the incidence of OSCC development. Immune checkpoint inhibitors that target the interaction of programmed death receptor 1 (PD-1) on T cells with the PD-1 ligand PD-L1 on cancer cells have been shown to extend the survival of patients with advanced OSCC. Here, we used the 4-Nitroquinoline-1-oxide (4-NQO) mouse model of oral carcinogenesis to test the hypothesis that PD-1 blockade may control the progression of OPLs. Mice were exposed to 4-NQO in their drinking water and then randomly assigned to two treatment groups that received either a blocking antibody for PD-1 or a control IgG. We found that anti-PD-1 treatment significantly reduced the number of oral lesions that developed in these mice, and prevented malignant progression. Low-grade dysplastic lesions responded to PD-1 blockade with a significant increase in the recruitment of CD8+ and CD4+ T cells and the accumulation of CTLA-4+ T cells in their microenvironment. Notably, PD-1 inhibition was accompanied by induction of interferon-γ, STAT1 activation and the production of the T cell effector granzyme B in infiltrating cells, and by the induction of apoptosis in the epithelial cells of the oral lesions, suggesting that T cell activation mediates the immunopreventive effects of anti-PD-1. These results support the potential clinical benefit of PD-1 immune checkpoint blockade to prevent OSCC development and progression and suggest that CTLA-4 inhibitors may enhance the preventive effects of anti-PD-1.
Keywords: Oral cancer, premalignant lesions, PD-1, immunoprevention, 4-NQO
INTRODUCTION
Oral cavity squamous cell carcinoma (OSCC) is an aggressive disease, with an overall survival rate of 50% that has remained unchanged for decades (1). Carcinomas of tongue are the most common OSCC (25–80%), with lower incidence for tumors that develop on the floor of the mouth, buccal mucosa, lips and palate (2–6). In addition to the high fatality rates, OSCC is associated with severe morbidity, and is complicated by the frequent multifocal tumor development and the high rates of recurrence and second primary tumor development (7). This field cancerization effect results from the molecular changes that accumulate in the oral epithelial cells upon exposure to carcinogens (8). Tobacco, alcohol, and/or betel nut exposure are well-established etiological factors for OSCC. While interruption of carcinogen exposure clearly reduces the oral cancer incidence, a subset of lesions may be at high risk of progression even after carcinogen cessation. Therefore, OSCC is a malignancy that may benefit from effective cancer prevention strategies that block or reverse the carcinogenesis process prior to malignant transformation (9).
While surgical resection of advanced oral premalignant lesions (OPLs) has become the standard of care, individuals with OPLs in multiple sites or with those arising in sites that are not amenable to surgical prevention by removal of at-risk tissues are in need of alternative prevention strategies, such as chemoprevention, in which medications are administered with the goal of attenuating or eliminating OPLs or preventing them from continuing down the multi-step pathway of carcinogenesis to frankly invasive cancers. Several chemopreventive agents have been tested over the past years for their efficacy in OSCC prevention (10–14). Despite initial promising results, chemoprevention has not been consistently reproduced and toxicity has been often a limiting factor. Therefore, chemoprevention strategies for OSCC are not yet available in the clinic.
Preclinical and clinical evidences have revealed that early transformed cells express antigens that allow the immune system to recognize them and initiate an immune response (15, 16). To overcome the antineoplastic effects of the immune system, tumor cells may transmit immune suppressive signals to the tumor microenvironment that enable cancer progression (17, 18).
PD-1 is a molecule expressed on several immune cells, including cytotoxic T cells, that attenuates the immune response after engagement with PD-L1, a PD-1 ligand that is expressed in the cancer cells. One particularly promising area of immunotherapy has been the generation of antibodies that can target immune checkpoint signals from tumor cells that interact with cytotoxic T cells, including the PD-L1/PD-1 axis (19). Blockade of PD-1-dependent immune checkpoints using monoclonal antibodies directed against PD-1 can alleviate the local immunosuppression and thereby, augment the T cell response against tumor cells. Notably, anti-PD-1 antibodies have been shown to be active agents in the treatment of OSCC (20). A recent report has shown that treatment of patients with platinum-refractory recurrent OSCC with the anti-PD-1 antibody, Nivolumab, results in longer survival than treatment with standard single agent chemotherapy, making this approach a new standard of care for these patients (21). Based on this exciting progress and the overall tolerability of the checkpoint inhibitors, we have become interested to know whether this could be a useful approach to prevent progression of OPLs to invasive OSCC.
The 4-Nitroquinoline-1-oxide (4-NQO) mouse model of oral carcinogenesis is an ideal model for cancer prevention studies because mice exposed to the carcinogen 4-NQO in their drinking water develop a range of oral lesions representative of all the stages of oral carcinogenesis. Several lines of evidence indicate that the histological and molecular changes induced by 4-NQO are similar to those found in human OPLs and OSCCs (22–25). The oral carcinogenesis process can be further accelerated by using mice with increased susceptibility to carcinogens, such as mice deficient for the tumor suppressor gene Trp53 (p53) (26, 27). Indeed, heterozygous p53 knockout mice (p53+/−) have been extensively used in chemoprevention studies for several carcinogen-induced malignancies (28).
In the current study, we investigated the preventive properties of a monoclonal anti-PD-1 antibody during 4-NQO–induced oral carcinogenesis in p53+/− mice, and we examined how PD-1 blockade affects the immune infiltrates of the oral lesions. We found that the anti-PD-1 antibody decreased the formation of oral dysplastic lesions, prevented their progression to SCC and induced specific patterns of expression of immune-modulatory receptors on the T cell infiltrates of OPLs, indicating that this is a prevention strategy that would be worthwhile evaluating in patients with OPLs at high risk of progression to oral cancer.
MATERIALS AND METHODS
Experimental animal model and treatments
Heterozygous p53 knockout mice (p53+/−) in a C57BL/6J background (females, 6–8 weeks old) were purchased from The Jackson Laboratory (Bar Arbor, ME, USA), strain #002101 (29). A stock solution of 4-NQO (50 mg/mL) was prepared by dissolving 4-NQO powder (Sigma-Aldrich, St Louis, MO, USA) in DMSO, and stored at −20°C until used. To administer mice with 4-NQO, the stock solution was added to the drinking water supplemented with 1% sucrose (Fisher Scientific, Pittsburg, PA, USA) at a final concentration of 100 µg/mL. Twenty-two p53+/− mice were given a fresh batch of 4-NQO-containing water every week for 8 weeks. In Vivo mAb anti-mouse PD-1 (RMP1–14, #BE0146) and the isotype control IgG2a (#BE0089) were purchased from Bio×Cell (West Lebanon, NH, USA), dissolved in PBS and stored at 4°C until used. Mice were injected intraperitoneally with anti-PD-1 (250µg/mouse) or IgG2a (250µg/mouse), twice a week for 4 weeks, as in previous studies (30–32). All animals underwent full oral cavity examination twice a week and euthanized for tissue retrieval five weeks after initiation of the antibody injection. All animal studies were carried out according to The University of Texas MD Anderson Cancer Center Institutional Animal Care and Use Committee (IACUC)-approved protocols.
Tissue dissection and sectioning
To harvest oral tissue, the mice were sacrificed following IACUC guidelines. Immediately after death, the tongues were excised and macroscopic lesions were counted and photographed. Quantified macroscopic lesions comprised those lesions that were visible, with a diameter of >1–2 mm, and usually presented with a whitish and papillary appearance. The tongues were longitudinally bisected, and fixed in 10% neutral-buffered formalin at room temperature overnight. Then, the tissue was transferred to 70% ethanol and embedded in paraffin. Twenty 5-µm histological sections from each specimen were cut and the 10th slide was stained with hematoxylin and eosin (H&E) for histopathologic analysis. H&E sections were screened under the microscope for the presence of microscopic lesions. Individual lesions were quantified along the entire longitudinal section of the tongue. The rest of the sections were processed for immunohistochemistry.
Histopathological analysis of tongue lesions
H&E-stained sections obtained from the tongues of 4-NQO-exposed mice were examined by a pathologist blinded to treatment groups. Dysplasia was defined as loss of polarity in the epithelial cells, nuclear pleomorphism and hyperchromasia, hyperkeratosis and parakeratosis, and increased or abnormal mitoses. Lesions with alterations limited to the lower one third or two thirds of the epithelium thickness were classified as mild or moderate dysplasia, respectively, and considered as low-grade lesions. Lesions in which these changes involved over two thirds of the epithelium were graded as severe dysplasia, and those that involved the full-thickness epithelium were classified as carcinoma in situ (CIS) (33). Invasive carcinoma was defined as a lesion with invasion through the membrane basement into the subepithelial tissues. Severe dysplasias and carcinomas were considered high-grade lesions (33).
Immunohistochemistry
Immunohistochemical analyses (IHC) were carried out on unstained sections adjacent to those used for pathological assessment of the lesions. The anti-PD-1antibody (#135202) was obtained from BioLegend (San Diego, CA, USA). Antibodies for CD4 (#14-0042-85) and Foxp3 (#14-5773) were from eBioscience (Santa Clara, CA, USA). Anti-CD8a (#361003) was from Synaptic Systems (Göttingen, Germany). The anti-PD-L1 antibody (#17952-1-AP) was obtained from Proteintech Group (Chicago, IL, USA). The anti-4-1BB (#ab203391) and anti-OX40 (#ab203220) were from Abcam Inc. (Cambridge, MA, USA). The anti-CTLA-4 (#orb253158) was obtained from Biorbyt Ltd. (San Francisco, CA, USA). The anti-interferon-γ antibody (# AF-585-NA) was from R&D Systems (Minneapolis, MN, USA). The pSTAT1 antibody (# 14994) was obtained from Cell Signaling (Danvers, MA, USA) and the Anti-Granzyme B antibody (#14-8822-80) was from ThermoFisher Scientific (Waltham, MA, USA). IHC was performed using the Leica Bond Max automated stainer (Leica Biosystems Buffalo Grove, IL, USA) in the Division of Surgery Histology Laboratory (MD Anderson Cancer Center). All slides were stained using previously optimized conditions with appropriate positive and negative controls. Images were captured on a DMLA microscope equipped with a DFC310 FX camera (Leica Microsystems, Buffalo Grove, IL, USA). Staining for CD4, CD8, FOXP3, PD-1, PD-L1, OX40, CTLA-4, and 4-1BB was scored as density of cells, defined as the number of positive cells per mm2.
TUNEL Assay with K14 immunofluorescent double staining
For detection of apoptosis in the oral lesions, the terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) assay was performed. To identify apoptosis in the epithelial component of the oral lesions we performed double staining for TUNEL and Keratin 14 (K14), an intermediate filament protein expressed in stratified epithelia (34). The assay was carried out according the manufacturer’s manual (Promega, #G3250), slightly modified for the double staining. Briefly, histological sections from paraffin-embedded tissue were deparaffinized with xylene and rehydrated with alcohol series. The samples were fixed in a Methanol/acetone (3/1) solution, permeabilized with Triton X-100, and incubated with the Terminal Deoxynucleotidyl Transferase (TdT) and a nucleotide mix containing fluorescein-12-dUTP. The slides were then incubated with a rabbit anti-mouse-K14 antibody (Covance, #PRB-155P) and a polyclonal secondary antibody to mouse IgG labeled with Alexa Fluor® 594 (# A-21207, Thermofisher Scientific). The samples were mounted and counterstained with Vectashield mounting medium containing DAPI (#H-1500, Vector Laboratories, Burlingame, CA). The slides were examined on an Olympus IX71 fluorescence microscope (Olympus, Center Valley, PA, USA), and images were captured using a Hamamatsu ORCA-ER camera (Hamamatsu, Shizuoka, Japan). Positive cells were counted from 3 randomly selected high power fields (×40) from each section and the density of TUNEL was defined as the number of positive cells per mm2.
Statistical analysis
Data analysis was performed with GraphPad Prism version 6 for Windows (San Diego, CA, USA). Two-tailed, unpaired t test was used to analyze differences in multiplicity of the oral lesions and analysis of the expression of biomarkers. The incidence of oral lesions was evaluated with the Chi-square test. P value of less than 0.05 were considered statistically significant, defined as P≤0.05*, P≤ 0.01**, P≤ 0.001***.
RESULTS
A monoclonal anti-PD-1 antibody significantly inhibits malignant progression of 4-NQO-induced oral lesions
To assess the preventive potential of PD-1 immune checkpoint blockade in oral cancer development, we exposed 22 p53+/− mice to 4-NQO (100 µg/mL) in their drinking water for 8 weeks. One week after completion of the 4-NQO treatment all of the mice showed white patches on their tongues, indicating the appearance of hyperkeratotic and/or dysplastic lesions. Initiation of PD-1 antibody treatment was chosen at this time point because it mimics the clinical setting for preventive therapy. The mice were randomly distributed into treatment (12 mice) and control groups (10 mice), and they were injected with anti-PD-1 antibody (250µg/mouse) or an equal volume of an isotype control IgG2a (250µg/mouse) respectively, twice a week for 4 weeks. The mice were sacrificed one week later and their tongues were processed to compare the incidence of both low-grade and high-grade lesions in the control and anti-PD-1 antibody treated groups.
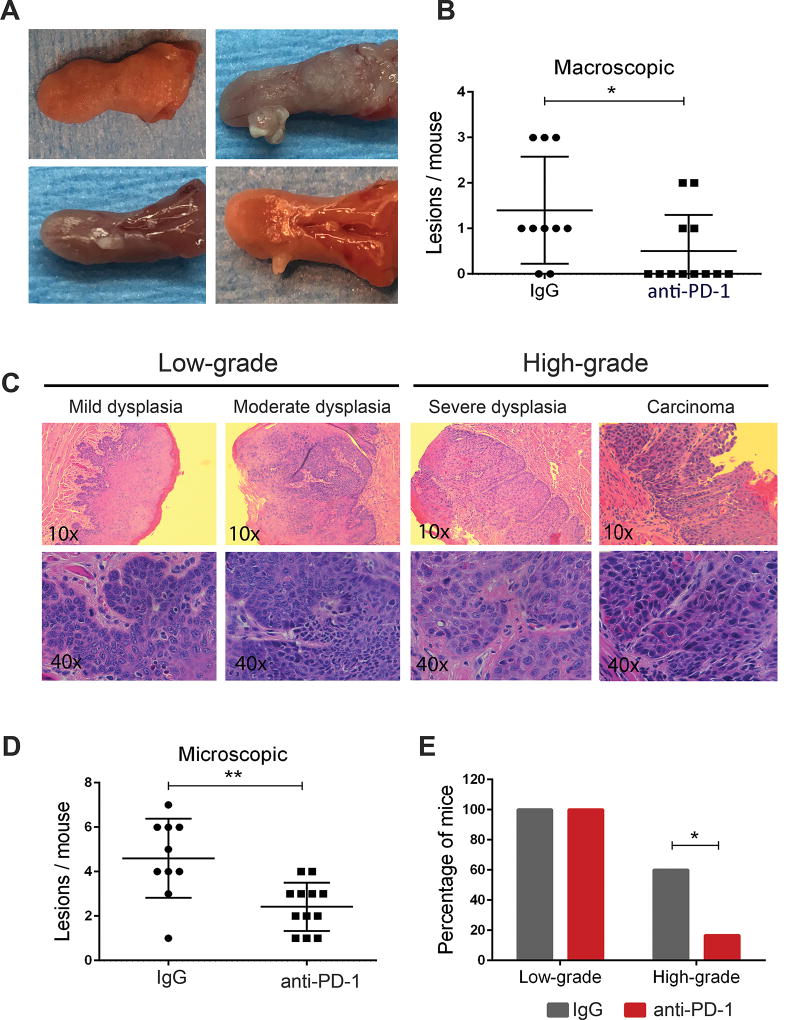
We observed that mice treated with anti-PD-1 had developed a significantly lower number of visible oral tongue lesions than control mice, suggesting that the PD-1 blockade prevented the growth of the early lesions that had developed in these mice (Fig. 1A and B). Similarly, we noted that anti-PD-1 treatment reduced the number of microscopic lesions compared with control mice (Fig. 1C and D; Table 1, P<0.05). Blinded histopathological assessment by two independent reviewers revealed that all of the mice in control and treatment groups had developed low-grade lesions, consisting of mild and/or moderate dysplasia (Fig. 1C and Table 1). In addition, 60% (6/10) of the mice in the control group were found to have lesions that are considered high-grade disease (severe dysplasia or carcinoma), whereas only 16.7% (2/12) of the anti-PD-1 treated mice had such lesions (Fig. 1E, Table 1, P<0.05). Of those, severe dysplasia was found in 50% (5/10) of the mice in the control group, compared to only 8.3% (1/12) of the mice treated with anti-PD-1 (Table 1, P<0.05). A similar trend was observed for carcinomas (CIS or invasive SCCs), which were observed on the tongues of 40% (4/10) of the control mice, but only in 8.3% (1/12) of the anti-PD-1 group, although this difference did not reach significance (Table 1, P=0.077). Overall, these observations represented a 43.3% decrease in high-grade lesions noted with the anti-PD-1 antibody treatment and suggest that anti-PD-1 treatment can prevent the malignant progression of OPLs.
Figure 1.
PD-1 blockade prevents oral cancer development. (A) Representative images of the gross appearance of the oral lesions that developed in mice exposed to 4-NQO. The top left panel shows the normal appearance of an untreated tongue and the rest of the panels show visible oral lesions induced by 4-NQO. (B) Quantification of the macroscopic lesions observed in control (IgG) and anti-PD-1 groups, at the completion of the study, 14 weeks after initiation of the 4-NQO treatment. (C) H&E staining of 4-NQO-induced oral lesions representative of different stages of carcinogenesis. (D) Number of microscopic lesions scored by counting the individual lesions detected in each mouse. (E) Incidence rate for low grade oral lesions (mild and moderate dysplasia) and high grade lesions (severe dysplasia and carcinoma) that developed in control and PD-1-treated mice. *P<0.05, **P<0.01.
Table 1.
Incidence and multiplicity of the 4-NQO-induced oral lesions according to their pathological grade and treatment groups.
| Low-grade
|
High-grade
|
Total
|
||||||
|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Total | Severe | Carcinoma | Total | |||
| Incidence# | Control-IgG | 10(100%) | 9 (90%) | 10(100%) | 5(50%) | 4(40%) | 6(60%) | 100% |
| PD-1 Ab | 12(100%) | 8(66.7%) | 10(100%) | 1(8.3%)* | 1(8.3%) | 2(16.7%)* | 100% | |
|
| ||||||||
| Multiplicity# | Control-IgG | 27(2.7) | 10(1) | 37(3.7) | 5(0.5) | 5(0.5) | 10(1) | 47(4.7) |
| PD-1 Ab | 23(1.91) | 3 (0.25) | 26(2.17) | 3(0.25) | 1(0.08) | 4(0.33) | 30(2.5)* | |
Control IgG (n=10). PD-1 Ab (n=12).
Mild, moderate, severe refer to mild dysplasia, moderate dysplasia and severe dysplasia, respectively.
Incidence is indicated as the number of mice with lesions of each pathological grade detected in each group. The percentage is shown in parentheses.
Multiplicity is indicated as the total number of lesions of each pathological grade detected in each group, with the mean indicated in parentheses.
P<0.05 for comparisons between the control-IgG and the corresponding PD-1 Ab group.
PD-1 blockade promotes T cell infiltration in 4-NQO-induced OPLs
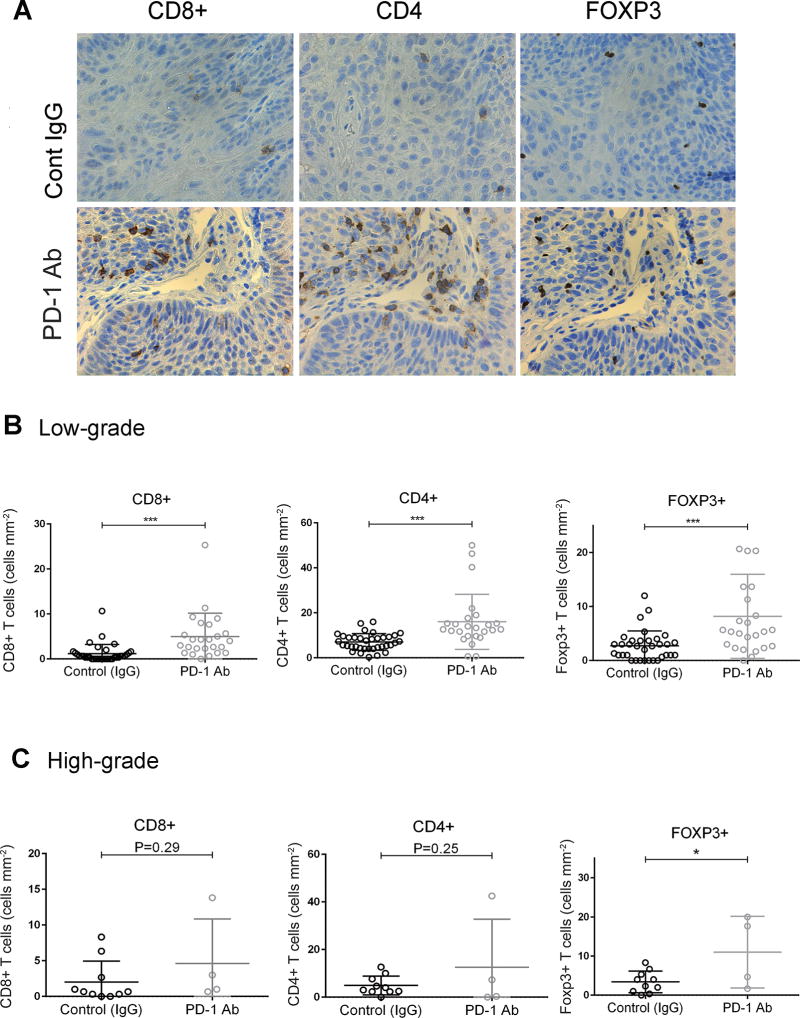
To determine whether anti-PD-1 treatment altered T cell infiltration in oral lesions induced by 4-NQO in mice we conducted an immunohistochemical analysis with specific antibodies for T cell markers. We observed a significant increase in CD8+ T cell infiltration in low-grade dysplastic lesions from mice that had been treated with the anti-PD-1 antibody, compared with those in the control group (Fig. 2A and B, P < 0.001). There was also a significant increase in the number of CD4+ and Foxp3+ cells in low-grade dysplastic samples in the PD-1 antibody treatment group, compared with the control group. (Fig. 2A and B, P<0.001). However, in high-grade lesions, there were no significant differences in CD8+ and CD4+ staining between samples from control and anti-PD-1-treated mice (Fig. 2C). Interestingly, the number of Foxp3+ cells was higher in high-grade lesions from mice treated with anti-PD-1 than in those from control mice (Fig. 2C, P < 0.05). These findings suggest that recruitment of CD8+ and CD4+ T cells in response to PD-1 blockade may contribute to prevent progression and/or eliminate low-grade lesions. The accumulation of Foxp3+ both in low and high-grade suggests that regulatory T cells (Tregs) may allow immune escape.
Figure 2.
Recruitment of CD8+, CD4+ and Foxp3+ cells into oral lesions in response to anti-PD-1 treatment. (A) Immunohistochemical staining for CD8, CD4 and Foxp3 in low-grade oral lesions induced by 4-NQO in control and anti-PD-1-treated mice. (B–C) Quantification of the number of positive cells for the indicated markers in (B) low-grade and (C) high-grade lesions. *P<0.05, ***P<0.001.
Immune checkpoint alterations in response to PD-1 blockade
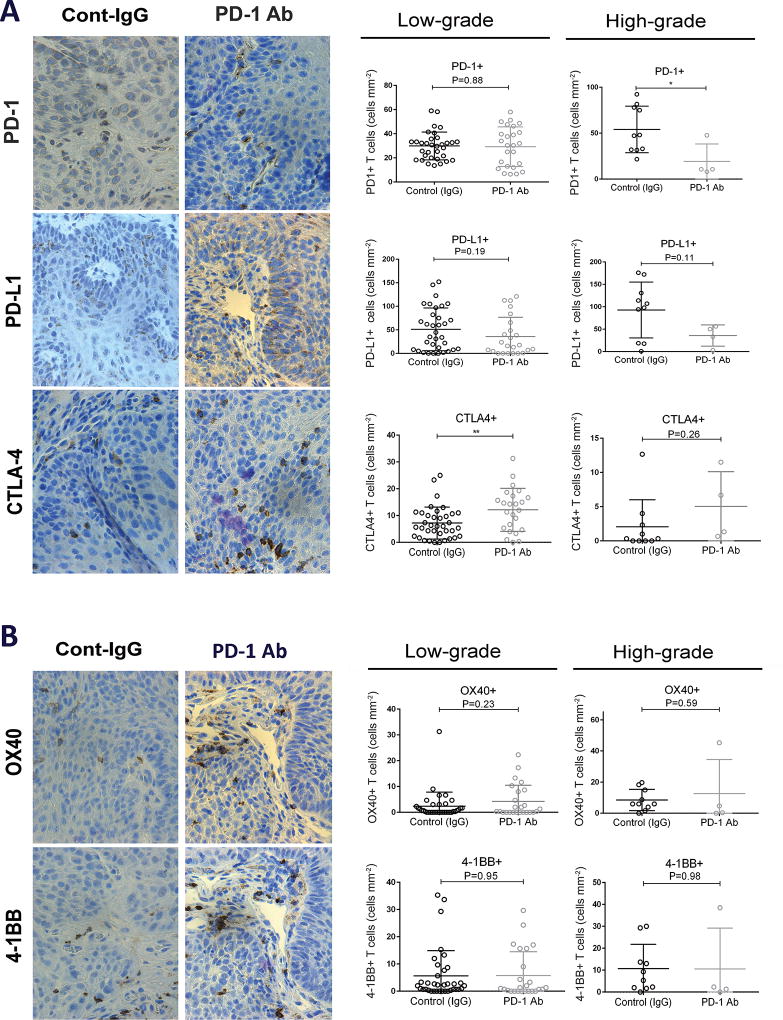
To assess the impact of PD-1 blockade on the expression of immune checkpoint proteins, we stained the oral lesions induced by 4-NQO with antibodies for PD-1, PD-L1 and CTLA-4. We found that PD-1+ T cell infiltration was similar in low-grade lesions from mice that had been exposed to the anti-PD-1 antibody and in control mice (Fig. 3A). Notably, the number of PD-1+ infiltrating cells was significantly lower in high-grade lesions that developed in mice treated with anti-PD-1 than in those form control mice (Fig. 3A). The expression of the PD-1 ligand PD-L1 did not change by the anti-PD-1 treatment, although a trend toward lower expression in high-grade lesions from mice treated with anti-PD-1 was observed (Fig. 3A). Of note, we observed that in control mice the number of both PD-1+ and PD-L1 + cells increased in high-grade lesions compared to low-grade dysplastic, whereas this trend was not observed in mice treated with the anti-PD-1 antibody, which may be explained by the lower levels of PD-1/PD-L1 in high–grade lesions from mice treated with anti-PD-1 (Supplementary Fig. S1A and S1B).
Figure 3.
Expression of immune checkpoint and costimulatory molecules in the immune infiltrates of oral lesions induced by 4-NQO. (A) Immunohistochemical images for the immune checkpoint molecules PD-1, PD-L1 and CTLA-4 in low-grade oral lesions, and the corresponding quantification in low-grade and high-grade lesions from control and anti-PD-1 groups. (B) Immunohistochemical analysis for the costimulatory molecules OX40 and 4-1BB in the indicated oral lesions. *P<0.05, **P<0.01.
We noted increased CTLA-4+ T cell infiltration in low-grade dysplasia from mice treated with anti-PD-1, compared to those from control mice (Fig. 3A), indicating that PD-1 checkpoint blockade was associated with the accumulation of CTLA-4+ T cells in early stage OPLs. On the other hand, high-grade lesions from control and PD-1-treated mice had similar levels of CTLA-4+ cells (Fig. 3A). Interestingly, high-grade dysplastic lesions showed significantly lower density of infiltrating CTLA-4+ T cells compared to that seen in low-grade dysplasia in control group, and a similar trend was observed in the anti-PD-1 treated mice (Supplementary Fig. S1C).
Lastly, we also analyzed the expression of the OX40 and 4-1BB, co-stimulatory molecules that promote T cell activation, and for which agonistic antibodies have shown strong anti-tumor effects in preclinical studies (35–37). We found that treatment with PD-1 antibody did not induce a significant change in the number of OX40+ or 4-1BB+ infiltrates in either low- or high-grade oral lesions (Fig. 3B), suggesting that these co-stimulatory molecules were not involved in the response to PD-1-blockade. The OX40+ staining of T cells significantly increased during progression of low-grade to high-grade lesions in the control and anti-PD-1 groups (Supplementary Fig. S2A). On the other hand, the number of 4-1BB+ cells remained unchanged during progression form low- to high-grade lesion in both experimental groups (Supplementary Fig. S2B).
PD-1 blockade promotes T cell activation and induction of apoptosis in oral lesions
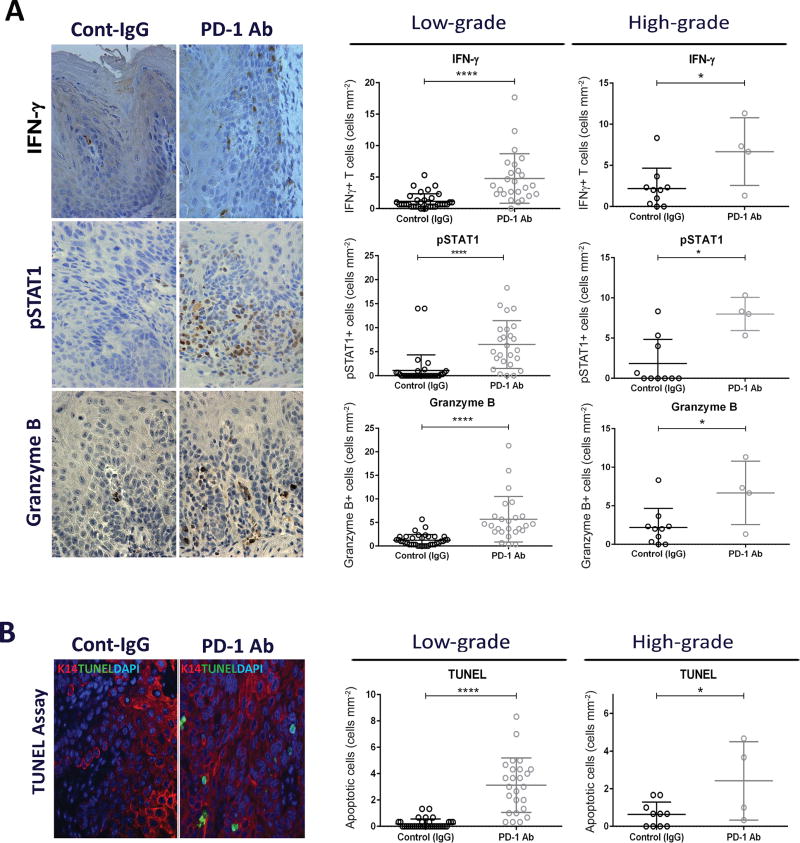
To determine whether the immunoprevention effects elicited by the anti-PD-1 treatment during oral carcinogenesis involved T cell activities, we analyzed the expression of interferon-γ (IFNγ), a cytokine produced by activated T cells that has been found to be upregulated in response to PD-1 blockade (38). We observed a significant increase in the number of infiltrating T cells expressing IFNγ in both low- and high-grade lesions from anti-PD-1-treated mice compared to their respective controls (Fig. 4A). The IFNγ activity in these lesions was further supported by the higher levels of pSTAT1, an immediate downstream mediator of IFNγ signaling (Fig. 4A) (39). Additionally, higher levels of the effector protease granzyme B were detected in low- and high-grade oral lesions from mice treated with the anti-PD-1 antibody compared to those from mice treated with control IgG, suggesting an increased cytotoxic T cell activity in response to anti-PD-1 treatment (Fig. 4A). The levels of IFNγ, pSTAT1 and granzyme B were similar in low- and high-grade lesions, for control and anti-PD-1 groups (Supplementary Fig. S3A–C).
Figure 4.
T cell activation and induction of apoptosis in epithelial cells of oral lesions induced by 4-NQO. (A) Immunohistochemical images for IFNγ, pSTAT1 and Granzyme B in low-grade oral lesions, and the corresponding quantification in low-grade and high-grade lesions from control and anti-PD-1 groups. (B) Double staining for TUNEL and K14 and quantification of apoptosis in oral lesions from control and anti-PD-1 groups. *P<0.05, **P<0.01, ***P<0.001, ***P<0.0001.
To assess whether the increased T cell activity was associated with induction of cell death, we performed double staining for TUNEL, to detect apoptosis, and for Keratin 14 (K14) to highlight the epithelial component of the oral lesions. Remarkably, we observed a significantly higher number of apoptotic cells in the oral lesions that developed in mice that had been treated with the anti-PD-1 antibody than in control lesions, for both low- and high-grade lesions (Fig. 4B). The K14 staining confirmed that the apoptotic cells were located in the epithelial component of the lesions. In addition, we observed that the number of apoptotic cells remained similar in low- and high-grade lesions from mice treated with anti-PD-1, suggesting a comparable sensitivity to apoptosis induced in response to anti-PD-1 (Supplementary Fig. S3D). In the control group, however, increased levels of apoptosis were found in high-grade lesions, which may reflect an increased cell turnover in high-grade lesions (Supplementary Fig. S3D). Overall, these observations suggests that PD-1 blockade resulted in T cell activation and the induction of apoptosis in the oral lesions induced by 4-NQO.
DISCUSSION
The recent clinical success of immune checkpoint blockade in the treatment of advanced OSCC is changing the standard of care of this malignancy (21). The efficacy of immunotherapies in a prevention setting, however, has not been well explored. The current study examined a monoclonal antibody for the immune checkpoint molecule PD-1 as a potential preventive therapy in OSCC development, using the 4-NQO model or oral carcinogenesis. Our data demonstrated a 43.3% decrease in high-grade lesions (carcinomas or severe dysplasia) in mice treated with the anti-PD-1 antibody, strongly supporting the concept of immunoprevention as a novel strategy to prevent malignant progression of oral premalignant lesions. As a prevention approach that involves the administration of medications, safety is a major concern to allow implementation in patients that have not yet developed malignant lesions (40). Although more data are necessary to evaluate the extent of the side effects in largely healthy patients, as those that can benefit from preventive interventions, concerns about secondary effects may be offset by the potential benefits in high risk populations, such as those with OPLs that exhibit LOH (14, 41). As the immunotherapy field is rapidly evolving, new more specific drugs are being designed, with expected lower side effects.
This study was designed to analyze the immediate impact of PD-1 immune checkpoint blockade in preventing progression of OPLs. For this reason, we terminated the study one week after completion of the anti-PD-1 treatment. We found that at the completion of the study, 14 weeks after initiation of 4-NQO treatment, all the mice had developed low-grade dysplasias. In addition to those, 60% of the control mice had developed high-grade lesions, compared to only 16.7% of the mice that had been treated with the anti-PD-1 antibody. These observations support the immunoprevention potential of PD-1 blockage in containing progression or OPLs. While the long-term benefits of this treatment could not be assessed in this study, accumulating evidence suggest that immunotherapies such as anti-PD-1 produce sustained responses in a variety of malignancies, including OSCC (42–44). Future studies will explore the long-term effects of preventive treatment with anti-PD-1 antibodies for OPLs.
The increased density of CD8+ and CD4+ immune infiltrates in low-grade dysplasia from mice that had been treated with anti-PD-1 antibody suggested that PD-1 blockade may prevent OPL progression by activating effector T cells, such as CD8+ and CD4+ T cells, to eliminate premalignant or malignant oral epithelial cells, a mechanism that has also been observed in cancer patients treated with immune checkpoint inhibitors (30, 45). Indeed, we detected increased T cell activation in oral lesions from mice that were treated with anti-PD-1, including the production of granzyme B which may contribute to the induction of apoptosis in the epithelial cells of the oral lesions.
Although we observed a positive response to PD-1 blockade, a subset of lesions continued to progress even after anti-PD-1 treatment. Interestingly, we found significantly lower levels of PD-1+ cells in high-grade lesions from mice that had received anti-PD-1 treatment compared to controls, suggesting that progression of OPLs with low levels of PD-1+ cells may not be blocked by the anti-PD-1 treatment. This is consistent with the increase levels of PD-1/PD-L1 found in melanoma patients that responded to PD-1 blockade, compared with non-responders (39, 45). Of note, the number of CD8+ and CD4+ infiltrating cells in high-grade lesions was similar in control and PD-1-treated mice, suggesting that the lower expression of PD-1 did not result from a general decrease in T cell infiltration. This observation, together with increased CD8+ and CD4+ infiltration in low-grade lesions from PD-1-treated mice, in the absence of PD-1 upregulation, suggests that PD-1 and CD8/CD4 are independently regulated in response to PD-1 blockade.
Additional mechanism of resistance to PD-1 blockade involve the upregulation of alternative immune checkpoints. We identified a potential compensatory mechanism that involves CTLA-4, another immune checkpoint protein that blocks the immune response in human tumors (46). Blocking anti-CTLA-4 antibodies have been shown to generate strong therapeutic response and extended overall survival in melanoma patients, as a single agent or in combination with PD-1 inhibitors (47–49). In our study, we found that low-grade dysplastic lesions from mice that had been treated with anti-PD-1 contained significantly higher levels of CTLA-4+ cells than those from control mice, suggesting that CTLA-4-dependent immune checkpoints induced in response to PD-1 blockade may compensate for PD-1 inhibition. These observations predict a potential benefit of combinatorial therapies with PD-1 and anti-CTLA-4 inhibitors to prevent progression of early stage OPLs.
In addition to immune checkpoint blockade, the anti-tumor immune response may be enhanced upon engagement of co-stimulatory receptors such as OX40 and 4-1BB (35–37, 50). We found that the anti-PD-1 treatment did not alter the infiltration of cells expressing OX40 and 4-1BB in OPLs, suggesting that these molecules are regulated by mechanisms independent of the PD-1/PD-L1 axis and may not contribute to the immune preventive effects of PD-1 blockade.
Overall, in this study we provide evidence for the potential clinical benefit of anti-PD-1 antibodies to prevent malignant progression of OPLs. The data strongly suggests that progression of emerging OPLs can be contained with PD-1 immune checkpoint inhibitors. Upregulation of CTLA-4 in response to PD-1 blockade suggests that combining PD-1 and CTLA-4 inhibitors may improve the outcomes. The preclinical data obtained with this experimental model or oral carcinogenesis provide knowledge that will help us to design effective trials for prevention treatment of patients considered at high risk of developing OSCCs.
Supplementary Material
Acknowledgments
We would like to thank Drs. Laura Bover and Michael Curran for their valuable advice in the anti-PD-1 administration.
Financial support: This work was supported by the Floyd and Kathleen Cailloux Research Endowment and the NIH grant R01DE014613 (JNM).
Footnotes
Conflict of interest: The authors declare no potential conflicts of interest.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Li R, Koch WM, Fakhry C, Gourin CG. Distinct epidemiologic characteristics of oral tongue cancer patients. Otolaryngol Head Neck Surg. 2013;148:792–6. doi: 10.1177/0194599813477992. [DOI] [PubMed] [Google Scholar]
- 3.Selvamani M, Yamunadevi A, Basandi PS, Madhushankari GS. Prevalence of oral squamous cell carcinoma of tongue in and around Davangere, Karnataka, India: A retrospective study over 13 years. J Pharm Bioallied Sci. 2015;7:S491–S494. doi: 10.4103/0975-7406.163511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andisheh-Tadbir A, Mehrabani D, Heydari ST. Epidemiology of squamous cell carcinoma of the oral cavity in Iran. J Craniofac Surg. 2008;19:1699–702. doi: 10.1097/SCS.0b013e31818c04cc. [DOI] [PubMed] [Google Scholar]
- 5.Bagan J, Sarrion G, Jimenez Y. Oral cancer: clinical features. Oral Oncol. 2010;46:414–7. doi: 10.1016/j.oraloncology.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Vigneswaran N, Williams MD. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;26:123–41. doi: 10.1016/j.coms.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Day GL, Blot WJ. Second primary tumors in patients with oral cancer. Cancer. 1992;70:14–9. doi: 10.1002/1097-0142(19920701)70:1<14::aid-cncr2820700103>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 8.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–8. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 9.Leeman RF, McKee SA, Toll BA, Krishnan-Sarin S, Cooney JL, Makuch RW, et al. Risk factors for treatment failure in smokers: relationship to alcohol use and to lifetime history of an alcohol use disorder. Nicotine Tob Res. 2008;10:1793–809. doi: 10.1080/14622200802443742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papadimitrakopoulou VA, William WN, Jr, Dannenberg AJ, Lippman SM, Lee JJ, Ondrey FG, et al. Pilot randomized phase II study of celecoxib in oral premalignant lesions. Clin Cancer Res. 2008;14:2095–101. doi: 10.1158/1078-0432.CCR-07-4024. [DOI] [PubMed] [Google Scholar]
- 11.Papadimitrakopoulou VA, Lee JJ, William WN, Jr, Martin JW, Thomas M, Kim ES, et al. Randomized trial of 13-cis retinoic acid compared with retinyl palmitate with or without beta-carotene in oral premalignancy. J Clin Oncol. 2009;27:599–604. doi: 10.1200/JCO.2008.17.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.William WN, Jr, Lee JJ, Lippman SM, Martin JW, Chakravarti N, Tran HT, et al. High-dose fenretinide in oral leukoplakia. Cancer Prev Res (Phila) 2009;2:22–6. doi: 10.1158/1940-6207.CAPR-08-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saba NF, Hurwitz SJ, Kono SA, Yang CS, Zhao Y, Chen Z, et al. Chemoprevention of head and neck cancer with celecoxib and erlotinib: results of a phase ib and pharmacokinetic study. Cancer Prev Res (Phila) 2014;7:283–91. doi: 10.1158/1940-6207.CAPR-13-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.William WN, Jr, Papadimitrakopoulou V, Lee JJ, Mao L, Cohen EE, Lin HY, et al. Erlotinib and the Risk of Oral Cancer: The Erlotinib Prevention of Oral Cancer (EPOC) Randomized Clinical Trial. JAMA Oncol. 2016;2:209–16. doi: 10.1001/jamaoncol.2015.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schumacher TN, Hacohen N. Neoantigens encoded in the cancer genome. Curr Opin Immunol. 2016;41:98–103. doi: 10.1016/j.coi.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Umar A. Cancer immunoprevention: a new approach to intercept cancer early. Cancer Prev Res (Phila) 2014;7:1067–71. doi: 10.1158/1940-6207.CAPR-14-0213. [DOI] [PubMed] [Google Scholar]
- 17.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–30. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 18.Ferris RL. Immunology and Immunotherapy of Head and Neck Cancer. J Clin Oncol. 2015;33:3293–304. doi: 10.1200/JCO.2015.61.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;19:1021–34. doi: 10.1158/1078-0432.CCR-12-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitz S, Machiels JP. Targeting the Tumor Environment in Squamous Cell Carcinoma of the Head and Neck. Curr Treat Options Oncol. 2016;17:37. doi: 10.1007/s11864-016-0412-6. [DOI] [PubMed] [Google Scholar]
- 21.Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375:1856–67. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanojia D, Vaidya MM. 4-nitroquinoline-1-oxide induced experimental oral carcinogenesis. Oral Oncol. 2006;42:655–67. doi: 10.1016/j.oraloncology.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Tang XH, Knudsen B, Bemis D, Tickoo S, Gudas LJ. Oral cavity and esophageal carcinogenesis modeled in carcinogen-treated mice. Clin Cancer Res. 2004;10:301–13. doi: 10.1158/1078-0432.ccr-0999-3. [DOI] [PubMed] [Google Scholar]
- 24.Zhou G, Hasina R, Wroblewski K, Mankame TP, Doci CL, Lingen MW. Dual inhibition of vascular endothelial growth factor receptor and epidermal growth factor receptor is an effective chemopreventive strategy in the mouse 4-NQO model of oral carcinogenesis. Cancer Prev Res (Phila) 2010;3:1493–502. doi: 10.1158/1940-6207.CAPR-10-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foy JP, Tortereau A, Caulin C, Le TV, Lavergne E, Thomas E, et al. The dynamics of gene expression changes in a mouse model of oral tumorigenesis may help refine prevention and treatment strategies in patients with oral cancer. Oncotarget. 2016 doi: 10.18632/oncotarget.8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z, Wang Y, Yao R, Li J, Lubet RA, You M. p53 Transgenic mice are highly susceptible to 4-nitroquinoline-1-oxide-induced oral cancer. Mol Cancer Res. 2006;4:401–10. doi: 10.1158/1541-7786.MCR-06-0028. [DOI] [PubMed] [Google Scholar]
- 27.Ide F, Kitada M, Sakashita H, Kusama K, Tanaka K, Ishikawa T. p53 haploinsufficiency profoundly accelerates the onset of tongue tumors in mice lacking the xeroderma pigmentosum group A gene. Am J Pathol. 2003;163:1729–33. doi: 10.1016/S0002-9440(10)63531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hursting SD, Perkins SN, Phang JM, Barrett JC. Diet and cancer prevention studies in p53-deficient mice. J Nutr. 2001;131:3092S–4S. doi: 10.1093/jn/131.11.3092S. [DOI] [PubMed] [Google Scholar]
- 29.Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 30.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–80. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morita M, Fujino M, Jiang G, Kitazawa Y, Xie L, Azuma M, et al. PD-1/B7-H1 interaction contribute to the spontaneous acceptance of mouse liver allograft. Am J Transplant. 2010;10:40–6. doi: 10.1111/j.1600-6143.2009.02859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bald T, Landsberg J, Lopez-Ramos D, Renn M, Glodde N, Jansen P, et al. Immune cell-poor melanomas benefit from PD-1 blockade after targeted type I IFN activation. Cancer Discov. 2014;4:674–87. doi: 10.1158/2159-8290.CD-13-0458. [DOI] [PubMed] [Google Scholar]
- 33.Speight PM. Update on oral epithelial dysplasia and progression to cancer. Head Neck Pathol. 2007;1:61–6. doi: 10.1007/s12105-007-0014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moll R, Divo M, Langbein L. The human keratins: biology and pathology. Histochem Cell Biol. 2008;129:705–33. doi: 10.1007/s00418-008-0435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisher TS, Kamperschroer C, Oliphant T, Love VA, Lira PD, Doyonnas R, et al. Targeting of 4-1BB by monoclonal antibody PF-05082566 enhances T-cell function and promotes anti-tumor activity. Cancer Immunol Immunother. 2012;61:1721–33. doi: 10.1007/s00262-012-1237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linch SN, McNamara MJ, Redmond WL. OX40 Agonists and Combination Immunotherapy: Putting the Pedal to the Metal. Front Oncol. 2015;5:34. doi: 10.3389/fonc.2015.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aspeslagh S, Postel-Vinay S, Rusakiewicz S, Soria JC, Zitvogel L, Marabelle A. Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer. 2016;52:50–66. doi: 10.1016/j.ejca.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 38.Peng W, Liu C, Xu C, Lou Y, Chen J, Yang Y, et al. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-gamma inducible chemokines. Cancer Res. 2012;72:5209–18. doi: 10.1158/0008-5472.CAN-12-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016;44:51–60. doi: 10.1016/j.ctrv.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, Poh CF, Williams M, Laronde DM, Berean K, Gardner PJ, et al. Loss of heterozygosity (LOH) profiles--validated risk predictors for progression to oral cancer. Cancer Prev Res (Phila) 2012;5:1081–9. doi: 10.1158/1940-6207.CAPR-12-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gildener-Leapman N, Ferris RL, Bauman JE. Promising systemic immunotherapies in head and neck squamous cell carcinoma. Oral Oncol. 2013;49:1089–96. doi: 10.1016/j.oraloncology.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roth P, Valavanis A, Weller M. Long-term control and partial remission after initial pseudoprogression of glioblastoma by anti-PD-1 treatment with nivolumab. Neuro Oncol. 2016 doi: 10.1093/neuonc/now265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lipson EJ, Sharfman WH, Drake CG, Wollner I, Taube JM, Anders RA, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19:462–8. doi: 10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen PL, Roh W, Reuben A, Cooper ZA, Spencer CN, Prieto PA, et al. Analysis of Immune Signatures in Longitudinal Tumor Samples Yields Insight into Biomarkers of Response and Mechanisms of Resistance to Immune Checkpoint Blockade. Cancer Discov. 2016;6:827–37. doi: 10.1158/2159-8290.CD-15-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–17. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montler R, Bell RB, Thalhofer C, Leidner R, Feng Z, Fox BA, et al. OX40, PD-1 and CTLA-4 are selectively expressed on tumor-infiltrating T cells in head and neck cancer. Clin Transl Immunology. 2016;5:e70. doi: 10.1038/cti.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.