Abstract
Grammatophyllum speciosum, a native species to Singapore, have become extinct mainly due to habitat loss. Recently, Singapore has reintroduced G. speciosum into the natural environment under the orchid conservation programme. In this study, leaves of G. speciosum grown under low light (LL) under natural conditions had faster expansion rate and higher specific leaf area than leaves grown under intermediate light (IL) and high light (HL). All leaves had more than 95% midday relative water content. Although midday Fv/Fm ratios were lower in HL leaves than in IL and LL leaves, none of them exhibited chronic photoinhibition. HL leaves had upregulated their light utilization through higher photochemical quantum yield (ΔF/Fm′) and greater electron transport rate. HL leaves also had higher non-photochemical quenching, indicating that they had higher capability to dissipate excess light as heat, which was supported by their lower chlorophyll but higher carotenoids content. Although there was a linear correction between leaf temperature and photosynthetic photon flux density (PPFD), no correlations were found between stomatal conductance (gs) and PPFD, gs and leaf temperature. Light-saturated photosynthetic CO2 assimilation rate (A sat) was significantly higher in HL leaves than those of IL and LL leaves. However, all leaves had similar light-saturated stomatal conductance. Although LL leaves had higher leaf total reduced nitrogen that those of IL and HL leaves, none of them seemed to suffer from nitrogen deficiency during the experimental period. To conclude, G. speciosum is able to survive under different growth irradiances without watering and adding fertilizers.
Keywords: CO2 assimilation, Photosynthetic pigments, Fv/Fm ratio, Leaf temperature, Relative water content, Specific leaf area, Stomatal conductance, Total reduced nitrogen
Background
Singapore houses 226 species of native tropical orchids. However, many of our natural habitats have been destroyed due to urbanisation. Besides the five common species, 178 species are extinct while the rest are either critically endangered or vulnerable (Ng 1994). One of the extinct species is Grammatophyllum speciosum, also known as the tiger orchid, which is the largest epiphytic C3 orchid plant in the world. Since 1995, The National Parks Board (NParks) in Singapore has been efficacious in propagating and reintroducing G. speciosum in its orchid conservation programme (Yam et al. 2010). More than 80% of native orchids planted on trees and ground in parks and nature reserve areas under different growth irradiances have survived (Yam 2013; Yam et al. 2011). Those could not have suffered from the complex interactions of high irradiances, high temperature, drought stress and nutrient deficiency.
Under tropical field conditions, orchids cope with variation of light intensities daily. Excessive light is one of the environmental stresses experienced by orchid plants (He et al. 1998, 2004, 2014; Koh et al. 1997; Tay et al. 2015). When they absorb excess light energy, if not dissipated as heat, it can be detrimental to photosystem II (PSII), known as dynamic or chronic photoinhibition (He et al. 1996). Dynamic photoinhibition is a reversible downregulation mechanism to reduce the light utilisation efficiency by diverting the excessive energy to the xanthophyll cycle so as to protect PSII reaction centres from photodamage (Demmig-Adams and Adam III 1992; Chow 1994; Osmond 1994). On the other hand, the excess energy could cause damage to the photosynthetic reaction centres, particularly of PSII, leading to sustained photoinhibition and then reduction of plant growth and productivity (Powles 1984; Osmond 1994; Barber 1995; Adams III et al. 2006). Chronic photoinhibition is the slower reversible loss of the function of PSII which is dependent on the repair and recovery rates of D1 protein in PSII reaction centres (Osmond 1994). Long term exposure to high irradiance would lead to irreversible oxidation of the chlorophyll (Chl) and destruction of chloroplasts due to formation of reactive oxygen species (ROS) (Lichtenthaler and Wellburn 1983). Thus, orchids grown under different growth irradiances, have different amount of Chl a, b and carotenoids (Car) (Lichtenthaler and Burkart 1999) with plants under high irradiance having more Car especially xanthophyll to photoprotect PSII from damage.
When the absorbed light energy exceeds the requirement of carbon fixation for light energy under high temperature or water deficit condition, photoinhibition is exacerbated (Lawlor and Tezara 2009). With water deficit, there is a reduction in leaf turgor potential and water content (Gomes et al. 2008); formation of ROS, which destroys the cell membrane, nucleic acid and protein (Ashraf 2009) such as Rubisco, an important carboxylase in C3 plants, thus decreasing carboxylation efficiency. Also, stomata close with water deficit to reduce transpiration which affects the leaf temperature (Farquhar and Sharkey 1982). Stomatal closure could also reduce photosynthesis by direct effects on the photosynthetic capacity of the mesophyll cells (Jones 1998). Closure of stomata results in reduced light-saturated stomatal conductance (g s sat) and photosynthetic CO2 assimilation, (A sat) affecting leaf growth and productivity (He et al. 2001).
Photoinhibition can also be aggravated by the lack of nutrients. Nitrogen (N) is often considered as the most limiting nutrient for the growth and yield of plants worldwide (Angus et al. 1993). N is a key component for protein synthesis and specifically for the maintenance of the abundant proteins associated with the photosynthetic apparatus (Loebl et al. 2010). Since PSII reaction centers are constantly damaged by high irradiances, cells need N to synthesis new proteins required for PSII repair (Nishiyama et al. 2006). If PSII repair cannot counteract photoinactivation due to N depletion, then the cells are subjected to chronic photoinhibition (Ragni et al. 2008).
Very little is known about the growth and photosynthetic performances of G. speciosum grown under natural tropical conditions. Furthermore, leaf photosynthetic gas exchange of plants subjected to mild drought stress may be largely due to stomatal limitation, rather than biochemical factors such as Rubisco protein (Lawlor and Cornic 2002; Bota et al. 2004; Flexas et al. 2006). Since all G. speciosum plants grown under natural conditions used for this project were maintenance free without watering and fertilization, it was often questioned that if N deficiency had occurs in these plants. Thus, using the native orchid species, G. speciosum, this project aimed to study (1) the effects of different growth irradiances on leaf growth, water relations and photosynthetic gas exchanges, (2) photosynthetic utilization of light energy and, (3) the concentration of leaf total reduced nitrogen (N) from leaves grown under different growth irradiances.
Methods
Plant materials
Grammatophyllum speciosum plants were planted on the grounds of National Institute of Education, Singapore in October 2012 by NParks under different growth irradiances, namely high light (HL, open field), intermediate light (IL) and low light (LL) respectively. The IL and LL conditions were obtained from different levels of shading by the surrounding trees. The average maximal photosynthetic photon flux density (PPFD) during midday at the top of the canopy were ca. 2000, 1200 and 600 under HL, IL and LL, respectively on sunny days. About 30 plants were planted under each of the light conditions. The 5th fully expanded leaves from the top of the plants were tagged for measurements from January to November 2013. These orchids were neither watered nor fertilized.
Measurement of leaf length and specific leaf area (SLA)
The lengths of the tagged leaves were measured thrice by inserting the stick abaxially. Six weeks later, leaves were harvested. To estimate SLA, six leaf discs (3.4 cm2) per individual condition were collected with a cork borer. The leaf’s midveins were avoided to reduce variations. The discs were dried to constant mass at 80 °C, and then weighed. Dry weight (DW) was used to calculate the SLA, the ratio of leaf area to DW. The measurements were carried out from February to March 2013.
Measurement of midday leaf relative water content (RWC)
On sunny days during March and October, leaves were harvested during midday. Six small squares (1 cm by 1 cm) were cut from one leaf before weighing the fresh weight (FW) instantly with an analytical balance (Sartorius). The samples were floated on water in the dark for 24 h before measuring their saturated weight (SW). They were then dried in the oven at 80 °C for 72 h before DW was obtained. RWC was calculated as RWC = (FW − DW)/(SW − DW) × 100%.
Measurements of photosynthetic photon flux density (PPFD), predawn and midday Fv/Fm ratios
PPFDs were measured using a photosynthetically available radiation quantum sensor and reading unit (Skye Instruments Ltd, Llandrindod, UK). They were measured from six different positions above the leaves for HL, IL and LL respectively. Chl fluorescence Fv/Fm ratios measured the potential efficiency of excitation energy captured by PSII. The non-destructive measurements were carried out predawn and during midday (1200–1300 h) with the Plant Efficiency Analyser, PEA (Hansatech Instruments Ltd, England) on sunny days in March and October. Attached leaves were pre-darkened with clips for 15 min before measurements. Dark-adapted leaves were placed under the light pipe and irradiated with the pulsed lower intensity-measuring beam to measure F0, basal Chl fluorescence. Fm, maximum Chl fluorescence was assessed by 0.8 s of saturated pulse (> 6000 mol m−2 s−1). The variable fluorescence yield, Fv, was determined by Fm–Fo. The efficiency of excitation energy captured by open PSII reaction centres in dark adapted leaves was estimated by the fluorescence Fv/Fm ratio.
Measurements of different Chl fluorescence parameters
These measurements were carried out in October. Newly fully expanded leaves were harvested at 0800 h for Chl fluorescence analysis. The effective photochemical quantum yield (ΔF/Fm′), electron transport rate (ETR) and non-photochemical quenching (NPQ) of Chl fluorescence were determined using the Imaging PAM Chl Fluorometer (Walz, Effeltrich, Germany) at 25 °C in the laboratory. Leaf discs were pre-darkened for 15 min before the measurements. Images of fluorescence emission from the PAM Chl Fluorometer, were digitized within the camera via a Firewire interface (400 Mb/s) (Firewire-1394, Austin, TX, USA) to a personal computer for storage and analysis. The details of measuring light pulses, actinic illumination and saturation pulses were described previously (He et al. 2011). Rapid light curve measurements in the presence of actinic illuminations (Schreiber et al. 1997) were obtained through the application of a series of 10-s light exposures with increasing irradiance from 1 to 1585 µmol photons m−2 s−1. In the presence of actinic illumination, the current fluorescence yield (Ft = F), and the maximum fluorescence (Fm′) at the steady state, were determined, from which the effective PSII quantum yield, ΔF/Fm′ [(Fm′ − F)/Fm′)] and ETR (PPFD × ΔF/Fm′ × 0.5 × 0.84) could be calculated. The use of two photons is necessary to transport one electron (factor 0.5). Correction factor 0.84 takes into account that only a fraction of incident light is really absorbed by photosynthesis. NPQ was defined as: NPQ = (Fm − Fm′)/Fm′ (Rascher et al. 2000).
Determination of photosynthetic pigments
These measurements were carried out in both March and October. Newly fully expanded leaves were harvested at 0800 h. Samples of 0.05 g were weighed and cut into very small pieces before soaking in 5 ml of N,N-dimethylformamide in the dark for 48 h at 4 °C. They were then quantified spectrophometrically (Du 650, Beckman, USA) using the procedure of Wellburn (1994).
Measurements of diurnal changes in gs and leaf temperature
The g s and leaf temperature were measured every 2 h from 0800 to 1800 h using the leaf porometer chamber (SC-1, Decagon, U.S.) with a fixed diffusion path to the leaf surface. These measurements were carried out in March on three sunny days.
Measurements of Asat and gs sat
These measurements were carried out in both March and October on three sunny days. They were measured simultaneously with an open infrared gas analysis system with a 6 cm2 chamber (LI-6400, Biosciences, US) between 0900 and 1030 h. Readings were measured with a LED light source, which supplied a saturated PPFD of 1000 mol m−2 s−1. The light source emitted light in the wavelength range of 660–675 nm. Average ambient [CO2] and relative humidity in the chamber were 400 ± 5 µmol mol−1 and 70%, respectively.
Determination of leaf total reduced nitrogen (TRN)
In October, the same leaves used for the measurements of A sat and g s sat were harvested immediately after recording the values of A sat and g s sat. The leaves were then used for the analysis of TRN. Leaf TRN concentration was determined by Kjeldahl digestion of dried samples in concentrated H2SO4. Dry samples of 0.15 were placed into a digestion tube with a Kjeldahl tablet and 2.5 ml of concentrated H2SO4. The mixture was then digested (about 90 min) until clear. After the digestion was completed, the mixture was allowed to cool for 30 min and the TRN was determined by with a Kjeltec auto 2300 analyser.
Statistical analysis
One-way ANOVA was used to test for significant differences among different growth irradiances, using Tukey’s multiple comparison tests to discriminate the means (MINITAB, Inc., Release 15, 2007).
Results
Leaf length, SLA and midday leaf RWC
All leaves increased gradually in length over 6 weeks of measurement. Leaves of G. speciosum plants grown under LL seemed to grow faster than leaves grown under IL and HL, which were the longest after 6 weeks (Fig. 1A, p < 0.05). However, there was no significant difference in leaf length between IL and HL leaves after 6 weeks of expansion (Fig. 1A, p > 0.05). SLA was not significantly different between leaves grown under HL and IL but LL grown leaves had significantly higher SLA (Fig. 1B). No significant differences in midday leaf RWC were observed across the plants grown under three different growth irradiances (Fig. 2, p > 0.05). Results of midday leaf RWC shown in Fig. 2 were obtained on sunny days in October. Same measurements were carried out on sunny days in March and all leaves had midday RWC greater than 95%.
Fig. 1.
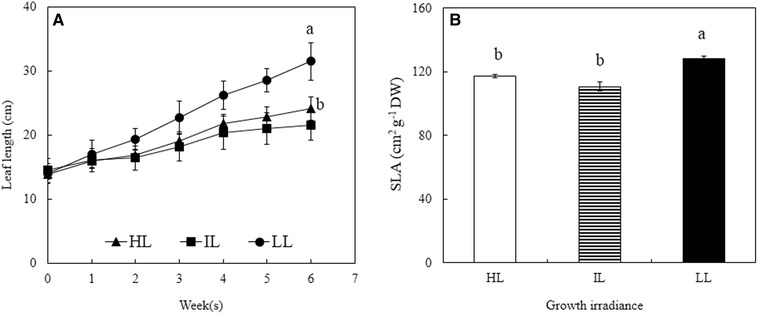
Leaf length (A) and SLA (B) of young expanding leaves of G. speciosum under different growth irradiances. Each value is the means from 6 different leaves of 6 different plants. Vertical bars represent SE. Means with same letter above the bars are not statistically different (p > 0.05) as determined by Tukey’s test
Fig. 2.
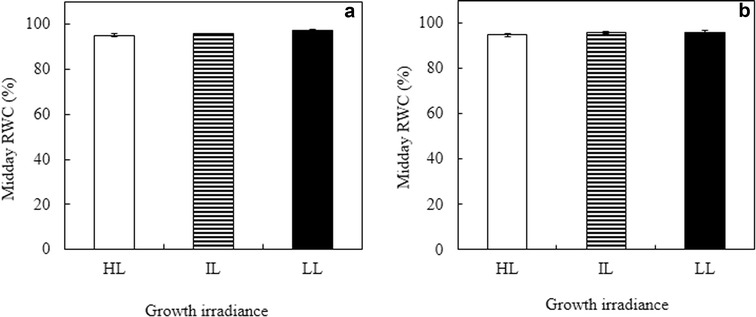
Midday RWC of young expanding (a) and fully expanded (b) leaves of G. speciosum under different growth irradiances. Each value is the mean from 6 different leaves of 6 different plants. Vertical bars represent SE
Midday and predawn Fv/Fm ratios measured under field conditions
These measurements were carried out in both March and October on sunny days. Similar results were obtained and thus, only data from October are shown in Fig. 3. Among all leaves, the lowest midday Fv/Fm ratio was recorded from the leaves that were grown under HL followed by those grown under IL and the highest Fv/Fm ratio was found in leaves exposed to LL (Fig. 3B, p < 0.05). Differences in midday Fv/Fm ratios corresponded with the levels of light to which they were exposed (Fig. 3A), that was, the higher the PPFD illuminated the leaves, the lower the midday Fv/Fm ratio. However, there were no significant differences in predawn Fv/Fm ratio and all the leaves had predawn Fv/Fm ratio ≥ 0.8 (Fig. 3C).
Fig. 3.
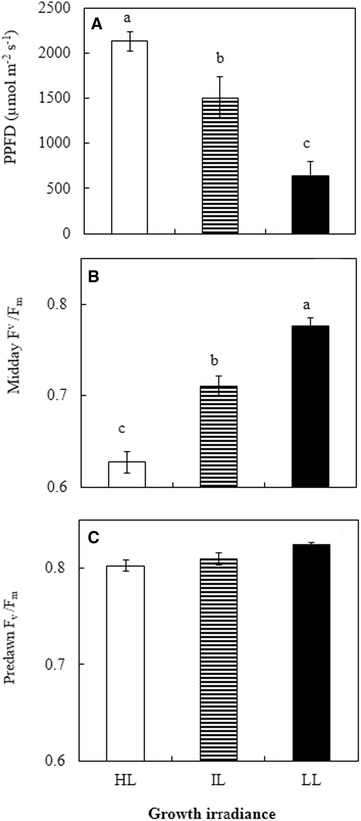
Midday PPFD (A), Midday (B) and predawn (c) Fv/Fm ratios and of leaves of G. speciosum under different growth irradiances on sunny days. Each value is the mean from 6 different leaves of 6 different plants measured from three sunny days. Vertical bars represent standard errors. Means with same letter above the bars are not statistically different (p > 0.05) as determined by Tukey’s test
Photochemical efficiency and photosynthetic pigments measured in the laboratory
Parameters such as F/Fm′ (photochemical quantum yield at actinic light) ETR and NPQ were used to explore the utilization of light energy by leaves grown under different growth irradiances. All results shown in Fig. 4 were obtained in October. The light response curves of these parameters were measured under different PPFDs in the laboratory from 1 to 1585 µmol m−2 s−1. When measured at the lowest PPFD of 1 µmol m−2 s−1, ΔF/Fm′ values were not significantly different among the different leaves ranging from 0.788 to 0.754. However, the values of ΔF/Fm′ decreased to 0.637, 0.549 and 0.468 measured at PPFD of 415 µmol m−2 s−1 for leaves grown under HL, IL and LL, respectively (Fig. 4a). The differences in ΔF/Fm′ among the different leaves at this PPFD were significantly different (p < 0.05). A further decreases of ΔF/Fm′ in all leaves were observed when PPFD was higher than 415 µmol m−2 s−1 with their value almost zero at PPFD of 1585 µmol m−2 s−1. However, the rate of deceases in ΔF/Fm′ was the fastest in leaves grown under LL followed by that of leaves under IL with increasing PPFD. Leaves grown under HL exhibited the slowest decrease rate of ΔF/Fm′ with increasing PPF. The HL leaves had the highest values of ΔF/Fm′ measured at higher PPFDs followed by the IL leaves and the LL leaves had the lowest values at each PPFD from 415 to 1255 µmol m−2 s−1 (Fig. 4a, p < 0.05).
Fig. 4.
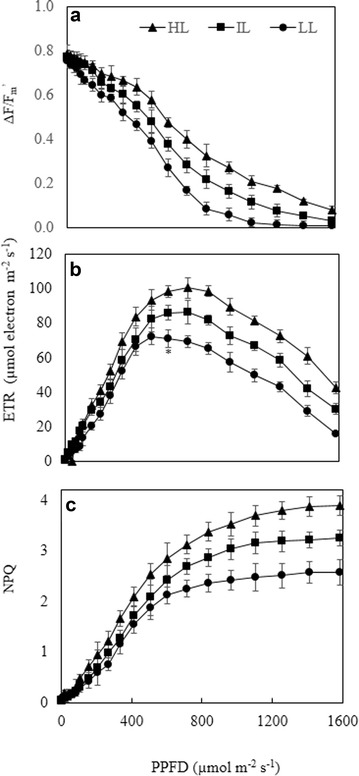
ΔF/Fm′ ratio (a), ETR (b) and NPQ (c) of leaves of G. speciosum grown under different growth irradiances. Each value is the mean from 30 readings of 6 different leaves from 6 different plants. Vertical bars represent standard errors. When the standard error bars cannot be seen, they are smaller than the symbols
Initially, there were steep increases of ETR values in all leaves with increasing PPFD until 715 µmol m−2 s−1, after which they all decreased sharply (Fig. 4b). Significant differences in ETR values were obtained among leaves grown under different grown irradiances from PPFD of 605 to 1585 µmol m−2 s−1 (p < 0.05). Although the changes of ETR to increasing PPFD were similar in all leaves, the ETR of LL leaves was the lowest at any given PPFD followed by that of IL leaves. The HL leaves had the highest ETR compared to those of IL and LL from 605 to 1585 µmol m−2 s−1.
There were gradual increases in NPQ of IL and HL leaves from 1 to 835 µmol photon m−2 s−1 after which they plateau around 2.69 and 3.12, respectively (Fig. 3C). The increase of NPQ was faster in HL leaves than in IL at the any given PPFD from 715 to 1585 µmol photon m−2 s−1 (p < 0.05). For LL leaves, although its NPQ also increased with increasing PPFD, it plateaus at much lower PPFD of 605 µmol photon m−2 s−1 compared to those of IL and HL leaves. At each given PPFD, LL leaves had the significantly lowest NPQ that those of IL and HL leaves from PPFD of 715 µmol photon m−2 s−1 onwards (p < 0.05).
Photosynthetic pigments of leaves of G. speciosum grown under different growth irradiances were analyzed in both March and October. Similar trends were observed and thus, only results obtained from Oct are presented in Fig. 5. The total Chl content was significantly higher in LL leaves than those of IL and HL leaves (Fig. 5A, p < 0.05). However, there was no significant difference in Chl a/b ratio across the different growth irradiances (Fig. 5B, p > 0.05). Total Car content of LL and IL leaves was not significantly different (p > 0.05) but they were much lower than that of HL leaves (Fig. 5C, p < 0.05). Chl/Car ratios of LL and IL leaves were higher than that of HL (Fig. 5D, p < 0.05) due to their higher Chl content (Fig. 5A) or lower Car content (Fig. 5C).
Fig. 5.
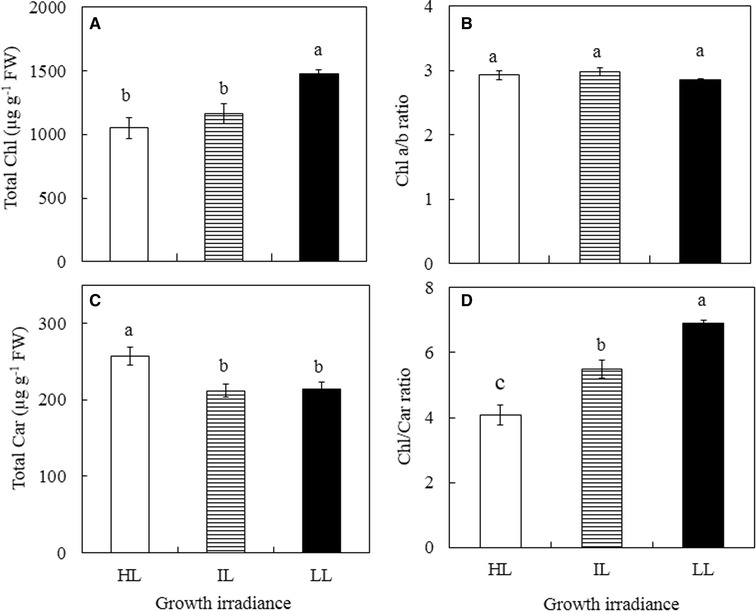
Total Chl content (A), Chl a/b ratio (B), total Car content (C) and Chl/Car ratio (D) of leaves of G. speciosum grown under different growth irradiances. Each value is the mean from 4 different leaves of 4 different plants. Vertical bars represent standard errors. Means with same letter above the bars are not statistically different (p > 0.05) as determined by Tukey’s test
Diurnal changes of PPFD, gs and leaf temperature and correlations among them
These measurements were carried out in March on three sunny days. Similar results were obtained among the three different sunny days. The values of PPFD (Fig. 6A) and leaf temperature (Fig. 6B) increased parallel from 0800 to 1400 h and then decreased after that. There were significant differences in PPFD (Fig. 6A), gs leaf temperature (Fig. 6B) and gs (Fig. 6C) across the different growth irradiances at 1000 h, when HL leaves had the highest values followed by those of IL leaves (p < 0.05). The LL leaves had the lowest leaf temperature and gs at the same given time compared to those of IL and HL leaves (p < 0.05). It was interesting to see that gs decreased from 1000 h onwards in all leaves. A close linear correlation between leaf temperature PPFD was established in leaves grown under different growth irradiance (Fig. 7a, r2 = 0.9106). However, there were no correlations between g s and PPFD (Fig. 7b, r2 = 0.2757), leaf temperature and g s (Fig. 7c, r2 = 0.1054).
Fig. 6.
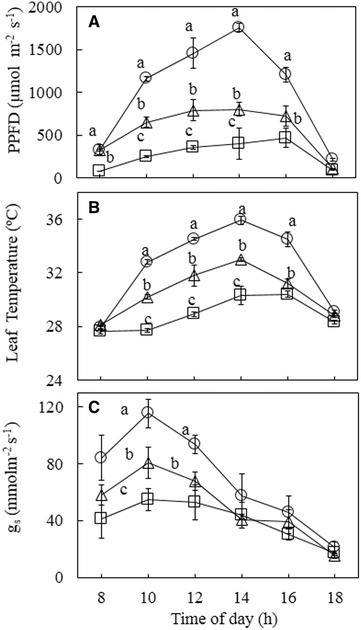
Diurnal changes in PPFD (A), leaf temperature (B) and gs (C) of leaves of G. speciosum grown under different growth irradiances. Each value is the mean from 6 different leaves of 6 different plants. Vertical bars represent standard errors. Means with same letter are not statistically different (p > 0.05) as determined by Tukey’s test
Fig. 7.
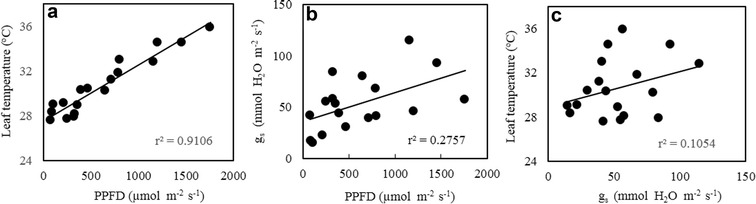
Correlations between leaf temperature and PPFD (a), gs and PPFD (b), leaf temperature and gs (c) of leaves of G. speciosum grown under different growth irradiances. All data derived from Fig. 6
Asat, gs sat and Leaf TRN
A sat of HL leaves was significantly higher than those of IL and LL leaves but not significantly different between IL and LL leaves (Fig. 8A, p < 0.05). However, there was no significant difference in the gs sat among all leaves (Fig. 8B, p > 0.05). For the leaf TRN concentration, there was no significant difference between HL and IL leaves (Fig. 8C, p > 0.05) but LL leaves had significant higher leaf TNR concentration compared to those of HL and IL leaves (Fig. 8C; p < 0.05). All results shown in Fig. 8 were obtained in October. A sat and g s sat were also measured in March and the trends were similar to those presented in Fig. 8A, B.
Fig. 8.
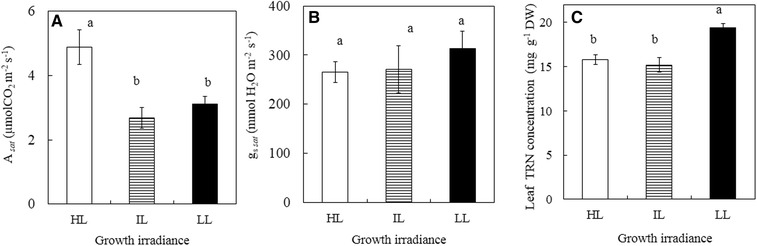
A sat (A), gs sat (B) and TRN (C) of leaves of G. speciosum grown under different growth irradiances. Each value is the mean from 4 different leaves of 4 different plants. Vertical bars represent standard errors. Means with same letter above the bars are not statistically different (p > 0.05) as determined by Tukey’s test
Discussion
The light environments for different leaves on the same plants vary according to the position of the sun and variable cloud cover. Furthermore, every leaf on the same plant was in different light environment due to its position on the plant in relation to the other leaves. These factors explained the differences in the variation in leaf development on the same plant. The extension zone of the G. speciosum, being a monocot, was enclosed by older leaves sheath shielding it from light (Dale 1988). This could be an added constraint. There might also be a wide variation in growth rate for leaves on the same plant. Too much light can cause detrimental damages to the photosynthetic apparatus (Lambers et al. 2008). On the other hand, insufficient light could reduce photosynthetic rates and a subsequent reduction in overall plant growth (He et al. 1998; Koh et al. 1997; He and Teo 2007). In the present study, leaves grown under LL seemed to expand significantly faster (Fig. 1A) and thinner reflected by higher SLA (Fig. 1B) than leaves grown under IL and HL. Under shady conditions, to optimise photosynthetic efficiency, SLA of leaves increased, resulting in longer and thinner leaves (Dale 1988). For plants grown under HL and IL conditions, reducing the surface area of leaves, leading to lesser water loss by evapotranspiration could be a strategy for water conservation (Tay et al. 2015).
Water deficit is one of the greatest limitations to the growth of epiphytic orchids (Laube and Zotz 2003; Zotz et al. 2010). However, in the present study, the midday RWC of all leaves grown under three growth irradiances were more than 95% for both young expanding (Fig. 2a) and fully expanded (Fig. 2b) leaves indicating that this orchid species did not suffer drought stress (Lizana et al. 2006; Lugojan and Ciulca 2011). Even though they were not irrigated, they could have a large supply of water from their pseudobulbs (Arditti 1992; He et al. 2011, 2014; Tay et al. 2015). Moreover, the study was carried out during the Northeast monsoon (October to November). The fat egg-shaped pseudobulbs of this tropical orchid store large amount of water during rainy season. Similar measurements were also carried out during the dry season (February to March). All leaves had more 95% midday RWC regardless of growth irradiances (data not shown). Stomatal regulation is one of the strategies preventing water loss (Yordanov et al. 2000). According to Cornic and Fresneau (2000), up to leaf RWC of about 70%, it is likely that stomatal closure plays the main role in preventing water loss (He et al. 2014; Tay et al. 2015). In the present study, decreased g s from 1000 h onwards (Fig. 6C) supported this postulation.
In this study, on sunny days, leaves grown under HL had much lower midday Fv/Fm ratio than those under IL and LL (Fig. 3B). HL leaves, being in open field, were exposed to maximum PPFD of 2133 μmol m−2 s−1, which were much higher than those of IL and LL leaves (Fig. 3A). The lowest midday Fv/Fm ratio due to higher PPFD indicated that dynamic PSII photoinhibition was much more severe in HL leaves than in IL and LL leaves. Photoinhibition could even occur at moderate or low light when other adverse conditions such as high temperature is present (He et al. 1996, 1998; He and Teo 2007). Leaf temperature (Fig. 6B) increased parallel with PPFD (Fig. 3A) from 0800 to 1400 h and the close linear correlation between leaf temperature and PPFD (Fig. 7a), suggested that dynamic PSII photoinhibition under tropical natural conditions resulted from the combination of high PPFD and high leaf temperature (He et al. 1996, 1998). It was reported that a large supply of water allowed leaves under all growth irradiances to modulate their leaf temperature, preventing them from overheating achieved through the evaporation of water from stomata known transpiration (He et al. 2001; Mohotti and Lawlor 2002; Hetherington and Woodward 2003; Crawford et al. 2012) and thus, protecting their PSII from photoinhibitory damages (Jagtap et al. 1998; He et al. 2001). However, in the present study, g s (Fig. 6C) increased from 0800 to 1000 h with increasing PPFD (Fig. 6A) in all leaves and it did not increase further with increasing PPFD and leaf temperature from 1000 h onwards. This result implied that G. speciosum had developed the avoidance of drought stress by widely opening stomata only for a short period of time in the early morning to conserve water (Ort 2001). Thus, the results of the present study did not support strategy of transpiration cooling as stomata of all leaves were partially closed with decreased gs (Fig. 6C) under high temperatures during midday (Fig. 6B). Although there were no correlations between the stomatal conductance and PPFD (Fig. 7b); or leaf temperature (Fig. 7c); across the different irradiances and across the whole day, g s (Fig. 6C) increased from 0800 to 1000 h with increasing PPFD (Fig. 6A). Stomata remain closed in the afternoon and opening only in the morning may play the main role in preventing water loss for this epiphytic tropical orchid (He et al. 2014; Tay et al. 2015). Furthermore, other environmental conditions such as humidity (Grantz 1990; Peak and Mott 2011) and vapour pressure deficit (VPD) (Day 2000; Shirke and Pathre 2004) may play crucial roles in regulating stomatal conductance.
Stomatal closure during midday could result in potential depletion of internal CO2. Low availability of CO2 at high light intensities may lead to the decrease of photosynthetic electron consumption, causing a dynamic photoinhibition (Osmond 1994; Cornic and Fresneau 2000). In the present study, all leaves had experienced dynamic photoinhibition measured by midday Fv/Fm ratios that were below 0.8 (Fig. 3B). However, all leaves completely recovered through the night from dynamic photoinhibition as they all had predawn Fv/Fm ratios greater than 0.8 (Osmond 1994). Rapidly reversible decreases in maximal PS II efficiency (i.e. lowered Fv/Fm ratios of pre-darkened leaves) is a photoprotective energy dissipation process, even though rates of photosynthetic electron transport remain maximal and photosynthesis is not inhibited (Adams et al. 1999). In the present study, leaves grown under HL had greater dynamic photoinhibition, these leaves, however, had higher ΔF/Fm′ (Fig. 4a), ETR (Fig. 4b), and NPQ (Fig. 4c), compared to those grown under IL and LL exhibiting their higher capacities of utilizing and dissipating light energy (He and Lee 2004; He et al. 2014). When a proportionally greater amount of the absorbed light cannot be utilized in photochemical activity at higher light levels, leaves normally upgregulated their Car level to dissipate excess photons through the xanthophyll cycle (Adams and Demmig-Adams, 1992; Demmig-Adams and Adams III 1992, 2006). When leaves grown under HL, there was an accompanied increase in Car (Fig. 5C), decrease in Chl (Fig. 5A) and thus, higher ratio of Car/Chl (Fig. 5D) to offer photoprotection (Armstrong and Hearst 1996; Puthur 2005). Shading increased Chl content of LL grown leaves (Fig. 5A, Anderson et al. 1991; Newman and Follett 1998). Plants adapted to HL are well known to have high Chl a/b ratio (Anderson 1986; Anderson and Osmond 1987). However, no significant differences were observed in Chl a/b ratios among all leaves. The low Chl content in leaves of HL plants exposing to maximal PPFD above 1500 μmol m−2 s−1 could be a result of adaptation (Anderson 1986).
Photosynthesis of healthy leaves increases proportionally to the increases in PPFD until its rate begins to saturate. Increases of photosynthetic CO2 assimilation rate with increasing PPFD for G. speciosum grown under all light conditions were very slow and they all began to show saturation under a PPFD about 600 µmol photon m−2 s−1 (data not shown). Using tropical crop species, Da Matta and colleagues (2001) studied the actual photosynthetic rate (A). They reported that A, determined under non-limiting light at ambient temperature and CO2, varied from 5.0 up to 26.3 µmol CO2 m−2 s−1. It was also reported previously, on an area basis, A covers a wide range, from less than 2 up to 70 µmol CO2 m−2 s−1 (Larcher 1995) and may even higher with a value of 80 µmol CO2 m−2 s−1 from Amaranthus retroflexus (Pearcy and Ehleringer 1984). In the present study, A sat (Fig. 8A) was higher in leaves grown under HL than under IL and LL from G. speciosum, a tropical native C3 orchid species. Higher leaf photosynthetic rate normally parallels its higher stomatal conductance at either high or low VPD environments (Shirke and Pathre 2004). In this study, g s sat did not seem to increase with increasing growth irradiance (Fig. 8B). VPD could affect the internal CO2 concentration (C i) however, all leaves had similar C i with average values around 280–300 mol CO2 mol−1 between 0900 and 1030 h (data not shown). A sat of 5 µmol CO2 m−2 s−1 (Fig. 8A) from HL grown G. speciosum leaves was much lower than most tropical C3 plants. The differences in A among species depend on the proportion of the absorbed excitation energy that is used for photosynthesis (Adams and Demmig-Adams 1992; Adams et al. 2002; Demmig-Adams and Adams 1992). On other hand, N availability is a major factor limiting growth and development of plants (Kraiser et al. 2011). It is a key component for protein synthesis and for the maintenance of the abundant proteins associated with the photosynthetic apparatus (Loebl et al. 2010). Although the LL grown leaves had higher total leaf TRN compared to HL and IL plants (Fig. 8C), none of them was suffered from N deficiency as all plants that had more than 1.5% N without supplying fertilizers to plants, during the experiment period (12 months after transplanting). Lower A sat was not caused by degradation of Rubisco either as all leaves had similar levels of Rubisco protein (data not show). Based on the above discussion, low photosynthetic rate and slow growth of G. speciosum could be mainly due to the limitation of internal CO2 concentration resulting from closing stomata for a long period of time during the day to conserve water (Ort 2001).
Conclusion
Leaves grown under LL expanded faster and they were thinner than those grown under IL and HL. Water deficit was not observed in any leaves as they could avoid drought stress by opening their stomata only during early morning and closing or partially closing them from midday onwards. Although HL grown leaves experienced dynamic photoinhibition but not chronic photoinhibition as these leaves had higher capacities of utilizing and dissipating light energy. Low photosynthetic rate of all leaves of G. speciosum was mainly due to the limitation of internal CO2 concentration instead of N deficiency. Slow but healthy growth of all G. speciosum under natural conditions was due to their different physiological acclimations to different growth environments.
Authors’ contributions
JH and TWY proposed the project based on TWY’s project on “Orchid conservation in Singapore”. JH and TWY carried out the first field study. JH supervised the laboratory analysis and repeated certain analysis. She also plotted all figures and wrote the manuscript. RMPL and SHJD, as MSc students carried out the experiments designed by HJ and TWY and analyzed the data. TWY planted all materials and also corrected the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This project was supported by the teaching vote, Ministry of Education, Singapore. We also thank Singapore Botanic Gardens and National Parks Board for providing the plant materials.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
My institution is still in the process of setting up the Data Repository. We will deposit our final data sets in our repository and inform you the URL after the paper is accepted for publication.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This project was supported by the teaching vote, Ministry of Education, Singapore.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- Asat
light-saturated photosynthetic CO2 assimilation
- Car
carotenoids
- Chl
chlorophyll
- DW
dry weigh
- ETR
electron transport rate
- Fo
minimal fluorescence yield of a “dark-adapted” sample
- Fm and Fv
maximal and variable fluorescence yields obtained from a dark-adapted sample upon application of a saturation pulse of radiation, respectively
- Fm′
the maximum fluorescence at the steady state
- Ft
the current fluorescence yield
- F/Fm′
the effective photochemical quantum yield
- Fv/Fm
ratio to estimate the maximum portion of absorbed quanta used in PSII reaction centers
- FW
fresh weight
- HL
high light
- IL
intermediate light
- LL
low light
- gs
stomatal conductance
- gs sat
light-saturated stomatal conductance
- NPQ
non-photochemical quenching
- PPFD
photosynthetic photon flux density
- PS II
photosystem II
- SLA
specific leaf area
- RWC
relative water content
- TRN
total reduced nitrogen
Contributor Information
Jie He, Email: jie.he@nie.edu.sg.
Regina M. P. Lim, Email: faithfulnessgrace@gmail.com
Sabrina H. J. Dass, Email: newsalem_13@yahoo.com
Tim W. Yam, Email: YAM_TIM_WING@nparks.gov.sg
References
- Adams WW, III, Demmig-Adams B. Operation of the xanthophyll cycle in higher plants in response to diurnal changes in incident sunlight. Planta. 1992;186:390–398. doi: 10.1007/BF00195320. [DOI] [PubMed] [Google Scholar]
- Adams WW, III, Demmig-Adams B, Logan BA, Barker DH, Osmond CB. Rapid changes in xanthophyll cycledependent energy dissipation and photosystem II efficiency in two vines, Stephania japonica and Smilax australis, growing in the understory of an open Eucalyptus forest. Plant Cell Environ. 1999;22:125–136. doi: 10.1046/j.1365-3040.1999.00369.x. [DOI] [Google Scholar]
- Adams WW, III, Demmig-Adams B, Rosenstiel TN, Brightwell AK, Ebbert V. Photosynthesis and photoprotection in overwintering plants. Plant Biol. 2002;4:545–557. doi: 10.1055/s-2002-35434. [DOI] [Google Scholar]
- Adams WW, III, Zarter CR, Mueh KE, Amiard V, Demmig-Adams B. Energy dissipation and photoinhibition: a continuum of photoprotection. In: Demmig-Adams B, Adams WW, Mattoo AK, editors. Photoprotection, photoinhibition, gene regulation, and environment. The Netherlands: Springer; 2006. pp. 49–64. [Google Scholar]
- Anderson JM. Photoregulation of the composition, function, and structure of thylakoid membranes. Annu Rev Plant Physiol. 1986;37:93–136. doi: 10.1146/annurev.pp.37.060186.000521. [DOI] [Google Scholar]
- Anderson JM, Osmond CB. Shade-sun responses: compromises between acclimation and photoinhibition. In: Kyle DJ, Osmond CB, Arntzen CJ, editors. Photoinhibition. Amsterdam: Elsevier; 1987. pp. 1–38. [Google Scholar]
- Anderson PC, Norcini JG, Knox GW. Influence of irradiance on leaf physiology and plant growth characteristics of Rhododendron x ‘Pink Ruffles’. J Amer Soc Hort Sci. 1991;116:881–887. [Google Scholar]
- Angus JF, Bowden JW, Keating BA. Modelling nutrient responses in the field. Plant Soil. 1993;156:57–66. doi: 10.1007/BF00024984. [DOI] [Google Scholar]
- Arditti J. Fundamentals of orchid biology. New York: Wiley; 1992. [Google Scholar]
- Armstrong GA, Hearst JE. Carotenoids 2: genetics and molecular biology of carotenoid pigment biosynthesis. FASEB J. 1996;10:228–237. doi: 10.1096/fasebj.10.2.8641556. [DOI] [PubMed] [Google Scholar]
- Ashraf M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotech Adv. 2009;27:84–93. doi: 10.1016/j.biotechadv.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Barber J. Molecular-basis of the vulnerability of photosystem-II to damage by light. Aust J Plant Physiol. 1995;22:201–208. doi: 10.1071/PP9950201. [DOI] [Google Scholar]
- Bota J, Medrano H, Flexas J. Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytol. 2004;162:67–681. doi: 10.1111/j.1469-8137.2004.01056.x. [DOI] [PubMed] [Google Scholar]
- Chow WS. Photoprotection and photoinhibitory damage. Adv Mol Cell Biol. 1994;10:15–196. [Google Scholar]
- Cornic G, Fresneau C. Photosynthetic carbon reduction and carbon oxidation cycles are the main electron sinks for photosystem II activity during a mild drought. Ann Bot. 2000;89:887–894. doi: 10.1093/aob/mcf064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AJ, Mclachlan DH, Herington AM, Franklin KA. High temperature exposure increases plant cooling capacity. Curr Biol. 2012;22:R396–R397. doi: 10.1016/j.cub.2012.03.044. [DOI] [PubMed] [Google Scholar]
- Da Matta FM, Loos RA, Rodrigues R, Barros RS. Actual and potential photosynthetic rates of tropical crop species. Rev Bras Fisiol Veg. 2001;13:24–32. doi: 10.1590/S0103-31312001000100003. [DOI] [Google Scholar]
- Dale JE. The control of leaf expansion. Ann Rev Plant Physiol Plant Mol Biol. 1988;39:267–295. doi: 10.1146/annurev.pp.39.060188.001411. [DOI] [Google Scholar]
- Day MD. Influence of temperature and leaf-to-air vapor pressure deficit on net photosynthesis and stomatal conductance in red spruce (Picea rubens) Tree Physiol. 2000;20:57–63. doi: 10.1093/treephys/20.1.57. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW., III Photoprotection and other responses of plants to high light stress. Ann Rev Plant Physiol Plant Mol Biol. 1992;43:599–626. doi: 10.1146/annurev.pp.43.060192.003123. [DOI] [Google Scholar]
- Demmig-Adams B, Adams WW., III Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. New Phytol. 2006;172:11–21. doi: 10.1111/j.1469-8137.2006.01835.x. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Sharkey TD. Stomatal conductance and photosynthesis. Ann Rev Plant Physiol Plant Mol Biol. 1982;33:317–345. doi: 10.1146/annurev.pp.33.060182.001533. [DOI] [Google Scholar]
- Flexas J, Ribas-Carbó M, Bota J, Galmés J, Henkle M, Martínez-Cañellas S, Medrano H. Decreased Rubisco activity during water stress is induced by stomatal closure, not by decreased relative water content. New Phytol. 2006;172:73–82. doi: 10.1111/j.1469-8137.2006.01794.x. [DOI] [PubMed] [Google Scholar]
- Gomes FP, Oliva MA, Mielke MS, Almeida AAFD, Leite HG, Aquino LA. Photosynthetic limitations in leaves of young Brazilian green dwarf coconut (Cocos nucifera L.‘nana’) palm under well-watered conditions or recovering from drought stress. Environ Exp Bot. 2008;62:195–204. doi: 10.1016/j.envexpbot.2007.08.006. [DOI] [Google Scholar]
- Grantz DA. Plant response to atmospheric humidity. Plant Cell Environ. 1990;13:667–679. doi: 10.1111/j.1365-3040.1990.tb01082.x. [DOI] [Google Scholar]
- He J, Lee SK. Photosynthetic utilization of radiant energy by temperate lettuce grown under natural tropical condition with manipulation of root-zone temperature. Photosynthetica. 2004;42:457–463. doi: 10.1023/B:PHOT.0000046166.29815.94. [DOI] [Google Scholar]
- He J, Teo LCD. Susceptibility of CAM Dendrobium Burana Jade green leaves and green flower petals to high light under tropical natural conditions. Photosynthetica. 2007;45:214–221. doi: 10.1007/s11099-007-0035-z. [DOI] [Google Scholar]
- He J, Chee CW, Goh CJ. “Photoinhibition” of Heliconia under natural tropical conditions—importance of leaf orientation for light interception and leaf temperature. Plant Cell Environ. 1996;19:1238–1248. doi: 10.1111/j.1365-3040.1996.tb00002.x. [DOI] [Google Scholar]
- He J, Kooh GH, Hew CS. Susceptibility of CAM Dendrobium leaves and flowers to high light and high temperature under natural tropical conditions. Environ Exp Bot. 1998;40:255–264. doi: 10.1016/S0098-8472(98)00042-2. [DOI] [Google Scholar]
- He J, Lee SK, Dodd IC. Limitations to photosynthesis of lettuce grown under tropical conditions: alleviation by root-zone cooling. J Exp Bot. 2001;52:1323–1330. doi: 10.1093/jexbot/52.359.1323. [DOI] [PubMed] [Google Scholar]
- He J, Ouyang W, Chia TF. Growth and photosynthesis of virus-infected and virus-eradicated orchid plants exposed to different growth irradiances under natural tropical conditions. Physiol Plant. 2004;121:612–619. doi: 10.1111/j.1399-3054.2004.00365.x. [DOI] [Google Scholar]
- He J, Tan BHG, Qin L. Source-to-sink relationship between green leaves and green pseudobulbs of C3 orchid in regulation of photosynthesis. Photosynthetica. 2011;49:209–218. doi: 10.1007/s11099-011-0023-1. [DOI] [Google Scholar]
- He J, Teh ZY, Yam TW. Orchid conservation in Singapore under natural conditions: responses of Grammatophyllum speciosum to growth irradiances. Plant Sci Interna. 2014;1:11–23. doi: 10.12735/psi.v1n1p11. [DOI] [Google Scholar]
- Hetherington AM, Woodward I. The role of stomata in sensing and driving environmental changes. Nature. 2003;424:901–908. doi: 10.1038/nature01843. [DOI] [PubMed] [Google Scholar]
- Jagtap V, Bhargava S, Streb P, Feierabend J. Comparative effect of water, heat and light stresses on photosynthetic reactions in Sorghum bicolor (L.) Moench. J Exp Bot. 1998;49:1715–1721. [Google Scholar]
- Jones HG. Stomatal control of photosynthesis and transpiration. J Exp Bot. 1998;49:387–398. doi: 10.1093/jxb/49.Special_Issue.387. [DOI] [Google Scholar]
- Koh GH, He J, Hew CS. Photosynthetic light utilization by CAM Dendrobium flowers. Photosyntetica. 1997;34:367–376. doi: 10.1023/A:1006807900507. [DOI] [Google Scholar]
- Kraiser T, Gras DE, Gutiérrez AG, González B, Gutiérrez RA. A holistic view of nitrogen acquisition in plants. J Exp Bot. 2011;62:1455–1466. doi: 10.1093/jxb/erq425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H, Chapin FS, III, Pons TL. Plant physiological ecology. The Netherlands: Springer; 2008. pp. 75–81. [Google Scholar]
- Larcher W. Physiological plant ecology. Berlin: Springer; 1995. [Google Scholar]
- Laube S, Zotz G. Which abiotic factors limit vegetative growth in a vascular epiphyte? Funct Ecol. 2003;17:598–604. doi: 10.1046/j.1365-2435.2003.00760.x. [DOI] [Google Scholar]
- Lawlor DW, Cornic G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002;25:275–294. doi: 10.1046/j.0016-8025.2001.00814.x. [DOI] [PubMed] [Google Scholar]
- Lawlor DW, Tezara W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Ann Bot. 2009;103:561–579. doi: 10.1093/aob/mcn244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK, Burkart S. Photosynthesis and high light stress. Bulg J Plant Physiol. 1999;25:3–16. [Google Scholar]
- Lichtenthaler HK, Wellburn AR. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans. 1983;11:591–592. doi: 10.1042/bst0110591. [DOI] [Google Scholar]
- Lizana C, Wentworth M, Martinez JP, Villegas D, Meneses R, Murchis EH, Pastenes C, Lercari B, Vernieri P, Horton P, Pinto M. Differential adaptation of two varieties of common bean to abiotic stress. Effects of drought on yield and photosynthesis. J Exp Bot. 2006;57:685–697. doi: 10.1093/jxb/erj062. [DOI] [PubMed] [Google Scholar]
- Loebl M, Cockshutt AM, Campbell DA, Finkel ZV. Physiological basis for high resistance to photoinhibition under nitrogen depletion in Emiliania huxleyi. Limnol Oceanogr. 2010;55:2150–2160. doi: 10.4319/lo.2010.55.5.2150. [DOI] [Google Scholar]
- Lugojan C, Ciulca S. Evaluation of relative water content in winter wheat. J Horti For Biotech. 2011;15:173–177. [Google Scholar]
- Mohotti AJ, Lawlor DW. Diurnal variation of photosynthesis and photoinhibition in tea: effects of irradiance and nitrogen supply during growth in the field. J Exp Bot. 2002;53:313–322. doi: 10.1093/jexbot/53.367.313. [DOI] [PubMed] [Google Scholar]
- Newman SE, Follett MW. Irrigation frequency and shading influences on water relations and growth of container-growth Euonymus japonica ‘Aureo-marginat’. J Environ Horti. 1998;6:96–100. [Google Scholar]
- Ng PKL. The Singapore red data book: Threatened plants and animals of Singapore, A Community Service Project by Asia Pacific Brewaries. Singapore: Nature Society; 1994. [Google Scholar]
- Nishiyama Y, Allakhverdiev S, Murata N. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. BBA Bioenerg. 2006;1757:742–749. doi: 10.1016/j.bbabio.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Ort DR. When there is too much light. Plant Physiol. 2001;125:29–32. doi: 10.1104/pp.125.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond CB (1994) What is photoinhibition? Some insights from comparisons of shade and sun plants. In: Baker NR, Bowyer, JR (eds) Photoinhibition of photosynthesis in the field. Oxford: Bios Scientific Publishing Oxford, pp 1–24
- Peak D, Mott KA. A new, vapour-phase mechanism for stomatal responses to humidity and temperature. Plant Cell Environ. 2011;34:162–178. doi: 10.1111/j.1365-3040.2010.02234.x. [DOI] [PubMed] [Google Scholar]
- Pearcy RW, Ehleringer J. Comparative ecophysiology of C3 and C4 plants. Plant Cell Environ. 1984;7:1–13. doi: 10.1111/j.1365-3040.1984.tb01194.x. [DOI] [Google Scholar]
- Powles SB. Photoinhibition of photosynthesis induced by visible-light. Ann Rev Plant Physiol Plant Mol Biol. 1984;35:15–44. doi: 10.1146/annurev.pp.35.060184.000311. [DOI] [Google Scholar]
- Puthur J. Influence of light intensity on growth and crop productivity of Vanilla planifolia Andr. Gen Appl Plant Physiol. 2005;31:215–224. [Google Scholar]
- Ragni M, Airs R, Leonardos N, Geider R. Photoinhibition of PSII in Emiliania huxleyi (Haptophyta) under highlight stress: the roles of photoacclimation, photoprotection and photorepair. J Phycol. 2008;44:670–683. doi: 10.1111/j.1529-8817.2008.00524.x. [DOI] [PubMed] [Google Scholar]
- Rascher U, Liebig M, Lüttge U. Evaluation of instant light-response curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll fluorometer on site in the field. Plant Cell Environ. 2000;23:1397–1405. doi: 10.1046/j.1365-3040.2000.00650.x. [DOI] [Google Scholar]
- Schreiber U, Gademann R, Ralph PJ, Larkum AWD. Assessment of photosynthetic performance of Prochloron in Lissoclinum patella by in situ and in hospite chlorophyll fluorescence measurements. Plant Cell Physiol. 1997;38:945–951. doi: 10.1093/oxfordjournals.pcp.a029256. [DOI] [Google Scholar]
- Shirke PA, Pathre UV. Influence of leaf-to-air vapour pressure deficit (VPD) on the biochemistry and physiology of photosynthesis in Prosopis juliflora. J Exp Bot. 2004;55:2111–2120. doi: 10.1093/jxb/erh229. [DOI] [PubMed] [Google Scholar]
- Tay S, He J, Yam TY. Photosynthetic light utilization efficiency, water relations and leaf growth of C3 and CAM tropical orchids under natural conditions. Am J Plant Sci. 2015;6:2949–2959. doi: 10.4236/ajps.2015.618290. [DOI] [Google Scholar]
- Wellburn AR. The spectral determination of chlorophylls A and B, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol. 1994;144:307–313. doi: 10.1016/S0176-1617(11)81192-2. [DOI] [Google Scholar]
- Yam TW. Native orchids of Singapore. Division, identification and conservation. Singapore: National Parks Board; 2013. [Google Scholar]
- Yam TW, Chua J, Tay F, Ang P. Conservation of the native orchids through seedling culture and reintroduction—A Singapore experience. Bot Rev. 2010;76:263–274. doi: 10.1007/s12229-010-9050-z. [DOI] [Google Scholar]
- Yam TW, Tay F, Ang P, Soh W. Conservation and reintroduction of native orchids of Singapore—the next phase. Eur J Environ Sci. 2011;1:27–38. [Google Scholar]
- Yordanov I, Velikova V, Tsonev T. Plant responses to drought, acclimation, and stress tolerance. Photosynthetica. 2000;38:171–186. doi: 10.1023/A:1007201411474. [DOI] [Google Scholar]
- Zotz G, Bogusch W, Hietz P, Ketteler N. Growth of epiphytic bromeliads in a changing world: the effects of CO2, water and nutrient supply. Acta Oecol. 2010;36:659–665. doi: 10.1016/j.actao.2010.10.003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
My institution is still in the process of setting up the Data Repository. We will deposit our final data sets in our repository and inform you the URL after the paper is accepted for publication.


