Abstract
Objective
To investigate whether treatment with anti-VEGF (vascular endothelial growth factor) neutralizing antibodies can reduce pain and voiding dysfunction in the cyclophosphamide (CYP) cystitis model of bladder pain in mice.
Materials and Methods
Adult female mice received anti-VEGF neutralizing antibodies (10 mg/kg intraperitoneal B20-4.1.1 VEGF mAb) or saline (control) pre-treatment, followed by CYP (150 mg/kg intraperitoneal) to induce acute cystitis. Pelvic nociceptive responses were assessed by applying von Frey filaments to the pelvic area. Spontaneous micturition was assessed using the void spot assay.
Results
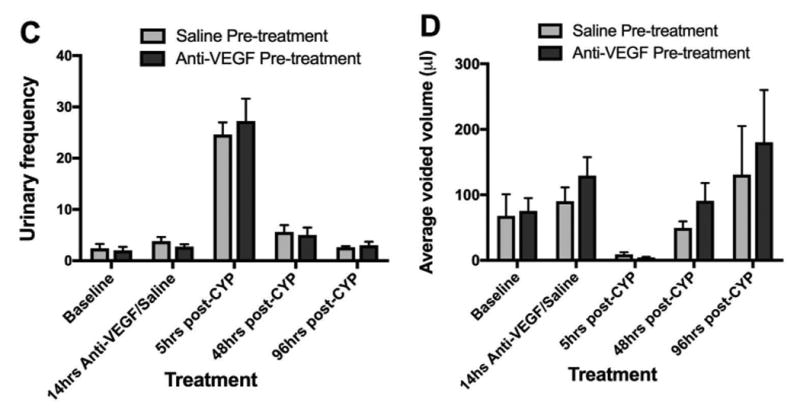
Systemic anti-VEGF neutralizing antibodies treatment significantly reduced the pelvic nociceptive response to CYP cystitis compared to control (saline). In the anti-VEGF pre-treatment group, there was a significant increase in pelvic hypersensitivity measured by the area under the curve (AUC) with von Frey filaments at 5 hours post-CYP (p=0.0035). However by 48 and 96 hours post-CYP, the pelvic hypersensitivity have reduced by 54% and 47% respectively compared to the 5 hours post-CYP time point, and were no longer significantly different from the baseline (p=0.22 and 0.17 respectively). There was no difference in urinary frequency and mean voided volume between the two pre-treatment groups.
Conclusion
Systemic blockade of VEGF signaling with anti-VEGF neutralizing antibodies was effective in reducing pelvic/bladder pain in the CYP cystitis model of bladder pain. Our data support the further investigation of the use of anti-VEGF antibodies to manage bladder pain or visceral pain.
Keywords: bladder pain, interstitial cystitis, vascular endothelial growth factor, anti-VEGF, cyclophosphamide cystitis
Introduction
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a clinical syndrome characterized by pain, pressure, discomfort perceived to be related to the urinary bladder, associated with lower urinary tract symptoms, in the absence of infection of other identifiable causes.(1) Our understanding of the etiology and pathophysiology of IC/BPS is poor; treatments are often ineffective and lack durable response over time. An objective of the MAPP Research Network animal studies is to study mechanistic pathways and investigate potential therapeutic targets using animal models of pelvic pain.(2) This paper described our findings on anti-VEGF neutralizing antibodies.
Vascular endothelial growth factor (VEGF) is a pleiotropic cytokine traditionally known for its angiogenic activity.(3) Recent clinical studies showed that bladder and urinary VEGF expression is elevated among IC/BPS patients,(4-6) and the bladder and urinary expression levels correlated to the severity of pain.(6, 7) VEGF expression was reduced after onabotulinumtoxinA (Botox) injection into the bladder and improvement of the pain.(8) The potential link between VEGF and bladder pain is further supported by animal studies. Instillation of VEGF into the bladder lumen resulted in the development of pelvic hypersensitivity in mice.(9) Up-regulation of VEGF in the bladder has also been observed in animals with cyclophosphamide cystitis.(10) Taken together, these clinical and basic studies suggest that VEGF signaling might play a role in modulating bladder pain and bladder function. In this study, we shall test the hypothesis that treatment with anti-VEGF neutralizing antibodies can reduce the pain and voiding dysfunction in the cyclophosphamide cystitis model of bladder pain in mice.
Materials and Methods
Animals
Adult female C57BL/6J mice (8-10 weeks, 18–23 g, Jackson Laboratory, Bar Harbor, ME) were bred in the animal housing facility. Animals were maintained on a 12-hour light/dark cycle with free access to food and water. All experiments were performed in accordance with the guidelines of the NIH were approved by the Washington University Animal Care and Use Committee.
Anti-VEGF or saline pre-treatment followed by CYP injection
Mice were divided into 2 groups. One group (n=15) was pre-treated with intraperitoneal anti-VEGF antibodies (10 mg/kg i.p. B20-4.1.1 VEGF mAb, provided courtesy of Genentech, South San Francisco, CA) while the other group (n=12) received saline pre-treatment (same volume and same time as anti-VEGF). A single intraperitoneal injection of cyclophosphamide (CYP 150 mg/kg i.p. from Sigma Aldrich, St. Louis, MO) was then administered 36 hours later to induce CYP cystitis. Behavioral assessments were performed at baseline, 14 hours after anti-VEGF or saline (control) injection, 5 hours, 48 hours and 96 hours after CYP administration. All behavioral testing was performed by observers blind to treatment. Additional functional and structural information of the B20-4 anti-VEGF neutralizing antibodies was described in Fuh et al (2006).(11)
Behavioral tests
Pelvic nociceptive response (referred mechanical allodynia) using von Frey filaments
Mice were placed individually in Plexiglas cubicles on a wire grid on a raised platform in a room with white noise emitted from a noise generator. Mice were allowed to acclimate to the environment for a minimum of 30 min before behavioral testing. Mechanical stimulation was applied vertically to the lower abdominal/pelvic area close to the bladder, using 4 graduated von Frey filaments with strengths of 0.02g, 0.08g, 0.32g, and 1.28g (North Coast Medical, San Jose, CA), in ascending order of strength. Each filament was applied for 1-2 seconds, for a total of 10 times, at intervals of 5-10 seconds apart. Different areas within this suprapubic region were stimulated to avoid desensitization or “wind-up”. Withdrawal or retraction of the lower abdominal/pelvic area from the filament stimulation was considered a positive nociceptive response. Data were expressed as the number of positive response out of 10 stimulations with each filament. To access the changes in withdrawal response to the whole range of filaments, the area under the curve (AUC) was also calculated for each animal and the average AUC for each treatment group was calculated. The AUC was calculated using the area of the trapezoid (the sum of the rectangle and triangle) between the points for each filament as previously described.(12) For this study, we used referred hypersensitivity instead of the visceromotor response(13) to test for pelvic hypersensitivity since the visceromotor response does not permit repeated measurements of the same animal over time.
Spontaneous micturition assessment using void spot assays (VSA)
Urinary frequency and mean voided volume were studied using the void spot assay (VSA).(14) Since C56BL/6J mice tend to urinate repeatedly in the corner of rectangular cages,(14) we modified the VSA technique to a circular area. Individual mice were gently removed from standard polycarbonate communal cages and placed into a circular mouse metabolic cage with precut circular-shaped filter paper (Fisher Scientific, Pittsburgh, PA, catalog no. 05-714-4) taped to the bottom. Mice were provided with standard dry mouse chow for the duration of the assay, but water was withheld during the testing period because water dripping from the bottle was found to impair the integrity of the filter paper and altered urine spot dimensions. The micturition cages were kept in a quiet area for 2 hours. Placement of mice into and removal from cages was done calmly to avoid startling the animal and causing reflex urination. At the end of the 2 hours, the mouse was returned to normal housing, the filter paper was retrieved from the cage, and urine spots on the filter paper were imaged using ultraviolet light on a trans illuminator (Fig 1A). Since urinary frequency of C57BL/6J mice varies with respect to the time of day (e.g., increased frequency at night),(14) we performed the VSA experiments at approximately the same time every afternoon between 1 and 5 PM.
Fig. 1.
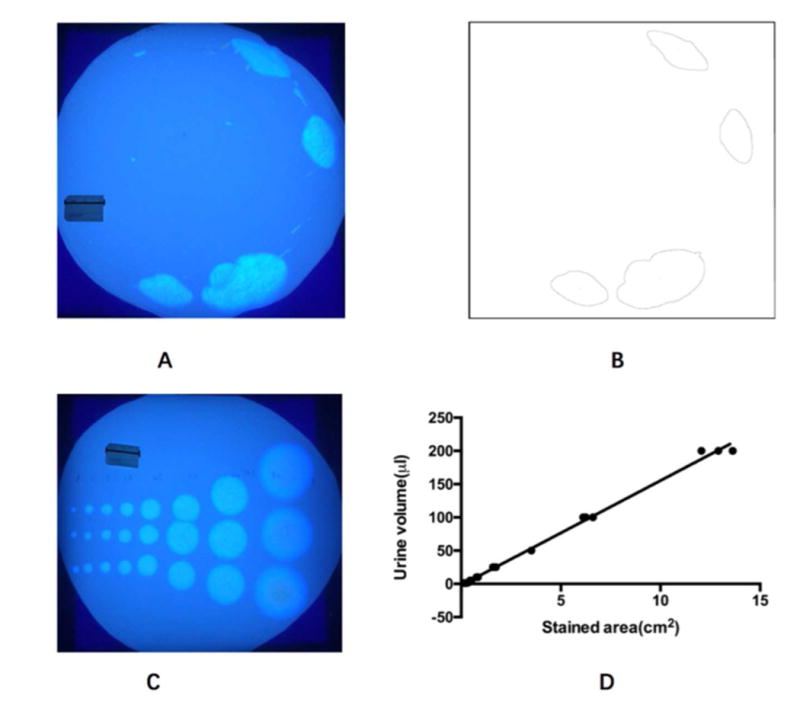
Void spot assay (VSA) was used to record spontaneous micturition. A: photographic image of a urine-stained filter paper on an ultraviolet illuminator (Scale ruler: 2.4 cm). B: Imaging processing of A. C: To construct the standard curve, urine was first collected from a mouse. Urine in different volumes (1, 2, 5, 10, 25, 50, 100 and 200 μl) were pipetted onto filter paper repeated in triplicate (Scale plate: 2.4 cm). D: There was a linear correlation between liquid volume and stained area on the filter paper up to a volume of 200 μL (R2 = 0.9962). The formula Y (volume) = 15.75*X (spot area) - 2.238 was subsequently used to calculate the area of each individual void on the filter paper.
Image analysis of VSA data
Images were analyzed using the Fiji version of ImageJ software. Scale was determined from images using a scale ruler (2.4 cm long, see Fig 1A). The global option was selected, and images were converted to a stack, inverted, auto-thresholded, and converted to binary. Thresholded images in the stack were inspected for artifacts and were individually corrected if thresholding had omitted areas of urine or highlighted tape marks (Fig 1B). Using the “analyze particles” function, excluding all particles with area less than 0.08 cm2 (which corresponded to 0.5 μL of urine) faithfully captured urine spots. This threshold area was chosen to exclude small bright spots or “particles” that might be from claw marks, tooth marks, footprints or tail dragging, as previously described by Yu et al (2014).(14) Urinary frequency was defined as the number of urine spots identified on a filter over the 2 hour duration of testing. Urinary frequency data were quantified by two independent observers and averaged. Micturition volumes were determined using an area-to-volume standard curve as shown in Fig 1C & 1D. We used VSA instead of awake cystometry for this study since VSA permits noninvasive and repeated measurement of spontaneous micturition of the same animal over time and does not involve surgical implantation of a catheter or the development of bladder inflammation. The VSA was used instead of metabolic cages because in our experience mice with CYP-induced cystitis void at very small volumes (≤10 μL) that are often below the level of detection of a standard mouse metabolic cage.
Statistical analysis
The results are expressed as mean ± SEM. Analyses were performed using Prism version 6 (GraphPad, San Diego, CA). Repeated measure one-way ANOVA was used to compare the 5 time points within each treatment group (Fig 2B, 2D). p-values were adjusted for multiple comparison using the Tukey's test. Multiple t-test comparison was used to compare anti-VEGF versus saline pre-treatment at each individual time point (Fig 2E, 3C, 3D), p-values were corrected for multiple comparisons using the Holm-Sidak method. p<0.05 was considered statistically significant.
Fig. 2.
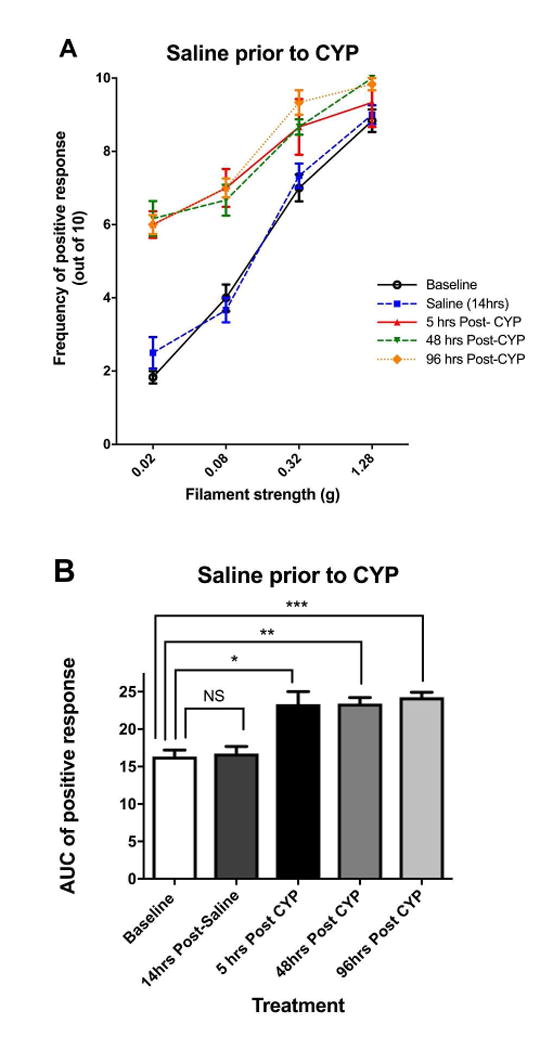
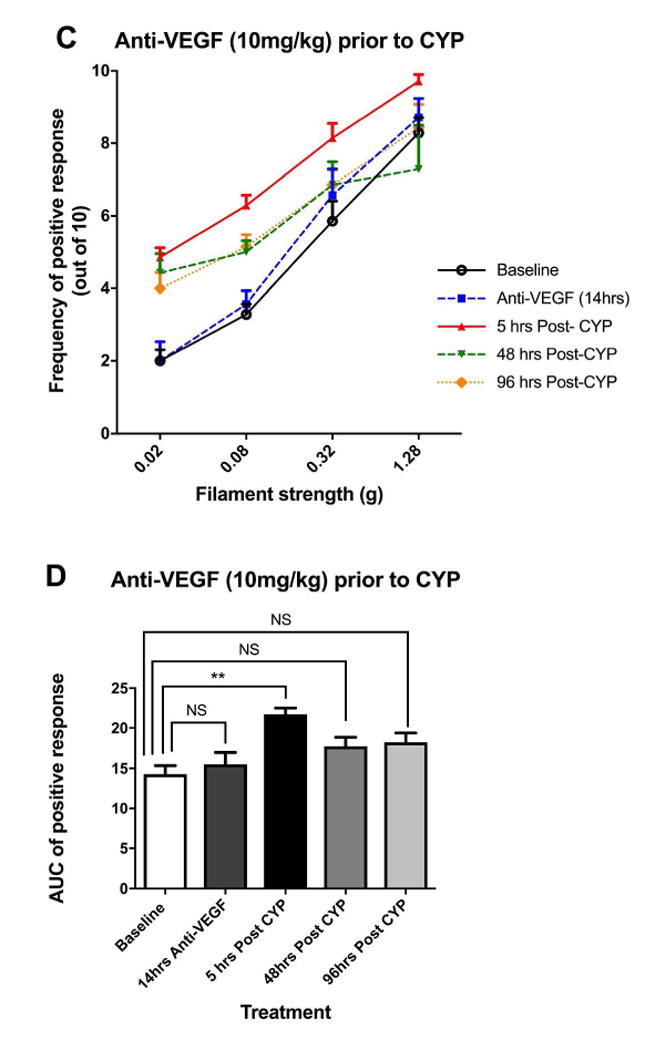
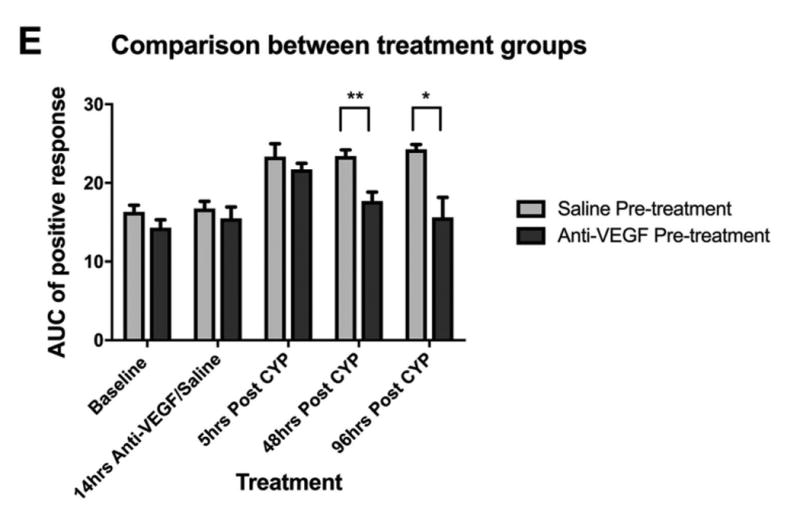
Effects of anti-VEGF pre-treatment on cyclophosphamide (CYP)-induced pelvic pain. A: Effect of CYP (150 mg/kg i.p.) on referred pelvic allodynia assessed using von Frey filaments (n=6). Note that the saline injected prior to CYP was used as the control arm of anti-VEGF pre-treatment. B: The area under the curve (AUC) of A. C: Effect of anti-VEGF pre-treatment (10 mg/kg i.p.) followed by CYP (150 mg/kg i.p.) on pelvic nociceptive response (n=7). D: The area under the curve (AUC) of C. E: Comparison of AUC between anti-VEGF and saline pre-treatment groups at various time points. Anti-VEGF pre-treatment was effective in reducing pelvic pain 48 and 96 hours after CYP compared to saline pre-treatment. *p<0.05, **p<0.005, ***p<0.001.
Fig. 3.
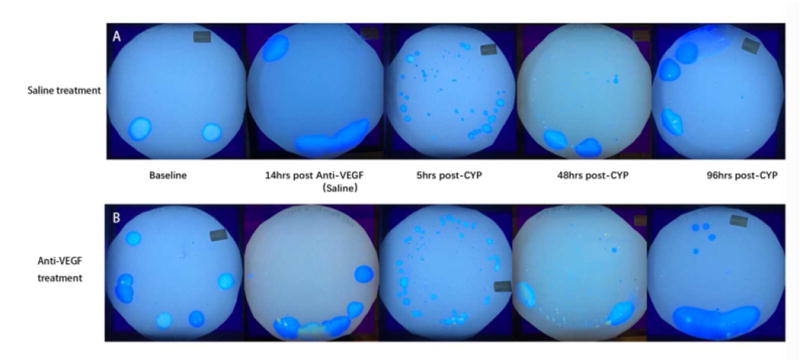
Effects of anti-VEGF pre-treatment on cyclophosphamide (CYP)-induced changes in spontaneous micturition behavior. A: Sample VAS blots from a saline pre-treated mouse. B: Sample blots from an anti-VEGF pre-treatment mouse. C: Comparison of urinary frequency with anti-VEGF (10 mg/kg) pre-treatment versus saline pre-treatment, prior to CYP (150 mg/kg i.p.) administration. Data over the course of two hours were plotted for each time-point. D: Comparison of mean voided volume. There was no difference between the saline (n=6) and anti-VEGF (n=8) pre-treatment groups at the various time points (p>0.05 in all comparison).
Results
Cyclophosphamide (CYP) Cystitis Induced Pelvic Hypersensitivity
Fig. 2A and 2B plotted the stimulus-response curve to von Frey filaments and the area under the curve (AUC) in the saline pre-treatment (control) experiments (n=6). A single intraperitoneal (i.p.) administration of CYP induced referred mechanical allodynia in the suprapubic area at 5 hours, 48 hours, and 96 hours post-CYP (Fig. 2A). The pelvic hypersensitivity was reflected as a significant increase in the area under the curve (AUC) in Fig. 2B at 5 hours, 48 hours, and 96 hours compared to the baseline (p=0.026, 0.0031, and 0.0003 respectively). There was no attenuation of AUC from 5 to 48 and 96 hours post-CYP (p>0.05).
Anti-VEGF Pre-treatment Reduced CYP-Induced Pelvic Hypersensitivity
Fig. 2C and 2D plotted the stimulus-response curve and the AUC in the anti-VEGF pre-treatment experiments (n=7). Anti-VEGF itself has no impact on pelvic hypersensitivity (no difference in AUC from baseline, p=0.89, Fig 2D). Compared to baseline, there was a significant increase in AUC at 5 hours post-CYP (p=0.0035). However by 48 and 96 hours post-CYP, the pelvic hypersensitivity have reduced by 54% and 47% respectively compared to the 5 hours post-CYP time point, and were no longer significantly different from the baseline (p=0.22 for the 48 hours vs. baseline comparison; and p=0.17 for 96 hours vs. baseline).
Comparison between the Anti-VEGF and Saline Pre-Treatment Groups
Fig 2E compared the AUC between the two treatment groups at various time points. There were no differences between the groups at baseline and 5 hours post-CYP. However, mice pre-treated with anti-VEGF had significantly less pelvic hypersensitivity at 48 and 96 hours post-CYP compared to mice pre-treated with saline (p=0.0021 at 48 hours, p=0.011 at 96 hours). In conclusion, the pelvic hypersensitivity induced by CYP cystitis was significantly reduced by pre-treatment with anti-VEGF antibodies compared to saline (control).
The Effect of Anti-VEGF on Spontaneous Micturition Behavior
When we compared the effects of anti-VEGF versus saline pre-treatment on spontaneous micturition behavior using the VSA, we found no difference in urinary frequency or mean voided volume between the two groups post-CYP (n=8 for anti-VEGF group, n=6 for saline group, p>0.05, Fig. 3C, 3D). Representative blots are shown in Fig 3A and 3B. Thus, anti-VEGF pre-treatment did not reduce CYP-induced micturition dysfunction.
Discussion
One challenge to the clinical management of IC/BPS is poor understanding of the mechanistic pathways and therapeutic targets involved in pelvic pain. The MAPP Research Network supports animal studies to address this research gap.(2) This pre-clinical study showed that systemic treatment with anti-VEGF antibodies was effective in reducing referred hypersensitivity in the acute CYP cystitis model of bladder pain. Anti-VEGF antibodies have traditionally been studied within the context of anti-angiogenesis and cancer treatment. To our knowledge, this is the first study that demonstrated the potential of anti-VEGF neutralizing antibodies in the treatment of visceral pain in general, and bladder pain specifically. Anti-VEGF monoclonal antibodies (bevacizumab) and antibody derivatives (ranibizumab) are clinically approved and currently available for the treatment of certain human conditions such as advanced cancers and macular degeneration,(15, 16) making these compounds of particular interest for translational research. Our proof-of-concept data support further investigation of the use of anti-VEGF antibodies to manage bladder pain or visceral pain conditions in future studies.
VEGF receptors (VEGF-R1, VEGF-R2) and their co-receptors, the neuropilins (Nrp-1, Nrp-2) are expressed at high levels in the human bladder urothelium.(17) Recent clinical studies showed that tissue and urinary VEGF levels are elevated among patients with IC/BPS, and VEGF levels are positively correlated to the severity of bladder pain in patients.(4, 5, 7, 18) Intravesical instillation of VEGF resulted in an increase in bladder sensory fiber density, up-regulation of voltage-gated sodium channels in lumbosacral dorsal root ganglia, and development of pelvic hypersensitivity in mice.(9) Up-regulation of VEGF in the bladder has also been observed in animals with CYP cystitis.(10) Acrolein, the urinary metabolite of CYP, damages the urothelial integrity and causes chemical cystitis of the bladder. In this study, systemic inhibition of VEGF signaling with anti-VEGF neutralizing antibodies reduced the pelvic hypersensitivity in an inflammatory model of bladder pain in mice. Collectively, these data are consistent with the hypothesis that VEGF signaling plays a mechanistic role in modulating bladder nociception.
Exactly how VEGF modulates bladder pain remains to be elucidated. VEGF is a pleiotropic cytokine traditionally known for its angiogenic activity.(3) VEGF levels are usually elevated at the site of tissue inflammation. VEGF enhances endothelial permeability, vasodilation, tissue edema, and macrophage recruitment, all part of the inflammatory response. VEGF is also involved in the pathogenesis of chronic inflammatory conditions such as atherosclerosis and inflammatory bowel disease.(19, 20) Up-regulation of VEGF-164, VEGF-R2 receptors, and Npn-1, Npn-2 co-receptors have been observed after acute and chronic CYP cystitis.(10)
Besides inflammation, tissue hypoxia is the main physiological stimulus that up-regulates VEGF. Bladder VEGF has been shown to be elevated in an animal model of pelvic ischemia.(21) Prior bladder blood flow studies using endoscopic laser Doppler flowmetry also showed that bladder perfusion decreases with bladder filing in patients with IC/BPS but increases in control subjects without IC/BPS.(22, 23) These findings raise the questions whether IC/BPS might represent a chronic microvascular ischemic condition, and the hypoxia that develops during bladder filling might contribute to visceral pain and VEGF expression in patients.
Other animal studies have also shown that VEGF may modulate pelvic pain by enhancing peripheral neuroplasticity. Intravesical instillation of VEGF has been shown to be associated with increased density of both sensory (TRPV-1) and motor (ChAT) nerve fibers in the bladder, and up-regulation of voltage-gated sodium channels in lumbosacral dorsal root ganglia innervating the bladder.(9)
At this time it is unclear mechanistically how anti-VEGF neutralizing antibodies might modulate pelvic pain. Most of the published literature on anti-VEGF antibodies has focused on anti-angiogenesis and cancer treatment. Surprisingly there is very little literature on the use of anti-VEGF to treat bladder pain or visceral pain. Blockade of VEGF signaling shows some promise to manage inflammatory joint pain. Intra-articular injection of bevacizumab is effective in the treatment of osteoarthritis.(24) Pre-treatment with anti-VEGF (B20) also blunted the chemical injury effects from intravesical bacillus Calmette-Guerin (BCG) instillation.(25) In our study, pre-treatment with anti-VEGF (B20) prior to the CYP administration was effective in reducing the nociceptive response to von Frey filaments stimulation while pre-treatment with saline (control) was not effective.
It is less clear whether VEGF might also play a role in modulating voiding function. In our study, systemic anti-VEGF was not effective in normalizing the increased urinary frequency or increase in small voided volumes that developed after CYP cystitis. However, Malykhina et al (2012) have shown that local instillation of VEGF into the bladder lumen of naïve mice reduced inter-micturition interval and bladder capacity during awake cystometry.(9) In their study, the effects on cystometry were observed after two weeks of intravesical VEGF instillation but not after one week of intravesical VEGF instillation. It is unclear whether the differences between the two studies might be due to different experimental paradigms (local VEGF stimulation versus systemic anti-VEGF inhibition), different models used (naïve mice versus CYP cystitis), different methodologies to measure micturition (cystometry versus spontaneous voiding measured by VSA, see discussion below), different duration of exposure (biweekly VEGF instillation versus a single acute exposure of anti-VEGF), or that VEGF signaling is differentially involved in the bladder pain versus voiding.
The current study has several limitations: (i) It is acknowledged that the CYP cystitis model is not an animal model of IC/BPS, however it is one of the most widely used and best characterized models to study inflammatory bladder pain. The CYP model recapitulates many of the key symptomatology of IC/BPS, for example, pain associated with bladder distention,(13) referred tactile allodynia to the suprapubic area,(26, 27) spontaneous painful behaviors,(26) urinary frequency,(26, 28) and bowel-bladder cross-talk(28) (see reference (2) for more detailed discussion of the validity of the model). We chose this model for our initial proof-of-concept experiments since up-regulation of VEGF signaling has previously been demonstrated in CYP cystitis,(10) thus this it is a reasonable model to examine the effects of anti-VEGF treatment, recognizing that the findings may not translate to the human condition (IC/BPS). In future studies we plan to examine the anti-VEGF effects in other bladder pain models. (ii) Although the VSA method is advantageous to metabolic cages (e.g., sensitive to volumes ≤10 μL) or awake cystometry (e.g., no invasive surgery to implant suprapubic catheter and no bladder inflammation), the VSA method has its own limitations. For example, overlapping voids in the same area of the filter paper may not be quantified correctly, and the VSA does not provide information on bladder pressures. Although a previous study showed that VSA patterns correlated reasonably well to baseline pressure, threshold pressure and intermicturition pressure,(29) caveats in the methodologies (VSA versus cystometry) may explain the differences in micturition results between our study and those from Malykhina et al (2012).(9) (iii) we have not examined the bladder histology or urinary markers of inflammation and hypoxia in the current study. These will be investigated in the future to characterize the histologic and urinary effects of anti-VEGF treatment.
Conclusions
Systemic treatment with anti-VEGF neutralizing antibodies was effective in reducing pelvic/bladder pain in the CYP cystitis model of bladder pain in mice. Our data support further investigation of the use of anti-VEGF antibodies to manage bladder pain or visceral pain in future studies.
Acknowledgments
This study is funded by the National Institutes of Health grants U01-DK-082315 (Multi-Disciplinary Approach to the Study of Chronic Pelvic Pain, MAPP, Research Network) to HL and RG, and K08-DK-094964 to HL. Dr. Shen's visiting scholarship to Washington University was supported by the National Natural Science Foundation of China (grant 81400758). Dr. Zhang's visiting scholarship to Washington University was supported by China Scholarship Council (grant 2013081110286) and Research Fund for the Doctoral Program of Higher Education (grant 20120001120056). We would like to thank Dr. Aaron Mickle and Maria Payne for assistance with the experiments. The anti-VEGF antibodies were provided with courtesy of Genentech Inc.
Abbreviations
- AUC
area under the curve
- CYP
cyclophosphamide
- IC/BPS
interstitial cystitis/bladder pain syndrome
- i.p
intraperitoneal
- MAPP
Multidisciplinary Approach to the Study of Chronic Pelvic Pain
- VEGF
vascular endothelial growth factor
- VSA
void spot assays
References
- 1.Hanno PM, Erickson D, Moldwin R, Faraday MM. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol. 2015;193(5):1545–53. doi: 10.1016/j.juro.2015.01.086. [DOI] [PubMed] [Google Scholar]
- 2.Lai H, Gereau RWt, Luo Y, O’Donnell M, Rudick CN, Pontari M, et al. Animal Models of Urologic Chronic Pelvic Pain Syndromes: Findings From the Multidisciplinary Approach to the Study of Chronic Pelvic Pain Research Network. Urology. 2015;85(6):1454–65. doi: 10.1016/j.urology.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nature medicine. 2003;9(6):669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 4.Tamaki M, Saito R, Ogawa O, Yoshimura N, Ueda T. Possible mechanisms inducing glomerulations in interstitial cystitis: relationship between endoscopic findings and expression of angiogenic growth factors. J Urol. 2004;172(3):945–8. doi: 10.1097/01.ju.0000135009.55905.cb. [DOI] [PubMed] [Google Scholar]
- 5.Lee JD, Lee MH. Increased expression of hypoxia-inducible factor-1alpha and vascular endothelial growth factor associated with glomerulation formation in patients with interstitial cystitis. Urology. 2011;78(4):971 e11–5. doi: 10.1016/j.urology.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 6.Dagher A, Curatolo A, Sachdev M, Stephens AJ, Mullins C, Landis JR, et al. Identification of novel non-invasive biomarkers of urinary chronic pelvic pain syndrome: findings from the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network. BJU Int. 2017 doi: 10.1111/bju.13832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiuchi H, Tsujimura A, Takao T, Yamamoto K, Nakayama J, Miyagawa Y, et al. Increased vascular endothelial growth factor expression in patients with bladder pain syndrome/interstitial cystitis: its association with pain severity and glomerulations. BJU Int. 2009;104(6):826–31. doi: 10.1111/j.1464-410X.2009.08467.x. discussion 31. [DOI] [PubMed] [Google Scholar]
- 8.Peng CH, Jhang JF, Shie JH, Kuo HC. Down regulation of vascular endothelial growth factor is associated with decreased inflammation after intravesical OnabotulinumtoxinA injections combined with hydrodistention for patients with interstitial cystitis--clinical results and immunohistochemistry analysis. Urology. 2013;82(6):1452 e1–6. doi: 10.1016/j.urology.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Malykhina AP, Lei Q, Erickson CS, Epstein ML, Saban MR, Davis CA, et al. VEGF induces sensory and motor peripheral plasticity, alters bladder function, and promotes visceral sensitivity. BMC physiology. 2012;12:15. doi: 10.1186/1472-6793-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheppudira BP, Girard BM, Malley SE, Schutz KC, May V, Vizzard MA. Upregulation of vascular endothelial growth factor isoform VEGF-164 and receptors (VEGFR-2, Npn-1, and Npn-2) in rats with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol. 2008;295(3):F826–36. doi: 10.1152/ajprenal.90305.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuh G, Wu P, Liang WC, Ultsch M, Lee CV, Moffat B, et al. Structure-function studies of two synthetic anti-vascular endothelial growth factor Fabs and comparison with the Avastin Fab. J Biol Chem. 2006;281(10):6625–31. doi: 10.1074/jbc.M507783200. [DOI] [PubMed] [Google Scholar]
- 12.Mickle AD, Shepherd AJ, Loo L, Mohapatra DP. Induction of thermal and mechanical hypersensitivity by parathyroid hormone-related peptide through upregulation of TRPV1 function and trafficking. Pain. 2015;156(9):1620–36. doi: 10.1097/j.pain.0000000000000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai HH, Qiu CS, Crock LW, Morales ME, Ness TJ, Gereau RWt. Activation of spinal extracellular signal-regulated kinases (ERK) 1/2 is associated with the development of visceral hyperalgesia of the bladder. Pain. 2011;152(9):2117–24. doi: 10.1016/j.pain.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu W, Ackert-Bicknell C, Larigakis JD, MacIver B, Steers WD, Churchill GA, et al. Spontaneous voiding by mice reveals strain-specific lower urinary tract function to be a quantitative genetic trait. Am J Physiol Renal Physiol. 2014;306(11):F1296–307. doi: 10.1152/ajprenal.00074.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bivacizumab treatment for metastatic colorectal cancer, advanced nonsquamous non-small cell lung cancer, metastatic kidney cancer, and gioblastoma. [Accessed on October 22, 2013]; http://www.avastin.com/patient.
- 16.Ranibizumab injection for diabetic macular edema and wet age-related macular degeneration. [Accessed October 22, 2013]; http://www.lucentis.com/lucentis.
- 17.Saban R, Saban MR, Maier J, Fowler B, Tengowski M, Davis CA, et al. Urothelial expression of neuropilins and VEGF receptors in control and interstitial cystitis patients. Am J Physiol Renal Physiol. 2008;295(6):F1613–23. doi: 10.1152/ajprenal.90344.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dagher A, Curatolo A, Sachdev M, Stephens AJ, Mullins C, Landis JR, et al. Identification of Novel Non-invasive Biomarkers of Urinary Chronic Pelvic Pain Syndrome (UCPPS): Findings from the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network. BJU Int. 2017 doi: 10.1111/bju.13832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chidlow JH, Jr, Shukla D, Grisham MB, Kevil CG. Pathogenic angiogenesis in IBD and experimental colitis: new ideas and therapeutic avenues. Am J Physiol Gastrointest Liver Physiol. 2007;293(1):G5–G18. doi: 10.1152/ajpgi.00107.2007. [DOI] [PubMed] [Google Scholar]
- 20.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 21.Azadzoi KM, Chen BG, Radisavljevic ZM, Siroky MB. Molecular reactions and ultrastructural damage in the chronically ischemic bladder. J Urol. 2011;186(5):2115–22. doi: 10.1016/j.juro.2011.06.047. [DOI] [PubMed] [Google Scholar]
- 22.Pontari MA, Hanno PM, Ruggieri MR. Comparison of bladder blood flow in patients with and without interstitial cystitis. J Urol. 1999;162(2):330–4. [PubMed] [Google Scholar]
- 23.Irwin P, Galloway NT. Impaired bladder perfusion in interstitial cystitis: a study of blood supply using laser Doppler flowmetry. J Urol. 1993;149(4):890–2. doi: 10.1016/s0022-5347(17)36253-5. [DOI] [PubMed] [Google Scholar]
- 24.Nagai T, Sato M, Kobayashi M, Yokoyama M, Tani Y, Mochida J. Bevacizumab, an anti-vascular endothelial growth factor antibody, inhibits osteoarthritis. Arthritis Res Ther. 2014;16(5):427. doi: 10.1186/s13075-014-0427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saban MR, Davis CA, Avelino A, Cruz F, Maier J, Bjorling DE, et al. VEGF signaling mediates bladder neuroplasticity and inflammation in response to BCG. BMC physiology. 2011;11:16. doi: 10.1186/1472-6793-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boudes M, Uvin P, Kerselaers S, Vennekens R, Voets T, De Ridder D. Functional characterization of a chronic cyclophosphamide-induced overactive bladder model in mice. Neurourol Urodyn. 2011;30(8):1659–65. doi: 10.1002/nau.21180. [DOI] [PubMed] [Google Scholar]
- 27.Bon K, Lichtensteiger CA, Wilson SG, Mogil JS. Characterization of cyclophosphamide cystitis, a model of visceral and referred pain, in the mouse: species and strain differences. J Urol. 2003;170(3):1008–12. doi: 10.1097/01.ju.0000079766.49550.94. [DOI] [PubMed] [Google Scholar]
- 28.Bielefeldt K, Lamb K, Gebhart GF. Convergence of sensory pathways in the development of somatic and visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol. 2006;291(4):G658–65. doi: 10.1152/ajpgi.00585.2005. [DOI] [PubMed] [Google Scholar]
- 29.Hodges SJ, Zhou G, Deng FM, Aboushwareb T, Turner C, Andersson KE, et al. Voiding pattern analysis as a surrogate for cystometric evaluation in uroplakin II knockout mice. J Urol. 2008;179(5):2046–51. doi: 10.1016/j.juro.2007.12.039. [DOI] [PubMed] [Google Scholar]


