ABSTRACT
Select agents (SA) pose unique challenges for licensing vaccines and therapies. In the case of toxin-mediated diseases, HHS assigns guidelines for SA use, oversees vaccine and therapy development, and approves animal models and approaches to identify mechanisms for toxin neutralization. In this commentary, we discuss next-generation vaccines and therapies against ricin toxin and botulinum toxin, which are regulated SA toxins that utilize structure-based approaches for countermeasures to guide rapid response to future biothreats.
KEYWORDS: botulinum toxin, RTA, RVEc, RiVax, antibodies, mass spectrometry, ricin
INTRODUCTION
This commentary addresses studies by Mantis and coworkers, who in this issue of Clinical and Vaccine Immunology describe the continued mapping of the neutralizing epitopes within ricin toxin (RTX) and relate these studies to current research on the botulinum neurotoxins (BoNTs) (1, 2). Our goal is to provide a perspective on how protein structure has facilitated development of vaccines and therapies against regulated select agent (SA) toxins. An overview of the properties of ricin toxin and botulinum neurotoxin is presented in Fig. 1. Ricin toxin and the botulinum neurotoxins are HHS and USDA Select Agents and Toxins (7 CFR part 331, 9 CFR part 121, and 42 CFR part 73).
FIG 1.
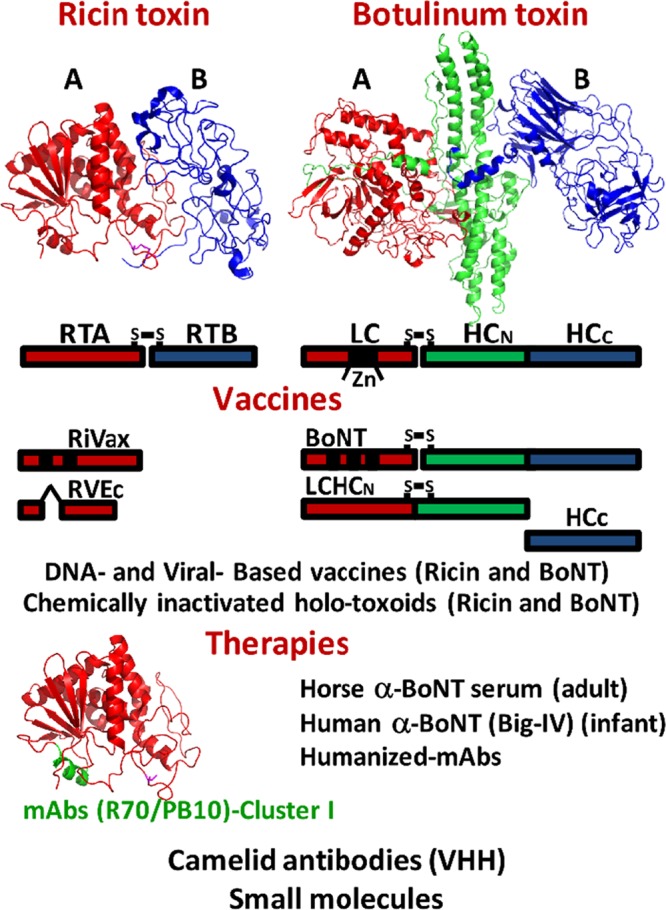
Ricin toxin and botulinum toxin: structure-function organization and vaccines and therapies under investigation. (Upper panel) Crystal structures of ricin toxin (PDB: 2AAI) and botulinum toxin serotype A (PDB: 3BTA) are shown. The A domains (catalytic domains; red) and B domains (binding domains; green and blue) are indicated. The schematics represent the following: ricin toxin (RTA, 267 amino acids [aa]; disulfide linked to RTB, 262 aa); botulinum toxin (LC, 443 aa; disulfide linked to HC [HCN], 448 to 872 aa; HCC, 877 to 1,295 aa). (Middle panel) Vaccines. Ricin protein-based vaccines, including RiVax and RVEc, are RTA derivatives modified for optimized production and attenuation and appear to have similar potencies in rodents and nonhuman primates, while viral and chemically inactivated holotoxoid vaccines are less extensively developed. BoNT protein-based vaccines include catalytically inactive BoNT and LCHCN (due to potency) and HCC (due to ease of production), along with chemically inactivated holotoxoid vaccines. A bivalent (A/B) HCC derivative is currently in clinical trials; numerous DNA and viral vectors express HCC. (Lower panel) Therapies. Several ricin antibody-based MAbs and, subsequently, camelid (VHH) antibodies have been identified that neutralize ricin within RTA in four distinct clusters. Cluster I comprises 97 to 105 aa, where two neutralizing MAbs (R70/PB10) bind. Camelid therapies and small-molecule inhibitors have been developed for ricin and botulism therapies. Current approved botulism antibody therapies include treatment of adult botulism with pooled horse α-BoNT(ABCDE) serum, while infant botulism is treated with human botulism immune globulin intravenous (Big-IV).
AB toxin structure/function.
AB toxins contain modular domains with distinct functional activities (3). The A domain encodes an enzymatic activity which targets a host protein to modulate host cell physiology, often inactivating a biological process within cells, leading to cell death or occasionally enhancing the action of a cellular process. The B domain often binds a host receptor, such as a protein or glycolipid, via interactions that bind directly to a specific host receptor or that bind a carbohydrate present on a glycosylated host receptor. AB toxins are often synthesized as single-chain proteins in an inactive form and are converted to an active di-chain by proteolysis between the A and B domains, which remain connected by an interchain disulfide (4, 5).
RTX is a category B bioterrorism toxigenic lectin enriched in seeds of the castor bean plant Ricinus communis. RTX is posttranslationally processed by host glycosylation and activated by cleavage between A and B domains (6, 7). The ricin toxin A domain (RTA) is a type II ribosome-inactivating protein with N-glycosidase activity, which depurinates 28S rRNA within the 60S host ribosome (8). Depurination stalls protein synthesis and induces a ribotoxic stress response (9). Ricin toxin B domain (RTB) contains at least two homologous lectin binding sites specific for galactose and binds galactose-containing lipids and glycoproteins (10). While binding of galactose results in nonproductive uptake of RTX, binding to mannose moieties on host mannose-containing receptors results in RTX retrograde trafficking to the endoplasmic reticulum, where RTA is delivered into the cytosol to target the ribosome (11). RTX exploits receptors on multiple cell types; therefore, the symptoms vary based on the route of intoxication and include pulmonary edema, respiratory distress, low blood pressure, seizures, and vomiting (12).
BoNTs are category A bioterrorism agents produced by Clostridium botulinum, or by related clostridia, which comprise seven serotypes (namely, serotypes A to G) defined by serotype-specific neutralizing antibodies (Abs) (13). BoNTs are activated by proteolysis. The B domain (HC) contains a receptor binding domain (HCC) and a translocation domain (HCN). BoNTs bind dual neuronal receptors: a sialic acid-decorated glycolipid known as a ganglioside and a synaptic vesicle protein or a second ganglioside (14–20). BoNTs enter neurons via synaptic vesicles where HCN facilitates pH-dependent delivery of the catalytic A domain (light chain [LC]) into the cytosol (21). LCs are Zn2+ metalloproteases which target SNARE-family vesicle fusion complex proteins involved in neurotransmitter release. During translocation, the intact interchain disulfide is reduced to facilitate LC delivery into the host cell cytosol to target the SNARE substrates within peripheral motor neurons (5, 21, 22). The proteolysis of SNARE substrates inhibits acetylcholine release at the neuromuscular junction, a requirement for muscle contraction. This results in the flaccid paralysis of botulism (23).
Structure-based analysis and complementary methods.
Design of effective vaccines requires mounting a protective immune response to epitopes within the toxin which stimulates the production of neutralizing antibodies. Serum-derived antibodies are characterized by antigen specificity and affinity and by neutralization of the protein toxin. Biochemical assays, namely, the enzyme-linked immunosorbent assay (ELISA), immunoblotting, and surface plasmon resonance (SPR) analysis, have been used to demonstrate immune response, antigen reactivity, and binding affinity. More recently, structural techniques such as X-ray crystallography and, as described in the studies reported in this issue of Clinical and Vaccine Immunology by Mantis and coworkers, hydrogen-deuterium exchange mass spectrometry (HD-X MS) (1, 2) have been used to resolve antibody and antigen complexes (24). The shift from methods that predominantly identify linear epitopes via denatured antigens or small peptides to native, conformational epitopes limits artifacts that arise in solid-phase binding of antigen, as noted by Mantis and coworkers. Additionally, competition ELISA is complicated by steric hindrance and exposure or masking of epitopes in the solid-phase assays. The use of antigenic peptides as screens employing phage or solid-phase assays can also be problematic, since the peptide conformation may not resemble the epitope (25, 26).
The availability of several antibody-antigen structures revealed by X-ray crystallography and provided by Mantis and coworkers permitted structure-based analysis of antibody-antigen complexes, including analysis of the molecular interactions within individual epitopes. Additionally, classification of indirect, structural residues and those involved in direct binding has illuminated interactions involved in neutralization (24). Understanding antibody-antigen contacts at the molecular level will ultimately aid in the engineering of antibodies of higher affinity to their antigens, which has been correlated with neutralization (27, 28) On the other hand, crystal structures of antibody-antigen complexes are not always available and can limit the solid-phase binding analysis to a static snapshot of a complex. Furthermore, molecular contacts do not resolve affinity or predict neutralization. When available, antigen structures can be used effectively to model and interpret both affinity and neutralization data.
Previously, peptide arrays, competitive ELISA, and phage display have been utilized to map B cell epitopes on ricin (29, 30). These methods focused on primary sequences or linear epitopes, while other mapping strategies such as alanine-scanning mutagenesis required cautious interpretation due to potential secondary structure disruption (24). Mantis and coworkers utilized HD-X MS to identify dominant epitopes within ricin for serum analysis of antibody response using competitive ELISA. The four epitope clusters identified by murine monoclonal antibodies (MAb) were further assigned as neutralizing epitopes by employing a library of camelid VHH antibodies, again using the structure of a subset of these VHH antibodies in complex with RTA solved by X-ray crystallography as a reference (31, 32). HD-X MS defines epitope regions via detection of a decreased rate of exchange of deuterium for amide hydrogens in the presence of antibody. Though the rate of exchange depends on many factors, identification of tryptic peptides with reduced exchange can lead to identification of broad epitopes. HD-X MS validated X-ray crystallographic data in the camelid study; however, the authors acknowledge that interpretation of intermediate protection was complicated, as one MAb, PB10, affected deuterium exchange on areas distal to the epitope when mapped to the crystal structure. This underscores the importance of utilizing multiple methods to assess protection and to map antigenic regions.
For the botulinum toxins, epitope mapping studies have used single-chain variable-fragment phage libraries which recognize native structure, MAbs, and solid-phase ELISA to confirm reactivity while using competition with short peptides to confirm domain specificity (33–35). One study compared BoNT/A, which contained such a structure, alone or in complex with nontoxic nonhemagglutinins, which are accessory proteins that lacked a crystal structure. The epitopes predominantly mapped to LC and HCN when BoNT/A was in complex, which is in agreement with current models of the BoNT complex (36, 37), which have shown the HCC to be shielded. The isolated translocation domain of the botulinum neurotoxin subunit also contains multiple epitopes, demonstrating the potential for multiple mechanisms of neutralization (35).
Subunit vaccines versus whole-protein vaccines.
Factors considered in vaccine design include ease of production, protein stability, potency of the protective immune response to toxin challenge, and potential off-target toxicity. As separation of AB toxins into either the A domain or the B domain results in atoxic fragments, the idea of the use of subunit vaccines is attractive to minimize risks to hosts, and minimal immunogenic domains may facilitate protein production. Mantis and coworkers used the RTA-derived vaccine RiVax to map ricin-neutralizing MAbs. RiVax elicits an antibody response in humans, mice, and macaques and protects macaques from challenge with a lethal dose of aerosolized ricin (38). Note that RTA was demonstrated to be more protective than RTB in active immunization and passive protection in mice and in cellular assays (39). The failure of the RTB vaccine to neutralize RTX is surprising; however, blocking toxin binding and entry is only one of several mechanisms that can neutralize toxin action (40, 41).
Previous studies identified neutralizing epitopes against BoNTs that were present in the receptor binding domain. Additionally, vaccination with HCC protects against challenge with holotoxin in one-dose vaccination (42). On this principle, an HCC subunit vaccine directed toward seven serologically unique botulinum toxins, toxins A to G, protected immunized mice from challenge with homologous botulinum holotoxins (43). On the other hand, LCHCN has also proved to be a potent neutralizing vaccine (44). The use of subunit vaccines has shown mixed effectiveness against heterologous BoNT subtypes. For example, high-dose vaccination was required to protect against challenge with heterologous BoNT/A subtypes (45). Vaccination with a holotoxin may require fewer vaccinations if neutralizing epitopes are present in multiple domains. A recent study demonstrated that BoNT holotoxins are more potent vaccines than their subunit vaccine equivalents (46).
Improving the safety of vaccines against ricin and botulinum also focused on engineering mutations within the toxin subunits. RiVax and RVEc are composed of atoxic ricin catalytic domains whose sequences extend through residue 267 and are truncated after residue 199, respectively. An additional mutation within a tripeptide (L74-D75-V76) at V76M in RiVax eliminates symptoms associated with vascular leak syndrome without disrupting structural integrity, while RVEc was engineered to remove a hydrophobic loop and was truncated to stabilize the protein (47). For the subunit vaccines utilizing HCC of BoNT/A, a mutation in the receptor binding pocket (W1266A) retained vaccine potency, showing that receptor binding function is not required to maintain vaccine potency (48). Thus, utilizing protein structure and function allows the use of reverse genetic approaches to optimize vaccine potency.
Chemically inactivated formalin toxoid vaccines continue to be used to neutralize diphtheria toxin and tetanus toxin. This strategy was used for botulinum toxoids, but long-term storage resulted in decreased potency and removal of the vaccine from application. Catalytically inactive holotoxins and subunits produced in Escherichia coli and yeast have gained favor due to their ease of purification and engineering (49). Ricin toxoids have demonstrated protection in mice but, given concerns over reversion of cytotoxic effects and delivery, would benefit from recombinant production (50). Analysis of BoNT/A in complex with nontoxic nonhemagglutinin resulted in the mapping of 44 single-chain variable fragments from a phage library of the complex. In this analysis, 2 were identified in the receptor binding domain, 15 in the light chain, and 3 in the translocation domain. Although not focused on identification of neutralizing epitopes, that study supported the idea of the utility of recombinant holotoxin vaccines.
Neutralizing versus nonneutralizing serum.
Eradication of many communicable diseases has relied on both an effective vaccine and a campaign to target susceptible populations. The primary approaches have included vaccination and population monitoring; however, diseases resulting from ingestion of seeds of the castor bean plant or toxigenic clostridial species are not considered to represent transmittable infectious diseases. Human exposure to ricin or botulinum toxin is uncommon and is primarily due to ingestion of contaminated foodstuffs. Thus, strategies for effective vaccines and therapies anticipate targeting populations at risk.
Detection of serum antibodies corresponding to purified antigen can be readily performed using ELISA. However, for RTX and BoNT, the ELISA titer does not correspond to neutralization capacity, especially in comparisons between serotypes. For this reason, animal model systems have been utilized to correlate protection data for both toxins. While recombinant ricin vaccines generate an immune response and show promise as vaccine candidates in animal models, screening of B cell hybridoma libraries generated from immunized mice indicated <10% neutralizing antibodies (51). The lack of correlation between detection of an immune response and the presence of neutralizing antibodies necessitates understanding how to shift this balance in humans. As a result, attempting to predict protection in an immunized human group by antibody reactivity alone is not likely to prove helpful. In contrast, the use of tetanus toxoid vaccine has almost completely eradicated tetanus in developed nations (52). Found in soil, Clostridium tetani is a prevalent cause of maternal and infant mortality due to spastic paralysis in most of the unvaccinated developing world (53). The toxoid was created before a structure of tetanus toxin was available for use in conducting structure-based analysis; however, tetanus toxoid has proven to be effective because it elicits a protective response. This correlation between protection and the presence of antibody reactivity in immunized populations has allowed accurate monitoring and disease eradication in many developing countries (54).
Overall, access to protein structure provides a reference point for integrating affinity and mapping data to accurately locate binding sites for neutralizing antibodies within a targeted protein toxin, as described by Mantis and coworkers, in comparison to the results previously possible using static approaches such as competitive solid-phase binding assay. We anticipate the utilization of additional high-resolution approaches to continue to enhance dissection of the molecular aspects of host-pathogen interactions beyond that of an antibody-antigen interaction with the continued enhanced efficiency of structural biological approaches, including cryo-electron microscopy (55) and structure-based protein informatics (56), as well as development of novel molecular tools such as camelid-based antibodies (57, 58).
ACKNOWLEDGMENT
This commentary was supported by AI118389.
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
Footnotes
For the articles discussed, see https://doi.org/10.1128/CVI.00236-17 and https://doi.org/10.1128/CVI.00237-17.
REFERENCES
- 1.Vance DJ, Tremblay JM, Rong Y, Angalakurthi SK, Volkin DB, Middaugh CR, Weis DD, Shoemaker CB, Mantis NJ. 2017. High-resolution epitope positioning of a large collection of neutralizing and nonneutralizing single-domain antibodies on the enzymatic and binding subunits of ricin toxin. Clin Vaccine Immunol 24:e00236-17. doi: 10.1128/CVI.00236-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toth RT IV, Angalakurthi SK, Van Slyke G, Vance DJ, Hickey JM, Joshi SB, Middaugh CR, Volkin DB, Weis DD, Mantis NJ. 2017. High-definition mapping of four spatially distinct neutralizing epitope clusters on RiVax, a candidate ricin toxin subunit vaccine. Clin Vaccine Immunol 24:e00237-17. doi: 10.1128/CVI.00237-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smyth CJ. 2006. Clinical infectious disease, p 669 In Alouf JE, Popoff MR (ed), The comprehensive sourcebook of bacterial protein toxins. Academic Press, San Diego, CA. [Google Scholar]
- 4.Falnes PO, Sandvig K. 2000. Penetration of protein toxins into cells. Curr Opin Cell Biol 12:407–413. doi: 10.1016/S0955-0674(00)00109-5. [DOI] [PubMed] [Google Scholar]
- 5.Schiavo G, Papini E, Genna G, Montecucco C. 1990. An intact interchain disulfide bond is required for the neurotoxicity of tetanus toxin. Infect Immun 58:4136–4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blum JS, Fiani ML, Stahl PD. 1991. Proteolytic cleavage of ricin A chain in endosomal vesicles. Evidence for the action of endosomal proteases at both neutral and acidic pH. J Biol Chem 266:22091–22095. [PubMed] [Google Scholar]
- 7.Mohanraj D, Ramakrishnan S. 1995. Cytotoxic effects of ricin without an interchain disulfide bond: genetic modification and chemical crosslinking studies. Biochim Biophys Acta 1243:399–406. doi: 10.1016/0304-4165(94)00166-U. [DOI] [PubMed] [Google Scholar]
- 8.Endo Y, Tsurugi K. 1987. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J Biol Chem 262:8128–8130. [PubMed] [Google Scholar]
- 9.Iordanov MS, Pribnow D, Magun JL, Dinh TH, Pearson JA, Chen SL, Magun BE. 1997. Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the alpha-sarcin/ricin loop in the 28S rRNA. Mol Cell Biol 17:3373–3381. doi: 10.1128/MCB.17.6.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frankel AE, Burbage C, Fu T, Tagge E, Chandler J, Willingham MC. 1996. Ricin toxin contains at least three galactose-binding sites located in B chain subdomains 1α, 1β, and 2γ. Biochemistry 35:14749–14756. doi: 10.1021/bi960798s. [DOI] [PubMed] [Google Scholar]
- 11.Simmons BM, Stahl PD, Russell JH. 1986. Mannose receptor-mediated uptake of ricin toxin and ricin A chain by macrophages. Multiple intracellular pathways for a chain translocation. J Biol Chem 261:7912–7920. [PubMed] [Google Scholar]
- 12.Audi J, Belson M, Patel M, Schier J, Osterloh J. 2005. Ricin poisoning: a comprehensive review. JAMA 294:2342–2351. doi: 10.1001/jama.294.18.2342. [DOI] [PubMed] [Google Scholar]
- 13.Rossetto O, Pirazzini M, Montecucco C. 2014. Botulinum neurotoxins: genetic, structural and mechanistic insights. Nat Rev Microbiol 12:535–549. doi: 10.1038/nrmicro3295. [DOI] [PubMed] [Google Scholar]
- 14.Chen C, Fu Z, Kim JJ, Barbieri JT, Baldwin MR. 2009. Gangliosides as high affinity receptors for tetanus neurotoxin. J Biol Chem 284:26569–26577. doi: 10.1074/jbc.M109.027391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozaki S, Kamata Y, Watarai S, Nishiki T-i, Mochida S. 1998. Ganglioside GT1b as a complementary receptor component for Clostridium botulinum neurotoxins. Microb Pathog 25:91–99. doi: 10.1006/mpat.1998.0214. [DOI] [PubMed] [Google Scholar]
- 16.Peng L, Berntsson RPA, Tepp WH, Pitkin RM, Johnson EA, Stenmark P, Dong M. 2012. Botulinum neurotoxin D-C uses synaptotagmin I and II as receptors, and human synaptotagmin II is not an effective receptor for type B, D-C and G toxins. J Cell Sci 125:3233–3242. doi: 10.1242/jcs.103564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng L, Tepp WH, Johnson EA, Dong M. 2011. Botulinum neurotoxin D uses synaptic vesicle protein SV2 and gangliosides as receptors. PLoS Pathog 7:e1002008. doi: 10.1371/journal.ppat.1002008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rummel A, Hafner K, Mahrhold S, Darashchonak N, Holt M, Jahn R, Beermann S, Karnath T, Bigalke H, Binz T. 2009. Botulinum neurotoxins C, E and F bind gangliosides via a conserved binding site prior to stimulation-dependent uptake with botulinum neurotoxin F utilising the three isoforms of SV2 as second receptor. J Neurochem 110:1942–1954. doi: 10.1111/j.1471-4159.2009.06298.x. [DOI] [PubMed] [Google Scholar]
- 19.Strotmeier J, Lee K, Volker AK, Mahrhold S, Zong Y, Zeiser J, Zhou J, Pich A, Bigalke H, Binz T, Rummel A, Jin R. 2010. Botulinum neurotoxin serotype D attacks neurons via two carbohydrate-binding sites in a ganglioside-dependent manner. Biochem J 431:207–216. doi: 10.1042/BJ20101042. [DOI] [PubMed] [Google Scholar]
- 20.Verderio C, Rossetto O, Grumelli C, Frassoni C, Montecucco C, Matteoli M. 2006. Entering neurons: botulinum toxins and synaptic vesicle recycling. EMBO Rep 7:995–999. doi: 10.1038/sj.embor.7400796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montecucco C, Schiavo G. 1994. Mechanism of action of tetanus and botulinum neurotoxins. Mol Microbiol 13:1–8. doi: 10.1111/j.1365-2958.1994.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 22.Zuverink M, Chen C, Przedpelski A, Blum FC, Barbieri JT. 2015. A heterologous reporter defines the role of the tetanus toxin interchain disulfide in light-chain translocation. Infect Immun 83:2714–2724. doi: 10.1128/IAI.00477-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popoff MR, Poulain B. 2010. Bacterial toxins and the nervous system: neurotoxins and multipotential toxins interacting with neuronal cells. Toxins 2:683–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abbott WM, Damschroder MM, Lowe DC. 2014. Current approaches to fine mapping of antigen–antibody interactions. Immunology 142:526–535. doi: 10.1111/imm.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammers CM, Stanley JR. 2014. Antibody phage display: technique and applications. J Invest Dermatol 134:1–5. doi: 10.1038/jid.2013.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Kennedy R, Byrne M, O'Fagain C, Berns G. 1990. Experimental section: a review of enzyme-immunoassay and a description of a competitive enzyme-linked immunosorbent assay for the detection of immunoglobulin concentrations. Biochem Educ 18:136–140. doi: 10.1016/0307-4412(90)90219-E. [DOI] [Google Scholar]
- 27.Maynard J, Georgiou G. 2000. Antibody engineering. Annu Rev Biomed Eng 2:339–376. doi: 10.1146/annurev.bioeng.2.1.339. [DOI] [PubMed] [Google Scholar]
- 28.Hagenaars AM, Van Delft RW, Nagel J. 1984. Comparison of ELISA and toxin neutralization for the determination of tetanus antibodies. J Immunoassay 5:1–11. doi: 10.1080/01971528408062995. [DOI] [PubMed] [Google Scholar]
- 29.O'Hara JM, Kasten-Jolly JC, Reynolds CE, Mantis NJ. 2014. Localization of non-linear neutralizing B cell epitopes on ricin toxin's enzymatic subunit (RTA). Immunol Lett 158:7–13. doi: 10.1016/j.imlet.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vance DJ, Mantis NJ. 2012. Resolution of two overlapping neutralizing B cell epitopes within a solvent exposed, immunodominant alpha-helix in ricin toxin's enzymatic subunit. Toxicon 60:874–877. doi: 10.1016/j.toxicon.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudolph MJ, Vance DJ, Cassidy MS, Rong Y, Mantis NJ. 2017. Structural analysis of single domain antibodies bound to a second neutralizing hot spot on ricin toxin's enzymatic subunit. J Biol Chem 292:872–883. doi: 10.1074/jbc.M116.758102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudolph MJ, Vance DJ, Cheung J, Franklin MC, Burshteyn F, Cassidy MS, Gary EN, Herrera C, Shoemaker CB, Mantis NJ. 2014. Crystal structures of ricin toxin's enzymatic subunit (RTA) in complex with neutralizing and non-neutralizing single chain antibodies. J Mol Biol 426:3057–3068. doi: 10.1016/j.jmb.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atassi MZ, Dolimbek BZ. 2004. Mapping of the antibody-binding regions on the HN-domain (residues 449–859) of botulinum neurotoxin A with antitoxin antibodies from four host species. Full profile of the continuous antigenic regions of the H-chain of botulinum neurotoxin A. Protein J 23:39–52. [DOI] [PubMed] [Google Scholar]
- 34.Atassi MZ, Dolimbek BZ, Hayakari M, Middlebrook JL, Whitney B, Oshima M. 1996. Mapping of the antibody-binding regions on botulinum neurotoxin H-chain domain 855–1296 with antitoxin antibodies from three host species. J Protein Chem 15:691–700. doi: 10.1007/BF01886751. [DOI] [PubMed] [Google Scholar]
- 35.Ayyar BV, Tajhya RB, Beeton C, Atassi MZ. 2015. Antigenic sites on the HN domain of botulinum neurotoxin A stimulate protective antibody responses against active toxin. Sci Rep 5:15776. doi: 10.1038/srep15776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen F, Kuziemko GM, Amersdorfer P, Wong C, Marks JD, Stevens RC. 1997. Antibody mapping to domains of botulinum neurotoxin serotype A in the complexed and uncomplexed forms. Infect Immun 65:1626–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gu S, Jin R. 2013. Assembly and function of the botulinum neurotoxin progenitor complex. Curr Top Microbiol Immunol 364:21–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roy CJ, Brey RN, Mantis NJ, Mapes K, Pop IV, Pop LM, Ruback S, Killeen SZ, Doyle-Meyers L, Vinet-Oliphant HS, Didier PJ, Vitetta ES. 2015. Thermostable ricin vaccine protects rhesus macaques against aerosolized ricin: epitope-specific neutralizing antibodies correlate with protection. Proc Natl Acad Sci U S A 112:3782–3787. doi: 10.1073/pnas.1502585112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maddaloni M, Cooke C, Wilkinson R, Stout AV, Eng L, Pincus SH. 2004. Immunological characteristics associated with the protective efficacy of antibodies to ricin. J Immunol 172:6221–6228. doi: 10.4049/jimmunol.172.10.6221. [DOI] [PubMed] [Google Scholar]
- 40.Foxwell BM, Detre SI, Donovan TA, Thorpe PE. 1985. The use of anti-ricin antibodies to protect mice intoxicated with ricin. Toxicology 34:79–88. doi: 10.1016/0300-483X(85)90080-0. [DOI] [PubMed] [Google Scholar]
- 41.Zucker DR, Murphy JR. 1984. Monoclonal antibody analysis of diphtheria toxin–I. Localization of epitopes and neutralization of cytotoxicity. Mol Immunol 21:785–793. [DOI] [PubMed] [Google Scholar]
- 42.Tavallaie M, Chenal A, Gillet D, Pereira Y, Manich M, Gibert M, Raffestin S, Popoff MR, Marvaud JC. 2004. Interaction between the two subdomains of the C-terminal part of the botulinum neurotoxin A is essential for the generation of protective antibodies. FEBS Lett 572:299–306. doi: 10.1016/j.febslet.2004.06.094. [DOI] [PubMed] [Google Scholar]
- 43.Baldwin MR, Tepp WH, Przedpelski A, Pier CL, Bradshaw M, Johnson EA, Barbieri JT. 2008. Subunit vaccine against the seven serotypes of botulism. Infect Immun 76:1314–1318. doi: 10.1128/IAI.01025-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shone C, Agostini H, Clancy J, Gu M, Yang HH, Chu Y, Johnson V, Taal M, McGlashan J, Brehm J, Tong X. 2009. Bivalent recombinant vaccine for botulinum neurotoxin types A and B based on a polypeptide comprising their effector and translocation domains that is protective against the predominant A and B subtypes. Infect Immun 77:2795–2801. doi: 10.1128/IAI.01252-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henkel JS, Tepp WH, Przedpelski A, Fritz RB, Johnson EA, Barbieri JT. 2011. Subunit vaccine efficacy against botulinum neurotoxin subtypes. Vaccine 29:7688–7695. doi: 10.1016/j.vaccine.2011.07.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Webb RP, Smith TJ, Smith LA, Wright PM, Guernieri RL, Brown JL, Skerry JC. 3 September 2017. Recombinant botulinum neurotoxin Hc subunit (BoNT Hc) and catalytically inactive Clostridium botulinum holoproteins (ciBoNT HPs) as vaccine candidates for the prevention of botulism. Toxins (Basel) doi: 10.3390/toxins9090269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Legler PM, Brey RN, Smallshaw JE, Vitetta ES, Millard CB. 2011. Structure of RiVax: a recombinant ricin vaccine. Acta Crystallogr D Biol Crystallogr 67:826–830. doi: 10.1107/S0907444911026771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Przedpelski A, Tepp WH, Kroken AR, Fu Z, Kim J-JP, Johnson EA, Barbieri JT. 2013. Enhancing the protective immune response against botulism. Infect Immun 81:2638–2644. doi: 10.1128/IAI.00382-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith LA. 2009. Botulism and vaccines for its prevention. Vaccine 27:D33–D39. doi: 10.1016/j.vaccine.2009.08.059. [DOI] [PubMed] [Google Scholar]
- 50.Yan C, Rill WL, Malli R, Hewetson J, Naseem H, Tammariello R, Kende M. 1996. Intranasal stimulation of long-lasting immunity against aerosol ricin challenge with ricin toxoid vaccine encapsulated in polymeric microspheres. Vaccine 14:1031–1038. doi: 10.1016/0264-410X(96)00063-1. [DOI] [PubMed] [Google Scholar]
- 51.O'Hara JM, Neal LM, McCarthy EA, Kasten-Jolly JA, Brey RN, Mantis NJ. 2010. Folding domains within the ricin toxin A subunit as targets of protective antibodies. Vaccine 28:7035–7046. doi: 10.1016/j.vaccine.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thwaites CL, Loan HT. 2015. Eradication of tetanus. Br Med Bull 116:69–77. doi: 10.1093/bmb/ldv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fauveau V, Mamdani M, Steinglass R, Koblinsky M. 1993. Maternal tetanus: magnitude, epidemiology and potential control measures. Int J Gynaecol Obstet 40:3–12. doi: 10.1016/0020-7292(93)90765-O. [DOI] [PubMed] [Google Scholar]
- 54.Annadurai K, Danasekaran R, Mani G. 2017. Elimination of maternal and neonatal tetanus in India: a triumph tale. Int J Prev Med 8:15. doi: 10.4103/ijpvm.IJPVM_392_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cressey D, Callaway E. 2017. Cryo-electron microscopy wins chemistry Nobel. Nature 550:167. doi: 10.1038/nature.2017.22738. [DOI] [PubMed] [Google Scholar]
- 56.Fieldhouse RJ, Turgeon Z, White D, Merrill AR. 2010. Cholera- and anthrax-like toxins are among several new ADP-ribosyltransferases. PLoS Comput Biol 6:e1001029. doi: 10.1371/journal.pcbi.1001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herrera C, Tremblay JM, Shoemaker CB, Mantis NJ. 2015. Mechanisms of ricin toxin neutralization revealed through engineered homodimeric and heterodimeric camelid antibodies. J Biol Chem 290:27880–27889. doi: 10.1074/jbc.M115.658070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tremblay JM, Kuo CL, Abeijon C, Sepulveda J, Oyler G, Hu X, Jin MM, Shoemaker CB. 2010. Camelid single domain antibodies (VHHs) as neuronal cell intrabody binding agents and inhibitors of Clostridium botulinum neurotoxin (BoNT) proteases. Toxicon 56:990–998. doi: 10.1016/j.toxicon.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


