Abstract
Purpose
To characterize the epidemiology of fluid overload and its association with mortality and duration of extracorporeal membrane oxygenation (ECMO) in children treated with ECMO.
Design
Retrospective cohort study.
Setting
Six tertiary children’s hospital intensive care units.
Patients
756 children < 18 years of age treated with ECMO for ≥ 24 hours from January 1, 2007 to December 31, 2011.
Results
Overall survival to ECMO decannulation and hospital discharge was 74.9% (n=566) and 57.7% (n=436), respectively. Median fluid overload at ECMO initiation was 8.8% (IQR 0.3, 19.2) and it differed between hospital survivors and non-survival, though not between ECMO survivors and non-survivors. Median peak fluid overload on ECMO was 30.9% (IQR 15.4, 54.8). During ECMO, 84.8% had a peak fluid overload ≥ 10%; 67.2% of patients had a peak fluid overload of ≥ 20% and 29% of patients had a peak fluid overload of ≥ 50%. The median peak fluid overload was lower in patients who survived ECMO (27.2% vs. 44.4%, p<0.0001) and survived to hospital discharge (24.8% vs. 43.3%, p<0.0001). After adjusting for acute kidney injury, pH at ECMO initiation, non-renal complications, ECMO mode, support type, centre and patient age, the degree of fluid overload at ECMO initiation (p =0.05) and the peak fluid overload on ECMO (p<0.0001) predicted duration of ECMO in survivors. Multivariable analysis showed that peak fluid overload on ECMO (aOR 1.09, 95% CI 1.04–1.15) predicted mortality on ECMO; fluid overload at ECMO initiation (aOR 1.13, 95% CI 1.05–1.22) and peak fluid overload (aOR 1.18, 95% CI 1.12–1.24) both predicted hospital morality.
Conclusions
Fluid overload occurs commonly and is independently associated with adverse outcomes including increased mortality and increased duration of ECMO in a broad pediatric ECMO population. These results suggest that fluid overload is a potential target for intervention to improve outcomes in children on ECMO.
Keywords: Fluid Overload, Pediatric, Extracorporeal Membrane Oxygenation, Extracorporeal Life Support, Acute Kidney Injury
Introduction
Extracorporeal membrane oxygenation (ECMO) provides short-term life-saving therapy for pediatric patients with severe refractory cardiac and respiratory failure. Multiple studies have shown that the development of acute kidney injury (AKI) during ECMO is common in children and is associated with increased mortality (1–8).
Fluid overload (FO) is an important complication of AKI. In children treated with continuous renal replacement therapy (CRRT) (4, 9–13), children undergoing cardiac surgery (14–16), and non-cardiac surgery children admitted to the intensive care unit (ICU) (17, 18), FO has been shown to be associated with adverse outcomes including ICU mortality, prolonged length of stay and worse oxygenation. Children treated with ECMO are typically the most critically ill patients in the ICU and are likely to be at high risk for FO. Several single-centre studies in children and neonates treated with ECMO have suggested an association between FO and adverse outcomes, including duration of ECMO and mortality (6, 19–21). Given the consistent finding of the negative effect of FO on outcomes in patients treated with ECMO, FO may represent a clinically important target for interventions (such as CRRT) to improve patient outcomes. However, the extent to which the FO-outcome associations are independent of or modified by the presence of AKI remains unclear. It is thus important to understand the temporal patterns of FO during ECMO at clinically relevant time points (e.g., ECMO initiation or discontinuation; when during ECMO treatment that FO is most severe) in order to understand the most critical potential FO intervention targets. Moreover, there is a need for a large multi-centre evaluation of the FO-outcome association to enable generalizability of findings and control for other factors associated with outcomes.
The Kidney Interventions During Extracorporeal Membrane Oxygenation (KIDMO) study group represents six centres in the United States and Canada established to study the epidemiology and impact of AKI, FO, and renal support therapy (RST) in children during ECMO (22). With the overarching hypothesis that FO in children supported with ECMO is associated with higher morbidity and mortality, the aim of this study was to characterize FO in the broadest pediatric ECMO patient population reported to date. We sought to determine the independent associations of FO at ECMO initiation and peak FO during ECMO on survival and duration of ECMO. In addition, we report the temporal pattern of FO, including the timing of peak FO and change in FO during ECMO.
Methods
Study Population
This study is a retrospective observational cohort study of pediatric patients on ECMO at 6 centres from January 1, 2007 to December 31, 2011. All patients < 18 years of age at the time of ECMO initiation were included. Patients with multiple independent ECMO treatment episodes (ECMO runs) during a hospitalization were excluded from analysis. Patients for whom data from the retrospective chart review could not be matched with data from the Extracorporeal Life Support Organization (ELSO) international registry and those with inadequate fluid balance data were also excluded. A detailed description of the inclusion/exclusion criteria and study population are outlined in Supplemental Figure 1. Investigational Review Board or Research Ethics Board approval for data collection and study was obtained and maintained at each individual participating centre. Waivers were obtained for patient consent at each site.
Data Collection
Data sources for this study included the ELSO Registry (ELSO, Ann Arbor Michigan) and data obtained retrospectively from individuals’ medical records. Data from the ELSO registry was merged with the retrospective database, utilizing a 4-point matching scheme (ELSO ID, date of birth, date of ECMO initiation, and date of ECMO discontinuation) as previously reported (1). Data collected from the ELSO registry included: a) demographic/baseline: age at ECMO initiation (categorized as neonates (0 to 30 days) vs. pediatric (31 days to 18 years)), weight at hospital admission, gender, centre of ECMO support; b) Pre-ECMO variables: serum pH, vasoactive medications, oxygenation index; c) ECMO variables: indication, support mode, duration of ECMO, and number of non-renal complications (1). Medical charts were reviewed for AKI and FO-specific variables including all serum creatinine (SCr) values, RST, detailed FO data before, during, and at ECMO discontinuation (Table 1 and 2).
Table 1.
Baseline patient characteristics overall and by hospital survival status
| Variable | Overall | In Hospital Mortality | p-value | |
|---|---|---|---|---|
| N=756 | Yes (N =320) | No (N =436) | ||
| Age (days) | 10 (2, 221.5) | 10 (3, 144) | 9 (1, 342.5) | 0.70 |
| Neonate (Age < 30 days) | 450 (59.5) | 202 (63.1) | 248 (56.9) | 0.08 |
| Pediatric | 306 (40.5) | 118 (36.9) | 188 (43.1) | |
| Female | 313 (41.6) | 134 (41.9) | 179 (41.1) | 0.85 |
| ICU Admission Weight | 3.5 (3, 7) | 3.3 (2.8, 4.9) | 3.8 (3.1, 8.5) | <.0001 |
| AKI during ECMO | 571 (75.8) | 278 (86.9) | 293 (67.2) | <.0001 |
| Renal Replacement Therapy | 380 (50.4) | 213 (66.5) | 167 (38.3) | <.0001 |
| ECMO Mode | ||||
| VA ECMO | 540 (71.4) | 279 (87.2) | 261 (59.9) | <.0001 |
| VV ECMO | 206 (27.3) | 39 (12.2) | 167 (38.3) | |
| Other | 10 (1.3) | 2 (0.6) | 8 (1.8) | |
| pH at ECMO initiation (N=706) | 7.20 (7.07, 7.31) | 7.19 (7.05, 7.29) | 7.21 (7.09, 7.32) | 0.02 |
| Oxygenation Index at initiation (N=501) | 44.9 (28, 66.7) | 45.5 (29.3, 69.6) | 44.6 (27.6, 63.2) | 0.42 |
| Inotropes | 663 (87.7) | 288 (90.0) | 375 (86.0) | 0.10 |
| ELSO Non Renal Complication | 672 (88.9) | 310 (96.9) | 362 (83.0) | <.0001 |
| ECMO Indication | ||||
| Pulmonary | 424 (56.1) | 136 (42.5) | 288 (66.1) | <.0001 |
| Cardiac | 197 (26.1) | 110 (34.4) | 87 (20.0) | |
| ECPR | 135 (17.9) | 74 (23.1) | 61 (14.0) | |
| Centre | ||||
| A | 199 (26.3) | 70 (21.9) | 129 (29.6) | <.0001 |
| B | 48 (6.4) | 28 (8.8) | 20 (4.6) | |
| C | 104 (13.8) | 38 (11.9) | 66 (15.1) | |
| D | 174 (23.0) | 92 (28.8) | 82 (18.8) | |
| E | 218 (28.8) | 82 (25.6) | 136 (31.2) | |
| F | 13 (1.7) | 10 (3.1) | 3 (0.7) | |
Continuous variables are expressed as median (interquartile range). Categorical variables are expressed as count (%). Abbreviations: Intensive Care Unit (ICU), Acute Kidney Injury (AKI), Extracorporeal membrane oxygenation (ECMO), Extracorporeal Life Support Organization (ELSO), veno-arterial (VA), veno-venous (VV), Extracorporeal cardiopulmonary resuscitation (ECPR)
Table 2.
Fluid Overload Severity Distribution
| N=756 | ||
|---|---|---|
| Percent Fluid Overload at ECMO Initiation | N | % of Total Patients |
| <10% | 405 | 53.6 |
| 10–19.9% | 169 | 22.4 |
| 20–29.9% | 67 | 8.9 |
| 30–39.9% | 48 | 6.3 |
| 40–49.9% | 25 | 3.3 |
| >=50% | 42 | 5.6 |
| Peak Fluid Overload on ECMO | N | % of Total Patients |
| <10% | 115 | 15.2 |
| 10–19.9% | 133 | 17.6 |
| 20–29.9% | 123 | 16.3 |
| 30–39.9% | 96 | 12.7 |
| 40–49.9% | 70 | 9.3 |
| >=50% | 219 | 29.0 |
All serum creatinine (SCr) values measured before and during ECMO were evaluated. AKI was defined using the SCr criteria of the Kidney Disease Improving Global Outcome (KDIGO) AKI definition with two variations. The Stage 3 criterion of estimated glomerular filtration rate < 35 ml/min/1.73 m2 at any time was not applied, because a large proportion of patients were neonates and as has been previously demonstrated, this criterion cannot be applied equally across patient ages (23). Urine output criteria were not utilized in this study because hourly urine output was not consistently available. Baseline SCr was defined as the lowest SCr in the 3 months prior to ECMO cannulation. In neonates, SCr decreases physiologically in the first week of life. For this reason, AKI in the neonatal patients was adjudicated according to the neonatal modified KDIGO definition by two authors independently (DJA and DTS) (24). In the case of difference in AKI status between the two authors, a third author (MZ) broke the tie.
Fluid Overload
Daily cumulative percent FO was determined using daily intake and output for the 28 days prior to ECMO cannulation and the first 21 days following cannulation (9). Cumulative percent FO was calculated according to previously published definition:
We also determined the cumulative FO at ECMO cannulation and at ECMO discontinuation. We calculated the change in FO between ECMO initiation and discontinuation as follows:
Outcome
The primary outcome was in hospital mortality. ECMO mortality and duration of ECMO served as secondary outcomes. ECMO mortality was defined as death while on ECMO or within 24 hours of ECMO decannulation.
Statistical Methods
Descriptive statistics were presented for the entire cohort and by in-hospital mortality status and mortality at ECMO decannulation. Median and inter-quartile range (IQR) were reported for continuous variables and Wilcoxon rank-sum test was used to test differences between the mortality groups. Frequency counts and percentages were reported for categorical variables and differences between the mortality groups were tested using Chi-square/Fisher’s exact test. Six categories (<10% to ≥50%) each were formed for %FO at ECMO initiation and %peak FO (highest cumulative FO during ECMO) to evaluate the relationship between increasing %FO and mortality. The two independent variables, %FO initiation and %peak FO, were examined as 10% change. Univariate and multivariable logistic regression (adjusting for age, centre, mode, support type, patient complications, and AKI [yes/no] and pH) was used to examine the relations (Odd ratios [OR] and 95% CI) between %FO at ECMO initiation and %peak FO with ECMO mortality and hospital mortality. In these analyses, %FO was rescaled so that each unit increase in the rescaled value represented an absolute 10% increase in FO. The relationship between %FO and duration of ECMO was examined using linear regression. In these analyses, ECMO duration was log transformed because of positive skewness and results were presented after back transforming the log estimates for better interpretability. Two-sided analyses were performed with p≤ 0.05 considered to be statistically significant. All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Patient Characteristics
During the study period 1,009 patients supported with ECMO were identified by study sites. After exclusions, 756 patients were included in this analysis (Supplemental Figure 1). Five hundred and forty (71.4%) patients received venoarterial ECMO and 450 (59.5%) patients were ≤ 1 month of age (median age 10 days (IQR 2, 221.5 days). Characteristics of the study population by hospital mortality are summarized in Tables 1 and by ECMO mortality in Supplemental Table 1. In general, AKI, RST during ECMO and VA ECMO (vs. VV ECMO) were more common in patients who died and patients who died had increased morbidity and illness markers (e.g., complications, lower pH). Mortality also differed by ECMO centre (details in Tables 1 and Supplemental Table 1).
Distribution of Fluid Overload Severity and Temporal Pattern
FO severity distribution during ECMO is presented in Table 2. At ECMO initiation, 46.4% of the cohort had ≥ 10% FO. Median FO at ECMO initiation for the entire cohort was 8.8% (IQR = 0.3, 19.2) (Table 3). During ECMO, 84.8% developed a peak FO ≥ 10%; 67.2% of patients developed a peak FO ≥ 20% and 29% of patients developed a peak FO ≥ 50% (Table 2). The median peak FO on ECMO was 30.9% (IQR 15.4, 54.8) (Table 3). The median day of peak FO attainment on ECMO was day 3 (IQR 1, 8). The median FO at ECMO discontinuation or death was 25.3% (IQR 9.7, 51.2) and median change in FO on ECMO was positive 13.6% (IQR 1.8, 32.4) (Table 3).
Table 3.
Fluid overload among ECMO patients overall and by survival status.
| Variable | Overall | ECMO Mortality | p-value | |
|---|---|---|---|---|
| N = 756 | Yes(N =190) | No(N =566) | ||
| Fluid Overload at ECMO Initiation (%) | 8.8 (0.3, 19.2) | 11.2 (0, 26.6) | 8.2 (1, 17.4) | 0.26 |
| Peak Fluid Overload on ECMO (%) | 30.9 (15.4, 54.8) | 44.4 (21.8, 70) | 27.2 (14.6, 48.9) | <.0001 |
| Day of ECMO of peak Fluid Overload | 3 (1, 8) | 3.5 (1, 11) | 3 (1, 8) | 0.07 |
| Fluid Overload at ECMO Discontinuation/Death(%) | 25.3 (9.7, 51.2) | 41.7 (17.8, 67.9) | 21.9 (8.2, 44.9) | <.0001 |
| Change in Fluid Overload on ECMO | 13.6 (1.8, 32.4) | 21.8 (7.8, 45.0) | 10.3 (0.5, 29.3) | <.0001 |
| Variable | Overall | In Hospital Mortality | p-value | |
| N = 756 | Yes(N = 320) | No(N = 436) | ||
| Fluid Overload at ECMO Initiation (%) | 8.8 (0.3, 19.2) | 11.9 (0, 26.2) | 7.9 (1, 16.0) | 0.04 |
| Peak Fluid Overload on ECMO (%) | 30.9 (15.4, 54.8) | 43.3 (21.7, 72.6) | 24.8 (13.8, 41.4) | <.0001 |
| Day of ECMO of peak Fluid Overload | 3 (1, 8) | 4 (1.5, 12) | 3 (1, 6) | <.0001 |
| Fluid Overload at ECMO Discontinuation/Death (%) | 25.3 (9.7, 51.2) | 40.2 (15, 68.6) | 19.3 (7.5, 38.3) | <.0001 |
| Change in Fluid Overload on ECMO | 13.6 (1.8, 32.4) | 20.7 (5.1, 46.6) | 8.7 (0.2, 25.0) | <.0001 |
| Variable | Overall | Renal Support Therapy | p-value | |
| N = 756 | Yes(N = 380) | No(N = 374) | ||
| Fluid Overload at ECMO Initiation (%) | 8.8 (0.3, 19.2) | 11.3 (0, 24.3) | 7.7 (0.8, 15.7) | 0.02 |
| Peak Fluid Overload on ECMO (%) | 30.9 (15.4,54.8) | 36.1 (18.4,63.8) | 25.2 (13.9, 48.2) | <.0001 |
| Day of ECMO of peak Fluid Overload | 3 (1, 8) | 4 (2, 9) | 3 (1, 8) | 0.01 |
| Fluid Overload at ECMO Discontinuation/Death (%) | 25.3 (9.7, 51.2) | 32.5 (11.4, 56.2) | 20.9 (7.9,46.7) | 0.003 |
| Change in Fluid Overload on ECMO | 13.6 (1.8, 32.4) | 15.7 (3.5 – 34.1) | 10.5 (1.1, 30.3) | 0.02 |
| Variable | Overall | Age Group | p-value | |
| N = 756 | Neonate(N = 450) | Pediatric(N = 306) | ||
| Fluid Overload at ECMO Initiation (%) | 8.8 (0.3, 19.2) | 8.2 (0, 18.1) | 9.4 (2.1,22.4) | 0.09 |
| Peak Fluid Overload on ECMO (%) | 30.9 (15.4,54.8) | 35.3 (18.8, 60.1) | 26.3 (11.9, 49.8) | 0.0003 |
| Day of ECMO of peak Fluid Overload | 3 (1, 8) | 3 (1, 8) | 3 (1, 8) | 0.81 |
| Fluid Overload at ECMO Discontinuation/Death (%) | 25.3 (9.7, 51.2) | 27.7 (10.1, 54.3) | 23.2 (8.4, 46.6) | 0.09 |
| Change in Fluid Overload on ECMO | 13.6 (1.8, 32.4) | 16.6 (1.8, 39.3) | 10.6 (1.8, 24.3) | 0.004 |
Variables are expressed as median (interquartile range)
The characteristics of FO were examined separately in two distinct populations including those that received RST (N=380) and the neonatal population (N=450) and are also presented in Table 3. Those that received RST differed significantly from those that did not in each of the FO characteristics examined including a higher degree of FO at ECMO initiation (p=0.02), higher peak FO on ECMO (p<0.0001), and later date of peak FO (p=0.01). The neonatal population only differed significantly from pediatric patients by having a higher peak FO on ECMO (p=0.0003) and a greater change in FO on ECMO (p=0.004).
Association of Fluid Overload and Mortality
Survival to ECMO decannulation and hospital discharge was 74.9% (n=566) and 57.8% (n=436), respectively. The associations of different measures of FO with mortality are shown in Table 3. Peak FO, FO at ECMO discontinuation and FO change during ECMO were all higher in patients who died on ECMO. FO at ECMO initiation was not associated with ECMO mortality. FO at ECMO initiation, peak FO, FO at ECMO discontinuation and change in FO on ECMO were all significantly higher in patients who died during hospitalization (Table 3). Figures 1a and 1b show that in general there was a graded increase in both ECMO and hospital mortality with increasing FO (by 10% intervals). There was a somewhat sharp increase in mortality at the 20% ECMO initiation FO severity stratum; the inflection point for increased mortality for peak FO during ECMO was at 30% peak FO (Figures 1a and 1b).
Figure 1.
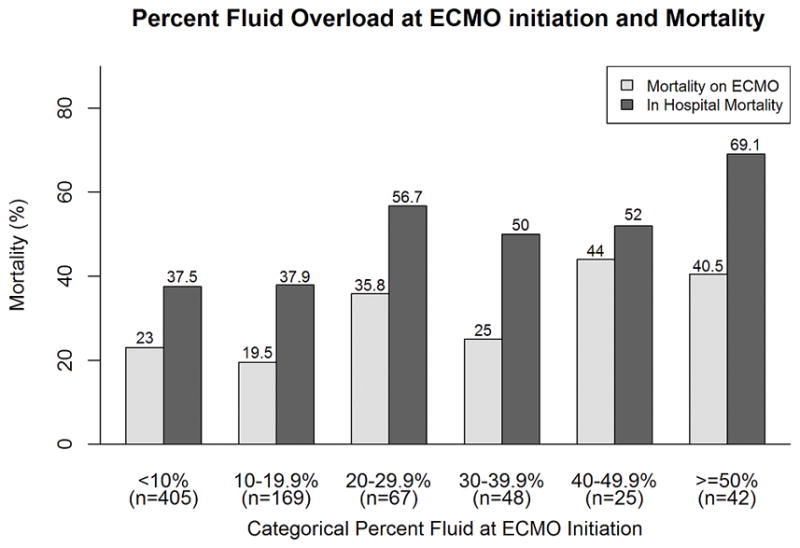
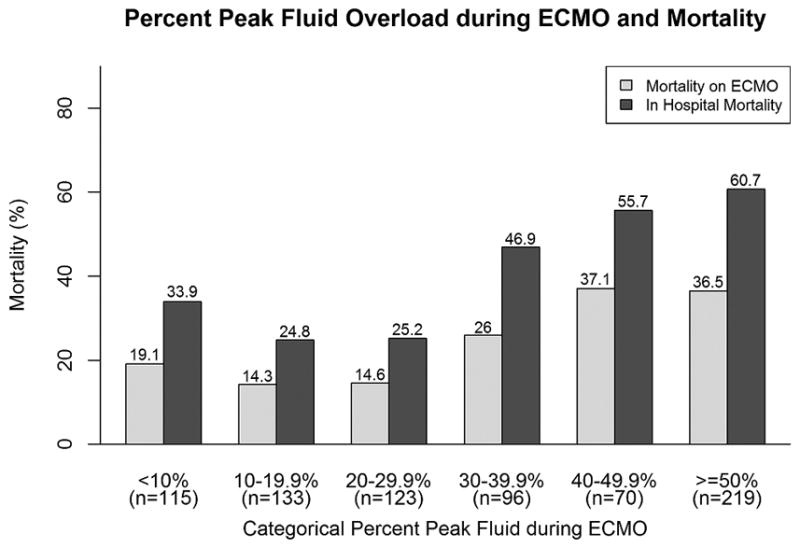
Association of increasing fluid overload and mortality.
Histograms depicting the relationship between increasing 10% fluid overload categories (x-axes; a) % fluid overload at ECMO initiation; b) % peak fluid overload during ECMO) and mortality (y-axes; dark bars ECMO mortality, light bars hospital mortality). Numbers above the bars are the proportions of patients who died within that category.
Abbreviations: ECMO: extracorporeal membrane oxygenation; FO: fluid overload.
When the degree of FO was examined as a dichotomous variable, a FO level of greater than 20% at ECMO initiation was associated with increased ECMO mortality (35.2% vs. 22%, p= 0.0003) and increased hospital mortality (57.1% vs. 37.6%, p= 0.0001). Similarly, a peak FO level of greater than 30% during ECMO was associated with increased ECMO mortality (34% vs. 15.9%, p<0.0001) and increased hospital mortality (56.4% vs. 27.8%, p<0.0001).
Univariate logistic regression analysis showed that each 10% rise in FO at ECMO initiation was associated with significantly higher hospital mortality risk (OR 1.12, 95% CI 1.04, 1.19, p = 0.002), but not ECMO mortality (OR 1.06, 95% CI 0.99, 1.13, p = 0.11). The peak FO on ECMO was associated with both increased ECMO mortality (OR 1.10, 95% CI 1.05, 1.14, p < 0.001) and hospital mortality (OR 1.16, 95% CI 1.11, 1.22, p < 0.001). In multivariable analyses (controlled for presence of AKI, pH at ECMO initiation, non-renal complications, ECMO mode, support type, centre and age; shown in Table 4), FO at ECMO initiation was associated with increased hospital mortality but not with ECMO mortality. As shown in Table 4, Peak FO during ECMO was associated with both ECMO mortality (each 10% rise in peak FO was associated with a 9% increased odds of ECMO mortality) and hospital mortality (each 10% rise in peak FO associated with a 17% increased odds of hospital mortality), independent of other variables. Of note, in all multivariable models, the presence of AKI more than doubled the odds of both ECMO and hospital mortality (Table 4). Examining neonatal and pediatric populations separately, the multivariable results in each population were similar to those of the whole cohort (Supplemental Table 2).
Table 4.
Multivariable analysis of association between fluid overload at ECMO initiation and peak fluid overload, with ECMO and hospital mortality.
| Outcome | FO at ECMO Initiation* |
Pediatric** | ECMO Mode (Other)*** |
ECMO Mode VV *** |
Support Type: Cardiac**** |
Support Type: ECPR**** |
Non-Renal Patient Complications |
pH at initiation | AKI |
|---|---|---|---|---|---|---|---|---|---|
| ECMO Mortality | 1.04 (0.97,1.11) | 0.93 (0.62,1.39) | 0.65 (0.13,3.34) | 0.3 (0.16,0.56) ## | 1.27 (0.76,2.14) | 1.47 (0.86,2.51) | 6.07 (1.99,18.50) ## | 0.12 (0.04,0.35) ## | 2.4 (1.29,4.46) # |
| Hospital Mortality | 1.13 (1.05,1.22) ## | 0.52 (0.36,0.76) ## | 0.21 (0.04,1.06) | 0.26 (0.16,0.43) ## | 1.27(0.79,2.05) | 1.22(0.74,2.01) | 6.27 (2.78,14.14) ## | 0.31 (0.12,0.79) # | 2.6 (1.59,4.27) ## |
| Peak FO on ECMO* | Pediatric** | ECMO Mode (Other)*** | ECMO Mode VV *** | Support Type: Cardiac**** | Support Type: ECPR**** | Non-Renal Patient Complications | pH at initiation | AKI | |
| ECMO Mortality | 1.09 (1.04,1.15) ## | 0.99 (0.66,1.49) | 0.7 (0.13, 3.68) | 0.32 (0.17, 0.6) ## | 1.42 (0.84,2.42) | 1.7 (0.99,2.94) | 5.98 (1.89,18.88) ## | 0.13 (0.05,0.38) ## | 2.24 (1.2,4.2) # |
| Hospital Mortality | 1.18 (1.12,1.24) ## | 0.59 (0.4,0.86) # | 0.23 (0.04,1.25) | 0.27 (0.16,0.45) ## | 1.55 (0.94,2.53) | 1.57 (0.93, 2.63) | 5.8 (2.45,13.71) ## | 0.38 (0.14,1.00) | 2.47 (1.48,4.11) ## |
All models adjusted for centre in addition; all data presented as Odds Ratio (95% Confidence interval)
Examines per 10% rise in FO
Reference neonates;
Reference VA ECMO,
Reference Respiratory
p<0.05;
p<0.005
In order to begin to evaluate the importance of fluid balance on ECMO the same multivariable models were evaluated replacing the FO term with a dichotomous term evaluating those with a positive fluid balance on ECMO. In these multivariable analyses (controlled for presence of AKI, pH at ECMO initiation, non-renal complications, ECMO mode, support type, centre and age), a positive fluid balance on ECMO was associated with increased ECMO mortality (aOR 2.94, 95% CI 1.66, 5.22, p= 0.0002) and hospital mortality (aOR 2.28, 95% CI 1.44, 3.63, p= 0.001).
An analysis evaluating the outcomes in the 79 patients who were excluded after ELSO matching showed that those that were excluded had significantly higher ECMO mortality (47.4% vs 25.1%, p<0.0001) and hospital mortality (65.8% vs. 42.3%, p<0.0001).
Association Between Fluid Overload and ECMO Duration
The median duration of ECMO was 147 hours (IQR 84, 262.5). The median duration of ECMO in survivors was 143.5 hours (IQR 88, 241). In order to evaluate the impact of FO on duration of ECMO only ECMO survivors were included in the analysis (N=566). In multivariable analysis (Table 5), both FO at ECMO initiation and Peak FO were associated with longer ECMO duration (hours), independent of other factors. Specifically, each 10% rise in FO at ECMO initiation was associated with a 2% relative increase in the number of hours on ECMO (p=0.05); each 10% rise in peak FO on ECMO was associated with a 6% relative increase in the number of hours on ECMO (p<0.0001) (Table 5). Examining neonatal and pediatric populations separately the results in each population did not differ meaningfully from the whole cohort (Supplemental Table 3).
Table 5.
Linear Regression Models Predicting Duration of ECMO in ECMO survivors (N=566)
| Fluid Overload at ECMO Initiation | |||
|---|---|---|---|
| Variable | Relative Change | CI | p-value |
| Fluid Overload at ECMO Initiation (%)* | 1.02 | 1.00,1.05 | 0.05 |
| Pediatric** | 1.00 | 0.89,1.13 | 0.94 |
| ECMO Mode (Other)*** | 0.82 | 0.52,1.3 | 0.4 |
| Mode VV *** | 0.79 | 0.68, 0.91 | 0.001 |
| Support type: Cardiac**** | 0.53 | 0.45,0.63 | <0.0001 |
| Support type: ECPR**** | 0.45 | 0.37,0.54 | <.0001 |
| Patient Complications | 1.44 | 1.21, 1.77 | <0.0001 |
| pH at initiation | 1.05 | 0.77, 1.43 | 0.76 |
| AKI | 1.42 | 1.22, 1.65 | <0.0001 |
| Peak Fluid Overload on ECMO | |||
| Variable | Relative Change | CI | p-value |
| Peak Fluid Overload on ECMO (%)* | 1.06 | 1.05, 1.08 | <0.0001 |
| Pediatric** | 1.05 | 0.93, 1.17 | 0.45 |
| ECMO Mode (Other)*** | 0.9 | 0.58, 1.4 | 0.65 |
| Mode VV *** | 0.81 | 0.71, 0.93 | 0.002 |
| Support type: Cardiac**** | 0.58 | 0.49,0.68 | <0.0001 |
| Support type: ECPR**** | 0.51 | 0.43,0.60 | <0.0001 |
| Patient Complications | 1.35 | 1.14, 1.60 | 0.001 |
| pH at initiation | 1.03 | 0.77, 1.39 | 0.82 |
| AKI | 1.47 | 1.29, 1.68 | <0.0001 |
All models adjusted for centre in addition;
Examines per 10% rise in FO
Reference neonates,
Reference VA ECMO,
Reference Support type: Pulmonary
Discussion
This study is the first comprehensive multi-centre evaluation of the temporal and severity patterns of FO and FO association with patient outcomes in children treated with ECMO. Our study shows that severe FO commonly occurs in children on ECMO and that worse FO during ECMO is associated with mortality and ECMO duration, independent of other factors, including the presence of AKI.
In a variety of adult and pediatric patient populations FO has been shown to be associated with adverse outcomes including prolonged mechanical ventilation, increased length of stay, and increased mortality. Swaniker et al first demonstrated the importance of FO in 128 children treated with ECMO for respiratory failure. This group showed that 41% of patients had significant FO (>10% above their dry weight) at ECMO initiation (19). The physiology of FO in children on ECMO was investigated using radio-labeled isotopes, showing an elevation in both the total body water and extracellular fluid (25). In neonates treated with ECMO for pulmonary hypertension, the degree of FO and improvement were key determinants of ECMO duration (26). In the two decades since these publications, there have been few publications describing the epidemiology of FO in children on ECMO. We found that almost half of ECMO-treated children have ≥10% FO at ECMO initiation, strongly suggesting that future studies should evaluate factors leading to FO and interventions to reduce pre-ECMO FO. Peak FO on ECMO was very high in our cohort with almost all attaining peak FO ≥10% and over half with peak FO ≥ 30%. Considering that in non-ECMO children in the ICU ≥10% FO has been suggested as an intervention threshold (27), and that ≥20% FO has been repeatedly been shown to be associated with poor outcomes (11), this level of FO in patients on ECMO is concerning and may benefit from intervention. While no FO management guidelines exist for ECMO, the Extracorporeal Life Support Organization makes the following statement about fluid status in ECMO, “the goal of fluid management is to return the extracellular volume to normal (dry weight) and maintain it there” (ELSO, 2016). Future research should now evaluate potential interventions to reduce FO on ECMO (such as diuresis, early initiation of RST) to determine the extent to which FO improvement on ECMO is feasible, and hopefully associated with better outcomes.
The independent contribution of FO to adverse outcomes has become clear across a variety of patient populations (3, 4, 9, 11–15, 17, 28–30). The impact of FO on outcomes in children has been robustly demonstrated in children undergoing CRRT, where the degree of FO at CRRT initiation has been consistently shown to be associated with increased mortality (3, 4, 9, 11–13). The impact of FO on outcomes has since demonstrated in a number of other critically ill populations including term neonates, children with sepsis, those undergoing cardiac surgery, and mechanically ventilated children (14, 15, 17, 28–30). Most studies on FO in ECMO patients have been single-centre. Recently, Selewski et al demonstrated an association between FO at CRRT initiation and increased mortality in 53 pediatric ECMO patients (3). To date there has not been a systematic evaluation of the impact of FO on outcomes in a general pediatric ECMO population. We found that the peak FO during ECMO is a significant predictor of ECMO mortality and in-hospital mortality. Furthermore, the degree of peak FO is associated with significant increases in duration of ECMO. This is first study to demonstrate the impact of FO at ECMO initiation and during ECMO on mortality in an inclusive pediatric ECMO patient population. Our data raise important questions about FO as a target for therapeutic intervention and provides a fundamental building block describing the epidemiology of FO.
Early in the history of ECMO, the potential impact of FO change during ECMO on outcomes in children was recognized. In 2000 Swaniker et al showed that FO decreased from 9 to 4% from ECMO initiation to discontinuation (respectively) in ECMO survivors, versus an increase in FO from 25 to 35% in non-survivors (19). A recent adult study by Schmidt et al echoed these findings, showing that survivors had a lower fluid balance on ECMO day 3–5 (21). The current study shows that the change in FO is associated with mortality. Importantly, we found that over 75% of patients had a positive fluid balance while on ECMO, suggesting that FO and the ability to achieve a negative fluid balance are potentially important therapeutic targets. Furthermore, we have also shown on multivariable analysis that the ability to achieve a negative fluid balance on ECMO is associated with improved survival. This raises the possibility of utilizing CRRT as a potential intervention in patients on ECMO, with FO as an indication for CRRT initiation (3, 4, 19, 26). CRRT provides flexibility and control in fluid management, and has been shown to enhance the ability to achieve dry weight and negative fluid balance during ECMO (31, 32). Our current study shows that children that received RST had higher degrees of peak FO on ECMO and a later peak FO, which may be interpreted as a need for earlier intervention. Taken together with the epidemiology of FO and its independent association with adverse outcomes in this study, these results suggest that a trial utilizing CRRT to manage fluid in children on ECMO may be warranted. The current study provides data from which a trial on fluid management during ECMO may be designed.
The biggest strengths of this study is the multi-centre approach that captured ample sample size to explore the impact of confounders in the association with outcomes. Despite these strengths, we acknowledge several limitations. Efforts were made to adjust for severity of illness, but the dataset did not have the data points necessary to calculate recently published severity of illness scores. Another limitation was that we were unable to merge approximately 20% of our identified centre patients who received ECMO with the ELSO data. Also patients that were excluded after matching had higher mortality than the study cohort potentially biasing our results to lower mortality. Another limitation is that data was not available describing which patients were post-operative cardiac surgery patients, which prevented their separate evaluation. This study also did not note the types of fluids (colloid vs crystalloid vs blood products) that patients received, which may potentially impact patient outcomes independent of fluid amount. The authors also acknowledge the limitations of solely utilizing serum creatinine (without urine output data) to calculate AKI, which is not only a delayed marker of AKI, but may be confounded by the presence of fluid overload. Although our study is multi-centre, generalizability may be limited for centres performing very little ECMO or with general ICU practice patterns differing substantially from our study centres. Within our own cohort, outcomes differed across centres; however due to the retrospective nature of this study, it was not possible to evaluate the effect of individual physician practice differences, which may also impact the FO-outcome association. Further study is warranted to identify centre specific practices and characteristics that impact outcomes, with a goal of standardizing fluid management practice, developing guidelines and evaluating impact of centre-variability reduction of improving patient outcomes.
Conclusion
In this five year retrospective multi-centre cohort study we show that FO occurs commonly in children on ECMO. Furthermore, we demonstrate the association between FO and adverse outcomes including increased mortality and increased length of ECMO in a broad pediatric ECMO population. The results of this study suggest that FO is a potential target for intervention to improve outcomes in children on ECMO.
Supplementary Material
Supplemental Figure 1. Flow diagram of subject inclusion, exclusion and database merging.
Abbreviations: ELSO: Extracorporeal Life Support Organization; ECMO: extracorporeal membrane oxygenation.
Acknowledgments
Funding Support: RedCap is supported by UL1 TR000445 from NACTS/NIH. Dr. Askenazi receives funding from the NIH (R01 DK13608-01) and the Pediatric and Infant Center for Acute Nephrology (PICAN), which is sponsored by Children’s of Alabama and the University of Alabama at Birmingham (UAB) School of Medicine, as well as by the Department of Pediatrics, and Center for Clinical and Translational Science (CCTS) under award number UL1TR00165. MZ receives research salary support from the Fonds de Recherche de Québec-Santé (FRQ-S).
Elaine Cooley (University of Michigan), Heart Institute Research Core (CCHMC)
Footnotes
Copyright form disclosure: Dr. Askenazi received funding from Baxter and AKI Foundation; he disclosed off-label product use in that acute CRRT is not FDA approved in the US for children <20 kg with any device. Dr. Fleming’s institution received funding from the National Center for Advancing Translational Sciences/National Institutes of Health (NIH); he received funding from Society of Critical Care Medicine (faculty at Pediatric Board Review Course July 2016); and he received support for article research from the NIH. Dr. Paden disclosed off-label product use in that all ECMO and CRRT use is off label in children. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Conflict of Interest: The authors report no conflict of interest with funding support for RedCap. Dr. Askenazi reports no conflict of interest with funding support as these sources were not used directly in the execution of this study.
Bibliography
- 1.Fleming GM, Sahay R, Zappitelli M, et al. The Incidence of Acute Kidney Injury and Its Effect on Neonatal and Pediatric Extracorporeal Membrane Oxygenation Outcomes: A Multicenter Report From the Kidney Intervention During Extracorporeal Membrane Oxygenation Study Group. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2016 doi: 10.1097/PCC.0000000000000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zwiers AJ, de Wildt SN, Hop WC, et al. Acute kidney injury is a frequent complication in critically ill neonates receiving extracorporeal membrane oxygenation: a 14-year cohort study. Critical care. 2013;17(4):R151. doi: 10.1186/cc12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selewski DT, Cornell TT, Blatt NB, et al. Fluid overload and fluid removal in pediatric patients on extracorporeal membrane oxygenation requiring continuous renal replacement therapy. Crit Care Med. 2012;40(9):2694–2699. doi: 10.1097/CCM.0b013e318258ff01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selewski DT, Cornell TT, Lombel RM, et al. Weight-based determination of fluid overload status and mortality in pediatric intensive care unit patients requiring continuous renal replacement therapy. Intensive Care Med. 2011;37(7):1166–1173. doi: 10.1007/s00134-011-2231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gadepalli SK, Selewski DT, Drongowski RA, et al. Acute kidney injury in congenital diaphragmatic hernia requiring extracorporeal life support: an insidious problem. J Pediatr Surg. 2011;46(4):630–635. doi: 10.1016/j.jpedsurg.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 6.Weber TR, Connors RH, Tracy TF, Jr, et al. Prognostic determinants in extracorporeal membrane oxygenation for respiratory failure in newborns. Ann Thorac Surg. 1990;50(5):720–723. doi: 10.1016/0003-4975(90)90669-w. [DOI] [PubMed] [Google Scholar]
- 7.Smith AH, Hardison DC, Worden CR, et al. Acute renal failure during extracorporeal support in the pediatric cardiac patient. ASAIO J. 2009;55(4):412–416. doi: 10.1097/MAT.0b013e31819ca3d0. [DOI] [PubMed] [Google Scholar]
- 8.Askenazi DJ, Ambalavanan N, Hamilton K, et al. Acute kidney injury and renal replacement therapy independently predict mortality in neonatal and pediatric noncardiac patients on extracorporeal membrane oxygenation. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2011;12(1):e1–6. doi: 10.1097/PCC.0b013e3181d8e348. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein SL, Currier H, Graf C, et al. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics. 2001;107(6):1309–1312. doi: 10.1542/peds.107.6.1309. [DOI] [PubMed] [Google Scholar]
- 10.Foland JA, Fortenberry JD, Warshaw BL, et al. Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med. 2004;32(8):1771–1776. doi: 10.1097/01.ccm.0000132897.52737.49. [DOI] [PubMed] [Google Scholar]
- 11.Sutherland SM, Zappitelli M, Alexander SR, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010;55(2):316–325. doi: 10.1053/j.ajkd.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein SL, Somers MJ, Baum MA, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 2005;67(2):653–658. doi: 10.1111/j.1523-1755.2005.67121.x. [DOI] [PubMed] [Google Scholar]
- 13.Hayes LW, Oster RA, Tofil NM, et al. Outcomes of critically ill children requiring continuous renal replacement therapy. J Crit Care. 2009;24(3):394–400. doi: 10.1016/j.jcrc.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Seguin J, Albright B, Vertullo L, et al. Extent, risk factors, and outcome of fluid overload after pediatric heart surgery*. Crit Care Med. 2014;42(12):2591–2599. doi: 10.1097/CCM.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 15.Hassinger AB, Wald EL, Goodman DM. Early postoperative fluid overload precedes acute kidney injury and is associated with higher morbidity in pediatric cardiac surgery patients. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2014;15(2):131–138. doi: 10.1097/PCC.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 16.Hazle MA, Gajarski RJ, Yu S, et al. Fluid overload in infants following congenital heart surgery. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2013;14(1):44–49. doi: 10.1097/PCC.0b013e3182712799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flori HR, Church G, Liu KD, et al. Positive fluid balance is associated with higher mortality and prolonged mechanical ventilation in pediatric patients with acute lung injury. Critical care research and practice. 2011;2011:854142. doi: 10.1155/2011/854142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arikan AA, Zappitelli M, Goldstein SL, et al. Fluid overload is associated with impaired oxygenation and morbidity in critically ill children. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2012;13(3):253–258. doi: 10.1097/PCC.0b013e31822882a3. [DOI] [PubMed] [Google Scholar]
- 19.Swaniker F, Kolla S, Moler F, et al. Extracorporeal life support outcome for 128 pediatric patients with respiratory failure. J Pediatr Surg. 2000;35(2):197–202. doi: 10.1016/s0022-3468(00)90009-5. [DOI] [PubMed] [Google Scholar]
- 20.Heiss KF, Pettit B, Hirschl RB, et al. Renal insufficiency and volume overload in neonatal ECMO managed by continuous ultrafiltration. ASAIO Trans. 1987;33(3):557–560. [PubMed] [Google Scholar]
- 21.Schmidt M, Bailey M, Kelly J, et al. Impact of fluid balance on outcome of adult patients treated with extracorporeal membrane oxygenation. Intensive Care Med. 2014;40(9):1256–1266. doi: 10.1007/s00134-014-3360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Askenazi DJ, Selewski DT, Paden ML, et al. Renal replacement therapy in critically ill patients receiving extracorporeal membrane oxygenation. Clinical journal of the American Society of Nephrology : CJASN. 2012;7(8):1328–1336. doi: 10.2215/CJN.12731211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Work DF, Schwartz GJ. Estimating and measuring glomerular filtration rate in children. Curr Opin Nephrol Hypertens. 2008;17(3):320–325. doi: 10.1097/MNH.0b013e3282fb77f2. [DOI] [PubMed] [Google Scholar]
- 24.Jetton JG, Askenazi DJ. Update on acute kidney injury in the neonate. Curr Opin Pediatr. 2012;24(2):191–196. doi: 10.1097/MOP.0b013e32834f62d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson HL, 3rd, Coran AG, Drongowski RA, et al. Extracellular fluid and total body water changes in neonates undergoing extracorporeal membrane oxygenation. J Pediatr Surg. 1992;27(8):1003–1007. doi: 10.1016/0022-3468(92)90547-k. discussion 1007–1008. [DOI] [PubMed] [Google Scholar]
- 26.Kelly RE, Jr, Phillips JD, Foglia RP, et al. Pulmonary edema and fluid mobilization as determinants of the duration of ECMO support. J Pediatr Surg. 1991;26(9):1016–1022. doi: 10.1016/0022-3468(91)90665-g. [DOI] [PubMed] [Google Scholar]
- 27.Brierley J, Carcillo JA, Choong K, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37(2):666–688. doi: 10.1097/CCM.0b013e31819323c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Askenazi DJ, Koralkar R, Hundley HE, et al. Fluid overload and mortality are associated with acute kidney injury in sick near-term/term neonate. Pediatric nephrology. 2013;28(4):661–666. doi: 10.1007/s00467-012-2369-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhaskar P, Dhar AV, Thompson M, et al. Early fluid accumulation in children with shock and ICU mortality: a matched case-control study. Intensive Care Med. 2015;41(8):1445–1453. doi: 10.1007/s00134-015-3851-9. [DOI] [PubMed] [Google Scholar]
- 30.Valentine SL, Sapru A, Higgerson RA, et al. Fluid balance in critically ill children with acute lung injury. Crit Care Med. 2012;40(10):2883–2889. doi: 10.1097/CCM.0b013e31825bc54d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoover NG, Heard M, Reid C, et al. Enhanced fluid management with continuous venovenous hemofiltration in pediatric respiratory failure patients receiving extracorporeal membrane oxygenation support. Intensive Care Med. 2008;34(12):2241–2247. doi: 10.1007/s00134-008-1200-y. [DOI] [PubMed] [Google Scholar]
- 32.Sell LL, Cullen ML, Whittlesey GC, et al. Experience with renal failure during extracorporeal membrane oxygenation: treatment with continuous hemofiltration. J Pediatr Surg. 1987;22(7):600–602. doi: 10.1016/s0022-3468(87)80107-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Flow diagram of subject inclusion, exclusion and database merging.
Abbreviations: ELSO: Extracorporeal Life Support Organization; ECMO: extracorporeal membrane oxygenation.


