Abstract
Purpose
Understanding the spectrum of pathogens in a given geographic region is important when deciding upon empiric antibiotic therapy. Here, we evaluate the spectrum of bacterial organisms cultured from corneal samples as well as their antibiotic sensitivities to guide initial treatment of keratitis.
Methods
We performed a retrospective case review of cultures from suspected infectious keratitis cases at the Francis I. Proctor Foundation, University of California, San Francisco from 1996 through 2015. Logistic regression models were used to assess the risk of culturing methicillin-resistant Staphylococcus aureus from ulcers over time as well as the association between year cultured and moxifloxacin resistance.
Results
A total of 522 of 2,203 (23.7%) cultures grew bacterial organisms thought to be the etiology of infection, and with available antibiotic sensitivity data. Of these, 338 (65.3%) grew gram-positive organisms with the most common being methicillin-sensitive staphylococcus aureus (20.1%, N =105). One hundred eighty (34.7%) grew gram-negative species with Pseudomonas aeruginosa as the most prevalent organism (10.9%, N = 57). There was 1.13 increased odds of culturing methicillin-resistant Staphylococcus aureus for each 1-year increase in culture date (P=0.01) and 1.26 increased odds of culturing an organism resistant to moxifloxacin with each 1-year increase in culture date after controlling for infectious organism (P<0.001).
Conclusions
Gram-positive organisms are the most commonly identified etiology of microbial keratitis in this series. Approximately 35% of cultured organisms had variable susceptibility to moxifloxacin and resistance appears to be increasing over time. The risk of culturing methicillin-resistant Staphylococcus aureus increased over time.
Keywords: Infectious keratitis, antibiotic resistance
INTRODUCTION
Understanding the spectrum of pathogens in a given geographic region is important when deciding upon initial antibiotic therapy.1–5 Causes of infectious keratitis vary depending on climate and other risk factors such as contact lens wear, or risk of trauma with vegetative material.6 Because untreated bacterial keratitis can lead to corneal perforation, endophthalmitis, and eventual loss of the eye, empiric antibiotic treatment is initiated before a specific causative organism can be identified.
In the literature and within our own institution there has been debate regarding the use of commercially available fourth generation fluoroquinolones versus compounded fortified antibiotics. Several randomized controlled trials have shown equivalency between these therapies.7–11 Notably these trials were conducted in developing countries where resistant organisms are less common. The use of fortified antibiotics in the United States remains prevalent.12,13
In this study we report the spectrum of bacterial organisms isolated from corneal cultures and their antibiotic resistance patterns from one tertiary care center in Northern California: the Francis I. Proctor microbiology laboratory at the University of California, San Francisco.
METHODS
After institutional research board approval was obtained from the University of California, San Francisco (UCSF), records from all corneal cultures performed and sent to the Francis I. Proctor microbiology laboratory from January 1st 1996 through September 31st 2015 were retrospectively reviewed. Corneal samples from infectious keratitis cases at the Proctor Foundation and at UCSF cornea clinics were collected in a standardized manner by performing Gram stain, and aerobic culture by inoculating blood and chocolate agar plates. Culture plates were incubated at 35°C in 10% CO2 and examined daily for up to seven days.
Cultures were considered positive when they met the following criteria: (1) the same organism was isolated on two or more media (2) an isolate was present on one media and associated with the identification of the same organism on gram stained direct smears. (3) heavy growth at the inoculation site on one solid media. (4) isolation of organisms consistent with normal flora such as coagulase negative Staphylococcus and Corynebacterium spp was considered significant only if there was moderate or numerous growth on two solid media. Antibiotic susceptibilities of microbiological isolates were determined according to disc susceptibility testing guidelines set forth by the Clinical and Laboratory Standards Institute (CLSI) and categorized as sensitive, intermediate, or resistant. In circumstances when CLSI interpretive standards were not available, Federal Drug Administration approved zone diameters were used. When applicable, CLSI Interpretive standards for Staphylococcus were used for antibiotics without interpretive standards for Streptococcus spp. and interpretive standards for Enterobacteriaceae were used to interpret antibiotics without interpretive standards for gram-negative rods. Susceptibility testing was conducted for the following antibiotics: bacitracin, cephalothin, erythromycin, sulfasoxazole, oxacillin, tetracycline, tobramycin, vancomycin, trimethoprim, ciprofloxacin, moxifloxacin, gatifloxacin, ofloxacin, neomycin, tobramycin, ampicillin, ceftazidime, gentamicin, and polymyxin.
Isolated organisms were grouped into one of ten classes for the purpose of our analysis: 1. Staphylococcus species 2. Streptococcus species (including Streptococcus pneumonia, Streptococcus viridans, Non-hemolytic streptococci, Nutritionally variant Streptococci, Beta hemolytic Streptococcus Group A, Beta hemolytic Streptococcus Group B) 3. Enterics (including Proteus penneri, Proteus mirabilis, Klebsiella pneumonia, Klebsiella oxytoca, Serratia species, Serratia marcescens, Serratia liquefaciens, Enterobacter species, Enterobacter cloacae, Enterobacter aerogenes, Escherichia coli, Morganella morganii, Citrobacter species 4. Pseudomonas species 5. Nocardia species 6. Mycobacterium species 7. Coagulase negative staphylococcus 8. Haemophilus species 9. Gram negative rods 10. Diphtheroids.
A logistic regression model was used to assess whether there was an increase in the risk of culturing MRSA among Staphylococcus ulcers over time. Similarly, a logistic regression model was used to evaluate the association between year cultured and moxifloxacin resistance after controlling for infectious organism. An alpha of <0.05 was considered to be statistically significant. All statistical analyses were conducted using Stata version 14.0.
RESULTS
A total 2,203 aerobic corneal cultures were performed over a 19-year time period from 1996 to 2015. Of these, 1,342 (62.2%) resulted in no isolation of aerobic bacteria, 27 (1.3%) isolated normal flora thought to represent contamination, 10 grew acid fast bacteria (0.4%), and 302 (14%) grew in culture but did not grow during sensitivity testing and therefore had no sensitivity data available. Four (0.008%) cultures grew fungus and were not included in the subsequent analyses. Therefore 522 cultures (23.7%) grew bacteria that were thought to be causing infectious keratitis (i.e. not a result of contamination), had available sensitivity data and were included in this analysis. Table 1 outlines the five most commonly isolated gram-positive organisms and their antibiotic susceptibility profiles. Methicillin-sensitive staphylococcus aureus (MSSA) represented approximately 20% (N = 105) of all isolates in our series. These organisms were susceptible to the majority of commonly used antibiotics. Methicillin-resistant staphylococcus aureus (MRSA) were cultured about 5% (N = 26) of the time. These organisms had inconsistent in vitro susceptibility to commonly used antibiotics such as fluoroquinolones. All oxacillin-resistant Staphylococcus are considered resistant to all current beta-lactam antimicrobial agents regardless of in vitro results, with the exception of the newer cephalosporins with anti-MRSA activity. MRSA isolates had good in vitro sensitivity to trimethoprim (96%) and sulfasoxazole (100%) and, as expected, they were 100% sensitive to vancomycin. Coagulase-negative staphylococci (CNS) were also commonly isolated (N = 52) and had variable sensitivity to cephalosporins (65%) and fluoroquinolones (42 – 100%). Moxifloxacin provided good coverage against most other tested gram-positive organisms.
Table 1.
Antibiotic Sensitivities of Isolated Gram Positive Organisms
| Organism | Bacitracin | Cephalothin | Erythromycin | Vancomycin | Sulfasoxazole | Trimethoprim | Moxifloxacin | Ciprofloxacin | Ofloxacin |
|---|---|---|---|---|---|---|---|---|---|
|
Oxacillin Sensitive S. aureus N = 105 |
95/104 (90%) |
105/105 (100%) |
75/104 (72%) |
81/81 (100%) |
100/105 (95%) |
101/105 (96%) |
54/59 (92%) |
96/104 (92%) |
– |
|
Oxacillin Resistant S. aureus N = 26 |
17/26 (65%) |
0/0* (0%) |
1/25 (4%) |
18/18ϕ (100%) |
26/26 (100%) |
25/26 (96%) |
2/21 (10%) |
2/25 (8%) |
– |
|
S. pneumonia N = 51 |
51/51 (100%) |
– | 39/51 (76%) |
51/51 (100%) |
47/51 (92%) |
30/51 (59%) |
24/24 (100%) |
– | 46/46 (100%) |
|
S. viridans N = 69 |
53/53 (100%) |
– | 30/62 (48%) |
63/63 (100%) |
46/47 (98%) |
29/47 (62%) |
33/34 (97%) |
– | 41/48 (85%) |
|
Oxacillin Sensitive Coag neg staph N = 52 |
26/32 (81%) |
32/32 (100%) |
18/32 (56%) |
30/30 (100%) |
29/32 (91%) |
23/32 (72%) |
6/9 (67%) |
26/31 (84%) |
2/2 (100%) |
|
Oxacillin Resistant Coag neg staph N = 55 |
43/55 (78%) |
9/53 (17%) |
14/55 (25%) |
44/45 (98%) |
48/55 (87%) |
37/54 (69%) |
4/31 (13%) |
9/48 (19%) |
9/9 (100%) |
Most frequently isolated gram positive organisms isolated in culture. The numerator displays the number of samples sensitive to the listed antibiotic while the denominator represents the total number of the organism isolated. Percent sensitivity to the antibiotic is shown in the parenthesis.
Of note Ofloxacin was tested in Streptococcus, and Ciprofloxacin was tested against all other gram positive organisms.
Were not tested as they were assumed to be resistant
In 2010, CLSI stopped reporting vancomycin susceptibilities with regards to MRSA as disc diffusion was not shown to be effective in determining susceptibility
Table 2 outlines the five most commonly isolated gram-negative bacteria and their antibiotic susceptibility profiles. Pseudomonas species (N = 57) were the most commonly cultured gram-negative organisms. Pseudomonas exhibited excellent in vitro sensitivity to fluoroquinolones (94 – 98%) as did Serratia, Moraxella and other enteric organisms. Non-Moraxella gram negative rods (N = 46) demonstrated better in vitro susceptibility to ceftazidime (100%) than ttobramycin (30%) or moxifloxacin (67%).
Table 2.
Antibiotic Sensitivities of Isolated Gram Negative Organisms
| Organism | Ampicillin | Gentamicin | Neomycin | Ceftazidime | Polymyxin | Sulfasoxazole | Trimethoprim | Tetracycline | Tobramycin | Moxifloxacin | Ciprofloxacin |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Pseudomonas sp. N = 57 |
0/0 (0%) |
49/52 (94%) |
29/51 (56%) |
51/52 (98%) |
51/52 (98%) |
3/50 (6%) |
0/0 (0%) |
0/0 (0%) |
49/52 (94%) |
28/29 (97%) |
51/52 (98%) |
|
Serratia sp. N = 32 |
5/12 (42%) |
12/12 (100%) |
12/12 (100%) |
12/12 (100%) |
5/12 (42%) |
12/12 (100%) |
12/12 (100%) |
7/12 (58%) |
12/12 (100%) |
5/5 (100%) |
12/12 (100%) |
|
Moraxella sp. N = 33 |
26 (96%) |
29/29 (100%) |
29/29 (100%) |
29/29 (100%) |
29/29 (100%) |
27/27 (100%) |
1/25 (4%) |
29/29 (100%) |
29/29 (100%) |
11/11 (100%) |
29/29 (100%) |
|
Other Enterics N = 51 |
3/16 (19%) |
17/17 (100%) |
17/17 (100%) |
17/17 (100%) |
11/17 (65%) |
12/16 (75%) |
15/17 (88%) |
13/17 (76%) |
17/17 (100%) |
11/11 (100%) |
17/17 (100%) |
|
Other Gram Negative Rods N = 46 |
3/10 (30%) |
5/10 (50%) |
4/10 (40%) |
10/10 (100%) |
7/10 (70%) |
9/10 (90%) |
2/11 (22%) |
9/10 (90%) |
3/10 (30%) |
2/3 (67%) |
8/10 (80%) |
Most frequently isolated gram negative organisms isolated in culture. The numerator displays the number of samples sensitive to the listed antibiotic while the denominator represents the total number of the organism isolated. Percent sensitivity to the antibiotic is shown in the parenthesis.
The risk of culturing MRSA from corneal cultures appeared to increase over time in this urban tertiary care center. Among Staphylococcus species there was 1.13 increased odds of culturing MRSA for each 1-year increase in culture date (P = 0.01). Resistance to moxifloxacin also increased over time since its introduction to our laboratory in 2005 with a 1.26 fold increase in culturing an organism that was resistant to moxifloxacin with each 1-year increase in culture date even after controlling for the type of organism (P < 0.001). A sensitivity analysis accounting for autocorrelation did not appreciably change our results. Figure 1 demonstrates the percentage of isolated Streptococcus and Staphylococcus samples with moxifloxacin resistance as parsed by year.
Figure 1.
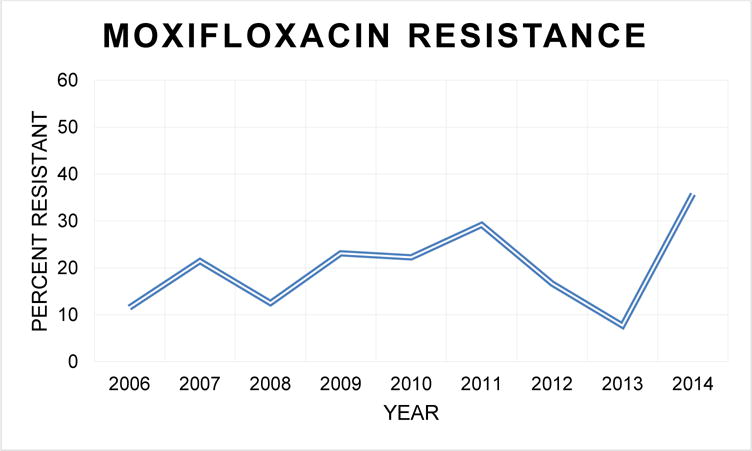
Proportion of moxifloxacin resistant Streptococcus and Staphylococcus species samples, organized by year. Overall, resistance to moxifloxacin increases with time.
DISCUSSION
Here, we report the spectrum of bacterial organisms isolated from corneal cultures at the microbiology laboratory of one tertiary ophthalmic care center in Northern California. We found that 65.3% of isolated organisms with sensitivity data were gram-positive with MSSA being the most common. Although MRSA only represented approximately 5% of isolated organism in our series, the risk of culturing MRSA also seemed to be increasing in our population. Approximately 26% of isolated gram-positive organisms had inconsistent susceptibility to moxifloxacin and other fluoroquinolones and the risk of culturing organisms resistant to moxifloxacin appeared to increase over time. Therefore, fluoroquinolone monotherapy, may not be appropriate empiric treatment in an urban tertiary hospital such as ours. However, the choice between empiric fortified antibiotics versus fluoroquinolone monotherapy in the treatment of bacterial ulcers is complex and depends on drug availability, practice setting (university versus private), geographic location (city versus rural) and risk of MRSA in the community.
Given the results of our study, two recommendations can be made: 1) it is important to culture corneal ulcers so that the infectious organism and antibiotic sensitivities can be properly identified and 2) whether starting with fortified antibiotics or with fluoroquinolone monotherapy initially, follow up of culture results and clinical response is essential. If the bacteria is resistant to fluoroquinolone monotherapy on culture or the infection is not responding clinically, the patient must be switched to fortified antibiotics. If the patient was initially started on fortified antibiotics but cultures reveal an organism that is sensitive to fluoroquinolones, switching is prudent given the ocular surface toxicity associated with fortified antibiotics. It is our strong recommendation that no one should be on high doses of fortified antibiotics for more than one week without either tapering or tailoring therapy.
Our results appear consistent with prior studies demonstrating a predominance of gram-positive organisms, and in particular MSSA. Staphylococcus aureus has been reported to be the most common cause of bacterial keratitis in the US,14 UK,15 and in Brazil.16 Increasing rates of MRSA in ocular infections have also been reported in other series, representing even up to 45% of Staphylococcus aureus isolates.17 Increased resistance of these organisms to fluoroquinolones has also been reported previously.18–23 In our hands, pseudomonas displayed excellent sensitivity to fluoroquinolones. This is in contrast to other studies which suggested that pseudomonas are also developing resistance.24,25
The strengths of this study include the fact that we report corneal culture results and antibiotic susceptibilities on a large number of cultures over a long time period at an ophthalmology specific microbiology laboratory. Limitations are that these results report in vitro antibiotic susceptibilities and we did not correlate this with clinical response to treatment. However, clinical outcomes such as visual acuity and scar size have been associated with in vitro susceptibility patterns previously.26 These organisms were cultured from a tertiary care center in an urban part of Northern California and may not be generalizable to other regions of the United States or to rural settings. Our rate of bacterial culture positivity was 37.8%, which is relatively low. We attribute this to the fact that many patients with infectious keratitis are started on antibiotics prior to being referred to our institution. We also maintain strict protocols to distinguish between true culture positive results and contaminants, which dropped the rate of organism recovery. Given referral bias, some patients may have had positive cultures despite antibiotic therapy, therefore organisms with antibiotic resistance may be over-represented in our series.
In summary, we describe a retrospective review of positive corneal cultures performed in one tertiary care center in Northern California, which demonstrates a predominance of gram-positive organisms. Over time we observed an increase in fluoroquinolone resistant organisms, which we could not attribute to the increase in prevalence of MRSA alone.
Acknowledgments
Financial Support: NIH K23EY025025, RPB
Footnotes
No conflicting relationship exists for any author.
References
- 1.Mah-Sadorra JH, Yavuz SG, Najjar DM, Laibson PR, Rapuano CJ, Cohen EJ. Trends in contact lens-related corneal ulcers. Cornea. 2005;24:51–58. doi: 10.1097/01.ico.0000138839.29823.57. [DOI] [PubMed] [Google Scholar]
- 2.Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27:22–27. doi: 10.1097/ICO.0b013e318156caf2. [DOI] [PubMed] [Google Scholar]
- 3.Alexandrakis G, Alfonso EC, Miller D. Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology. 2000;107:1497–1502. doi: 10.1016/s0161-6420(00)00179-2. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim YW, Boase DL, Cree IA. Epidemiological characteristics, predisposing factors and microbiological profiles of infectious corneal ulcers: the Portsmouth corneal ulcer study. Br J Ophthal. 2009;93:1319–1324. doi: 10.1136/bjo.2008.151167. [DOI] [PubMed] [Google Scholar]
- 5.Cruciani F, Cuozzo G, Di Pillo S, Cavallaro M. Predisposing factors, clinical and microbiological aspects of bacterial keratitis: a clinical study. La Clinica terapeutica. 2009;160:207–210. [PubMed] [Google Scholar]
- 6.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 7.Constantinou M, Daniell M, Snibson GR, Vu HT, Taylor HR. Clinical efficacy of moxifloxacin in the treatment of bacterial keratitis: a randomized clinical trial. Ophthalmology. 2007;114:1622–1629. doi: 10.1016/j.ophtha.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Hanet MS, Jamart J, Chaves AP. Fluoroquinolones or fortified antibiotics for treating bacterial keratitis: systematic review and meta-analysis of comparative studies. Can J Ophthalmol. 2012;47:493–499. doi: 10.1016/j.jcjo.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Shah VM, Tandon R, Satpathy G, et al. Randomized clinical study for comparative evaluation of fourth-generation fluoroquinolones with the combination of fortified antibiotics in the treatment of bacterial corneal ulcers. Cornea. 2010;29:751–757. doi: 10.1097/ICO.0b013e3181ca2ba3. [DOI] [PubMed] [Google Scholar]
- 10.Sharma N, Goel M, Bansal S, et al. Evaluation of moxifloxacin 0.5% in treatment of nonperforated bacterial corneal ulcers: a randomized controlled trial. Ophthalmology. 2013;120:1173–1178. doi: 10.1016/j.ophtha.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Panda A, Ahuja R, Sastry SS. Comparison of topical 0.3% ofloxacin with fortified tobramycin plus cefazolin in the treatment of bacterial keratitis. Eye (Lond) 1999;13:744–747. doi: 10.1038/eye.1999.220. [DOI] [PubMed] [Google Scholar]
- 12.Hsu HY, Nacke R, Song JC, Yoo SH, Alfonso EC, Israel HA. Community opinions in the management of corneal ulcers and ophthalmic antibiotics: a survey of 4 states. Eye Contact Lens. 2010;36:195–200. doi: 10.1097/icl.0b013e3181e3ef45. [DOI] [PubMed] [Google Scholar]
- 13.McLeod SD, DeBacker CM, Viana MA. Differential care of corneal ulcers in the community based on apparent severity. Ophthalmology. 1996;103:479–484. doi: 10.1016/s0161-6420(96)30668-4. [DOI] [PubMed] [Google Scholar]
- 14.Jeng BH, Gritz DC, Kumar AB, et al. Epidemiology of ulcerative keratitis in Northern California. Arch Ophthalmol. 2010;128:1022–1028. doi: 10.1001/archophthalmol.2010.144. [DOI] [PubMed] [Google Scholar]
- 15.Orlans HO, Hornby SJ, Bowler IC. In vitro antibiotic susceptibility patterns of bacterial keratitis isolates in Oxford, UK: a 10-year review. Eye. 2011;25:489–493. doi: 10.1038/eye.2010.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Passos RM, Cariello AJ, Yu MC, Hofling-Lima AL. Microbial keratitis in the elderly: a 32-year review. Arq Brasileiros Oftal. 2010;73:315–319. doi: 10.1590/s0004-27492010000400002. [DOI] [PubMed] [Google Scholar]
- 17.Sand D, She R, Shulman IA, Chen DS, Schur M, Hsu HY. Microbial keratitis in los angeles: the doheny eye institute and the los angeles county hospital experience. Ophthalmology. 2015;122:918–924. doi: 10.1016/j.ophtha.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein MH, Kowalski RP, Gordon YJ. Emerging fluoroquinolone resistance in bacterial keratitis: a 5-year review. Ophthalmology. 1999;106:1313–1318. [PubMed] [Google Scholar]
- 19.Lalitha P, Manoharan G, Karpagam R, et al. Trends in antibiotic resistance in bacterial keratitis isolates from South India. Br J Ophthal. 2016 doi: 10.1136/bjophthalmol-2016-308487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ni N, Nam EM, Hammersmith KM, et al. Seasonal, geographic, and antimicrobial resistance patterns in microbial keratitis: 4-year experience in eastern Pennsylvania. Cornea. 2015;34:296–302. doi: 10.1097/ICO.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 21.Elsahn AF, Yildiz EH, Jungkind DL, et al. In vitro susceptibility patterns of methicillin-resistant Staphylococcus aureus and coagulase-negative Staphylococcus corneal isolates to antibiotics. Cornea. 2010;29:1131–1135. doi: 10.1097/ICO.0b013e3181d2ce25. [DOI] [PubMed] [Google Scholar]
- 22.Asbell PA, Colby KA, Deng S, et al. Ocular TRUST: nationwide antimicrobial susceptibility patterns in ocular isolates. Am J Ophthalmol. 2008;145:951–958. doi: 10.1016/j.ajo.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 23.Chang VS, Dhaliwal DK, Raju L, Kowalski RP. Antibiotic Resistance in the Treatment of Staphylococcus aureus Keratitis: a 20-Year Review. Cornea. 2015;34:698–703. doi: 10.1097/ICO.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lomholt JA, Kilian M. Ciprofloxacin susceptibility of Pseudomonas aeruginosa isolates from keratitis. Br J Ophthalmol. 2003;87:1238–1240. doi: 10.1136/bjo.87.10.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knauf HP, Silvany R, Southern PM, Jr, Risser RC, Wilson SE. Susceptibility of corneal and conjunctival pathogens to ciprofloxacin. Cornea. 1996;15:66–71. [PubMed] [Google Scholar]
- 26.Srinivasan M, Mascarenhas J, Rajaraman R, et al. Corticosteroids for bacterial keratitis: the Steroids for Corneal Ulcers Trial (SCUT) Arch Ophthalmol. 2012;130:143–150. doi: 10.1001/archophthalmol.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]


