Abstract
Large childhood body size has been consistently shown to be associated with decreased breast cancer risk. However, it is important to consider the effects of a large childhood body size on other adult diseases. It is not clear if the associations between childhood body size and adult diseases will persist if they later attain healthy weight. The associations between body size at age 7 and 17 adverse outcomes in adulthood were examined using Cox models in a Swedish study of 65,057 women. Large body size at age 7, when compared to small body size, was associated with decreased risk for breast cancer (HR [95% CI]: 0.81 [0.70–0.93]) and increased risks for anorexia (2.13 [1.63–2.77]) and bulimia (1.91 [1.35–2.70]). Neither adjusting for adult BMI nor restricting the dataset to lean adults (BMI < 25 kg/m2) attenuated the associations. While large body size at age 7 by itself was positively associated with increased risks of diabetes (1.34 [1.16–1.55]), PCOS (1.69 [1.13–2.51]) and hypertension (before age 60), the associations were no longer significant after controlling for adult BMI. No clear associations were found with the remaining adverse outcomes (cervical, uterine, melanoma, colon cancer, depression, ovarian cyst, stroke, hyperlipidemia, heart failure, myocardial infarction, and angina pectoris).
Introduction
Obesity is classified as a chronic disease by the World Health Organization (WHO) (2000 report “Obesity: Preventing and Managing the Global Epidemic”), described as being prevalent in both developed and developing countries, and affecting children as well as adults. Various measures of both adult and childhood anthropometry such as BMI, height, waist-to-hip ratio, and body size have been extensively studied as risk factors for many adult diseases. In particular, research on the effect of childhood obesity on clinical disease in adulthood is of public health interest, as the potential for early intervention is high.
Childhood body size, in particular, is an interesting early life risk factor, as it has been consistently shown to confer protection against both pre- and postmenopausal breast cancer1,2. This counterintuitive link has caught media attention and sparked public interest. However, larger children are at greater risk of being overweight or obese when they get older, which may in turn confer a greater risk of poor health due to other causes and premature death in adulthood3,4. Diabetes, stroke, hypertension, angina and heart diseases which are commonly associated with adult obesity have also been demonstrated to be significantly associated with childhood body size5–13. In addition, potential links have been found between large childhood body size and increased risks of several cancers, which includes malignancies in the endometrium, colon, thyroid, liver, brain, and spine14–20. Childhood stature has also been associated with higher predisposition to adult melanoma21.
While it is unclear if estimates of simple body size are better indicators of adiposity than BMI, the use of somatotypes to measure childhood anthropometry has several advantages over childhood BMI. For example, BMI is generally defined in adults as an index of adiposity that is largely independent of stature; but BMI in children is highly influenced by height22. In addition, BMI is not only correlated with total body fat and percent body fat23–26, but also with fat-free mass22. Childhood BMI has been shown to perform poorly to moderately well in identifying children with excess adiposity, thus missing a large proportion of those who are truly overweight, as determined from percent body fat27–29. Women may also find it easier to recall approximate body size during childhood than BMI.
Although childhood body size is recognized as an early-life risk factor for many adult diseases, most studies to date have presented the associations with different diseases separately in different reports. However, most of these articles do not emphasize enough that associations is not causation, and that while large body size during childhood may decrease the risk of certain conditions such as breast cancer, it could also elevate the risks of other negative health outcomes. With the rise of large cohort studies, it is timely to examine the effect of childhood body size on multiple adult health conditions in the same study to give a well-rounded overview of the risk factor. We thus examined a wide range of long-term consequences of a large body size during childhood on later life well-being in a large Swedish study comprising 65,057 women. The main aim of this study was to clarify the association between childhood body size and different diseases using the same set of women. We also looked at whether childhood body size is an independent risk factor after adult BMI is taken into account. As it is unclear if the associations between childhood body size and adult diseases will persist if the children later attain healthy weight, we performed subset analyses in women who were not overweight (<25 kg/m2) in adult life and also in women who were neither overweight nor of a large body size during adolescence (age 18).
Methods
Study population
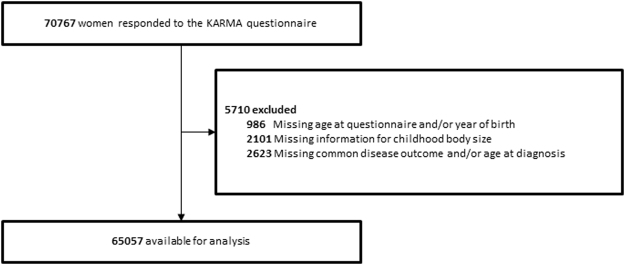
The KARMA (KARolinska MAmmography Project for Risk Prediction of Breast Cancer, http://karmastudy.org/) study is a well-characterized breast cancer cohort study in Sweden30. All women who underwent screening or clinical mammography between January 2011 and March 2013 at four hospitals (Stockholm South General Hospital, Helsingborg Hospital, Skåne University Hospital and Landskrona Hospital) were invited to participate. The mean ages of women at time of invitation and recruitment for the parent cohort were 53.7 (standard deviation [SD] 9.9) and 54.6 (SD 10.0) years, respectively. Approximately 53% of the recruited women were postmenopausal. Each participant gave informed consent and this study was approved by the ethical review board at Karolinska Institutet. A flow diagram of how the analytical cohort of 65,057 women was derived after the exclusion of 5,710 women with missing information on age, childhood body size and common diseases is shown in Fig. 1.
Figure 1.
Flow diagram of how the analytical cohort was derived.
Assessment of childhood body size
Body size at ages 7 and 18 were assessed using the Stunkard Figure Rating Scale31 in the KARMA questionnaire. The well-validated nine-level pictogram illustrates body sizes ranging from extreme thinness (category 1) to obesity (category 9)32,33, which we grouped into three major categories (small [categories 1 and 2], medium [categories 3 and 4], large [categories 5 and above])34 based on visual assessments of how similar the body sizes were. The major categories of childhood body size (small, medium and large) were treated as a semi-continuous variable (coded 0, 1, and 2) to estimate linear trend. Childhood body size was also evaluated as a continuous variable (nine levels). Childhood BMI was not collected.
Linkage to Swedish Cancer Register
Linkage to the Swedish Cancer Register (last updated 2013–12–31) was performed using Personal Identity Numbers, a ten–digit number that is unique for every resident in Sweden and used in all health-based registers35. Established in 1958, the Swedish Cancer Register is known for its high quality and coverage36. Although the Swedish Cancer Register includes all cancers (n = 5,346 in our study), we included only five specific sites (breast [n = 2,733], cervix uteri [n = 222], corpus uteri [n = 259], melanoma [n = 551] and colon [n = 235]) in our analyses in view of the number of events available for meaningful analyses.
Self-reported adverse outcomes derived from questionnaire
Participants were asked “Have you ever been diagnosed with any of the following by a medical doctor?” for 13 non-cancer adverse outcomes: diabetes, hypertension, hyperlipidemia, myocardial infarction, angina pectoris, heart failure, stroke, pre-eclampsia, polycystic ovary syndrome, ovarian cysts, depression, bulimia and anorexia. Corresponding years of diagnosis were also self-reported. Pre-clampsia was not included as an outcome of interest as it is a condition that occurs only during pregnancy.
Statistical analysis
Using Cox proportional hazards regression models, the associations between childhood body size (small, medium and large) and 17 adverse outcomes (5 cancers and 12 common adult diseases) were assessed. As the risk factors for pre- and postmenopausal breast cancer may be different37, we performed stratification by menopausal status. Chronological age was chosen as the underlying time scale. All analyses were stratified by year of birth (≤1950, 1951–1960, 1961 and later) to control for cohort effects. Women enter the analysis at age 7 (left-truncation). For each outcome trait studied, follow-up time was until age at diagnosis of the trait or age at questionnaire. The proportionality assumption of Cox regression was verified using scaled Schoenfeld residuals (cox.zph). The assumption of proportionality was violated for one outcome – hypertension. The time-dependent hazard ratios for hypertension were therefore estimated using flexible parametric survival models (stpm2 module in STATA).
To investigate whether the associations between childhood body size and common diseases in adulthood were independent of adult BMI, we adjusted for adult BMI. To investigate whether childhood body size was associated with common diseases in adulthood for women who were not overweight in adult life, we repeated the analyses in a subset of women with adult BMI < 25 kg/m2. To investigate whether childhood body size was associated with common diseases in adulthood for women who were neither overweight in adult life nor of a large body size during adolescence, we further repeated the analyses in a subset of women with adult BMI < 25 kg/m2 and of either a small or medium body size at age 18. In summary, we ran four models for each adverse outcome: no adjustment (*), adjusted for BMI at questionnaire (continuous, kg/m2) (†), no adjustment for a subset of women with BMI less than 25 kg/m2 at questionnaire (‡) and no adjustment for a subset of women with adult BMI less than 25 kg/m2 and either small or medium body size at age 18 (§). The described analyses were performed in R (version 3.3.1)38 unless otherwise stated. All analyses were performed in accordance with relevant institutional and national guidelines and regulations.
Restricting analysis to breast cancer controls
In Sweden, the proportion of breast cancer among all incident cancer cases (2010–2014) is 27.6%39. As women attending clinical mammography were also invited to participate in the KARMA study in addition to women attending screening, breast cancer cases are overrepresented in the KARMA study, contributing to over half of all cancer cases studied (Supplementary Table 1). In this study, the overlap between women with any common disease and breast cancer ranges from 5.5% to 9.6%. Given that childhood body size has been reported to be associated with breast cancer risk in opposite directions to those of other health outcomes, we repeated all analyses on a subset of 62,324 non-breast cancer participants.
Results
Participant characteristics
Table 1 describes participant characteristics of the 65,057 women included in the study. The average age at study entry was 55.1 years. One in five women had a diagnosis of hypertension, with the average age at diagnosis at 50.8 years. Other common health outcomes include depression (14.8%), hyperlipidemia (10.6%) and breast cancer (4.2%). Mean ages at diagnosis for diabetes, stroke, angina pectoris, myocardial infarction and heart failure were 48.9, 54.5, 55.2, 56.4 and 56.8 years, respectively. Health conditions with mean ages at diagnosis below 45 years include depression (41.5 years), ovarian cyst (36.1 years), PCOS (29.4 years), bulimia (25.3 years) and anorexia (19.6 years).
Table 1.
Characteristics of study population (n = 65,057) from Karolinska Mammography Project for Risk Prediction of Breast Cancer (KARMA), recruited between 2011 and 2013.
| Age at entry (years), mean (SD) | 55.1 (10.0) | |
| Age at menarche (years), mean (SD) | 13.1 (1.5) | |
| Irregular menstrual cycle during adult life, n (%) | 7626 (11.3) | |
| Physical activity at age 18 (h/week), n (%) | ||
| 0 | 2749 (4.2) | |
| <1 | 7294 (11.2) | |
| 1–2 | 17207 (26.4) | |
| 3–5 | 17377 (26.7) | |
| 5+ | 14570 (22.4) | |
| Education, n (%) | ||
| Elementary | 7518 (11.6) | |
| Intermediate | 17795 (27.4) | |
| College | 30012 (46.1) | |
| Other | 9559 (14.7) | |
| Number of children, n (%) | ||
| 0 | 8304 (12.8) | |
| 1 | 9622 (14.8) | |
| 2 | 30996 (47.6) | |
| 3+ | 16126 (24.8) | |
| Ever regular smoke, n (%) | 34423 (52.9) | |
| Body mass index at questionnaire (BMI, kg/m2), mean (SD) | 25.2 (4.2) | |
| Body size at age 7, n (%) | ||
| 1 | 15074 (23.2) | |
| 2 | 20032 (30.8) | |
| 3 | 14091 (21.7) | |
| 4 | 9433 (14.5) | |
| 5 | 4592 (7.1) | |
| 6 | 1511 (2.3) | |
| 7 | 260 (0.4) | |
| 8 | 53 (0.1) | |
| 9 | 11 (<0.1) | |
| Cancer outcomes, n (%) | Average age at diagnosis (years), mean (SD) | |
| Breast | 2733 (4.2) | 55.4 (10.1) |
| Cervical cancer | 222 (0.3) | 41.9 (10.9) |
| Uterine | 259 (0.4) | 58.8 (7.5) |
| Melanoma | 551 (0.8) | 47.8 (13.1) |
| Colon | 235 (0.4) | 55.0 (14.3) |
| Non-cancer outcomes, n (%) | ||
| Depression | 9642 (14.8) | 41.5 (11.4) |
| Anorexia | 498 (0.8) | 19.6 (7.1) |
| Bulimia | 318 (0.5) | 25.3 (9.7) |
| PCOS | 217 (0.3) | 29.4 (6.8) |
| Ovarian cyst | 4325 (6.6) | 36.1 (12.7) |
| Diabetes | 1805 (2.8) | 48.9 (15.5) |
| Hypertension | 12695 (19.5) | 50.8 (11.7) |
| Hyperlipidemia | 6869 (10.6) | 54.3 (10.1) |
| Stroke | 689 (1.1) | 54.5 (11.8) |
| Heart failure | 369 (0.6) | 56.8 (13.0) |
| Myocardial infarction | 479 (0.7) | 56.4 (9.4) |
| Angina pectoris | 570 (0.9) | 55.2 (10.7) |
Table 2 shows the associations between childhood body size and five common cancers in adulthood (breast, cervical, uterine, skin and colon). A significant dose-dependent inverse relationship was observed between childhood body size and breast cancer. Compared to small childhood body size, large childhood body size was associated with a 19% decrease in breast cancer risk in the unadjusted model (HRlarge vs small*: 0.81 [0.70 to 0.93], Pcontinuous* = 0.005). This association was found to be independent of adult BMI (HRlarge vs small†: 0.79 [0.68 to 0.91], Pcontinuous† = 0.001). Neither restricting the dataset to adults who were not overweight (<25 kg/m2) (HRlarge vs small‡: 0.73 [0.59 to 0.92], Pcontinuous‡ = 0.011) nor further restricting the dataset to adults who were not of a large body size at age 18 (HRlarge vs small §: 0.65 [0.49 to 0.86], Pcontinuous § = 0.013) attenuated the association. The effect sizes of the associations between childhood body size and adult breast cancer risk stratified by menopausal status were appreciably different in the crude and BMI-adjusted analyses. However, the reduced breast cancer risk appeared to be more pronounced for postmenopausal breast cancer in a subset of women who were lean during adolescence and adulthood (HRlarge vs smal § l: 0.55 [0.38 to 0.81], Pcontinuous § = 0.050). No clear association was observed between childhood body size and any of the other cancers studied (cervical, uterine, melanoma and colon).
Table 2.
Hazard ratios (HR) and corresponding 95% confidence intervals (CI) for the associations between childhood body size and different cancers in 65,057 women.
| Cancer Outcome | Body size | n | Crude | Adjusted for BMI | n‡ | Subset, adult BMI < 25 kg/m2, i.e. lean during adulthood | n§ | Subset, adult BMI < 25 kg/m2 and small/medium at 18, i.e. lean during adolescence and adulthood |
|---|---|---|---|---|---|---|---|---|
| HR (95% CI)* | HR (95% CI)† | HR (95% CI)‡ | HR (95% CI)§ | |||||
| Breast | Small | 1573 | 1.00 (Reference) | 1.00 (Reference) | 862 | 1.00 (Reference) | 852 | 1.00 (Reference) |
| Medium | 942 | 0.94 (0.87 to 1.02) | 0.93 (0.86 to 1.01) | 428 | 0.93 (0.83 to 1.05) | 399 | 0.94 (0.84 to 1.06) | |
| Large | 218 | 0.81 (0.70 to 0.93) | 0.79 (0.68 to 0.91) | 84 | 0.73 (0.59 to 0.92) | 51 | 0.65 (0.49 to 0.86) | |
| Trend | 2733 | 0.92 (0.86 to 0.97) | 0.90 (0.85 to 0.96) | 1374 | 0.89 (0.82 to 0.97) | 1302 | 0.88 (0.80 to 0.97) | |
| Continuous | 2733 | 0.96 (0.93 to 0.99) | 0.95 (0.93 to 0.98) | 1374 | 0.95 (0.91 to 0.99) | 1302 | 0.94 (0.90 to 0.99) | |
| Breast | Small | 514 | 1.00 (Reference) | 1.00 (Reference) | 306 | 1.00 (Reference) | 302 | 1.00 (Reference) |
| (Premenopausal) | Medium | 325 | 0.95 (0.83 to 1.09) | 0.95 (0.83 to 1.10) | 154 | 0.94 (0.78 to 1.15) | 143 | 0.96 (0.78 to 1.17) |
| Large | 80 | 0.84 (0.67 to 1.07) | 0.84 (0.67 to 1.07) | 35 | 0.89 (0.63 to 1.27) | 23 | 0.84 (0.55 to 1.29) | |
| Trend | 919 | 0.93 (0.84 to 1.03) | 0.93 (0.84 to 1.03) | 495 | 0.95 (0.82 to 1.09) | 468 | 0.94 (0.80 to 1.10) | |
| Continuous | 919 | 0.96 (0.91 to 1.00) | 0.96 (0.91 to 1.00) | 495 | 0.96 (0.89 to 1.03) | 468 | 0.95 (0.88 to 1.03) | |
| Breast | Small | 1044 | 1.00 (Reference) | 1.00 (Reference) | 545 | 1.00 (Reference) | 539 | 1.00 (Reference) |
| (Postmenopausal) | Medium | 608 | 0.94 (0.85 to 1.04) | 0.92 (0.83 to 1.02) | 269 | 0.93 (0.80 to 1.08) | 251 | 0.94 (0.81 to 1.10) |
| Large | 138 | 0.80 (0.67 to 0.96) | 0.78 (0.65 to 0.93) | 49 | 0.66 (0.50 to 0.89) | 28 | 0.55 (0.38 to 0.81) | |
| Trend | 1790 | 0.91 (0.85 to 0.98) | 0.90 (0.84 to 0.97) | 863 | 0.87 (0.78 to 0.97) | 818 | 0.85 (0.76 to 0.96) | |
| Continuous | 1790 | 0.97 (0.93 to 1.00) | 0.96 (0.92 to 0.99) | 863 | 0.95 (0.90 to 1.00) | 818 | 0.94 (0.89 to 1.00) | |
| Cervical | Small | 130 | 1.00 (Reference) | 1.00 (Reference) | 78 | 1.00 (Reference) | 78 | 1.00 (Reference) |
| Medium | 68 | 0.79 (0.59 to 1.06) | 0.80 (0.59 to 1.07) | 31 | 0.74 (0.49 to 1.12) | 30 | 0.77 (0.51 to 1.17) | |
| Large | 24 | 1.03 (0.66 to 1.58) | 1.03 (0.66 to 1.60) | 15 | 1.49 (0.85 to 2.58) | 8 | 1.13 (0.55 to 2.35) | |
| Trend | 222 | 0.93 (0.76 to 1.14) | 0.93 (0.76 to 1.14) | 124 | 1.04 (0.79 to 1.36) | 116 | 0.91 (0.67 to 1.25) | |
| Continuous | 222 | 0.96 (0.87 to 1.06) | 0.96 (0.87 to 1.06) | 124 | 1.00 (0.87 to 1.14) | 116 | 0.93 (0.79 to 1.08) | |
| Uterine | Small | 151 | 1.00 (Reference) | 1.00 (Reference) | 70 | 1.00 (Reference) | 67 | 1.00 (Reference) |
| Medium | 89 | 0.94 (0.72 to 1.22) | 0.87 (0.67 to 1.13) | 30 | 0.80 (0.52 to 1.22) | 23 | 0.68 (0.42 to 1.09) | |
| Large | 19 | 0.76 (0.47 to 1.22) | 0.66 (0.41 to 1.06) | 6 | 0.63 (0.27 to 1.44) | 3 | 0.47 (0.15 to 1.49) | |
| Trend | 259 | 0.90 (0.74 to 1.09) | 0.83 (0.69 to 1.01) | 106 | 0.79 (0.58 to 1.09) | 93 | 0.68 (0.46 to 1.00) | |
| Continuous | 259 | 0.97 (0.89 to 1.07) | 0.94 (0.85 to 1.03) | 106 | 0.93 (0.80 to 1.08) | 93 | 0.86 (0.72 to 1.03) | |
| Melanoma | Small | 301 | 1.00 (Reference) | 1.00 (Reference) | 186 | 1.00 (Reference) | 182 | 1.00 (Reference) |
| Medium | 184 | 0.94 (0.78 to 1.13) | 0.95 (0.79 to 1.14) | 93 | 0.93 (0.73 to 1.20) | 84 | 0.93 (0.71 to 1.20) | |
| Large | 66 | 1.25 (0.95 to 1.63) | 1.26 (0.96 to 1.65) | 24 | 0.99 (0.64 to 1.51) | 18 | 1.08 (0.67 to 1.76) | |
| Trend | 551 | 1.06 (0.93 to 1.20) | 1.06 (0.94 to 1.21) | 303 | 0.97 (0.81 to 1.16) | 284 | 0.98 (0.81 to 1.19) | |
| Continuous | 551 | 1.02 (0.96 to 1.09) | 1.03 (0.96 to 1.09) | 303 | 0.98 (0.90 to 1.07) | 284 | 0.99 (0.90 to 1.09) | |
| Colon | Small | 125 | 1.00 (Reference) | 1.00 (Reference) | 67 | 1.00 (Reference) | 66 | 1.00 (Reference) |
| Medium | 83 | 1.06 (0.80 to 1.39) | 1.06 (0.80 to 1.40) | 40 | 1.12 (0.76 to 1.66) | 36 | 1.10 (0.74 to 1.66) | |
| Large | 27 | 1.28 (0.85 to 1.94) | 1.28 (0.84 to 1.95) | 15 | 1.69 (0.97 to 2.97) | 9 | 1.49 (0.74 to 2.98) | |
| Trend | 235 | 1.11 (0.92 to 1.34) | 1.11 (0.92 to 1.34) | 122 | 1.24 (0.96 to 1.61) | 111 | 1.17 (0.87 to 1.57) | |
| Continuous | 235 | 1.05 (0.96 to 1.15) | 1.05 (0.96 to 1.15) | 122 | 1.12 (0.99 to 1.28) | 111 | 1.11 (0.96 to 1.28) |
Statistically significant associations (P < 0.05) are presented in bold. *Stratified by year of birth (1950, 1951–1960, 1961 and later). †Stratified by year of birth and adjusted for body mass index (BMI, kg/m2, continuous) at questionnaire. ‡Subset of women with adult BMI less than 25 kg/m2, stratified by year of birth. §Subset of women with adult BMI less than 25 kg/m and either small or medium body size at age 18, stratified by year of birth.
Table 3 shows the associations between childhood body size and eleven common diseases (depression, anorexia, bulimia, PCOS, ovarian cyst, diabetes, hyperlipidemia, stroke, heart failure, myocardial infarction and angina pectoris). In the crude analyses, when compared to a small childhood body size, a large childhood body size was associated with nearly two-fold increased risks with both anorexia (HRlarge vs small*:2.13 [1.63 to 2.77], Pcontinuous* < 0.001) and bulimia (HRlarge vs small*:1.91 [1.35 to 2.70], Pcontinuous* < 0.001). Similarly, larger childhood body size was associated with increased risk of diabetes and PCOS in a dose-dependent manner (HRlarge vs small *: 1.34 [1.16 to 1.55], Pcontinuous * = 0.021 and HRlarge vs small*: 1.69 [1.13 to 2.51], Pcontinuous* = 0.007, respectively). The increased risks for diabetes and PCOS conferred by larger childhood body size were attenuated when adult BMI was taken into account (HRlarge vs small†: 0.97 [0.84 to 1.13], Pcontinuous† = 0.005 and HRlarge vs small†: 1.25 [0.83 to 1.88], Pcontinuous † = 0.331, respectively). No clear dose-dependent associations were observed for depression, ovarian cyst, stroke, hyperlipidemia, heart failure, myocardial infarction and angina pectoris.
Table 3.
Hazard ratios (HR) and corresponding 95% confidence intervals (CI) for the associations between childhood body size and self-reported common diseases in 65,057 women.
| Outcome | Body size | n | Crude | Adjusted for BMI | n‡ | Subset, adult BMI < 25 kg/m2, i.e. lean during adulthood | n§ | Subset, adult BMI < 25 kg/m2 and small/medium at 18, i.e. lean during adolescence and adulthood |
|---|---|---|---|---|---|---|---|---|
| HR (95% CI)* | HR (95% CI)† | HR (95% CI)‡ | HR (95% CI)§ | |||||
| Depression | Small | 5266 | 1.00 (Reference) | 1.00 (Reference) | 2973 | 1.00 (Reference) | 2905 | 1.00 (Reference) |
| Medium | 3369 | 0.95 (0.91 to 0.99) | 0.90 (0.86 to 0.94) | 1483 | 0.92 (0.87 to 0.98) | 1345 | 0.92 (0.86 to 0.98) | |
| Large | 1007 | 1.05 (0.98 to 1.12) | 0.95 (0.89 to 1.02) | 387 | 1.03 (0.92 to 1.14) | 263 | 1.02 (0.90 to 1.15) | |
| Trend | 9642 | 1.00 (0.97 to 1.03) | 0.95 (0.92 to 0.98) | 4843 | 0.97 (0.93 to 1.02) | 4513 | 0.96 (0.92 to 1.01) | |
| Continuous | 9642 | 1.00 (0.99 to 1.02) | 0.97 (0.96 to 0.99) | 4843 | 0.99 (0.96 to 1.01) | 4513 | 0.98 (0.96 to 1.01) | |
| Anorexia | Small | 194 | 1.00 (Reference) | 1.00 (Reference) | 156 | 1.00 (Reference) | 150 | 1.00 (Reference) |
| Medium | 228 | 1.74 (1.43 to 2.10) | 2.22 (1.83 to 2.69) | 196 | 2.36 (1.91 to 2.91) | 180 | 2.41 (1.94 to 3.00) | |
| Large | 76 | 2.13 (1.63 to 2.77) | 3.18 (2.44 to 4.16) | 57 | 2.94 (2.17 to 3.99) | 38 | 2.89 (2.02 to 4.13) | |
| Trend | 498 | 1.51 (1.34 to 1.71) | 1.86 (1.65 to 2.10) | 409 | 1.84 (1.61 to 2.10) | 368 | 1.89 (1.64 to 2.19) | |
| Continuous | 498 | 1.25 (1.18 to 1.33) | 1.40 (1.32 to 1.49) | 409 | 1.39 (1.30 to 1.48) | 368 | 1.43 (1.33 to 1.54) | |
| Bulimia | Small | 121 | 1.00 (Reference) | 1.00 (Reference) | 89 | 1.00 (Reference) | 79 | 1.00 (Reference) |
| Medium | 154 | 1.85 (1.46 to 2.34) | 1.92 (1.51 to 2.44) | 101 | 2.11 (1.58 to 2.80) | 84 | 2.13 (1.57 to 2.89) | |
| Large | 43 | 1.91 (1.35 to 2.70) | 2.04 (1.43 to 2.91) | 21 | 1.92 (1.19 to 3.09) | 16 | 2.36 (1.38 to 4.04) | |
| Trend | 318 | 1.48 (1.27 to 1.72) | 1.53 (1.31 to 1.79) | 211 | 1.58 (1.30 to 1.91) | 179 | 1.72 (1.39 to 2.13) | |
| Continuous | 318 | 1.24 (1.15 to 1.34) | 1.27 (1.17 to 1.37) | 211 | 1.31 (1.19 to 1.44) | 179 | 1.37 (1.24 to 1.53) | |
| PCOS | Small | 102 | 1.00 (Reference) | 1.00 (Reference) | 59 | 1.00 (Reference) | 57 | 1.00 (Reference) |
| Medium | 83 | 1.16 (0.87 to 1.55) | 1.00 (0.74 to 1.34) | 33 | 1.03 (0.67 to 1.58) | 27 | 0.94 (0.60 to 1.49) | |
| Large | 32 | 1.69 (1.13 to 2.51) | 1.25 (0.83 to 1.88) | 12 | 1.71 (0.92 to 3.18) | 10 | 2.11 (1.08 to 4.13) | |
| Trend | 217 | 1.26 (1.04 to 1.53) | 1.08 (0.89 to 1.32) | 104 | 1.21 (0.90 to 1.61) | 94 | 1.23 (0.89 to 1.70) | |
| Continuous | 217 | 1.14 (1.04 to 1.25) | 1.05 (0.95 to 1.16) | 104 | 1.08 (0.93 to 1.26) | 94 | 1.09 (0.92 to 1.29) | |
| Ovarian cyst | Small | 2376 | 1.00 (Reference) | 1.00 (Reference) | 1374 | 1.00 (Reference) | 1347 | 1.00 (Reference) |
| Medium | 1501 | 0.95 (0.89 to 1.01) | 0.93 (0.87 to 0.99) | 727 | 0.98 (0.90 to 1.08) | 663 | 0.98 (0.90 to 1.08) | |
| Large | 448 | 1.04 (0.94 to 1.15) | 1.00 (0.91 to 1.11) | 189 | 1.07 (0.92 to 1.25) | 141 | 1.17 (0.98 to 1.39) | |
| Trend | 4325 | 0.99 (0.95 to 1.04) | 0.98 (0.93 to 1.02) | 2290 | 1.01 (0.95 to 1.08) | 2151 | 1.03 (0.96 to 1.11) | |
| Continuous | 4325 | 1.00 (0.98 to 1.02) | 0.99 (0.97 to 1.01) | 2290 | 1.00 (0.97 to 1.04) | 2151 | 1.01 (0.97 to 1.04) | |
| Diabetes | Small | 958 | 1.00 (Reference) | 1.00 (Reference) | 296 | 1.00 (Reference) | 291 | 1.00 (Reference) |
| Medium | 623 | 1.01 (0.91 to 1.11) | 0.85 (0.77 to 0.94) | 140 | 0.88 (0.72 to 1.07) | 128 | 0.88 (0.71 to 1.08) | |
| Large | 224 | 1.34 (1.16 to 1.55) | 0.97 (0.84 to 1.13) | 36 | 0.92 (0.65 to 1.31) | 25 | 0.94 (0.62 to 1.41) | |
| Trend | 1805 | 1.11 (1.04 to 1.19) | 0.94 (0.88 to 1.01) | 472 | 0.93 (0.80 to 1.07) | 444 | 0.92 (0.78 to 1.08) | |
| Continuous | 1805 | 1.04 (1.01 to 1.08) | 0.95 (0.92 to 0.98) | 472 | 0.95 (0.88 to 1.02) | 444 | 0.95 (0.88 to 1.03) | |
| Hyperlipidemia | Small | 3864 | 1.00 (Reference) | 1.00 (Reference) | 1829 | 1.00 (Reference) | 1797 | 1.00 (Reference) |
| Medium | 2286 | 0.92 (0.87 to 0.97) | 0.86 (0.82 to 0.91) | 824 | 0.82 (0.76 to 0.89) | 760 | 0.83 (0.76 to 0.90) | |
| Large | 719 | 1.08 (0.99 to 1.17) | 0.96 (0.89 to 1.04) | 241 | 0.98 (0.86 to 1.12) | 175 | 1.05 (0.90 to 1.22) | |
| Trend | 6869 | 0.99 (0.96 to 1.03) | 0.94 (0.90 to 0.97) | 2894 | 0.92 (0.87 to 0.97) | 2732 | 0.92 (0.87 to 0.98) | |
| Continuous | 6869 | 0.99 (0.98 to 1.01) | 0.96 (0.95 to 0.98) | 2894 | 0.95 (0.92 to 0.98) | 2732 | 0.95 (0.92 to 0.98) | |
| Stroke | Small | 422 | 1.00 (Reference) | 1.00 (Reference) | 189 | 1.00 (Reference) | 186 | 1.00 (Reference) |
| Medium | 202 | 0.75 (0.64 to 0.89) | 0.71 (0.60 to 0.84) | 93 | 0.92 (0.72 to 1.18) | 85 | 0.92 (0.71 to 1.18) | |
| Large | 65 | 0.90 (0.70 to 1.17) | 0.81 (0.62 to 1.06) | 22 | 0.88 (0.56 to 1.36) | 13 | 0.75 (0.43 to 1.32) | |
| Trend | 689 | 0.87 (0.77 to 0.98) | 0.82 (0.73 to 0.93) | 304 | 0.93 (0.78 to 1.11) | 284 | 0.89 (0.73 to 1.09) | |
| Continuous | 689 | 0.94 (0.88 to 0.99) | 0.91 (0.86 to 0.96) | 304 | 0.96 (0.88 to 1.05) | 284 | 0.94 (0.85 to 1.04) | |
| Heart failure | Small | 204 | 1.00 (Reference) | 1.00 (Reference) | 81 | 1.00 (Reference) | 74 | 1.00 (Reference) |
| Medium | 130 | 1.02 (0.82 to 1.27) | 0.91 (0.73 to 1.14) | 43 | 0.99 (0.68 to 1.43) | 39 | 1.05 (0.72 to 1.55) | |
| Large | 34 | 1.00 (0.70 to 1.44) | 0.82 (0.57 to 1.18) | 7 | 0.64 (0.30 to 1.39) | 4 | 0.58 (0.21 to 1.59) | |
| Trend | 368 | 1.01 (0.86 to 1.18) | 0.91 (0.78 to 1.06) | 131 | 0.89 (0.67 to 1.17) | 117 | 0.92 (0.68 to 1.25) | |
| Continuous | 368 | 1.01 (0.94 to 1.09) | 0.96 (0.89 to 1.04) | 131 | 0.95 (0.83 to 1.09) | 117 | 0.97 (0.83 to 1.13) | |
| Myocardial infarction | Small | 277 | 1.00 (Reference) | 1.00 (Reference) | 103 | 1.00 (Reference) | 103 | 1.00 (Reference) |
| Medium | 146 | 0.83 (0.68 to 1.02) | 0.76 (0.62 to 0.93) | 50 | 0.90 (0.64 to 1.26) | 43 | 0.83 (0.58 to 1.18) | |
| Large | 56 | 1.20 (0.90 to 1.60) | 1.00 (0.74 to 1.33) | 14 | 1.00 (0.57 to 1.75) | 9 | 0.93 (0.47 to 1.83) | |
| Trend | 479 | 1.00 (0.87 to 1.15) | 0.91 (0.79 to 1.04) | 167 | 0.96 (0.76 to 1.22) | 155 | 0.89 (0.68 to 1.17) | |
| Continuous | 479 | 0.99 (0.93 to 1.06) | 0.94 (0.88 to 1.01) | 167 | 0.95 (0.84 to 1.07) | 155 | 0.91 (0.79 to 1.04) | |
| Angina pectoris | Small | 339 | 1.00 (Reference) | 1.00 (Reference) | 128 | 1.00 (Reference) | 127 | 1.00 (Reference) |
| Medium | 180 | 0.84 (0.70 to 1.01) | 0.77 (0.64 to 0.92) | 58 | 0.84 (0.62 to 1.15) | 51 | 0.80 (0.58 to 1.10) | |
| Large | 51 | 0.89 (0.66 to 1.19) | 0.75 (0.55 to 1.00) | 7 | 0.40 (0.19 to 0.86) | 3 | 0.25 (0.08 to 0.78) | |
| Trend | 570 | 0.90 (0.79 to 1.02) | 0.82 (0.72 to 0.94) | 193 | 0.74 (0.58 to 0.95) | 181 | 0.69 (0.53 to 0.91) | |
| Continuous | 570 | 0.94 (0.89 to 1.00) | 0.90 (0.84 to 0.96) | 193 | 0.85 (0.76 to 0.96) | 181 | 0.82 (0.72 to 0.94) |
Statistically significant associations (P < 0.05) are presented in bold. *Stratified by year of birth (1950, 1951–1960, 1961 and later). †Stratified by year of birth and adjusted for body mass index (BMI, kg/m2, continuous) at questionnaire. ‡Subset of women with adult BMI less than 25 kg/m2, stratified by year of birth. §Subset of women with adult BMI less than 25 kg/m and either small or medium body size at age 18, stratified by year of birth.
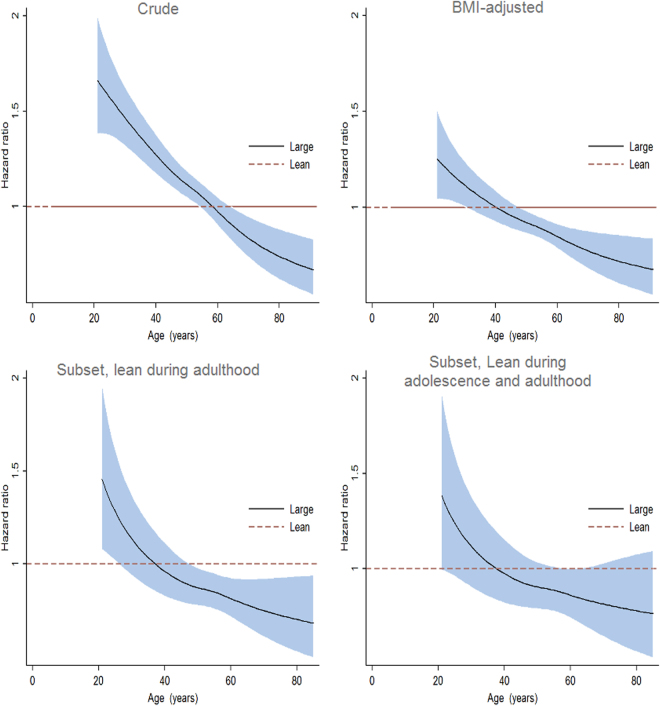
The HR and corresponding 95% CI changed over time for the association between childhood body size and hypertension (proportional hazards assumption violated for this outcome), and was thus examined using flexible parametric models (Fig. 2). In the crude analyses, childhood body size was associated with increased risk of hypertension before age 60, but not afterwards. The increased risk was attenuated when adjusted for adult BMI. Similarly, larger childhood body size did not appreciably elevate adult hypertension risk among women who were lean during adolescence or adulthood.
Figure 2.
Age-dependent hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) for hypertension, large versus small childhood body size. HRs were estimated from flexible parametric survival models adjusting for year of birth (crude); adjusting for year of birth and BMI (BMI-adjusted); restricted to a subset of women with adult BMI less than 25 kg/m2 and adjusting for year of birth (lean during adulthood); and restricted to subset of women with adult BMI less than 25 kg/m2 and either small or medium body size at age 18 (lean during adolescence and adulthood).
In the sensitivity analyses, we did not find any appreciable changes to the results associated with non-breast cancer outcomes when restricting to non-breast cancer participants (Supplementary Tables 2 and 3).
Discussion
In summary, our results suggest that childhood body size is an important risk factor for breast cancer and eating disorders that is independent of adult BMI. The associations between childhood body size with other adult diseases such as diabetes, PCOS and hypertension have to be considered in the light of adult BMI.
An inverse relationship between childhood body size and breast cancer has been consistently shown1. As adult obesity is an established risk factor for postmenopausal but not premenopausal breast cancer, the apparent protective effect that a large childhood body size has over a small childhood body size for both pre- and postmenopausal breast cancer has been much discussed40–43. Considering that childhood fatness is typically associated with earlier menarche, which is in turn associated with high breast cancer risk, the inverse correlation between childhood body size and breast cancer risk is counterintuitive. However, the inverse association has been shown not to be mediated by adult BMI or menstrual characteristics44,45, which suggests that an excess of adiposity during childhood might prevent tumorigenesis through other mechanisms. A possible explanation is that the increased exposure to estrogen from the extra adipose tissue at a young age induces a persistent upregulation of tumor suppressor genes46.
While much interest and attention is generated by the conclusion that a large childhood body size reduces breast cancer risk, it is important to evaluate childhood body size alongside other health outcomes. Not unexpectedly, obesity in childhood has been shown to be a retrospective correlate of eating disorders such as bulimia in later years47. In a large UK-based study, Micali et al. identified body dissatisfaction in children as young as eight years old to be prospectively associated with dieting and purging behaviors during adolescence48. Discontent with the size and shape of their body might subject children with larger body size to undue pressure from media, family and peers, putting them at risk of eating disorders in their teens.
Other common diseases that are hypothesized to have beginnings in early life include a range of cancers (other than breast cancer) and several adult obesity-related morbidities3. A high BMI in childhood has been shown to frequently persist into adult life, where obese children were five-fold more likely to remain obese later on in life compared to those who were not obese49,50. As our data showed that the increased risks of diabetes and hypertension conferred by larger childhood body size do not persist after controlling for adult BMI, it is likely that the associations between childhood body size and these diseases are driven by adult BMI. Similarly, in the Nurses’ Health Study, it was observed that children who were of large body size were not associated with an increased risk of diabetes if they became lean in later years51.
Unexpectedly, no clear link between childhood body size and cardiovascular diseases was found in our study. However, a systematic review and meta-analysis of more than 30 studies by Llewellyn et al. suggested that childhood BMI is of limited accuracy in predicting adult morbidity, reason being that less than a third of the adult morbidities developed in children who were overweight or obese3. On the contrary, the majority of adult chronic diseases (~70%) occurred in children who were of normal weight3,49, which could possibly explain the absence of associations between childhood body size and cardiovascular diseases in adulthood. Other studies have also reported that an independent contribution of childhood body size in affecting blood lipid status, insulin levels, metabolic syndrome or cardiovascular disease is unlikely52,53.
The main strength of this study lies in its large sample size of close to 70,000 women. Other works that have simultaneously examined multiple long-term health risk outcomes with respect to childhood anthropometry in a single study include smaller studies such as the Scottish Mental Survey of 1947 (4,620 participants, of which approximately half are female), a Swedish prospective study of 504 children (271 girls) with 40 years of follow-up and the Harvard Growth Study of 1922 to 1935 (508 lean or overweight adolescents aged between 13 to 18)54–56. An additional strength of this study is that cancer outcomes were identified through the long-standing nation-wide Swedish Cancer Registry, to which the reporting of all newly diagnosed cancer cases is compulsory. The Swedish Cancer Registry was founded in 1958 and shows an almost complete coverage due to extensive monitoring and requests for missing data36.
While the five cancer outcomes were retrieved from Swedish registries, it should be noted that the study used self-reported medical histories of the participants to study seven other common diseases, for example, hypertension and diabetes, which are typically diagnosed in primary health care57. Unlike inpatient or hospital discharge records (complete coverage since 198758) or hospital-based outpatient physician visits (from 2011) which can be retrieved from the Swedish National Patient Register, primary health care data is not tracked on a national level. However, good agreement has been shown between patient-reported comorbidity events and information abstracted from medical records59. As with all studies using survey-based data regarding events or experiences from the past, recall error due to time and memory failure is a limitation and can bias our estimates towards the null. This is especially true for the conditions which usually affect younger women, such as polycystic ovary syndrome, ovarian cysts, depression, bulimia and anorexia. However, individuals are unlikely to forget if they have ever been diagnosed with one of these conditions. It is also possible that women participating in a mammography screening program may have a differential recall of non-breast related health events. Another point to note is that since KARMA comprises participants of a nation-wide mammography screening program, the generalizability of our results may be limited to a more health-conscious group of women. However, since the Swedish health care system is mainly government-funded and decentralized and all women essentially have the same access, the study should represent women from a wide range of income levels and backgrounds. As childhood BMI was not collected, it was not possible to validate how closely recalled somatotypes aligned with childhood BMI z-score. However, this measure is well-validated by others32,33 and is currently used to assess recalled somatotypes in several large, ongoing prospective studies, including the Nurses’ Health Study60.
Our findings provide perspective for the wide range of long-term consequences of childhood body size on later life well-being. While further research on inverse association between childhood body size and breast cancer will help us to better understand the etiology of the disease, other negative health outcomes of childhood obesity in adult life need to be weighed simultaneously in the light of the apparent protective effect against breast cancer.
Availability of data and Materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethical approval and consent to participate
Each participant gave informed consent and this study has been approved by the ethical review board at Karolinska Institutet. All analyses were performed in accordance with relevant institutional and national guidelines and regulations.
Electronic supplementary material
Acknowledgements
The KARMA study was supported by the Märit and Hans Rausing Initiative Against Breast Cancer, the Swedish Research Council (2014–2271); Swedish Cancer Society (CAN 2016/684); and FORTE (2016–00081). This work was also supported by a research grant from Karolinska Institutet (Karolinska Institutets forskningsbidrag (2016fobi47643)). JL is a recipient of a Singapore National Research Foundation Fellowship. The funding agencies had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. We thank the reviewers for their careful reviews and helpful comments.
Author Contributions
J.L. and P.H. designed the study. M.E. provided technical assistance with data acquisition. W.H. and K.C. provided “epidemiological” expertise. J.L. performed all data analyses and wrote the manuscript. All authors discussed and interpreted the data and revised the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-17258-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ahlgren M, Melbye M, Wohlfahrt J, Sorensen TI. Growth patterns and the risk of breast cancer in women. The New England journal of medicine. 2004;351:1619–1626. doi: 10.1056/NEJMoa040576. [DOI] [PubMed] [Google Scholar]
- 2.Felix JF, et al. Genome-wide association analysis identifies three new susceptibility loci for childhood body mass index. Human molecular genetics. 2016;25:389–403. doi: 10.1093/hmg/ddv472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llewellyn A, Simmonds M, Owen CG, Woolacott N. Childhood obesity as a predictor of morbidity in adulthood: a systematic review and meta-analysis. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2016;17:56–67. doi: 10.1111/obr.12316. [DOI] [PubMed] [Google Scholar]
- 4.Serdula MK, et al. Do obese children become obese adults? A review of the literature. Prev Med. 1993;22:167–177. doi: 10.1006/pmed.1993.1014. [DOI] [PubMed] [Google Scholar]
- 5.Calcaterra V, et al. Prevalence of metabolic syndrome (MS) in children and adolescents with varying degrees of obesity. Clinical endocrinology. 2008;68:868–872. doi: 10.1111/j.1365-2265.2007.03115.x. [DOI] [PubMed] [Google Scholar]
- 6.Lo JC, et al. Severe obesity in children: prevalence, persistence and relation to hypertension. International journal of pediatric endocrinology. 2014;2014:3. doi: 10.1186/1687-9856-2014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. The Journal of pediatrics. 2007;150:12–17 e12. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 8.Rank M, et al. The cardio-metabolic risk of moderate and severe obesity in children and adolescents. The Journal of pediatrics. 2013;163:137–142. doi: 10.1016/j.jpeds.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Baker JL, Olsen LW, Sorensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. The New England journal of medicine. 2007;357:2329–2337. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biro FM, Wien M. Childhood obesity and adult morbidities. The American journal of clinical nutrition. 2010;91:1499S–1505S. doi: 10.3945/ajcn.2010.28701B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skinner AC, Perrin EM, Moss LA. & Skelton, J. A. Cardiometabolic Risks and Severity of Obesity in Children and Young Adults. The New England journal of medicine. 2015;373:1307–1317. doi: 10.1056/NEJMoa1502821. [DOI] [PubMed] [Google Scholar]
- 12.Sabo RT, Wang A, Deng Y, Sabo CS, Sun SS. Relationships between childhood growth parameters and adult blood pressure: the Fels Longitudinal Study. J Dev Orig Health Dis. 2017;8:113–122. doi: 10.1017/S2040174416000520. [DOI] [PubMed] [Google Scholar]
- 13.Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. International journal of obesity. 2010;35:891–898. doi: 10.1038/ijo.2010.222. [DOI] [PubMed] [Google Scholar]
- 14.Jeffreys M, Smith GD, Martin RM, Frankel S, Gunnell D. Childhood body mass index and later cancer risk: a 50-year follow-up of the Boyd Orr study. International journal of cancer. 2004;112:348–351. doi: 10.1002/ijc.20423. [DOI] [PubMed] [Google Scholar]
- 15.Kitahara CM, Gamborg M, Berrington de Gonzalez A, Sorensen TI, Baker JL. Childhood height and body mass index were associated with risk of adult thyroid cancer in a large cohort study. Cancer research. 2014;74:235–242. doi: 10.1158/0008-5472.CAN-13-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitahara CM, Gamborg M, Rajaraman P, Sorensen TI, Baker JL. A prospective study of height and body mass index in childhood, birth weight, and risk of adult glioma over 40 years of follow-up. American journal of epidemiology. 2014;180:821–829. doi: 10.1093/aje/kwu203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berentzen TL, Gamborg M, Holst C, Sorensen TI, Baker JL. Body mass index in childhood and adult risk of primary liver cancer. Journal of hepatology. 2014;60:325–330. doi: 10.1016/j.jhep.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Aarestrup J, Gamborg M, Ulrich LG, Sorensen TI, Baker JL. Childhood body mass index and height and risk of histologic subtypes of endometrial cancer. International journal of obesity. 2016;40:1096–1102. doi: 10.1038/ijo.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, et al. Early life body fatness and risk of colorectal cancer in u.s. Women and men-results from two large cohort studies. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24:690–697. doi: 10.1158/1055-9965.EPI-14-0909-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dougan MM, et al. Prospective study of body size throughout the life-course and the incidence of endometrial cancer among premenopausal and postmenopausal women. International journal of cancer. 2015;137:625–637. doi: 10.1002/ijc.29427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyle KD, Gamborg M, Holmich LR, Baker JL. Associations between childhood height and morphologically different variants of melanoma in adulthood. European journal of cancer. 2016;67:99–105. doi: 10.1016/j.ejca.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garn SM, Leonard WR, Hawthorne VM. Three limitations of the body mass index. Am J Clin Nutr. 1986;44:996–997. doi: 10.1093/ajcn/44.6.996. [DOI] [PubMed] [Google Scholar]
- 23.Goran MI, Driscoll P, Johnson R, Nagy TR, Hunter G. Cross-calibration of body-composition techniques against dual-energy X-ray absorptiometry in young children. Am J Clin Nutr. 1996;63:299–305. doi: 10.1093/ajcn/63.3.299. [DOI] [PubMed] [Google Scholar]
- 24.Daniels SR, Khoury PR, Morrison JA. The utility of body mass index as a measure of body fatness in children and adolescents: differences by race and gender. Pediatrics. 1997;99:804–807. doi: 10.1542/peds.99.6.804. [DOI] [PubMed] [Google Scholar]
- 25.Roche AF, Sievogel RM, Chumlea WC, Webb P. Grading body fatness from limited anthropometric data. Am J Clin Nutr. 1981;34:2831–2838. doi: 10.1093/ajcn/34.12.2831. [DOI] [PubMed] [Google Scholar]
- 26.Pietrobelli A, et al. Body mass index as a measure of adiposity among children and adolescents: a validation study. J Pediatr. 1998;132:204–210. doi: 10.1016/S0022-3476(98)70433-0. [DOI] [PubMed] [Google Scholar]
- 27.Warner JT, Cowan FJ, Dunstan FD, Gregory JW. The validity of body mass index for the assessment of adiposity in children with disease states. Ann Hum Biol. 1997;24:209–215. doi: 10.1080/03014469700004942. [DOI] [PubMed] [Google Scholar]
- 28.Lazarus R, Baur L, Webb K, Blyth F. Body mass index in screening for adiposity in children and adolescents: systematic evaluation using receiver operating characteristic curves. Am J Clin Nutr. 1996;63:500–506. doi: 10.1093/ajcn/63.4.500. [DOI] [PubMed] [Google Scholar]
- 29.Himes JH, Bouchard C. Validity of anthropometry in classifying youths as obese. Int J Obes. 1989;13:183–193. [PubMed] [Google Scholar]
- 30.Gabrielson, M. et al. Cohort profile: The Karolinska Mammography Project for Risk Prediction of Breast Cancer (KARMA). Int J Epidemiol, doi:10.1093/ije/dyw357 (2017). [DOI] [PMC free article] [PubMed]
- 31.Stunkard AJ, Sorensen T, Schulsinger F. Use of the Danish Adoption Register for the study of obesity and thinness. Research publications - Association for Research in Nervous and Mental Disease. 1983;60:115–120. [PubMed] [Google Scholar]
- 32.Must A, Willett WC, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. American journal of epidemiology. 1993;138:56–64. doi: 10.1093/oxfordjournals.aje.a116777. [DOI] [PubMed] [Google Scholar]
- 33.Koprowski C, Coates RJ, Bernstein L. Ability of young women to recall past body size and age at menarche. Obes Res. 2001;9:478–485. doi: 10.1038/oby.2001.62. [DOI] [PubMed] [Google Scholar]
- 34.Li J, et al. Effects of childhood body size on breast cancer tumour characteristics. Breast cancer research: BCR. 2010;12:R23. doi: 10.1186/bcr2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. European journal of epidemiology. 2009;24:659–667. doi: 10.1007/s10654-009-9350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barlow L, Westergren K, Holmberg L, Talback M. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta oncologica. 2009;48:27–33. doi: 10.1080/02841860802247664. [DOI] [PubMed] [Google Scholar]
- 37.Clavel-Chapelon F, Group ENE. Differential effects of reproductive factors on the risk of pre- and postmenopausal breast cancer. Results from a large cohort of French women. Br J Cancer. 2002;86:723–727. doi: 10.1038/sj.bjc.6600124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. (2016).
- 39.Engholm G, et al. NORDCAN–a Nordic tool for cancer information, planning, quality control and research. Acta oncologica. 2010;49:725–736. doi: 10.3109/02841861003782017. [DOI] [PubMed] [Google Scholar]
- 40.Baer HJ, Colditz GA, Willett WC, Dorgan JF. Adiposity and sex hormones in girls. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16:1880–1888. doi: 10.1158/1055-9965.EPI-07-0313. [DOI] [PubMed] [Google Scholar]
- 41.Magnusson C, et al. Body size in different periods of life and breast cancer risk in post-menopausal women. International journal of cancer. 1998;76:29–34. doi: 10.1002/(SICI)1097-0215(19980330)76:1<29::AID-IJC6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 42.Weiderpass E, et al. A prospective study of body size in different periods of life and risk of premenopausal breast cancer. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2004;13:1121–1127. [PubMed] [Google Scholar]
- 43.Magnusson CM, Roddam AW. Breast cancer and childhood anthropometry: emerging hypotheses? Breast cancer research: BCR. 2005;7:83. doi: 10.1186/bcr1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baer HJ, et al. Body fatness during childhood and adolescence and incidence of breast cancer in premenopausal women: a prospective cohort study. Breast cancer research: BCR. 2005;7:R314–325. doi: 10.1186/bcr998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shawon MSR, Eriksson M, Li J. Body size in early life and risk of breast cancer. Breast cancer research: BCR. 2017;19:84. doi: 10.1186/s13058-017-0875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cabanes A, et al. Prepubertal estradiol and genistein exposures up-regulate BRCA1 mRNA and reduce mammary tumorigenesis. Carcinogenesis. 2004;25:741–748. doi: 10.1093/carcin/bgh065. [DOI] [PubMed] [Google Scholar]
- 47.Goncalves S, Machado BC, Martins C, Hoek HW, Machado PP. Retrospective Correlates for Bulimia Nervosa: A Matched Case-Control Study. European eating disorders review: the journal of the Eating Disorders Association. 2016;24:197–205. doi: 10.1002/erv.2434. [DOI] [PubMed] [Google Scholar]
- 48.Micali N, et al. Adolescent eating disorder behaviours and cognitions: gender-specific effects of child, maternal and family risk factors. The British journal of psychiatry: the journal of mental science. 2015;207:320–327. doi: 10.1192/bjp.bp.114.152371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2016;17:95–107. doi: 10.1111/obr.12334. [DOI] [PubMed] [Google Scholar]
- 50.Simmonds M, et al. The use of measures of obesity in childhood for predicting obesity and the development of obesity-related diseases in adulthood: a systematic review and meta-analysis. Health technology assessment. 2015;19:1–336. doi: 10.3310/hta19430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yeung EH, Zhang C, Louis GM, Willett WC, Hu FB. Childhood size and life course weight characteristics in association with the risk of incident type 2 diabetes. Diabetes care. 2010;33:1364–1369. doi: 10.2337/dc10-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lloyd LJ, Langley-Evans SC, McMullen S. Childhood obesity and risk of the adult metabolic syndrome: a systematic review. International journal of obesity. 2012;36:1–11. doi: 10.1038/ijo.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Antonisamy, B. et al. Weight Gain and Height Growth during Infancy, Childhood, and Adolescence as Predictors of Adult Cardiovascular Risk. The Journal of pediatrics, doi:10.1016/j.jpeds.2016.09.059 (2016). [DOI] [PMC free article] [PubMed]
- 54.Batty GD, Calvin CM, Brett CE, Cukic I, Deary IJ. Childhood body weight in relation to morbidity from cardiovascular disease and cancer in older adulthood: 67-year follow-up of participants in the 1947 Scottish Mental Survey. American journal of epidemiology. 2015;182:775–780. doi: 10.1093/aje/kwv154. [DOI] [PubMed] [Google Scholar]
- 55.Mossberg HO. 40-year follow-up of overweight children. Lancet. 1989;2:491–493. doi: 10.1016/S0140-6736(89)92098-9. [DOI] [PubMed] [Google Scholar]
- 56.Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. The New England journal of medicine. 1992;327:1350–1355. doi: 10.1056/NEJM199211053271904. [DOI] [PubMed] [Google Scholar]
- 57.Wandell P, et al. Most common diseases diagnosed in primary care in Stockholm, Sweden, in 2011. Fam Pract. 2013;30:506–513. doi: 10.1093/fampra/cmt033. [DOI] [PubMed] [Google Scholar]
- 58.Ludvigsson JF, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ye F, et al. Comparison of Patient Report and Medical Records of Comorbidities: Results From a Population-Based Cohort of Patients With Prostate Cancer. JAMA Oncol. 2017;3:1035–1042. doi: 10.1001/jamaoncol.2016.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baer HJ, Tworoger SS, Hankinson SE, Willett WC. Body fatness at young ages and risk of breast cancer throughout life. American journal of epidemiology. 2010;171:1183–1194. doi: 10.1093/aje/kwq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.