Abstract
Prostate cancer remains dependent on androgen receptor signaling even after castration. Aberrant androgen receptor signaling in castration resistant prostate cancer is mediated by mechanisms such as alterations in the androgen receptor and activation of interacting signaling pathways. Clinical evidence confirms that resistance to the next generation anti-androgen, enzalutamide, may be mediated to a large extent by alternative splicing of the androgen receptor to generate constitutively active splice variants such as AR-V7. The splice variants AR-V7 and Arv567es have been implicated in the resistance to not only enzalutamide, but also to abiraterone and other conventional therapeutics such as taxanes. Numerous studies including ours suggest that splicing factors such as hnRNPA1 promote the generation of AR-V7, thus contributing to enzalutamide resistance in prostate cancer cells. In the present study, we discovered that quercetin, a naturally occurring polyphenolic compound, reduces the expression of hnRNPA1, and consequently, that of AR-V7. The suppression of AR-V7 by quercetin resensitizes enzalutamide-resistant prostate cancer cells to treatment with enzalutamide. Our results indicate that quercetin downregulates hnRNPA1 expression, downregulates the expression of AR-V7, antagonizes androgen receptor signaling, and resensitizes enzalutamide-resistant prostate cancer cells to enzalutamide treatment in vivo in mouse xenografts. These findings demonstrate that suppressing the alternative splicing of the androgen receptor may have important implications in overcoming the resistance to next-generation anti-androgen therapy.
Keywords: Quercetin, hnRNPA1, AR-V7, Enzalutamide, Androgen Receptor
Introduction
Prostate cancer remains one of the cancers with high rates of incidence and mortality among men in the US. While responsive to androgen deprivation therapy (ADT) initially, most patients exhibit progression to castration resistance, which poses the principal challenge to researchers. Castration-resistant prostate cancer (CRPC) evades androgen ablation by activating androgen receptor (AR)-dependent signaling through alternative mechanisms. The development of androgen axis-targeted therapeutics such as abiraterone and enzalutamide marked the continuing success of ADT, but like earlier ADT, these new therapies have a short duration of efficacy due to primary or acquired resistance. A major mechanism of therapy resistance in prostate cancer is the generation of truncated AR splicing variants that lack the ligand-binding-domain and are constitutively active, thus evading binding of anti-androgens.
Many AR splice variants are constitutively nuclear and active even in the absence of androgens, thus indicating their potential role in the acquisition of the CRPC phenotype. Expression of most of these variants arises from the inclusion of cryptic exons located in introns 2 and 3 of the AR gene, which inserts premature stop codons and termination sites, yielding shorter AR proteins of 75–80 kDa lacking the androgen-binding domain (1–3). The selective knockdown of truncated AR variants such as AR-V7 and ARv567es suppresses the androgen-independent growth of CRPC cells (4). The variants confer resistance to not only AR targeted therapies such as enzalutamide and abiraterone (5, 6), but also to conventional chemotherapeutics such as taxanes used as first line therapies against CRPC (7, 8). The mechanisms mediating increased expression of aberrant AR splice variants in PCa are being actively investigated, the possible causes being either genomic rearrangement and/or intragenic deletions of the AR locus (9) or aberrant expression/recruitment of specific splicing factors (10, 11).
We reported previously that the splicing factor hnRNPA1 plays a major role in the alternative splicing of the AR to generate AR-V7 (11). Based on the hypothesis that the generation of AR-V7 is one of the principal mechanisms of resistance to enzalutamide and targeting alternative splicing would be beneficial in reducing the levels of AR-V7 and overcoming therapy resistance, we set out to identify compounds that target the expression of the splicing factor hnRNPA1. We found that a previous report used chemical proteomics to show that hnRNPA1 is a target of quercetin, a naturally occurring polyphenolic compound. Hence, in this study, we examined the activity of quercetin in inhibiting hnRNPA1 and overcoming therapy resistance in enzalutamide-resistant prostate cancer cells.
Men at high risk for prostate cancer frequently supplement their diet with combinations of vitamins, minerals, and fruit/seed extracts, and more than 25% consume three or more supplements. Nearly one in five men at high risk for prostate cancer use fruit and seed extracts either alone or in combination with vitamins. Quercetin is one of the components of fruits and seeds that are being actively studied for their anti-proliferative effects. In vitro studies using prostate cancer cell lines as well as in vivo studies in mice have found that quercetin provides chemoprotection, induces apoptosis, and increases the activity of antioxidant enzymes in cancer cells (12–14). An in vitro study reported that quercetin regulates insulin-like growth factor signaling and induces apoptosis in androgen-independent PC-3 prostate cancer cells (15). Quercetin was found to reverse epidermal growth factor-induced epithelial-to-mesenchymal transition and prevent or delay prostate cancer metastases in a PC-3 prostate cancer cell xenograft model (16). Supplementation with quercetin was found to enhance the chemopreventive effects of green tea against prostate cancer cell xenografts (17). In addition, quercetin was shown to improve chronic prostatitis/chronic pelvic pain in a significant proportion of men (18). Treatment with a combination of green tea and quercetin sensitized prostate cancer cells to docetaxel (19). Thus, accumulating evidence points to a significant anti-proliferative role for quercetin in prostate cancer.
In this study, we examined the effects of quercetin on the splicing factor hnRNPA1 and consequently on the expression of AR-V7, the most abundant splice variant of the AR. Our results indicate that quercetin downregulates the expression of hnRNPA1, which leads to suppression of the expression of AR-V7 and consequently of sustained ligand-independent signaling mediated by AR-V7. We also demonstrate that co-treatment with quercetin and enzalutamide resensitizes enzalutamide-resistant prostate cancer cells to enzalutamide, presumably due to the suppression of AR-V7 levels. These findings may have significant implications for the optimization of the efficacy of androgen receptor antagonists such as enzalutamide.
Materials and Methods
Cell lines and reagents
HEK293 and CWR22Rv1 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and were cultured in DMEM or RPMI containing 10% complete FBS and penicillin/streptomycin, respectively. All experiments with cell lines were performed either within 6 months of receipt from ATCC or resuscitation after cryopreservation. ATCC uses Short Tandem Repeat (STR) profiling for testing and authentication of cell lines. C4-2B cells were kindly provided and authenticated by Dr. Leland Chung, Cedars-Sinai Medical Center, Los Angeles, CA. 22Rv1 and C4-2B cells resistant to enzalutamide (22Rv1-Enza-R and C4-2B-Enza-R) were described previously (20, 21). Antibodies against AR (441; mouse monoclonal), Tubulin, and PolII were from Santa Cruz Biotechnologies. Antibodies against the splicing factor hnRNPA1 (9H10) were from Sigma-Aldrich. Antibodies against AR-V7 were obtained from Precision Antibody. Sso FastTM Eva Green qPCR Supermix was from Bio-Rad. Quercetin (chemical structure in Suppl. Fig. 1A) was obtained from Sigma. Enzalutamide was generously supplied by Medivation, Inc. The luciferase reporter construct that contained the 5′-flanking region (−5400 to +580) of the full length human AR promoter was kindly provided by Drs. Wen-Chin Huang and Leland Chung, Cedars-Sinai Medical Center, Los Angeles, CA (22). All other reagents were of analytical grade and obtained from local suppliers.
Cell growth assays
Plasmid and oligonucleotide transfections were performed using Lipofectamine 2000 transfection reagent (Invitrogen). Viable cell numbers were determined using a Coulter cell counter (Beckman Coulter).
Western Blot Analysis
Cells were lysed in high salt buffer containing 50 mM Hepes pH 7.9, 250 mM NaCl, 1 mM EDTA, 1% NP-40, 1 mM PMSF, 1 mM Na Vanadate, 1 mM NaF and protease inhibitor cocktail (Roche). Total protein was estimated using the Coomassie Protein Assay Reagent (Pierce). Equal amounts of protein were loaded on 10% SDS–PAGE and transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat milk in PBST (1× PBS+0.1% Tween-20) and probed with the indicated primary antibodies in 1% BSA. The signal was detected by ECL (Millipore) after incubation with the appropriate HRP-conjugated secondary antibodies.
Real-Time quantitative RT-PCR
Total RNAs were extracted using TriZOL reagent (Invitrogen). cDNAs were prepared after digestion with RNase-free RQ1 DNase (Promega). The cDNAs were subjected to real-time reverse transcription-PCR (RT-PCR) using Sso FastTM Eva Green Supermix (Bio-Rad) as described previously (23). Each reaction was normalized by co-amplification of actin. Triplicates of samples were run on a Bio-Rad CFX-96 real-time cycler. Primers used were: AR-full length: 5′-AAG CCA GAG CTG TGC AGA TGA, 3′-TGT CCT GCA GCC ACT GGT TC; AR-V7: 5′-AAC AGA AGT ACC TGT GCG CC, 3′-TCA GGG TCT GGT CAT TTT GA; PSA: 5′-GCC CTG CCC GAA AGG, 3′-GAT CCA CTT CCG GTA ATG CA; Actin: 5′-AGA ACT GGC CCT TCT TGG AGG, 3′-GTT TTT ATG TTC CTC TAT GGG; hnRNPA1: 5′-TGG AGG TGG TGG AAG CTA CA, 3′-TAG CTA CTG CTG CTG CTG GA; NKX3.1: 5′-CCG AGA CGC TGG CAG AGA CC, 3′-GCT TAG GGG TTT GGG GAA G; UBE2C: 5′-TGG TCT GCC CTG TAT GAT GT, 3′-AAA AGC TGT GGG GTT TTT CC.
Chromatin Immunoprecipitation assay (ChIP)
DNA-protein complexes in the cells were crosslinked with 1% formaldehyde for 10 min at 37°C. Cells were washed and harvested and cell lysates prepared by sonication at 4°C. 10 μg of the total protein was immunoprecipitated with anti-AR antibodies. Isotype matched IgG was used as control. The immunoprecipitated protein-DNA complexes were washed, eluted, the cross linking was reversed with NaCl for 4 h at 65°C and subjected to proteinase K digestion at 37°C overnight. Bound DNA was extracted with phenol:chloroform:isoamylalcohol and precipitated with the addition of ammonium acetate, glycogen, and 100% ethanol. Dissolved DNA was subjected to PCR with primers spanning the proximal AREs of the PSA promoter. The primer sequences used were: ARE-I/II: Forward: 5′-CCTAGATGAAGTCTCCATGAGCTACA-3′ and ARE-I/II: Reverse: 5′-GGGAGGGAGAGCTAGCACTTG-3′.
Luciferase Assays
Cells were transfected with the PSA-enhancer-promoter-luciferase reporter and treated as indicated in the figures. Cell lysates were subjected to luciferase assays with the Luciferase Assay System (Promega).
Clonogenic Assays
Anchorage-dependent clonogenic ability assays were performed as described previously (24). Briefly, C4-2B parental and C4-2B-Enza-R or 22Rv1 parental and 22Rv1-Enza-R cells were treated with vehicle, 10 or 20 μM quercetin, or a combination of quercetin and 20 μM enzalutamide for 24 h. Cells were trypsinized and replated at low densities (1000 cells/dish) in 10 cm culture plates. The plates were incubated at 37°C in RPMI1640 containing 10% FBS and were left undisturbed for 14 days. At the end of the experiment, cells were fixed with methanol, stained with 0.05% Crystal Violet and the numbers of colonies were counted.
Mouse xenograft assays
2×106 22Rv1 cells were injected with 1:1 matrigel sub-cutaneously into the flanks of male SCID mice and tumor growth monitored. When the tumors were palpable, mice were randomly divided into 4 groups (n=6) and treated with: 1) vehicle (0.5% Methocel 4AM), 2) 25 mg/kg enzalutamide via oral gavage daily for 5 days/week, 3) 20 mg/kg quercetin i.p. daily for 5 days/week, and 4) a combination of quercetin and enzalutamide daily for 5 days/week. Treatments were performed 5 days a week for 3 weeks and tumor growth was monitored using digital calipers. At the end of the experiment, tumor tissues were harvested and expression levels of full length AR, hnRNPA1, and Ki67 were analyzed using immunohistochemical staining and AR-V7 using RNA in situ hybridization.
Immunohistochemistry
Tumors were fixed using formalin and paraffin embedded tissue blocks were dewaxed, rehydrated, and endogenous peroxidase activity blocked. Antigen retrieval was performed in sodium citrate buffer (0.01 mol/L, pH 6.0) in a microwave oven at 1,000 W for 3 min and then at 100 W for 20 min. Nonspecific antibody binding was blocked by incubating with 10% fetal bovine serum in PBS for 30 min at room temperature. Slides were then incubated with anti-Ki-67 (NeoMarker), anti-AR (441) (Santa Cruz), and anti-hnRNPA1 (Sigma) at 4°C overnight. Slides were washed and incubated with biotin-conjugated secondary antibodies for 30 min, followed by incubation with avidin DH-biotinylated horseradish peroxidase complex for 30 min (Vectastain ABC Elite Kit, Vector Laboratories). The sections were developed with the diaminobenzidine substrate kit (Vector Laboratories) and counterstained with hematoxylin.
RNA in situ hybridization
RNA in situ hybridization was performed to detect the mRNA levels of AR-V7 using the ACD RNAscope 2.0 Brown kit (Advanced Cell Diagnostics). ACD target probes, a series of paired oligonucleotides forming a binding site for a preamplifier, are designed to detect RNA corresponding to the cryptic AR exon 3 sequence in the mature human AR-V7 transcript. FFPE tissue sections were baked for 1 h at 60°C, deparaffinized with xylene for 20 min at room temperature, and allowed to air dry after two rinses using 100% ethanol. After a series of pretreatment steps, the cells were permeabilized using protease to allow probe access to the RNA target. Hybridization of the probes to the RNA targets was performed by incubation for 2 h at 40°C. After two washes, the slides were processed for standard signal amplification according to the manufacturer’s instructions.
Statistical Analyses
Data are shown as means ± SD. Multiple group comparison was performed by one-way ANOVA followed by the Scheffe procedure for comparison of means. P≤0.05 was considered significant.
Results
Quercetin downregulates the expression of hnRNPA1
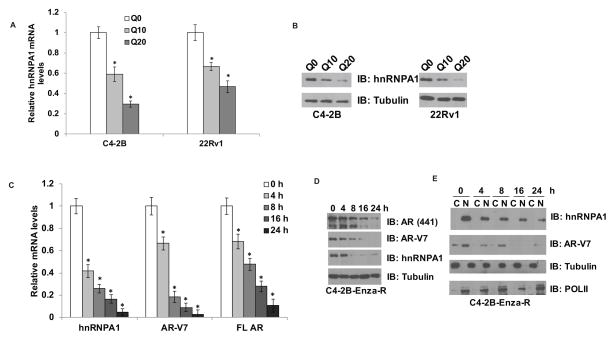
Our previous studies showed that the splicing factor, hnRNPA1, plays a critical role in the alternative splicing of the AR to generate splice variants such as AR-V7 (11). We also demonstrated that the suppression of hnRNPA1 expression using siRNA reduces the levels of AR-V7, thereby resensitizing enzalutamide-resistant cells to treatment with enzalutamide. Hence, we hypothesized that downregulation of hnRNPA1 expression may assist in suppressing the expression of AR-V7, and consequently in prolonging the effectiveness of enzalutamide. A recent report (25) disclosed that quercetin, a phytoflavonoid, exerts its anti-cancer effects by binding to and impairing the ability of hnRNPA1 to shuttle between the nucleus and cytoplasm, resulting in its cytoplasmic retention. Hence, we analyzed whether quercetin affects the expression of hnRNPA1. We treated C4-2B and 22Rv1 cells with 0, 10, or 20 μM quercetin and analyzed the expression of hnRNPA1 at mRNA and protein levels. The results showed that treatment with quercetin significantly downregulated the expression of hnRNPA1 (Fig. 1A & B). Since our previous findings indicated an essential role for hnRNPA1 in the alternative splicing of the AR to generate alternative splice variants such as AR-V7 (11), we analyzed the expression levels of full length AR and AR-V7 in C4-2B-Enza-R cells treated with 10 μM quercetin for 0, 4, 8, 16, and 24 h. The results demonstrated that the time-dependent suppression of hnRNPA1 expression by quercetin at mRNA and protein levels reduced the mRNA and protein levels of full length AR as well as AR-V7 (Fig. 1C & D). To test whether the effects of quercetin on the mRNA levels of the full length AR and AR-V7 were due to the inhibition of transcription, we transfected HEK293 cells with a luciferase reporter driven by the AR promoter region (−5400 to +580). Luciferase activity of the reporter was analyzed after treatment with 0, 5, or 10 μM quercetin for 24 h. As shown in Suppl. Fig. 1B, quercetin did not decrease the activity of the AR promoter region significantly, implying that quercetin may not affect the transcriptional activity of the AR promoter and the transcription of the AR pre-mRNA. While we cannot rule out the possibility, our data suggest that the reduction of full length AR mRNA by quercetin is unlikely to compound its effect on the AR-V7 mRNA via hnRNPA1. We also analyzed whether quercetin affects the sub-cellular localization of hnRNPA1 in C4-2B-Enza-R cells treated with 10 μM quercetin for 0, 4, 8, 16, and 24 h. Our results showed that the nuclear retention of hnRNPA1 was reduced in cells treated with quercetin (Fig. 1E). These results indicate that quercetin may exert anti-androgenic effects by antagonizing the expression of hnRNPA1, full length AR, and AR-V7.
Figure 1. Quercetin downregulates hnRNPA1, FL AR, and AR-V7.
C4-2B and CWR22Rv1 cells were treated with 10 or 20 μM quercetin and mRNA (A) and protein (B) levels of hnRNPA1 were analyzed using qRT-PCR and western blotting, respectively. Quercetin induced a dose dependent decrease in both mRNA and protein levels of hnRNPA1 in both cell lines tested. C4-2B-Enza-R cells were treated with 20 μM quercetin for 0, 4, 8, 16, or 24 h and mRNA (C) and protein (D) levels of hnRNPA1, FL AR, and AR-V7 were analyzed using qRT-PCR and western blotting, respectively. Quercetin induced a time-dependent decrease in hnRNPA1, FL AR, and AR-V7 levels. E) C4-2B-Enza-R cells were treated with 20 μM quercetin for 0, 4, 8, 16, or 24 h and cytoplasmic and nuclear extracts were prepared. Protein levels of hnRNPA1 and AR-V7 were analyzed in the extracts using western blotting. Quercetin induced a time-dependent decrease in the total levels of both hnRNPA1 and AR-V7. Reduction in the nuclear transport of hnRNPA1 accompanied by cytoplasmic retention was not observed as reported earlier. Results are presented as means ± SD of 3 independent experiments performed with triplicates. * denotes p ≤0.05.
Quercetin synergizes with enzalutamide
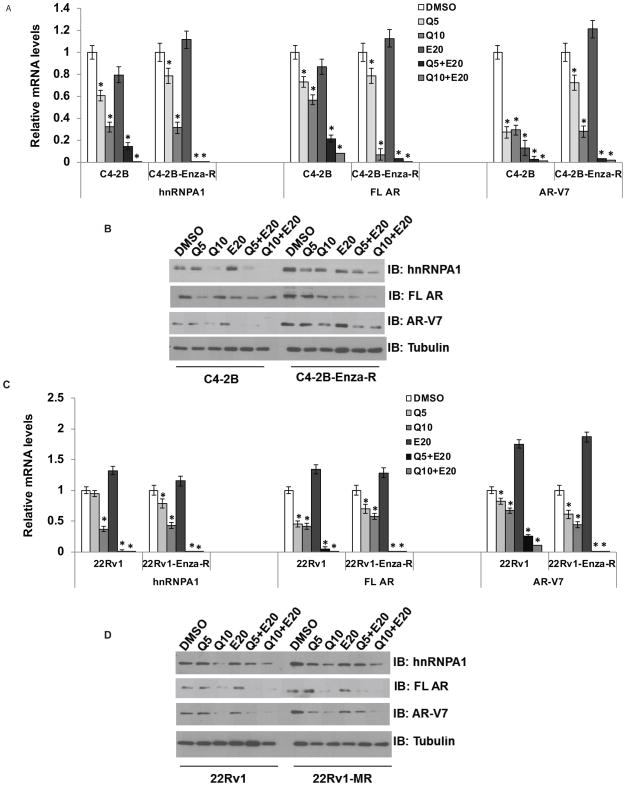
Next, we analyzed whether treatment with a combination of quercetin and enzalutamide affects the expression levels of hnRNPA1, full length AR, or AR-V7. We treated C4-2B and C4-2B-Enza-R or 22Rv1 and 22Rv1-Enza-R cells with 5 or 10 μM quercetin and 20 μM enzalutamide either singly or in combination. Total RNA was prepared and mRNA levels of hnRNPA1, FL AR, and AR-V7 were analyzed using qRT-PCR. Whole cell lysates were also analyzed for the protein levels of hnRNPA1, FL AR, and AR-V7. As shown in Fig. 2, treatment with quercetin alone reduced the expression levels of hnRNPA1, FL AR, and AR-V7 at both mRNA and protein levels. Importantly, the combination of quercetin with enzalutamide synergistically reduced the mRNA and protein levels of hnRNPA1, AR, and AR-V7 in the enzalutamide-resistant C4-2B-Enza-R and 22Rv1-Enza-R cell lines, indicating that treatment with quercetin may synergize with enzalutamide treatment.
Figure 2. Treatment with a combination of quercetin and enzalutamide synergistically downregulates hnRNPA1, FL AR, and AR-V7.
C4-2B and C4-2B-Enza-R cells were treated with 5 or 10 μM quercetin and 20 μM enzalutamide either singly or in combination. Total RNA or proteins were extracted and mRNA (A) and protein (B) levels of hnRNPA1, FL AR, and AR-V7 were analyzed using qRT-PCR and western blotting, respectively. Combination of quercetin and enzalutamide reduced the expression of hnRNPA1, FL AR, and AR-V7 synergistically. Similarly, 22Rv1 and 22Rv1-Enza-R cells were treated with 5 or 10 μM quercetin and 20 μM enzalutamide either singly or in combination. Total RNA or proteins were extracted and mRNA (C) and protein (D) levels of hnRNPA1, FL AR, and AR-V7 were analyzed using qRT-PCR and western blotting, respectively. Combination of quercetin and enzalutamide reduced the expression of hnRNPA1, FL AR, and AR-V7 synergistically. Results are presented as means ± SD of 3 independent experiments performed with triplicates. * denotes p ≤0.05.
Quercetin downregulates androgen receptor signaling
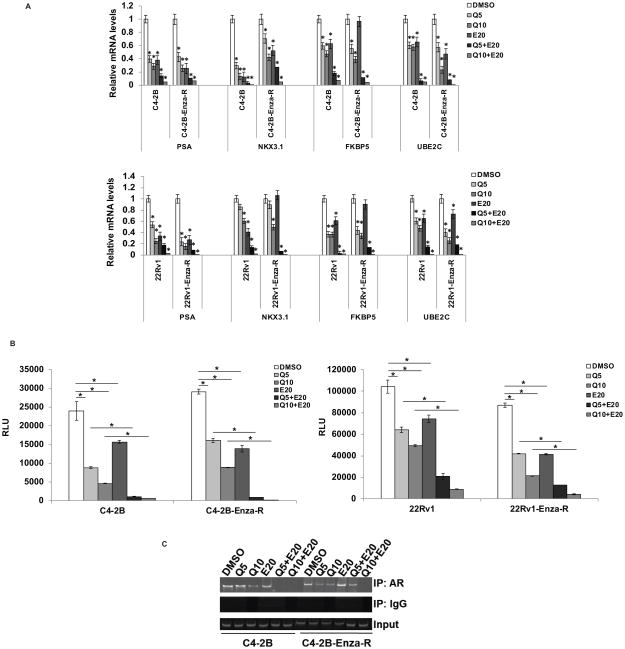
Next, we treated C4-2B parental and C4-2B-Enza-R or 22Rv1 and 22Rv1-Enza-R cells with quercetin and enzalutamide either singly or in combination and assessed the expression levels of known target genes of the AR signaling pathway, such as PSA, NKX3.1, FKBP5, and UBE2C. As shown in Fig. 3A, levels of all 4 AR target genes were reduced by treatment with quercetin or enzalutamide singly, while treatment with the combination of quercetin and enzalutamide exhibited synergy in suppressing their expression. To further verify synergism between quercetin and enzalutamide, we treated C4-2B and C4-2B-Enza-R cells with 5 doses of quercetin (0, 2.5, 5. 10, 20, or 50 μM) or 3 doses of enzalutamide (5, 10, or 20 μM) or their combinations and analyzed total RNAs for the expression levels of FL AR and AR-V7 as well as their target genes PSA, FKBP5, and UBE2C. As shown in Suppl. Fig. 2, the combination of quercetin and enzalutamide reduced the expression levels of all genes tested synergistically.
Figure 3. Combination treatment with quercetin and enzalutamide downregulates androgen receptor signaling synergistically.
A) C4-2B and C4-2B-Enza-R (upper panel) and 22Rv1 and 22Rv1-Enza-R (lower panel) cells were treated with 5 or 10 μM quercetin and 20 μM enzalutamide either singly or in combination. Expression levels of androgen receptor-regulated genes PSA, NKX3.1, FKBP5, and UBE2C were analyzed using qRT-PCR. Treatment with the combination of quercetin and enzalutamide reduced the mRNA levels of all 4 androgen receptor-regulated genes. B) C4-2B and C4-2B-Enza-R (left panel) and 22Rv1 and 22Rv1-Enza-R (right panel) cells were transfected with PSA-enhancer/promoter-luciferase reporter vector and treated with 5 or 10 μM quercetin and 20 μM enzalutamide either singly or in combination. Luciferase reporter activities were measured using a luminometer. Treatment with the combination of quercetin and enzalutamide reduced the activity of luciferase synergistically. C) C4-2B and C4-2B-Enza-R cells were treated with 5 or 10 μM quercetin and 20 μM enzalutamide either singly or in combination. Cell lysates were subjected to ChIP assays to examine the recruitment of the androgen receptor to the ARE-I/II region of the PSA promoter. Treatment with the combination of quercetin and enzalutamide reduced the recruitment of the AR to the PSA promoter synergistically. Results are presented as means ± SD of 3 independent experiments performed with triplicates. * denotes p ≤0.05.
We also tested the effects of treatment with a combination of quercetin and enzalutamide on AR transcriptional activity using luciferase assays. We transfected a reporter luciferase vector containing the enhancer and promoter regions of the PSA promoter (PSA-E/P-Luc) into C4-2B and C4-2B-Enza-R or 22Rv1 and 22Rv1-Enza-R cells, followed by treatment with quercetin and enzalutamide, either singly or in combination. As shown in Fig. 3B, AR transcriptional activity was suppressed by treatment with quercetin or enzalutamide alone, while the combination of quercetin and enzalutamide exerted synergistic effects in reducing luciferase activity, indicating that treatment with a combination of quercetin and enzalutamide reduces AR transcriptional activity. To verify synergism between quercetin and enzalutamide, we treated C4-2B and C4-2B-Enza-R cells with 5 doses of quercetin (0, 2.5, 5. 10, 20, or 50 μM) or 3 doses of enzalutamide (5, 10, or 20 μM) or their combinations after transfection with the PSA-E/P-Luc reporter and analyzed luciferase activities. As shown in Suppl. Fig. 3A, the combination of quercetin and enzalutamide reduced the transcriptional activity of the AR synergistically.
Next, we tested whether treatment with a combination of quercetin and enzalutamide affects the recruitment of AR to the promoter regions of target genes. We treated C4-2B and C4-2B-Enza-R cells with quercetin and enzalutamide either singly or in combination and subjected them to ChIP assay to assess the recruitment of AR to the PSA promoter region. As shown in Fig. 3C, the results showed that treatment with a combination of quercetin and enzalutamide synergistically inhibits the recruitment of AR to target gene promoters. These findings collectively indicate that quercetin and enzalutamide synergize with each other in the downregulation of AR signaling.
Quercetin resensitizes enzalutamide-resistant prostate cancer cells to enzalutamide
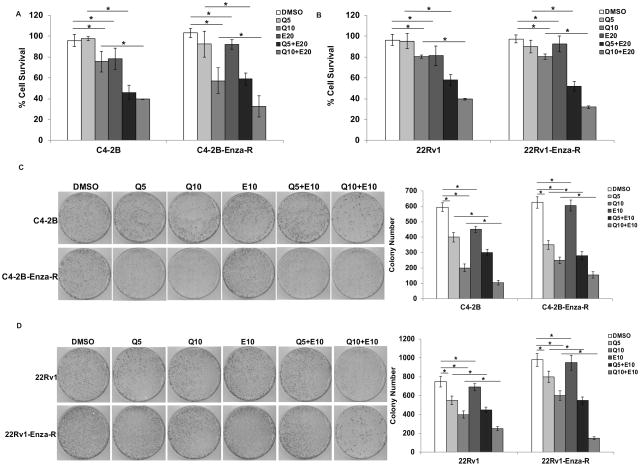
Based on the results that quercetin suppresses AR-V7 levels, we analyzed whether it can resensitize enzalutamide-resistant prostate cancer cells to enzalutamide. We treated C4-2B and C4-2B-Enza-R or 22Rv1 and 22Rv1-Enza-R cells with quercetin and enzalutamide either singly or in combination for 48 h and analyzed cell survival. As shown in Fig. 4A, treatment with quercetin or enzalutamide alone suppressed the survival of both C4-2B and C4-2B-Enza-R cells by a modest 20–30%, while the combination of quercetin with enzalutamide suppressed cell survival by >60% in both cell lines. Similar results were obtained with the 22Rv1 cells (Fig. 4B), indicating that quercetin resensitizes enzalutamide-resistant cells to enzalutamide treatment. To verify synergism between quercetin and enzalutamide, we treated C4-2B and C4-2B-Enza-R cells with 5 doses of quercetin (0, 2.5, 5. 10, 20, or 50 μM) or 3 doses of enzalutamide (5, 10, or 20 μM) or their combinations and analyzed cell survival. As shown in Suppl. Fig. 3B, the combination of quercetin and enzalutamide reduced cell survival synergistically.
Figure 4. Combination treatment with quercetin and enzalutamide resensitizes resistant cells to enzalutamide in vitro.
C4-2B and C4-2B-Enza-R (A) and 22Rv1 and 22Rv1-Enza-R (B) cells were treated with 5 or 10 μM quercetin and 20 μM enzalutamide either singly or in combination and cell survival was analyzed. Treatment with the combination of quercetin and enzalutamide reduced the cell survival of the enzalutamide-resistant C4-2B-Enza-R and 22Rv1-Enza-R cells significantly compared with treatment with either agent alone. C4-2B and C4-2B-Enza-R (C) and 22Rv1 and 22Rv1-Enza-R (D) cells were treated with 5 or 10 μM quercetin and 20 μM enzalutamide either singly or in combination for 24 h and the clonogenic ability of the surviving cells was analyzed. Treatment with the combination of quercetin and enzalutamide reduced the clonogenic ability of the enzalutamide-resistant C4-2B-Enza-R and 22Rv1-Enza-R cells significantly compared with either agent alone. Right panels show quantification of the numbers of colonies formed by cells in the respective treatment groups. Results are presented as means ± SD of 3 independent experiments performed with triplicates. * denotes p ≤0.05.
Next, we analyzed whether treatment with the combination of quercetin and enzalutamide affects the anchorage-dependent clonogenic ability of enzalutamide-resistant cells. As shown in Fig. 4C and D, the results showed that quercetin exerts a more significant suppressive effect on the clonogenic ability of enzalutamide-resistant C4-2B-Enza-R cells compared to the parental C4-2B cells, which may be due to the ability of quercetin to antagonize the generation of AR-V7 by suppressing the expression of hnRNPA1 (Fig. 4C). The clonogenic ability of enzalutamide-resistant C4-2B-Enza-R and 22Rv1-Enza-R cells (Fig. 4D) was reduced by >70% by treatment with a combination of quercetin and enzalutamide, indicating that quercetin may resensitize enzalutamide-resistant cells to enzalutamide by downregulation of the levels of AR-V7.
Quercetin resensitizes enzalutamide-resistant xenografts to enzalutamide in vivo
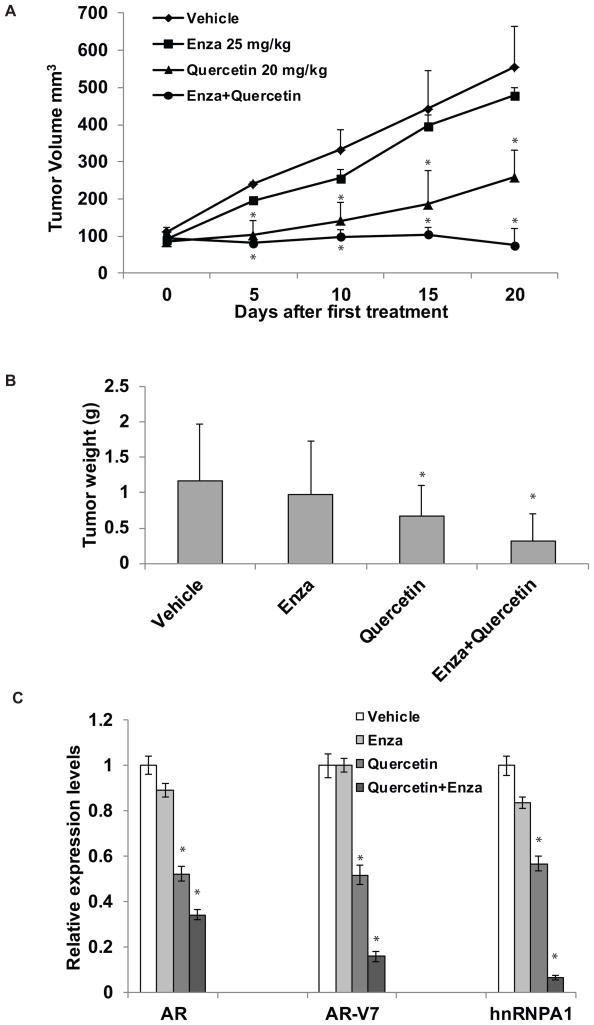
Next, we performed mouse xenograft assays to assess the synergism between quercetin and enzalutamide in vivo. 2×106 22Rv1 cells were injected sub-cutaneously into both flanks of SCID mice and tumor growth was monitored. When the tumors were palpable, the xenografts were treated with quercetin and enzalutamide either singly or in combination for 3 weeks and tumor growth was monitored. As shown in Fig. 5A and B, enzalutamide did not have a significant effect on the growth of 22Rv1 xenografts, while quercetin alone was able to suppress tumor growth. In addition, treatment with the combination of quercetin and enzalutamide abolished 22Rv1 tumors almost completely. qRT-PCR (Fig. 5C) and immunohistochemistry (Suppl. Fig. 3C) were performed to confirm the downregulation of hnRNPA1, AR, and AR-V7 mRNA and protein levels in the tumor tissues. Assessment of the proliferation marker Ki67 indicated that treatment with the combination of quercetin and enzalutamide suppressed the proliferation of 22Rv1 xenografts significantly (Suppl. Fig. 3C). These results collectively demonstrate that quercetin may synergize with enzalutamide treatment and may resensitize enzalutamide-resistant prostate cancer cells to enzalutamide.
Figure 5. Combination treatment with quercetin and enzalutamide resensitizes resistant cells to enzalutamide in vivo.
22Rv1 cells (2×106) were injected sub-cutaneously into both flanks of SCID mice and tumor growth was monitored. Once the tumors were palpable, the mice were randomly divided into 4 groups (n=6/group) and treated with: 1) vehicle (0.5% Methocel 4AM), 2) 25 mg/kg enzalutamide via oral gavage daily for 5 days/week, 3) 20 mg/kg quercetin i.p. daily for 5 days/week, and 4) a combination of quercetin and enzalutamide daily for 5 days/week. Tumors (n=12/group) were monitored twice a week and were harvested and weighed at the end of 3 weeks. Tumor volumes (A) and weights (B) of tumors treated with the combination of quercetin and enzalutamide were significantly reduced. C) Total RNAs derived from the tumor tissues were analyzed by qRT-PCR for the expression levels of hnRNPA1, FL AR, and AR-V7. Transcript levels of hnRNPA1, FL AR, and AR-V7 were reduced significantly in the tumor tissues by treatment with the combination of quercetin and enzalutamide. * denotes p ≤0.05.
Discussion
In this study, we show that quercetin inhibits the expression of hnRNPA1, FL AR, and AR-V7, downregulates AR signaling, and synergizes with enzalutamide in suppressing prostate cancer cell xenograft growth in vivo. Quercetin resensitizes enzalutamide-resistant cells to enzalutamide treatment, an observation which may augur well for the use of quercetin as an adjunct to anti-androgenic therapy in prostate cancer.
Phytoflavonoids such as quercetin are found in fruits and vegetables such as olives, onions, kale, cranberries, grapes etc. They have been shown to arrest prostate cancer cell growth, invasion, and metastasis in vitro and in vivo (15, 26–29). Flavonols are structurally similar to androgens and hence interaction with AR has been postulated as a mechanism for their anti-cancer effects in prostate cancer. Quercetin (3,3′,4′,5,7-pentahydroxyflavone) is commonly known for its anti-inflammatory, anti-oxidant, and anti-cancer activities. Quercetin has been shown to compete with testosterone at high concentrations, which are higher than those needed for cell growth inhibition, indicating that competition with testosterone is unlikely to be its main mechanism of action (30). Quercetin has been shown to have varying cell line-dependent effects on the expression of the AR (31, 32). Nevertheless, the consensus is that quercetin may inhibit expression and activity of the AR. Quercetin has been shown to suppress AR function by inhibiting protein-protein interactions between c-Jun, Sp1, and AR in LNCaP cells (33). Even though several previous studies have focused on the anti-androgenic activity of quercetin, the anti-cancer effects of quercetin cannot be attributed solely to its effects on the AR or its splice variants. The pro-apoptotic activity of quercetin in PC-3 and DU145 cells was found to be due to its inhibition of HSP90 (26). Quercetin can suppress the properties of prostate cancer stem cells and their metastatic potential (34). Quercetin can also modulate Wnt signaling in prostate cancer cells (35). Viewed together, these reports along with our results provide a rationale for efforts to enhance the effects of flavonols by modification of their structure (36).
Androgen receptor splice variants such as AR-V7 have been shown to play a central role in the occurrence of de novo or acquired resistance to androgen axis-targeted therapeutics such as enzalutamide and abiraterone (37). They have also been implicated in the failure of taxane-based therapies (38). Detection of AR-V7 in circulating tumor cells has also been shown to predict sensitivity to enzalutamide or abiraterone (39). These studies attest to the importance of alternatively spliced AR variants in therapy resistance. We have shown previously that the splicing factor hnRNPA1, via a regulatory pathway involving NF-kappaB2/p52 and c-Myc, regulates AR alternative splicing to generate AR-V7 (11). Downregulation of hnRNPA1 using siRNA resensitizes enzalutamide-resistant prostate cancer cells to enzalutamide. In view of these findings, efforts are underway to identify or develop novel molecules that can antagonize either the expression or activity of hnRNPA1. We analyzed published literature to identify potential candidates and found a report of an interaction between quercetin and hnRNPA1 (25). The study found that quercetin binds to and retains hnRNPA1 in the cytoplasm. Our preliminary studies found that quercetin may downregulate the expression of hnRNPA1 in C4-2B and 22Rv1 prostate cancer cells. This led us to analyze whether quercetin can resensitize enzalutamide-resistant cells to enzalutamide. As expected, our results confirmed that quercetin downregulates the expression of hnRNPA1 and AR-V7 in enzalutamide-resistant prostate cancer cells. The bioavailability of quercetin is low as it is present in glycosylated form, which can be readily metabolized by enzymes in the gut and liver, leading to low systemic concentrations in the body (40). It is also poorly water soluble. These limitations can be overcome by the use of quercetin-containing nanomicelles, which have been shown to increase the bioavailability of quercetin and inhibit the growth of PC-3 prostate cancer xenografts (41). Similarly, a naturally occurring cis-glycoside of quercetin, quercetin-6-C-b-D-glucopyranoside with better bioavailability was isolated from the bark of Ulmus wallichiana (42). Thus, identification of quercetin analogs with higher bioavailability and improved anti-cancer activity may be an attractive option.
In our study, quercetin was effective in the suppression of signaling by both FL AR and AR-V7 and resensitized enzalutamide-resistant cells to enzalutamide. Emergence of resistance after prolonged treatment has been one of the most concerning features of next generation AR-axis antagonists. In addition, studies have shown that patients with higher initial levels of variants such as AR-V7 may exhibit a blunted response to enzalutamide therapy. In this scenario adjunctive therapies that suppress the alternative splicing of the AR may help improve the efficacy of enzalutamide; moreover, suppressing the levels of AR-V7 before treatment with enzalutamide may result in a higher percentage of patients responding to enzalutamide therapy. Such hypotheses should be tested in rigorous clinical trials and should form a part of the drug optimization effort in the future.
In summary, we found that suppression of AR-V7 levels via targeting alternative splicing of the AR may synergize with and resentitize resistant prostate cancer cells to enzalutamide treatment. The recently concluded phase 4 PLATO trial that evaluated continued treatment with enzalutamide plus abiraterone and prednisone vs. abiraterone and prednisone alone in chemotherapy-naïve metastatic castration resistant prostate cancer (mCRPC) patients after disease progression on enzalutamide did not show an improvement in progression-free-survival (PFS) (http://press.pfizer.com/press-release/pfizer-and-astellas-announce-top-line-results-phase-4-plato-trial-xtandi-enzalutamide-). This attests to the importance of continued efforts to identify and develop new strategies to treat enzalutamide resistant prostate cancers. Our results serve as a proof of the principle that alternative splicing of the AR may be a viable target for the development of adjunctive therapies to augment the efficacy of AR antagonists.
Supplementary Material
Acknowledgments
Financial Support: DOD PC100502, NIH/NCI CA140468 (R. Tummala)
NIH/NCI CA140468, CA168601, CA179970, US Department of Veterans Affairs, ORD VA Merit Award I01BX0002653 (W. Lou)
NIH/NCI CA140468, CA168601, CA179970, DOD PC150229, US Department of Veterans Affairs, ORD VA Merit Award I01BX0002653 (A.C. Gao)
NIH/NCI CA202404, DOD PC100502 (N. Nadiminty)
This work was supported in part by grants NIH/NCI CA202404, DOD PC100502 (N. Nadiminty); and NIH/NCI CA140468, CA168601, CA179970, DOD PC150229, and US Department of Veterans Affairs, ORD VA Merit Award I01BX0002653 (A.C. Gao).
References
- 1.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a Novel Androgen Receptor Exon Generates a Constitutively Active Androgen Receptor that Mediates Prostate Cancer Therapy Resistance. Cancer Research. 2008;68:5469–77. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, et al. Ligand-Independent Androgen Receptor Variants Derived from Splicing of Cryptic Exons Signify Hormone-Refractory Prostate Cancer. Cancer Research. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu R, Isaacs WB, Luo J. A snapshot of the expression signature of androgen receptor splicing variants and their distinctive transcriptional activities. The Prostate. 2011;71:1656–67. doi: 10.1002/pros.21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun S, Sprenger CCT, Vessella RL, Haugk K, Soriano K, Mostaghel EA, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. The Journal of Clinical Investigation. 2010;120:2715–30. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, et al. Resistance to CYP17A1 Inhibition with Abiraterone in Castration-Resistant Prostate Cancer: Induction of Steroidogenesis and Androgen Receptor Splice Variants. Clinical Cancer Research. 2011;17:5913–25. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KAT, Dehm SM. Androgen Receptor Splice Variants Mediate Enzalutamide Resistance in Castration-Resistant Prostate Cancer Cell Lines. Cancer Research. 2013;73:483–9. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thadani-Mulero M, Portella L, Sun S, Sung M, Matov A, Vessella RL, et al. Androgen Receptor Splice Variants Determine Taxane Sensitivity in Prostate Cancer. Cancer Research. 2014;74:2270–82. doi: 10.1158/0008-5472.CAN-13-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan X, Cai C, Chen S, Chen S, Yu Z, Balk SP. Androgen receptor functions in castration-resistant prostate cancer and mechanisms of resistance to new agents targeting the androgen axis. Oncogene. 2013 doi: 10.1038/onc.2013.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Hwang TH, Oseth LA, Hauge A, Vessella RL, Schmechel SC, et al. AR intragenic deletions linked to androgen receptor splice variant expression and activity in models of prostate cancer progression. Oncogene. 2012;31:4759–67. doi: 10.1038/onc.2011.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu LL, Xie N, Sun S, Plymate S, Mostaghel E, Dong X. Mechanisms of the androgen receptor splicing in prostate cancer cells. Oncogene. 2013;33:3140–50. doi: 10.1038/onc.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nadiminty N, Tummala R, Liu C, Lou W, Evans CP, Gao AC. NF-κB2/p52:c-Myc:hnRNPA1 Pathway Regulates Expression of Androgen Receptor Splice Variants and Enzalutamide Sensitivity in Prostate Cancer. Molecular Cancer Therapeutics. 2015;14:1884–95. doi: 10.1158/1535-7163.MCT-14-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Firdous AB, Sharmila G, Balakrishnan S, RajaSingh P, Suganya S, Srinivasan N, et al. Quercetin, a natural dietary flavonoid, acts as a chemopreventive agent against prostate cancer in an in vivo model by inhibiting the EGFR signaling pathway. Food & Function. 2014;5:2632–45. doi: 10.1039/c4fo00255e. [DOI] [PubMed] [Google Scholar]
- 13.Sharmila G, Bhat FA, Arunkumar R, Elumalai P, Raja Singh P, Senthilkumar K, et al. Chemopreventive effect of quercetin, a natural dietary flavonoid on prostate cancer in in vivo model. Clinical Nutrition. 2014;33:718–26. doi: 10.1016/j.clnu.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Moon YJ, Wang X, Morris ME. Dietary flavonoids: Effects on xenobiotic and carcinogen metabolism. Toxicology in Vitro. 2006;20:187–210. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 15.Senthilkumar K, Elumalai P, Arunkumar R, Banudevi S, Gunadharini ND, Sharmila G, et al. Quercetin regulates insulin like growth factor signaling and induces intrinsic and extrinsic pathway mediated apoptosis in androgen independent prostate cancer cells (PC-3) Molecular and Cellular Biochemistry. 2010;344:173–84. doi: 10.1007/s11010-010-0540-4. [DOI] [PubMed] [Google Scholar]
- 16.Bhat FA, Sharmila G, Balakrishnan S, Arunkumar R, Elumalai P, Suganya S, et al. Quercetin reverses EGF-induced epithelial to mesenchymal transition and invasiveness in prostate cancer (PC-3) cell line via EGFR/PI3K/Akt pathway. The Journal of Nutritional Biochemistry. 2014;25:1132–9. doi: 10.1016/j.jnutbio.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Wang P, Heber D, Henning SM. Quercetin increased the antiproliferative activity of green tea polyphenol (−)-epigallocatechin gallate in prostate cancer cells. Nutrition and Cancer. 2012;64:580–7. doi: 10.1080/01635581.2012.661514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoskes DA, Nickel JC. Quercetin for Chronic Prostatitis/Chronic Pelvic Pain Syndrome. Urologic Clinics of North America. 2011;38:279–84. doi: 10.1016/j.ucl.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Wang P, Henning SM, Heber D, Vadgama JV. Sensitization to docetaxel in prostate cancer cells by green tea and quercetin. The Journal of Nutritional Biochemistry. 2015;26:408–15. doi: 10.1016/j.jnutbio.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu C, Lou W, Zhu Y, Nadiminty N, Schwartz CT, Evans CP, et al. Niclosamide Inhibits Androgen Receptor Variants Expression and Overcomes Enzalutamide Resistance in Castration-Resistant Prostate Cancer. Clinical Cancer Research. 2014;20:3198–210. doi: 10.1158/1078-0432.CCR-13-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadiminty N, Tummala R, Liu C, Yang J, Lou W, Evans CP, et al. NF-κB2/p52 Induces Resistance to Enzalutamide in Prostate Cancer: Role of Androgen Receptor and Its Variants. Molecular Cancer Therapeutics. 2013;12:1629–37. doi: 10.1158/1535-7163.MCT-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang W-C, Zhau HE, Chung LWK. Androgen Receptor Survival Signaling Is Blocked by Anti-β2-microglobulin Monoclonal Antibody via a MAPK/Lipogenic Pathway in Human Prostate Cancer Cells. Journal of Biological Chemistry. 2010;285:7947–56. doi: 10.1074/jbc.M109.092759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nadiminty N, Lou W, Sun M, Chen J, Yue J, Kung H-J, et al. Aberrant Activation of the Androgen Receptor by NF-kB2/p52 in Prostate Cancer Cells. Cancer Research. 2010;70:3309–19. doi: 10.1158/0008-5472.CAN-09-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nadiminty N, Chun JY, Lou W, Lin X, Gao AC. NF-κB2/p52 enhances androgen-independent growth of human LNCaP cells via protection from apoptotic cell death and cell cycle arrest induced by androgen-deprivation. The Prostate. 2008;68:1725–33. doi: 10.1002/pros.20839. [DOI] [PubMed] [Google Scholar]
- 25.Ko C-C, Chen Y-J, Chen C-T, Liu Y-C, Cheng F-C, Hsu K-C, et al. Chemical Proteomics Identifies Heterogeneous Nuclear Ribonucleoprotein (hnRNP) A1 as the Molecular Target of Quercetin in Its Anti-cancer Effects in PC-3 Cells. Journal of Biological Chemistry. 2014;289:22078–89. doi: 10.1074/jbc.M114.553248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aalinkeel R, Bindukumar B, Reynolds JL, Sykes DE, Mahajan SD, Chadha KC, et al. The Dietary Bioflavonoid, Quercetin, Selectively Induces Apoptosis of Prostate Cancer Cells by Down-Regulating the Expression of Heat Shock Protein 90. The Prostate. 2008;68:1773–89. doi: 10.1002/pros.20845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knowles LM, Zigrossi DA, Tauber RA, Hightower C, Milner JA. Flavonoids suppress androgen-independent human prostate tumor proliferation. Nutr Cancer. 2000;38:116–22. doi: 10.1207/S15327914NC381_16. [DOI] [PubMed] [Google Scholar]
- 28.Senthilkumar K, Arunkumar R, Elumalai P, Sharmila G, Gunadharini DN, Banudevi S, et al. Quercetin inhibits invasion, migration and signaling molecules involved in cell survival and proliferation of prostate cancer cell line (PC-3) Cell Biochem Funct. 2011;29:87–95. doi: 10.1002/cbf.1725. [DOI] [PubMed] [Google Scholar]
- 29.Lee D-H, Szczepanski M, Lee YJ. Role of Bax in quercetin-induced apoptosis in human prostate cancer cells. Biochemical Pharmacology. 2008;75:2345–55. doi: 10.1016/j.bcp.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kampa M, Hartzoglou A, Notas G, Damianaki A, Bakogeorgou E, Gemetzi C, et al. Wine antioxidant polyphenols inhibit the proliferation of human prostate cancer cell lines. Nutr Cancer. 2000;37:223–33. doi: 10.1207/S15327914NC372_16. [DOI] [PubMed] [Google Scholar]
- 31.Xing N, Chen Y, Mitchell SH, Young CYF. Quercetin inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. Carcinogenesis. 2001;22:409–14. doi: 10.1093/carcin/22.3.409. [DOI] [PubMed] [Google Scholar]
- 32.HSIEH T-C, WU JM. Targeting CWR22Rv1 Prostate Cancer Cell Proliferation and Gene Expression by Combinations of the Phytochemicals EGCG, Genistein and Quercetin. Anticancer Research. 2009;29:4025–32. [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan H, Young CYF, Tian Y, Liu Z, Zhang M, Lou H. Suppression of the androgen receptor function by quercetin through protein–protein interactions of Sp1, c-Jun, and the androgen receptor in human prostate cancer cells. Molecular and Cellular Biochemistry. 2010;339:253–62. doi: 10.1007/s11010-010-0388-7. [DOI] [PubMed] [Google Scholar]
- 34.TSAI P-H, CHENG C-H, LIN C-Y, HUANG Y-T, LEE L-T, KANDASWAMI CC, et al. Dietary Flavonoids Luteolin and Quercetin Suppressed Cancer Stem Cell Properties and Metastatic Potential of Isolated Prostate Cancer Cells. Anticancer Research. 2016;36:6367–80. doi: 10.21873/anticanres.11234. [DOI] [PubMed] [Google Scholar]
- 35.Baruah MM, Khandwekar AP, Sharma N. Quercetin modulates Wnt signaling components in prostate cancer cell line by inhibiting cell viability, migration, and metastases. Tumor Biology. 2016;37:14025–34. doi: 10.1007/s13277-016-5277-6. [DOI] [PubMed] [Google Scholar]
- 36.Boam T. Anti-androgenic effects of flavonols in prostate cancer. ecancermedicalscience. 2015;9:585. doi: 10.3332/ecancer.2015.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antonarakis ES. Current understanding of the resistance to enzalutamide and abiraterone in advanced prostate cancer. Clinical Advances in Hematology and Oncology. 2016;14:316–9. [PubMed] [Google Scholar]
- 38.Antonarakis ES, Lu C, Luber B, et al. ANdrogen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncology. 2015;1:582–91. doi: 10.1001/jamaoncol.2015.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and Resistance to Enzalutamide and Abiraterone in Prostate Cancer. New England Journal of Medicine. 2014;371:1028–38. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Németh K, Plumb WG, Berrin J-G, Juge N, Jacob R, Naim YH, et al. Deglycosylation by small intestinal epithelial cell β-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. European Journal of Nutrition. 42:29–42. doi: 10.1007/s00394-003-0397-3. [DOI] [PubMed] [Google Scholar]
- 41.Zhao J, Liu J, Wei T, Ma X, Cheng Q, Huo S, et al. Quercetin-loaded nanomicelles to circumvent human castration-resistant prostate cancer in vitro and in vivo. Nanoscale. 2016;8:5126–38. doi: 10.1039/c5nr08966b. [DOI] [PubMed] [Google Scholar]
- 42.Hamidullah, Kumar R, Saini KS, Kumar A, Kumar S, Ramakrishna E, et al. Quercetin-6-C-β-d-glucopyranoside, natural analog of quercetin exhibits anti-prostate cancer activity by inhibiting Akt-mTOR pathway via aryl hydrocarbon receptor. Biochimie. 2015;119:68–79. doi: 10.1016/j.biochi.2015.10.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.