Abstract
Granulocyte transfusions (GTXs) have been used to treat and prevent infections in neutropenic patients for more than 40 years, despite persistent controversy regarding their efficacy. This narrative review attempts to complement recent systematic reviews by the Cochrane Collaboration and provide both historical context and critical assessment of the most significant clinical studies published over the years. The data suggest that properly collected and promptly infused granulocytes are active against infections, both bacterial and fungal. The most important question that remains unanswered is in which patients the administration of granulocytes will be beneficial. The preponderance of evidence suggests that granulocyte transfusions may be efficacious in few select cases as a temporizing measure to control an infection that is expected (or proven) to be refractory to optimal antimicrobial treatment, and that could otherwise be controlled by marrow recovery, which is expected to happen. In this regard, they are best considered a “bridge” that grants enough time for the recipient to develop their own response to the infection. The challenges to use GTXs successfully are both clinical, in terms of timely identifying the patients who may benefit, and logistical, in terms of optimal selection of donors and collection technique.
Keywords: Granulocyte transfusion, invasive fungal infection, pulmonary reaction, neutropenia, granulocytopenia, gram-negative bacteremia
There are many more published reviews on granulocyte transfusions (GTXs) than clinical trials: a simple PubMed search with the words “granulocyte transfusion” OR “granulocyte transfusions” in the “Title” field shows 47 Clinical Trials and 85 Reviews (search performed May 8, 2017). The aim of the current addition to the already overcrowded literature is to provide practicing clinicians with a succinct critical assessment of the data from the standpoint of an Infectious Disease practitioner who has worked for more than two decades in one of the institutions that pioneered this therapeutic modality.
Unbiased, systematic reviews have recently been published by the Cochrane Collaboration on the use of GTXs for prophylaxis and treatment. The conclusions were that there is low quality evidence suggesting GTX may work for prophylaxis [1] and that there is not enough evidence to decide on treatment efficacy [2]. These systematic reviews include, for methodological reasons, only 12 and 10 papers, respectively. In this review I will comment on most controlled trials on GTX, starting with the most recent ones, as well as on some case series that provide additional information. After discussing efficacy I will address toxicity. Finally, I will try to make recommendations for use and for research based on the evidence presented.
Brief History of Granulocyte Transfusions
Granulocyte transfusions may be considered the oldest form of cell therapy. Injection of “buffy coat” preparations to treat neutropenic states was initially reported back in 1934 [3]. Subsequent studies showed that granulocytes infused into aplastic dogs migrated to sites of infection [4]. Animal models showed that GTXs could help in the management of bacterial infections [5]. However, obtaining enough neutrophils from healthy donors to produce a measurable increase in absolute neutrophil count (ANC) was challenging. This prompted using as donors patients with chronic myelogenous leukemia, who had ANC of up to 300,000 /μL [6]. The subsequent development of the continuous blood-flow separator in 1969 provided a way to obtain enough granulocytes from healthy volunteers to establish granulocyte transfusion as a viable procedure (for a review of the history of the device see [7]). Case series and case reports suggesting a favorable effect in neutropenic patients with infection were published [8], and subsequently randomized controlled trials involving patients with (predominantly) bacterial infection during neutropenia were performed. Some of the studies showed improved outcomes [9–11], but others were negative [12,13]. Besides conflicting evidence regarding efficacy, data showing significant toxicity in the form of lethal pulmonary reactions also appeared [14]. The result was that by the 1990s the use of GTXs had decreased based on the widespread belief that GTXs did not add significant efficacy to optimal antimicrobial therapy and the practice became less common. For a critique of these early studies see [15].
Renewed interest in GTXs followed the availability of colony-stimulating factors (granulocyte colony-stimulating factor (G-CSF) was approved by the FDA in 1991) which, when administered to the donor, could result in much higher yields of granulocytes for transfusion [16]. If the reason for the negative results of some trials was insufficient dose, as some experts had postulated [15], the use of G-CSF stimulation should overcome the problem. The addition of dexamethasone to G-CSF was shown to increase the yield even more (by a factor of 1.5x) [17]. Since then, the approach in many U.S. centers has been to use the combination of G-CSF and dexamethasone [18]. This approach is not universal, however, and several centers in Europe do not use G-CSF [19]. Overall, there seem to be significant technical differences from center to center nationally and internationally [18,19] and these could be a persistent source of differences in observed outcomes.
Technical Considerations
There is general agreement that at least 1 x 1010 granulocytes (or 1.5 x 108 granulocytes/kg) should be given per transfusion to expect efficacy, although there is only scant clinical evidence that this is the case. Many experts believe that even higher numbers are necessary or desirable — at least 4 x 1010. The term “high-dose” granulocyte transfusion has been used to refer to ≥ 0.6 x 109 granulocytes/kg (which, in a 70 kg recipient, would give the 4 x 1010 mentioned above) [20]. The usual method to obtain granulocytes for transfusion in the US is by single-donor apheresis (intermittent or continuous centrifugation leukapheresis, using an agent like dextran or heptastarch to facilitate separation of the red blood cells). An adult therapeutic dose of granulocytes obtained by apheresis contains between 1.5 x 108 and 3 x 108 granulocytes/kg body weight of the designated recipient [21].
Besides apheresis, granulocytes may be obtained from the blood by centrifugation and collection of the “buffy coat” (the layer between the red cells and plasma) which results in a product rich in platelets and less abundant in granulocytes. A modification of this approach results in less contamination with red cells and plasma, and has been shown to be safe in a multicenter trial Massey 2012: The UK National Health System offers “Leucocytes, Buffy Coat, Irradiated”. Each pack is approximately 50ml, has a hematocrit of 45%, contains 1–2 x109 white cells, 90x109 platelets and 9.5 g of hemoglobin [22].
Finally, filtration leukapheresis was used in several of the original studies of GTX, because it allowed the collection of large quantities of granulocytes [9–11]. The cells showed impaired phagocytic activity in vitro [23] and transfusion of granulocytes obtained by filtration apheresis was associated with more side effects, so the procedure seems to have been abandoned.
Studies on the activity of granulocytes collected for transfusion suggest the cells generally remain functional for a few hours [23–25], although many timed variations in gene expression may be found depending on the collection method (stimulation of donors with dexamethasone or G-CSF or both) and storage [26], and some abnormalities in function (e.g., impaired killing of Candida yeast forms) can be detected in vitro [27]. Ideally, transfusion should take place less than 6 hours after collection. It is customary to irradiate the cells before transfusion to prevent transfusion-associated graft versus host disease (TA-GVHD) which could potentially be caused by lymphocytes in the collected product. Some experts, however, believe this compromises neutrophil function and unirradiated granulocytes can be employed safely. A controlled trial of irradiated vs nonirradiated GTX did not find any difference and there were no cases of TA-GVHD [28].
GTX in current clinical practice: case report
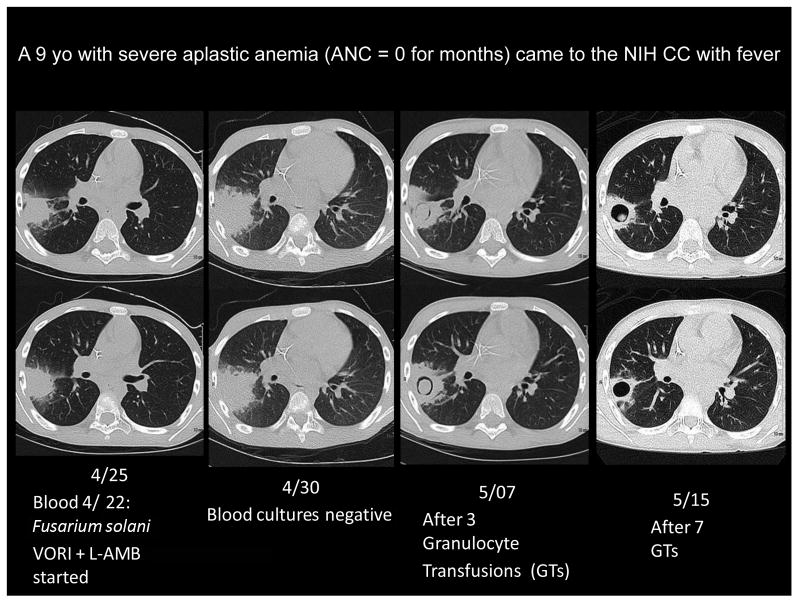
Figure 1 illustrates the effect of GTXs on a proven invasive fungal infection that seemed to be progressing on antifungal therapy. A 9 year-old child with severe aplastic anemia presented with fever, positive blood cultures for Fusarium solani and a wedge-shaped, dense pulmonary infiltrate that showed branching septate hyphae on a fine-needle aspirate. The apparent lack of response of the infection to the combination of voriconazole and amphotericin B, and the subsequent resolution after a few GTXs (with appearance of the crescent sign, which is associated with neutrophil recovery) are evident from the images (this patient was included in the series of Fusarium infections reported by NIH investigators [29]). Unfortunately, Fusarium was never cleared from his joints and bones, and alloimmunization after 32 GTXs may have contributed to his rejecting a first cord blood transplant. Despite a subsequent successful second transplant (4 moths after admission) the patient finally succumbed to infection with Phaeoacremonium, another refractory mold.
Figure 1.
Effect of Granulocyte Transfusions (GTXs) on a fungal infection that was progressing despite combination therapy with voriconazole (VORI) and liposomal amphotericin B (L-AMB). The antifungal agents sufficed to clear the blood cultures, but the pulmonary infiltrate continued worsening until granulocytes were administered.
This case illustrates many of the issues associated with GTXs, including the difficulties of performing conclusive randomized controlled trials. Many of the physicians involved in the care of this patient at our institution would have been reluctant to randomize him: his infection seemed to be progressing, Fusarium solani is known to be refractory to antifungal agents and bone marrow recovery was not likely to happen for many weeks. Regarding outcome, the patient was alive at 3 and 6 weeks (criteria for success in all the controlled trials) but his infection was not cured and he did not live to hospital discharge. He also developed alloimmunization against some of the HLA antigens of the cord unit he received as stem cell transplant, had primary graft failure and required a second transplant. It is likely that the increased immunosuppression administered to ensure a successful second transplant facilitated the infection with Phaeoacremonium that took his life. In some respects this case can be used to advocate GTXs (the patient survived a refractory mold infection for 4 months, long enough for transplant) and to decry them (the patient did not survive to discharge).
GTX for treatment of infection: Review of Recent Studies (post G-CSF availability)
Controlled Studies: no evidence of efficacy
There are only three controlled studies of GTXs obtained by stimulating donors with G-CSF: a single-center case-control study [30] and two randomized, multicenter phase III trials, one from Europe [31] and the other from the U.S. [20]. (Table 1). The three studies found no evidence of effectiveness of GTX, but they have different limitations that may make their conclusions less than definitive.
Table 1.
Modern (post G-CSF) Controlled Studies of Therapeutic Granulocyte Transfusions
| Post-GCSF | |||||
|---|---|---|---|---|---|
| Reference Year | Control | GTX | Main Finding | Other findings | Caveat |
| Response to therapy n/N (%) | |||||
| Documented Infections | Documented Infections | ||||
| [30] 2002 |
45/74 (61%) Bacterial infection: 17/18 Yeast infection: 20/30 Mold infection: 8/26 |
32/74 (43%) Bacterial infection: 4/18 Yeast infection: 20/30 Mold infection: 8/26 |
No efficacy of GTX | GTX from community donors were initiated earlier and resulted in similar outcome as family donors | Case-control study Patients may have been too ill. |
| [31] 2008* |
28/34 (82%) Bacterial/unknown: 6/6 Fungal: 21/28 |
32/38 (84%) Bacterial/unknown: 10/11 Fungal: 22/27 |
No efficacy of GTX | Underpowered Patients not ill enough Low dose of granulocytes low number of GTX |
|
| [20] 2015* |
21/49 (43%) Bacterial infection: 8/25 (Bacteremia: 2/11) Fungal Infection: 13/24 Per protocol: 16/39 |
20/48 (42%) Bacterial infection: 9/26 (Bacteremia: 4/14) Fungal Infection: 11/22 Per protocol: 17/35 |
No efficacy of GTX | Underpowered 1/3 received “low dose” GTX |
Single center case-control study
The case control study (which is not included in the Cochrane review due to its design) compared two prospective trials, one that obtained GTXs from family members and another in which GTXs were obtained from community donors. The patients were awaiting or had received hematopietic stem cell transplants (HCT) at the Fred Hutchinson Cancer Research Center (FHCRC) between 1990 and 1999. The two trials aimed to determine the feasibility, toxicity and response to GTXs and included 74 patients (34 received GTXs from related donors and 40 from community donors). The investigators decided to match each patient with a control who was awaiting or had received HCT at the FHCRC between 1983 and 1999. The matching criteria were type of infection (mold, yeast, bacterial), number of transplants before infection and presence or absence of relapse. Family donors received G-CSF only, all unrelated donors received G-CSF and half (20 of 40) also received dexamethasone. As previously described, the addition of dexamethasone resulted in higher number of collected granulocytes as well as higher post-transfusion increments in absolute neutrophil count (ANC). An important finding regarding feasibility was that the median delay between diagnosis of infection and initiation of GTX was shorter when using unrelated community donors (3 vs 5 days, ranges 0–14 vs 0–25, p=0.01). The overall survival was not significantly different between recipients of related or unrelated donors, and not different from the control patients who did not receive GTX. The only statistically significant difference in infection outcome was a lower rate of progressive or fatal bacterial infections in the control (untransfused) patients (raw numbers calculated from the information on the paper are shown on Table 1). In their discussion, the authors suggest the possibility that by including only patients with microbiologically documented infection they may have selected a group that was too advanced for the intervention to be of benefit (overall 42 of 74 patients (57%) had progressive infection or death, whereas the controls had “only” 40% progressive infection or death). This argument has been used to explain other negative trials and is at the crux of the controversy: if the infection is too advanced, GTX cannot help but if the infection is too mild GTX cannot improve the excellent outcomes obtained by antimicrobials alone. How can the clinician determine the infection that is “just right”?
Randomized multicenter trials
The European multicenter randomized trial [31] did not show any benefit of GTX either, and it can be critiziced precisely for including patients who were not ill enough: the success rate was over 80% both in the recipients of GTX and the untransfused controls. Investigators in five centers in Austria and Germany randomized 74 patients between 1999 and 2005, and finalized the study with less than 50% of the expected sample size due to a decline in recruitment. The patients did not have to have a microbiologically documented infection. Other limitations of the study included low number of transfusions (17 of 39 patients randomized to GTX received only 1 or 2 GTX before neutrophil recovery) and significant crossover between arms. The doses of granulocytes per infusion were also lower than desired: 16% of the transfusions had less than the recommended neutrophil dose of 3 x 108 per kg (which is still significantly less than the 4 x 1010 dose recommended by some experts). Of interest, there was no evidence of a correlation between dose and efficacy or dose and increment in the neutrophil count. No subgroup could be identified that seemed to benefit more than any other. In fact, if one accepts that GTX can be effective only when high doses are used, this study adds absolutely no evidence to the debate. What it demonstrates is that controls are necessary to interpret the results of an interventional study. Without a control arm, an efficacy of 84% with (relatively) low dose of GTX would look outstanding—but it was the same in the control arm. This point must be considering when assessing all uncontrolled studies. As a case in point, some of the same investigators of this randomized trial published their case series in 59 children and young adults who received GTX between 1995 and 2005 [32] and documented a 28-day survival rate of 72%. How can this result be interpreted?
The RING (Resolving Infection in Neutropenia with Granulocytes) study attempted to overcome the limitations of prior trials, but only partially accomplished its goal [20]. As in the European study, accrual was too low: only 114 patients were randomized, when the target sample size was 236. The slow accrual rate forced a change in the inclusion criteria to allow patients with presumed infection (originally proven infection was required), and the time between meeting eligibility and randomization was extended from the original 24h after diagnosis to one week. Of note, these two modifications could result in the inclusion of patients less like to respond to GTX: the “presumed infection group” could include patients without an infection (and so unlikely to derive benefit from neutrophils) and the patients included late could have an infection too advanced for any intervention to be effective. The primary endpoint was clinical success, defined as survival to day 42 plus clinical response of the infection (stable infection was considered a failure).The adjudicating panel was blinded to the subject’s study arm. Donors were stimulated with 480 mg of G-CSF and 8 mg of dexamethasone orally. Continuous flow apheresis with hydroxyethil starch as a sedimenting agent was used, processing 7 to 10 L of blood. The goal was to collect 4 x 1010 granulocytes per transfusion (0.6 x 109/kg), but this was not always achieved: more than one-quarter of the subjects received a lower dose (the median dose, however, was 54.9 x 109, which was above the target). The dose differences were said to be site-specific and not due to dose or timing of G-CSF or dexamethasone, nor to amount of blood processed during the collection.
There was no difference in success rate between the GTX and control groups, whether analyzed per protocol (PP: 49% vs 41%) or by Modified Intention to Treat (MITT: 42% vs 43%). There was no discernible difference in response rate based on the type of infection (bacterial vs fungal), location of infection or risk category. A model that adjusted for baseline prognostic factors like Zubrod score or mechanical ventilation did not find any difference either. Overall it is a very well done convincingly negative study, but the question is whether it could have found a benefit with the small sample size. The planned sample size of 118 patients per arm would have had 80% power to detect an absolute difference of 20% (success rate with GTX 70% vs 50% in controls).
In their discussion the investigators pointed out that the low power does not allow us to exclude with certainty a beneficial effect of GTX. They added a post-hoc analysis where they compared subjects who received high dose as intended with those who received low dose and found a significant difference in success rate (high dose vs low dose: 59% vs 15%, p < 0.01) [20]. When looking at this difference, however, one must remember that the authors explain earlier in the paper that “Whether or not subjects received high-dose or lowdose transfusions was not a random occurrence but was largely sitespecific.” If the dose was not a random occurrence and was site-specific it seems arbitrary to attribute any difference in outcomes between the low-dose and the high-dose recipients merely to the dose itself. Why not attribute it to the site? And even if this finding would support the notion that really high doses of neutrophils are truly effective it also brings with it the possibility of a potentially negative effect of “lower dose” GTX, because the 15% success rate of that subgroup was also lower than the 41–43% of control group (p = 0.16). Besides, even the high-dose subgroup outcome was NOT statistically significantly superior to the control (p=0.11). These possibilities have been discussed at length in Editorials [33] and Review Articles [34], although it is well known that such a post-hoc analysis can at best be only hypothesis-generating. To add to these concerns, a single-center, retrospective study from Italy found that recipients of both low-dose (<1.5 x 108 cells/kg) and high-dose (> 3 x 108 cells/kg) GTX had higher infection-related mortality than recipients of “standard doses” (1.5–3 x 108/kg) [35].
A third phase III, randomized controlled trial (Granulozytentransfusionen bei Patienten mit febriler Neutropenie, GRANITE) is still ongoing in Germany but the inclusion criteria allows for suspected infection (fever resistant to therapy for > 96h) and the expected sample size (200) may be too low to provide a definitive answer http://apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2009-010700-28-DE
Assessment of the modern controlled studies
The fact that contemporary controlled studies have found no evidence of a positive effect of GTX is troubling. It may not rule out a favorable effect of GTXs, but at a minimum it suggests that investigators have not been able to identify the appropriate design to show the effect. Ultimately, there is no controlled evidence that granulocyte transfusions improve the outcome of current standard treatment of infections during neutropenia. All the positive evidence thus lies on the older trials, which 1) Included mainly bacterial infections 2) Precede modern antibiotics like third-generation cephalosporins and carbapenems 3) Precede the era of growth factors that may shorten the duration of neutropenia and 3) Precede the use of new antifungals like voriconazole, posaconazole or echinocandins. Of particular interest, the control groups in the early positive controlled trials documented the mortality of gram-negative bacteremia to be 70–100%, which seem to be much worse than current practice: the mortality of gram-negative bacteremia in neutropenic patients currently reported varies between 5% for susceptible bacteria and 20% for resistant bacteria [36,37].
Uncontrolled Studies
Experience at the NIH Clinical Center
In contrast to the multicenter controlled studies, single-institution case series continue to be reported. Investigators from the National Institutes of Health, arguably the institution with longest experience in the procedure, have published their experience using GTX in multiple settings. In chronic granulomatous disease (CGD) 58 courses of GTXs administered to treat 58 refractory infections in 40 patients over 29 years were reviewed [38]. Most cases (88%) had definitive microbiological confirmation, and most had fungal infections. The success rate was high: 42 cleared and 9 partially cleared infections (88% overall, 51 of 58). Granulocytes were mobilized with dexamethasone before 1995 and with dexamethasone + G-CSF after 1995. All patients received ≥ 0.6 x 109 granulocytes/kg per transfusion. There was no difference in response rate based on the type of infection (bacterial vs fungal). Comparing the responders (partial or complete clearing) to the non-responders showed the responders received more and more frequent transfusions and were younger. Early start of GTX (≤1 month after the beginning of the infection) was associated with a better response. The GTX were generally safe: 31 adverse events in 1954 transfusions administered, with only one case of transfusion-associated lung injury (TRALI). It should be noted, however, that these investigators had previously reported a more detaliked analysis of 8 transfusion reactions in 10 of the patients in this cohort [39]. A striking institutional decline in the use of GTXs for CGD after 2002 is acknowledged (2.8 GTX courses per year before, 0.7 after 2002), with concomitant decline in the success rate (only 43% after 2002). The authors speculate that the increasing use of hematopietic stem cell transplantation (HCT) as treatment for refractory infections in CGD at their institution is the main contributor to the decline.
The experience at the NIH in 32 patients with aplastic anemia transfused between 1997 and 2007 has also been published [40]. In this paper the overall survival to hospital discharge was 58% (only 44% for the 18 patients with invasive fungal infection). Of particular interest are the inclusion criteria: ANC < 200, proven or probable invasive fungal infection or ”a bacterial infection which, in the experience of our center, was associated with greater than 90% mortality” and no response to appropriate antibiotic or antifungal therapy for 24–48h. These criteria are much more restrictive than those used in the modern randomized clinical trials and much more likely to identify a subset of patients who could benefit from granulocytes. This paper also failed to identify any dose-response effect, whether by measuring granulocyte dose administered, ANC increment or ANC “area under the curve” (AUC), an estimate of the time the patient was with a “protective” ANC. Although there were five cases of acute lung injury (also called “pulmonary reaction” in the GTX literature) early in the course of the study, the authors reported no events during the last 7 years.
Another report from the NIH focusing on a particularly refractory fungal infection, fusariosis, has been published [29]. We chose to analyze what is generally considered a refractory fungal infection [41] as clinical scenario where the beneficial effect of GTXs would be obvious. In this article, the response rate of 90% (10 of 11 patients) seemed superior to the available literature (30%) but the survival to hospital discharge was still low (45%) emphasizing the fact that granulocyte transfusions in neutropenic patients are usually only a temporizing measure until hematologic recovery occurs. Importantly, transfusion reactions (identified as transfusion associated circulatory overload, TACO) were thought to have contributed to two of the 6 deaths.
Experience at other centers
The experience of GTXs for febrile neutropenia at the Seoul National University Hospital between 1999 and 2009 was published in 2011 [42]. Here the authors decided to analyze only 138 episodes of febrile neutropenia in 128 patients who received at least three GTXs per episode. The patients received daily GTX from G-CSF and dexamethasone-mobilized donors at a very high dose 0.96X 109 /kg per transfusion (range: 0.47 – 1.80 10X9 /kg) and all had clinically or microbiologically documented infections. The median ANC increment on the fourth day post-transfusion was > 1000 /μL. Control of the infection was achieved in 73 of the 138 episodes (52.9%) and, as in many other reports, there was no correlation between dose of of granulocytes or ANC increment and clinical success. The prognostic factors that remained significant by multivariate analysis were underlying refractory disease and septic shock. There were 13 severe pulmonary complications (respiratory failure, hemoptysis or both). These did not seem related to the use of amphotericin B, but rather to daily infusion of more than 250 mL of GTX and azotemia.
Recent retrospective reports from the M.D. Anderson Cancer Center have focused on Candida and Aspergillus infections. The experience in Candida seemed to be positive [43] and the experience in aspergillosis negative [44]. The most recent report from the same institution is a chart review of 74 patients who received GTX between 2004 and 2007 [45]. Donors were stimulated with G-CSF and dexamethasone, and the median transfusion dose was high: 5.6 x 1010 (range, 4.0–10 x 1010 ) granulocytes per transfusion dose, with patients receiving a median of 4 GTX (range 1–50 GTX). The authors report responses in 34 of the 74 patients (46%). They classified the patients as having severe infections or not, and report that the responses were better in patients with severe infections. Unfortunately the heterogeneity of the population (10 patients had viral disease), the variation in treatment (some patients received G-CSF, some interferon-gamma (IFNg), some corticosteroids) the retrospective nature of the study and the lack of a control group make it impossible to determine whether GTX were beneficial.
Two additional uncontrolled case series are of particular interest. The “Granulocytes in neutropenia 1” (GIN 1) [46], a multicenter study from the U.K., evaluated the safety of transfusing pooled, whole blood-derived granulocytes. The product (“granulocytes in additive solution and plasma”: GASP) is obtained by combining 10 buffy coats (obtained from 10 blood donations) with 400 mL of platelet additive solution and re-centrifuging [25]. The main advantage of this method lies in its availability. The packs transfused in the study had a mean volume of 207 ml and contained an average of 1 x 1010 granulocytes, and were given to 13 children and and 17 adults either therapeutically or as secondary prophylaxis. The survival at 30 days was 100 and 88% for children and adults, respectively, although it should be emphasized that this was a safety study without a control group. The product was well tolerated: fever happened following 9% of transfusions, hypotension 2.9% and hypoxia 2.2%. One case of TACO required admission to the intensive care unit for 48h.
Another uncontrolled single-center repot in aplastic anemia used non-mobilized granulocytes to treat fifty-six patients with severe infections (31 fungal, 25 bacterial) [47]. The amount of granulocytes given was, as expected, significantly less (the mean dose in concentrates was 9.2 ±4.7 x 109 cells) and resulted in lower ANC increase (0.27 ± 0.21 / μL) than studies using G-CSF or dexamethasone mobilized donors, but patients received them daily or every other day (median 18, range 3–75) and had a favorable outcome at 180 days of 52% in fungal infections and 84% in bacterial infections. The authors contrast these results with their prior experience of 61% mortality of fungal infection in severe aplastic anemia. As in other papers, survival was contingent on hematopoietic recovery
Assessment of the uncontrolled studies
Unfortunately, even if the uncontrolled case series provide useful descriptive information they cannot truly attest to efficacy. A review of these reports shows that a number of experienced hematology/oncology and infectious diseases specialists believe both that 1) GTXs work and that 2) they can identify patients who will benefit from them. But there is no proof of efficacy in any of these reports, no matter how impressive the results may appear.
GTX for treatment of infection: Summary of Older Studies
A review of the early studies of GTX (Table 2) is necessary for two reasons. First, some of these [9–11] are the only controlled studies that have shown efficacy—without them there would be no question about GTX, as all subsequent controlled studies have been negative. Second, they involve mainly patients with gram-negative bacterial infections treated with relatively ineffective antibiotics, and in the current era of multidrug-resistant pathogens this use may become relevant again. Overall the studies found that the “response” (variously defined) in the transfused group ranged between 46 and 88%, and in the control (untransfused) between 15 and 83%. These ranges suggest the populations studies were not quite homogeneous. When the control group had very poor responses, the transfused patients did significantly better. These papers were thoroughly discussed over the years, and a very insightful meta-analysis addressing them was published in 1997 by Vamvakas and Pineda [48]. The meta-analysis found that GTX were efficacious in bacterial sepsis during neutropenia when 1) The dose was high 2) The control group had very low survival and 3) Compatible granulocytes were used. The first two criteria (dose and poor prognosis of the control group), however, could not be disentangled: the controlled studies that used high dose [9–11] were the same that had very poor survival in the control group [9–11] (26%, 36% and 15%). Conversely, the two studies with high control survival rates [12,13] administered relatively low doses of granulocytes
Table 2.
Older (pre-granulocyte colony stimulating factor (G-CSF))Controlled Studies of GTX
| Pre GCSF | |||||||
|---|---|---|---|---|---|---|---|
| Reference Year | Control | GTX | Main Finding | Other findings | Caveat | ||
| Success n/N (%) | Success n/N (%) | ||||||
| Documented Infections | Presumed infection | Documented Infections | Presumed infection | ||||
| [8] 1972 | 11/37 (30%) | N/A | 18/39 (46%) | N/A | High mortality in control patients Patients had to have documented Gram-negative bacteremia |
||
| [9] 1975* | 5/19 (26%) | N/A | 15/17 (88%) | N/A | GTX improve survival in infections during neutropenia | No correlation between HLA- matching and clinical effect. Anti-leucocyte antibodies developed in 65% of GTX recipients Granulocytes obtained by filtration leukapheresis seem to work clinically. Fever happened after 59% of GTX |
High mortality in control patients Only patients failing antibiotics were included. Average dose of GTX was 2.2 x 1010 per transfusion |
| [12] 1975 | 16/21 (76%) | 12/17 (71%) | |||||
| [10] 1977* |
5/14 (36%) With marrow recovery: 5/6 Without marrow recovery: 0/8 |
N/A |
12/16 (75%) With marrow recovery: 4/4 Without marrow recovery: 8/12 |
N/A | GTX improve survival in Gram- negative bacteremia | No difference in efficacy between granulocytes obtained by filtration leukapheresis or continuos centrifugation; no evidence of effect of anti- leucocyte antibodies | The mortality in control patients is high for today’s standards No effect when there was marrow recovery |
| [52] 1977* |
10/19 (53%) With marrow recovery: 8/9 Without marrow recovery: 2/10 |
15/19 |
11/14 (79%) With marrow recovery: 5/6 Without marrow recovery: 6/8 |
7/8 | Prolongation of median survival time in patients without marrow recovery | Granulocytes obtained by filtration leukapheresis seemed functional. No effect on fever without documented infection. No effect on superinfections. Only one episode of candidemia (received GTX and died) |
The difference was only in patients with documented infection and persistent marrow failure. GTX dose was 5 x 1010 daily |
| [11] 1977* |
2/13 (15%) Patients with marrow recovery: 2/2 Patients without marrow recovery: 0/11 |
10/17 (59%) Patients with marrow recovery: 5/6 Patients without marrow recovery: 5/11 |
Documented bacteremia (and 1 candidemia and 1 herpes simplex in the control group) worsening after 3 days Granulocyte dose was low |
||||
| [49] | 10/12 (83%) | 10/13 (77%) | |||||
| [13] 1982* |
30/48 (63%) Gram-negative bacteremia: 19/32 Gram-positive bacteremia 4/5 Pneumonia 1/4 Cellulitis/abscess 6/7 |
N/A |
34/47 (72%) Gram-negative bacteremia: 23/36 Gram-positive bacteremia 3/3 Pneumonia 5/5 Cellulitis/abscess 3/3 |
N/A | Granulocyte transfusions do not add any benefit to optimal antimicrobial treatment | Granulocyte dose was low: 0.5 x 1010 per transfusion (range: 0.1 x 1010– 2.7 x1010) | |
| [50] | Gram-negative bacteremia: 6/7 | 18/23 (78%) | Gram-negative bacteremia: 3/4 | 11/16 (69%) | The early administration of GTX does not improve the response rate | ||
| [51] 1984* | 7/11 (64%) | 8/13 (62%) | Pulmonary complications were equally common in both groups | No relationship between amphotericin B administration and the appearance of pulmonary complications | |||
| [53] 1984 |
15/22 (68%) (29 episodes) With marrow recovery: 13/14 Without marrow recovery: 2/15 |
41/53 (775)(58 episodes of infection) With marrow recovery: 26/26 Without marrow recovery: 15/32 |
GTX provide no benefit if bone marrow recovery takes place |
The other controlled trials included in the Cochrane review but not considered in the earlier meta-analysis were all negative, and all of them had very good success rates in the control group: 83% [49], 78% [50] and 64% [51]. One of these studies only randomized 24 patients with possible infection (all proven infections received GTX) although most of them ended up having a documented infection by the end of the 21-day study period [51] and administered low number of granulocytes (0.87 ± 0.35 x 1010 granulocytes per transfusion), and focused on the pulmonary complications. The other two studies did administer high dose of granulocytes, but started them within 24 hours of initiating antibiotics, precisely to test whether early initiation of GTX in patients with suspected infection would be beneficial[50].
Considered together the early studies do not support the routine use of GTX in neutropenic patients with infection. They suggest, however, that the subset of patients with very high risk of death—which on antibiotics alone would have asurvival of 30% of less-- could possibly benefit from GTXs. Benefit seems more likely if the dose is high and the transfused granulocytes are compatible with the donor, although these two conditions are not proven.
GTX for Prophylaxis of Infection
Two early trials of prophylactic GTX showed decreased frequency of infection, but no survival benefit [54,55]. Subsequent studies confirmed the lack of effect on overall outcome but did not show the decreased infection risk [56–59] and highlighted complications of the procedure, including CMV infection [56,57], pulmonary reactions [57] and transfusion reactions with alloimmunization [60]. The largest study showed lower frequency of septicemia in the transfused group, but this was offset by higher frequency of pneumonia, very high frequency of fever, chills, dyspnea or wheezing (72%), pulmonary infiltrates (37%) and higher mortality in the transfused group (almost twice as high, even if not statistically significant 22% vs 13%). Although a subsequent study showed more favorable results [61] another compared GTX with prophylactic antibiotics and found no difference in any of the outcome variables measured [62]. The results of the trials and the subsequent availability of growth factors probably contributed to the decline of this practice [63]. A 1997 meta-analysis concluded that prophylactic GTX could “no longer be recommended”, arguing that the the effect on prevention of infections detected by the first studies would be negligible in the presence of more effective antibiotics and colony growth factors [64].
Only three controlled studies of prophylactic GTX have been published after 2000 [65–67]. On a phase I/II trial investigators administered G-CSF mobilized granulocytes to 16 neutropenic patients and compared them with 16 controls who were neutropenic secondary to chemotherapy or HCT but had no compatible donor for GTX [65]. No difference regarding infectious parameters could be demonstrated. The other two studies are in allogeneic peripheral blood stem cell transplantation (allo-PBSCT) recipients. Patients received prophylactic GTX or not depending on the ABO-compatibility of their PBSC donors (the authors call this “biological randomization”) The first trial addressed whether transfusion of two GTX influenced the subsequent risk of CMV viremia after allo-PBSCT, it did not address other infections. The study did not find that the two GTX added significantly to the risk of CMV conferred by the PBSCT (the GTX and the stem cells came from the same donor).. The second trial also used PBSC donors as granulocyte donors for transplant recipients [67]. The investigators accrued 151 donor-recipient pairs and compared the 53 pairs in who GTX was possible with the 98 pairs in which it was considered not feasible (due to ABO mismatch, CMV serocompatibility mismatch, failure to meet American Association of Blood Banks criteria to donate blood products or poor PBSC collection). The patients received just two high-dose GTXs (mean around 5 x 1010granulocytes per transfusion) on Days +3 and +6 or +5 and +7 post transplant. The patients who received GTXs had less days of profound (ANC < 100/μL) neutropenia (1.5 vs. 3.2, p <0.0001), fewer of them experienced bacteremia (13.2% vs 29.6%), and both the number of days of fever and the number of days on antibiotics were lower in the transfused group. However, there was no difference in length of the initial hospital stay or 100-day survival. A multivariate analysis supported the notion that the fewer days of neutropenia were due to the GTX and correlated directly with the decrease in infections, fever and antibiotic use. The authors don’t report any significant toxicity. Of note, no prophylactic antibacterial or antifungal agents were used in either group.
Taken together, the studies on prophylaxis support similar conclusions than the studies on treatment: transfused granulocytes work appropriately as granulocytes, i.e., they fight pathogens. It is hard to believe they add anything to optimal anti-infective prophylaxis (or treatment) as long as this optimal anti-infective prophylaxis is reasonably successful. The latest Cochrane Review on the use of GTX for prophylaxis of infections [1] reviewed all 12 randomized trials and concluded that “there is low-quality evidence that prophylactic granulocyte transfusion lead to a reduction in the number of people developing a bacterial or fungal infection, especially if the dose is at least 1.0 x 1010 per day.” The question then becomes, when is it appropriate to use GTX to prevent infection? If the risk of infection is low or the mortality of an infection that could happen is low, the use of GTX does not seem justified. But what if a patient is at particular risk for a particularly lethal or untreatable infection?
Several case series and case reports reporting the use of GTX as prophylaxis for previously existent infection (secondary prophylaxis) have also appeared [68,69]. In a 10-year single-center experience Nikolajeva et al report the data on 28 children who underwent allogeneic HCT and received GTX because they were considered to be at high risk of infection or had persistent infection at the time of transplant [70]. 18 patients survived, and only 2 deaths were caused by progression of infection. Unfortunately, these are uncontrolled studies, and probably even more prone to publication bias than controlled trials. It is not possible to conduct a randomized trial of this practice, but one could argue that this is precisely the setting where GTXs could be most effective: If a patient is known to be at very high risk for a lethal, untreatable pathogen responsive to neutrophils, the use of prophylactic GTXs seems entirely reasonable. Examples would include refractory molds or MDR Gram-negative bacilli with no good antibiotic options. At our institution we occasionally use them for this indication, most recently on a patient who was to undergo allo-HCT after having had disseminated fusariosis during the induction of remission of his leukemia (Sheela S. et al, Leukemia Research Reports 2017, in press).
Toxicity of Granulocyte Transfusions
The main complications of GTX include fever, HLA sensitization, pulmonary reactions and (if CMV+ donors are used) CMV infection.
Fever
Fever occurs commonly after GTX and seems to be related more to the recipient than to the product itself: Hubel et al documented an increase in temperature ≥ 1.5 C after 3.75% of transfusions, but in 17.5% of patients [30]. The RING study reported “mild to moderate” transfusion reactions (most commonly fever, chills, and/or modest changes in blood pressure) in 41% of subjects. Other studies report much lower frequency of fever, and some do not even mention febrile reactions.
Alloimmunization
Following GTX, recipients may develop antibodies against HLA antigens and anti-neutrophil antibodies (against non-HLA antigens). Although these antibodies have shown to be associated with diminishing increment in the ANC and shorter duration of detectable neutrophil counts in animal models [71], an early report on 187 GTX transfusions to 19 neutropenic cancer patients failed to identify a statistically significant correlation between the presence of antibodies and the response to the transfusions [72], and testing for the presence of these antibodies is not part of the routine procedure at most institutions [18,19]. Alloimmuniization to HLA and neutrophil seems to occur very frequently (depending on how many GTX are administered). NIH investigators reported that 70% (14 of 18) very heavily transfused patients with chronic granulomatous disease (median 45 GTX per patient) developed alloimmunization detectable by at least one method [73]. These investigators subsequently determined that lack of HLA antibodies was associated with longer survival of transfused granulocytes and absence of pulmonary reactions [39]. Development of HLA immunization may also result in primary rejection of a subsequent allo-HCT [74].
Pulmonary toxicity
“Pulmonary reactions”, the apparition of pulmonary infiltrates and hypoxemia following GTX, were not mentioned in the first controlled trials of GTX. They became a focus of attention after a chart review of all the patients who had received GCX at the NIH Clinical Center between 1973 and 1980 showed that 14 of 22 (64%) patients receiving amphotericin B during GTX had developed respiratory deterioration, whereas only 2 of 35 (6%) who did not receive amphotericin experienced a similar process [14]. The investigators refer specifically to an acute respiratory distress syndome developing in temporal association with amphotericin, and declared it “quite uncharacteristic” of the toxic reactions previously associated with transfusions or with amphotericin B. Although this particular association has not been proven or reproduced, to this day it is customary to temporally separate amphotericin B products from the GTX by a few hours. Clearly some patients do develop hypoxemia and pulmonary infiltrates after GTX. The current frequency in prospective trials seems to be around 10–15% [20], [30]). Retrospective chart reviews may show higher prevalence: up 53% in the study of GTX in aspergillosis from the MD Anderson Cancer Center [44]. During a chart review it may difficult to tease out reactions truly caused by the simultaneous use of granulocytes and amphotericin from other causes of pulmonary problems like fluid overload, infection, TRALI and TACO. Regardless of the true incidence, investigators from multiple institutions have reported potentially fatal pulmonary complications of GTX, and this factor should be considered the most important potential toxicity of the procedure
CMV infection
Finally, CMV infection and disease has been reported following GTX [56,65]. The surveys on GTX practices from Europe [19] and the US [18] Show that most (but not all) use CMV negative donors preferentially, if not exclusively, at least for CMV-negative recipients. This was also the practice in the RING trial.
Conclusion
Transfused granulocytes have activity against infectious agents, but may cause transfusion reactions (including severe, even fatal, pulmonary reactions), alloimmunization that could contribute to rejection of a subsequent HCT and (unless they are obtained from CMV-seronegative donors) CMV infection. Regarding prevention of infection, there is enough (low quality, but consistent) evidence to suggest that prophylactic GTX may result in decreased infection, but there is no evidence they would be better than prophylactic antimicrobials, and overall survival has never been affected. Limited data from case series and case reports suggest GTX as prophylaxis may have a limited role as secondary prophylaxis of refractory fungal infections. The key concept here is the refractoriness of the pathogen to the available antimicrobial agents.
Regarding the therapeutic use of GTX for established infections, all modern controlled studies have failed to show clinical benefit. The negative result of the RING study is particularly troublesome, because it is difficult to envision how it could have been modified to provide a more definitive answer. Although it is possible, as the authors suggested and some experts have argued, that there was indeed an effect (limited to the patients who received large doses of granulocytes) but the study could not demonstrate it due to lack of power, the simpler explanation is that GTXs, given to the patient population identified by the inclusion criteria of the RING study, do not add any benefit to optimal antimicrobial treatment. This interpretation is consistent also with the findings of other recent controlled studies [30,31]. This is easy to understand—if someone had an infection that was bound to respond to antimicrobial therapy anyway, the GTX would not show any benefit (in fact, they could only cause harm).
Patient selection becomes then the significant limitation of the studies. On one end of the spectrum you have GTX administered to patients who had an 85% chance of recovering from their infection with antimicrobial agents alone [31]. On the other end, you have the NIH published practice of administering GTX only to patients with refractory mold or those with bacterial infections “expected to result in mortality rate higher than 90%” [40]. These are not comparable groups. With a patient population similar to the former, it will be almost impossible to recruit enough patients to show a difference (if 85% were bound to do well, only 15% of recruited patients could derive benefit from the intervention, which would make accruing the necessary sample size impossible). On the other extreme, if one considers randomizing patients with a 90% chance of dying (the NIH threshold to use GTX in bacterial infections) it seems logical to question the ethics of withholding a potentially life-saving intervention.
The difficulties in accrual are unlikely to go away, which means most likely no RCT will ever be performed to provide the definitive answer the field has been demanding for decades. Is there another way of obtaining high-quality evidence? Retrospective studies that involve reviewing decade-old charts and attempting to match suitable controls cannot provide an answer, just generate hypothesis—and the hypothesis (GTX help to control infections) already exists. A possible design that should be considered would be some variation of the methodology used by the VITAL study of isavuconazole for the treatment of mucormycosis [75]: a single-arm, open label, multicenter trial where cases were matched with contemporaneous control patients from an international registry treated with the standard of care (in the case of VITAL, amphotericin-based regimens). The establishment of an international registry following the model proposed by Vrielink et al [76], perhaps under the auspices of the CIBMTR, should also be considered.
Pending new data, it is still unclear how to use GTXs. Modern, properly controlled trials have shown no benefit. Uncontrolled observations and historical controls may be notoriously misleading. Overall, the quantity and quality of evidence are such that it seems justifiable to designate GTXs as “investigational procedure” better left to institutions that have had experience with it and describe good results. That said, there are a few reports that are compelling enough to believe that this intervention may be life-saving under some circumstances, which means centers who take care of patients with prolonged neutropenia should at least consider GTXs. The technical aspects of the procedure must be carefully implemented: obtaining the largest amount of granulocytes, transfusing them within 8 hours and aiming for an ANC increase in the 500–1000/μL should be minimum goals. Family donors did not show any advantage over unrelated donors and, in fact, unrelated donors showed significant logistical advantages and earlier initiation of therapy. Testing for alloimmunization to select the most compatible donor seems appropriate, but not all centers do it. Most papers report on the use of single donors, but GTX from pooled donors are available in the UK. Reports on daily or every-other day transfusions don’t show any consistent difference.
Regarding the indication, even physicians experienced with the use of GTXs frequently disagree in individual cases, even if they do agree on the theoretical indication. All the following criteria should be met (modified from Massey et al [22]): 1) Severe neutropenia, defined as ANC <0.5 x 109 /L due to congenital or acquired bone marrow failure syndromes (or neutrophil dysfunction); 2) Proven or highly probable fungal or bacterial infection that is unresponsive to appropriate antimicrobial therapy as demonstrated by visible spreading lesions on skin, mucosa or radiological examination and 3) Neutrophil recovery (to ANC > 0.5 x 109 /L) is anticipated. To these it may be added (or considered implicit) that the patient should be under active treatment in an attempt to achieve disease remission (i.e., there should still be curative intent, not palliative mode).
The first requirement does not require elaboration. The second is the most controversial in individual cases, because determining when an infection is not responding but still could “turn around” is ultimately a judgment call where reasonable, experienced practitioners may disagree. GTX for secondary prophylaxis of refractory infections would be included by it. The third is key: even the early controlled studies that showed the benefit of GTX showed success only when there was ultimate myeloid recovery. This was also true in the fusarium series of the NIH: a 90% response rate ended up being a 45% survival rate due to lack of recovery of marrow function [29]. GTXs are, at best, a “bridge” procedure, that may keep a patient alive until myeloid recovery ensues.
Ideally, advances in antimicrobial therapeutics would make GTX obsolete. However, the evolution of resistant bacteria and the increase in immunosuppressed patients vulnerable to a broad range of uncommon pathogens may contribute to the persistence of this niche intervention, at least in a few institutions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Estcourt LJ, Stanworth S, Doree C, Blanco P, Hopewell S, Trivella M, Massey E. Granulocyte transfusions for preventing infections in people with neutropenia or neutrophil dysfunction. Cochrane Database Syst Rev. 2015;6:CD005341. doi: 10.1002/14651858.CD005341.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Estcourt LJ, Stanworth SJ, Hopewell S, Doree C, Trivella M, Massey E. Granulocyte transfusions for treating infections in people with neutropenia or neutrophil dysfunction. Cochrane Database Syst Rev. 2016;4:CD005339. doi: 10.1002/14651858.CD005339.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strumia MM. The effect of leukocytic cream injections in the treatment of the neutropenias. American Journal of Medical Sciences. 1934;187:527–544. [Google Scholar]
- 4.Brecher G, Wilbur KM, Cronkite EP. Transfusion of separated leukocytes into irradiated dogs with aplastic marrows. Proc Soc Exp Biol Med. 1953;84:54–6. doi: 10.3181/00379727-84-20539. [DOI] [PubMed] [Google Scholar]
- 5.Epstein RB, Clift RA, Thomas ED. The effect of leukocyte transfusions on experimental bacteremia in the dog. Blood. 1969;34:782–90. [PubMed] [Google Scholar]
- 6.Freireich Levin, Whang Carbone, Bronson, Morse The function and fate of transfused leukocytes from donors with chronic myeloid leukaemia in leukopenic recipients. Ann N Y Acad Sci. 1964;113:1081–9. doi: 10.1111/j.1749-6632.1964.tb40726.x. [DOI] [PubMed] [Google Scholar]
- 7.Freireich EJ. Leukocyte transfusion and the development of the continuous-flow blood cell separator. Transfus Med Rev. 2011;25:344–50. doi: 10.1016/j.tmrv.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Graw RG, Herzig G, Perry S, Henderson ES. Normal granulocyte transfusion therapy: treatment of septicemia due to gram-negative bacteria. N Engl J Med. 1972;287:367–71. doi: 10.1056/NEJM197208242870801. [DOI] [PubMed] [Google Scholar]
- 9.Higby DJ, Yates JW, Henderson ES, Holland JF. Filtration leukapheresis for granulocyte transfusion therapy. Clinical and laboratory studies. N Engl J Med. 1975;292:761–6. doi: 10.1056/NEJM197504102921501. [DOI] [PubMed] [Google Scholar]
- 10.Herzig RH, Herzig GP, Graw RG, Bull MI, Ray KK. Successful granulocyte transfusion therapy for gram-negative septicemia. A prospectively randomized controlled study. N Engl J Med. 1977;296:701–5. doi: 10.1056/NEJM197703312961301. [DOI] [PubMed] [Google Scholar]
- 11.Vogler WR, Winton EF. A controlled study of the efficacy of granulocyte transfusions in patients with neutropenia. Am J Med. 1977;63:548–55. doi: 10.1016/0002-9343(77)90200-5. [DOI] [PubMed] [Google Scholar]
- 12.Fortuny IE, Bloomfield CD, Hadlock DC, Goldman A, Kennedy BJ, McCullough JJ. Granylocyte transfusion: a controlled study in patients with acuute nonlymphocytic leukemia. Transfusion. 1975;15:548–58. doi: 10.1046/j.1537-2995.1975.15676082229.x. [DOI] [PubMed] [Google Scholar]
- 13.Winston DJ, Ho WG, Gale RP. Therapeutic granulocyte transfusions for documented infections. A controlled trial in ninety-five infectious granulocytopenic episodes. Ann Intern Med. 1982;97:509–15. doi: 10.7326/0003-4819-97-4-509. [DOI] [PubMed] [Google Scholar]
- 14.Wright DG, Robichaud KJ, Pizzo PA, Deisseroth AB. Lethal pulmonary reactions associated with the combined use of amphotericin B and leukocyte transfusions. N Engl J Med. 1981;304:1185–9. doi: 10.1056/NEJM198105143042001. [DOI] [PubMed] [Google Scholar]
- 15.Strauss RG. Therapeutic granulocyte transfusions in 1993. Blood. 1993;81:1675–8. [PubMed] [Google Scholar]
- 16.Bensinger WI, Price TH, Dale DC, Appelbaum FR, Clift R, Lilleby K, Williams B, Storb R, Thomas ED, Buckner CD. The effects of daily recombinant human granulocyte colony-stimulating factor administration on normal granulocyte donors undergoing leukapheresis. Blood. 1993;81:1883–8. [PubMed] [Google Scholar]
- 17.Stroncek DF, Yau YY, Oblitas J, Leitman SF. Administration of G--CSF plus dexamethasone produces greater granulocyte concentrate yields while causing no more donor toxicity than G--CSF alone. Transfusion. 2001;41:1037–44. doi: 10.1046/j.1537-2995.2001.41081037.x. [DOI] [PubMed] [Google Scholar]
- 18.Strauss RG, Klein HG, Leitman SF, Price TH, Lichtiger B, Martinez F, Reesink HW, Panzer S. Preparation of granulocyte concentrates by apheresis: collection modalities in the USA. Vox Sang. 2011;100:426–33. doi: 10.1111/j.1423-0410.2010.01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leitner G, Panzer S, Reesink HW, Stiegler G, Fischer-Nielsen A, Dickmeiss E, Einsele H, Reinhardt P, Schrezenmeier H, Wiesneth M, Coluccia P, Nygell UA, Halter J, Sigle J, Gratwohl A, Buser AS, Ozturk G, Anak S. Preparation of granulocyte concentrates by apheresis. Vox Sanguinis. 2010;98:567–575. doi: 10.1111/j.1423-0410.2010.01315.x. [DOI] [PubMed] [Google Scholar]
- 20.Price TH, Boeckh M, Harrison RW, McCullough J, Ness PM, Strauss RG, Nichols WG, Hamza TH, Cushing MM, King KE, Young JH, Williams E, McFarland J, Holter Chakrabarty J, Sloan SR, Friedman D, Parekh S, Sachais BS, Kiss JE, Assmann SF. Efficacy of transfusion with granulocytes from G-CSF/dexamethasone treated donors in neutropenic patients with infection. Blood. 2015;126:2153. doi: 10.1182/blood-2015-05-645986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Conseil D. Guide to the preparation, use and quality assurance of blood components : recommendation No. R (95) 15. Council of Europe; Strasbourg: 2015. [Google Scholar]
- 22.Massey E. CLINICAL GUIDELINES FOR THE USE OF GRANULOCYTE TRANSFUSIONS. Granulocyte WorkingGroup; UK: 2012. [Google Scholar]
- 23.Strauss RG. Function of granulocytes collected for transfusion. Prog Clin Biol Res. 1982;88:9–17. [PubMed] [Google Scholar]
- 24.Bashir S, Cardigan R. Granulocyte concentrates: how can we assess their quality? Transfus Med. 2003;13:245–57. doi: 10.1046/j.1365-3148.2003.00451.x. [DOI] [PubMed] [Google Scholar]
- 25.Bashir S, Stanworth S, Massey E, Goddard F, Cardigan R. Neutrophil function is preserved in a pooled granulocyte component prepared from whole blood donations. Br J Haematol. 2008;140:701–11. doi: 10.1111/j.1365-2141.2008.06996.x. [DOI] [PubMed] [Google Scholar]
- 26.Cugno C, Deola S, Filippini P, Stroncek DF, Rutella S. Granulocyte transfusions in children and adults with hematological malignancies: benefits and controversies. J Transl Med. 2015;13:362. doi: 10.1186/s12967-015-0724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gazendam RP, van de Geer A, van Hamme JL, Tool AT, van Rees DJ, Aarts CE, van den Biggelaar M, van Alphen F, Verkuijlen P, Meijer AB, Janssen H, Roos D, van den Berg TK, Kuijpers TW. Impaired killing of Candida albicans by granulocytes mobilized for transfusion purposes: a role for granule components. Haematologica. 2016;101:587–96. doi: 10.3324/haematol.2015.136630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freireich EJ, Lichtiger B, Mattiuzzi G, Martinez F, Reddy V, Kyle Wathen J. A prospective, randomized, double-blind study, comparing unirradiated to irradiated white blood cell transfusions in acute leukemia patients. Leukemia. 2013;27:861–5. doi: 10.1038/leu.2012.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kadri SS, Remy KE, Strich JR, Gea-Banacloche J, Leitman SF. Role of granulocyte transfusions in invasive fusariosis: systematic review and single-center experience. Transfusion. 2015 doi: 10.1111/trf.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hübel K, Carter RA, Liles WC, Dale DC, Price TH, Bowden RA, Rowley SD, Chauncey TR, Bensinger WI, Boeckh M. Granulocyte transfusion therapy for infections in candidates and recipients of HPC transplantation: a comparative analysis of feasibility and outcome for community donors versus related donors. Transfusion. 2002;42:1414–21. doi: 10.1046/j.1537-2995.2002.00249.x. [DOI] [PubMed] [Google Scholar]
- 31.Seidel MG, Peters C, Wacker A, Northoff H, Moog R, Boehme A, Silling G, Grimminger W, Einsele H. Randomized phase III study of granulocyte transfusions in neutropenic patients. Bone Marrow Transplant. 2008;42:679–84. doi: 10.1038/bmt.2008.237. [DOI] [PubMed] [Google Scholar]
- 32.Seidel MG, Minkov M, Witt V, Matthes-Martin S, Pötschger U, Worel N, Leitner G, Stary J, Gadner H, Peters C. Granulocyte transfusions in children and young adults: does the dose matter? J Pediatr Hematol Oncol. 2009;31:166–72. doi: 10.1097/MPH.0b013e318196a6f9. [DOI] [PubMed] [Google Scholar]
- 33.Cancelas JA. Granulocyte transfusion: questions remain. Blood. 2015;126:2082–2083. doi: 10.1182/blood-2015-09-669085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strauss RG. Neutrophil/granulocyte transfusions collected from G-CSF + dexamethasone-stimulated donors. Curr Opin Hematol. 2015 doi: 10.1097/MOH.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 35.Teofili L, Valentini CG, Di Blasi R, Orlando N, Fianchi L, Zini G, Sica S, De Stefano V, Pagano L. Dose-Dependent Effect of Granulocyte Transfusions in Hematological Patients with Febrile Neutropenia. PLoS One. 2016;11:e0159569. doi: 10.1371/journal.pone.0159569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Mahallawy HA, El-Wakil M, Moneer MM, Shalaby L. Antibiotic resistance is associated with longer bacteremic episodes and worse outcome in febrile neutropenic children with cancer. Pediatr Blood Cancer. 2011;57:283–8. doi: 10.1002/pbc.22926. [DOI] [PubMed] [Google Scholar]
- 37.Lee JH, Kim SK, Kim SK, Han SB, Lee JW, Lee DG, Chung NG, Cho B, Jeong DC, Kang JH, Kim HK. Increase in Antibiotic-Resistant Gram-Negative Bacterial Infections in Febrile Neutropenic Children. Infect Chemother. 2016;48:181–189. doi: 10.3947/ic.2016.48.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marciano BE, Allen ES, Cantilena CC, Kristosturyan E, Klein HG, Fleisher TA, Holland SM, Malech HL, Rosenzweig SD. Granulocyte transfusions in patients with chronic granulomatous disease and refractory infections: the NIH experience. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heim KF, Fleisher TA, Stroncek DF, Holland SM, Gallin JI, Malech HL, Leitman SF. The relationship between alloimmunization and posttransfusion granulocyte survival: experience in a chronic granulomatous disease cohort. Transfusion. 2011;51:1154–62. doi: 10.1111/j.1537-2995.2010.02993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quillen K, Wong E, Scheinberg P, Young NS, Walsh TJ, Wu CO, Leitman SF. Granulocyte transfusions in severe aplastic anemia: an eleven-year experience. Haematologica. 2009;94:1661–8. doi: 10.3324/haematol.2009.010231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guarro J. Fusariosis, a complex infection caused by a high diversity of fungal species refractory to treatment. Eur J Clin Microbiol Infect Dis. 2013;32:1491–500. doi: 10.1007/s10096-013-1924-7. [DOI] [PubMed] [Google Scholar]
- 42.Kim KH, Lim HJ, Kim JS, Kim BS, Bang SM, Kim I, Han KS, Kim BK, Lee SM, Yoon SS. Therapeutic granulocyte transfusions for the treatment of febrile neutropenia in patients with hematologic diseases: a 10-year experience at a single institute. Cytotherapy. 2011;13:490–8. doi: 10.3109/14653249.2010.529889. [DOI] [PubMed] [Google Scholar]
- 43.Safdar A, Hanna HA, Boktour M, Kontoyiannis DP, Hachem R, Lichtiger B, Freireich EJ, Raad II. Impact of high-dose granulocyte transfusions in patients with cancer with candidemia: retrospective case-control analysis of 491 episodes of Candida species bloodstream infections. Cancer. 2004;101:2859–65. doi: 10.1002/cncr.20710. [DOI] [PubMed] [Google Scholar]
- 44.Raad II, Chaftari AM, Al Shuaibi MM, Jiang Y, Shomali W, Cortes JE, Lichtiger B, Hachem RY. Granulocyte transfusions in hematologic malignancy patients with invasive pulmonary aspergillosis: outcomes and complications. Ann Oncol. 2013 doi: 10.1093/annonc/mdt110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Safdar A, Rodriguez G, Zuniga J, Al Akhrass F, Pande A. Use of healthy-donor granulocyte transfusions to treat infections in neutropenic patients with myeloid or lymphoid neoplasms: experience in 74 patients treated with 373 granulocyte transfusions. Acta Haematol. 2014;131:50–8. doi: 10.1159/000351174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Massey E, Harding K, Kahan BC, Llewelyn C, Wynn R, Moppett J, Robinson SP, Green A, Lucas G, Sadani D, Liakopoulou E, Bolton-Maggs P, Marks DI, Stanworth S. The granulocytes in neutropenia 1 (GIN 1) study: a safety study of granulocytes collected from whole blood and stored in additive solution and plasma. Transfus Med. 2012;22:277–84. doi: 10.1111/j.1365-3148.2012.01152.x. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Wu Y, Fu R, Qu W, Ruan E, Wang G, Liu H, Song J, Xing L, Guan J, Li L, Liu C, Shao Z. Granulocyte transfusion combined with granulocyte colony stimulating factor in severe infection patients with severe aplastic anemia: a single center experience from China. PLoS One. 2014;9:e88148. doi: 10.1371/journal.pone.0088148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vamvakas EC, Pineda AA. Meta-analysis of clinical studies of the efficacy of granulocyte transfusions in the treatment of bacterial sepsis. J Clin Apher. 1996;11:1–9. doi: 10.1002/(SICI)1098-1101(1996)11:1<1::AID-JCA1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 49.Scali G, von Felten A, Fehr J, Sauter C, Gmür J. Granulocyte substitution in febrile leukemia patients with bone marrow aplasia. 1. Results of a prospective study. Schweiz Med Wochenschr. 1978;108:1583–5. [PubMed] [Google Scholar]
- 50.Klastersky JA. Early granulocyte transfusions in high risk febrile neutropenic patients. Schweiz Med Wochenschr Suppl. 1983;14:46–8. [PubMed] [Google Scholar]
- 51.Bow EJ, Schroeder ML, Louie TJ. Pulmonary complications in patients receiving granulocyte transfusions and amphotericin B. Can Med Assoc J. 1984;130:593–7. [PMC free article] [PubMed] [Google Scholar]
- 52.Alavi JB, Root RK, Djerassi I, Evans AE, Gluckman SJ, MacGregor RR, Guerry D, Schreiber AD, Shaw JM, Koch P, Cooper RA. A randomized clinical trial of granulocyte transfusions for infection in acute leukemia. N Engl J Med. 1977;296:706–11. doi: 10.1056/NEJM197703312961302. [DOI] [PubMed] [Google Scholar]
- 53.Matsue K, Harada M, Nakao S, Ueda M, Kondo K, Odaka K, Mori T, Hattori K. Controlled study of therapeutic granulocyte transfusions in granulocytopenic patients with severe infections. Jpn J Clin Oncol. 1984;14:21–30. [PubMed] [Google Scholar]
- 54.Clift RA, Sanders JE, Thomas ED, Williams B, Buckner CD. Granulocyte transfusions for the prevention of infection in patients receiving bone-marrow transplants. N Engl J Med. 1978;298:1052–7. doi: 10.1056/NEJM197805112981904. [DOI] [PubMed] [Google Scholar]
- 55.Mannoni P, Rodet M, Vernant JP, Brun B, Coquin-Radeau EI, Bracq C, Rochant H, Dreyfus B. Efficiency of prophylactic granulocyte transfusions in preventing infections in acute leukaemia. Rev Fr Transfus Immunohematol. 1979;22:503–18. [PubMed] [Google Scholar]
- 56.Winston DJ, Ho WG, Young LS, Gale RP. Prophylactic granulocyte transfusions during human bone marrow transplantation. Am J Med. 1980;68:893–7. doi: 10.1016/0002-9343(80)90223-5. [DOI] [PubMed] [Google Scholar]
- 57.Winston DJ, Ho WG, Gale RP. Prophylactic granulocyte transfusions during chemotherapy of acute nonlymphocytic leukemia. Ann Intern Med. 1981;94:616–22. doi: 10.7326/0003-4819-94-5-616. [DOI] [PubMed] [Google Scholar]
- 58.Strauss RG, Connett JE, Gale RP, Bloomfield CD, Herzig GP, McCullough J, Maguire LC, Winston DJ, Ho W, Stump DC, Miller WV, Koepke JA. A controlled trial of prophylactic granulocyte transfusions during initial induction chemotherapy for acute myelogenous leukemia. N Engl J Med. 1981;305:597–603. doi: 10.1056/NEJM198109103051101. [DOI] [PubMed] [Google Scholar]
- 59.Ford JM, Cullen MH, Roberts MM, Brown LM, Oliver RT, Lister TA. Prophylactic granulocyte transfusions: results of a randomized controlled trial in patients with acute myelogenous leukemia. Transfusion. 1982;22:311–6. doi: 10.1046/j.1537-2995.1982.22482251217.x. [DOI] [PubMed] [Google Scholar]
- 60.Schiffer CA, Aisner J, Daly PA, Schimpff SC, Wiernik PH. Alloimmunization following prophylactic granulocyte transfusion. Blood. 1979;54:766–74. [PubMed] [Google Scholar]
- 61.Gomez-Villagran JL, Torres-Gómez A, Gomez-Garcia P, Martinez-Guibelalde F, Velasco-Jimena F. A controlled trial of prophylactic granulocyte transfusions during induction chemotherapy for acute nonlymphoblastic leukemia. Cancer. 1984;54:734–8. doi: 10.1002/1097-0142(1984)54:4<734::aid-cncr2820540424>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 62.Petersen FB, Buckner CD, Clift RA, Nelson N, Counts GW, Meyers JD, Thomas ED. Prevention of nosocomial infections in marrow transplant patients: a prospective randomized comparison of systemic antibiotics versus granulocyte transfusions. Infect Control. 1986;7:586–92. doi: 10.1017/s0195941700065437. [DOI] [PubMed] [Google Scholar]
- 63.Menitove JE, Abrams RA. Granulocyte transfusions in neutropenic patients. Crit Rev Oncol Hematol. 1987;7:89–113. doi: 10.1016/s1040-8428(87)80016-1. [DOI] [PubMed] [Google Scholar]
- 64.Vamvakas EC, Pineda AA. Determinants of the efficacy of prophylactic granulocyte transfusions: a meta-analysis. J Clin Apher. 1997;12:74–81. doi: 10.1002/(sici)1098-1101(1997)12:2<74::aid-jca4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 65.Illerhaus G, Wirth K, Dwenger A, Waller CF, Garbe A, Brass V, Lang H, Lange W. Treatment and prophylaxis of severe infections in neutropenic patients by granulocyte transfusions. Ann Hematol. 2002;81:273–81. doi: 10.1007/s00277-002-0439-6. [DOI] [PubMed] [Google Scholar]
- 66.Vij R, DiPersio JF, Venkatraman P, Trinkaus K, Goodnough LT, Brown RA, Khoury HJ, Devine SM, Oza A, Shenoy S, Blum W, Adkins D. Donor CMV serostatus has no impact on CMV viremia or disease when prophylactic granulocyte transfusions are given following allogeneic peripheral blood stem cell transplantation. Blood. 2003;101:2067–9. doi: 10.1182/blood-2002-07-2110. [DOI] [PubMed] [Google Scholar]
- 67.Oza A, Hallemeier C, Goodnough L, Khoury H, Shenoy S, Devine S, Augustin K, Vij R, Trinkaus K, Dipersio JF, Adkins D. Granulocyte-colony-stimulating factor-mobilized prophylactic granulocyte transfusions given after allogeneic peripheral blood progenitor cell transplantation result in a modest reduction of febrile days and intravenous antibiotic usage. Transfusion. 2006;46:14–23. doi: 10.1111/j.1537-2995.2005.00665.x. [DOI] [PubMed] [Google Scholar]
- 68.Mousset S, Hermann S, Klein SA, Bialleck H, Duchscherer M, Bomke B, Wassmann B, Böhme A, Hoelzer D, Martin H. Prophylactic and interventional granulocyte transfusions in patients with haematological malignancies and life-threatening infections during neutropenia. Ann Hematol. 2005;84:734–41. doi: 10.1007/s00277-005-1055-z. [DOI] [PubMed] [Google Scholar]
- 69.Grigull L, Beilken A, Schmid H, Kirschner P, Sykora KW, Linderkamp C, Donnerstag F, Goudeva L, Heuft HG, Welte K. Secondary prophylaxis of invasive fungal infections with combination antifungal therapy and G-CSF-mobilized granulocyte transfusions in three children with hematological malignancies. Support Care Cancer. 2006;14:783–6. doi: 10.1007/s00520-005-0910-8. [DOI] [PubMed] [Google Scholar]
- 70.Nikolajeva O, Mijovic A, Hess D, Tatam E, Amrolia P, Chiesa R, Rao K, Silva J, Veys P. Single-donor granulocyte transfusions for improving the outcome of high-risk pediatric patients with known bacterial and fungal infections undergoing stem cell transplantation: a 10-year single-center experience. Bone Marrow Transplant. 2015;50:846–9. doi: 10.1038/bmt.2015.53. [DOI] [PubMed] [Google Scholar]
- 71.Appelbaum FR, Trapani RJ, Graw RG. Consequences of prior alloimmunization during granulocyte transfusion. Transfusion. 1977;17:460–4. doi: 10.1046/j.1537-2995.1977.17578014584.x. [DOI] [PubMed] [Google Scholar]
- 72.Ungerleider RS, Appelbaum FR, Trapani RJ, Deisseroth AB. Lack of Predictive Value of Antileukocyte Antibody Screening in Granulocyte Transfusion Therapy. Transfusion. 1979;19:90–94. doi: 10.1046/j.1537-2995.1979.19179160275.x. [DOI] [PubMed] [Google Scholar]
- 73.Stroncek DF, Leonard K, Eiber G, Malech HL, Gallin JI, Leitman SF. Alloimmunization after granulocyte transfusions. Transfusion. 1996;36:1009–15. doi: 10.1046/j.1537-2995.1996.36111297091747.x. [DOI] [PubMed] [Google Scholar]
- 74.O’Donghaile D, Childs RW, Leitman SF. Blood consult: granulocyte transfusions to treat invasive aspergillosis in a patient with severe aplastic anemia awaiting mismatched hematopoietic progenitor cell transplantation. Blood. 2012;119:1353–5. doi: 10.1182/blood-2011-10-345751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marty FM, Ostrosky-Zeichner L, Cornely OA, Mullane KM, Perfect JR, Thompson GR, Alangaden GJ, Brown JM, Fredricks DN, Heinz WJ, Herbrecht R, Klimko N, Klyasova G, Maertens JA, Melinkeri SR, Oren I, Pappas PG, Ráčil Z, Rahav G, Santos R, Schwartz S, Vehreschild JJ, Young JA, Chetchotisakd P, Jaruratanasirikul S, Kanj SS, Engelhardt M, Kaufhold A, Ito M, Lee M, Sasse C, Maher RM, Zeiher B, Vehreschild MJ VITAL and FungiScope Mucormycosis Investigators. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016;16:828–37. doi: 10.1016/S1473-3099(16)00071-2. [DOI] [PubMed] [Google Scholar]
- 76.Vrielink H, Meijer B, van’t Ende E, Ball LM, Brand A, Zwaginga JJ. Granulocyte transfusions for pediatric patients and the establishment of national treatment guidelines and donor registry. Transfus Apher Sci. 2009;41:73–6. doi: 10.1016/j.transci.2009.05.016. [DOI] [PubMed] [Google Scholar]