Abstract
Background
Tamoxifen is a medication often used for the treatment and prevention of breast cancer. It is classically associated with several gynecological side effects to include a thickened endometrial stripe, increased uterine polyp formation, and an increased risk of uterine cancer. Rarely tamoxifen use has been associated with proliferation of endometriosis often severe enough to mimic a late-stage gynecologic malignancy.
Case
A 62-year-old Gravida 0 postmenopausal female with a medical history of severe obesity, infertility, and preventative tamoxifen use presented for evaluation of gross hematuria. A CT urogram was performed and demonstrated findings concerning for carcinomatosis, likely gynecologic in origin. Cervical cancer screening was up-to-date and she had a negative colonoscopy within the prior 2 years. Serum tumor markers were remarkable only for a mildly elevated CA125 of 37.6. Diagnostic laparoscopy demonstrated apparent operable carcinomatosis limited to the pelvis. The procedure was converted to an exploratory laparotomy, where radical tumor cytoreduction was performed to no gross residual disease. Frozen sections performed intraoperatively were unclear of origin but suggestive of low malignant potential. Final pathology resulted for endometriosis.
Conclusion
This case illustrates a presentation of endometriosis in a postmenopausal woman mimicking advanced mullerian malignancy. The patient's estrogenic state from obesity in combination with the agonist action of the tamoxifen likely contributed to her rare presentation. While findings such as a thickened endometrial stripe are typical of tamoxifen use, such widespread proliferation of endometriosis resulting in a pelvic mass, genito-urinary obstruction, and plaque-like pelvic spread are not.
Précis
Endometriosis is a benign estrogen dependent condition rarely problematic in a postmenopausal patient. Tamoxifen use in the setting of an obese patient may contribute to a proliferation of pre-existing endometriosis which resembles an aggressive late-stage gynecological malignancy.
Highlights
-
•
Apparent carcinomatosis in an obese postmenopausal tamoxifen user.
-
•
Radical tumor cytoreduction performed as primary treatment.
-
•
Endometriosis, not malignant process, revealed on final pathology.
-
•
Obesity and tamoxifen likely contributed to polypoid postmenopausal endometriosis.
1. Case
A 62-year-old Gravida 0 postmenopausal female presented to her primary care physician for evaluation of gross hematuria. A CT urogram and pelvic ultrasound were performed during workup and demonstrated findings concerning for a gynecologic malignancy: a right ovarian mass, carcinomatosis and a thickened endometrial stripe (Fig. 1 and Fig. 2). She had up-to-date cervical cancer screening, and a negative colonoscopy 2 years prior Fig. 3, Fig. 4. Her medical history was significant for hypertension, type 2 diabetes (HbA1C 6.4), morbid obesity (BMI 41), infertility, and atypical ductal hyperplasia for which she had been taking 20 mg of tamoxifen daily for the past 43 months (25.8 g cumulative) for breast cancer risk-reduction. She denied any postmenopausal bleeding, abdominal pain, or any other changes to her baseline health. Tumor markers (CEA, CA-125, CA19-9) were only notable for a mildly elevated CA125 of 37.6.
Fig. 1.
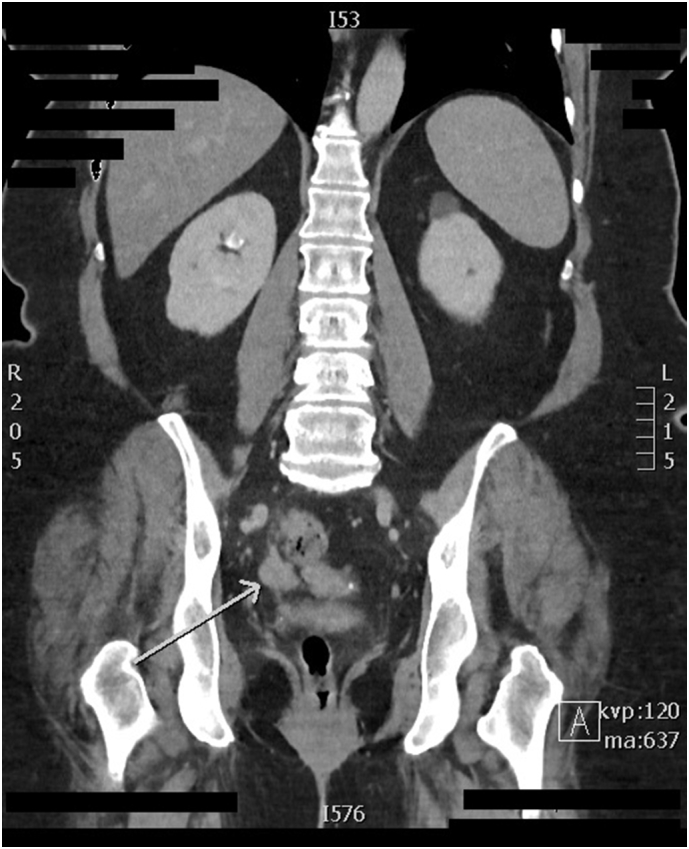
CT Urogram demonstrating intraabdominal soft tissue nodules.
Fig. 2.
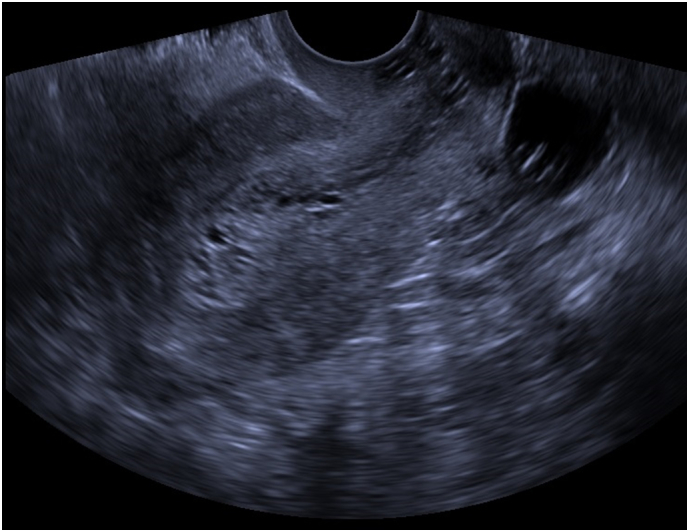
Pelvic Ultrasound demonstrating a thickened, heterogeneous endometrial stripe.
Fig. 3.
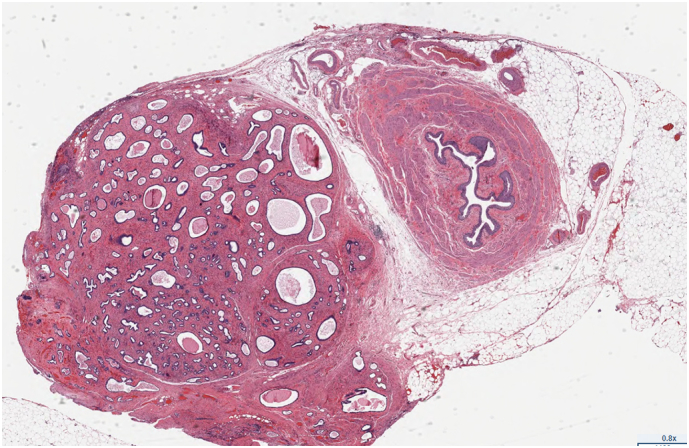
Nodule of Endometriosis(left) adjacent to Ureter(right) (E&M stain).
Fig. 4.
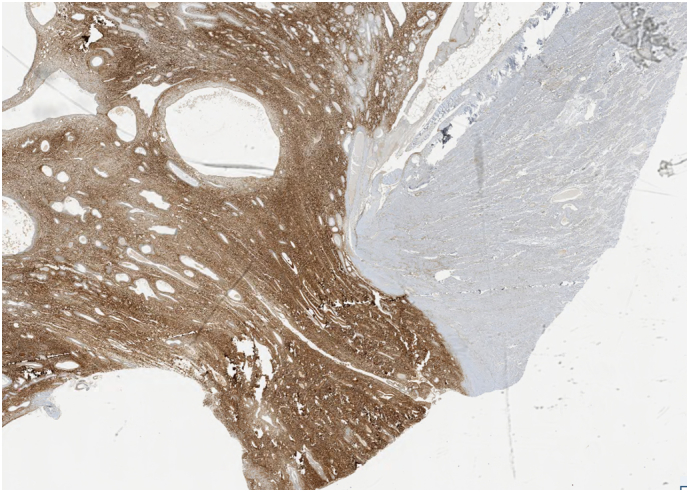
CD10 Immunohistochemical stain positivity(left) on parametria.
The patient was evaluated by Gynecology Oncology at our facility and her case reviewed in a multidisciplinary tumor board. Under the high suspicion for advanced gynecological malignancy the patient then underwent a diagnostic laparoscopy showing multiple confluent plaque-like implants on bilateral ovaries, bladder peritoneum, and diffusely over the posterior cul-de-sac with several rectosigmoid colon implants. Her right ureter was densely adherent to the right ovarian tumor. Her appendix was also enlarged, but frozen section favored low grade disease of mullerian origin. In order to achieve complete cytoreduction the case was converted intraoperatively to a laparotomy and a posterior pelvic supra-levator posterior exenteration with end-to-end colo-rectal anastomosis was performed. The distal right ureter was resected and ureteroneocystotomy performed. An enlaraged right para-aortic lymph node was resected, along with bilateral pelvic lymph node dissection.
Of note, final pathology revealed endocervical polypoid lesions, four 1.5–3.5 cm bulbous endometrial polyps, and multiple polypoid nodules 0.2–3 cm on the posterior uterine serosal surface. Multiple 1-2 cm nodules covered both ovaries, similar sized nodules as well as an aggregated fibroadenomatous 5.5 × 2.5 × 0.9 cm mass covered the segment of recto-sigmoid colon. The tissue samples were sent to Joint Pathology Center (Silver Spring, MD) for definitive diagnosis of the biphasic mullerian process with differentials including endometriosis and low grade mullerian tumor. Final pathology resulted for a fibroid uterus with endometriosis on uterus, ovaries, left fallopian tube, distal colon, peritoneum, appendix, and soft tissue adjacent to ureter.
2. Discussion
Endometriosis is a benign estrogen responsive disease process involving endometrial tissue outside the endometrium (Olive and Schwartz, 1993). It is most commonly encountered in the third and fourth decade of life, typically a slowly progressing condition, presenting as pelvic pain or infertility (Olive and Schwartz, 1993). This case exemplifies a rare presentation of clinically progressive endometriosis in a postmenopausal female, possibly exacerbated by severe obesity and tamoxifen use.
Tamoxifen is a selective estrogen modulator used in the treatment and prevention of breast cancer. It is an estrogen receptor antagonist in the breast, but an estrogen receptor agonist in the endometrium. As a result, tamoxifen can increase the risk of endometrial cancer, and users are monitored closely for abnormal uterine and postmenopausal bleeding (Choi et al., 2015). Several factors from the patient's medical history, to include infertility are suggestive of a previous history of endometriosis. While endometriosis classically becomes suppressed in the low-estrogen state of menopause, our patient displayed a clinical presentation more consistent with progressive disease. Taken together, the high estrogen milieu from the adipose pathway of obesity in combination with the agonist action of tamoxifen most likely contributed to the clinically progressive symptoms resulting from the patient's endometriosis.
Our review of the literature suggests that extensive surgical cytoreduction, often to include hysterectomy, bilateral salpingo-oophorectomy, bowel or ureter resection is frequently performed for the treatment of extensive endometriosis (Karimi et al., 2009, Bese et al., 2003). Effects of aggressive endometriosis often include bowel obstruction or, as in our case, impact on urinary function requiring surgical management. Biopsy confirmation of the endometriosis, as opposed to malignancy, could theoretically also allow for a less extensive debulking effort. Even with a benign preoperative biopsy, it is hard to imagine the management of this case would have changed due to disease distribution and concern for occult malignancy.
An additional factor to consider in the management of patients with extensive endometriosis is not only concern for an occult cancer, but also their increased risk for developing ovarian cancer (Parker et al., 2004, Pearce et al., 2012). Endometriosis is a risk factor for the development of invasive epithelial ovarian cancer, especially that of low-grade serous, endometrioid, and clear-cell ovarian cancer (Parker et al., 2004, Pearce et al., 2012). Endometriosis can be thought of as a precursor lesion for certain subtypes of ovarian cancer (Parker et al., 2004, Pearce et al., 2012). This should be taken into consideration in both the workup and treatment of invasive presentations of endometriosis.
There are few reported cases of endometriosis mimicking cancer and even fewer with an association to tamoxifen use (Choi et al., 2015, Parker et al., 2004, Chang et al., 2003, Laird et al., 2004, Schlessinger and Silverberg, 1999, Naufel et al., 2014, Matsuura et al., 2009). This case represents an unusually aggressive presentation of endometriosis in a postmenopausal woman from our literature review. Parker et al. described 24 cases of polypoid endometriosis – a rare form of endometriosis where polypoid masses form which resemble malignant tumors (Parker et al., 2004). Many of these cases also demonstrated difficulty in differentiation from low-grade mullerian neoplasm as in our case (Parker et al., 2004). The characteristic pathological features described by Parker et al. match those demonstrated in our case, confirming the diagnosis of polypoid endometriosis (Parker et al., 2004). Only one of the Parker cases featured a patient using tamoxifen; in this case the polypoid endometriosis was incidentally discovered and limited to the uterus and ovaries (Parker et al., 2004). Additionally, our literature review revealed 9 cases of severe postmenopausal endometriosis with associated tamoxifen use (Choi et al., 2015, Chang et al., 2003, Laird et al., 2004, Schlessinger and Silverberg, 1999, Naufel et al., 2014). Most presentations of tamoxifen effect were in the form of an isolated pelvic or adnexal mass, typically measuring 4-10 cm (Choi et al., 2015, Chang et al., 2003, Laird et al., 2004, Schlessinger and Silverberg, 1999, Naufel et al., 2014). Tamoxifen use ranged from a 7.3–116.8 g cumulative dose, with no clear correlation between tamoxifen dose and tumor burden noted on review (Schlessinger and Silverberg, 1999). The bulk of these cases were described in a case series of polypoid endometriosis by Schesinger and Silverberg: 12 of their 17 cases had a history of tamoxifen use, but only 6 involved postmenopausal patients with findings concerning for malignancy (Schlessinger and Silverberg, 1999). They postulate, as do we, that tamoxifen may exert an agonistic effect on endometriosis in both pre and postmenopausal patients, potentially resulting in either malignant or proliferative disease (Schlessinger and Silverberg, 1999). We identified a total of 4 invasive postmenopausal endometriosis cases with associated tamoxifen use since 1999. Even systemic endometriosis, such as periurethral, rectal and thoracic disease was noted associated with tamoxifen use in perimenopausal and postmenopausal patients (Thiels et al., 2016, Matsuura et al., 2009, Hajjar et al., 1993).
Considerations for alternative management strategies we could have employed include: diagnostic laparoscopy with biopsies followed by more conservative management with tamoxifen discontinuation, more focused resection using a minimally invasive platform, or the use of GnRH agonists. The difficulty, as demonstrated in our case, is in obtaining adequate sampling to safely assure a benign diagnosis in order to avoid more extensive surgery. This patient ultimately underwent appropriate radical resection of endometriosis and resultant return of normal bowel and bladder function, with prevention of potential endometriosis-associated invasive cancer. Suspicion for concurrent malignant progression when endometriosis is found in peri- and post-menopausal patients should remain high. A definitive surgical management strategy and appropriate comprehensive pre-operative counseling is advised. More research is needed to refine the current understanding and characterization of tamoxifen function and direct effects on endometriosis using molecular tools.
Funding
This project has not received any funding.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
Acknowledgments
None.
Informed consent
Written and Verbal Informed consent has been obtained from the patient and can be provided upon request.
References
- Bese T., Smsek Y., Bese N., Ilvan S., Arvas M. Extensive pelvic endometriosis with malignant change in tamoxifen-treated postmenopausal women. Int. J. Gynecol. Cancer. 2003 May–Jun;13(3):376–380. doi: 10.1046/j.1525-1438.2003.13188.x. [DOI] [PubMed] [Google Scholar]
- Chang C., Chen P., Leu F. Florid polypoid endometriosis exacervated by tamoxifen therapy in breast cancer. Obstst. Gynecol. 2003:1127e30. doi: 10.1016/s0029-7844(03)00628-8. [DOI] [PubMed] [Google Scholar]
- Choi I.H., Jin S., Jeen Y.M., Lee Y.M., Lee J.J., Kim D.W. Tamoxifen-associated polypoid endometriosis mimicking an ovarian neoplasm. Obstet. Gynecol. Sci. 2015;58(4):327–330. doi: 10.5468/ogs.2015.58.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar L.R., Kim W. Intestinal and pelvic endometriosis presenting as a tumor and associated with tamoxifen therapy: report of a case. Obstet. Gynecol. 1993;(4 pt 2 Suppl):624. [PubMed] [Google Scholar]
- Karimi Z.M., Behtash N., Sekhavat L., Dehghan A. Effects of tamoxifen on the cervix and uterus in women with breast cancer: experience with Iranian patients and a literature review. Asian Pac. J. Cancer Prev. 2009;10(4):595–598. [PubMed] [Google Scholar]
- Laird L.A., Hoffman J.S., Omrani A. Multifocal polypoid endometriosis presenting as huge pelvic masses causing deep vein thrombosis. Arch. Pathol. Lab. Med. 2004;128(5):561–564. doi: 10.5858/2004-128-561-MPEPAH. [DOI] [PubMed] [Google Scholar]
- Matsuura M., Fujiwara T. Catamenial pneumothorax with breast cancer treated successfully by goserelin acetate. Kyobu Geka. 2009;62(11):1015–1018. [PubMed] [Google Scholar]
- Naufel D.Z., Penachim T.J. Atypical retroperitoneal endometriosis and use of tamoxifen. Radiol. Bras. 2014;47(5):323–325. doi: 10.1590/0100-3984.2013.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive D.L., Schwartz L.B. Endometriosis. N. Engl. J. Med. 1993;328:1759. doi: 10.1056/NEJM199306173282407. [DOI] [PubMed] [Google Scholar]
- Parker R.L., Dadmanesh F., Young R.H., Clement P.B. Polypoid endometriosis: a Clinicopathologic analysis of 24 cases and a review of the literature. Am. J. Surg. Pathol. 2004;28:285–297. doi: 10.1097/00000478-200403000-00001. [DOI] [PubMed] [Google Scholar]
- Pearce C.L., Templeman C., Rossing M.A., Lee A., Near A.M. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case–control studies. Lancet Oncol. 2012;13(4):385–394. doi: 10.1016/S1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger C., Silverberg S.G. Tamoxifen-associated polyps (basalomas)arising in multiple endometriotic foci: a case report and review of the literature. Gynecol. Oncol. 1999;73(2):305–311. doi: 10.1006/gyno.1998.5305. [DOI] [PubMed] [Google Scholar]
- Thiels C.A., Shenoy C.C., Ubl D.S., Haberman E.B., Kelley S.R., Mathis K.L. Rates, trends, and short-term outcomes of colorectal resections for endometriosis: an ACS-NSQIP review. Int. J. Surg. 2016;31:5–9. doi: 10.1016/j.ijsu.2016.05.039. [DOI] [PubMed] [Google Scholar]


