Abstract
Previous studies have shown that excessive alcohol drinking is associated with chronic pain development; however, the molecular mechanism underlying this association is poorly understood. In this study, we investigated the effect of chronic alcohol consumption on plantar incision-induced postsurgical pain. We observed that 4-week ethanol drinking significantly prolonged plantar incision-induced mechanical pain, but not thermal pain. The chronic alcohol consumption enhanced plantar incision-produced α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor GluA1 phosphorylation at the Ser831 site in the spinal cord. The targeted mutation of the GluA1 phosphorylation site in GluA1 S831A mutant mice significantly inhibited the incisional pain prolongation produced by chronic alcohol consumption. Moreover, chronic alcohol consumption combined with plantar incision markedly increased AMPA receptor-mediated miniature excitatory postsynaptic currents in the spinal dorsal horn neurons, and this effect was diminished significantly in the GluA1 S831A mutant mice. Our results suggest that chronic alcohol consumption may promote the development of persistent postsurgical pain by enhancing AMPA receptor GluA1 Ser831 phosphorylation. We identified chronic alcohol consumption as a risk factor for pain chronification after surgery.
Keywords: Chronic alcohol consumption, Postsurgical pain, AMPA receptor phosphorylation, Pain chronification
Introduction
Both alcohol use and pain are highly prevalent in the world. Although low-to-moderate alcohol consumption may inhibit the development of chronic pain [1, 2], excessive alcohol consumption could be associated with the onset of different painful conditions [3, 4]. An integrative review has indicated that chronic alcohol drinking appears to produce deleterious pain-related outcomes (e.g., greater pain severity) [5]. However, the mechanisms underlying the interrelations between pain and alcohol drinking remain to be illustrated.
It has been demonstrated that chronic alcohol consumption produces a sustained increase in stress hormones (epinephrine and corticosterone) and induces painful peripheral neuropathy [6]. On the other hand, stress hormones have been shown to play an important role in α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor phosphorylation and trafficking in the central nervous system (CNS) [7–10]. AMPA receptor phosphorylation contributes to synaptic plasticity in the CNS [11], and stress hormone-enhanced AMPA receptor phosphorylation facilitates long-term potentiation induction [8], which is the basis for central sensitization during chronic pain. Our previous study [10] suggests that increased spinal AMPA receptor phosphorylation underlies stress-induced transition from acute to chronic pain [10]. In the present study, we examined whether chronic alcohol consumption affects plantar incision-induced postsurgical pain by regulating AMPA receptor phosphorylation. We also used AMPA receptor phospho-deficient mutant mice to further investigate the relationship between chronic alcohol consumption and pain chronification after surgery.
Materials and Methods
Animals
C57BL/6 male wild-type (WT) mice were purchased from Charles River Laboratories (Chicago, IL); AMPA receptor GluA1 S831A phospho-deficient mutant mice with C57BL/6 background [11] were obtained from Dr. Richard Huganir’s Laboratory at Johns Hopkins University School of Medicine. All animals weighing 25–30 g were housed up to four per cage on a standard 12-h light/dark cycle, with water and food pellets available ad libitum. All efforts were made to minimize pain or discomfort and to reduce the number of animals used. All animal procedures were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals and were approved by the Texas A&M University College of Dentistry Institutional Animal Care and Use Committee.
Chronic Alcohol Consumption and Plantar Incision
Chronic alcohol consumption was conducted as described previously [12]. After acclimation, mice received 10% (w/v) ethanol as their sole source of drinking water for two days, followed by five days of 15% (w/v) ethanol. After the seven-day ramp period, mice were given unlimited access to drinking 20% (w/v) ethanol and maintained at this concentration for three weeks. Averagely each mouse consumed about 4 ml of ethanol per day and the amounts of ethanol consumption in all the mice were similar (4.033 ± 0.211). On the day following 4-week ethanol drinking, a 5-mm longitudinal plantar incision was made through the skin and fascia in the left hindpaw under isoflurane anesthesia according to previous studies [10, 13]. The incision began 2 mm from the proximal edge of the heel and extended toward the toes. The underlying muscle was elevated with a curved forceps, leaving the muscle origin and insertion intact. The body weight of mice had no significant difference between ethanol drinking groups and non-ethanol drinking groups during the entire experimental period. All the mice did not show locomotor dysfunction after ethanol drinking and/or plantar incision.
Pain Behavioral Testing
Mechanical and thermal pain hypersensitivities were measured as described in previous studies [10, 13–16] at 1 day before incision surgery as baseline and different days after surgery. 1) Mechanical pain testing: Mice were placed on an elevated wire mesh floor and were covered with a clear Plexiglas chamber. After acclimation for 30 min, their paw withdrawal responses to mechanical stimuli were determined using calibrated von Frey filaments (0.08, 0.15, 0.25, 0.41, 0.7, 1.2, and 2.0 g). Each monofilament was applied five times to the plantar side of the hindpaw for approximately 1 s with a 10-s interval, starting with a force of 0.08 g and continuing in an ascending order. A stimulus-related withdrawal was considered a positive response. The paw withdrawal threshold was calculated as the force at which the positive response occurred in three out of five stimuli. 2) Thermal pain testing: Mice were placed in a Plexiglas chamber on a glass plate under which a light box was located. A radiant heat stimulus was applied by aiming a beam of light through a hole in the light box to each hind paw through the glass plate. The light beam was turned off when the mouse lifted the paw, allowing the measurement of time between the start of the light beam and the paw lift. This time was defined as the paw withdrawal latency. The stimulus intensity was adjusted to give 10–12 s withdrawal latency in the normal mouse. A cutoff time of 20 s was used to avoid tissue damage to the hindpaw. All pain behavioral tests were done by an investigator blinded to animal treatment groups.
Western Blotting
The mice were sacrificed and ipsilateral lumbar spinal cord tissues were harvested either immediately following 4-week ethanol drinking or day 15 after incision/ethanol treatment. AMPA receptor phosphorylation in the spinal cord was analyzed with quantitative Western blotting. Proteins from the lumbar enlargement segments of mouse spinal cords were extracted as previously described [10, 17]. The affinity-purified anti-GluA1 and anti-phospho-GluA1 at Ser831 antibodies were used to assess the expression of total GluA1 and phosphorylated GluA1 at Ser831 site, respectively. The specificity of the antibodies has been validated in our previous study[10] by using several approaches: 1) The antibody only produces one band in the expected molecular weight for the target protein; 2) The addition of relevant blocking peptide results in loss of the band the antibody detected; 3) The specificity of the anti-phospho-GluA1 at Ser831 antibody has been confirmed with the tissue from GluA1 Ser831 mutant mice. The intensity of Western blots was quantified using densitometry. The signals of the phospho-specific GluA1 antibody were normalized to total GluA1 in phosphorylation analysis.
Electrophysiology
In our electrophysiological experiments, pyramidal projection neurons in the laminae II (substantia gelatinosa) of the spinal cord dorsal horn were chosen for recording under a microscope. AMPA receptor-mediated miniature excitatory postsynaptic currents (mEPSCs) were recorded using whole cell patch-clamping in spinal cord slices prepared from different groups of mice on day 15 after incision/ethanol treatment. Spinal cord slices were preincubated with artificial cerebrospinal fluid for 30 min. Spinal mEPSCs were recorded for 10 min, and the data from last 5 min of recording was used for statistical analysis. Tetrodotoxin (0.5 μM) was included in perfusion solution. The mEPSCs were recorded at a holding potential of −70 mV, at which NMDA receptors are presumably blocked by Mg2+ ions. The following mEPSC characteristics were determined: frequency and peak amplitude. Bicuculline (10 μM) and strychnine (1 μM) were added to the perfusion solution to block inhibitory synaptic transmission in all experiments. AMPA receptor-mediated synaptic responses were isolated by treatment with APV (100 μM) and confirmed by blocking with GYKI 53655 (20 μM), a selective AMPA receptor antagonist.
Statistical Analysis
The data are expressed as the mean ± SEM. Comparisons among groups were performed by one-way and two-way analyses of variance followed by the Student-Newman-Keuls method. The differences founded with P < 0.05 were considered statistically significant.
Results
Chronic Alcohol Consumption Prolongs Plantar Incision-induced Postsurgical Pain
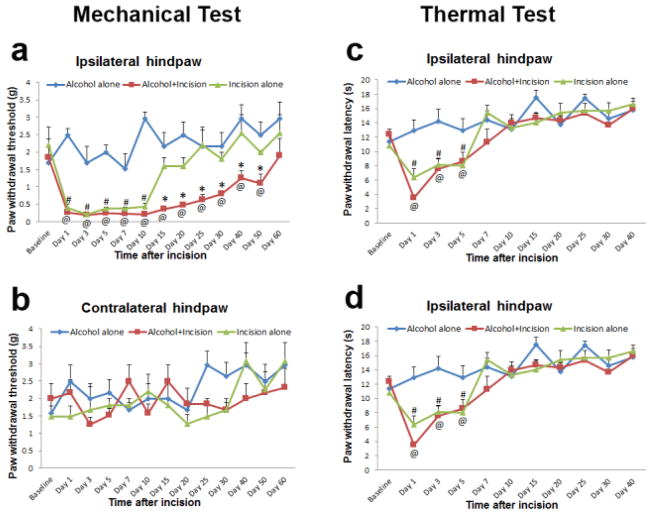
In this study, we found that chronic alcohol consumption significantly prolonged plantar incision-induced mechanical pain in the ipsilateral hindpaw of WT mice (Fig. 1a, *P < 0.05 vs. incision alone group) but had no effect on plantar incision-induced thermal pain (Fig. 1c). Alcohol drinking alone did not produce either mechanical or thermal pain behaviors (Fig. 1a, c). In the plantar incision alone group, the paw withdrawal threshold at the ipsilateral side returned to baseline level on day 15 after surgery (Fig. 1a, #P < 0.05 vs. baseline), and the paw withdrawal latency at the ipsilateral side returned to baseline level on day 7 after surgery (Fig. 1c, #P < 0.05 vs. baseline). However, in the alcohol drinking plus plantar incision group, the plantar incision-induced mechanical pain was prolonged and the paw withdrawal threshold at the ipsilateral side did not return to baseline level until day 60 after surgery (Fig. 1a, @P < 0.05 vs. baseline), but the plantar incision-induced thermal pain was not significantly altered, and the paw withdrawal latency at the ipsilateral side still returned to baseline level on day 7 after surgery (Fig. 1c, @P < 0.05 vs. baseline). In addition, both mechanical and thermal pain were not induced by plantar incision and/or alcohol drinking in the contralateral hindpaw (Fig. 1b, d).
Figure 1.
Chronic alcohol consumption prolongs plantar incision-induced postsurgical pain in WT mice (n = 10 for each group). a 4-week ethanol drinking significantly prolonged mechanical pain after plantar incision in the ipsilateral hindpaw and ethanol drinking alone did not produce mechanical pain (*P < 0.05 vs incision alone group; #P < 0.05 vs baseline in incision alone group; @P < 0.05 vs baseline in alcohol plus incision group). b No mechanical pain was observed in the contralateral hindpaws of all WT mice. c 4-week ethanol drinking had no effect on plantar incision-induced thermal pain in the ipsilateral hindpaw, and ethanol drinking alone did not produce thermal pain (#P < 0.05 vs baseline in incision alone group; @P < 0.05 vs baseline in alcohol plus incision group). d No thermal pain was observed in the contralateral hindpaws of all WT mice.
Chronic Alcohol Consumption Enhances Plantar Incision-induced AMPA Receptor GluA1 Phosphorylation in the Spinal Cord
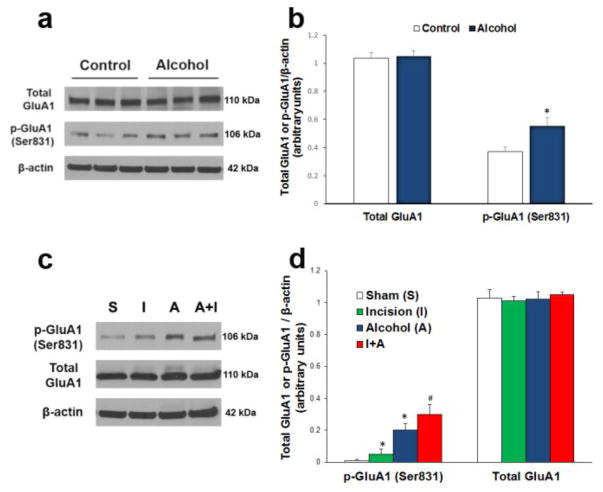
To reveal whether chronic alcohol consumption affects incisional pain through regulating AMPA receptor activities in the spinal cord, we harvested ipsilateral lumbar spinal cord tissues either immediately following 4-week ethanol drinking (Fig. 2a, b) or day 15 after incision/ethanol treatment (Fig. 2c, d). Western blot analysis showed that 4-week ethanol drinking significantly increased GluA1 phosphorylation at the Ser831 site (Fig. 2a, b, *P < 0.05 vs. control group), but the chronic alcohol consumption had no effect on the expression of total GluA1 in the spinal cord (Fig. 2a, b).
Figure 2.
Chronic alcohol consumption enhances plantar incision-induced AMPA receptor GluA1 phosphorylation in the spinal cord (n = 3 for each group in a and b; n = 6 for each group in c and d). a 4-week ethanol drinking increased GluA1 phosphorylation at Ser831 site, but had no effect on the expression of total GluA1 in the spinal cord. b Statistical analysis of the data in a. *P < 0.05 vs. control group. c On day 15 after incision/ethanol treatment, both incision (I) and ethanol drinking (A) increased GluA1 phosphorylation at the Ser831 site compared to the sham group (S), and chronic alcohol consumption further enhanced plantar incision-induced GluA1 Ser831 phosphorylation (A+I). None of the treatments changed the total GluA1 level in the spinal cord. d Statistical analysis of the data in c. *P < 0.05 vs. sham control group; #P < 0.05 vs. incision group. β-actin served as a loading control in all Western blot experiments.
Moreover, on day 15 after the incision/ethanol treatment, both incision and ethanol drinking increased GluA1 phosphorylation at the Ser831 site (Fig. 2c, d, *P < 0.05 vs. sham control group), and chronic alcohol consumption further enhanced plantar incision-induced GluA1 Ser831 phosphorylation (Fig. 2c, d, #P < 0.05 vs. incision group). None of the treatments changed the total GluA1 level in the spinal cord (Fig. 2c, d).
Spinal AMPA Receptor GluA1 Phosphorylation Controls Chronic Alcohol Consumption-produced Prolongation of Postsurgical Pain
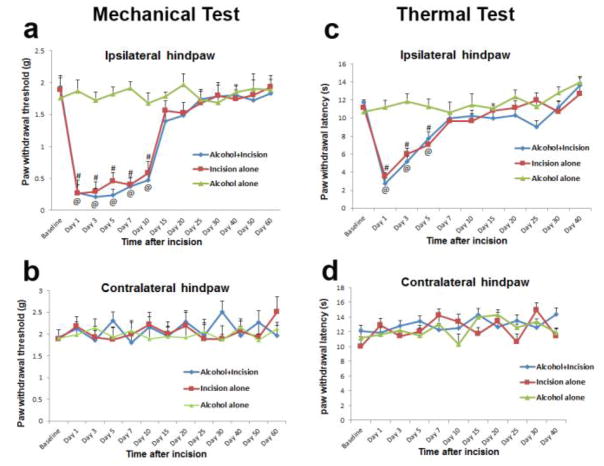
To further investigate if spinal GluA1 Ser831 phosphorylation contributes to the effect of chronic alcohol consumption on incisional pain, AMPA receptor GluA1 S831A phospho-deficient mutant mice were used. In the mutant mice, GluA1 phosphorylation site Ser831 is mutated to alanine using a gene knock-in technique to prevent the phosphorylation of this site in vivo. Interestingly, we found that the targeted mutation of GluA1 phosphorylation site Ser831 significantly disrupted the chronic alcohol consumption-produced prolongation of incisional mechanical pain in the ipsilateral hindpaw. Therefore, the paw withdrawal thresholds in both incision alone group and the alcohol plus incision group returned to baseline levels on day 15 after incision in the GluA1 S831A phospho-deficient mutant mice (Fig. 3a, #P < 0.05 vs. baseline for the incision alone group and @P < 0.05 vs. baseline for the alcohol plus incision group). Paw withdrawal latency in thermal pain testing also showed a similar time course in both incision alone group and the alcohol plus incision group (Fig. 3c, #P < 0.05 vs. baseline for the incision alone group and @P < 0.05 vs. baseline for the alcohol plus incision group). Neither mechanical pain nor thermal pain was significantly different between the incision alone group and the alcohol plus incision group in the GluA1 S831A mutant mice, suggesting that GluA1 Ser831 phosphorylation plays a key role in chronic alcohol consumption-produced prolongation of postsurgical pain. In addition, no pain behaviors were observed in the contralateral hindpaw of GluA1 Ser831A mutant mice (Fig. 3b, d).
Figure 3.
Chronic alcohol consumption does not prolong plantar incision-induced postsurgical pain in AMPA receptor GluA1 Ser831 phospho-deficient mutant mice (n = 10 for each group). a Targeted mutation of GluA1 Ser831 phosphorylation significantly inhibited chronic alcohol consumption-induced prolongation of mechanical pain after plantar incision and ethanol drinking alone did not produce mechanical pain (#P < 0.05 vs baseline in incision alone group; @P < 0.05 vs baseline in alcohol plus incision group). b No mechanical pain was observed in the contralateral hindpaws of all mutant mice. c Chronic alcohol consumption had no effect on plantar incision-induced thermal pain in the ipsilateral hindpaw and ethanol drinking alone did not produce thermal pain (#P < 0.05 vs baseline in incision alone group; @P < 0.05 vs baseline in alcohol plus incision group). d No thermal pain was observed in the contralateral hindpaws of all mutant mice.
Chronic Alcohol Consumption Increases AMPA Receptor-mediated Synaptic Activities via Regulating GluA1 Ser831 Phosphorylation in the Spinal Dorsal Horn Neurons
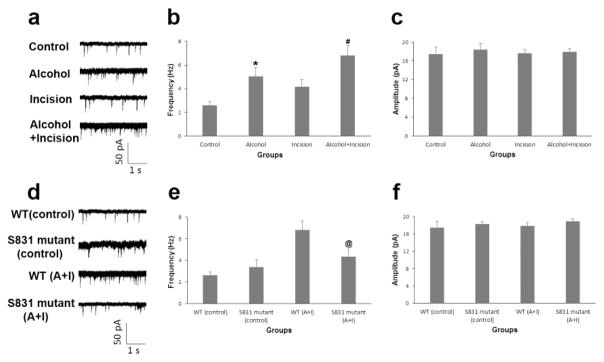
To reveal the effect of chronic alcohol consumption on AMPA receptor-mediated synaptic activities in the spinal dorsal horn neurons, AMPA receptor-mediated mEPSCs were recorded in the spinal cord slices prepared from different groups of mice on day 15 after incision/ethanol treatment. Whole cell patch-clamping was carried out according to our previous study [10]. We found that chronic alcohol consumption not only significantly increased the frequency of AMPA receptor-mediated mEPSCs in the spinal cord neurons (Fig. 4a, b, *P < 0.05 vs. control group), but also further enhanced the spinal AMPA receptor-mediated electrical activities in the alcohol plus incision group (Fig. 4a, b, #P < 0.05 vs. the incision group). None of these treatments had significant effect on the current peak amplitude (Fig. 4c).
Figure 4.
Chronic alcohol consumption increases AMPA receptor-mediated synaptic activities via the regulation of GluA1 Ser831 phosphorylation in the spinal dorsal horn neurons (n = 16 for control group, n = 13 for alcohol group, n = 14 for incision group, and n = 13 for alcohol plus incision group in a, b and c; n = 16 for WT control group, n = 11 for S831 mutant control group, n = 13 for WT alcohol plus incision (A+I) group, and n = 12 for S831 mutant A+I group in d, e and f). a On day 15 after incision and/or ethanol treatment, chronic alcohol consumption not only increased the frequency of AMPA receptor-mediated mEPSCs in the spinal cord neurons (Alcohol group), but also further enhanced incision-induced spinal AMPA receptor-mediated electrical activities (Alcohol+Incision group). b Statistical analysis of the data in a. *P < 0.05 vs. control group; #P < 0.05 vs. incision group. c None of these treatments had significant effect on the current peak amplitude. d AMPA receptor GluA1 Ser831 phospho-deficient mutant mice showed similar basal currents as those in WT mice under normal conditions, but the GluA1 S831A mutation significantly diminished the chronic alcohol consumption-produced increase in the spinal AMPA receptor-mediated mEPSCs. e Statistical analysis of the data in d. @P < 0.05 vs. WT mice. f There was no significant change in the current amplitude after these treatments in the GluA1 S831A phospho-deficient mutant mice.
To clarify the role of AMPA receptor GluA1 Ser831 phosphorylation in the above-mentioned effect, we compared the effect of chronic alcohol consumption on spinal AMPA receptor-mediated mEPSCs in both WT and GluA1 S831A phospho-deficient mutant mice. We found that both types of mice showed similar basal currents under normal conditions (Fig. 4d, e), but GluA1 S831A mutation significantly diminished the chronic alcohol consumption-produced increase in spinal AMPA receptor-mediated mEPSCs (Fig. 4d, e, @P < 0.05 vs. WT mice). We also observed that there was no significant change in the current amplitude after these treatments in the GluA1 S831A phospho-deficient mutant mice (Fig. 4f). Our data indicate that spinal AMPA receptor-mediated electrical activity can be enhanced by chronic alcohol consumption through regulating AMPA receptor phosphorylation.
DISCUSSION
Although pain and alcohol use disorder may share overlapping neural circuitry [4, 18], the relationship between pain and alcohol use is not fully understood. Previous studies have shown that acute administration of ethanol produces a modest degree of antinociception in different pain models [19, 20]; however, chronic alcohol consumption induces painful neuropathy [6] and marked hyperalgesia has been observed during alcohol withdrawal in both animals and humans [21, 22]. Both analgesic effect of acute alcohol and alcohol withdrawal-induced hyperalgesia contribute to alcohol misuse and alcohol addiction. Alcohol dependence could be a major predictor for pain severity after injury [23, 24]. Because the association between alcohol dependence and chronic pain has been reported [4, 25] and both of them are serious issues in public health, it is very important to understand the mechanisms by which they interact with each other. In the present study, we examined the effect of chronic alcohol consumption on postsurgical pain. Persistent postsurgical pain is a suitable model to clarify the pathogenic mechanisms underlying chronic pain development. We found that chronic alcohol consumption significantly prolongs plantar incision-induced mechanical pain (but not thermal pain) by enhancing spinal AMPA receptor phosphorylation. Our results suggest that chronic alcohol consumption is a risk factor involved in pain chronification after surgery.
Our previous study demonstrated that AMPA receptor phosphorylation contributes to the transition from acute to chronic pain after surgery [10]. It has been proved that AMPA receptor GluA1 phosphorylation at both Ser831 and Ser845 plays a critical role in synaptic plasticity of the CNS [11, 26]. GluA1 phospho-deficient mutant mice with knock-in mutations (S831A and S845A) that block phosphorylation at both Ser845 and Ser831 sites of GluA1 show disturbances in synaptic plasticity and learning [11], suggesting that the phosphorylation of AMPA receptor GluA1 at both Ser845 and Ser831 sites is sufficient to increase the probability of synaptic plasticity. In the present study, we found that chronic alcohol consumption increases the expression of phospho-GluA1 at Ser831 in the spinal cord and enhances plantar incision-induced spinal GluA1 Ser831 phosphorylation. In addition, we previously observed that plantar incision does not alter GluA1 phosphorylation at the Ser845 site [10]. In our preliminary experiments, we also observed that chronic alcohol consumption does not affect GluA1 Ser845 phosphorylation (data not shown). Therefore, it is reasonable to postulate that spinal GluA1 phosphorylation at the Ser831 site, but not the Ser845 site, is involved in the effect of chronic alcohol consumption on pain chronification after surgery. To verify this hypothesis, GluA1 S831A phospho-deficient mice were employed in the present study. Our data show that the targeted mutation of the GluA1 Ser831 phosphorylation site completely blocks the prolongation of postsurgical pain produced by 4-week ethanol drinking. Taken together, these results indicate that chronic ethanol consumption may prolong postsurgical pain by enhancing AMPA receptor phosphorylation in the spinal cord.
In the present study, we found that 4-week ethanol drinking only protracts plantar incision-induced mechanical pain, but has no effect on the incision-induced thermal pain. Our data also showed that the chronic ethanol consumption-prolonged incisional pain is mediated by spinal AMPA receptor GluA1 phosphorylation at the Ser831 site. These results suggest that the regulation of GluA1 Ser831 phosphorylation in the spinal cord is required for the pathogenesis of postsurgical mechanical pain, but not thermal pain after surgery. Moreover, we further observed that both WT and GluA1 S831A phospho-deficient mutant mice show a similar time course of plantar incision-induced thermal pain, though the two types of mice display a significant difference in the time course of mechanical pain after plantar incision plus alcohol dinking, which supports above-mentioned notion.
Furthermore, our electrophysiological experiments demonstrated that chronic alcohol consumption enhances AMPA receptor-mediated synaptic activities in a GluA1 Ser831 phosphorylation-dependent manner. We found that 4-week ethanol drinking increases the frequency of AMPA receptor-mediated mEPSCs in the spinal dorsal horn neurons, but has no effect on the mEPSC amplitude. Previous studies [27–30] have shown that the amplitude of mEPSCs is dependent on both vesicle size of neurotransmitter and number of postsynaptic receptors; however, the frequency of mEPSCs is interpreted as presynaptic release probability that is dependent on the numbers of active zones and docked vesicles per active zone. Therefore, our data suggest that chronic alcohol consumption could affect the probability of presynaptic glutamate release, but may not influence the number of postsynaptic AMPA receptors and glutamate vesicle size, as shown in our Western blotting experiments, in which we observed that chronic alcohol consumption enhances plantar incision-induced GluA1 Ser831 phosphorylation, but does not affect the expression of the total AMPA receptors in the spinal cord. In the CNS, AMPA receptors have been shown to localize in presynaptic axon terminals to mediate neurotransmitter release [31, 32]. Our electrophysiological data showed that mutation of AMPA receptor GluA1 Ser831 phosphorylation inhibits the effect of chronic alcohol consumption on frequency of AMPA receptor-mediated mEPSCs, which suggests that GluA1 phosphorylation at presynaptic terminals may contribute to chronic alcohol consumption-induced synaptic facilitation by enhancing presynaptic release of glutamate (indicated as increase of mEPSC frequency).
In conclusion, our study provides experimental evidence to show a direct relationship between alcohol use and pain chronification after surgery. We identified chronic alcohol consumption as a risk factor for the development of persistent postsurgical pain by regulating AMPA receptor phosphorylation and synaptic activities in the spinal cord. Our findings will help us understand the molecular mechanism by which excessive alcohol drinking contributes to persistent postsurgical pain development; this information may guide physicians to predict that patients with chronic alcohol consumption could be at greater risk for developing chronic pain after surgical procedures. Therefore, monitoring alcohol concentration in the blood might be used to improve pain management in high-risk patients.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 DE022880 (F.T.) and K02 DE023551 (F.T.) as well as National Natural Science Foundation of China 81500962 (S.L.). The authors thank Dr. Richard Huganir (Johns Hopkins University School of Medicine) for providing AMPA receptor GluA1 S831A phospho-deficient mutant mice.
Footnotes
Compliance with Ethical Standards: All animal procedures were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals and were approved by the Texas A&M University College of Dentistry Institutional Animal Care and Use Committee.
Potential Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Skillgate E, Vingard E, Josephson M, Holm LW, Alfredsson L. Is smoking and alcohol consumption associated with long-term sick leave due to unspecific back or neck pain among employees in the public sector? Results of a three-year follow-up cohort study. J Rehabil Med. 2009;41:550–556. doi: 10.2340/16501977-0370. [DOI] [PubMed] [Google Scholar]
- 2.Bergman S, Herrstrom P, Jacobsson LT, Petersson IF. Chronic widespread pain: a three year followup of pain distribution and risk factors. J Rheumatol. 2002;29:818–825. [PubMed] [Google Scholar]
- 3.Brennan PL, Schutte KK, SooHoo S, Moos RH. Painful medical conditions and alcohol use: a prospective study among older adults. Pain Med. 2011;12:1049–1059. doi: 10.1111/j.1526-4637.2011.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egli M, Koob GF, Edwards S. Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev. 2012;36:2179–2192. doi: 10.1016/j.neubiorev.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zale EL, Maisto SA, Ditre JW. Interrelations between pain and alcohol: An integrative review. Clin Psychol Rev. 2015;37:57–71. doi: 10.1016/j.cpr.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dina OA, Khasar SG, Alessandri-Haber N, Green PG, Messing RO, Levine JD. Alcohol-induced stress in painful alcoholic neuropathy. The European journal of neuroscience. 2008;27:83–92. doi: 10.1111/j.1460-9568.2007.05987.x. [DOI] [PubMed] [Google Scholar]
- 7.Groc L, Choquet D, Chaouloff F. The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nature neuroscience. 2008;11:868–870. doi: 10.1038/nn.2150. [DOI] [PubMed] [Google Scholar]
- 8.Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, Malinow R. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Krugers HJ, Hoogenraad CC, Groc L. Stress hormones and AMPA receptor trafficking in synaptic plasticity and memory. Nat Rev Neurosci. 2010;11:675–681. doi: 10.1038/nrn2913. [DOI] [PubMed] [Google Scholar]
- 10.Li C, Yang Y, Liu S, Fang H, Zhang Y, Furmanski O, Skinner J, Xing Y, Johns RA, Huganir RL, et al. Stress induces pain transition by potentiation of AMPA receptor phosphorylation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:13737–13746. doi: 10.1523/JNEUROSCI.2130-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, et al. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631–643. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- 12.Ballas ZK, Cook RT, Shey MR, Coleman RA. A dynamic flux in natural killer cell subsets as a function of the duration of alcohol ingestion. Alcohol Clin Exp Res. 2012;36:826–834. doi: 10.1111/j.1530-0277.2011.01678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pogatzki EM, Raja SN. A mouse model of incisional pain. Anesthesiology. 2003;99:1023–1027. doi: 10.1097/00000542-200310000-00041. [DOI] [PubMed] [Google Scholar]
- 14.Tao F, Su Q, Johns RA. Cell-permeable peptide Tat-PSD-95 PDZ2 inhibits chronic inflammatory pain behaviors in mice. Mol Ther. 2008;16:1776–1782. doi: 10.1038/mt.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tao F, Tao YX, Mao P, Zhao C, Li D, Liaw WJ, Raja SN, Johns RA. Intact carrageenan-induced thermal hyperalgesia in mice lacking inducible nitric oxide synthase. Neuroscience. 2003;120:847–854. doi: 10.1016/s0306-4522(03)00362-2. [DOI] [PubMed] [Google Scholar]
- 16.Tao F, Tao YX, Zhao C, Dore S, Liaw WJ, Raja SN, Johns RA. Differential roles of neuronal and endothelial nitric oxide synthases during carrageenan-induced inflammatory hyperalgesia. Neuroscience. 2004;128:421–430. doi: 10.1016/j.neuroscience.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 17.Tao F, Skinner J, Su Q, Johns RA. New role for spinal Stargazin in alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor-mediated pain sensitization after inflammation. J Neurosci Res. 2006;84:867–873. doi: 10.1002/jnr.20973. [DOI] [PubMed] [Google Scholar]
- 18.Apkarian AV, Neugebauer V, Koob G, Edwards S, Levine JD, Ferrari L, Egli M, Regunathan S. Neural mechanisms of pain and alcohol dependence. Pharmacol Biochem Behav. 2013;112:34–41. doi: 10.1016/j.pbb.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gatch MB, Lal H. Effects of ethanol and ethanol withdrawal on nociception in rats. Alcohol Clin Exp Res. 1999;23:328–333. [PubMed] [Google Scholar]
- 20.Campbell VC, Taylor RE, Tizabi Y. Antinociceptive effects of alcohol and nicotine: involvement of the opioid system. Brain Res. 2006;1097:71–77. doi: 10.1016/j.brainres.2006.04.054. [DOI] [PubMed] [Google Scholar]
- 21.Potter JS, Prather K, Weiss RD. Physical pain and associated clinical characteristics in treatment-seeking patients in four substance use disorder treatment modalities. Am J Addict. 2008;17:121–125. doi: 10.1080/10550490701862902. [DOI] [PubMed] [Google Scholar]
- 22.Dhir A, Naidu PS, Kulkarni SK. Protective effect of cyclooxygenase-2 (COX-2) inhibitors but not non-selective cyclooxygenase (COX)-inhibitors on ethanol withdrawal-induced behavioural changes. Addict Biol. 2005;10:329–335. doi: 10.1080/13556210500352964. [DOI] [PubMed] [Google Scholar]
- 23.Castillo RC, MacKenzie EJ, Wegener ST, Bosse MJ. Prevalence of chronic pain seven years following limb threatening lower extremity trauma. Pain. 2006;124:321–329. doi: 10.1016/j.pain.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 24.Holmes A, Williamson O, Hogg M, Arnold C, Prosser A, Clements J, Konstantatos A, O’Donnell M. Predictors of pain severity 3 months after serious injury. Pain Med. 2010;11:990–1000. doi: 10.1111/j.1526-4637.2010.00890.x. [DOI] [PubMed] [Google Scholar]
- 25.Katon W, Egan K, Miller D. Chronic pain: lifetime psychiatric diagnoses and family history. Am J Psychiatry. 1985;142:1156–1160. doi: 10.1176/ajp.142.10.1156. [DOI] [PubMed] [Google Scholar]
- 26.Makino Y, Johnson RC, Yu Y, Takamiya K, Huganir RL. Enhanced synaptic plasticity in mice with phosphomimetic mutation of the GluA1 AMPA receptor. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:8450–8455. doi: 10.1073/pnas.1105261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Auger C, Marty A. Quantal currents at single-site central synapses. The Journal of physiology. 2000;526(Pt 1):3–11. doi: 10.1111/j.1469-7793.2000.t01-3-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishikawa T, Sahara Y, Takahashi T. A single packet of transmitter does not saturate postsynaptic glutamate receptors. Neuron. 2002;34:613–621. doi: 10.1016/s0896-6273(02)00692-x. [DOI] [PubMed] [Google Scholar]
- 29.Liu G, Choi S, Tsien RW. Variability of neurotransmitter concentration and nonsaturation of postsynaptic AMPA receptors at synapses in hippocampal cultures and slices. Neuron. 1999;22:395–409. doi: 10.1016/s0896-6273(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 30.Oertner TG, Sabatini BL, Nimchinsky EA, Svoboda K. Facilitation at single synapses probed with optical quantal analysis. Nature neuroscience. 2002;5:657–664. doi: 10.1038/nn867. [DOI] [PubMed] [Google Scholar]
- 31.Fujiyama F, Kuramoto E, Okamoto K, Hioki H, Furuta T, Zhou L, Nomura S, Kaneko T. Presynaptic localization of an AMPA-type glutamate receptor in corticostriatal and thalamostriatal axon terminals. The European journal of neuroscience. 2004;20:3322–3330. doi: 10.1111/j.1460-9568.2004.03807.x. [DOI] [PubMed] [Google Scholar]
- 32.Pittaluga A, Feligioni M, Longordo F, Luccini E, Raiteri M. Trafficking of presynaptic AMPA receptors mediating neurotransmitter release: neuronal selectivity and relationships with sensitivity to cyclothiazide. Neuropharmacology. 2006;50:286–296. doi: 10.1016/j.neuropharm.2005.09.004. [DOI] [PubMed] [Google Scholar]