Abstract
C-Phycocyanin (CP) is a biliprotein enriched in blue-green algae that is known to possess antioxidant, anti-apoptosis, anti-inflammatory, and radical-scavenging properties in somatic cells. However, the protective effect of CP on porcine embryo developmental competence in vitro remains unclear. In the present study, we investigated the effect of CP on the development of early porcine embryos as well as its underlying mechanisms. Different concentrations of CP (2, 5, 8, 10 μg/mL) were added to porcine zygote medium 5 during in vitro culture. The results showed that 5 μg/mL CP significantly increased blastocyst formation and hatching rate. Blastocyst formation and quality were significantly increased in the 50 μM H2O2 treatment group following 5 μg/mL CP addition. CP prevented the H2O2-induced compromise of mitochondrial membrane potential, release of cytochrome c from the mitochondria, and reactive oxygen species generation. Furthermore, apoptosis, DNA damage level, and autophagy in the blastocysts were attenuated by supplementation of CP in the H2O2-induced oxidative injury group compared to in controls. These results suggest that CP has beneficial effects on the development of porcine parthenotes by attenuating mitochondrial dysfunction and oxidative stress.
Introduction
Pigs are important for both agricultural production and biomedical research1. In vitro-produced porcine embryos are extensively used for studying the mechanism of embryonic development and animal reproductive technologies, including cryopreservation, cloning, and transgenesis2. The micromilieu surrounding preimplantation embryos during in vitro culture is important for the developmental competence of the embryos. Embryos produced in vitro show some differences from in vivo-derived embryos because when embryos are manipulated and cultured under in vitro environmental conditions, exposure to light, greater oxygen stress and culture medium composition has been correlated with oxidative stress generated by the production of reactive oxygen species (ROS)3,4. Oxidative stress brings about a number of types of embryo damage. ROS can pass through cell membrane and induce impairment of nucleic acids and lipids proteins. These alterations have numerous effects, including mitochondrial dysfunction, ATP depletion, apoptosis and embryo arrest3. Therefore, the use of substances with antioxidant properties during the in vitro production of embryos may prevent excessive ROS production and improve embryo developmental competence5,6.
C-Phycocyanin (CP) is a major biliprotein found in blue-green algae, such as Spirulina platensis 7. Numerous beneficial effects of CP have been demonstrated, including antioxidant, anti-apoptosis, anti-inflammatory, and radical-scavenging properties in somatic cells7–10. Owing to its health benefits, CP has been used as a blue colorant for food, cosmetics and pharmaceutical industries11. Previous studies have shown that CP inhibits ROS production, reverses caspase-3 activity, reduces apoptosis cell population, and prevent mitochondrial dysfunction12. A previous study also showed that D-galactose-induced compromise of female reproductive capability can be partially rescued by the antioxidant and anti-apoptosis effects of CP13. However, the effect of CP on embryo development during in vitro culture has not been examined.
H2O2 can affect cell viability by disrupting the biological membrane and homeostasis of extracellular matrix14,15. Thus, H2O2 is used to imitate the situation of oxidative stress to examine the potency of antioxidants in alleviating cellular injury8. In this study, we first detected the dose-effect of CP on early porcine embryos cultured in vitro. Next, the antioxidative effect of CP on porcine embryo development was examined after pre-treatment with H2O2.
Results
Effects of treatment with various concentrations of CP on in vitro development of porcine embryos
After parthenogenetic activation, one-cell-stage parthenotes were cultured with various concentrations of CP. Figure 1A showed that cleavage was not affected by addition of 2, 5, 8, or 10 μg/mL CP (p > 0.05). Blastocyst formation was also not affected by addition of 2 μg/mL CP (p > 0.05). In addition, addition of 5 μg/mL CP significantly increased the blastocyst rate compared to in other groups (p < 0.05), while high doses of CP (8 and 10 μg/mL) negatively affected blastocyst formation (p < 0.001). Addition of 5 μg/mL CP significantly increased the hatching rate of blastocysts compared to controls (Fig. 1B, p < 0.05). The mRNA expression of hatching-related genes, COX2, FN1, and ITGA5, significantly increased in CP group (Fig. 1C, p < 0.01). Thus, the concentration of CP used in subsequent experiments was 5 μg/mL.
Figure 1.
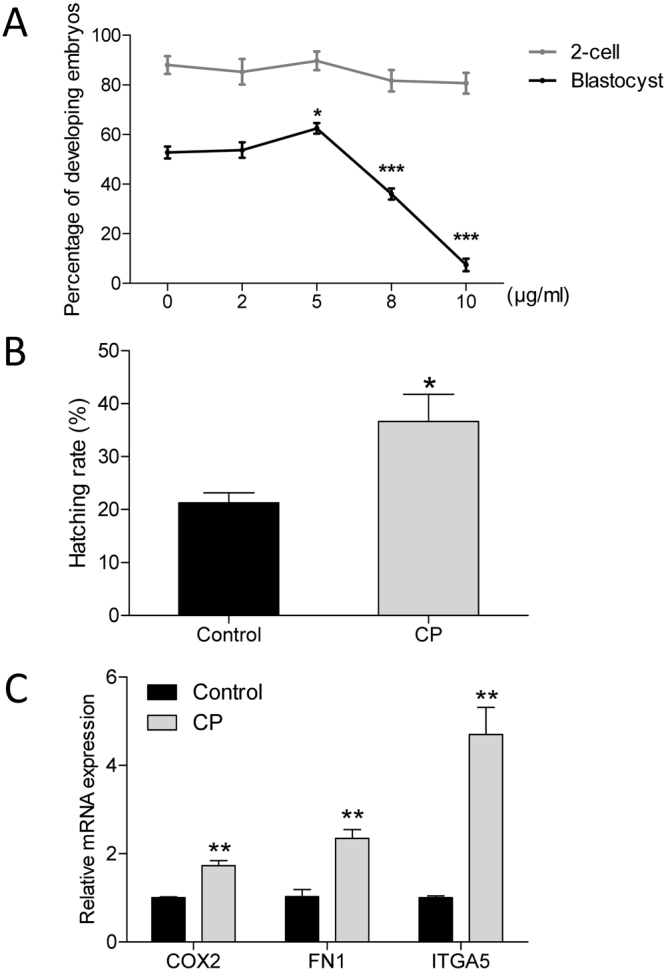
Effects of CP on in vitro development of parthenogenetic porcine embryos. (A) Cleavage and blastocyst formation of porcine embryos cultured in the presence of CP at different concentrations. (B) Percentage of blastocyst hatching in the presence or absence of 5 µg/mL CP in the culture medium. (C) Relative expression of hatching-related genes in blastocysts. Data are expressed as the mean ± SEM. In addition, *p < 0.05, **p < 0.01, and ***p < 0.001 indicate significant differences between different groups. The experiments were replicated three times with 612 embryos.
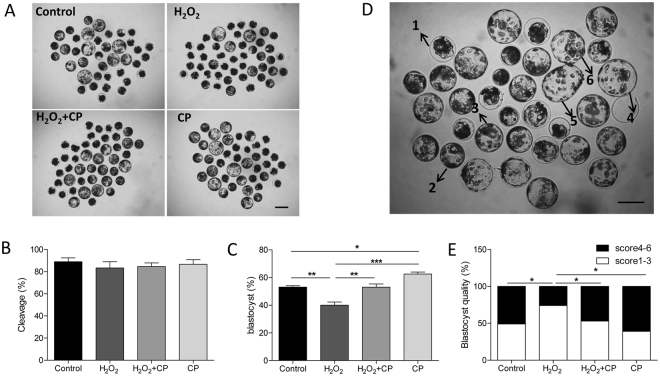
To induce oxidative stress, H2O2 was used to treat porcine one-cell-stage parthenotes. When the parthenotes were exposed to 50 μM H2O2, the rates of blastocyst formation were significantly reduced (Fig. 2C, p < 0.01). Addition of 5 μg/mL CP provided notable protection as indicated by an increase in the percentage of embryos that reached the blastocyst stage compared to those treated with H2O2 alone (Fig. 2C, p < 0.01). The cleavage rate was not affected by H2O2 treatment alone or together with CP addition (Fig. 2B, p > 0.05).
Figure 2.
Addition of CP promoted early porcine embryo development in present of H2O2. Images (A), cleavage (B), and blastocyst formation (C) of embryos in control, H2O2, H2O2 + CP, and CP groups. (D) Blastocysts were graded on a scale from 1 to 6 as illustrated. Different scores are indicated by the respective numbers. (E) Blastocyst quality was examined in the control, H2O2, H2O2 + CP, and CP groups. Data are expressed as the mean ± SEM. In addition, *p < 0.05, **p < 0.01, and ***p < 0.001 indicate significant differences between different groups. Bar = 200 µm. The experiments were replicated six times with 1226 embryos.
Blastocysts were graded on a scale from 1 to 6 depending on their degree of expansion as shown in Fig. 2D. The rate of good-quality blastocysts (score 4–6) was significantly lower in the H2O2 treatment group than in the control, H2O2 + CP, and CP groups (Fig. 2E, p < 0.05).
CP prevented H2O2-induced production of ROS
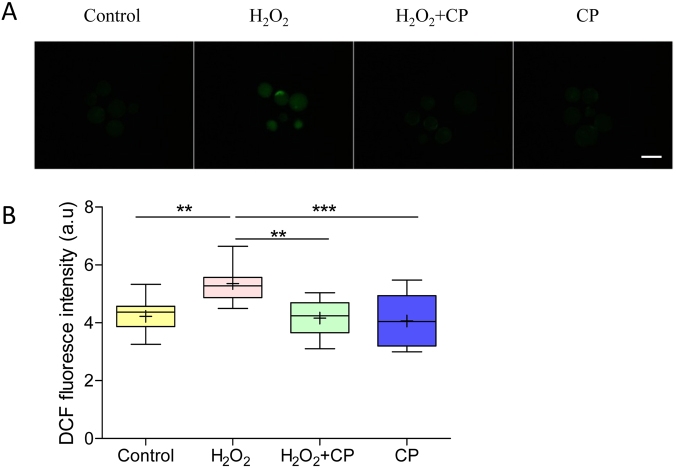
To determine antioxidation of CP in early porcine embryos, ROS level in blastocysts was measured using the DCFH fluorescent reaction. As shown in Fig. 3A, the DCFH fluorescence intensity was much higher in the H2O2-treated group compared with the control, H2O2 + CP, and CP groups. Quantification analysis showed that present of CP significantly decreased the level of intracellular ROS in blastocysts pre-treated with H2O2 (p < 0.01, Fig. 3B), suggesting that CP prevents H2O2-induced production of ROS.
Figure 3.
CP decreased H2O2-induced intracellular ROS level in early porcine blastocysts. (A) Fluorescence signals of ROS in blastocysts. (B) Intracellular ROS levels in blastocysts. Data are expressed as the mean ± SEM. In addition, **p < 0.01 and ***p < 0.001 indicate significant differences between different groups. Bar = 200 µm. The experiments were replicated three times with 187 blastocysts.
CP prevented mitochondrial dysfunction induced by H2O2 in porcine embryos
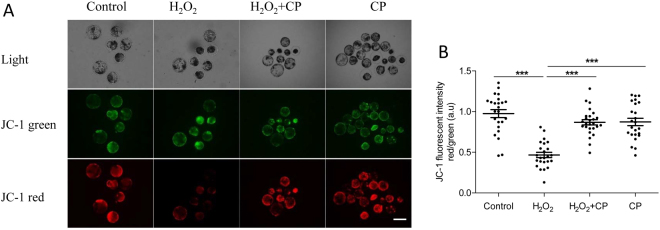
Because mitochondria dysfunction is one of major reason that induces increase of ROS level and compromises embryo development16,17, we further explored mitochondrial membrane potential (MMP) in blastocysts using the JC-1 fluorescent reaction. As shown in Fig. 4A, H2O2 treatment enhanced green fluorescence and attenuated red fluorescence compared with that in the control, H2O2 + CP, and CP groups. Quantification analysis showed that the ratio of fluorescence intensity (red/green) decreased nearly 50% after H2O2 treatment, whereas it was recovered to levels similar to that in the control and CP alone groups in present of CP (p < 0.001, Fig. 4B).
Figure 4.
CP prevented H2O2-induced mitochondrial dysfunction in porcine blastocysts. (A) Representative fluorescence images of JC-1 staining in blastocysts. (B) Quantification of the ratio of fluorescence intensity (red/green) of JC-1 in blastocysts. Data are expressed as the mean ± SEM. In addition, ***p < 0.001 indicate significant differences between different groups. Bar = 200 µm. The experiments were replicated three times with 232 blastocysts.
CP prevented H2O2-induced apoptosis
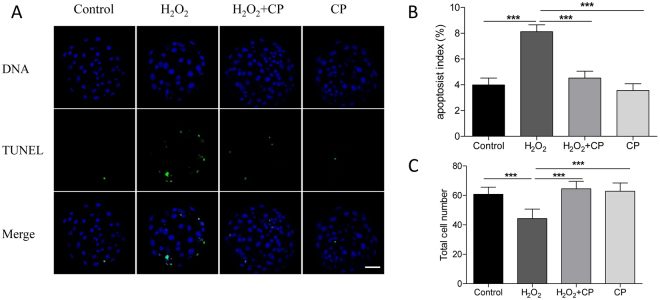
Apoptosis rate in blastocysts developed from H2O2-treated parthenotes in the presence or absence of CP was determined by TUNEL assay. As shown in Fig. 5, apoptosis rate of blastocyst pre-treated with H2O2 was significantly increased and the total cell number was decreased compared with control and CP alone groups (p < 0.001). However, blastocysts in the H2O2 + CP group had more cells than those treated only with H2O2 (p < 0.001). Furthermore, the number of TUNEL-positive cells decreased significantly in blastocysts derived from H2O2 + CP group as well (p < 0.001), suggesting a protective effect of CP against apoptosis in blastocysts.
Figure 5.
CP prevents H2O2-induced apoptosis in porcine blastocysts. (A) Representative fluorescence images of TUNEL staining in blastocysts. Apoptosis index (B) and total cell number (C) in blastocysts. Data are expressed as the mean ± SEM. In addition, *p < 0.05 and ***p < 0.001 indicate significant differences between different groups. Bar = 40 µm. The experiments were replicated four times with 334 blastocysts.
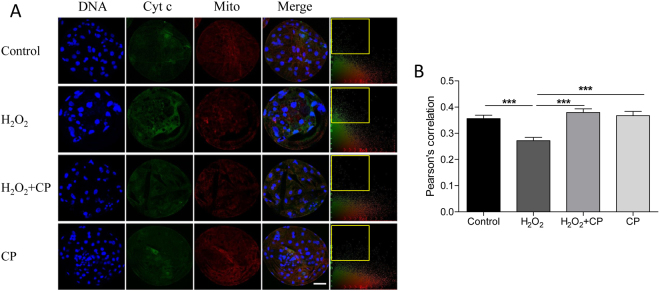
CP prevented H2O2-induced release of cytochrome c from mitochondria
To determine how CP decreased H2O2-induced apoptosis, colocalization of the mitochondria and cytochrome c was analyzed, as shown in Fig. 6. In H2O2-treated blastocysts, more cytochrome c was dispersed throughout the cytoplasm than in the control, H2O2 + CP, and CP groups. Pearson’s correlation showed that CP can prevent H2O2-induced cytochrome c release (p < 0.001).
Figure 6.
Colocalization of cytochrome c and mitochondria in blastocysts in different groups. (A) Blastocysts were labeled with a cytochrome c specific antibody (green) and Mito Tracker Red. (B) Colocalization was analyzed using Image-Pro Plus; frame indicates released cytochrome c. Pearson’s correlation was analyzed using Image-Pro Plus. Data are expressed as the mean ± SEM. In addition, ***p < 0.001 indicates significant differences between different groups. Bar = 40 µm. The experiments were replicated three times with 246 blastocysts.
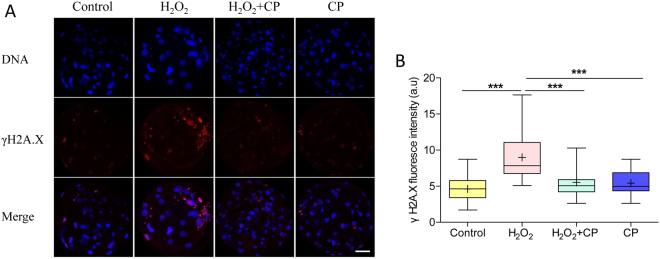
CP prevented H2O2-induced DNA damage
The effect of CP on DNA damage (as indicated by γH2A.X) in porcine parthenogenetic embryos was shown in Fig. 7. Compared with control and CP groups, DNA damage in blastocysts pre-treated with H2O2 was much higher, whereas damage was markedly decreased in the presence of CP (p < 0.001).
Figure 7.
CP attenuated H2O2-induced DNA damage in porcine blastocysts. (A) Representative fluorescence images showing the presence of γH2A.X protein in the nuclei of porcine blastocysts. (B) Fluorescence intensity of γH2AX in blastocysts. Data are expressed as the mean ± SEM. In addition, ***p < 0.001 indicates significant differences between different groups. Bar = 40 µm. The experiments were replicated three times with 168 blastocysts.
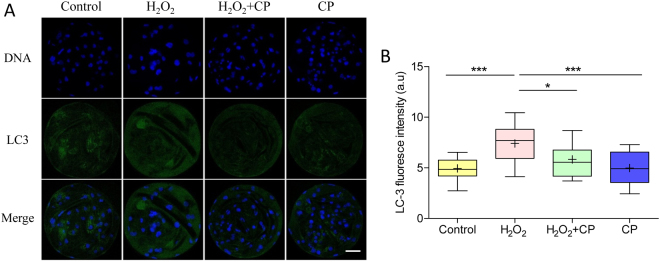
CP prevented H2O2-induced autophagy
Oxidative stress can induce cell autophagy. Therefore, the expression level of LC3 was examined after treatment with or without H2O2 or CP. Immunofluorescence staining revealed a significant increase of LC3 in fluorescence intensity of H2O2-treated embryos (p < 0.001), which was markedly decreased in the presence of CP (Fig. 8, p < 0.05).
Figure 8.
CP attenuated H2O2-induced autophagy in porcine parthenogenetic blastocysts. (A) Representative fluorescence images showing the presence of LC3 protein in porcine blastocysts. (B) Fluorescence intensity of LC3 in blastocysts. Data are expressed as the mean ± SEM. In addition, ***p < 0.001 indicates significant differences between different groups. Bar = 40 µm. The experiments were replicated three times with 205 blastocysts.
Discussion
During in vitro production of preimplantation embryos, oxidative stress impairs embryo development and quality18. The present study showed that CP promoted the porcine embryo development from one-cell stage parthenotes to the blastocyst stage pre-treated with or without H2O2 and decrease apoptosis in blastocysts in present of H2O2, suggesting CP had a protective effect against oxidative stress on porcine preimplantation embryos.
Previous studies showed that CP has antioxidative capacity in several other cell types, including 2D and 3D astrocytes19, Madin-Darby canine kidney cells8, and chondrocytes cells12; however, the present study firstly evaluates the effect of CP on the development of early porcine embryos under oxidative stress. Our data showed addition of 5 μg/mL CP consumedly improved the development and quality of early porcine embryos, as indicated by increased blastocyst formation and hatching, reduced apoptosis, and less DNA damage and autophagy. These effects of CP on the development of preimplantation embryos may be related to the antioxidant and anti-apoptosis properties of CP in porcine embryos.
In biological systems, ROS are natural byproduct of the normal metabolism of oxygen and have important roles in cell of organism20. However, too much ROS caused by environmental stress impair cell structures, which finally induces apoptotic cell death in various cell types21. Previous study showed that CP notably bated apoptosis in Madin-Darby canine kidney cells by reducing intracellular ROS8. Moreover, CP also effectively prevented H2O2-induced ROS accumulation and inhibited apoptotic signals in 2D and 3D astrocytes19. Consistently, our data showed that supplementation of CP in culture media not only prevented production of ROS and decreased the number of apoptosis cells, but also significantly increased the total cell numbers in blastocysts.
In addition to preventing the direct production of ROS, maintaining stable mitochondrial function also appears to be a powerful antioxidant property of CP. During early mouse embryo development, mitochondrial dysfunction caused by treatment with H2O2 induce both cell cycle arrest and apoptosis22. MMP is an important parameter of mitochondrial function. Dissipation of MMP has been shown to promote cytochrome c release into the cytosol under oxidative stress. As a result, a series of caspase related pathway are activated23. In present study, we found that treatment with H2O2 led to depolarizing the MMP; this effect was prevented by treatment with CP. These results are consistent with those of previous studies8,24. Furthermore, to detect whether apoptosis occur in porcine blastocysts was caused by releasing of cytochrome c from mitochondria, we also tested the colocalization of mitochondria and cytochrome c. Our results clearly revealed that mitochondria and cytochrome c did not colocalize in the H2O2 treatment alone group, indicating that cytochrome c was released into the cytosol. However, CP prevented H2O2-induced cytochrome c release.
DNA damage is another harmful effect of oxidative stress because DNA damage can induce apoptosis in several cells3,25. In the present study, DNA damage occurred in porcine blastocysts derived from H2O2 treated parthenotes, while CP prevented this H2O2-induced DNA damage. This may explain how CP attenuated oxidative stress-induced apoptosis.
Autophagy, a natural, regulated mechanism of the cell that degrades unnecessary proteins and dysfunctional organelles, represents a general cellular and tissue response to oxidative stress26,27. Under oxidative stress, chaperone-mediated autophagy was activated and oxidized proteins with higher susceptibility was removed to protect the cells from ROS-induced damage28. However, when oxidative stress reaches a level beyond control of protective respond of autophagy, cell death occurs through necrosis, apoptosis, or autophagic cell death29,30. In the present study, the autophagy marker LC3 was used to estimate the level of autophagy in porcine blastocysts. H2O2-induced oxidative stress increased autophagy and apoptosis in porcine blastocysts, whereas autophagy and apoptosis in porcine blastocysts in the control, H2O2 + CP, and CP groups with low oxidative stress were lower. Furthermore, apoptosis and autophagic cell death under high oxidative stress levels may contribute to a low total cell number in present of H2O2. The protective effect of CP increased the total cell number by preventing oxidative stress.
In conclusion, although embryonic cleavage was not affected by addition of CP, 5 μg/mL CP significantly increased blastocyst formation and the hatching rate during early porcine embryo development. As shown in Fig. 9, CP prevented H2O2-induced production of ROS and depolarization of MMP and release of cytochrome c from the mitochondria. Furthermore, apoptosis, DNA damage level, and autophagy in the blastocysts were attenuated by supplementation with CP after H2O2 treatment. Taken together, these results suggest that CP has beneficial effects on the development of porcine parthenotes by attenuating mitochondrial dysfunction and oxidative stress.
Figure 9.
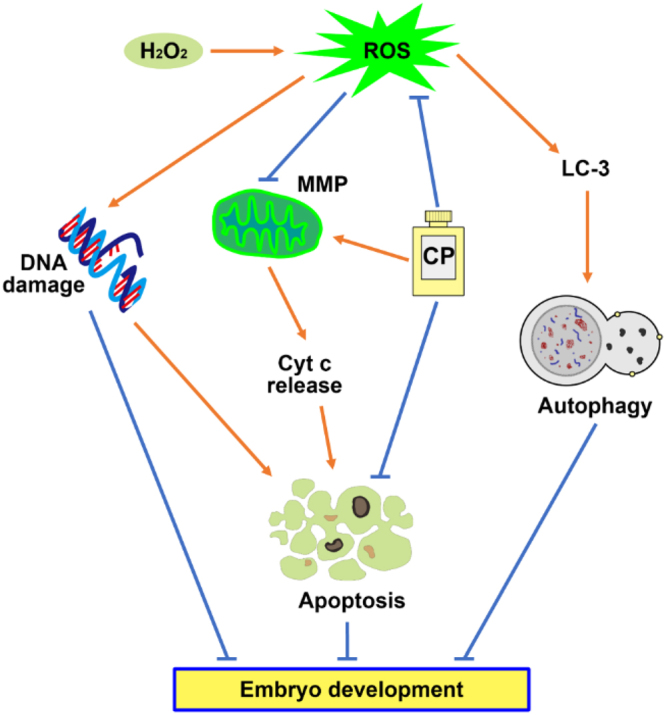
Schematic representation of the protective effect of CP during porcine embryonic development. H2O2 exposure causes the generation of oxidative stress, resulting in DNA damage, autophagy, and mitochondrial dysfunction. Apoptosis was induced by the release of cytochrome c from the mitochondria because of the compromise of MMPs. Addition of CP promotes embryo development by attenuating H2O2-induced oxidative stress, apoptosis, and compromised MMP because of the antioxidative, anti-apoptosis, and protection mitochondrial dysfunction properties.
Materials and Methods
All chemicals were purchased from Sigma-Aldrich Co., Inc. (St. Louis, MO, USA) unless otherwise indicated. All manipulations were performed on a heated stage adjusted to 38.5 °C unless otherwise indicated.
Collection of porcine oocytes and in vitro maturation
Ovaries from pre-pubertal gilts were collected from a local slaughterhouse (Farm story dodarm B&F, Umsung, Chungbuk, Korea) and transported to the laboratory at 37 °C in saline supplemented with 75 mg/mL penicillin G and 50 mg/mL streptomycin sulfate. Follicles 3–6 mm in diameter were aspirated. Cumulus–oocyte complexes were selected according to their morphologic characteristics, i.e., those showing at least three layers of compact cumulus cells and evenly granulated ooplasm. Briefly, the COCs were washed three times with TL-HEPES supplemented with 0.1% polyvinyl alcohol (PVA, w/v) and 0.05 g/L gentamycin. Next, the COCs were washed three times in maturation medium (TCM-199 supplemented with 0.1 g/L sodium pyruvate, 0.6 mM L-cysteine, 10 ng/mL epidermal growth factor, 10% porcine follicular fluid, 10 IU/mL luteinizing hormone, and 10 IU/mL follicle-stimulating hormone) and then transferred to maturation medium. Maturation was performed by culturing approximately 50 COCs in 500 μL of maturation medium in 4-well dishes. The medium was covered with mineral oil and the plates were incubated at 38.5 °C in a humidified atmosphere of 5% CO2 for 44 h.
Parthenogenetic activation and in vitro culture
After removing cumulus cells by repeated pipetting in 1 mg/mL hyaluronidase, denuded oocytes were parthenogenetically activated by 2 direct-current pulses of 120 V for 60 µs in 297 mM mannitol (pH 7.2) containing 0.1 mM CaCl2, 0.05 mM MgSO4, 0.01% PVA (w/v), and 0.5 mM HEPES. These oocytes were cultured in bicarbonate-buffered porcine zygote medium 5 (PZM-5) containing 4 mg/mL BSA and 7.5 µg/mL cytochalasin B (CB) for 3 h to suppress extrusion of the pseudo-second polar body. Next, the oocytes were thoroughly washed and cultured in bicarbonate-buffered PZM-5 supplemented with 4 mg/mL BSA in 4-well plates for 7 days at 38.5 °C and 5% CO2. To determine the dose-effect of CP on early porcine embryos cultured in vitro, the activated embryos were cultured with CP at various concentrations (2, 5, 8, 10 µg/mL). In addition, to determine the protective effect of CP against oxidative stress, the activated embryos were pre-incubated with CP for 3 h. After pre-incubation, H2O2 (50 µM) was added for 30 min. Then, the embryos were washed three times and transferred into PZM-5 medium with or without CP. Cleavage and blastocyst formation rates were examined on days 2 and 7 after activation. After 7 days of culture, the quality of the blastocysts was evaluated according to Gardner’s criteria31. Blastocysts were graded on a scale from 1 to 6 depending on their degree of expansion as follows: Grade 1, Blastocyst cavity occupies less than half of the blastocoel volume of the embryo; Grade 2, Blastocyst cavity occupies more than half of the blastocoel volume of the embryo; Grade 3, Blastocyst cavity completely fills the embryo; Grade 4, Blastocyst cavity occupies more than the blastocoel volume of the embryo, with a thinning zona pellucida; Grade 5, Hatching out of the zona pellucida; Grade 6, Hatched out of the zona pellucida. To determine the total cell number, day 7 blastocysts were randomly collected and stained with 10 mg/mL Hoechst 33342 in PBS for 5 min.
Measurement of ROS contents
ROS in blastocysts were detected using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA, Cat #D399, Molecular Probes Invitrogen, Eugene, OR) as previously described32. Briefly, blastocysts were incubated for 15 min in PBS/PVA containing 10 µM H2DCF-DA at room temperature. After incubation, blastocysts were washed three times with PBS/PVA. Fluorescent signals were captured as a TIFF file using a digital camera (DP72; Olympus, Tokyo, Japan) connected to a fluorescence microscope (IX70, Olympus). Quantification of ROS levels was performed by analyzing the fluorescence intensity in blastocysts using Image J version 1.44 g software (National Institutes of Health, Bethesda, MD, USA).
Terminal deoxynucleotidyl transferase-mediated 2′-deoxyuridine 5′-triphosphate (dUTP) nick-end labeling (TUNEL) assay
The intracellular apoptosis level of blastocysts was measured in a TUNEL assay using an in situ cell death detection kit (Cat #11684795910, Roche) as described previously33. After washing three times with PBS/PVA, blastocysts were fixed in 3.7% paraformaldehyde for 30 min at room temperature and subsequently permeabilized by incubation in 0.5% Triton X-100 for 30 min at room temperature. The embryos were incubated with fluorescein-conjugated dUTP and terminal deoxynucleotidyl transferase enzyme for 1 h, and then washed three times with PBS/PVA. Embryos were treated with 10 µg/mL Hoechst 33342 for 5 min, washed three times with PBS/PVA, and mounted onto glass slides. Images were captured using a confocal microscope (Zeiss LSM 710 META, Jena, Germany). The apoptosis index was calculated as the percentage of TUNEL-positive nuclei.
Immunofluorescence and confocal microscopy
After washing three times with PBS/PVA, embryos were fixed in 3.7% paraformaldehyde for 30 min at room temperature, permeabilized with PBS/PVA containing 0.5% Triton X-100 at room temperature for 30 min, and incubated in PBS/PVA containing 1.0% bovine serum albumin at room temperature for 1 h. These embryos were incubated overnight at 4 °C with anti-LC3 (1:100; Cat #ab58610, Abcam, Cambridge, UK), anti-cytochrome c (1:100; Cat #ab110325, Abcam, Cambridge, UK), and anti-γH2A.X (1:100; Cat #2577, Ser139, Cell Signaling Technology, Danvers, MA, USA) diluted 1:100 in blocking solution. After washing three times with PBS/PVA, the embryos were incubated at room temperature for 1 h with goat anti-rabbit IgG, rabbit anti-goat IgG, or anti-mouse IgG. The oocytes and embryos were stained with 10 µg/mL Hoechst 33342 for 5 min, washed three times with PBS/PVA, mounted onto slides, and examined using a confocal microscope (Zeiss LSM 710 META). Images were processed using Zen software (version 8.0, Zeiss).
Assay of mitochondrial membrane potential (∆Ψm)
Day-7 blastocysts were incubated in PZM-5 containing 2.5 µM 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide (JC-1) (Cat #M34152, Invitrogen, Carlsbad, CA, USA) at 38.5 °C in 5% CO2 for 30 min. Membrane potential was calculated as the ratio of red florescence, which corresponded to activated mitochondria (J-aggregates), to green fluorescence, which corresponded to less-activated mitochondria (J-monomers)34. Fluorescence was visualized using an epifluorescence microscope (Nikon Corp., Tokyo, Japan).
Colocalization assay of mitochondria and cytochrome c
To investigate the colocalization of mitochondria and cytochrome c, blastocysts were incubated with 500 nM MitoTracker Red CMXRos (Cat #M7512, Invitrogen, Eugene, OR, USA) at 38.5 °C for 30 min. After three washes with PZM-5, staining of cytochrome c was carried out as described in Immunofluorescence and confocal microscopy.
Real time reverse transcriptase-polymerase chain reaction (RT-PCR)
Day-7 blastocysts were collected and mRNA was extracted from a pool of 10 blastocysts per group using the DynaBeads mRNA Direct Kit (Cat #61012, Dynal Asa, Oslo, Norway) according to the manufacturer’s instructions. cDNA was obtained by reverse transcription of mRNA using the Oligo (dT)12–18 primer and SuperScript III Reverse Transcriptase (Invitrogen). Amplification was conducted as follows: 95 °C for 3 min followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 20 s, and final extension at 72 °C for 5 min. Target genes were COX2, FN1, and ITGA5. GAPDH was used as a reference gene. The primers used to amplify each gene are shown in Table 1 35. mRNA quantification data were analyzed using the 2−ΔΔCT method36.
Table 1.
Primer sequences used in real time RT-PCR.
| Gene | Primer sequence (5′-3′) | Product Size (bp) |
|---|---|---|
| COX2 | F:GGCTGCGGGAACATAATAGA | 183 |
| R:GCAGCTCTGGGTCAAACTTC | ||
| FN1 | F:AGGGCGATGAACCACAGT | 221 |
| R:GCTCCAGCGAACGACAAT | ||
| ITGA5 | F:TGATGACAGTTATTTGGGCTAC | 100 |
| R: CAAAGTCCTCGCTGCTCT | ||
| GAPDH | F:TTCCACGGCACAGTCAAG | 117 |
| R:ATACTCAGCACCAGCATCG |
F: forward; R: reverse.
Statistical analysis
All data were subjected to one-way analysis of variance. Differences among treatments were examined using the Duncan multiple range test. All percentage data were subjected to arcsine transformation prior to statistical analysis and presented as the mean ± SEM. Significance was set at p < 0.05. All calculations were performed using SPSS software v.19 (SPSS, Inc., Chicago, IL, USA). All box plots show the median (line), mean (+), and 25th and 75th percentiles (boxes) and the whiskers show the minimum to maximum values.
Data Availability
All data generated or analysed during this study are included in this published article.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2015R1D1A1A01057629), Republic of Korea.
Author Contributions
Xiang-Shun Cui, Wen-Fa Lu and Ying-Jie Niu designed the experiment. Ying-jie Niu and Wenjun Zhou conducted the experiments, analyzed the results and wrote the article. Jing Guo, Kyung-Tae Shin conducted part of experiments. Zheng-Wen Nie helped revise the figures. Xiang-Shun Cui and Nam-Hyung Kim assisted in the analyses of the results and helped revise the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Ying-Jie Niu and Wenjun Zhou contributed equally to this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wen-Fa Lv, Email: wenfa2004@163.com.
Xiang-Shun Cui, Email: xscui@cbnu.ac.kr.
References
- 1.Prather R, Hawley R, Carter D, Lai L, Greenstein J. Transgenic swine for biomedicine and agriculture. Theriogenology. 2003;59:115–123. doi: 10.1016/S0093-691X(02)01263-3. [DOI] [PubMed] [Google Scholar]
- 2.Day BN. Reproductive biotechnologies: current status in porcine reproduction. Animal reproduction science. 2000;60-61:161–172. doi: 10.1016/S0378-4320(00)00079-8. [DOI] [PubMed] [Google Scholar]
- 3.Guerin P, El Mouatassim S, Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Human reproduction update. 2001;7:175–189. doi: 10.1093/humupd/7.2.175. [DOI] [PubMed] [Google Scholar]
- 4.Yu S, et al. Protective effect of quercetin on the development of preimplantation mouse embryos against hydrogen peroxide-induced oxidative injury. PloS one. 2014;9:e89520. doi: 10.1371/journal.pone.0089520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Matos DG, Gasparrini B, Pasqualini SR, Thompson JG. Effect of glutathione synthesis stimulation during in vitro maturation of ovine oocytes on embryo development and intracellular peroxide content. Theriogenology. 2002;57:1443–1451. doi: 10.1016/S0093-691X(02)00643-X. [DOI] [PubMed] [Google Scholar]
- 6.Kere M, Siriboon C, Lo NW, Nguyen NT, Ju JC. Ascorbic acid improves the developmental competence of porcine oocytes after parthenogenetic activation and somatic cell nuclear transplantation. The Journal of reproduction and development. 2013;59:78–84. doi: 10.1262/jrd.2012-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pleonsil P, Soogarun S, Suwanwong Y. Anti-oxidant activity of holo- and apo-c-phycocyanin and their protective effects on human erythrocytes. International journal of biological macromolecules. 2013;60:393–398. doi: 10.1016/j.ijbiomac.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Farooq SM, et al. C-phycocyanin confers protection against oxalate-mediated oxidative stress and mitochondrial dysfunctions in MDCK cells. PloS one. 2014;9:e93056. doi: 10.1371/journal.pone.0093056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Y, et al. Effects of phycocyanin on INS-1 pancreatic beta-cell mediated by PI3K/Akt/FoxO1 signaling pathway. International journal of biological macromolecules. 2016;83:185–194. doi: 10.1016/j.ijbiomac.2015.11.054. [DOI] [PubMed] [Google Scholar]
- 10.Romay C, Gonzalez R, Ledon N, Remirez D, Rimbau V. C-phycocyanin: a biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Current protein & peptide science. 2003;4:207–216. doi: 10.2174/1389203033487216. [DOI] [PubMed] [Google Scholar]
- 11.Kuddus M, Singh P, Thomas G, Al-Hazimi A. Recent developments in production and biotechnological applications of C-phycocyanin. BioMed research international. 2013;2013:742859. doi: 10.1155/2013/742859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young IC, Chuang ST, Hsu CH, Sun YJ, Lin FH. C-phycocyanin alleviates osteoarthritic injury in chondrocytes stimulated with H2O2 and compressive stress. International journal of biological macromolecules. 2016;93:852–859. doi: 10.1016/j.ijbiomac.2016.09.051. [DOI] [PubMed] [Google Scholar]
- 13.Li YJ, et al. C-phycocyanin protects against low fertility by inhibiting reactive oxygen species in aging mice. Oncotarget. 2016;7:17393–17409. doi: 10.18632/oncotarget.8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heijink A, et al. Biomechanical considerations in the pathogenesis of osteoarthritis of the knee. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 2012;20:423–435. doi: 10.1007/s00167-011-1818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nature reviews. Molecular cell biology. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 16.Igosheva N, et al. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PloS one. 2010;5:e10074. doi: 10.1371/journal.pone.0010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thouas GA, Trounson AO, Wolvetang EJ, Jones GM. Mitochondrial dysfunction in mouse oocytes results in preimplantation embryo arrest in vitro. Biology of reproduction. 2004;71:1936–1942. doi: 10.1095/biolreprod.104.033589. [DOI] [PubMed] [Google Scholar]
- 18.Lopes, A. S., Lane, M. & Thompson, J. G. Oxygen consumption and ROS production are increased at the time of fertilization and cell cleavage in bovine zygotes. Human reproduction25, 2762–2773, 10.1093/humrep/deq. 221 (2010). [DOI] [PubMed]
- 19.Min SK, et al. Assessment of C-phycocyanin effect on astrocytes-mediated neuroprotection against oxidative brain injury using 2D and 3D astrocyte tissue model. Scientific reports. 2015;5:14418. doi: 10.1038/srep14418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devasagayam TP, et al. Free radicals and antioxidants in human health: current status and future prospects. The Journal of the Association of Physicians of India. 2004;52:794–804. [PubMed] [Google Scholar]
- 21.Bayir H, Kagan VE. Bench-to-bedside review: Mitochondrial injury, oxidative stress and apoptosis–there is nothing more practical than a good theory. Critical care. 2008;12:206. doi: 10.1186/cc6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L, Trimarchi JR, Keefe DL. Involvement of mitochondria in oxidative stress-induced cell death in mouse zygotes. Biology of reproduction. 2000;62:1745–1753. doi: 10.1095/biolreprod62.6.1745. [DOI] [PubMed] [Google Scholar]
- 23.Kimura N, et al. Intrinsic oxidative stress causes either 2-cell arrest or cell death depending on developmental stage of the embryos from SOD1-deficient mice. Molecular human reproduction. 2010;16:441–451. doi: 10.1093/molehr/gaq007. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Rojas B, et al. C-phycocyanin prevents cisplatin-induced mitochondrial dysfunction and oxidative stress. Molecular and cellular biochemistry. 2015;406:183–197. doi: 10.1007/s11010-015-2436-9. [DOI] [PubMed] [Google Scholar]
- 25.Gartner A, Milstein S, Ahmed S, Hodgkin J, Hengartner MO. A conserved checkpoint pathway mediates DNA damage–induced apoptosis and cell cycle arrest in C. elegans. Molecular cell. 2000;5:435–443. doi: 10.1016/S1097-2765(00)80438-4. [DOI] [PubMed] [Google Scholar]
- 26.Klionsky DJ. Autophagy revisited: a conversation with Christian de Duve. Autophagy. 2008;4:740–743. doi: 10.4161/auto.6398. [DOI] [PubMed] [Google Scholar]
- 27.Ryter SW, Choi AM. Regulation of autophagy in oxygen-dependent cellular stress. Current pharmaceutical design. 2013;19:2747–2756. doi: 10.2174/1381612811319150010. [DOI] [PubMed] [Google Scholar]
- 28.Kiffin R, Christian C, Knecht E, Cuervo AM. Activation of chaperone-mediated autophagy during oxidative stress. Molecular biology of the cell. 2004;15:4829–4840. doi: 10.1091/mbc.E04-06-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Azad MB, Gibson SB. Superoxide is the major reactive oxygen species regulating autophagy. Cell death and differentiation. 2009;16:1040–1052. doi: 10.1038/cdd.2009.49. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species. Journal of cell science. 2007;120:4155–4166. doi: 10.1242/jcs.011163. [DOI] [PubMed] [Google Scholar]
- 31.Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertility and sterility. 2000;73:1155–1158. doi: 10.1016/S0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 32.Nasr-Esfahani MH, Aitken JR, Johnson MH. Hydrogen peroxide levels in mouse oocytes and early cleavage stage embryos developed in vitro or in vivo. Development. 1990;109:501–507. doi: 10.1242/dev.109.2.501. [DOI] [PubMed] [Google Scholar]
- 33.Ferris J, Mahboubi K, MacLusky N, King WA, Favetta LA. BPA exposure during in vitro oocyte maturation results in dose-dependent alterations to embryo development rates, apoptosis rate, sex ratio and gene expression. Reproductive toxicology. 2016;59:128–138. doi: 10.1016/j.reprotox.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Sutton-McDowall ML, Feil D, Robker RL, Thompson JG, Dunning KR. Utilization of endogenous fatty acid stores for energy production in bovine preimplantation embryos. Theriogenology. 2012;77:1632–1641. doi: 10.1016/j.theriogenology.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Liang S, Niu YJ, Shin KT, Cui XS. Protective Effects of Coenzyme Q10 on Developmental Competence of Porcine Early Embryos. Microscopy and microanalysis: the official journal of Microscopy Society of America, Microbeam Analysis Society, Microscopical Society of Canada. 2017;23:849–858. doi: 10.1017/S1431927617000617. [DOI] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.