Abstract
Purpose
To assess the effect of dry eye disease (DED) in graft donors on dendritic cell (DC) maturation, host T cell sensitization and corneal allograft rejection.
Methods
Corneas of control (healthy donor) and DED mice (C57BL/6) were transplanted onto fully allogeneic naïve BALB/c recipients (n=10 mice/group). Long-term allograft survival was evaluated for 8 weeks. Corneas and draining lymph nodes (dLNs) were harvested at day 14 post-transplantation (n=5 mice/group). The frequencies of MHCIIhigh CD11c+DCs in the donor corneas and host dLNs, and the frequencies of IFN-γ+ and IL-17+ CD4+ T cells and Foxp3 expression by Tregs in host dLNs were investigated using flow cytometry. Enzyme-linked immunospot (ELISPOT) assay was used to assess host T cell allosensitization via direct and indirect pathways (n=3/group).
Results
Recipients of DED donor corneas showed significantly reduced graft survival (10%) compared to control mice (50% survival, p=0.022), and had significantly increased frequencies of mature DCs in the grafted cornea (DED donor 44.0±0.36% vs. healthy donor 35.4±0.5%; p<0.0001) and host dLNs (DED donor 25.1±0.66% vs. healthy donor 13.7±1.6%; p=0.005). Frequencies of IFN-γ+ and IL-17+ T cells were increased in the dLNs of recipients of DED corneas, while the expression (mean fluorescence intensity) of Foxp3 in Tregs was decreased significantly in these mice (DED donor 6004±193 vs. healthy donor 6806±81; p=0.0002). ELISPOT analysis showed that the direct pathway of allosensitization was significantly amplified in recipients of grafts with DED (p=0.0146).
Conclusions
Our results indicate that DED in the donor is a significant risk factor for subsequent corneal allograft rejection.
Keywords: Dry eye disease, corneal transplantation, graft rejection, dendritic cell, allosensitization
INTRODUCTION
Corneal transplantation is one of the most common tissue grafting procedures worldwide, with over 65,000 cases performed annually.1 It is widely recognized that the condition of the recipients’ host bed can affect graft survival: corneal grafts performed in avascular “low-risk” host beds enjoy a high survival rate as a result of corneal immune privilege;2 in contrast, over 50% of grafts are rejected in “high-risk” hosts whose graft beds are characterized by neovascularization and inflammation.3 However, very little is known about how the state of the donor ocular surface can affect the outcome of corneal transplantation. Donor factors that are currently assessed as possibly affecting graft outcome include endothelial cell density, history of infection, disseminated cancer, ocular surgery, and possibly age. However, a history of ocular surface disease or previous corneal inflammation is not assessed in the eye bank screening process.
Inflammation and cell death are considered as major contributors to dry eye disease (DED)-associated loss of corneal integrity and barrier function.4, 5 Dry eye disease is one of the most common ocular disorders affecting tens of millions of people worldwide,6, 7 and is associated with disturbance of the ocular surface immunohomeostasis.8 Aging, hormonal changes, environmental factors and prolonged work with visual display terminals 9 have contributed to the growing DED population. The high prevalence of DED is at least of theoretical concern in the context of transplantation, since it has been established that DED promotes maturation of antigen presenting cells (APCs),10 T cell activation, and lymphangiogenesis11, 12, all of which can amplify immune reactivity and possibly pose a significant risk to corneal grafts if the tissue is transplanted.
Herein, we investigated the effects of DED in donor grafts on APC maturation, pro-inflammatory cytokine production and graft survival using a validated murine model of corneal transplantation.
MATERIALS AND METHODS
Mice and Anesthesia
Six- to eight-week-old C57BL/6 (H-2b) and BALB/c (H-2d) female mice were purchased from Charles River Laboratories (Wilmington, MA, USA). Mice were housed in a specific pathogen-free environment at the Schepens Eye Research Institute animal facility. All animals were treated according to the guidelines established by the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and Public Health Review, and all procedures were approved by the Institutional Animal Care and Use Committee. Anesthesia was administered intraperitoneally (ketamine/xylazine solution at a dose of 120 mg/kg body weight and 20 mg/kg body weight, respectively).
Induction of Dry Eye Disease in Donor Corneal Tissue
Acute DED was induced by exposing C57BL/6 mice to the controlled-environment chamber (CEC) with a relative humidity of <20%, airflow of 15 L/min and a constant temperature of 21–23°C for 14 consecutive days as previously described.13
Orthotropic Corneal Transplantation and Assessment of Graft Survival
The procedure for corneal orthotropic transplantation, in which corneal grafts from C57BL/6 mice are transplanted to BALB/c recipient mice, has been well established and described previously.14 Briefly, central cornea was marked with a 2 mm diameter trephine and excised from a donor C57BL/6 mouse using Vannas scissors (Storz Instruments, San Dimas, CA). The graft bed was prepared by excising a 1.5 mm central cornea button from a BALB/c mouse. The donor button was then placed onto the recipient bed and secured with eight interrupted 11-0 nylon sutures. Corneal sutures were removed 7 days after surgery. Graft survival was evaluated once a week using a slit-lamp biomicroscope for 8 weeks. A standardized opacity-grading (range, 0–5+) system was used to define rejection and corneas with an opacity score of 2+ for two consecutive examinations were considered rejected.15
In Vivo Confocal Microscopy
In vivo confocal microscopy (IVCM), the Heidelberg Retina Tomograph II Rostock Cornea Module (Heidelberg Engineering GmbH, Heidelberg, Germany), was used to examine dendritic cells in the cornea. Healthy or DED donor mice were anesthetized and placed on the microscope stand and the eyes were coated with Genteal gel (Novartis, St. Louis, MO, USA). Images were taken covering a corneal section of 400×400 μm2 and transverse optical resolution of 1 μm/pixel. Number of dendritic cells, morphologically identified as bright dendritiform cells, was analyzed quantitatively using ImageJ.
Flow Cytometry Analysis
Cervical draining lymph nodes (dLNs) were harvested and single-cell suspensions were prepared. To avoid non-specific staining, cells were blocked with an anti-FcR blocking antibody (eBioscience, San Diego, CA, USA). Mature DCs were stained with anti-CD11c Alexa 488 (N418, BioLegend), anti-CD45 PE (30-F11, eBioscience) and anti-I-A/I-E PeCy7 (M5/114.15.2, BioLegend). Tregs were stained with anti-CD4 FITC (RM4-5), anti-CD25 PE (PC61) and anti-Foxp3 PECy7 (FJK-16s) (BioLegend, San Diego, CA, USA). For intracellular IFN-γ and IL-17 staining, cells were stimulated with 50 ng/ml phorbol 12-myristate 13-acetate (PMA) and 500 ng/ml inomycin (Sigma-Aldrich, St. Louis, MO, USA) for 12 hours at 37°C and 5% CO2 in the presence of GolgiStop (0.7μl per 100 μl cell culture media; BD Biosciences, San Jose, CA) to inhibit cytokine secretion. Cells were then stained with anti-CD4 FITC, anti-IFN-γ APC (XMG1.2) and anti-IL-17 PECy7 (TC11-18H10.1) (Biolegend). All antibodies and their matched isotype controls and fixation and permeabilization buffers were purchased from eBioscience. Stained cells were examined using the LSRII Flow Cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and analyzed using FlowJo software X 10.0.7. (FlowJo LLC, Ashland, OR, USA).
Magnetic Cell Sorting
CD4+CD25− conventional T cells (Tconv) and CD4+CD25+ regulatory T cells (Tregs) from BALB/c mice and APCs from C57BL/6 mice were isolated by magnetic cell sorting (MACS) using Treg and CD90.2 isolation kits, respectively, according to the manufacturer’s instructions (Miltenyi Biotec, Bergisch-Gladbach, Germany).
Suppression Assay
Conventional T cells (Tconv; 1×105) isolated from the dLNs of naïve BALB/c mice were co-cultured with Tregs (5×104) from transplant recipients (at day 14 post-transplantation), T cell-depleted allogeneic splenocytes from C57BL/6 mice (1×105), and 1 μg/ml anti-CD3 antibody (145-2C11, BioLegend) for 3 days. Proliferation of CD3-stimulated Tconv cells without adding Tregs served as the control with 0% suppression. Proliferation was measured using the Bromodeoxyuridine (BrdU) incorporation assay (EMD Millipore, Billerica, MA, USA), and percent suppression was calculated using the following formula: % suppression = [(Tconv proliferation without Tregs − Tconv proliferation with Tregs)/(Tconv proliferation without Tregs)] ×100.
Enzyme-linked Immunospot Assay
The enzyme-linked immunospot assay (ELISPOT) was performed to measure the number of spots of directly and indirectly primed T cells, as described previously.16 Briefly, 96-well ELISPOT plates (Whatman Polyfiltronics, Newton, MA, USA) were coated with 4 μg/mL primary anti-IFN-γ mAb (BD Pharmingen) in sterile PBS for 48 hours. Then, plates were washed and blocked for 1.5 hours with PBS containing 1% bovine serum albumin and cells were added to these plates. Purified T cells (5 × 105) sorted by CD90.2-positive MACS from the dLNs of the grafted BALB/c mice (five in each group, 2 weeks post-transplantation of C57BL/6 donor corneas) were pooled and then incubated in triplicates with C57BL/6 antigen presenting cells (APCs; 5 × 105, CD90.2-negative, MACS sorted splenocytes) for 48 hours to quantify frequencies of directly allosensitized T cells. To quantify frequencies of indirectly allosensitized T cells, syngeneic BALB/c APCs (5 × 105) pulsed with sonicated donor antigen (2 × 107, C57BL/6 APCs/mL) were incubated with BALB/c recipient T cells harvested from dLNs of BALB/c recipients with healthy C57BL/6 donor cornea serving as the control. After washing, biotinylated anti-IFN-γ detection mAb was added at 2 μg/mL (BD Pharmingen) and incubated for 2 hours at room temperature. Plates were then washed, incubated for 1.5 hours with avidin-HRP and developed using 3-amino-9-ethylcarbazole substrate for 30 minutes (MN 51-2577KC, BD Biosciences). The resulting spots were analyzed using the computer-assisted ELISPOT image analyzer (Cellular Technology Ltd., Cleveland, OH, USA).
Statistical Analysis
Student’s t test was used to compare the means between two groups. The two-way ANOVA test was used to compare graft opacity scores between the groups at different time points (Bonferroni post-test). Kaplan-Meier analysis was used to construct survival curves, and the log-rank test was used to compare corneal graft survival. Data are presented as mean ± standard error of mean (SEM) and considered statistically significant at *p <0.05, ** p <0.01, *** p < 0.001.
RESULTS
Recipients of grafts with dry eye disease demonstrate reduced allograft survival
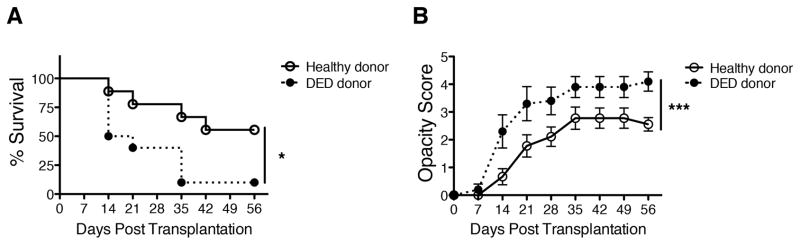
Fully MHC- and minor H- allodisparate dry eye or control (healthy) donor corneas derived from C57BL/6 mice were grafted onto naïve BALB/c recipients, and graft survival was assessed until week 8 post-transplantation. We found that graft survival was significantly lower (Figure 1A, 10% in DED donor group vs. 50% in healthy control donors; log-rank test, n=10, *p=0.0215) and graft opacity scores were significantly higher (Figure 1B, n=10, ***p<0.0001) in hosts who had received tissue from DED donors compared to healthy tissue recipients.
Figure 1. Recipients of grafts with dry eye disease demonstrate reduced allograft survival.
Corneal grafts were harvested from C57BL/6 mice with dry eye disease (DED) or from healthy C57BL/6 donors as the control. Corneal grafts were transplanted into healthy BALB/c host beds, and observed weekly over 8 weeks. (A) Kaplan-Meier survival curves showing graft survival of mice with healthy (control) vs. DED grafts (n=10/group, *p=0.0215); (B) Graft opacity scores were assessed using biomicroscopy (two-way ANOVA with Bonferroni post-test, n=10/group, ***p<0.0001).
Dry eye disease in the donor tissue promotes dendritic cell maturation in the cornea and draining lymph nodes of the host
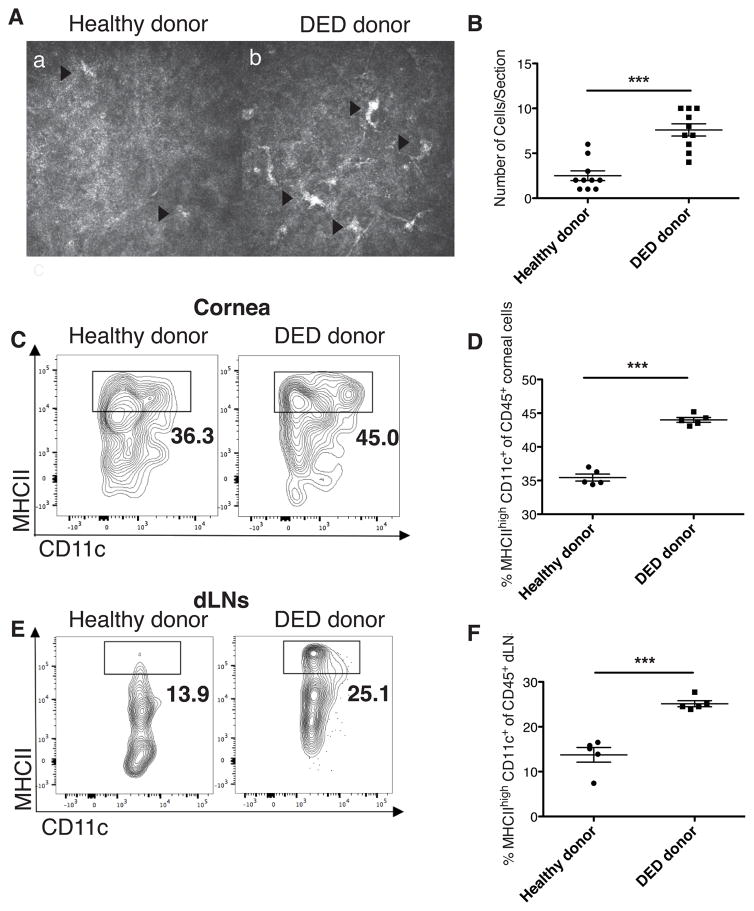
Using IVCM, we first evaluated the density of dendritic cells in the cornea of healthy and DED donor mice prior to transplantation. Dendritic cells were identified morphologically as bright dendritiform cells.17 There was a markedly increased number of dendritic cells in the examined corneal sections of mice with DED compared to healthy controls (Figures 2A and 2B, DED cornea 7.6±0.7 cells vs. healthy cornea 2.5±0.5 cells, n=10, ***p<0.001). APCs in the ocular surface and lymphoid tissues, including dLNs, play a critical role in priming host CD4 effector T cells in corneal transplantation.18 Thus, we next determined the frequencies of mature CD45+ CD11c+ MHCIIhigh DCs in the corneas and dLNs by flow cytometry 14 days after corneal transplantation (Figures 2C–F). The frequencies of mature DCs were significantly increased in both the grafted cornea (Figures 2C and 2D, n=5, ***p<0.001) and dLNs (Figures 2E and 2F, n=5, *p<0.05) of recipients of DED grafts compared to those receiving healthy control donor tissue. The frequencies of immature (MHC II−) DCs remained similar in both groups (data not shown).
Figure 2. Dry eye disease in donor promotes dendritic cell maturation in graft recipient.
(A) Representative in vivo confocal microscopy (IVCM) images displaying dendritic cells in the cornea of mice with DED and in healthy donors (n=10/group). The size of image is 400 × 400 μm2. (B) Number of dendritic cells, identified as bright dendritiform cells, per section in DED and healthy donor corneas (***p <0.001). (C) Representative flow cytometry plots showing mature CD11c+ MHCIIhigh dendritic cells (DCs) in cornea 14 days post-transplantation. (D) Mean frequencies of mature CD11c+ MHCIIhigh DCs among CD45+ cells in the cornea were assessed using flow cytometry (n=5, ***p<0.001). (E) Representative flow cytometry plots showing mature CD11c+ MHCIIhigh DCs in the draining lymph nodes (dLNs). (F) Mean frequencies of mature CD11c+ MHCIIhigh DCs among CD45+ cells in the dLNs were assessed using flow cytometry (n=5, *p<0.05). p values were calculated using the Student’s t-test and error bars represent standard error of mean.
Dry eye disease in donor tissue leads to significantly increased Th1 and Th17 frequencies and decreased Treg function in the recipient
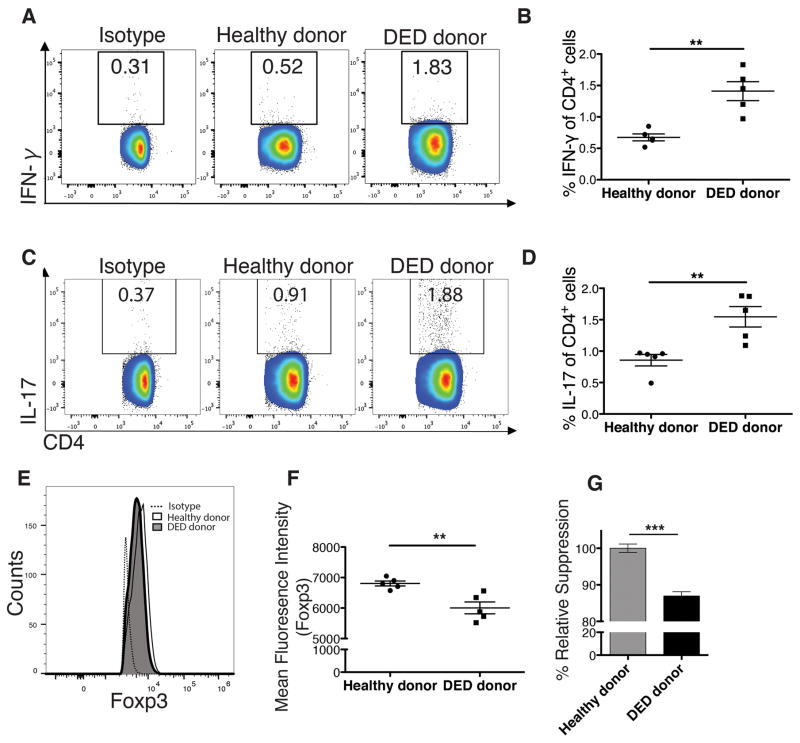
Interferon-γ (IFN-γ)-producing CD4+ T cells are the principal mediators of acute allograft rejection.19 We thus analyzed the frequencies of total IFN-γ-producing CD4+ T cells in the dLNs 14 days post-transplantation. We observed significantly higher frequencies of IFN-γ+ CD4+ T cells in dLNs of recipients of DED donor corneas compared to healthy tissue recipients (Figures 3A and 3B, n=5, **p=0.0017). Because the pro-inflammatory cytokine IL-17 has also been implicated in the acute corneal allograft response 20 and DED,8, 21 we analyzed the frequencies of IL-17–producing T cells in the dLNs 14 days post-transplantation. Our results showed that recipients of DED donor corneas had significantly higher frequencies of Th17 cells in dLNs compared to healthy tissue recipients (Figures 3C and 3D, n=5, **p=0.0061).
Figure 3. Dry eye disease in donor leads to increased Th1 and Th17 frequencies and decreased regulatory T (Treg) function.
Host T cell responses in draining lymph nodes (dLNs) of transplant recipients was assessed 14 days post-transplantation using flow cytometry. (A) Representative flow cytometry plot showing CD4+ IFN-γ + Th1 cells. (B) Mean Th1 cell frequencies in the dLNs of recipients with healthy vs. dry eye donor corneas are shown (n=5, **p=0.0017). (C) Representative plots showing the frequencies of CD4+ IL-17A+ Th17 cells. (D) Mean Th17 cell frequencies in the dLNs of recipients with healthy vs. dry eye donor corneas are shown (n=5, p=0.0061). (E & F) The mean fluorescence intensity (MFI) of Foxp3 in Tregs from dLNs of recipients with healthy vs. dry eye donor corneas are shown, demonstrating significantly lower levels of Foxp3 expression by Tregs in recipients of grafts from dry eye donors (n=5, **p=0.0051). (G) The suppressive function of Tregs harvested from hosts with grafts derived from dry eye vs. normal healthy donors was compared using the T cell proliferation assay. Results show a modest, though statistically significant decrease in the suppressive function of Tregs in recipients of grafts from dry eye donors compared to healthy controls (54.8% vs. 63.1%, n=6, ***p<0.0001; p values were calculated using the Student’s t-test and error bars represent standard error of mean.
Tregs are crucial for allograft survival as they attenuate multiple facets of alloimmunity,22 and Foxp3 is the key transcription factor that maintains their immunosuppressive function. We have previously shown that the level of expression of Foxp3 is a critical determinant of Treg immunosuppression activity in transplantation.23 Thus, we evaluated Treg frequencies and Foxp3 expression in dLNs of grafted hosts at day 14 after transplantation. While the frequencies of Tregs were similar in both groups (data not shown), we observed a 10–12% decrease in the mean fluoresence intensity (MFI) of Foxp3 (Figures 3E and 3F, n=5, **p=0.0051) in recipients of DED donor corneas compared to healthy graft recipients. To assess Treg suppressive function in vitro, Tregs isolated from the dLNs of DED grafted recipients were cultured with naive T cells in the presence of donor APCs, and their capacity to suppress T cell proliferation was measured.23 Tregs from recipients grafted with DED donor tissue displayed a moderately reduced suppressive function compared to Tregs harvested from recipients of healthy (non-DED) corneal tissue (Figure 3G, n=6, ***p<0.0001).
Dry eye disease in donor cornea activates host T cells through the direct pathway of allosensitization
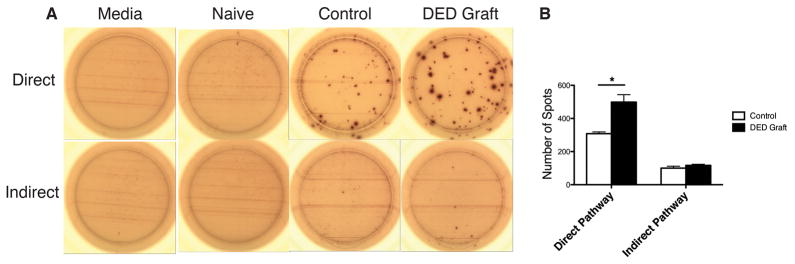
Given that host T cells are allosensitized either directly by donor APCs or indirectly by host APCs,19 we investigated the frequencies of both directly and indirectly primed IFN-γ-producing T cells using the ELISPOT assay at day 14 post-transplantation (Figure 4A and 4B). The frequencies of directly allosensitized T cells in hosts recieving DED donor tissue were significantly higher than recipients of healthy control tissue (Figure 4B; n=3, p=0.015). No differences were observed in the levels of indirectly primed host T cells between the two groups (Figure 4B; n=3, p=0.33).
Figure 4. Dry eye disease in graft promotes host T cell allosensitization through the direct pathway.
T cell allosensitization was assessed using the ELISPOT assay. The assay was carried out 14 days after transplantation to analyze CD4+ IFN-γ+ T cell sensitization through direct and indirect pathways. (A) Representative images of spots are shown (n=3). (B) Mean number of spots representing directly and indirectly allosensitized CD4+IFN-γ+ T cells in recipients grafted with healthy vs. dry eye donor corneas are shown (Direct pathway: *p=0.015; Indirect pathway: p=0.33; p values were calculated using the Student’s t-test and error bars represent standard error of mean.
DISCUSSION
Previous clinical and experimental studies have shown that transplant survival is significantly influenced by the host graft bed microenvironment.3, 24 In this study, we show for the first time that grafting corneal tissue harvested from DED donors onto uninflamed healthy host beds significantly reduces graft survival, clearly indicating that DED donor status portends an increased risk for graft rejection, even when grafted onto healthy uninflamed recipient beds.
To determine the mechanisms that mediate the heightened alloimmunity observed in recipients of DED donor tissues, we evaluated several factors, including APC maturation, effector T cell responses, and Treg suppressive function. Previous work by our group has shown that donor-derived APCs residing in graft tissue and graft-infiltrating host APCs can mediate the direct and indirect pathways of allosensitization, respectively.14, 24 These APCs can bear graft antigens, migrate to host lymphoid tissues, where they prime naïve host T cells and promote alloreactivity. In general, mature MHC class IIhigh DCs, the most potent APCs, promote T cell immunity, whereas immature DCs (MHC class II−) can contribute to T cell tolerance.25 Higher density of DCs has been shown in various inflammatory conditions such as DED.17 Using IVCM, we similarly observed increased number of DCs in donors with DED compared to healthy controls prior to transplantation. We found that the frequencies of mature CD11c+ MHCIIhigh DCs are significantly increased in the grafted corneas and the dLNs of recipients of grafts from DED donors. These data are in accord with those from a previous publication from our group where we reported increased corneal leukocytic (CD45+ CD11b+) infiltration and activation in dry eye corneas.10 Recent findings have shown that mature MHCIIhigh DCs produce high levels of pro-inflammatory cytokines, such as IL-12, IL-1β and IL-6 25 through which they promote host T cell responses. Based on our results, we hypothesize that the increased frequencies and activation (maturation) of passenger leukocytes present in DED corneas promote T cell allosensitization and acute rejection in hosts bearing these grafted tissues. In accord with this hypothesis, we demonstrate that the direct pathway dominates in recipients of grafts from DED donors.
Recent studies, including those from our group, have suggested that DED is a T cell-mediated autoimmune disorder.26 Increased expression of Th1-associated IFN-γ and Th17-associated IL-17,21 and dysfunctional Tregs, have been associated with DED severity. In accord with these observations, our results show increased Th1 and Th17 responses, as well as reduced Treg function, in mice transplanted with DED corneas. Moreover, Th1-associated IFN-γ-producing T cells are the principal mediators of acute corneal allograft rejection19 whose function is kept in check by regulatory T cells that promote allotolerance by suppressing host T cell sensitization and expansion.27 Thus, the balance between Th1 and Treg cells plays a critical role in determining graft survival.23, 28 We observed significantly decreased Foxp3 expression, a transcription factor whose expression level highly correlates with Treg function,23 suggesting that increased effector Th1 cell activation and Treg dysfunction act in concert, leading to high rejection rates observed in recipients of grafts from donors with DED.
In summary, our findings demonstrate that corneal tissue derived from DED donors hamper graft survival by augmenting T-cell driven inflammation in the host. It is noteworthy that the amplified immune response to grafted tissue from DED donors documented in this study was seen in donors whose DED was not treated pre- or postoperatively with steroids or other immunomodulatory therapies. Since our previous work has shown significant reduction of corneal immune cell infiltration in response to a variety of topical immune modulators,10, 29, 30 we speculate that immunomodulatory treatments that suppress corneal inflammation, either before donor death/tissue procurement or after grafting, can dampen the effect of pre-existing immune cell activation seen in DED donors on graft rejection. Based on the mouse derived data in our experiments, we can speculate that, similarly, eye bank eyes from donors with moderate to severe dry eye disease may predispose grafted hosts to higher rejection rates. Since the presence of DED in the donor leads primarily to immune cell mobilization and activation in the donor stroma, we expect that the effect of DED on graft outcome will be far more impactful in penetrating full-thickness grafting as compared to endothelial keratoplasty. These results warrant further exploration and confirmation in clinical studies, given the very high prevalence of DED in the population. If confirmed, it would mean that a significant minority of tissue donors might harbor a condition that portends a poor prognosis for hosts grafted with their corneas.
Acknowledgments
Grant information: This study was supported by the National Institutes of Health/National Eye Institute Grants EY012963 and EY020889 (RD) and the Eye Bank Association of America (JH/RD).
The authors would like to thank Dr. Susanne Eiglmeier for assistance in editing the manuscript and Dr. Qiang Zhang for technical support.
Footnotes
Financial Disclosure: The authors declare no financial conflicts of interest.
References
- 1.Williams KA, Brereton HM, Coster DJ. Prospects for genetic modulation of corneal graft survival. Eye. 2009;23:1904–9. doi: 10.1038/eye.2008.378. [DOI] [PubMed] [Google Scholar]
- 2.Niederkorn JY. Corneal transplantation and immune privilege. International reviews of immunology. 2013;32:57–67. doi: 10.3109/08830185.2012.737877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dana MR, Qian Y, Hamrah P. Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea. 2000;19:625–43. doi: 10.1097/00003226-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Luo L, Li DQ, Doshi A, et al. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 5.Stern ME, Beuerman RW, Fox RI, et al. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17:584–9. doi: 10.1097/00003226-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Schaumberg DA, Dana R, Buring JE, et al. Prevalence of dry eye disease among US men: estimates from the Physicians’ Health Studies. Arch Ophthalmol. 2009;127:763–8. doi: 10.1001/archophthalmol.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaumberg DA, Sullivan DA, Buring JE, et al. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318–26. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 8.Barabino S, Chen Y, Chauhan S, et al. Ocular surface immunity: homeostatic mechanisms and their disruption in dry eye disease. Prog Retin Eye Res. 2012;31:271–85. doi: 10.1016/j.preteyeres.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchino M, Yokoi N, Uchino Y, et al. Prevalence of dry eye disease and its risk factors in visual display terminal users: the Osaka study. Am J Ophthalmol. 2013;156:759–66. doi: 10.1016/j.ajo.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 10.Rashid S, Jin Y, Ecoiffier T, et al. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol. 2008;126:219–25. doi: 10.1001/archophthalmol.2007.61. [DOI] [PubMed] [Google Scholar]
- 11.Goyal S, Chauhan SK, El Annan J, et al. Evidence of corneal lymphangiogenesis in dry eye disease: a potential link to adaptive immunity? Arch Ophthalmol. 2010;128:819–24. doi: 10.1001/archophthalmol.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chauhan SK, Jin Y, Goyal S, et al. A novel pro-lymphangiogenic function for Th17/IL-17. Blood. 2011;118:4630–4. doi: 10.1182/blood-2011-01-332049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barabino S, Shen L, Chen L, et al. The controlled-environment chamber: a new mouse model of dry eye. Invest Ophthalmol Vis Sci. 2005;46:2766–71. doi: 10.1167/iovs.04-1326. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Hamrah P, Zhang Q, et al. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. The Journal of experimental medicine. 2002;195:259–68. doi: 10.1084/jem.20010838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inomata T, Mashaghi A, Di Zazzo A, et al. Ocular surgical models for immune and angiogenic responses. J Biol Methods. 2015:2. doi: 10.14440/jbm.2015.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hattori T, Saban DR, Emami-Naeini P, et al. Donor-derived, tolerogenic dendritic cells suppress immune rejection in the indirect allosensitization-dominant setting of corneal transplantation. Journal of leukocyte biology. 2012;91:621–7. doi: 10.1189/jlb.1011500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kheirkhah A, Rahimi Darabad R, Cruzat A, et al. Corneal Epithelial Immune Dendritic Cell Alterations in Subtypes of Dry Eye Disease: A Pilot In Vivo Confocal Microscopic Study. Invest Ophthalmol Vis Sci. 2015;56:7179–85. doi: 10.1167/iovs.15-17433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamagami S, Dana MR, Tsuru T. Draining lymph nodes play an essential role in alloimmunity generated in response to high-risk corneal transplantation. Cornea. 2002;21:405–9. doi: 10.1097/00003226-200205000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Boisgerault F, Liu Y, Anosova N, et al. Role of CD4+ and CD8+ T cells in allorecognition: lessons from corneal transplantation. Journal of immunology. 2001;167:1891–9. doi: 10.4049/jimmunol.167.4.1891. [DOI] [PubMed] [Google Scholar]
- 20.Yin XT, Zobell S, Jarosz JG, et al. Anti-IL-17 therapy restricts and reverses late-term corneal allorejection. Journal of immunology. 2015;194:4029–38. doi: 10.4049/jimmunol.1401922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Chauhan SK, Lee HS, et al. Chronic dry eye disease is principally mediated by effector memory Th17 cells. Mucosal Immunol. 2014;7:38–45. doi: 10.1038/mi.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sagoo P, Lombardi G, Lechler RI. Relevance of regulatory T cell promotion of donor-specific tolerance in solid organ transplantation. Frontiers in immunology. 2012;3:184. doi: 10.3389/fimmu.2012.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chauhan SK, Saban DR, Lee HK, et al. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. Journal of immunology. 2009;182:148–53. doi: 10.4049/jimmunol.182.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huq S, Liu Y, Benichou G, et al. Relevance of the direct pathway of sensitization in corneal transplantation is dictated by the graft bed microenvironment. Journal of immunology. 2004;173:4464–9. doi: 10.4049/jimmunol.173.7.4464. [DOI] [PubMed] [Google Scholar]
- 25.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends in immunology. 2002;23:445–9. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 26.Niederkorn JY, Stern ME, Pflugfelder SC, et al. Desiccating stress induces T cell-mediated Sjogren’s Syndrome-like lacrimal keratoconjunctivitis. Journal of immunology. 2006;176:3950–7. doi: 10.4049/jimmunol.176.7.3950. [DOI] [PubMed] [Google Scholar]
- 27.Cunnusamy K, Paunicka K, Reyes N, et al. Two different regulatory T cell populations that promote corneal allograft survival. Invest Ophthalmol Vis Sci. 2010;51:6566–74. doi: 10.1167/iovs.10-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan X, Zeng H, Jie Y, et al. CD154 blockade modulates the ratio of Treg to Th1 cells and prolongs the survival of allogeneic corneal grafts in mice. Exp Ther Med. 2014;7:827–34. doi: 10.3892/etm.2014.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dana R. Comparison of topical interleukin-1 vs tumor necrosis factor-alpha blockade with corticosteroid therapy on murine corneal inflammation, neovascularization, and transplant survival (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2007;105:330–43. [PMC free article] [PubMed] [Google Scholar]
- 30.Sadrai Z, Stevenson W, Okanobo A, et al. PDE4 inhibition suppresses IL-17-associated immunity in dry eye disease. Invest Ophthalmol Vis Sci. 2012;53:3584–91. doi: 10.1167/iovs.11-9110. [DOI] [PMC free article] [PubMed] [Google Scholar]