SUMMARY
Background
In coagulopathic blood, circulating thrombin may drive platelet dysfunction.
Methods/Results
Using calcium dye-loaded platelets, the effect of thrombin exposure and soluble fibrin generation on subsequent platelet GPVI function was investigated. Exposure of apixaban-treated platelet-rich plasma (12% PRP) to thrombin (1–10 nM), but not ADP or thromboxane mimetic U46619 exposure, dramatically blocked subsequent GPVI activation by convulxin, collagen-related peptide, or fibrillar collagen. Consistent with soluble fibrin multimerizing and binding GPVI, the onset of convulxin-insensitivity required 200–500 seconds of thrombin exposure, was not mimicked by exposure to PAR-1/4 activating peptides, was not observed with washed platelets, and was blocked by fibrin polymerization inhibitor (GPRP) or Factor XIIIa inhibitor (T101). PAR-1 signaling through Gαq was not required since vorapaxar blocked thrombin-induced calcium mobilization but had no effect on the ability of thrombin to impair GPVI-signaling. Convulxin-insensitivity was unaffected by the metalloprotease inhibitor GM6001 or the αIIbβ3 antagonist GR144053, indicating negligible roles for GPVI shedding or αIIbβ3 binding of fibrin. Thrombin treatment of washed platelets resuspended in purified fibrinogen also produced convulxin-insensitivity that was prevented by GPRP. Exposure of apixaban/PPACK-treated whole blood to thrombin-treated fibrinogen resulted in >50% decrease in platelet deposition in a collagen microfluidic assay that required soluble fibrin assembly.
Conclusions
Conversion of only 1% plasma fibrinogen in coagulopathic blood would generate 90 nM soluble fibrin, far exceeding ~1 nM GPVI in blood. Soluble fibrin, rather than thrombin-induced platelet activation through PAR-1 and PAR-4, downregulated GPVI-signaling in response to stimuli, and may lead to subsequent hypofunction of endogenous or transfused platelets.
Keywords: coagulopathy, fibrin, GPVI, platelet, thrombin, trauma
INTRODUCTION
Thrombin generation within the systemic circulation can drive complex changes in blood associated with coagulopathy. During trauma, for example, major changes in blood biochemistry occur due to hemorrhagic shock, release of tissue factor (TF) into the vasculature, endothelial release of tPA, endothelial glycocalyx shedding, and systemic inflammatory events [1–3]. Following trauma, thrombin and plasmin are generated in the systemic circulation as indicated by elevated plasma levels of thrombin-antithrombin, fibrin degradation products, and activated protein C [4–7].
Soluble fibrin monomer or soluble fibrin in various states of multimerization can circulate for several hours [8] and reach levels of 100 nM in trauma-induced coagulopathy (TIC) patients [9], 40 nM in Day 0 trauma patients (estimated from fibrinopeptide A levels) [10], 90 nM in neck fracture patients [11], 54 nM in sepsis patients [12], 42 nM in coronary artery disease patients [13], 30–300 nM in ECMO patients [14], and up to 67 nM in post-operative AAA patients [15], all relative to a healthy human baseline of ~7.5 nM soluble fibrin monomer [13]. Also, soluble fibrin exceeds 600 nM (50× baseline) in the rat model of Noble-Collip drum trauma [16].
Platelets obtained from trauma patients can display a hypofunctional phenotype, potentially contributing to TIC [17,18]. Platelet dysfunction after trauma has been detected by aggregometry [7,19], by thromboelastography [17], and by microfluidic assay of platelet deposition on collagen [20]. Interestingly, trauma patients often display no significant differences in baseline platelet count or platelet P-selectin levels compared to healthy individuals [7].
Platelet GPVI is an immunoglobulin superfamily receptor [21] present at about 4000 copies/platelet [22], corresponding to about 1 nM concentration in platelet-rich plasma (PRP). Known GPVI-activating ligands include collagen, collagen-related peptide (CRP), and convulxin [23,24], as well as fibronectin [23], vitronectin [24], and laminins [25]. Within a forming clot and on fibrin-coated surfaces, insoluble fibrin has recently been described as a ligand and agonist of platelet GPVI signaling [21,26]. Platelet GPVI binding to fibrin surfaces increases platelet procoagulant activity [21], amplifies collagen-independent thrombin generation and platelet recruitment at the clot surface [26], and contributes to thrombus growth and stabilization [21,27].
However, the function of soluble fibrin species on platelets in suspension is less well understood, since circulating platelets may eventually encounter a wound site presenting various adhesive matrix stimuli such as collagen, vitronectin, and laminin. Fibrinogen can be activated to desA and desB soluble fibrin monomer which will immediately bind fibrinogen (an assembly also considered, confusingly, as soluble fibrin monomer). Additionally, thrombin activity results in assemblies of sub-micron soluble fibrin multimers (<50 monomer units) which are easily detectable by light scattering. In diluted apixaban-treated PRP where added thrombin is consumed without further thrombin generation, these dilute, soluble multimeric fibrin species are stable in suspension and never reach a concentration to form long fibrin strands, laterally aggregated bundles, or fibrin gels (insoluble fibrin). We use the term “soluble fibrin” to refer to sub-micron desA/B fibrin multimeric assemblies that may also contain bound fibrinogen. The fibrin polymerization inhibitor Gly-Pro-Arg-Pro (GPRP) keeps soluble fibrin monomer from binding fibrinogen or assembling into longer multimer units.
We hypothesized that platelet hypofunction can result from exposure to low levels of thrombin and/or thrombin-generated plasma species such as soluble fibrin. Since elevated soluble fibrin levels have been implicated in trauma and disseminated intravascular coagulation (DIC) [16,28–30], understanding GPVI signaling after soluble fibrin exposure has clinical implications. We developed approaches using apixaban-treated, diluted PRP that allows addition of low-dose thrombin (t1/2~1 minute via inhibition by antithrombin) to trigger limited fibrin monomer generation without formation of an insoluble fibrin gel for studies of platelet GPVI signaling in the presence of soluble fibrin species.
MATERIAL AND METHODS
Platelet calcium assays
Apixaban and GM6001 (SelleckChem), Fluo-4 NW dye and probenecid (Invitrogen), ADP, GPRP, PGE1, apyrase, and Factor Xa (Sigma-Aldrich), convulxin (Cayman Chemical), thrombin and human fibrinogen (Haematologic Technologies Inc.), PAR-1/4 agonist peptides (Bachem), U46619 (Tocris Bioscience), T101 (Zedira), vorapaxar (Ryan Scientific), and GR144053 (R&D Systems) were stored and used according to manufacturers’ instructions. Whole blood was drawn by venipuncture from healthy donors with University of Pennsylvania Institutional Review Board approval into a syringe containing apixaban (final concentration, 250 nM) to prevent Factor Xa-driven generation of thrombin. Donors self-reported to be free of any medications or alcohol use for three days prior to the blood draw. Female donors self-reported not using oral contraceptives.
Platelet calcium measurements were conducted in 384-well plate assay as previously described [31]. Briefly, 2 mL PRP was obtained from whole blood (120g centrifugation, 10 min) and incubated with a vial of Fluo-4 NW dye mixture reconstituted with 7.8 mL of sterile 20 mM HEPES (N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid) buffered saline (HBS, pH 7.4) and 200 µL of 77 mg/mL probenecid for 30 minutes. In some experiments, GPRP and/or vorapaxar were added with the calcium dye to give final concentrations of 500 µM and/or 100 nM, respectively. Additionally, a 384-well plate containing platelet agonists was assembled, including thrombin, ADP, U46619 (a stable thromboxane analog), SFLLRN and AYPGKF (PAR-1 and PAR-4 receptor agonists), as well as convulxin (a potent and specific GPVI activator). Dye-loaded PRP was then dispensed into a 384-well plate. Both plates were loaded into a FlexStation 3 (Molecular Devices, Inc.) fluorescence reader. Agonists were dispensed column-wise to PRP, where dynamic fluorescence intensity F(t) was read and normalized by the pre-dispense baseline (Fo). For all experiments, 10 µL of agonist was added to 30 µL of PRP in each well, followed by a subsequent addition of 10 µL of convulxin at a later specified time. In each well, the final concentration of PRP after agonist addition was 12% PRP by volume. The fluorescence was read for 20 seconds before first dispense, and readings were taken every 2.5 seconds (Ex: 485 nm; Em: 525 nm). In previous tests, there was no evidence for autocrine signaling in the dilute PRP conditions of the experiment [31]. In calcium experiments using washed platelets instead of PRP, 500 nM human fibrinogen was added. In this case, the platelet pellet from 2 mL PRP was resuspended in 1.1 mL of HBS to obtain a washed platelet suspension. For calcium assay using type 1 fibrillar collagen (Chronolog), PRP was prepared as described, however the small FlexStation automation pipettes resulted in variable delivery, thus requiring manual pipetting and assay using a FluoroSkan Ascent 384-well plate reader.
Microfluidic assays
Fluorescent human fibrinogen (Thermo Fisher Scientific, Alexa Fluor® 647 conjugate) was reacted with thrombin (2.5 nM final concentration) for 5 minutes after a 15-minute incubation with either 5 mM GPRP or HBS (to prevent or allow fibrin polymerization, respectively), after which D-Phenylalanyl-prolyl-arginyl Chloromethyl Ketone (PPACK; Haematologic Technologies Inc.) was added (100 µM final concentration) to inhibit thrombin. The mixture was then diluted 10-fold into whole blood that had been drawn into PPACK (100 µM) and apixaban (1 µM). Platelets were labeled using PE fluorescent anti-CD61 (BD Biosciences). The 8-channel microfluidic device was fabricated out of polydimethyl-siloxane (PDMS) (Ellsworth Adhesives) as previously described [32]. The device was blocked with 0.5% bovine serum albumin (BSA; Sigma-Aldrich) for 30 minutes before sample perfusion (each channel: 60 µm high, 250 µm wide). Apixaban/PPACK-treated whole blood was perfused through the device using a syringe pump (Harvard Apparatus) at a wall shear rate of 200 s−1 over a patterned 250-µm long strip of 1 mg/mL fibrillar collagen type 1 (Chronolog). The deposited platelet and fibrin fluorescence intensities were recorded every minute for 6 minutes.
RESULTS
Thrombin, but not ADP or U46619, attenuated subsequent platelet GPVI signaling
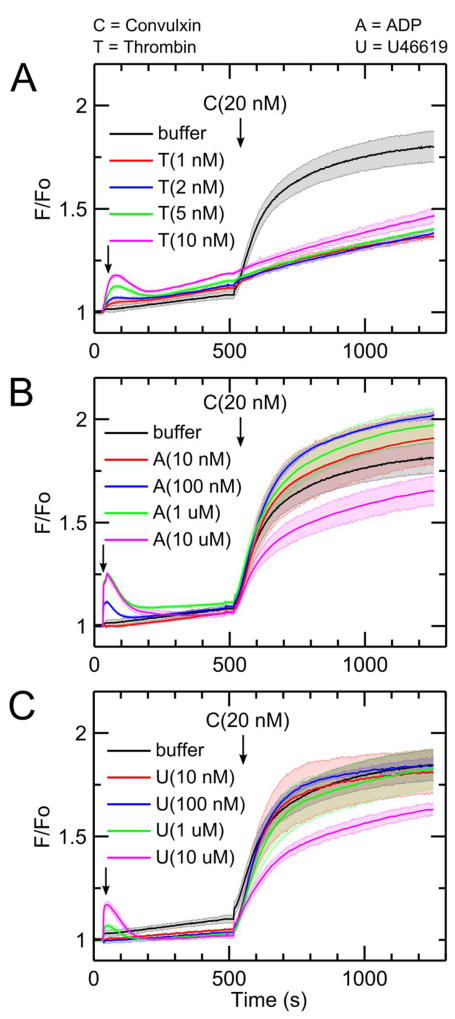
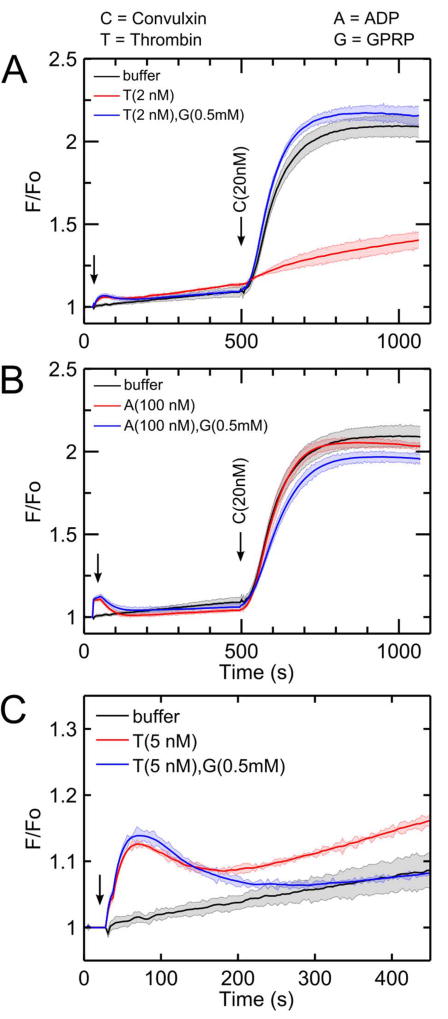
Addition of thrombin (1–10 nM) to diluted apixaban-treated PRP caused a dose-dependent calcium mobilization (Figure 1A). Pretreatment with thrombin, however, resulted in a marked inhibition of calcium mobilization when 20 nM convulxin was added 480 seconds after thrombin (Figure 1A). In the absence of thrombin pretreatment, convulxin drives a massive and sustained calcium mobilization lasting over 700 seconds in the measurement. Dasatinib, a Syk inhibitor, blocked convulxin-induced GPVI signaling with minimal effect on Gαq agonists of calcium mobilization (via thrombin, U46619, and ADP), thus confirming that convulxin is activating GPVI with concomitant calcium mobilization dependent on Syk signaling (Supplemental Figure S1). Even the lowest dose of thrombin (1 nM), which caused minimal calcium mobilization, had significant inhibitory effect on GPVI activation by convulxin. However, convulxin-insensitivity was not observed when ADP or U46619 were added instead of thrombin (Figure 1B–C) even though both agonists caused dose-dependent calcium mobilizations similar to that evoked by thrombin treatment.
Figure 1. Thrombin but not ADP or U46619 blocks subsequent platelet GPVI activation by convulxin.
(A) Platelet activation by thrombin for 480 seconds causes a significant reduction in subsequent convulxin-induced calcium response. This effect was apparent for doses of thrombin (1 – 10 nM) treatment of diluted (12%), apixaban-treated PRP. (B) Platelet activation by ADP does not significantly attenuate subsequent convulxin-induced calcium response. (C) Platelet activation by the thromboxane analog, U46619, did not significantly attenuate subsequent convulxin-induced calcium response. (C, convulxin; T, thrombin; A, ADP; U, U46619).
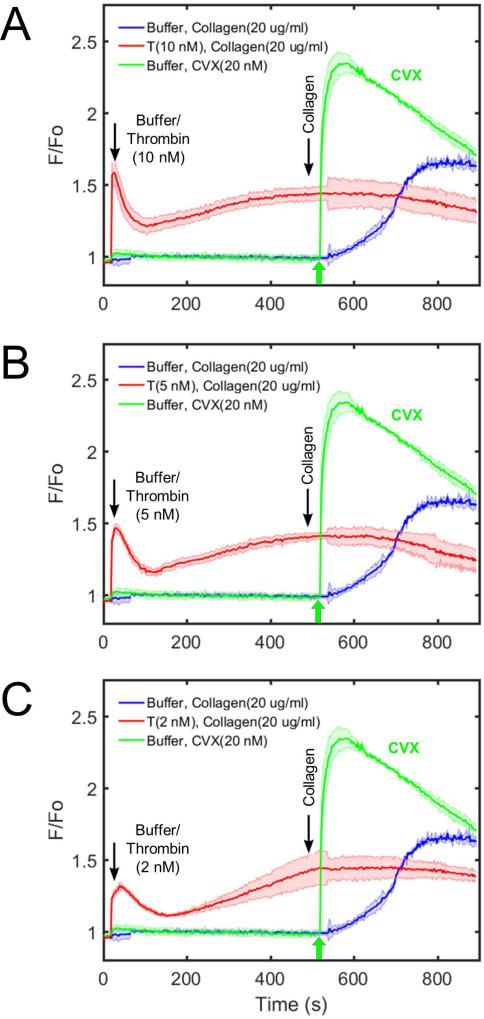
When ADP was added instead of thrombin, a very low dose of ADP (10 nM) did not affect subsequent calcium response to convulxin compared to the control condition (Figure 1B). Low to medium doses of ADP (100 nM–1 µM) slightly increased subsequent calcium response to convulxin. A high dose of ADP (10 µM) resulted in a detectable reduction in subsequent final platelet calcium response to convulxin, but not nearly to the extent seen in the case of thrombin. When U46619 was used as the first stimulus, very low to medium doses of U46619 (10 nM–1 µM) did not affect subsequent calcium response to convulxin compared to the control condition (Figure 1C). Similarly, as seen in the case of ADP, a high dose of U46619 (10 µM) resulted in a detectable reduction in subsequent final platelet calcium response to convulxin, but not nearly to the extent caused by any dose of thrombin. In a related experiment, thrombin treatment of apixaban-treated PRP caused a marked loss in platelet sensitivity to CRP (Supplemental Figure S2), although 25 µg/mL CRP was not as potent as 20 nM convulxin in activating platelet GPVI. Type I fibrillar collagen (20 µg/mL) was tested as a platelet agonist instead of convulxin. In this unstirred reaction, the platelet calcium response to collagen alone was slower than that observed with convulxin alone. As seen with convulxin and CRP, pretreatment of the PRP with thrombin (2–10 nM) resulted in a complete insensitivity to subsequent exposure to fibrillar collagen (Figure 2A–C).
Figure 2. Thrombin treatment of platelets blocks subsequent activation via fibrillar collagen when measuring calcium mobilization.
Various doses of thrombin (A: 10 nM; B: 5 nM; C: 2 nM) prevent further downstream platelet activation via collagen (Chrono-log; 10 µg/mL), fully consistent with the previous findings using convulxin. Additionally, collagen and convulxin show significantly different kinetic profiles of GPVI signaling which can be attributed to the molecular composition of each species. Collagen, a larger and more fibrillar molecule, activates GPVI in a slower but more sustained manner, while convulxin elicits a rapid and transient response.
Thrombin-induced GPVI-signaling defect was time-dependent
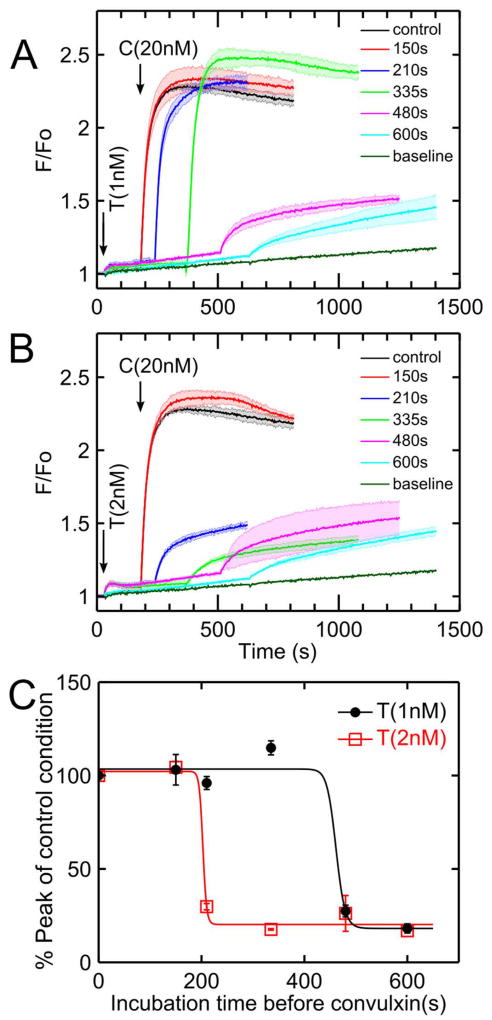
Convulxin (20 nM) was added to PRP at varying times after thrombin addition (1–2 nM). When convulxin was added after low-dose thrombin (1 nM) incubation times of 150, 210, and 335 seconds, the calcium signal in response to convulxin was comparable to the control condition in which PRP was not first activated by thrombin (Figure 3A). However, when the incubation time with 1 nM thrombin increased to 480 seconds, subsequent calcium responses to convulxin decreased significantly. When a slightly higher dose of thrombin (2 nM) was first added to PRP, subsequent calcium responses to convulxin were not attenuated until incubation times increased to 210 seconds or higher (Figure 3B). The onset time of convulxin-insensitivity depended on thrombin dose (Figure 3C). In comparing peak calcium response to convulxin (no thrombin pretreatment) with that obtained with prior thrombin treatment, an incubation with 2 nM thrombin for 220 seconds or incubation with 1 nM thrombin for 500 seconds led to a marked 80% reduction in convulxin-triggered GPVI signaling.
Figure 3. Effect of thrombin dose and exposure time to drive convulxin-insensitivity.
(A) When platelets were treated with low dose thrombin (1 nM), the reduction of convulxin-sensitivity was time-dependent with strong onset detected between 335 and 480 sec of thrombin incubation. (B) When platelets were activated by a slightly higher thrombin dose (2 nM), thrombin-induced convulxin-insensitivity was detected after 200 sec of thrombin incubation. (C) Sensitivity to convulxin decreased with thrombin incubation time (data fitted with a Hill function) with more rapid onset observed at higher thrombin dose. (C, convulxin; T, thrombin).
Activating PAR-1 and PAR-4 does not result in attenuation of GPVI signaling
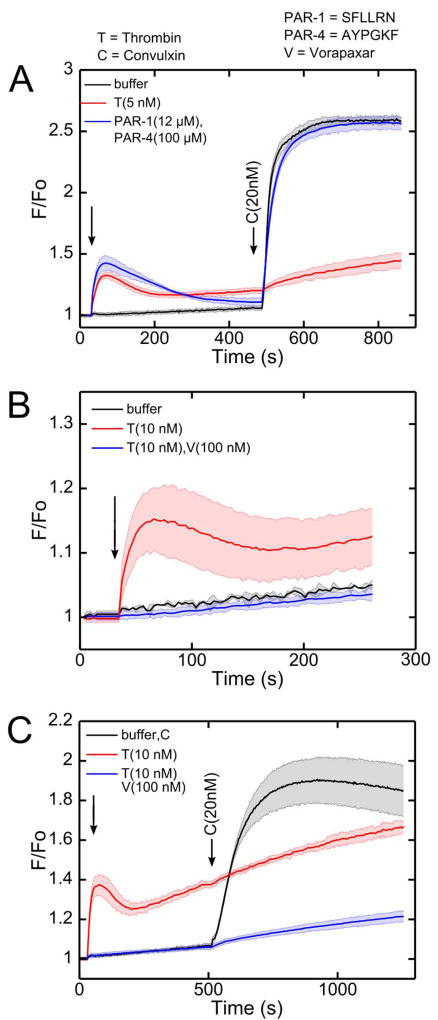
Thrombin signals through Gαq-linked PAR-1 and PAR-4 receptors in human platelets, but when PAR-1 and PAR-4 agonists (SFLLRN and AYPGKF) were added to PRP (instead of active thrombin), the subsequent platelet calcium response to convulxin was unaffected compared to the control condition (Figure 4A). In combination with the observations with ADP and U46619, this result indicated that thrombin activity played a unique role other than through Gαq-dependent signaling through PAR-1/4. When vorapaxar, a PAR-1 specific inhibitor was added, calcium signaling in response to 10 nM thrombin was fully blocked (Figure 4B). However, when thrombin was added to PRP incubated with vorapaxar, the lack of thrombin-induced calcium mobilization had no effect on the subsequent thrombin-dependent GPVI-signaling defect when convulxin was added (Figure 4C). In separate calcium mobilization experiments using PAR-1 and PAR-4 activating peptides, vorapaxar was found to be a PAR-1 selective inhibitor (Supplemental Figure S3), as expected.
Figure 4. Attenuation of convulxin sensitivity was not observed by pretreatment with PAR-1 and PAR-4 agonist peptides.
(A) Despite calcium mobilization through PAR-1 and PAR-4, platelet activation by PAR-1 and PAR-4 specific agonist peptides (SFLLRN and AYPGKF) did not reduce subsequent convulxin-insensitivity, as was observed with thrombin. (B) Vorapaxar, a PAR-1 antagonist, blocked thrombin-induced calcium signaling. (C) The convulxin-insensitivity after thrombin treatment was still apparent in the presence of vorapaxar. (PAR-1=SFLLRN activation peptide; PAR-4=AYPGKF activation peptide; V, vorapaxar; T, thrombin; C, convulxin).
Soluble fibrin caused convulxin-insensitivity independent of receptor shedding or fibrin binding by αIIbβ3
The results shown in Figures 1–4 are fully consistent with thrombin acting on a plasma element to attenuate platelet sensitivity to GPVI agonists. This was confirmed in an assay comparing the effect of thrombin pretreatment of washed platelets versus PRP. Thrombin pretreatment of washed platelets caused substantial calcium mobilization, but had little effect on the GPVI response to convulxin (Supplemental Figure S4), confirming the role of thrombin on a plasma component. We next tested the ability of GPRP to block soluble fibrin multimerization and rescue GPVI function in the presence of active thrombin. When thrombin was first added to PRP incubated with GPRP, subsequent calcium response to convulxin was completely normal when compared to the control condition where no thrombin was first added (Figure 5A). This demonstrated that soluble fibrin assembly was essential for the thrombin-induced ablation of platelet sensitivity to convulxin. GPRP rescued GPVI sensitivity to convulxin for thrombin pretreatment concentrations ranging from 2–20 nM (Supplemental Figure S5). Unlike thrombin, the P2Y1/P2Y12 agonist ADP does not induce polymerization of fibrinogen into fibrin. Therefore, as expected, when the experiment in Figure 5A was repeated with ADP instead of thrombin, GPRP had no effect on the subsequent calcium response to convulxin (Figure 5B). Notably, in the calcium responses to thrombin alone, the calcium signal displayed an upward trend, consistent with soluble fibrin acting as a weak GPVI activator [21]. However, in cases with GPRP present, the thrombin-induced calcium mobilization was transient and eventually returned to baseline (Figure 5C).
Figure 5. Inhibition of fibrin polymerization with GPRP prevents thrombin-induced convulxin-insensitivity.
(A) The thrombin-induced attenuation of platelet calcium GPVI signaling was not observed when GPRP was present. (B) Because platelet activation by ADP does not cause fibrinogen to polymerize into fibrin, GPRP has no effect on subsequent convulxin response. (C) Following thrombin stimulation of platelets, fibrin results in a sustained calcium mobilization that returned to unstimulated levels in the presence of GPRP.
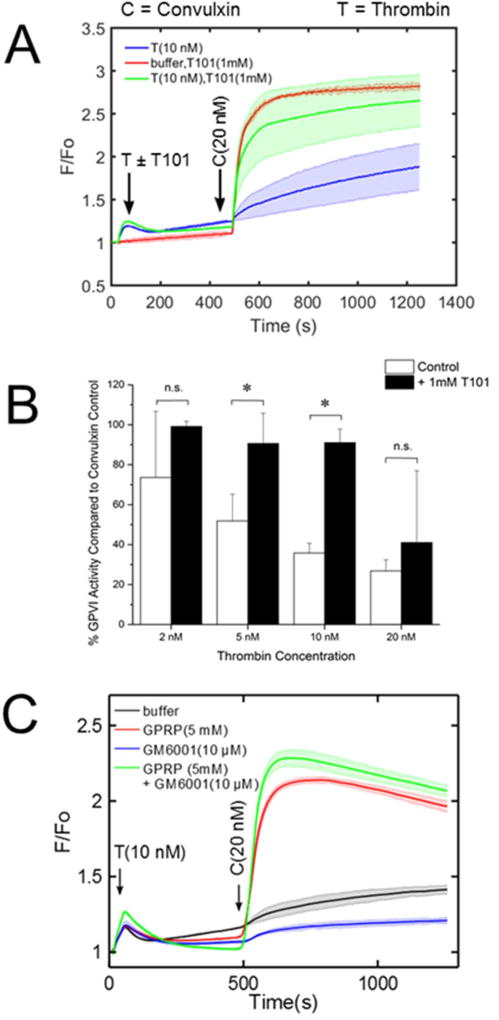
Since fibrin assembly was required for convulxin insensitivity, we tested if FXIIIa activity played a role in thrombin-induced GPVI attenuation. Interestingly, the FXIIIa inhibitor T101 had little effect on platelet response to thrombin, but caused a marked reduction in the thrombin-induced convulxin insensitivity (Figure 6A–B). Additionally, T101 alone had little effect on the convulxin response of platelets without thrombin pretreatment. T101 was unable to overcome the attenuation of GPVI at the highest thrombin concentration tested (20 nM).
Figure 6. Inhibition of cross-linking enzyme FXIIIa with T101 results in significant restoration of platelet GPVI activity and inhibition of ADAM10 shows no effect of GPVI shedding.
(A) Calcium dye-loaded platelets were incubated with 1 mM T101 for 10 minutes prior to activation with thrombin (2–20 nM). In the presence of the FXIIIa inhibitor, fibrin still polymerizes but cannot cross-link to form the traditional mesh network which is crucial for clot stabilization. The plot shows the effect of T101 towards greatly restoring convulxin-induced platelet activation. (B) Platelet GPVI exhibits a marked increase in sensitivity, especially at intermediate concentrations of thrombin (n=4 donors, * p<0.05). (C) Thrombin-induced convulxin-insensitivity does not require GPVI shedding. Activation of platelets by thrombin resulted in attenuation of subsequent convulxin-induced calcium mobilization even in the presence of GM6001, which blocks GPVI shedding. When GPRP was present to block fibrin polymerization, thrombin treatment had no effect convulxin sensitivity, even in the presence of GM6001 which blocks GPVI shedding.
Since GPVI activators like CRP (as well as thrombin) can cause GPVI dimerization [33,34] and shedding by metalloproteases such as a-disintegrin-and-metalloproteinase 10 (ADAM10), we tested the role of shedding in thrombin-induced GPVI-signaling deficiency. Treatment of PRP with the metalloprotease inhibitor GM6001 had little effect on the ability of thrombin to attenuate platelet response to convulxin (Figure 6C). GPRP maintained its ability to prevent thrombin-induced convulxin-insensitivity in the presence of GM6001.
Use of the αIIbβ3 inhibitor, GR144053, inhibited fibrinogen-dependent platelet aggregation (via aggregometry) as expected, however GR144053 did not alter the thrombin-mediated attenuation of convulxin-induced signaling (Supplemental Figure S6). This finding eliminates the potential role of fibrin(ogen)-driven integrin αIIbβ3 outside-in signaling in the observed deficiency in response to GPVI agonists. These results indicate that GPVI shedding and αIIbβ3-mediated fibrin binding were not the cause of convulxin-insensitivity in the presence of soluble fibrin, even when soluble fibrin served as a relatively weak agonist for platelet GPVI. When soluble fibrin is formed in the presence of vorapaxar (to prevent thrombin activation), there is little change in calcium signal (Figure 4C), indicating that the generation of soluble fibrin is not strongly activating in this system. Still, in this experiment with vorapaxar present, a marked convulxin insensitivity was observed (an effect fully reversed by GPRP and indicative of soluble fibrin driving the GPVI-signaling deficiency).
Thrombin treatment of washed platelets in purified fibrinogen display convulxin-insensitivity
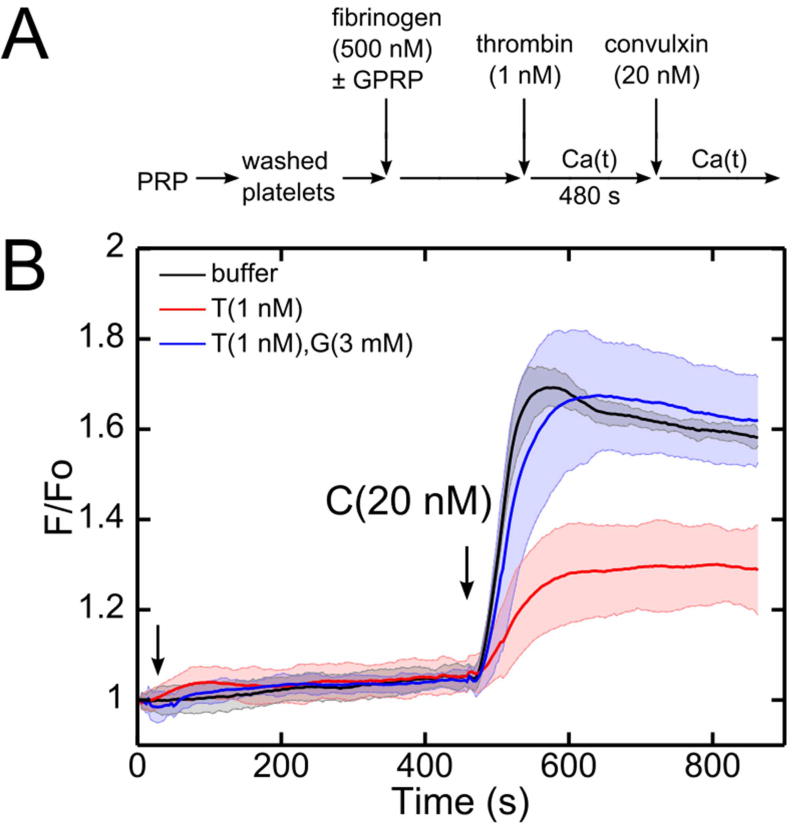
Since thrombin can act on numerous proteins in plasma, washed platelets were placed in a buffer containing purified fibrinogen to explore the role of soluble fibrin generation. Distinct from PRP, this buffer does not contain plasma antithrombin or FXIII. The washed platelets were incubated with human fibrinogen (500 nM) with and without GPRP for 10 minutes before the assay (Figure 7A). Exposure for 480 seconds of the washed platelets in purified fibrinogen to a low dose of thrombin (1 nM) caused a marked attenuation of the subsequent convulxin response (Figure 7B). Similar to the PRP experiments, GPRP prevented the thrombin-dependent signaling defect in response to convulxin. Soluble fibrin assemblies reached a size of 1.095±0.347 µm after 10 min of thrombin-fibrinogen reaction, as detected by light scattering (Supplemental Figure S7).
Figure 7. Thrombin activation of washed platelets in purified fibrinogen reduced subsequent activation by convulxin, an effect blocked by GPRP.
(A) Schematic of experimental protocol. (B) In a washed platelet and fibrinogen (500 nM) mixture, low dose of thrombin (1 nM) at 480 seconds incubation time significantly attenuated subsequent convulxin response.
Presence of soluble fibrin reduces platelet deposition to collagen under flow
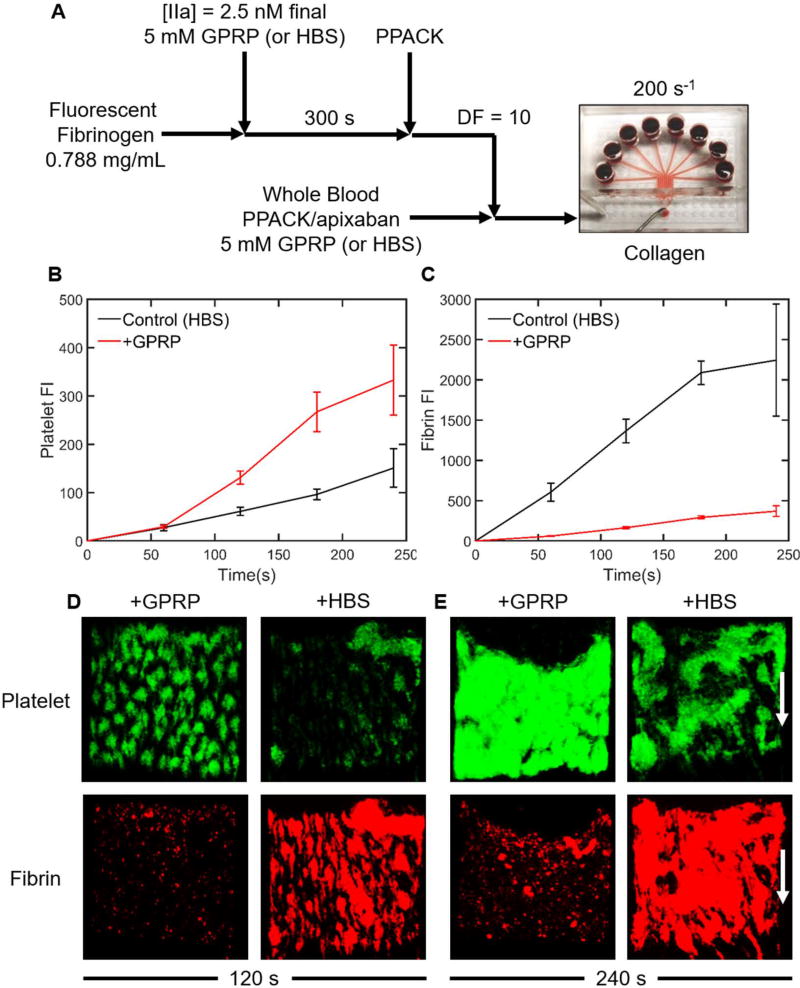
Fluorescent fibrinogen (0.788 mg/mL) was incubated in either 5 mM GPRP or HBS (control condition) for 10 min. Following incubation, 2.5 nM thrombin was added to generate either soluble fibrin monomer (GPRP present) or soluble fibrin polymer (HBS control). After 300 seconds, thrombin activity was inhibited with 100 µM PPACK. The resulting solutions were diluted by a factor of 10 in PPACK/apixaban-treated whole blood (treated to prevent endogenous thrombin production or thrombin activity). Platelet deposition from whole blood with 230 nM soluble fibrin monomer (GPRP condition) or 230 nM soluble fibrin polymer (HBS control condition), nominal concentrations assuming 100% fibrinopeptide release by thrombin, was then tested on collagen at a wall shear rate of 200 s−1 (Figure 8A).
Figure 8. Under thrombin-free conditions, presence of soluble fibrin in whole blood reduces platelet adhesion on collagen under flow.
(A) Schematic of experimental protocol. Fluorescent fibrinogen was exposed to 2.5 nM thrombin with either 5 mM GPRP (inhibit fibrin formation) or HBS (control). After 300 s, the thrombin was quenched with 100 µM PPACK. This reacted fibrinogen/fibrin solution was diluted by a factor of 10 into PPACK/apixaban-inhibited whole blood with either 5 mM GPRP or HBS and then perfused through an 8-channel microfluidic device at 200 s−1 over a collagen surface. (B) GPRP increased platelet deposition on the collagen surface after 60 s. (C) Fibrin co-deposition with platelets was significantly decreased with 5 mM GPRP. (D) At 120 s, soluble fibrin monomer (GPRP present) resulted in more platelet deposition to collagen, while soluble fibrin polymer (no GPRP) resulted in less platelet adhesion to collagen. (E) At 240 s, platelet deposition and aggregation on collagen was quite pronounced in the presence of GPRP which blocked fibrin co-deposition, as expected.
As expected, 5 mM GPRP substantially blocked the amount of fibrin that co-deposited with the platelets (Figure 8C–E). This indicated that ~8000 nM fibrinogen in whole blood outcompeted 230 nM fibrin monomer for platelet binding. In contrast, 230 nM soluble fibrin polymer added to whole blood co-deposited with platelets, even in the presence of normal levels of fibrinogen. When GPRP is present, there still appears to be a small amount of fibrin detected by fluorescence, which can be attributed to platelet-bound fibrinogen or soluble fibrin monomer. Consistent with a defect in collagen-induced platelet activation via GPVI, the presence of soluble fibrin polymer (HBS control condition) caused a substantial reduction of platelet deposition on collagen (Figure 8B–E), especially after ~100 seconds when ADP and thromboxane release are especially important for platelet buildup [35].
DISCUSSION
In coagulopathic blood, low levels of thrombin in the systemic circulation may generate soluble fibrin monomers and small submicron-scale soluble protofibrils (<10–50 monomers) that never reach the point of gelation. Several lines of evidence are presented that soluble fibrin, not thrombin cleavage of PARs, was the cause of GPVI-signaling deficiency in response to convulxin, CRP, and collagen. PAR-1 and PAR-4 activating peptides that trigger calcium mobilization had no effect on convulxin response. Vorapaxar, which blocked thrombin-induced calcium signaling, had no effect on thrombin-mediated convulxin-insensitivity. Agonists such as ADP and U46619, which similarly trigger signaling through Gαq-coupled receptors, were not able to alter subsequent convulxin sensitivity. Importantly, GPRP blocked the ability of thrombin to attenuate platelet sensitivity to convulxin. Also, the time of thrombin exposure and kinetics of onset were consistent with fibrin monomer generation and soluble fibrin multimerization. Thrombin treatment of washed platelets did not induce convulxin-insensitivity, unless platelets were supplemented with purified fibrinogen. Both the calcium mobilization assay with fibrillar collagen (Figure 2) and microfluidic platelet deposition from flowing whole blood on collagen-coated surfaces (Figure 8) indicated that exposure of plasma to thrombin drives an attenuation of platelet GPVI signaling in response to collagen.
To test the findings under non-dilute PRP conditions, we used aggregometry to generate soluble fibrin under non-static conditions and challenge GPVI through addition of convulxin. The results were consistent with those observed in the calcium mobilization experiments: a low dose of thrombin with stirring (1200 rpm) permitted fibrin polymerization and subsequent addition of GPVI agonists shows a significant (~35%) reduction in aggregation compared to the control, a result which is completely reversed with GPRP (Supplemental Figure S8). Platelet aggregation in PRP and platelet deposition from whole blood in a microfluidic device were reduced by a similar amount when fibrin was allowed to polymerize, an effect reversed by GPRP. Given the multimeric nature of most GPVI activators, it was not unexpected that fibrin monomer which exists in the presence of GPRP was insufficient to block GPVI activation.
GPVI-deficiency is extremely rare and not linked to a strong bleeding phenotype in healthy individuals. However, GPVI-deficient patients can display spontaneous bleeds [36]. The potential risk of genotypic GPVI-deficiency in combination with trauma is unknown. Importantly, a combined deficiency in mouse platelet PAR-4 and GPVI causes a severe bleeding phenotype in the tail bleed assay [37]. A level of only 1% conversion of fibrinogen (90 nM soluble fibrin) would be sufficient to overwhelm platelet GPVI which exists at ~1 nM in PRP, as the avidity of binding between polyvalent soluble fibrin and clustering receptors on platelets may be much stronger relative to the Kd of the monovalent affinity involving soluble forms of ligand and receptor. Since soluble fibrin can circulate without rapid clearance and GPVI signaling through fibrin is fairly weak compared to that driven by collagen, platelet hypofunction of endogenous or transfused platelets may be a cofactor in certain coagulopathies.
We are much obliged to highlight recent work demonstrating platelet GPVI as a receptor for fibrin, which was also found to bind to a distinct configuration of GPVI [38]. In that study, sonicated crosslinked fibrin (but not D-dimer) caused <10% washed platelet aggregation over 6 min, while 30 µg/mL D-dimer substantially inhibited platelet aggregation by subsequent challenge by 0.1–0.3 µg/mL collagen (but interestingly not higher doses of collagen). As noted in [38], an agent that selectively blocks the interaction of fibrin but not collagen with GPVI has the potential as antithrombotic therapy with reduced bleeding risk. Such an agent might hypothetically also protect circulating platelet function in trauma patients with circulating levels of soluble fibrin polymer and lytic assemblies of D-dimer.
Our results and prior studies of fibrin activation of GPVI during thrombosis [21,26] are not particularly discordant on close examination. To mimic systemic blood changes and subsequent local hemostatic response, we challenge GPVI with a subsequent agonist after pre-exposure to soluble fibrin. In studies of thrombus generation at a wound site, there is no additional GPVI ligand added to the assay other than the original fibrin generated in the clot [21,26]. Also, there is no reason to expect sub-micron soluble fibrin multimers bound to a platelet in suspension to induce the same signaling as a spread platelet experiencing large expanses of solid-phase fibrin gel. Since platelets (and many other cell types) can sense the rigidity of their adhesive-mechanical environment [39], we suggest that platelet signaling on solid fibrin presented on glass/plastic or in a clot may be different from platelets in suspension interacting with soluble fibrin. We also observed that soluble fibrin multimers induce a weak signal in our assay that was blocked by GPRP (Figure 5C).
We found GPVI attenuation occurred acutely at ~200 seconds with 2 nM thrombin treatment (Figure 3B–C) and was not blocked by a metalloprotease inhibitor (Figure 6C). This timescale of GPVI attenuation and the potency of GPRP to block convulxin-insensitivity was fully consistent with the known dynamics of fibrin polymerization, which typically requires <5–10 minutes. Several prior studies have demonstrated minimal soluble GPVI release over 60 min exposure of PRP to tissue factor (200 nM peak thrombin) or to thrombin (1 U/ml), both of which generate fibrin in PRP [40,41]. Thrombin alone does not release soluble GPVI from washed platelets [42], fully consistent with an absence of convulxin-insensitivity following strong thrombin stimulation of washed platelets (Supplemental Figure S4). Even strong agonists like collagen, convulxin, or Factor Xa typically require 60 min for full release of soluble GPVI. The exposure of washed platelets to one of the most potent inducers of shedding, Factor Xa, did not phenocopy the kinetics or severity of GPVI-signaling defects (Supplementary Figure S9) that we observed with soluble fibrin generation. Thus, the observed rapid and acute attenuation of convulxin-sensitivity is highly unlikely to be caused by fibrin-induced GPVI shedding, which is also fully consistent with the lack of effect of GM6001 (Figure 6C). GM6001 is a known inhibitor of ADAM-mediated GPVI shedding [40–43]. Platelet GPVI shedding is regulated primarily by ADAM10 [44], and may also be facilitated by other members of the a-disintegrin-and-metalloproteinase family (such as ADAM17) [45]. The GM6001 result supports the conclusion that soluble fibrin polymer blocks other more potent ligands from binding GPVI, an effect not requiring GPVI shedding. Since fibrin in clots is known to bind GPVI, soluble fibrin is likely serving as a receptor antagonist to sterically block other ligands from binding. The observed data do not prove mechanism, but are most consistent with (1) steric hindrance of GPVI by soluble fibrin species bound to the receptor and/or (2) soluble fibrin-triggered GPVI signaling that desensitizes the receptor. The observations were not consistent with GPVI shedding, Gαq-dependent GPVI attenuation, or αIIbβ3-mediated GPVI attenuation as mechanisms of the observed phenotype.
Distinct from the role of intrathrombus generation of fibrin, low levels of non-gelling, soluble fibrin may function differently in the context of trauma to cause an acquired GPVI-signaling defect in the systemic circulation. Generation of low and transient levels of thrombin in coagulopathic blood may generate circulating soluble fibrin able to bind platelet GPVI to cause platelet insensitivity to stronger GPVI agonists such as collagen. Soluble fibrin is a long-lived species once it is generated, so platelet transfusion therapies for high-risk trauma patients may become affected by these pre-existing species in the systemic circulation. If transfused platelets display an acquired deficiency in GPVI-signaling in trauma patients with elevated soluble fibrin, the extraordinary hemostatic demands essential for survival may not be fully achieved.
Supplementary Material
ESSENTIALS.
Collagen and thrombin when used simultaneously generate highly activated platelets.
The effect of thrombin stimulation on subsequent glycoprotein VI (GPVI) function was observed.
Soluble fibrin, but not protease activated receptor (PAR) activation, prevented GPVI activation.
Circulating soluble fibrin in coagulopathic blood may cause an acquired GPVI signaling defect.
Acknowledgments
This study was supported by NIH R01 HL103419 (S.L.D.), 5T32HL007954-15 (B.A.H.), 5T32HL007954-18 (C.C.V), and NIH UM1 HL120877 (TACTIC Consortium), and U01 HL131053 (S.L.D.)
Footnotes
Contributions: M. Y. Lee, C. C. Verni, B. A. Herbig, and S. L. Diamond designed research, analyzed and interpreted the data, and wrote the manuscript; M. Y. Lee, C. C. Verni, and B. A. Herbig performed the research.
Conflict-of-interest disclosure: No conflicts to disclose.
References
- 1.Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y, Mackway-Jones K, Parr MJ, Rizoli SB, Yukioka T, Hoyt DB, Bouillon B. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65:748–54. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 2.Ostrowski SR, Johansson PI. Endothelial glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. J Trauma Acute Care Surg. 2012;73:60–66. doi: 10.1097/TA.0b013e31825b5c10. [DOI] [PubMed] [Google Scholar]
- 3.Lenz A, Franklin GA, Cheadle WG. Systemic inflammation after trauma. Injury. 2007;38:1336–45. doi: 10.1016/j.injury.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Boldt J, Papsdorf M, Rothe A, Kumle B, Piper S. Changes of the hemostatic network in critically ill patients--is there a difference between sepsis, trauma, and neurosurgery patients? Crit Care Med. 2000;28:445–50. doi: 10.1097/00003246-200002000-00026. [DOI] [PubMed] [Google Scholar]
- 5.Conti A, Sanchez-Ruiz Y, Bachi A, Beretta L, Grandi E, Beltramo M, Alessio M. Proteome study of human cerebrospinal fluid following traumatic brain injury indicates fibrin(ogen) degradation products as trauma-associated markers. J Neurotrauma. 2004;21:854–63. doi: 10.1089/0897715041526212. [DOI] [PubMed] [Google Scholar]
- 6.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–30. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 7.Ramsey MT, Fabian TC, Shahan CP, Sharpe JP, Mabry SE, Weinberg JA, Croce MA, Jennings LK. A prospective study of platelet function in trauma patients. J Trauma Acute Care Surg. 2016;80:726–33. doi: 10.1097/TA.0000000000001017. [DOI] [PubMed] [Google Scholar]
- 8.Iversen LH, Thorlacius-Ussing O, Okholm M. Soluble fibrin in plasma before and after surgery for benign and malignant colorectal disease. Thromb Res. 1995;79:471–81. doi: 10.1016/0049-3848(95)00137-g. [DOI] [PubMed] [Google Scholar]
- 9.Yanagida Y, Gando S, Sawamura A, Hayakawa M, Uegaki S, Kubota N, Homma T, Ono Y, Honma Y, Wada T, Jesmin S. Normal prothrombinase activity, increased systemic thrombin activity, and lower antithrombin levels in patients with disseminated intravascular coagulation at an early phase of trauma: comparison with acute coagulopathy of trauma-shock. Surgery. 2013;154:48–57. doi: 10.1016/j.surg.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Gando S, Nanzaki S, Sasaki S, Kemmotsu O. Significant correlations between tissue factor and thrombin markers in trauma and septic patients with disseminated intravascular coagulation. Thromb Haemost. 1998;79:1111–5. [PubMed] [Google Scholar]
- 11.Sakai H, Nishihara H, Kakemizu M, Imai M, Igarashi K, Okazaki A. Discrepancy between soluble fibrin and D-dimer levels among sampling sites in elderly patients with femoral neck fracture. J Anesth. 2009;23:308–9. doi: 10.1007/s00540-008-0731-2. [DOI] [PubMed] [Google Scholar]
- 12.Toh JMH, Ken-Dror G, Downey C, Abrams ST. The clinical utility of fibrin-related biomarkers in sepsis. Blood Coagul Fibrinolysis. 2013;24:839–43. doi: 10.1097/MBC.0b013e3283646659. [DOI] [PubMed] [Google Scholar]
- 13.Giannitsis E, Siemens HJ, Mitusch R, Tettenborn I, Wiegand U, Schmucker G, Sheikhzadeh A, Stierle U. Prothrombin fragments F1+2, thrombin-antithrombin III complexes, fibrin monomers and fibrinogen in patients with coronary atherosclerosis. Int J Cardiol. 1999;68:269–74. doi: 10.1016/s0167-5273(98)00256-3. [DOI] [PubMed] [Google Scholar]
- 14.Westerlund E, Woodhams BJ, Eintrei J, Söderblom L, Antovic JP. The evaluation of two automated soluble fibrin assays for use in the routine hospital laboratory. Int J Lab Hematol. 2013;35:666–71. doi: 10.1111/ijlh.12117. [DOI] [PubMed] [Google Scholar]
- 15.Hosaka A, Miyata T, Aramoto H, Shigematsu H, Nakazawa T, Okamoto H, Shigematsu K, Nagawa H. Clinical implication of plasma level of soluble fibrin monomer-fibrinogen complex in patients with abdominal aortic aneurysm. J Vasc Surg. 2005;42:200–5. doi: 10.1016/j.jvs.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 16.Hayakawa M, Gando S, Ono Y, Wada T, Yanagida Y, Sawamura A, Ieko M. Noble-Collip drum trauma induces disseminated intravascular coagulation but not acute coagulopathy of trauma-shock. Shock. 2015;43:261–7. doi: 10.1097/SHK.0000000000000281. [DOI] [PubMed] [Google Scholar]
- 17.Wohlauer MV, Moore EE, Thomas S, Sauaia A, Evans E, Harr J, Silliman CS, Ploplis V, Castellino FJ, Walsh M. Early platelet dysfunction: an unrecognized role in the acute coagulopathy of trauma. J Am Coll Surg. 2012;214:739–46. doi: 10.1016/j.jamcollsurg.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis PK, Musunuru H, Walsh M, Cassady R, Yount R, Losiniecki A, Moore EE, Wohlauer MV, Howard J, Ploplis VA, Castellino FJ, Thomas SG. Platelet dysfunction is an early marker for traumatic brain injury-induced coagulopathy. Neurocrit Care. 2013;18:201–8. doi: 10.1007/s12028-012-9745-6. [DOI] [PubMed] [Google Scholar]
- 19.Kutcher ME, Redick BJ, McCreery RC, Crane IM, Greenberg MD, Cachola LM, Nelson MF, Cohen MJ. Characterization of platelet dysfunction after trauma. J Trauma Acute Care Surg. 2012;73:13–9. doi: 10.1097/TA.0b013e318256deab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li R, Elmongy H, Sims C, Diamond SL. Ex vivo recapitulation of trauma-induced coagulopathy and preliminary assessment of trauma patient platelet function under flow using microfluidic technology. J Trauma Acute Care Surg. 2016;80:440–9. doi: 10.1097/TA.0000000000000915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alshehri OM, Hughes CE, Montague S, Watson SK, Frampton J, Bender M, Watson SP. Fibrin activates GPVI in human and mouse platelets. Blood. 2015;126:1601–8. doi: 10.1182/blood-2015-04-641654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dütting S, Bender M, Nieswandt B. Platelet GPVI: a target for antithrombotic therapy?! Trends Pharmacol Sci. 2012;33:583–90. doi: 10.1016/j.tips.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Bültmann A, Li Z, Wagner S, Peluso M, Schönberger T, Weis C, Konrad I, Stellos K, Massberg S, Nieswandt B, Gawaz M, Ungerer M, Münch G. Impact of glycoprotein VI and platelet adhesion on atherosclerosis-A possible role of fibronectin. J Mol Cell Cardiol. 2010;49:532–42. doi: 10.1016/j.yjmcc.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Schönberger T, Ziegler M, Borst O, Konrad I, Nieswandt B, Massberg S, Ochmann C, Jürgens T, Seizer P, Langer H, Münch G, Ungerer M, Preissner KT, Elvers M, Gawaz M. The dimeric platelet collagen receptor GPVI-Fc reduces platelet adhesion to activated endothelium and preserves myocardial function after transient ischemia in mice. Am J Physiol Cell Physiol. 2012;303:C757–66. doi: 10.1152/ajpcell.00060.2012. [DOI] [PubMed] [Google Scholar]
- 25.Inoue O, Suzuki-Inoue K, McCarty OJT, Moroi M, Ruggeri ZM, Kunicki TJ, Ozaki Y, Watson SP. Laminin stimulates spreading of platelets through integrin alpha6beta1-dependent activation of GPVI. Blood. 2006;107:1405–12. doi: 10.1182/blood-2005-06-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mammadova-Bach E, Ollivier V, Loyau S, Schaff M, Dumont B, Favier R, Freyburger G, Latger-Cannard V, Nieswandt B, Gachet C, Mangin PH, Jandrot-Perrus M. Platelet glycoprotein VI binds to polymerized fibrin and promotes thrombin generation. Blood. 2015;126:683–91. doi: 10.1182/blood-2015-02-629717. [DOI] [PubMed] [Google Scholar]
- 27.Nieswandt B, Pleines I, Bender M. Platelet adhesion and activation mechanisms in arterial thrombosis and ischaemic stroke. J Thromb Haemost. 2011;9:92–104. doi: 10.1111/j.1538-7836.2011.04361.x. [DOI] [PubMed] [Google Scholar]
- 28.Bredbacka S, Edner G. Soluble fibrin and d-dimer as detectors of hypercoagulability in patients with isolated brain trauma. J Neurosurg Anesthesiol. 1994;6:75–82. doi: 10.1097/00008506-199404000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Scherer RU, Spangenberg P. Procoagulant activity in patients with isolated severe head trauma. Crit Care Med. 1998;26:149–56. doi: 10.1097/00003246-199801000-00031. [DOI] [PubMed] [Google Scholar]
- 30.Wada H, Sakuragawa N, Shiku H. Hemostatic molecular markers before onset of disseminated intravascular coagulation in leukemic patients. Semin Thromb Hemost. 1998;24:293–7. doi: 10.1055/s-2007-995857. [DOI] [PubMed] [Google Scholar]
- 31.Lee MY, Diamond SL. A human platelet calcium calculator trained by pairwise agonist scanning. PLoS Comput Biol. 2015;11:1–24. doi: 10.1371/journal.pcbi.1004118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maloney SF, Brass LF, Diamond SL. P2Y12 or P2Y1 inhibitors reduce platelet deposition in a microfluidic model of thrombosis while apyrase lacks efficacy under flow conditions. Integr Biol. 2010;2:183–92. doi: 10.1039/b919728a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arthur JF, Shen Y, Kahn ML, Berndt MC, Andrews RK, Gardiner EE. Ligand binding rapidly induces disulfide-dependent dimerization of glycoprotein VI on the platelet plasma membrane. J Biol Chem. 2007;282:30434–41. doi: 10.1074/jbc.M701330200. [DOI] [PubMed] [Google Scholar]
- 34.Jung SM, Moroi M, Soejima K, Nakagaki T, Miura Y, Berndt MC, Gardiner EE, Howes JM, Pugh N, Bihan D, Watson SP, Farndale RW. Constitutive dimerization of glycoprotein VI (GPVI) in resting platelets is essential for binding to collagen and activation in flowing blood. J Biol Chem. 2012;287:30000–13. doi: 10.1074/jbc.M112.359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stalker TJ, Traxler EA, Wu J, Wannemacher KM, Cermignano SL, Voronov R, Diamond SL, Brass LF. Hierarchical organization in the hemostatic response and its relationship to the platelet signaling network. Blood. 2013;121:1875–85. doi: 10.1182/blood-2012-09-457739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arai M, Yamamoto N, Moroi M, Akamatsu N, Fukutake K, Tanoue K. Platelets with 10% of the normal amount of glycoprotein VI have an impaired response to collagen that results in a mild bleeding tendency. Br J Haematol. 1995;89:124–30. doi: 10.1111/j.1365-2141.1995.tb08900.x. [DOI] [PubMed] [Google Scholar]
- 37.Bynagari-Settipalli YS, Cornelissen I, Palmer D, Duong D, Concengco C, Ware J, Coughlin SR. Redundancy and interaction of thrombin- and collagen-mediated platelet activation in tail bleeding and carotid thrombosis in mice. Arterioscler Thromb Vasc Biol. 2014;34:2563–9. doi: 10.1161/ATVBAHA.114.304244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Onselaer M-B, Hardy AT, Wilson C, Sanchez X, Babar AK, Miller JLC, Watson CN, Watson SK, Bonna A, Philippou H, Herr AB, Mezzano D, Ariens RAS, Watson SP. Fibrin and D-dimer bind to monomeric GPVI. Blood Advances. 2017;1:1495–504. doi: 10.1182/bloodadvances.2017007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu Y, Brown AC, Myers DR, Sakurai Y, Mannino RG, Tran R, Ahn B, Hardy ET, Kee MF, Kumar S, Bao G, Barker TH, Lam WA. Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation. Proc Natl Acad Sci USA. 2014;111:14430–5. doi: 10.1073/pnas.1322917111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Tamimi M, Grigoriadis G, Tran H, Paul E, Servadei P, Berndt MC, Gardiner EE, Andrews RK. Coagulation-induced shedding of platelet glycoprotein VI mediated by factor Xa. Blood. 2011;117:3912–21. doi: 10.1182/blood-2010-08-301523. [DOI] [PubMed] [Google Scholar]
- 41.Gardiner EE, Karunakaran D, Shen Y, Arthur JF, Andrews RK, Berndt MC. Controlled shedding of platelet glycoprotein (GP)VI and GPIb-IX-V by ADAM family metalloproteinases. J Thromb Haemost. 2007;5:1530–7. doi: 10.1111/j.1538-7836.2007.02590.x. [DOI] [PubMed] [Google Scholar]
- 42.Bergmeier W, Rabie T, Strehl A, Piffath CL, Prostredna M, Wagner DD, Nieswandt B. GPVI down-regulation in murine platelets through metalloproteinase-dependent shedding. Thromb Haemost. 2004;91:951–8. doi: 10.1160/TH03-12-0795. [DOI] [PubMed] [Google Scholar]
- 43.Stephens G, Yan Y, Jandrot-Perrus M, Villeval JL, Clemetson KJ, Phillips DR. Platelet activation induces metalloproteinase-dependent GP VI cleavage to down-regulate platelet reactivity to collagen. Blood. 2005;105:186–91. doi: 10.1182/blood-2004-07-2842. [DOI] [PubMed] [Google Scholar]
- 44.Facey A, Pinar I, Arthur JF, Qiao J, Jing J, Mado B, Carberry J, Andrews RK, Gardiner EE. A-Disintegrin-And-Metalloproteinase (ADAM) 10 activity on resting and activated platelets. Biochemistry. 2016;55:1187–94. doi: 10.1021/acs.biochem.5b01102. [DOI] [PubMed] [Google Scholar]
- 45.Bender M, Hofmann S, Stegner D, Chalaris A, Bösl M, Braun A, Scheller J, Rose-John S, Nieswandt B. Differentially regulated GPVI ectodomain shedding by multiple platelet-expressed proteinases. Blood. 2010;116:3347–55. doi: 10.1182/blood-2010-06-289108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.