ABSTRACT
Haloferax volcanii is polyploid and contains about 20 genome copies under optimal conditions. However, the chromosome copy number is highly regulated and ranges from two during phosphate starvation to more than 40 under conditions of phosphate surplus. The aim of this study was the characterization of the influence of two replication origins on the genome copy number. The origin repeats and the genes encoding origin recognition complex (ORC) proteins were deleted. The core origin oriC1-orc1 (ori1) deletion mutant had a lower genome copy number and a higher level of fitness than the wild type, in stark contrast to the oriC2-orc5 (ori2) deletion mutant. The genes adjacent to ori1 could not be deleted, and thus, at least two of them are probably essential, while deletion of the genes adjacent to ori2 was possible. Various fragments of and around the origins were cloned into a suicide plasmid to generate haloarchaeal artificial chromosomes (HACs). The copy number of the oriC1-orc1 HAC was much higher than that of the oriC2-orc5 HAC. The addition of adjacent genes influenced both the HAC copy number and the chromosome copy number. The results indicate that the origins of H. volcanii are not independent but that the copy number is regulated via a network of genes around the origins.
IMPORTANCE Several species of archaea have more than one origin of replication on their major chromosome and are thus the only known prokaryotic species that allow the analysis of the evolution of multiorigin replication. The widely studied Haloferax volcanii H26 strain has a major chromosome with four origins of replication. Two origins, ori1 and ori2, were chosen for an in-depth analysis using deletion mutants and haloarchaeal artificial chromosomes. The analysis was not restricted to the core origin regions; origin-adjacent genes were also included. Because H. volcanii is polyploid, the effects on the chromosome copy number were of specific importance. The results revealed extreme differences between the two origins.
KEYWORDS: Archaea, Haloarchaea, Haloferax volcanii, polyploidy, multiorigin replication, deletion mutants, haloarchaeal artificial chromosomes, origin recognition complex, haloarchaea artificial chromosomes, origin recognition proteins, replication initiation, chromosome copy number control, origin of replication
INTRODUCTION
It was long thought that prokaryotes are typically monoploid, with a few long-known exceptions like Deinococcus radiodurans. In stark contrast, during the last decade it became clear that the majority of prokaryotic species are oligoploid (up to 10 genome copies) or polyploid (more than 10 genome copies) (1–5). Many different evolutionary advantages that might have led to the evolution of polyploidy in different groups of prokaryotes for different reasons at different times have been previously described (6). In 2006, Breuert et al. reported that two species of halophilic archaea, Haloferax volcanii and Halobacterium salinarum, are polyploid (7). Recently, the genome copy number has been quantified for three additional, freshly isolated species of haloarchaea, which also turned out to be polyploid (8). This is also true for Haloferax mediterranei (K. Zerulla and J. Soppa, unpublished results), and thus polyploidy seems to be typical for and widespread in haloarchaea. Several evolutionary advantages such as a low mutation rate, desiccation resistance, survival over geological times, and growth in the absence of external phosphate have been experimentally shown to be true for haloarchaea (8–10).
In most species, the chromosome copy number is tightly regulated. Under constant environmental conditions, the chromosome copy number is kept at a constant level, e.g., at one copy in monoploid species and a certain number of copies in oliploid and polyploid species. Changing conditions can lead to an increase or a decrease of the copy number. In many species, the copy number is growth phase regulated and is higher in exponential than in stationary phase (Table 1). The genome copy numbers are also influenced by environmental parameters and can differ greatly. For example, H. volcanii contains only 2 genome copies after growth in the absence of phosphate and more than 40 genome copies after the addition of a surplus of phosphate (10). In the cyanobacterium Synechocystis sp. strain PCC 6803, the genome copy number is influenced not only by the phosphate concentration but also by the light intensity (11). The number of genome copies is determined by the frequency of initiation of replication per cell cycle; i.e., for a constant copy number, each origin of replication has to fire once and only once per cell cycle. For an increase in genome copy number, the number of initiation events per cell cycle must be higher than 1; for a decrease, the number must be lower than 1. Therefore, we characterized the influence of two replication regions and their adjacent genes on the genome copy number of H. volcanii.
TABLE 1.
Comparison of genome copy numbers under different conditions
| Growth condition(s) | No. of gene copies ± SDa |
Source | |
|---|---|---|---|
| exp | stat | ||
| Complex medium | 39.9 ± 5.3 | 27.9 ± 5.4 | 9 |
| Synthetic medium + Casamino Acids | 31.4 ± 3.1 | 20.1 ± 3.2 | |
| Synthetic medium + acetate | 30.0 ± 4.2 | 26.2 ± 3.3 | |
| Synthetic medium + glucose | 35.6 ± 12.7 | 32.6 ± 3.3 | |
| Synthetic medium + glucose − phosphate | 13.7 ± 2.1 | 1.9 ± 0.4 | 10 |
exp, exponential phase; stat, stationary phase.
The genome of H. volcanii has been sequenced and is comprised of one major chromosome, three minor chromosomes, and one small plasmid (12). In the widely used H26 laboratory strain, the small plasmid has been removed, and one of the minor chromosomes, pHV4, has been found to be integrated into the major chromosome, resulting in a cell with three replicons (13). Using marker frequency analysis, it was shown that the major chromosome in H26 contains four origins of replication, three on the original chromosome and one on pHV4 (13). Because the minor chromosomes contain one origin each, the total number of origins in the cell is six. H. volcanii is not the only archaeal species with more than one origin in the major chromosome. The presence of multiple origins was first discovered in two species of Sulfolobus, which were shown to have three replication origins on their chromosome (14, 15).
An even higher number of four origins has been mapped for Pyrobaculum calidifontis (16). To date, no bacterial species with more than one replication origin per chromosome has been found; these archaea are the only models that can be used to study the evolution of origin multiplicity in prokaryotes and to characterize the properties of different origins located on one replicon.
In archaea, replication origins are comprised of (i) a noncoding region containing “origin recognition boxes” (ORBs) and “DNA unwinding elements” (DUE) and (ii) an adjacent gene encoding an “origin recognition complex” (ORC) protein (17, 18). A recent review gives a very good overview of the current knowledge about DNA replication in Archaea (19).
The proximity of ORB boxes and orc genes is analogous, not homologous, to the proximity of the origin oriC and the gene encoding the replication protein DnaA in bacteria (20, 21). Instead, the archaeal ORC protein is homologous to the eukaryotic ORC proteins as well as to the eukaryotic Cdc6 protein. Therefore, several different designations for the archaeal protein, e.g., ORC, ORC1 (the most similar of several eukaryotic ORC proteins that form a heteromeric complex), Cdc6, and ORC/Cdc6, are in use. For simplicity, we use the name “ORC” in this paper.
The six proteins that form the ORC are all essential in the budding yeast, Saccharomyces cerevisiae, and in fission yeast, Schizosaccharomyces pombe; however, ORC1 and ORC2 of the human ORC protein were recently found to be nonessential (22). In Halobacterium salinarum NRC-I, only 2 of 10 orc genes were found to be essential (23). The importance of the orc genes of H. volcanii is described in another paper (K. Ludt and J. Soppa, submitted for publication).
The physical interaction of ORC proteins with the adjacent origin has been shown to occur in Sulfolobus, which nicely explains the high degree of conservation of the proximity of ORBs and initiator protein in archaea (25). Also, it has been postulated that the ORC protein and the adjacent origins form specialized pairs that are autonomous and independent from other pairs in Haloarcula hispanica and other Haloacula species (26, 27). This is in contrast to eukaryotes, which harbor a limited set of ORC proteins but many thousands of replication origins.
Active replication origins of H. volcanii were first identified by cloning “autonomously replicating sequences” (ARS) from a library of the genome (28), even before the genome sequence became available (12). Two different origins, one from the major chromosome and one from a minor chromosome, could be cloned, and they were shown to differ in their ARS activity. After the genome sequence became available, a very elegant and comprehensive deletion analysis of origins was performed (13). Single-deletion mutants of all four origins on the major replicon could be generated, showing that none of them is essential for growth. Very surprisingly, a quadruple-deletion mutant could also be constructed, and that mutant even grew 7.5% faster than the wild-type H26. Marker frequency analysis excluded the possibility of the activation of a hitherto-silent, additional origin but showed that the chromosome was origin free. It was postulated that another mechanism of replication, probably based on homologous recombination, had taken over and that this mechanism might be ancient, preceding the invasion of the chromosome by different genetic elements that later became replication origins. However, survival in the absence of origins is not universally possible in haloarchaea; the removal of all three primary origins in Haloferax mediterranei led to the activation of a hitherto-dormant origin, and the generation of a quadruple-deletion mutant was not possible (29).
We selected the two major origins of the original chromosome of H. volcanii, origin 1 and origin 2, for an analysis of their activities. We expanded our analysis to the genes that are localized 5′ and 3′ to the core origins to unravel whether they might also be important for copy number control. Two different approaches were used. On the one hand, precise markerless deletions were generated, and on the other hand, genomic fragments around the origins were cloned into a suicide vector to generate “haloarchaeal artificial chromosomes” (HACs). Competitive growth of the wild-type strain and deletion mutants was performed, and the chromosome copy numbers and HAC copy numbers were quantified by real-time PCR.
RESULTS
The core replication origins origin 1 and 2 and adjacent genes.
In this paper, the term “origin” is used to describe the pair of repeat regions with DUEs and ORBs and the adjacent orc gene; e.g., oriC1-orc1 is denoted origin 1 (ori1), and oriC2-orc5 is named origin 2 (ori2). Origin 1 was chosen for the analysis because it is highly conserved in archaea and because it has been postulated that it had already been present in the last universal archaeal ancestor (26). Origin 2 was chosen because it is not conserved and therefore is probably evolutionary younger than origin 1. Their comparison should reveal similarities and differences between different haloarchaeal origins.
Figure 1A gives an overview of the genomic organization of origin 1 and the adjacent genes. HVO_3010 encodes the Hef endonuclease (helicase-associated endonuclease for fork-structured DNA), which is known to be involved in replication (30, 31). In archaea, Hef was discovered as a novel endonuclease in Pyrococcus furiosus (32). As the name implies, it acts at structured DNA, and thus it is involved in the resolution of stalled replication forks and recombination intermediates (31, 32). In H. volcanii, Hef is not essential in the wild type, because Hef and Hjc have redundant functions, but it becomes essential when Hjc is absent (31). The localization of genes HVO_3011 to HVO_3013 directly upstream of origin 1 is highly conserved in all halophilic archaea, indicating that they might be important for (the regulation of) replication. Their functions are not known; only HVO_3013 has the very general annotation that it is likely to be a probable GTP-binding protein. The genes downstream of origin 1 encode a signal peptidase, a subunit of the DNA polymerase, and an oxidoreductase. The regions that were chosen for the generation of deletion mutants are indicated in Fig. 1A.
FIG 1.
Schematic overviews of the genomic organizations around replication origins ori1 (A) and ori2 (B). The core origins are comprised of (i) noncoding sequences (oriC) containing the origin repeat boxes (ORBs) and DNA unwinding elements (DUE) and (ii) a paralog of an orc gene. The gene designations of the adjacent genes (HVO_no.) are included. The regions that were selected for deletions are indicated.
Figure 1B shows the genomic organization of origin 2 and the adjacent genes. The upstream genes encode the helicase Rad25 (HVO_1723) and a conserved hypothetical protein (HVO_1724). The genes downstream of origin 2 encode another conserved hypothetical protein and the basal transcription initiation factor TATA box binding protein (TBP). Of course, it is tempting to speculate that the two hypothetical proteins adjacent to origin 2 might be important for its function. The regions that were chosen for the generation of deletion mutants (see below) are indicated in Fig. 1B.
The effects of deleting core origins 1 and 2.
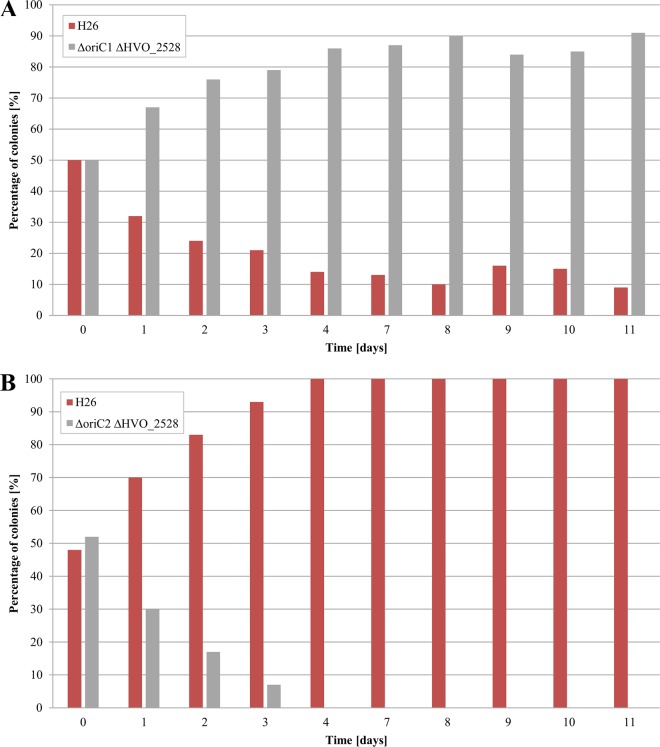
At first, the core origin regions were deleted. The so called pop-in–pop-out method (33) was used to ensure markerless deletions of the oriC-ORC pairs without affecting any adjacent genes. The exact genomic locations of the deleted regions are included in Table 2. The successful deletions in all chromosomal copies of the polyploid H. volcanii were verified by Southern blotting analyses (data not shown). The genome copy numbers were quantified in exponentially growing cultures as well as in stationary-phase cultures, and the results are shown in Fig. 2. Deletion of origin 1 led to a slight decrease in copy number in both growth phases. In stark contrast, deletion of origin 2 resulted in massive increases in copy numbers to about 70 copies in exponential phase and 40 copies in stationary phase. Competitive growth experiments were performed to unravel whether these changes in genome copy number had any effect on the fitness of H. volcanii. To this end, a markerless in-frame deletion mutant of gene HVO_2528, which encodes phytoene dehydrogenase, was generated, resulting in cells that were white, in contrast to the red cells that are proficient in carotinoid biosynthesis. Equal numbers of white cells with all replication origins and of carotenoid-producing red origin-deletion mutants were mixed and grown together. Every day, aliquots of the overnight cultures were used to inoculate new medium. Serial dilutions of cultures from all days were plated to enable the quantification of the fractions of red and white cells. Figure 3A shows that the origin 1 deletion mutant outperformed the wild type and that its fraction increased from nearly 50% to more than 90% within 10 days. In stark contrast, the origin 2 deletion mutant exhibited a large growth disadvantage compared to the wild type, and its fraction decreased to nearly zero within 10 days (Fig. 3B).
TABLE 2.
Deleted regions and genes of deletion mutants
| Haloferax volcanii mutant name | Deleted region | Deleted gene(s) |
|---|---|---|
| Δ5′ori1 | 2841632–2842487 | HVO_3010, HVO_3012, HVO_3013, HVO_3014, HVO_0001 |
| Δori1 | 2847040–2487 | HVO_0001 |
| Δ3′ori1 | 2847040–5827 | HVO_0001, HVO_0002, HVO_0003, HVO_0004 |
| Δ5′ori2 | 1590510–1592908 | HVO_1723, HVO_1724, HVO_1725 |
| Δori2 | 1592908–1596454 | HVO_1725 |
| Δ3′ori2 | 1596454–1597516 | HVO_1725, HVO_1726, HVO_1727 |
| Δ2528 | 2394240–2395718 | HVO_2528 |
FIG 2.
Copy numbers of the major chromosome in the wild-type H26 strain and the deletion mutants of the core origin regions origins 1 and 2. The copy numbers were determined in exponentially growing cultures (exp) and stationary-phase cultures (stat). Average values of data from three biological replicates and their standard deviations are shown.
FIG 3.
Competitive growth of the white wild-type H26 strain and the red mutants of core origin regions origin 1 (A) and origin 2 (B). The strains were mixed in equal ratios and grown competitively. Every day, aliquots of the grown cultures were used to inoculate new cultures, and serial dilutions were plated to quantify the fractions of red and white cells.
To exclude the possibility that deletion of the carotenoid biosynthesis gene influenced the fitness of the wild type, the experiment was mirrored and gene HVO_2528 was additionally deleted in the two origin-deletion strains. Competitive growth experiments were performed with mixtures of the red wild-type and the white origin deletion mutants, and the results are shown in Fig. 4. The results were identical to those described above in these combinations also; i.e., the origin 1 deletion mutant outperformed the wild type, while the origin 2 deletion mutant had a severe growth disadvantage.
FIG 4.
Competitive growth of the red wild-type H26 strain and the white mutants of core origin regions origin 1 (A) and origin 2 (B). The strains were mixed in equal ratios and grown competitively. Every day, aliquots of the grown cultures were used to inoculate new cultures, and serial dilutions were plated to quantify the fractions of red and white cells.
Taken together, the data from the competitive growth experiments indicated that strains with higher genome copy numbers had a growth disadvantage under optimal conditions compared to strains with a lower genome copy number, probably due to the energetic burden of replication of a higher number of genomes during each cell cycle. Notably, deletion of the core regions of origins 1 and 2 had opposite effects on the copy number and the fitness of H. volcanii.
Copy numbers of HACs containing core origin 1 or 2.
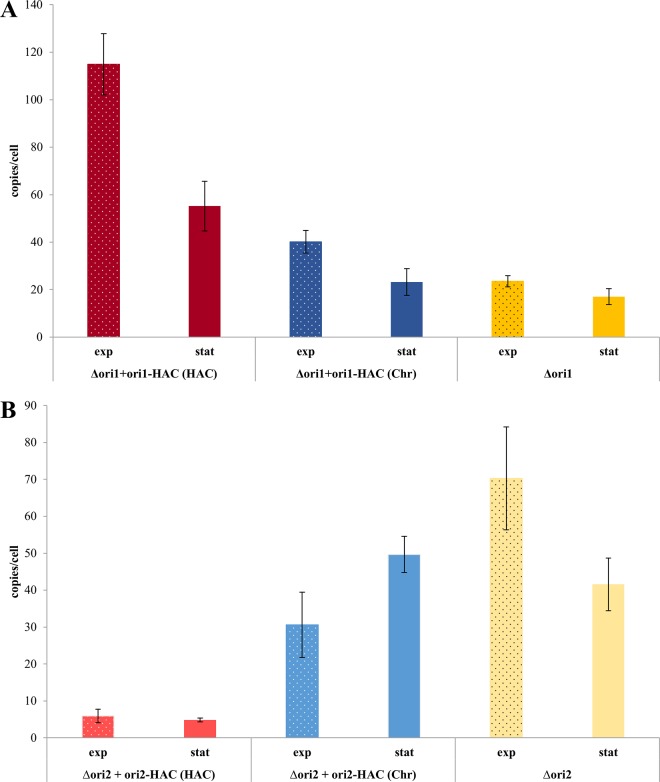
To complement the deletion experiments, the core regions of origins 1 and 2 were cloned into the pMH101 suicide plasmid (34). Plasmid pMH101 does not contain its own replication origin for haloarchaea, and attempts to transform H. volcanii with pMH101 did not result in any colony formation. In addition, the plasmid is routinely used for the generation of deletion mutants, and the absence of a native replication origin is a prerequisite for this application (see, e.g., reference 35). The cloning of origins 1 and 2 into pMH101 resulted in the haloarchaeal artificial chromosomes (HACs) ori1-HAC and ori2-HAC. The two HACs were used to transform the respective deletion mutants, and colony formation verified that autonomous replication had been successfully been transferred from the chromosome to pMH101. The copy numbers of the two HACs and the major chromosome were quantified. Figure 5 shows that the HAC copy numbers were extremely different, with nearly 120 copies per cell for ori1-HAC and less than 10 copies for ori2-HAC in exponential phase. The difference was a bit smaller in stationary phase but was still enormous. Notably, the core region of origin 1 was sufficient to transfer the growth-phase-dependent regulation from the chromosome to the HAC, while this was not the case for the ori2-HAC. In addition to the HAC copy number, the chromosome copy number in the complemented deletion mutants was quantified also. The results are also shown in Fig. 5 in comparison to the noncomplemented deletion mutants. A clear effect of the HACs on the chromosome in trans was observed. Again, the two core origins had opposite effects; the presence of ori1-HAC increased the copy number of the chromosome, while the presence of the ori2-HAC decreased the copy number of the chromosome. Furthermore, the direction of growth-phase-dependent regulation was retained by the ori1-HAC, while the presence of the ori2-HAC inverted the regulation. Taken together, the results obtained with the core origin HACs underscored the large difference between two replication origins and identified cross-regulation between different replicons in trans.
FIG 5.
The deletion mutants of core origins origin 1 (A) and origin 2 (B) were transformed with the HACs containing the respective core origin regions. The copy numbers of the HACs and the major chromosome were quantified in cultures in the exponential phase and stationary phase, as indicated. Average values of data from three biological replicates and their standard deviations are shown.
Generation of HACs containing core origin-adjacent genes.
Next, HACs were generated that contained origin-adjacent genes, in addition to the core origins. An overview of the four cloned regions is given in Fig. 6A. The HACs were used to transform the respective core origin deletion strains. Because the new HACs contained genes that were also present on the major chromosome, the possibility existed that homologous recombination could lead to an integration of the HACs into the chromosome. Therefore, prior to any other characterization of the strains, Southern blot analyses were performed to unravel whether the HACs had replicated autonomously or whether they had integrated into the chromosome. The results are shown in Fig. 6B; in each case, the respective deletion strain was analyzed prior to (−) and after (+) transformation with the respective HACs. The chromosome of the core origin 1 deletion strain remained unchanged, indicating that the two HACs with the adjacent genes replicated autonomously. In contrast, both HACs with core origin 2-adjacent genes had integrated into the chromosome. The results revealed another difference between the two origin regions and underscored that origin 2 is much weaker than origin 1. It was decided to delete the origin-adjacent genes from the chromosome first, to exclude the possibility of homologous recombination and enforce autonomous replication of the HACs.
FIG 6.
(A) Schematic overviews of the genomic regions that were cloned into a suicide plasmid to generate four HACs with origin-adjacent genes. For comparison, the core origin regions are also included. (B) Southern blot analyses to analyze whether the HACs replicated autonomously or were integrated into the major chromosome by homologous recombination. −, strains without HACs; +, HAC-containing strains.
Deletion of core origin-adjacent genes.
The regions chosen for deletions are indicated in Fig. 1; they coincided with the regions that had been cloned into the extended HACs. It turned out that the regions on both sides of core origin 1 could not be deleted. The pop-in clones could readily be generated, but after the pop-out selection more than 100 tested clones turned out to be wild type. Thus, it was concluded that the regions on both sides of the core origin 1 most probably contain at least one essential gene.
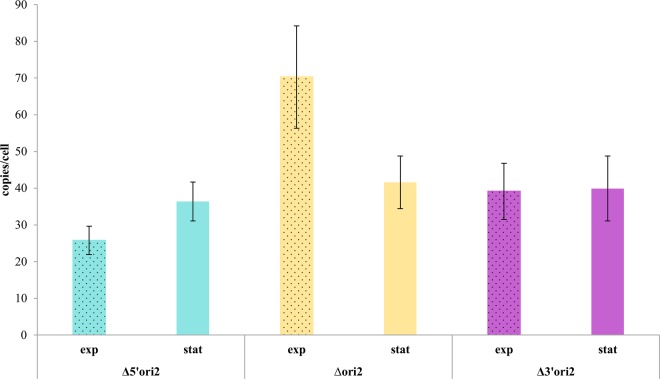
In contrast to origin 1, deletions could be generated on both sides of core origin 2, and thus none of the genes is essential. The effects on the chromosome copy number are shown in Fig. 7. In both cases, the deletion of adjacent genes led to a considerable decrease in copy number, compared to deletion of the core origin region alone. However, this effect was seen only in exponentially growing cells, while the copy number in stationary-phase cells remained unchanged. Thus, it seems that at least two of genes HVO_1723 to HVO_1726 have a positive effect on the chromosome copy number in the exponential growth phase.
FIG 7.
Copy numbers of the major chromosome in the deletion strains of core origin 2 and strains containing additional deletions of adjacent genes. Average values of data from three biological replicates and their standard deviations are shown.
Characterization of HACs containing core origin-adjacent genes.
The two expanded HACs with core origin 2-adjacent genes were transformed into the respective expanded deletion strains, and the copy numbers of HACs and the chromosome were determined. Figure 8A shows that the genes 5′ of core origin 2 did not have a large positive effect on the HAC copy number. In contrast, the genes 3′ to the core origin caused an approximately 4-fold increase of the HAC copy number, both in exponential phase and in stationary phase. Figure 8B summarizes the effects of the HACs on the copy number of the chromosome. The presence of the 5′-adjacent genes on the HAC did not much influence the chromosome copy number, while, in contrast, the presence of the 3′-adjacent genes on the HAC considerable decreased the copy number of the major chromosome. Again, this result showed that there is cross-regulation between different replicons in H. volcanii in trans.
FIG 8.
Copy numbers of the HACs (A) and the major chromosome (B) in three strains carrying deletions in the chromosome that were complemented with HACs containing the respective deleted fragments.
DISCUSSION
Characterization of deletion mutants.
In comparison to the wild-type strain results, the deletions of core origins 1 and 2 had opposite effects on the chromosome copy number as well as on the fitness of the resulting strains. Loss of the major and highly conserved origin 1 led to a reduced genome copy number and considerably enhanced fitness under optimal conditions. The repeat region oriC1 had been deleted previously and it had been reported that the mutant had a slight growth disadvantage of 1.6% compared to the wild type (13). However, in congruence with our results, deletion of the oriC1-orc1 pair led to a growth advantage of the deletion mutant of about 2% (D. Ausiannikava and T. Allers, personal communication). Deletion of core origin 2 led to a large increase in genome copy numbers and a decrease in fitness. In a previous study, the deletion of oriC2 alone had already resulted in a growth defect of 4% (13). Based on our results, it is tempting to speculate that oriC2 deletion in the previous study also resulted to an increase in chromosome copy number and that the increased genome copy number was the reason for the observed the growth defect.
In addition to the core origins, we also attempted to delete core origin-adjacent genes. This attempt failed for origin 1, showing that there is most probably at least one essential gene at both sides. The Hef helicase/nuclease (HVO_3010) could be deleted, and the mutant did not have phenotype during normal growth (31). Therefore, one or more of the highly conserved genes HVO_3011 to HVO_3013, which encode conserved hypothetical proteins, are essential. On the other side of the core origin, the genes for the signal peptide peptidase (HVO_0002) could not be deleted and it was concluded that it is essential (36). It can be safely assumed that DNA polymerase subunit PolD1 (HVO_0003) is also essential. Attempts to delete the polD1 gene in Halobacterium salinarum had failed, and it was concluded that it is one of only a few essential replication genes (23). The genomic localization of polD1 very close to origin 1 (with sec11 in between) is highly conserved in haloarchaea. PolD was found to be the only essential DNA polymerase in Thermococcus kodakaraensis and Methanococcus maripaludis and was postulated to be the primary replicative polymerase for both strands of the DNA (37).
In contrast, the genes adjacent to origin 2 could be deleted and thus none of the four genes is essential. However, the genome copy number is much higher in their presence, and therefore, at least one gene on both sides is involved in copy number regulation. Further single gene deletions will be needed to identify the genes that are responsible for the effect.
Generation of HACs to study copy number control.
The cloning of autonomously replicating sequences (ARS) has been used before to identify archaeal replication origins. To our knowledge, the first example was the cloning of an origin from H. salinarum in 2003 (38). For H. volcanii, this approach led to the identification of one origin from the major chromosome and of another origin which was thought to be identically present on two minor chromosomes (28). The ARS activity of 5 origin regions among 11 possible origin regions tested from Haloarcula hispanica could be confirmed (27). We extended the approach and did not use it for the isolation of ARS from random libraries or as an analysis tool for the in vivo activity of predicted origins. Instead, we used it to analyze the copy number regulation of two core origins (which were known before to have ARS activity) and adjacent genes. Therefore, the term HAC is used instead of ARS to underline the different aim compared to the previous applications. In addition, the term HAC is in line with “yeast artificial chromosomes” (YACs) and “bacterial artificial chromosomes” (BACs) and indicates that the many applications of artificial chromosomes in eukaryotes and bacteria will also be possible in haloarchaea. For example, future applications might include cloning of large pieces from genomes of other haloarchaeal species or the elucidation of predicted encoded metabolic pathways using H. volcanii as a host.
The construction of HACs and the quantification of copy numbers of HACs and the major chromosome turned out to be very informative. Marker gene analysis of the wild-type H26 strain had shown before that origin 1 is a stronger origin than origin 2 in the context of the major chromosome with four replication origins (13). However, the difference was not very large and only the analysis of single origins in different HACs enabled quantification of the greater than 20-fold difference in activity (Fig. 5).
For Haloarcula hispanica, results have been presented that indicate that each ORC protein specifically recognizes the corresponding ORB elements and thus that the distinct oriC-orc pairs act independently. It has been argued that distinct oriC-orc pairs help to minimize competition among multiple origins on one chromosome for initiator proteins and to maintain independent control of all origins (27, 39). The results of the HAC analyses have shown that the situation is very different in H. volcanii. The addition of ori-adjacent genes to the ori2-HAC not only influenced the copy number of the HAC but also resulted in a large change in the copy number of the major chromosome. This clearly showed that there is cross talk between different replicons in H. volcanii. In addition, in another paper we show that cross talk between different replicons also exists (Ludt and Soppa, submitted). In several cases, the deletion of one orc gene influenced the copy number of up to three replicons.
In addition, intrachromosomal cross talk between different origins also exists in H. volcanii. For example, the deletion of genes adjacent to origin 2 in the ori2 deletion mutant drastically affected the chromosome copy number and thus must have influenced one or more of the three other remaining replication origins.
Taken together, the results indicate that replicon copy numbers in H. volcanii are determined through a regulatory network that includes many ORC proteins as well as other players such as the three conserved hypothetical proteins encoded directly upstream of core origin 1. The HACs will be used to analyze the function and importance of individual gene products.
Our analyses revealed that the two replication origins on the major chromosome of H. volcanii, replication origins 1 and 2, are much more dissimilar than had been previously anticipated. The differences are summarized in Table 3. Taking the results together, it seems that origin 1 is much older than origin 2 and is much stronger. Origin 2 was probably integrated into the haloarchaeal chromosome much later and is much weaker. Today, however, the native genome copy number of H. volanii depends on the activity of both origins (and probably also on that of origin 3). There must be cross talk between the origins; otherwise, the discrepancy between the 20-fold difference in activity and the very similar peak heights in a marker frequency analysis (13) cannot be explained.
TABLE 3.
Comparison of ori1 and ori2
| Characteristic | Result |
|
|---|---|---|
| ori1 | ori2 | |
| Conserved in haloarchaea | Yes | No |
| Chromosome copy no. in deletion mutant compared to H26 (36/28)a | Lower (23/16)a | Higher (70/42)a |
| Copy no. of HAC | High (114/55)a | Low (5/5)a |
| Fitness of deletion mutant compared to H26 | Higher | Lower |
| Adjacent genes | Essential | Not essential but influence ploidy |
Data represent numbers of copies in exponential phase/stationary phase.
MATERIALS AND METHODS
Haloarchaeal and bacterial strains.
H. volcanii H26 was kindly provided by Thorsten Allers (University of Nottingham, Nottingham, United Kingdom) and used as the wild-type and parent strain for deletion mutants in this study. H26 is a pyrE deletion mutant and is therefore auxotrophic for uracil, providing a selection marker for deletion and complementation experiments (33). The deletion strains Δori1, Δori2, Δ3′ori2, and Δ5′ori2 were generated in this study (see below), and HVO_2528 was deleted in strains H26, Δori1, and Δori2 (see below). The complementation of deletion mutants with HACs (ori1-HAC, ori2-HAC, 3′ori2-HAC, and 5′ori2-HAC) is described in Results, and the construction of the HACs is described below.
Escherichia coli XL1-Blue MRF was obtained from Stratagene (Amsterdam, The Netherlands).
Media and culture conditions.
H. volcanii was grown in synthetic medium (40) (all ingredients were from VWR, Radnor, PA) supplemented with 8 μM FeSO4 (catalog no. P015.1; Roth, Karlsruhe, Germany), 0.1% (vol/vol) SL-6 trace element solution (http://www.haloarchaea.com/resources/halohandbook/Halohandbook_2008_v7.pdf) (all from Roth), 1 ml vitamin solution (catalog no. B6891; Sigma-Aldrich, St. Louis, MO), 10 mM NH4Cl as a N source (catalog no. 21236.267; VWR), 1 mM K2HPO4 as a P source (catalog no. 6878.2; Roth), 0.25% (wt/vol) Bacto Casamino Acids as a C source (catalog no. 223050; BD, Franklin Lakes, NJ), and 50 μg/ml uracil (catalog no. A0667; AppliChem, Darmstadt, Germany) (the uracil was omitted for the complementation mutants). Solid media contained 1.4% (wt/vol) Bacto agar (catalog no. 214010; BD). Cultures were grown in 100-ml Erlenmeyer flasks in a rotary shaker at 42°C and 250 rpm. Plates were incubated at 42°C.
E. coli XL1-Blue MRF was grown under standard conditions (41).
General molecular genetic techniques.
General molecular genetic techniques were performed according to the method described by Green and Sambrook (41). Genomic DNA was isolated from H. volcanii as previously described (42). Plasmid DNA was isolated using a GenElute Plasmid Midiprep kit (Sigma-Aldrich), and PCR fragments were purified either directly or from preparative agarose gels using a PCR-Kombi-kit (SeqLab, Göttingen, Germany). Restriction enzymes and T4 DNA ligase were supplied by Thermo Fisher Scientific (Waltham, MA), and, if not otherwise stated, the enzymes were used according to the instructions of the manufacturer. The Pfu polymerase and Taq polymerase were isolated in-house, and oligonucleotides were synthesized by Sigma-Aldrich. H. volcanii was transformed according to the method described by Cline et al. (43).
Construction of deletion mutants.
The deletion mutants were generated by the so-called pop-in–pop-out method as previously described (33). In short, about 500 nucleotides (nt) of the upstream region of the respective gene, including a short 5′ part of the open reading frame (ORF) and about 500 nt containing the 3′ part and downstream region, were amplified separately. The two fragments contained an overlap so that they could be combined in a third PCR, generating a fragment containing the upstream and downstream regions and an in-frame-deleted version of the ORF. All oligonucleotides are listed in Table S1 in the supplemental material. The suicide vector pMH101 (34) was digested with EcoRV, and the PCR fragment was cloned by restriction selection cloning. The sequences of the resulting plasmids were verified by sequencing. H. volcanii was transformed with the plasmids, and pop-in mutants were selected using growth in the absence of uracil. Pop-out mutants were selected by the addition of uracil and fluoroorotic acid (5-FOA). Deletion mutants were identified by colony PCR and verified by Southern blotting as described below. The deletion mutants, the extent of the deletions, and the deleted genes are summarized in Table 1.
Construction of HACs and complementation mutants.
For construction of so-called haloarchaeal artificial chromosomes (HACs), the pMH101 suicide vector was used (34). pMH101 contains the pyrE gene as a selection marker. First, the desired fragments were amplified by PCR using the oligonucleotides described in Table S2. After purification, the PCR fragments were ligated into the EcoRV-digested pMH101 suicide vector and E. coli XL1-Blue MRF was transformed. Positive clones were identified by PCR, the HACs were isolated as described above, and the sequence was verified by sequencing (GATC, Constance, Germany). Afterwards, the deletion mutants were transformed with their respective HACs.
Southern blot analysis for verification of deletion mutants and analysis of integration of HAC.
Southern blot hybridization was performed as previously described (44). Genomic DNA of the various mutants was digested with the restriction enzymes listed in Table S3 in the supplemental material. Table S3 also lists the oligonucleotides that were used to generate digoxigenin (DIG)-dUTP-labeled probes and the expected restriction fragment sizes for the wild-type and the deletion mutants. In all cases, the Southern blot analyses included an overexposure of the films to exclude the possibility that the deletion mutants had retained a few copies of the wild-type genome, in addition to the genome with the deletion variant of the respective gene.
Competitive growth to determine relative levels of fitness of strains.
For competitive growth analysis, the gene for phytoene dehydrogenase (HVO_2528) was deleted in strains Δori1, Δori2, and H26. Deletion of HVO_2528 results in a disruption of the carotenoid synthesis and thus in a colorless phenotype compared to the characteristically red colonies of the wild type. Oligonucleotides for the deletion and the Southern blot analysis are included in Table S1 and S3, respectively. In all three cases, markerless in-frame deletions of gene HVO_2528 were generated using the experimental approach described above.
After generation of the colorless mutants, 30 ml of medium was inoculated with 1 × 107 cells of a 1:1 mixture from exponentially growing cultures of the colorless H26 strain and a red origin deletion mutant. The mixed culture was incubated for 24 h, and then 100 μl was transferred into 30 ml of fresh medium. The mixed culture was further grown, and, every day, 1 × 107 cells were transferred into fresh medium. Every day, appropriate serial dilutions were plated, and, after an incubation period of 4 to 5 days, red and white colonies were counted and the results graphically depicted.
The experiment was repeated with the red H26 strain and colorless origin deletion mutants to ensure that the observed differences in growth were not the result of HVO_2528 deletion.
Quantification of genome and HAC copy numbers using quantitative real-time PCR (qPCR).
For quantification of genome and HAC copy numbers, a previously established real-time PCR technique was used (7, 10, 44). As a standard fragment for genome copy number determination, a 1-kbp fragment of leuB was amplified by PCR, purified from a preparative agarose gel as described above, and precipitated with ethanol. It was dissolved in Tris-EDTA (TE) buffer, and the DNA concentration was determined photometrically using a NanoDrop ND-1000 spectrophotometer. The concentration of the standard was adjusted to 100 to 200 ng/μl, and the number of molecules of fragment per microliter was calculated. For HAC copy number determinations, a 1-kbp fragment of pMH101 was amplified and treated as described above. All oligonucleotides used for standard fragment PCR and real-time PCR are described in Table S4.
For determination of genome and HAC copy numbers, 3 × 108 cells of early exponential cultures (1 × 108 to 3 × 108 cells/ml) and early stationary-phase cultures (1 × 109 to 3 × 109 cells/ml) were harvested by centrifugation at 13,000 rpm for 5 min. The supernatant was discarded, and the pellet was resuspended in 100 μl basal salt solution (medium without a C, N, or P source) and lysed by addition of 900 μl of distilled water. A 40-μl volume of cell extract was dialyzed for 3 h on membrane filters (VSWP01300; Merck Millipore, Darmstadt, Germany) (13-mm diameter) against distilled water. Serial dilutions of the standard fragment (10−3 to 10−8) in duplicate and four different dilutions of three biological replicates, also in duplicate, were used for qPCR, such that 24 technical replicates were used for copy number determination. A negative control with omission of template DNA was also performed. The qPCR mixtures contained 0.8 μM each oligonucleotide, 5.5 μl nuclease-free water, 12.5 μl Maxima SYBR green qPCR master mix (catalog no. K0223; Thermo Fisher Scientific), and 5 μl of standard or cell extract, reaching a total volume of 25 μl. qPCR conditions were 10 min at 96°C; 50 cycles of 30 s at 96°C, 30 s at 64°C, and 30 s at 72°C followed by 5 min at 72°C; and a melt curve analysis performed from 62 to 96°C in 1°C steps.
Supplementary Material
ACKNOWLEDGMENTS
We thank Fabian Weipert for initial results concerning the application of HACs for the characterization of replication origins.
This work was supported by the Deutsche Forschungsgemeinschaft (German Research Council) through grant SO 264/24.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00517-17.
REFERENCES
- 1.Pecoraro V, Zerulla K, Lange C, Soppa J. 2011. Quantification of ploidy in proteobacteria revealed the existence of monoploid, (mero-)oligoploid and polyploid species. PLoS One 6:e16392. doi: 10.1371/journal.pone.0016392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griese M, Lange C, Soppa J. 2011. Ploidy in cyanobacteria. FEMS Microbiol Lett 323:124–131. doi: 10.1111/j.1574-6968.2011.02368.x. [DOI] [PubMed] [Google Scholar]
- 3.Hildenbrand C, Stock T, Lange C, Rother M, Soppa J. 2011. Genome copy numbers and gene conversion in methanogenic archaea. J Bacteriol 193:734–743. doi: 10.1128/JB.01016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angert ET. 2012. DNA replication and genomic architecture of very large bacteria. Annu Rev Microbiol 66:197–212. doi: 10.1146/annurev-micro-090110-102827. [DOI] [PubMed] [Google Scholar]
- 5.Spaans SK, van der Oost J, Kengen SWM. 2015. The chromosome copy number of the hyperthermophilic archaeon Thermococcus kodakarensis KOD1. Extremophiles 19:741–750. doi: 10.1007/s00792-015-0750-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soppa J. 2014. About desiccation resistance, giant cell size, long-term survival, enforcement by a eukaryotic host and additional aspects. J Mol Microbiol Biotechnol 24:409–419. doi: 10.1159/000368855. [DOI] [PubMed] [Google Scholar]
- 7.Breuert S, Allers T, Spohn G, Soppa J. 2006. Regulated polyploidy in halophilic archaea. PLoS One 1:e92. doi: 10.1371/journal.pone.0000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaakkola ST, Zerulla K, Guo Q, Liu Y, Ma H, Yang C, Bamford DH, Chen X, Soppa J, Oksanen HM. 2014. Halophilic archaea cultivated from surface sterilized middle-late Eocene rock salt are polyploid. PLoS One 9:e110533. doi: 10.1371/journal.pone.0110533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zerulla K, Soppa J. 2014. Polyploidy in haloarchaea: advantages for growth and survival. Front Microbiol 5:274. doi: 10.3389/fmicb.2014.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zerulla K, Chimileski S, Näther D, Gophna U, Papke RT, Soppa J. 2014. DNA as a phosphate storage polymer and the alternative advantages of polyploidy for growth or survival. PLoS One 9:e94819. doi: 10.1371/journal.pone.0094819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zerulla K, Ludt K, Soppa J. 2016. The plody level of Synechocystis sp. PCC 6803 is highly variable and is influenced by chemical and physical external parameters. Microbiology 162:730–739. [DOI] [PubMed] [Google Scholar]
- 12.Hartman AL, Norais C, Badger JH, Delmas S, Haldenby S, Madupu R, Robinson J, Khouri H, Ren Q, Lowe TM, Maupin-Furlow J, Pohlschroder M, Daniels C, Pfeiffer F, Allers T, Eisen JA. 2010. The complete genome sequence of Haloferax volcanii DS2, a model archaeon. PLoS One 5:e9605. doi: 10.1371/journal.pone.0009605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawkins M, Malla S, Blythe MJ, Nieduszynski CA, Allers T. 2013. Accelerated growth in the absence of DNA replication origins. Nature 503:544–547. doi: 10.1038/nature12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lundgren M, Andersson A, Chen L, Nilsson P, Bernander R. 2004. Three replication origins in Sulfolobus species: synchronous initiation of chromosome replication and asynchronous termination. Proc Natl Acad Sci U S A 101:7046–7051. doi: 10.1073/pnas.0400656101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson MP, Dionne I, Lundgren M, Marsh VL, Bernander R, Bell SD. 2004. Identification of two origins of replication in the single chromosome of the archaeon Sulfolobus solfataricus. Cell 116:25–38. doi: 10.1016/S0092-8674(03)01034-1. [DOI] [PubMed] [Google Scholar]
- 16.Pelve EA, Lindas AC, Knöppel A, Mira A, Bernander R. 2012. Four chromosome replication origins in the archaeon Pyrobaculum calidifontis. Mol Microbiol 85:986–995. doi: 10.1111/j.1365-2958.2012.08155.x. [DOI] [PubMed] [Google Scholar]
- 17.Wu Z, Liu J, Yang H, Xiang H. 2014. DNA replication origins in archaea. Front Microbiol 5:179. doi: 10.3389/fmicb.2014.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson NP, Bell SD. 2005. Origins of DNA replication in the three domains of life. FEBS J 272:3757–3766. doi: 10.1111/j.1742-4658.2005.04768.x. [DOI] [PubMed] [Google Scholar]
- 19.Ausiannikava D, Allers T. 2017. Diversity of DNA replication in the Archaea. Genes (Basel) 8:E56. doi: 10.3390/genes8020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skarstad K, Katayama T. 2013. Regulating DNA replication in bacteria. Cold Spring Harb Perspect Biol 5:a012922. doi: 10.1101/cshperspect.a012922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zakrzewska-Czerwińska J, Jakimowicz D, Zawilak-Pawlik A, Messer W. 2007. Regulation of the initiation of chromosomal replication in bacteria. FEMS Microbiol Rev 31:378–387. doi: 10.1111/j.1574-6976.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 22.Shibata E, Kiran M, Shibata Y, Singh S, Kiran S, Dutta A. 2016. Two subunits of human ORC are dispensable for DNA replication and proliferation. Elife 5:e19084. doi: 10.7554/eLife.19084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berquist BR, DasSarma P, DasSarma S. 2007. Essential and non-essential DNA replication genes in the model halophilic archaeon, Halobacterium sp. NRC-I. BMC Genetics 8:31. doi: 10.1186/1471-2156-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reference deleted.
- 25.Samson RY, Xu Y, Gadelha C, Stone TA, Fagiri JN, Li D, Qin N, Pu F, Liang YX, She Q, Bell SD. 2013. Specificity and function of archaeal DNA replication initiator proteins. Cell Rep 3:485–496. doi: 10.1016/j.celrep.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Z, Liu J, Yang H, Liu H, Xiang H. 2014. Multiple replication origins with diverse control mechanisms in Haloarcula hispanica. Nucleic Acids Res 42:2282–2294. doi: 10.1093/nar/gkt1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Z, Liu H, Liu J, Liu X, Xiang H. 2012. Diversity and evolution of multiple orc/cdc6-adjacent replication origins in haloarchaea. BMC Genomics 13:478. doi: 10.1186/1471-2164-13-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norais C, Hawkins AL, Hartman AL, Eisen JA, Myllykallio H, Allers T. 2007. Genetic and physical mapping of DNA replication origins in Haloferax volcanii. PLoS Genet 3:e77. doi: 10.1371/journal.pgen.0030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang H, Wu Z, Liu J, Liu X, Wang L, Cai S, Xiang H. 2015. Activation of a dormant replication origin is essential for Haloferax mediterranei lacking the primary origins. Nat Commun 6:8321. doi: 10.1038/ncomms9321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lestini R, Delpech F, Myllykallio H. 2015. DNA replication restart and cellular dynamics of Hef helicase/nuclease protein in Haloferax volcanii. Biochimie 118:254–263. doi: 10.1016/j.biochi.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 31.Lestini R, Duan Z, Allers T. 2010. The archaeal Xpf/Mus81/FANCM homolog Hef and the Holliday junction resolvase Hjc define alternative pathways that are essential for cell viability in Haloferax volcanii. DNA Repair 9:994–1002. doi: 10.1016/j.dnarep.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Komori K, Fujikane R, Shinagawa H, Ishino Y. 2002. Novel endonuclease in Archaea cleaving various branched structure. Genes Genet Syst 77:227–241. doi: 10.1266/ggs.77.227. [DOI] [PubMed] [Google Scholar]
- 33.Allers T, Ngo HP, Mevarech M, Lloyd RG. 2004. Development of additional selectable markers for the halophilic archaeon Haloferax volcanii based on the leuB and trpA genes. Appl Environ Microbiol 70:943–953. doi: 10.1128/AEM.70.2.943-953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammelmann M, Soppa J. 2008. Optimized generation of vectors for the construction of Haloferax volcanii deletion mutants. J Microbiol Methods 75:201–204. doi: 10.1016/j.mimet.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 35.Jaschinski K, Babski J, Lehr M, Burmester A, Benz J, Dörr M, Marchfelder A, Soppa J. 2014. Generation and phenotyping of a collection of sRNA gene deletion mutants of the haloarchaeon Haloferax volcanii. PLoS One 9:e90763. doi: 10.1371/journal.pone.0090763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fine A, Irihimovitch V, Dahan I, Konrad Z, Eichler J. 2006. Cloning, expression, and purification of functional Sec11a and Sec11b, type I signal peptidases of the archaeon Haloferax volcanii. J Bacteriol 188:1911–1919. doi: 10.1128/JB.188.5.1911-1919.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenough L, Menin JF, Desai NS, Kelman Z. 2014. Characterization of family D DNA polymerase from Thermococcus sp. 9°N. Extremophiles 18:653–664. doi: 10.1007/s00792-014-0646-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berquist BR, DasSarma S. 2003. An archaeal chromosomal autonomously replicating sequence element from an extreme halophile, Halobacterium sp. strain NRC-1. J Bacteriol 185:5959–5966. doi: 10.1128/JB.185.20.5959-5966.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Z, Yang H, Liu J, Wang L, Xiang H. 2014. Association between the dynamics of multiple replication origins and the evolution of multireplicon genome architecture in haloarchaea. Genome Biol Evol 6:2799–2810. doi: 10.1093/gbe/evu219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nieuwlandt DT, Daniels CJ. 1990. An expression vector for the archaebacterium Haloferax volcanii. J Bacteriol 172:7104–7110. doi: 10.1128/jb.172.12.7104-7110.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Green MR, Sambrook J. 2012. Molecular cloning: a laboratory manual, 4th ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 42.Rosenshine I, Zusman T, Werzberger R, Mevarech M. 1987. Amplification of specific DNA sequences correlates with resistance of the archaebacterium Halobacterium volcanii to the dihydrofolate reductase inhibitors trimethoprim and methotrexate. Mol Gen Genetics 208:518–522. doi: 10.1007/BF00328149. [DOI] [Google Scholar]
- 43.Cline SW, Lam WL, Charlebois RL, Schalkwyk LC, Doolittle WF. 1989. Transformation methods for halophilic archaebacteria. Can J Microbiol 35:148–152. doi: 10.1139/m89-022. [DOI] [PubMed] [Google Scholar]
- 44.Lange C, Zerulla K, Breuert S, Soppa J. 2011. Gene conversion results in the equalization of genome copies in the polyploid haloarchaeon Haloferax volcanii. Mol Microbiol 80:666–677. doi: 10.1111/j.1365-2958.2011.07600.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.