Dear Editor,
Human infection of Rickettsia sibirica subsp. sibirica was first described in Russian Far East in the 1930s.1 Since then, this spotted fever group (SFG) rickettsiosis has been found across a large territory of north Asia, including Russia, China, and Mongolia and Kazakhstan.2, 3, 4, 5 Common clinical manifestations include fever, headache, weakness, myalgia, rash, eschar, and lymphadenopathy. The disease is usually mild, rarely related to severe complications, and nonfatal.
R. sibirica subsp. sibirica BJ-90, a variant of R. sibirica, was first isolated from Dermacentor sinicus in China in 1990,6, 7 and has recently been detected in a patient with multiorgan dysfunction.8 Here we report two cases with severe illness caused by R. sibirica subsp. sibirica BJ-90 in northern China.
An 81-year-old woman from Harbin City was admitted to Mudanjiang Forestry Central Hospital in northern China on June 2013 (Supplementary Figure S1), with fever (temperature, up to 39.0 °C), fatigue, myalgias, and arthralgia for 7 days, and a hyperpigmented maculopapular rash for 3 days (Figure 1). She reported having a tick bite at the waist when taking a walk in a public park 3 days before illness onset. During the third days of symptoms, she received treatment with cephalosporin and ibuprofen, but her condition did not improve. On admission, blood test abnormalities included: leukocytosis (white blood cell count, 18.1 × 109/L), thrombocytopenia (platelet count, 49 × 1012/L), neutrocytosis (neutrophil count, 13.8 × 109/L); increased levels of liver enzymes (alanine aminotransferase, 73 U/L; aspartate aminotransferase, 86 U/L), glutamyl transpeptidase (95 U/L), creatinine (525 μmol/L), urea nitrogen (44.2 mmol/L), uric acid (648 μmol/L), high-sensitivity C-reactive protein (72 mg/L), and β2-macroglobulin (25 mg/L); decreased levels of albumin (26.0 g/L), cholinesterase (3,192 U/L), calcium (1.6 mmol/L), and carbon dioxide combining power (15 mmol/L). Urine tests showed an elevated level of erythrocytes (80 cells per μL) and total protein (0.3 g/L). Chest radiograph showed increased lung markings, and ultrasonic examination revealed cholecystitis, and hepatic and renal diffuse changes. The patient was intravenously treated with doxycycline based on the clinical symptoms and the preceding tick bite. 2 days after admission, the patient’s leukocytosis, thrombocytopenia, neutrocytosis, blood biochemistry abnormalities, and hepatic and renal dysfunction did not improve (Supplementary Table S1); her condition deteriorated with oliguria, chest distress, rising heart rate, and oedema legs. The patient was subsequently administered with respirator and supportive treatment, but she deteriorated further and developed coma. The patient died 5 days after hospitalization.
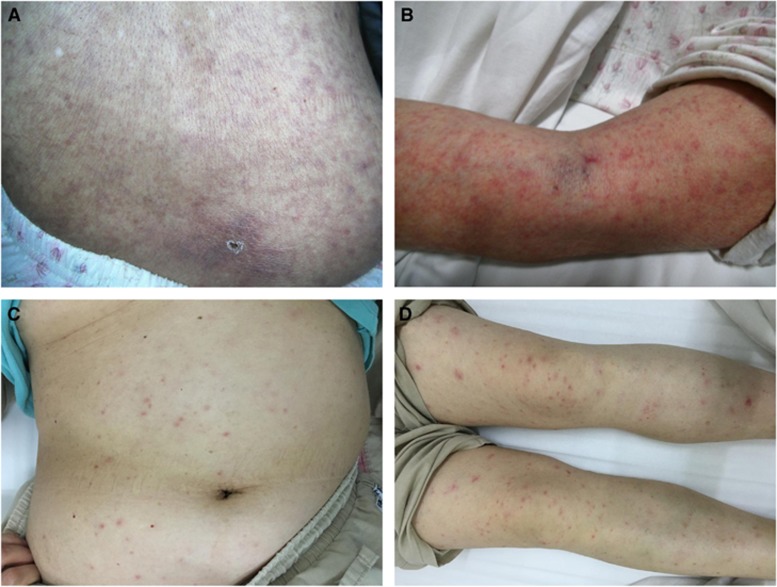
Figure 1.
Typical signs of the two patients with Rickettsia sibirica subspecies sibirica BJ-90 infection. Case 1: (A) shows a crusted eschar at the site of tick bite, and a maculopapular, hyperpigmented, non-itchy rash affecting the trunk; (B) shows a maculopapular, red, non-itchy rash on the arms. Case 2: (C) shows a sporadic petechial rash affecting the trunk; (D) shows a red petechial rash on the legs.
A 62-year-old woman from Beijing City was admitted to Beijing Chao-Yang Hospital in northern China on June 2016 (Supplementary Table S1), with fever (temperature, up to 39.0 °C), fatigue, dizziness, tinnitus, and chest distress for 6 days, and a sporadic petechial rash for 2 days (Figure 1). The patient reported that she noted a tick bite at the left armpit 2 days before illness onset. The laboratory findings on admission were as follows: leukopenia (white blood cell count, 3.7 × 109/L), thrombocytopenia (platelet count, 82 × 1012/L), lymphopenia (lymphocyte count, 0.4 × 109/L); increased levels of liver enzymes (alanine aminotransferase, 220 U/L; aspartate aminotransferase, 386 U/L), lactate dehydrogenase (772 U/L), hydroxybutyrate dehydrogenase (557 U/L), C-reactive protein (78 mg/L), and D-Dimer (6.31 mg/L); and decreased levels of albumin (34.8 g/L). Urine tests showed an elevated level of erythrocytes (10 cells/μL) and total protein (0.25 g/L). Computed tomography showed thickening of left pleura and slight hydropericardium, and ultrasonic examination revealed cyst of right kidney. 3 days later, she developed oedema legs. After treated with oral minocycline for 7 days, the patient’s clinical manifestations and laboratory abnormalities resolved, except slightly increased levels of lactate dehydrogenase and hepatic aminotransferases.
Because the patients presented with typical rash and had a recent history of tick bite, SFG rickettsiae was considered as the causative agent. 0.2 mL EDTA-anticoagulant blood samples were obtained from the patients before initiation of therapy, and DNA was extracted with the Blood Mini Kit (Qiagen, Germantown, USA) according to the manufacturer’s instructions. A nested PCR targeting the citrate synthase gene (gltA) was performed as previously described.9 For further confirmation, the outer membrane protein A gene (ompA) was amplified using external primers Rr190.70p and Rr190.602n,10 and internal primers 190.70-38s1M (5′-AAA GCC GCT TTA TTC ACC-3′) and 190.602-384r1M (5′-GGC AAC AAG TTA CCT CCC-3′) modified according to previous study.7 To obtain nearly entire length of both gltA and ompA genes, hemi-nested PCR assays were performed using the product of the first PCR round as templates. PCR fragments were sequenced on a 3730 DNA sequencer (Applied Biosystems, Foster City, CA, USA) and aligned by using ClustalW software.
Nucleotide sequences of both gltA (1290 bp) and ompA (533 bp) genes (GenBank accession numbers KY617774 and KY617775 for Case 1; KM288711 and KM288712 for Case 2) from the two patients were identical. Sequence analyses showed that the two gene sequences had 100% similarities to the corresponding sequences from Rickettsia sibirica subsp. sibirica BJ-90 (GenBank accession numbers JX945526 and AF179365, respectively).
Serum samples were tested by indirect immunofluorescence assay (IFA) for IgM and IgG against R. rickettsii by using a commercially IFA kit (Focus Diagnostics Inc., Cypress, USA). For Case 1, the serum sample collected at admission was available, and IgM and IgG titers were 256 and 512, respectively. For Case 2, IgM titers of 128 and IgG titers of 256 were observed in the serum sample obtained at admission, and IgM titers of 256 and IgG titers of 1024 were observed in the serum sample obtained 14 days after illness onset. In addition, PCR and IFA results for other tick-borne pathogens, including Anaplasma phagocytophilum, Babesia microti, Borrelia burgdorferi sensu lato, severe fever with thrombocytopenia syndrome bunyavirus, and tick-borne encephalitis virus, were negative.
In the current study, two cases of Rickettsia sibirica subsp. sibirica BJ-90 infection, including one fatal case, were identified. Both patients presented with severe clinical manifestations, including a spread rash around the trunk and extremities, chest distress, oedema legs, and multiorgan dysfunction. Moreover, one patient developed oliguria and coma, and died 12 days after illness onset. The fatal outcome may be associated with several factors, among which are old age, late start of effective treatment, and early laboratory abnormalities such as liver dysfunction, acute renal failure, hypoalbuminemia, and hypocalcemia. Based on the clinical data from our two patients and the previously reported patient,8 we suppose a high pathogenicity of Rickettsia sibirica subsp. sibirica BJ-90, which needs to be corroborated by further investigations from a large number of cases.
R. sibirica subsp. sibirica BJ-90 have been detected from D. sinicus in China and from D. silvarum in Russia,6, 11 indicating that Dermacentor ticks are potential vectors for the pathogen. Considering the wide distribution of Dermacentor ticks in northern China, it is deduced that the health burden of human infection with Rickettsia sibirica subsp. sibirica BJ-90 might be beyond our understanding, although only three cases have been reported until now. Extended investigation and case surveillance are required to understand the distribution of human infection with Rickettsia sibirica subsp. sibirica BJ-90.
Diagnosis of tick-borne SFG rickettsioses is confirmed almost exclusively by serologic methods in China,12 which is an effective tool but cannot identify the causative agent to species level. In northern China where complex circumstance of SFG rickettsiae is endemic, clinicians should be aware of the potential high pathogenicity of R. sibirica subspecies sibirica BJ-90, and molecular-based diagnostic approaches should be applied on suspected patients.
Acknowledgments
This study was supported by grants from the Natural Science Foundation of China (81290344 and 81472005), the National Key Research and Development Program of China (2016YFX1201905), and the Beijing Municipal Science & Technology Commission (Z15100004015029).
Footnotes
Supplementary Information for this article can be found on the Emerging Microbes & Infections website (http://www.nature.com/emi)
Supplementary Material
References
- Rehacek J, Tarasevich IV. Acari-borne Rickettsiae and Rickettsioses Eurasia, pp 128–145Veda, Publishing House of the Slovak Academy of Sciences: Bratislava, Slovakia. 1988. [Google Scholar]
- Fan MY, Walker D, Yu SR et al. Epidemiology and ecology of rickettsial diseases in the People’s Republic of China. Rev Infect Dis 1987; 9: 823–840. [PubMed] [Google Scholar]
- Balayeva NM, Eremeeva ME, Ignatovich VF et al. Biological and genetic characterization of Rickettsia sibirica strains isolated in the endemic area of the North Asian tick typhus. Am J Trop Med Hyg 1996; 55: 685–692. [DOI] [PubMed] [Google Scholar]
- Rudakov N, Shpynov S, Fournier PE et al. Ecology and molecular epidemiology of tick-borne rickettsioses and Anaplasmoses with natural foci in Russia and Kazakhstan. Ann NY Acad Sci 2006; 1078: 299–304. [DOI] [PubMed] [Google Scholar]
- Cao WC, Zhan L, De Vlas SJ et al. Molecular detection of spotted fever group Rickettsia in Dermacentor silvarum from a forest area of northeastern China. J Med Entomol 2008; 45: 741–744. [DOI] [PubMed] [Google Scholar]
- Yu XJ, Fan MY, Bi DZ. Primary identification of spotted fever group Rickettsiae isolated from Dermacentor sinicus in the Beijing area. Chin J Microbiol Immunol 1991; 11: 305. [Google Scholar]
- Zhang JZ, Fan MY, Yu XJ et al. Phylogenetic analysis of the Chinese Rickettsia isolate BJ-90. Emerg Infect Dis 2000; 6: 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia N, Jiang JF, Huo QB, Jiang BG, Cao WC. Rickettsia sibirica subsp. sibirica BJ-90 as a cause of human disease. N Engl J Med 2013; 369: 1176–1178. [DOI] [PubMed] [Google Scholar]
- Mediannikov OY, Sidelnikov Y, Ivanov L et al. Acute tick-borne rickettiosis caused by Rickettsia heilongjiangsis in Russian Far East. Emerg Infect Dis 2004; 10: 810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda H, Munderloh UG, Kurtti TJ. Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Appl Environ Microbiol 1997; 63: 3926–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpynov SN, Fournier PE, Rudakov NV et al. Molecular identification of a collection of spotted fever group Rickettsiae obtained from patients and ticks from Russia. Am J Trop Med Hyg 2006; 74: 440–443. [PubMed] [Google Scholar]
- Fang LQ, Liu K, Li XL et al. Emerging tick-borne infections in mainland China: an increasing public health threat. Lancet Infect Dis 2015; 15: 1467–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.