Abstract
Single nucleotide polymorphisms (SNPs) in FCγ-receptor genes FCGR3A (rs396991) and FCGR2A (rs1801274) influence the affinity of the Fc portion of anti-CD20 immunoglobulin G1 monoclonal antibody. Their roles in diffuse large B-cell lymphoma (DLBCL) treated with rituximab in combination with anthracycline-based chemotherapy remain controversial. To address this question, we genotyped FCGR2A and FCGR3A SNPs in two prospective DLBCL cohorts from Lymphoma Study Association (LYSA) trials (N=554) and Iowa/Mayo Specialized Program Of Research Excellence (SPORE) (N=580). Correlation with treatment response and hematological toxicity were assessed in LYSA. Correlation with event free survival (EFS) and overall survival (OS) was performed in both cohorts, followed by a meta-analysis to increase power. Our study shows the absence of correlation between these SNPs and treatment response. Grade 3–4 febrile neutropenia during treatment was more frequently observed in FCGR3A VV (39%) than VF (29%) and FF (32%) carriers (P=0.04). Our analysis for EFS and OS shows that FCGR3A was not associated with outcome. In a meta-analysis using an ordinal model, FCGR2A (per R allele) was associated with a better EFS (HR=0.87; 95%CI, 0.76–0.99; P=0.04) and OS (HR=0.86; 95%CI, 0.73–1.00; P=0.05) which was not altered after adjustment for the International Prognostic Index. Overall, our data demonstrate that DLBCL patients with the low affinity FCγRIIA RR had an unexpectedly better outcome than FCγRIIA H carriers. Whether rituximab efficacy is improved in FCγRIIA RR patients due a clearance reduction or other functions of FCγRIIA in DLBCL should be investigated (clinicaltrials.gov identifiers: NCT00135499, NTC00135499 NCT00140595, NCT00144807, NCT00144755, NCT01087424, NCT00301821).
Keywords: FCGR2A, FCGR3A, polymorphisms, immunochemotherapy, prognostic, DLBCL
Introduction
Rituximab in combination with anthracycline-based chemotherapy has improved the prognosis of diffuse large B-cell lymphoma (DLBCL) patients. Better understanding of factors that affect the response variability of rituximab remains an important therapeutic challenge [1]. Tumor burden had a prognostic impact for DLBCL patients treated with rituximab in combination with CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) regimen and gender and weight were shown to influence outcome by affecting rituximab clearance [2,3].
Antibody-dependent cellular cytotoxicity (ADCC) is one of the major hypothesized mechanisms of action of rituximab [1]. The affinity between Fc portion of anti-CD20 monoclonal antibody and FcG receptors (FCγR) that engages ADCC can be modulated by the presence of single nucleotide polymorphisms (SNPs) in FCGR genes. The FCGR3A rs396991 SNP leading to the substitution of a valine (V) for a phenylalanine (F), confers a higher affinity to FCγRIIIA 158V for IgG1 than FCγRIIIA 158F [4]. The FCGR2A rs1801274 SNP modifies an amino-acid at position 131 of FCγRIIA with either a histidine (H) or an arginine (R); FCγRIIA 131H has a higher affinity than FCγRIIA 131R [4]. With rituximab in monotherapy for follicular lymphoma (FL) patients, initial studies showed that FCGR3A VV patients had a better response rate than FCGR3A F carriers [5,6]. For DLBCL patients treated with immunochemotherapy, the data on the therapeutic impact of FCGR3A and FCGR2A are unclear based on a relatively small number (51 to 263) of DLBCL patients [7–12]. A trend for a higher event-free survival (EFS) was observed for FCGR3A VV compared to FCGR3A F patients treated by R-CHOP in the RICOVER-60 trial [13]. One important observation by the authors of the latter study was that all previous studies were underpowered to observe a statistically significant difference in outcome for FCGR3A or FCGR2A genotype [13].
We analyzed the prognostic value of FCGR2A and FCGR3A in two prospective cohorts (N=554 and 580) of newly diagnosed DLBCL patients treated with anthracycline-based chemotherapy and rituximab. We performed a meta-analysis based on these 1,134 patients to increase statistical power to clarify this important therapeutic question. We performed exploratory analyses to assess heterogeneity by sex, tumor bulk and the absolute lymphocyte count (ALC) at diagnosis, which are clinical characteristics known to affect rituximab clearance or efficacy [2,3,14].
Methods
Study population
LYSA cohort
The LNH03B program of the LYSA consisted of five prospective multicentric, controlled studies including 1,704 DLBCL patients older than 18 years of age [15–19]. Details of the treatment of 554 patients included in this study are presented in Table S1
SPORE cohort
Patients with newly diagnosed lymphoma were enrolled from 2002–2009 in the Molecular Epidemiology Resource (MER), a prospective cohort that is part of the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence (SPORE) [20]. Details of the treatment, which was based on routine practice, are provided in Table S1
This study was conducted in accordance with the Declaration of Helsinki. Ethics committees of Haute-Normandie (LYSA) and the SPORE study Human Subjects Institutional Review Board at Mayo Clinic and the University of Iowa approved this study. All patients provided written consent for participation. In accordance with French law, no mention of race or ethnicity was made.
SNP genotyping
DNA was extracted from peripheral blood. In the LYSA, FCGR3A (rs396991) and FCGR2A (rs1801274) genotyping used a complete assay containing primers, probes and TaqMan® Genotyping Master Mix from Applied Biosystems (Foster City, California, USA) on an ABI Prism 7000 Sequence Detection System (Applied Biosystems). Duplicate genotyping were performed for 10% of samples and agreement was 100%. In the SPORE, the FCGR2A SNP was genotyped as part of a larger project using a custom Illumina Infinium array (Illumina, San Diego, CA) and the FCGR3A SNP was genotyped using a custom designed pyrosequencing assay [21].
Statistical analysis
The correlation between FCGR genotypes and initial characteristics was assessed. Correlation between FCGR3A and FCGR2A genotype and response to treatment and toxicity (grade 3–4 anemia, grade 3–4 thrombocytopenia and grade 3–4 febrile neutropenia during treatment, at least one cycle delayed for 5 days or more) were only performed in the LYSA cohort in whom these data were prospectively collected in clinical trial setting. Tumor responses were classified based on the 1999 Cheson criteria [22]. EFS was evaluated from the date of randomization (LYSA) or the date of diagnosis (SPORE) to the date of disease progression, relapse, re-treatment or death from any cause. Overall survival was evaluated from the date of randomization (LYSA) or the date of diagnosis (SPORE) to the date of death from any cause. A Chi-square test was used to examine associations between genotypes and patient characteristics and treatment response. Survival was estimated by the Kaplan-Meier product limit method and compared using the log-rank test. The prognostic value of each SNP was evaluated for EFS and OS in ordinal (per FCGR3A V and FCGR2A R allele) and genotypic (dominant and recessive) models, first in each cohort, and then in a meta-analysis [23].
Results
The clinical characteristics of the 554 LYSA and 580 SPORE DLBCL patients are presented in Table S2. In the LYSA, the median age was 61 years (range, 18–93), 19% had a ECOG PS 0–1, 73% had an Ann Arbor stage III–IV and 50% had an aaIPI score of 2–3. In the SPORE, the median age was 62 years (range, 18–92), 17% had an ECOG PS 0–1. Compared to the LYSA cohort, fewer SPORE patients had Ann Arbor stage III–IV (59% vs 73%) and aaIPI score 2–3 (41% vs 50%). With a median follow-up of 39 months, the 3-year EFS and OS rates were 69.3% and 75.3%, respectively in the LYSA cohort. In the SPORE cohort, with a median follow-up of 59 months, the 3-year EFS and OS rates were 66.5% and 79.8%, respectively.
Genotyping data were available on 554 LYSA and 580 SPORE patients for FCGR2A and 552 patients for FCGR3A (Table S2). The distribution of the VV, VF and FF alleles for FCGR3A was 15%, 46% and 39%, respectively in the LYSA and 11%, 46% and 43%, respectively in the SPORE. The HH, HR and RR allele distribution for FCGR2A was 28%, 49% and 23%, respectively in the LYSA and 23%, 54% and 23% in the SPORE. These distributions were consistent with Hardy-Weinberg equilibrium. There was no difference in patient characteristics according to FCGR3A and FCGR2A genotypes in the LYSA (Table S3) or in the SPORE (Table S4).
FCGR3A and FCGR2A and response to treatment in LYSA patients
For the FCGR3A SNP, complete response (CR) and unconfirmed CR (CRu) after induction therapy was observed in 50 patients (63%) with the VV allele, in 168 patients (68%) with the VF allele and in 128 patients (63%) with the FF allele (P=0.44); for FCGR2A SNP, CR/CRu was observed in 93 patients (62%) with the HH allele, in 171 patients (66%) with the HR allele and 82 patients (67%) with the RR allele (P=0.63). At the end of initial treatment a CR/CRu was documented for 60 (73%), 195 (76%), 158 (74%) patients with respectively FCGR3A VV, VF, FF genotype (P=0.85) and 113 (73%), 202 (75%), 98 (76%) FCGR2A HH, HR, RR carriers, respectively (P=0.88). Results were similar when analyses were restricted to the 340 and 191 patients assessable for response treated by R-CHOP and R-ACVBP in induction therapy, respectively (data not shown).
EFS and OS according to FCGR3A and FCGR2A genotypes
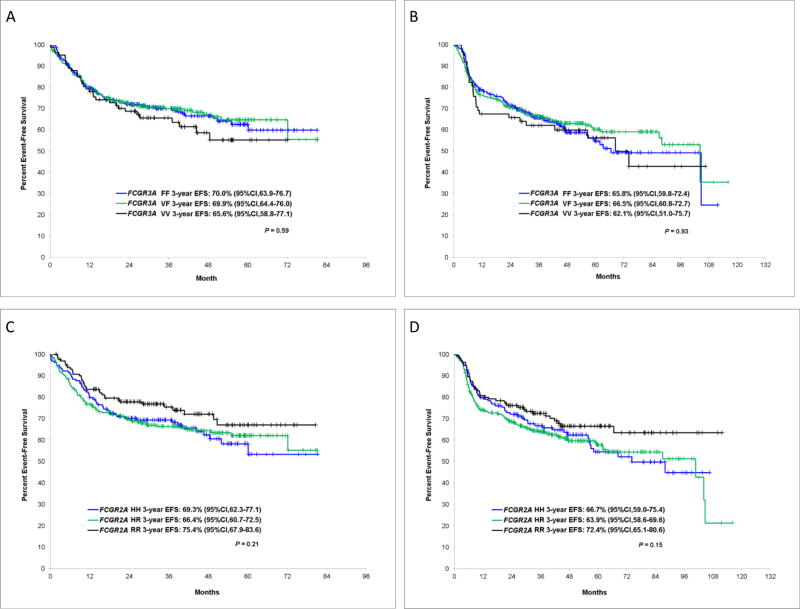
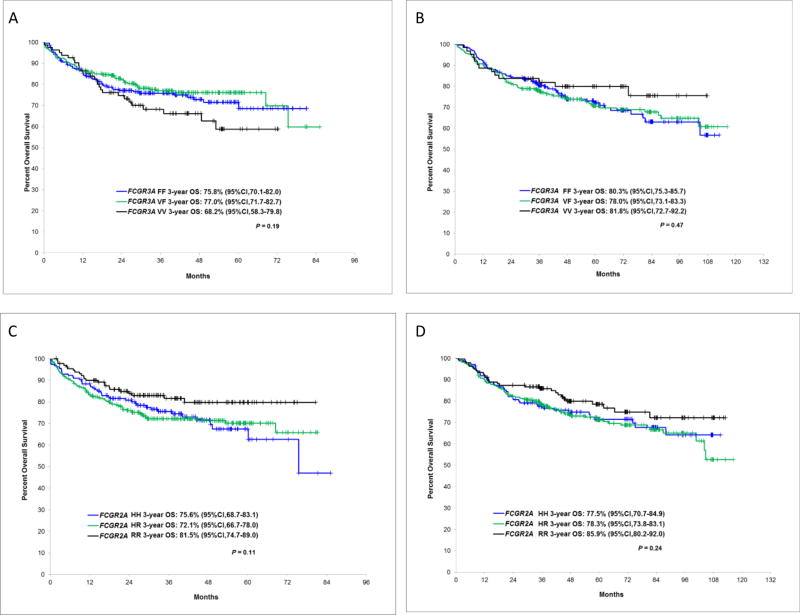
The 3-year EFS rates for patients with FCGR3A VV, VF and FF were not significantly different in LYSA (P=0.59) (Figure 1A) or the SPORE (P=0.93) cohort (Figure 1B). We also did not observe any difference for OS for this SNP in LYSA (P=0.19) (Figure 2A) and in the SPORE (P=0.47) (Figure 2B). The 3-year EFS between HH, HR and RR carriers of FCGR2A SNP was not significantly different in LYSA (P=0.21) (Figure 1C) or in the SPORE (P=0.15) (Figure 1D). However, we observed a trend of higher 3-year EFS rates for FCGR2A RR patients compared to FCGR2A H carriers in LYSA (75.4% [67.9%–83.6%] vs 67.4% [62.9%–72.2%], P=0.08) and in the SPORE (72.4% [65.1%–80.6%] vs 64.8% [60.3%–69.5%], P=0.06). Similarly, the OS was not significantly different between the three FCGR2A genotypes in LYSA (P=0.11) (Figure 2C) or in the SPORE (P=0.24) (Figure 2D), but we observed a better OS in LYSA (81.5% [74.7%–89%] vs 73.4% [69.1%–77.9%], P=0.04) and a trend in the SPORE (85.9% [80.2%–92%] vs 78% [74.2%–82%], P=0.10) for FCGR2A RR compared to FCGR2A H patients.
Figure 1.
Event free survival according to FCGR3A and FCGR2A alleles in LYSA and the SPORE cohorts. FCGR3A in LYSA (A) and in the SPORE (B), FCGR2A in LYSA (C) and in the SPORE (D)
Figure 2.
Overall survival according to FCGR3A and FCGR2A alleles in LYSA and the SPORE cohorts. FCGR3A in LYSA (A) and in the SPORE (B), FCGR2A in LYSA (C) and in the SPORE (D)
In a meta-analysis of the results for FCGR3A (N=1,134 patients), there was no association for EFS (hazard ratio [HR]=1.04; 95%CI, 0.90–1.19; P=0.61) or OS (HR=0.98; 95%CI, 0.83–1.15; P=0.78) using an ordinal (per allele) model (Table 1). We also did not observe any difference of outcome between patients who carried the high-affinity FCγRIIIA VV genotype compared to patients with the low affinity FCγRIIIA F allele (genotypic model) (Table S5). For FCGR2A, the combined analysis showed that this SNP (per allele) was associated with EFS using an ordinal model (HR=0.87; 95%CI, 0.79–0.99; P=0.04) and OS (HR=0.86; 95%CI, 0.73–1.00; P=0.05) (Table 2). These results retained significance after aaIPI adjustment for EFS (HR=0.85; 95%CI, 0.74–0.97; P=0.02) and for OS (HR=0.83; 95%CI, 0.71–0.97; P=0.02). While we did not observe any difference of outcome between the high affinity genotype (FCγRIIA HH) and the low affinity allele (FCγRIIA R) (genotypic model) (Table S6), we observed in the meta-analysis better prognosis of FCGR2A RR compared to FCGR2A H for EFS (HR=0.71; 95%CI, 0.56–0.91; P=0.01) and OS (HR=0.68; 95%CI, 0.51–0.91; P=0.01) (Table 3), which remained after aaIPI adjustment for both EFS (HR=0.70; 95%CI, 0.55–0.89; P=0.006) and OS (HR=0.83; 95%CI, 0.71–0.97; P=0.02).
Table 1.
Association of FCGR3A rs396991 V allele (ordinal or per allele model) with event-free and overall survival
| EFS | OS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| LYSA | SPORE | Meta-analysis | LYSA | SPORE | Meta-analysis | |||||||
|
|
|
|
|
|
|
|||||||
| Population | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P |
| All | 1.07 (0.87, 1.32) | 0.52 | 1.01 (0.82, 1.23) | 0.93 | 1.04 (0.90, 1.19) | 0.61 | 1.10 (0.86, 1.39) | 0.45 | 0.91 (0.72, 1.16) | 0.45 | 0.98 (0.83, 1.15) | 0.78 |
| R-CHOP* | 1.07 (0.85, 1.34) | 0.57 | 1.00 (0.81, 1.24) | 0.99 | 1.03 (0.88, 1.21) | 0.68 | 1.17 (0.91, 1.50) | 0.23 | 0.91 (0.70, 1.18) | 0.47 | 1.03 (0.86, 1.24) | 0.72 |
| R-CHOP | 1.07 (0.84, 1.35) | 0.60 | 1.01 (0.82, 1.23) | 0.93 | 1.03 (0.89, 1.20) | 0.69 | 1.10 (0.84, 1.44) | 0.51 | 0.91 (0.72, 1.16) | 0.45 | 0.99 (0.83, 1.19) | 0.91 |
| Bulky | 1.13 (0.76, 1.69) | 0.54 | 1.14 (0.69, 1.89) | 0.61 | 1.13 (0.83, 1.55) | 0.43 | 1.14 (0.73, 1.77) | 0.56 | 0.89 (0.51, 1.54) | 0.68 | 1.04 (0.73, 1.46) | 0.84 |
| Non bulky | 1.05 (0.82, 1.35) | 0.70 | 0.99 (0.79, 1.24) | 0.93 | 1.02 (0.86, 1.20) | 0.85 | 1.08 (0.81, 1.44) | 0.58 | 0.91 (0.69, 1.19) | 0.47 | 0.99 (0.81, 1.20) | 0.88 |
| Male | 1.25 (0.94, 1.66) | 0.12 | 0.95 (0.73, 1.25) | 0.73 | 1.08 (0.89, 1.32) | 0.41 | 1.37 (1.00, 1.88) | 0.05 | 0.82 (0.59, 1.13) | 0.22 | 1.06 (0.85, 1.34) | 0.59 |
| Female | 0.88 (0.64, 1.21) | 0.43 | 1.09 (0.80, 1.49) | 0.57 | 0.98 (0.79, 1.23) | 0.89 | 0.82 (0.56, 1.19) | 0.29 | 1.06 (0.73, 1.52) | 0.77 | 0.93 (0.72, 1.21) | 0.60 |
| Low ALC | 0.86 (0.63, 1.17) | 0.33 | 1.17 (0.87, 1.57) | 0.30 | 1.01 (0.81, 1.25) | 0.95 | 0.90 (0.64, 1.26) | 0.54 | 1.11 (0.78, 1.59) | 0.56 | 0.99 (0.78, 1.27) | 0.96 |
| High ALC | 1.40 (1.03, 1.91) | 0.03 | 0.80 (0.59, 1.09) | 0.16 | 1.06 (0.85, 1.32) | 0.59 | 1.53 (1.06, 2.20) | 0.02 | 0.69 (0.47, 0.99) | 0.05 | 1.03 (0.79, 1.33) | 0.85 |
Hazard Ratio (HR) is per V allele (ordinal model, modeled as 0, 1, or 2). A HR>1 corresponds to deleterious effect for event-free survival (EFS) or overall survival (OS) of the V allele; HR<1 correspond to protective effect.
EFS: event-free survival; OS: overall survival; HR: hazard ratio; CI: confidence interval; R-CHOP: rituximab, cyclophosphamide, vincristine and prednisone; ALC: absolute lymphocyte count. Low and high absolute lymphocyte count (ALC) are defined by ALC< and ≥1.0 × 109/L.
R-CHOP regimen including 60 patients older than 80 years old treated with R-low-dose-CHOP
Table 2.
Association of FCGR2A rs1801274 R allele (ordinal or per allele model) with event-free and overall survival
| EFS | OS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| LYSA | SPORE | Meta-analysis | LYSA | SPORE | Meta-analysis | |||||||
|
|
|
|
|
|
|
|||||||
| Population | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P |
| All | 0.86 (0.70, 1.05) | 0.15 | 0.88 (0.73, 1.07) | 0.20 | 0.87 (0.76, 0.99) | 0.04 | 0.82 (0.65, 1.04) | 0.10 | 0.88 (0.70, 1.10) | 0.25 | 0.86 (0.73, 1.00) | 0.05 |
| R-CHOP* | 0.88 (0.71, 1.10) | 0.27 | 0.87 (0.71, 1.07) | 0.19 | 0.88 (0.75, 1.02) | 0.09 | 0.81 (0.63, 1.04) | 0.10 | 0.89 (0.70, 1.13) | 0.34 | 0.85 (0.72, 1.01) | 0.07 |
| R-CHOP | 0.85 (0.67, 1.06) | 0.15 | 0.88 (0.73, 1.07) | 0.20 | 0.87 (0.75, 1.00) | 0.05 | 0.78 (0.60, 1.02) | 0.06 | 0.88 (0.70, 1.10) | 0.25 | 0.84 (0.70, 0.99) | 0.04 |
| Bulky | 0.85 (0.58, 1.26) | 0.42 | 1.18 (0.76, 1.83) | 0.46 | 0.98 (0.73, 1.31) | 0.90 | 0.72 (0.46, 1.12) | 0.14 | 1.29 (0.76, 2.20) | 0.34 | 0.91 (0.65, 1.28) | 0.60 |
| Non bulky | 0.87 (0.69, 1.11) | 0.27 | 0.84 (0.68, 1.04) | 0.10 | 0.85 (0.73, 1.00) | 0.05 | 0.88 (0.67, 1.16) | 0.37 | 0.82 (0.64, 1.06) | 0.13 | 0.85 (0.71, 1.02) | 0.09 |
| Male | 0.80 (0.62, 1.04) | 0.10 | 0.90 (0.70, 1.16) | 0.41 | 0.85 (0.71, 1.02) | 0.08 | 0.74 (0.55, 1.00) | 0.05 | 0.87 (0.64, 1.17) | 0.35 | 0.80 (0.65, 0.99) | 0.04 |
| Female | 0.94 (0.69, 1.30) | 0.73 | 0.89 (0.66, 1.18) | 0.41 | 0.91 (0.74, 1.13) | 0.40 | 0.97 (0.67, 1.42) | 0.89 | 0.90 (0.64, 1.27) | 0.57 | 0.93 (0.73, 1.20) | 0.60 |
| Low ALC | 0.91 (0.68, 1.23) | 0.55 | 0.87 (0.65, 1.16) | 0.33 | 0.89 (0.72, 1.09) | 0.27 | 0.88 (0.63, 1.22) | 0.43 | 0.85 (0.60, 1.21) | 0.36 | 0.86 (0.68, 1.10) | 0.23 |
| High ALC | 0.79 (0.59, 1.07) | 0.13 | 0.91 (0.68, 1.20) | 0.49 | 0.85 (0.69, 1.04) | 0.12 | 0.75 (0.52, 1.07) | 0.12 | 0.89 (0.63, 1.24) | 0.48 | 0.82 (0.64, 1.05) | 0.11 |
Hazard Ratio (HR) is per R allele (ordinal model, modeled as 0, 1, or 2). A HR>1 corresponds to deleterious effect for event-free survival (EFS) or overall survival (OS) of the R allele; HR<1 correspond to protective effect
EFS: event-free survival; OS: overall survival; HR: hazard ratio; CI: confidence interval; R-CHOP: rituximab, cyclophosphamide, vincristine and prednisone; ALC: absolute lymphocyte count. Low and high absolute lymphocyte count (ALC) are defined by ALC< and ≥1.0 × 109/L.
R-CHOP regimen including 60 patients older than 80 years old treated with R-low-dose-CHOP
Table 3.
Association of low-affinity FCγRIIA RR versus high-affinity FCγRIIA H with event-free and overall survival
| EFS | OS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| LYSA | SPORE | Meta-analysis | LYSA | SPORE | Meta-analysis | |||||||
|
|
|
|
|
|
|
|||||||
| Population | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P |
| All | 0.71 (0.49, 1.04) | 0.08 | 0.72 (0.52, 1.01) | 0.06 | 0.71 (0.56, 0.91) | 0.01 | 0.62 (0.40, 0.98) | 0.04 | 0.71 (0.48, 1.07) | 0.10 | 0.68 (0.51, 0.91) | 0.01 |
| R-CHOP* | 0.75 (0.50, 1.12) | 0.16 | 0.76 (0.53, 1.08) | 0.12 | 0.75 (0.58, 0.98) | 0.04 | 0.60 (0.37, 0.98) | 0.04 | 0.77 (0.51, 1.16) | 0.21 | 0.69 (0.51, 0.95) | 0.02 |
| R-CHOP | 0.75 (0.50, 1.14) | 0.18 | 0.72 (0.52, 1.01) | 0.06 | 0.74 (0.57, 0.95) | 0.02 | 0.64 (0.39, 1.06) | 0.09 | 0.71 (0.48, 1.07) | 0.10 | 0.69 (0.50, 0.94) | 0.02 |
| Bulky | 0.49 (0.22, 1.09) | 0.08 | 0.71 (0.30, 1.70) | 0.44 | 0.58 (0.32, 1.05) | 0.07 | 0.24 (0.07, 0.77) | 0.02 | 1.19 (0.49, 2.89) | 0.71 | 0.66 (0.32, 1.34) | 0.25 |
| Non bulky | 0.82 (0.54, 1.25) | 0.35 | 0.75 (0.52, 1.07) | 0.11 | 0.78 (0.59, 1.02) | 0.07 | 0.83 (0.51, 1.35) | 0.45 | 0.67 (0.43, 1.06) | 0.09 | 0.74 (0.53, 1.03) | 0.08 |
| Male | 0.65 (0.39, 1.07) | 0.09 | 0.77 (0.49, 1.20) | 0.25 | 0.71 (0.51, 0.99) | 0.05 | 0.57 (0.31, 1.03) | 0.06 | 0.69 (0.40, 1.21) | 0.19 | 0.63 (0.42, 0.95) | 0.03 |
| Female | 0.81 (0.46, 1.41) | 0.45 | 0.71 (0.43, 1.18) | 0.19 | 0.75 (0.52, 1.10) | 0.14 | 0.72 (0.36, 1.42) | 0.34 | 0.77 (0.43, 1.40) | 0.40 | 0.75 (0.48, 1.17) | 0.20 |
| Low ALC | 0.88 (0.52, 1.49) | 0.63 | 0.80 (0.50, 1.29) | 0.36 | 0.83 (0.59, 1.19) | 0.31 | 0.74 (0.40, 1.37) | 0.34 | 0.82 (0.47, 1.46) | 0.51 | 0.78 (0.51, 1.19) | 0.25 |
| High ALC | 0.55 (0.30, 1.00) | 0.05 | 0.63 (0.37, 1.08) | 0.09 | 0.60 (0.40, 0.89) | 0.01 | 0.49 (0.23, 1.03) | 0.06 | 0.61 (0.32, 1.16) | 0.13 | 0.56 (0.34, 0.90) | 0.02 |
With a genotypic model, the prognostic effect of the low-affinity receptor FCγRIIA RR was compared to the high-affinity receptor FCγRIIA H (HH or HR). A hazard ratio (HR)>1 corresponds to deleterious effect for event-free survival (EFS) or overall survival (OS) of FCγRIIA RR; a HR<1 correspond to protective effect.
EFS: event-free survival; OS: overall survival; HR: hazard ratio; CI: confidence interval; R-CHOP: rituximab, cyclophosphamide, vincristine and prednisone; ALC: absolute lymphocyte count. Low and high absolute lymphocyte count (ALC) are defined by ALC< and ≥1.0 × 109/L.
R-CHOP regimen including 60 patients older than 80 years old treated with R-low-dose-CHOP
Association of FCGR3A and FCGR2A with clinical characteristics
Sex
In LYSA, males with the FCGR3A VV genotype compared to FCGR3A F carriers had a poorer OS (HR=1.86; 95%CI, 1.09–3.19; P=0.02), but this result was not significant after aaIPI adjustment and was not confirmed in the SPORE (Table S5). In the meta-analysis, the beneficial effect on EFS of FCGR2A RR compared to FCGR2A H showed the same trend among men (HR=0.71; 95%CI, 0.51–0.99; P=0.05) and women (HR=0.75; 95%CI, 0.52–1.10; P=0.14). These results were also observed for OS among men (HR=0.63; 95%CI, 0.42–0.95; P=0.03) and women (HR=0.75; 95%CI, 0.48–1.17; P=0.20) (Table 3).
ALC
We hypothesized that FCGR polymorphisms could display a distinct role in patients with low or high lymphocyte counts. Among patients with a low ALC (<1.0 × 109/L), there was no association of the FCGR3A SNP with either EFS or OS (Table 1 and Table S5). For patients with a high ALC (≥ 1.0 × 109/L), those with FCGR3A VV genotype had a worse EFS (HR=1.60; 95%CI, 0.94–2.73; P=0.08) and OS (HR=2.10; 95%CI, 1.17–3.78; P=0.01) compared to FCGR3A F carriers in LYSA; however, we could not confirm the results in the SPORE cohort. In contrast, the FCGR2A RR was associated with a better EFS (HR=0.60; 0.40–0.89; P=0.01) and OS (HR=0.56; 0.34–0.90; P=0.02) for patients with a high ALC in the meta-analysis (Table 3), and these results remained significant after aaIPI adjustment for EFS (HR=0.57; 95%CI, 0.38–0.85; P=0.01) and OS (HR=0.55; 95%CI, 0.33–0.88; P=0.01). For patients with low ALC, the benefit effect of FCGR2A RR was weaker and not statistically significant for EFS (HR=0.83; 95%CI, 0.59–1.19; P=0.31) and OS (HR=0.78; 95%CI, 0.51–1.19; P=0.25).
Bulky disease
We assessed whether the FCGR3A and FCGR2A SNPs influenced the prognosis of patients with or without a bulky disease, as defined by the presence of a mass>10cm. We did not observe any association for the FCGR3A (Table 1 and Table S5) or the FCGR2A (Tables 2–3) SNPs when stratified on bulky disease.
R-CHOP patients
In LYSA and SPORE, 351 and 511 patients were treated by R-CHOP regimen, respectively. There was no association of the FCGR3A with either EFS or OS (Table 1 and Table S5). For FCGR2A, the meta-analysis of the ordinal model showed a suggestive trend for EFS (HR=0.88; 95%CI, 0.75–1.02; P=0.09) and OS (HR=0.85; 95%CI, 0.72–1.01; P=0.07) (Table 2). Compared to FCGR2A H carriers, FCGR2A RR patients had superior EFS (HR=0.75; 95%CI, 0.58–0.98; P=0.04) and OS (HR=0.69; 95%CI, 0.51–0.95; P=0.02) (Table 3), including after aaIPI adjustment for EFS (HR=0.73; 95%CI, 0.56–0.95; P=0.02) and OS (HR=0.67; 95%CI, 0.49–0.92; P=0.01). The results were similar when excluding the 60 patients treated with R-low-dose-CHOP.
Hematological toxicities during treatment
Previous reports showed that the degree of neutropenia could be influenced by the FCGR3A SNP for patients treated with rituximab monotherapy after autologous stem cell transplantation [24] or in combination with chemotherapy [8,25]. We observed that LYSA patients with FCGR3A VV genotype (N=32, 39%) were more likely to have at least one febrile neutropenia (grade 3–4) during treatment than FCGR3A VF (N=75, 29%) and FCGR3A FF (N=69, 32%) genotypes (P=0.04). The FCGR2A SNP was not associated with the rate of febrile neutropenia. Neither SNP was associated with anemia, thrombocytopenia, or the number of cycles applied with a delay ≥ 5 days.
Discussion
Eight smaller series (51 to 263 DLBCL patients), likely underpowered [13], reported weak and not statistically significant correlations between these two FCGR SNPs and prognosis after immunochemotherapy [7–12]. We performed a meta-analysis of two large and independent cohorts to overcome this limitation.
We found no association for the FCGR3A SNP with EFS or OS in DLBCL patients treated by rituximab and anthracycline-based chemotherapy, confirming five previous smaller studies [7,8,10–12]. The prognostic effect of the FCGR3A SNP has mainly been observed in FL patients treated by single agent rituximab in retrospective studies [5,6,14], although these results were not confirmed prospectively [26]. One explanation for the lack of prognostic value of FCGR3A SNP in the context of immunochemotherapy is that the association between chemotherapy and rituximab is deleterious for ADCC effectors. Another hypothesis is that the rituximab dose (375mg/m2) used in the immunochemotherapy schedule abrogated the rituximab activity modulation of FCGR3A SNP, which was only observed at low concentration (<0.01 µg/mL) in an in vitro study [27]. Our data are consistent with results from FL and chronic lymphocytic leukemia in which FCGR3A had no prognostic impact when patients were treated by immunochemotherapy [28,29]. With respect to hematological toxicity, FCGR3A VV patients had more frequent grade 3–4 neutropenia during immunochemotherapy, which is consistent with previous reports [8,25].
Our results for the FCGR2A SNP were unexpected, given that we observed that the low-affinity FCγRIIA 131RR genotype had a modest but significantly better outcome than the high-affinity FCγRIIA 131H carriers. In DLBCL, most studies have not observed any prognostic effect of the FCGR2A SNP for patients treated with immunochemotherapy [7,9,10,12,13]. An increase susceptibility to systemic lupus erythematous had been described for FCGR2A RR carriers in relation with a reduced ability of FCγRII 131RR to clear immune complexes leading to their increased depositions in tissues [31]. With anti-tumor necrosis factor-α monoclonal antibodies, patients with rheumatoid arthritis who carry FCγRII 131RR genotype had a better clinical response with a reduced clearance of infliximab in the circulation [32]. One hypothesis could be that FCγRII 131RR carriers have a reduced clearance of rituximab that increases anti-CD20 availability. Unfortunately, no rituximab dosage was available in our series to explore this hypothesis. In the study of Muller et al. assessing the rituximab clearance in DLBCL, they observed that after completion of immunochemotherapy, rituximab remained detectable in patient sera up to 9 months (median 1.1µg/mL, range 0–2.8) [3]. Whether FCGR2A SNP is able to modulate residual rituximab clearance after initial treatment should be investigated with the hypothesis being that FCGR2A RR compared to FCGR2A H patients retain higher rituximab serum levels that could be active on residual disease. We also observed this trend for FCGR2A SNP in FL patients included in PRIMA study [29]. After induction immunochemotherapy, among FL patients randomized in the observational arm, FCGR2A RR patients had a non-significant but clear trend for a better 3-year PFS (62.5%) compared to FCGR2A HR (52.7%) and FCGR2A HH (54.4%) carriers (P=0.26) but in the rituximab maintenance arm, the PFS curves of the three FCGR2A genotypes were all superimposed. One hypothesis is that the addition of rituximab every 2 months for 2 years abolished the effect of FCγRIIA 131RR observed after initial treatment in the observational arm.
FCγRIIA is the receptor of the C-reactive protein (CRP) and FCγRIIA 131RR has a better affinity with CRP than FCγRIIA 131H [33]. In pneumococcal diseases, patients with FCγRIIA 131RR have a higher affinity for CRP and an increase of proinflammatory cytokine response [34]. A study found a protective effect of FCγRIIA 131RR for patients with invasive pneumococcal diseases with lower hospital mortality [35]. It would be interesting to investigate whether FCγRIIA modulates inflammatory response in DLBCL. A role in lymphoma pathogenesis cannot be excluded, as the FCGR2A SNP has been implicated in lymphoma susceptibility [36].
While sex and tumor burden could affect rituximab clearance and efficacy in DLBCL [2,3], we did not observe any significant associations in analyses stratified by sex or by tumor burden, although power was lower for these analyses. We observed that the better prognosis of FCGR2A RR seemed more pronounced in high compared to low ALC patients. It would be interesting to have a larger patient sample size to investigate if FCGR2A RR patients with higher immune effectors have a better benefit of rituximab treatment compared to FCGR2A H patients.
One limitation of our study is that patients were not treated homogeneously. In the LYSA trials two types of induction treatment (R-CHOP and R-ACVBP) and different consolidation therapies were used. Patients in the SPORE lacked a controlled treatment trial setting, but both cohorts were very similar from clinical, genotype frequency and outcomes perspectives. We did not perform any correction test in our statistical analysis: the primary objective of the study was to test previously reported effect of FCGR3A and FCGR2A SNPs in two large prospective DLBCL cohorts and perform a meta-analysis to gain statistical power. Our results for the subgroup analyses should be viewed as exploratory.
In conclusion, in these two large prospective cohorts, we observed no association of the FCGR3A rs396991 SNP and DLBCL outcome. Somewhat unexpectedly, the FCGR2A rs1801274 SNP influenced DLBCL outcome. While the effect was relatively weak, it suggests a re-evaluation of the relation of FCγRIIA and rituximab clearance and its impact on DLBCL pathogenesis.
Supplementary Material
Acknowledgments
The authors would like to thank the staff of LYSARC (Lymphoma Study Association Research Organisation) for the LYSA operational management.
Funding: This work was supported by the Institut National du Cancer (INCa, Paris, France, 2008-020//CT D20743); Fondation de France (postdoctoral fellowship, H.G.); Philippe Foundation (postdoctoral fellowship, H.G.); grants CA97274 and CA129539 from the US National Institutes of Health and by the Predolin Foundation.
C.H., O.F. and G.S. received honoraria from Roche. G.S. and F.P. performed consulting for Roche.
Footnotes
Conflict of interest: The remaining authors declare no competing financial interests.
References
- 1.Cartron G, Trappe RU, Solal-Céligny P, Hallek M. Interindividual variability of response to rituximab: from biological origins to individualized therapies. Clin Cancer Res. 2011;17:19–30. doi: 10.1158/1078-0432.CCR-10-1292. [DOI] [PubMed] [Google Scholar]
- 2.Pfreundschuh M, Ho AD, Cavallin-Stahl E, et al. Prognostic significance of maximum tumour (bulk) diameter in young patients with good-prognosis diffuse large-B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: an exploratory analysis of the MabThera International Trial Group. Lancet Oncol. 2008;9:435–44. doi: 10.1016/S1470-2045(08)70078-0. [DOI] [PubMed] [Google Scholar]
- 3.Müller C, Murawski N, Wiesen MHJ, et al. The role of sex and weight on rituximab clearance and serum elimination half-life in elderly patients with DLBCL. Blood. 2012;119:3276–84. doi: 10.1182/blood-2011-09-380949. [DOI] [PubMed] [Google Scholar]
- 4.Bruhns P, Iannascoli B, England P, et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–25. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 5.Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–8. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 6.Weng W-K, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–7. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Fabisiewicz A, Paszkiewicz-Kozik E, Osowiecki M, Walewski J, Siedlecki JA. FcγRIIA and FcγRIIIA polymorphisms do not influence survival and response to rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone immunochemotherapy in patients with diffuse large B-cell lymphoma. Leuk Lymphoma. 2011;52:1604–6. doi: 10.3109/10428194.2011.574760. [DOI] [PubMed] [Google Scholar]
- 8.Keane C, Nourse JP, Crooks P, et al. Homozygous FCGR3A-158V alleles predispose to late onset neutropenia after CHOP-R for diffuse large B-cell lymphoma. Intern Med J. 2012;42:1113–9. doi: 10.1111/j.1445-5994.2011.02587.x. [DOI] [PubMed] [Google Scholar]
- 9.Levy D, Bellesso M, Oliveira-Souza P, et al. The H/R FcγRIIA-131 polymorphism and survival in patients with diffuse large B-cell lymphoma (DLBCL) treated with R-CHOP: a study in a genetically mixed population. Clinics (Sao Paulo) 2011;66:919–22. doi: 10.1590/S1807-59322011000500034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitroviç Z, Aurer I, Radman I, et al. FCgammaRIIIA and FCgammaRIIA polymorphisms are not associated with response to rituximab and CHOP in patients with diffuse large B-cell lymphoma. Haematologica. 2007;92:998–9. doi: 10.3324/haematol.10327. [DOI] [PubMed] [Google Scholar]
- 11.Váróczy L, Zilahi E, Gyetvai A, et al. Fc-gamma-receptor IIIa polymorphism and gene expression profile do not predict the prognosis in diffuse large B-cell lymphoma treated with R-CHOP protocol. Pathol Oncol Res. 2012;18:43–8. doi: 10.1007/s12253-011-9414-7. [DOI] [PubMed] [Google Scholar]
- 12.Kim DH, Jung H Du, Kim JG, et al. FCGR3A gene polymorphisms may correlate with response to frontline R-CHOP therapy for diffuse large B-cell lymphoma. Blood. 2006;108:2720–5. doi: 10.1182/blood-2006-01-009480. [DOI] [PubMed] [Google Scholar]
- 13.Ahlgrimm M, Pfreundschuh M, Kreuz M, et al. The impact of Fc-γ receptor polymorphisms in elderly patients with diffuse large B-cell lymphoma treated with CHOP with or without rituximab. Blood. 2011;118:4657–62. doi: 10.1182/blood-2011-04-346411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghielmini M, Rufibach K, Salles G, et al. Single agent rituximab in patients with follicular or mantle cell lymphoma: clinical and biological factors that are predictive of response and event-free survival as well as the effect of rituximab on the immune system: a study of the Swiss Group for Clinical Cancer Research (SAKK) Ann Oncol. 2005;16:1675–82. doi: 10.1093/annonc/mdi320. [DOI] [PubMed] [Google Scholar]
- 15.Ketterer N, Coiffier B, Thieblemont C, et al. Phase III study of ACVBP versus ACVBP plus rituximab for patients with localized low-risk diffuse large B-cell lymphoma (LNH03-1B) Ann Oncol. 2013;24:1032–7. doi: 10.1093/annonc/mds600. [DOI] [PubMed] [Google Scholar]
- 16.Delarue R, Tilly H, Mounier N, et al. Dose-dense rituximab-CHOP compared with standard rituximab-CHOP in elderly patients with diffuse large B-cell lymphoma (the LNH03-6B study): a randomised phase 3 trial. Lancet Oncol. 2013;14:525–33. doi: 10.1016/S1470-2045(13)70122-0. [DOI] [PubMed] [Google Scholar]
- 17.Fitoussi O, Belhadj K, Mounier N, et al. Survival impact of rituximab combined with ACVBP and upfront consolidation autotransplantation in high-risk diffuse large B-cell lymphoma for GELA. Haematologica. 2011;96:1136–43. doi: 10.3324/haematol.2010.038109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peyrade F, Jardin F, Thieblemont C, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2011;12:460–8. doi: 10.1016/S1470-2045(11)70069-9. [DOI] [PubMed] [Google Scholar]
- 19.Récher C, Coiffier B, Haioun C, et al. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH03-2B): an open-label randomised phase 3 trial. Lancet. 2011;378:1858–67. doi: 10.1016/S0140-6736(11)61040-4. [DOI] [PubMed] [Google Scholar]
- 20.Drake MT, Maurer MJ, Link BK, et al. Vitamin D insufficiency and prognosis in non-Hodgkin’s lymphoma. J Clin Oncol. 2010;28:4191–8. doi: 10.1200/JCO.2010.28.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charbonneau B, Wang AH, Maurer MJ, et al. CXCR5 polymorphisms in non-Hodgkin lymphoma risk and prognosis. Cancer Immunol Immunother. 2013;62:1475–84. doi: 10.1007/s00262-013-1452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 23.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 24.Weng W-K, Negrin RS, Lavori P, Horning SJ. Immunoglobulin G Fc receptor FcgammaRIIIa 158 V/F polymorphism correlates with rituximab-induced neutropenia after autologous transplantation in patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2010;28:279–84. doi: 10.1200/JCO.2009.25.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li S-C, Chen Y-C, Evens AM, et al. Rituximab-induced late-onset neutropenia in newly diagnosed B-cell lymphoma correlates with Fc receptor FcγRIIIa 158(V/F) polymorphism. Am J Hematol. 2010;85:810–2. doi: 10.1002/ajh.21818. [DOI] [PubMed] [Google Scholar]
- 26.Kenkre V, Hong F, Cerhan JR, et al. Fc gamma receptor 3A and 2A polymorphisms do not predict response to rituximab in follicular lymphoma. Clin Cancer Res. 2015 Oct 28; doi: 10.1158/1078-0432.CCR-15-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dall’Ozzo S, Tartas S, Paintaud G, et al. Rituximab-dependent cytotoxicity by natural killer cells: influence of FCGR3A polymorphism on the concentration-effect relationship. Cancer Res. 2004;64:4664–9. doi: 10.1158/0008-5472.CAN-03-2862. [DOI] [PubMed] [Google Scholar]
- 28.Dornan D, Spleiss O, Yeh R-F, et al. Effect of FCGR2A and FCGR3A variants on CLL outcome. Blood. 2010;116:4212–22. doi: 10.1182/blood-2010-03-272765. [DOI] [PubMed] [Google Scholar]
- 29.Ghesquières H, Cartron G, Seymour JF, et al. Clinical outcome of patients with follicular lymphoma receiving chemoimmunotherapy in the PRIMA study is not affected by FCGR3A and FCGR2A polymorphisms. Blood. 2012;120:2650–7. doi: 10.1182/blood-2012-05-431825. [DOI] [PubMed] [Google Scholar]
- 30.Rossi D, Bruscaggin A, La Cava P, et al. The genotype of MLH1 identifies a subgroup of follicular lymphoma patients that do not benefit from doxorubicin: FIL-FOLL05 study. Haematologica. 2015;100:517–24. doi: 10.3324/haematol.2014.108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dijstelbloem HM, Bijl M, Fijnheer R, et al. Fcgamma receptor polymorphisms in systemic lupus erythematosus: association with disease and in vivo clearance of immune complexes. Arthritis Rheum. 2000;43:2793–800. doi: 10.1002/1529-0131(200012)43:12<2793::AID-ANR20>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 32.Cañete JD, Suárez B, Hernández MV, et al. Influence of variants of Fc gamma receptors IIA and IIIA on the American College of Rheumatology and European League Against Rheumatism responses to anti-tumour necrosis factor alpha therapy in rheumatoid arthritis. Ann Rheum Dis. 2009;68:1547–52. doi: 10.1136/ard.2008.096982. [DOI] [PubMed] [Google Scholar]
- 33.Marnell L, Mold C, Du Clos TW. C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol. 2005;117:104–11. doi: 10.1016/j.clim.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Mold C, Du Clos TW. C-reactive protein increases cytokine responses to Streptococcus pneumoniae through interactions with Fc gamma receptors. J Immunol. 2006;176:7598–604. doi: 10.4049/jimmunol.176.12.7598. [DOI] [PubMed] [Google Scholar]
- 35.Bouglé A, Max A, Mongardon N, et al. Protective effects of FCGR2A polymorphism in invasive pneumococcal diseases. Chest. 2012;142:1474–81. doi: 10.1378/chest.11-2516. [DOI] [PubMed] [Google Scholar]
- 36.Hosgood HD, Purdue MP, Wang SS, et al. A pooled analysis of three studies evaluating genetic variation in innate immunity genes and non-Hodgkin lymphoma risk. Br J Haematol. 2011;152:721–6. doi: 10.1111/j.1365-2141.2010.08518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.