Abstract
Objectives
This purpose of the study was to evaluate TAVR outcomes at low, intermediate and high volume institutions.
Background
For the care of complex patients, volume-outcome effect is well described. The initial US TAVR experience was limited to a few centers of excellence. The impact of institutional volume on outcomes after TAVR has not been systematically studied.
Methods
Within the Banner Health system, TAVR is performed at 3 institutions-a low volume, an intermediate volume and a high volume institution. 181 consecutive patients undergoing TAVR within these 3 institutions were the study cohort. To adjust for bias and confounders between the 3 groups, risk-adjusted multivariate logistic regression and propensity score analysis was performed. The primary endpoint was a composite of mortality, dialysis-dependent renal failure, cerebrovascular accident, need for new permanent pacemaker and readmission within 30 days.
Results
The primary endpoint was reached in 38.8% of patients at the high volume institution and 76.2% of patients at the low volume institution (p < 0.01). Having a TAVR procedure at a larger volume institution was an independent predictor of having improved outcomes (OR 0.33, 95% CI 0.16–0.68; p = 0.003). These improved outcomes after the TAVR procedure noted at the large volume institution were seen in the most complex patients: age ≥80 years, BMI >30, diabetes, hypertension, prior CAD, CKD and NYHA class III/IV heart failure.
Conclusions
High-risk patients undergoing TAVR at a large volume institution have better 30-day outcomes compared to outcomes at intermediate and low volume centers.
Keywords: Health-care outcomes, Transcatheter-valve-interventions, Institutional-volume
1. Introduction
Transcatheter aortic valve replacement (TAVR) has rapidly transformed the treatment of severe aortic stenosis.1 Pivotal TAVR trials, were performed at a limited number of centers and favorable results led to the commercial approval of TAVR. New TAVR centers were opened as programs developed the requisite infra-structure like a hybrid OR and organized “heart teams”. This additional infrastructural requirement and cross specialty collaboration was distinct to TAVR sites and unlike from institutions that only offered PCI or CABG.2 Since initial approval by the Food and Drug Administration, TAVR is being performed at approximately 400 institutions in the United States (US).3 The Society of Thoracic Surgeons/American College of Cardiology (STS/ACC) TVT Registry audit has revealed significant variation in site volume and patient selection for TAVR. A survey of the National Inpatient Sample had suggested that unadjusted in-hospital mortality was higher at low-volume centers, performing <20 TAVR cases/year, when compared to intermediate and high-volume centers. However, annual TAVR volume did not predict lower mortality in the subsequent multivariate analysis of that dataset.4
Outcome variability for TAVR amongst institutions is large.5 Specific factors contributing to this variability, remain unknown. We sought to evaluate the impact of patient characteristics and institutional volume, on 30-day outcomes in patients undergoing TAVR.
2. Methods
2.1. Data source
This was a retrospective analysis of patients undergoing TAVR at Banner Health, a large hospital system in the US. Within this hospital system, TAVR is performed at 3 centers: a low-volume (<40 cases/year), intermediate-volume (40–75 cases/year), and high-volume center (>75 cases/year). A database of TAVR patients was compiled from an integrated electronic medical records system. Patients enrolled in clinical trials and who received a self-expanding valve commercially were excluded because these valves were not available at all sites.
2.2. Study population
196 consecutive patients undergoing TAVR with a Sapien XT Valve (Edwards Lifesciences, Irvine CA) for severe symptomatic aortic valve stenosis between January 1, 2014 and June 31, 2015 within the Banner Health System met criteria. One hundred patients underwent TAVR at the high volume center, seventy five underwent TAVR at the intermediate-volume center, and twenty-one patients underwent TAVR at the low volume center. Fifteen patients were excluded from final analysis due to either missing pre-TAVR work-up data or off label indication (2 from large volume, 13 from intermediate-volume center). A total of 181 patients were included in final analysis. For each patient undergoing TAVR, the medical records system and picture archive and communications system (PACS) were queried, and data abstraction performed by independent reviewers.
2.3. Procedural details
All patients underwent pre-procedural computed tomography angiography (CTA) scans for sizing of the aortic annulus and assessment of the peripheral vasculature. CT scans were interpreted by a staff radiologist at each institution. The valve size and access was decided locally at the institution where the procedure was performed. Other aspects of the procedure were standardized with all patients undergoing TAVR under general anesthesia in a hybrid operating room with transesophageal echocardiography and fluoroscopic guidance.
2.4. Outcome measures
The study cohort was divided into high-volume, intermediate-volume, and low-volume groups. The annual minimum institutional volume set by the ACC/STS is 20 TAVR’s/year or 40 TAVR’s over 2 years, and the numbers in this study are typical representations of low, intermediate and high-volume centers.6 Patient characteristics and procedural details were collected and classified by center volume. All the centers included in this study were part of a single health care system. As TAVR programs were rolled out at the low and intermediate volume centers, operators and staff from the low and intermediate-volume centers were invited to the high volume center for hands-on-TAVR training. This led to standardized processes and reduced variability amongst programs.
The primary objective of the study is to assess and compare the 30-day outcomes at the low, intermediate and high-volume centers. The primary endpoint was defined as a composite of all-cause mortality, new post-TAVR hemodialysis, new post-TAVR permanent pacemaker (PPM) implantation, and hospital readmission for cardiac causes with 30 days.
2.5. Statistical analysis
The Shapiro–Wilk test was used to check for normality for each continuous variable. Continuous variables that are normally distributed were described using means and standard deviations and non-normally-distributed variables were described as median and interquartile ranges. Categorical variables were described as proportions. Linear correlation was evaluated using Pearson correlation coefficient and Bland–Altman analysis. Comparison between low, intermediate and large-volume centers for continuous variables was made using either one-way ANOVA or Kruskal–Wallis test (based on normal or non-normal distribution respectively), and for categorical variables using chi-squared test. A significant result was followed with a pairwise comparison using student’s t-test, nonparametric Mann–Whitney test, chi squared or Fisher’s exact test as appropriate and adjusted for multiple comparisons using the Hommel procedure.
Univariate logistic regression models were used for evaluating associations between baseline characteristics and the primary endpoint. Separate bivariate logistic regression analysis was performed to identify baseline characteristics associated with TAVR outcomes at the large-volume center compared with intermediate and low-volume centers. To adjust for inherent bias and confounding variables introduced by the lack of randomization in this study, multivariate regression and propensity score adjustment methods were used. Separate logistic and cox regression models were developed within the constraints of overfitting. Variables with p-values < 0.1, variables known or thought to be associated with events, and unbalanced baseline characteristics amongst groups were entered into the multivariate regression model in a forward, stepwise fashion. Variables were allowed to enter the model as either continuous or dichotomized. P-value ≤0.05 was used to retain variables in the multivariate model. Different models were compared using area under the receiver operating characteristics analysis. To evaluate confounder-adjusted association of center volume with 30-day outcomes, we developed a propensity score accounting for differences in baseline, pre-TAVR patient characteristics for large vs intermediate and low volume centers. This propensity score was then introduced in the multivariate regression model as a covariate with 30-day primary outcome as dependent variable. The baseline characteristics retained in the final propensity score model were atrial fibrillation, left bundle branch block, LFEF < 50%, pulmonary hypertension, and STS PROM ≥ 12%. Time-to-event survival analysis was done using cox regression, with graphical representation using Kaplan–Meier estimator method, and event-free survival compared using log-rank test to generate p-values. A two-sided alpha of <0.05 was considered statistically significant.
3. Results
Ninety-eight patients (54.14%) underwent TAVR at the high-volume center, sixty-two (34.25%) at the intermediate-volume center, and twenty-one (11.6%) at the low-volume center. Baseline patient characteristics are reported in Table 1. Patients undergoing TAVR at the low-volume center were younger, more likely to have a history of smoking and atrial fibrillation. Conversely, patients undergoing TAVR at the large-volume center were more likely to be in NYHA Class IV heart failure.
Table 1.
Baseline demographics categorized by low, intermediate and large-volume hospital sites.
| Variable | Low Volume Site (n = 21) | Intermediate Volume Site (n = 62) | Large Volume Site (n = 98) | p-value |
|---|---|---|---|---|
| Age – Median (IQR) | 82 (80–85) | 86.5 (81–89) | 83 (75–87) | 0.01 |
| Gender – Male # (%) | 12 (57.1) | 25 (40.3) | 52 (53.1) | 0.22 |
| Race – White # (%) | 20 (95.2) | 56 (90.3) | 89 (90.8) | 0.61 |
| BMI – Median (IQR) | 27.9 (26–29.8) | 27.3 (23.8–31.2) | 26.7 (24.3–31.3) | 0.63 |
| Diabetes Mellitus type 2 # (%) | 7 (33.3) | 28 (45.2) | 36 (36.7) | 0.48 |
| IDDM # (%) | 6 (28.6) | 14 (22.6) | 9 (9.2) | 0.07 |
| HTN # (%) | 20 (95.2) | 55 (88.7) | 88 (89.7) | 0.83 |
| CVA/TIA # (%) | 6 (28.5) | 10 (16.1) | 15 (15.3) | 0.17 |
| CAD # (%) | 18 (85.7) | 44 (70.9) | 61 (62.2) | 0.09 |
| CABG # (%) | 7 (33.3) | 21 (33.9) | 24 (24.9) | 0.04 |
| PCI # (%) | 11 (52.3) | 26 (41.9) | 50 (51.0) | 0.49 |
| PVD # (%) | 14 (66.7) | 33 (53.2) | 58 (59.2) | 0.53 |
| COPD # (%) | 7 (33.3) | 15 (24.2) | 30 (30.4) | 0.43 |
| DLD # (%) | 18 (85.7) | 40 (64.5) | 76 (77.6) | 0.08 |
| CKD-Stage (GFR) # (%) | 0.76 | |||
| Stage I/II (>60) | 14 (66.7) | 44 (70.9) | 59 (60.6) | |
| Stage III (30–60) | 6 (28.6) | 15 (24.2) | 31 (31.9) | |
| Stage IV/V (<30) | 1 (4.8) | 3 (4.8) | 7 (7.45) | |
| Smoker # (%) | 15 (71.4) | 23 (37.1) | 47 (47.9) | 0.02 |
| Chronic Liver Disease – # (%) | 0 | 0 | 5 (5.1) | 0.20 |
| Immunosuppressed – # (%) | 2 (9.5) | 1 (1.6) | 23 (23.9) | <0.001 |
| Permanent Pacemake – # (%) | 4 (19.0) | 13 (21) | 16 (16.3) | 0.89 |
| Porcelain Aorta – # (%) | 0 | 2 (3.2) | 13 (13.3) | 0.03 |
| Hostile Mediastinum – # (%) | 0 | 0 | 8 (7.9) | 0.04 |
| Baseline EKG – # (%) | <0.001 | |||
| Afib | 12 (57.1) | 23 (37.1) | 14 (14.3) | |
| LBBB | 1 (4.8) | 0 | 9 (9.2) | |
| RBBB | 1 (4.8) | 0 | 6 (6.1) | |
| LVEF ≤40% – median (IQR) | 30 (20–40) | 38.5 (30–40) | 25 (25–40) | 0.26 |
| Pulmonary hypertension – # (%) | 3 (14.3) | 5 (8.1) | 34 (34.7) | <0.001 |
| RV dysfunction – # (%) | 7 (33.3) | 10 (16.1) | 2 (2.0) | <0.001 |
| NYHA Class – # (%) | <0.001 | |||
| Class I | 1 (4.8) | 0 | 2 (2.0) | |
| Class II | 1 (4.8) | 3 (4.8) | 4 (4.1) | |
| Class III | 14 (66.7) | 50 (80.6) | 45 (45.9) | |
| Class IV | 5 (23.8) | 9 (14.5) | 47 (47.9) | |
| STS Mortality Risk – median (IQR) | 5.3 (3.9–8.9) | 5 (3.6–7.1) | 8 (5.1–11.1) | <0.001 |
Abbreviations: IQR: Interquartile range (25–75 percentile), BMI: body mass index, IDDM: Insulin dependent diabetes mellitus, HTN: hypertension, CVA: cerebrovascular accident, TIA: transient ischemic attack, CAD: coronary artery disease, CABG: coronary artery bypass grafting, PCI: percutaneous coronary intervention, PVD: peripheral vascular disease, COPD: chronic obstructive pulmonary disease, DLD: dyslipidemia, CKD: chronic kidney disease, EKG: electrocardiogram, Afib: atrial fibrillation, LBBB: left bundle branch block, RBBB: right bundle branch block, LVEF: left ventricular ejection fraction, RV: right ventricle, NYHA Class: New York heart association class, STS: society of thoracic surgeons.
Patients at the high-volume center had a higher median STS-PROM score of 8%, (IQR 5.1–11.1%) versus 5% (IQR 3.6–7.1%) at the intermediate-volume and 5.3% (IQR 3.9–8.9%) at the low-volume center(p < 0.001).
Procedural details are reported in Table 2. The sites were well-matched in their procedural characteristics. Patients at the high volume center were more likely to have a trans-apical approach (51.02%, 30.65%, 47.62% respectively; p = 0.03) compared to the intermediate and low volume centers. Median contrast volume was significantly lower at the high-volume center (85 mL) than at the intermediate-volume (100 mL) and low-volume (125 mL) centers (p < 0.001).
Table 2.
Procedural details categorized by low, intermediate and large-volume hospital sites.
| Variable | Low Volume Site (N = 21) | Intermediate Volume Site (N = 62) | Large Volume Site (N = 98) | P-Value |
|---|---|---|---|---|
| Approach | 0.03 | |||
| Direct Aortic – # (%) | 0 | 2 (3.2) | 0 | |
| Trans Femoral – # (%) | 11 (52.4) | 41 (66.1) | 48 (49) | |
| Trans Apical – # (%) | 10 (47.6) | 19 (30.6) | 50 (51) | |
| # Valve-in-valve – # (%) | 9.5 (45.2) | 0 | 2.0 (2.0) | 0.12 |
| Number of Valves Used | 1.0 | |||
| Single – # (%) | 21 (100) | 61 (98.4) | 95 (96.9) | |
| Two – # (%) | 0 | 1 (1.6) | 3 (3.1) | |
| Valve Size Used – median (IQR) | 26 (23–26) | 26 (23–26) | 26 (23–26) | 0.13 |
| 23 mm – # (%) | 6 (28.6) | 29 (46.8) | 38 (38.8) | |
| 26 mm – # (%) | 11 (52.4) | 29 (46.8) | 41 (41.8) | |
| 29 mm – # (%) | 4 (19.1) | 4 (6.5) | 19 (19.4) | |
| Contrast Volume | 120 (100–130) | 100 (75–125) | 85 (80–85) | <0.001 |
There was no difference in 30-day mortality, CVA, dialysis dependent renal failure, or need for a new PPM between the 3 groups. The high-volume center, compared to intermediate and low-volume centers, had a higher incidence of AKI after TAVR (34.7%, 12.9%, 23.8% respectively; p = 0.007).
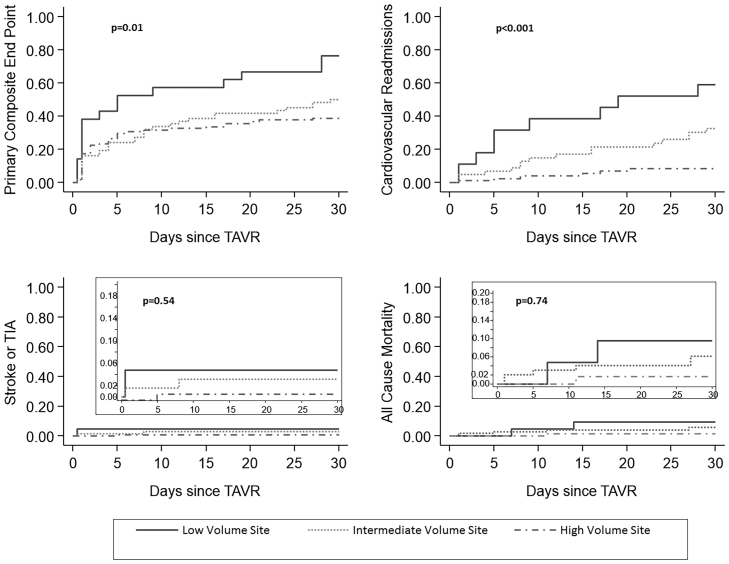
There was a significant difference in the all cause and cardiac readmissions between the high-volume center (9.2%) and the low-volume center (47.6%) (p < 0.001) at 30 days (Table 3). Kaplan–Meier curves for the primary endpoint; cardiac readmissions, all-cause mortality and CVA are depicted in Fig. 1.
Table 3.
Endpoints categorized by low, intermediate and large-volume hospital sites.
| Variable | Low Volume Site (N = 21) | Intermediate Volume Site (N = 62) | Large Volume Site (N = 98) | P-Value |
|---|---|---|---|---|
| Primary endpoint – # (%) | 16 (76.2) | 31 (50) | 38 (38.8) | 0.01 |
| All-Cause Mortality 30 day – # (%) | 2 (9.5) | 3 (4.8) | 6 (6.1) | 0.74 |
| Procedural Death – # (%) | 0 | 2 (3.2) | 0 | 0.21 |
| Readmission 30d | <0.001 | |||
| Readmission any reason – # (%) | 10 (47.6) | 20 (32.3) | 9 (9.2) | |
| Cardiac Readmission – # (%) | 9 (42.9) | 16 (25.8) | 6 (6.12) | |
| Second Valve – # (%) | 0 | 1 (1.6) | 3 (3.1) | 1.0 |
| CVA/TIA Post- # (%) | 1 (4.8) | 2 (3.2) | 2 (2.0) | 0.54 |
| Device Success – # (%) | 21 (100) | 61 (98.4) | 94 (96) | 0.81 |
| MACCE – # (%) | 4 (19.1) | 6(9.7) | 11 (11.2) | 0.44 |
| Major Vascular Complications – # (%) | 1 (4.8) | 6 (9.8) | 8 (8.2) | 0.96 |
| Bleeding (VARC2) – # (%) | 1 (4.8) | 3 (4.8) | 7 (7.1) | 0.05 |
| PVL – # (%) | 1 (4.8) | 0 | 3 (3.1) | 0.36 |
| Dialysis Post-TAVR – # (%) | 4 (19.1) | 5 (8.1) | 12 (12.2) | 0.33 |
| AKI Post – # (%) | 5 (23.8) | 8 (12.9) | 34 (34.7) | 0.007 |
| PPM new – # (%) | 4 (19.1) | 9 (14.5) | 13 (13.3) | 0.72 |
| LOS (days) | 3 (2–5) | 4 (3–6) | 4 (2–7) | 0.22 |
Abbreviations: Primary endpoint was obtained at 30 days and was defined as a composite of all-cause mortality, dialysis-dependent renal failure, post-procedural cerebrovascular accident, need for new permanent pacemaker and hospital readmission. Device success was defined as successful-access, retrieval of delivery system, device deployed in right location, one valve used and no embolization; CVA/TIA: cerebrovascular accident/transient ischemic attack, MACCE: major cardiovascular and cerebrovascular events, VARC2: valvular academic research consortium, PVL: perivalvular leak, AKI: acute kidney injury, PPM: permanent pacemaker, LOS: length of stay. Cardiac readmission included readmission for heart failure, cardiac arrest, bleeding, permanent pacemaker implant, stroke or TIA.
Fig. 1.
Kaplan–Meier curves demonstrating primary endpoints (A), cardiovascular readmissions (B), stroke or TIA (C), and all-cause mortality (D) 30 days post TAVR procedure.
In univariate analysis, having the TAVR at a large-volume center was associated with significantly reduced odds of reaching the primary endpoint (OR 0.48, 95% CI 0.26–0.87; p = 0.01) whereas a high STS PROM ≥ 12 (OR 5.07, 95% CI 2.06–13.85, p = 0.01) was associated with significantly increased odds of meeting the primary endpoint. Many patient characteristics were associated with a trend towards worse outcomes but did not meet statistical significance (p-value 0.05–0.09), including female gender (OR 1.7, p = 0.08), ejection fraction ≤35% (OR 2.24, p = 0.07), chronic kidney disease (OR 1.81, p = 0.06), alternative access (OR 1.17, p = 0.07), peripheral vascular disease (OR 1.68, p = 0.08). In multivariate analysis, having a TAVR at a large-volume center remained an independent predictor for a better outcome (OR 0.33, 95% CI 0.16–0.65, p = 0.001), whereas female gender (OR 1.99, p = 0.04) and STS ≥ 12 (OR 5.72, 95% CI 2.25–14.5, p < 0.01) were independent predictors of worse outcome (Table 4).
Table 4.
Univariate and Multivariate Predictors of the Primary Endpoint.
| Variable | Univariate Analysis |
Multivariate Analysis |
||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P-Value | Odds Ratio | 95% CI | P-Value | |
| Large-volume Site | 0.48 | 0.26–0.87 | 0.01 | 0.33 | 0.16–0.65 | 0.001 |
| Female Gender | 1.67 | 0.93–3.02 | 0.08 | 1.99 | 1.01–3.93 | 0.04 |
| RBBB | 2.93 | 0.55–15.55 | 0.20 | 5.10 | 0.77–33.5 | 0.08 |
| LVEF ≤ 35% | 2.24 | 0.93–5.38 | 0.07 | 2.65 | 0.96–7.32 | 0.06 |
| NYHA Class III/IV | 0.31 | 0.07–1.21 | 0.09 | 0.26 | 0.05–1.16 | 0.07 |
| STS ≥ 12 | 5.07 | 2.06–13.85 | <0.01 | 5.72 | 2.25–14.51 | <0.01 |
| Age ≥ 80 | 0.84 | 0.44–1.59 | 0.59 | 0.62 | 0.28–1.35 | 0.23 |
| BMI ≥ 30 | 1.12 | 0.59–2.13 | 0.71 | 1.37 | 0.66–2.82 | 0.39 |
| Diabetes Mellitus | 1.28 | 0.70–2.32 | 0.41 | 1.26 | 0.63–2.55 | 0.50 |
| Hypertension | 1.14 | 0.43–3.03 | 0.76 | 1.43 | 0.48–4.22 | 0.51 |
| Prior CVA | 1.37 | 0.61–3.07 | 0.44 | 1.29 | 0.52–3.17 | 0.57 |
| CAD | 1.17 | 0.61–2.27 | 0.62 | 1.39 | 0.65–3.00 | 0.38 |
| CKD (GFR < 60) | 1.81 | 0.96–3.37 | 0.06 | 1.59 | 0.48–10.79 | 0.29 |
| Afib | 1.56 | 0.76–3.2 | 0.24 | 1.57 | 0.73–3.38 | 0.24 |
| LBBB | 1.13 | 0.31–4.07 | 0.84 | 1.84 | 0.44–8.24 | 0.42 |
| PHT | 1.17 | 0.58–2.33 | 0.65 | 2.01 | 0.86–4.68 | 0.10 |
| Alternate Access | 1.17 | 0.94–3.09 | 0.07 | 1.61 | 0.81–3.19 | 0.16 |
| PVD | 1.68 | 0.92–3.06 | 0.08 | 1.66 | 0.84–3.27 | 0.14 |
| COPD | 0.98 | 0.52–1.85 | 0.95 | 1.08 | 0.52–2.22 | 0.53 |
Abbreviations: RBBB: right bundle branch block, LVEF: left ventricular ejection fraction, NYHA: New York Heart Association, STS: Society of Thoracic Surgery, BMI: body mass index, CVA: cerebrovascular accident, CAD: coronary artery disease, CKD: chronic kidney disease, Afib: atrial fibrillation, LBBB: left bundle branch block, PHT: pulmonary hypertension, PVD: peripheral vascular disease, COPD: chronic obstructive pulmonary disease.
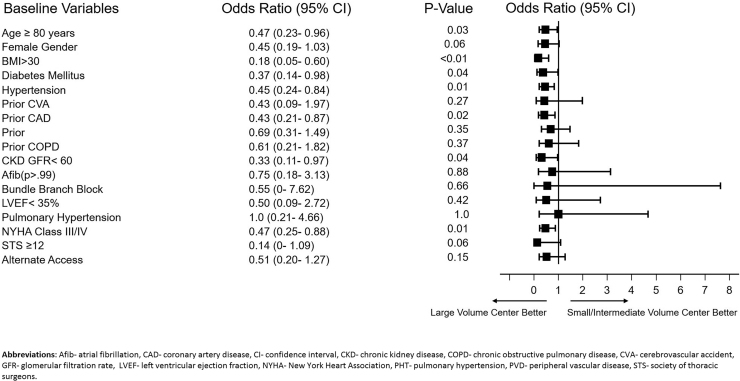
In a subgroup bivariate analysis, TAVR outcomes at a large volume center were better than at a low and intermediate-volume center for the most complex and sick patients: octogenarians (OR 0.47, 95% CI 0.23–0.96, p = 0.03), patients with a BMI >30 kg/m2 (OR 0.18, 95% CI 0.05–0.60, p < 0.01), patients with concomitant CAD (OR 0.43, 95% CI 0.21–0.87, p = 0.02), CKD (OR 0.33, 95% CI 0.11–0.97, p = 0.04), and NYHA III/IV heart failure (OR 0.47, 95% CI 0.25–0.88, p = 0.01) (Fig. 2).
Fig. 2.
Bivariate analysis and forest plot demonstrating that odds of reaching the primary endpoint were significantly lower when TAVR was performed at a large-volume center compared to low and intermediate-volume centers.
4. Discussion
Transcatheter aortic valve replacement (TAVR) has transformed the treatment of aortic stenosis in patients at high and extreme risk for open surgery. Although there has been rapid dispersion of TAVR, there is significant variation in procedural volume at different centers. The relationship between institutional TAVR volume and outcome remains uncertain.3 While volume alone does not assure high-quality care, high-volume centers are more likely to have the infrastructure for delivering complex care. There is published data for complex cardiovascular procedures with a positive correlation between volume and outcomes.7, 8, 9 Volume-outcome relationships have not been systematically explored for patients undergoing TAVR.
The results of this study demonstrate that TAVR outcomes at 30 days are improved at centers with a large procedural volume (>75 TAVR/year) compared to centers doing fewer cases annually. These differences are driven primarily by the 30-day readmission rate, despite the fact that patients at the large volume center have higher STS-PROM scores and are more often in NYHA Class IV heart failure. We believe that the improved outcomes may be related to the experience of the heart team and processes at the large volume center for early identification and prevention of potential complications.
The 30-day cardiac readmission rate at the low volume center was 42.9%. It is unclear why the cardiac readmission rate are higher in the low-volume center. Hospital readmissions are the largest driver of post-discharge resource use.10 Hospitals have been subjected up to a 3% penalty for higher than expected readmission rates for a given diagnosis. Although bundled payments and financial penalties are unique to the US healthcare system, hospital readmissions negatively impact the quality of life of patients undergoing TAVR worldwide.
This study also aims to identify which patients might benefit from undergoing TAVR at a high-volume center. In the bivariate analysis, TAVR outcomes at a large volume center were better than at a low and intermediate-volume center for the most complex and sick patients. These included patients with a STS-PROM ≥ 12, patients over the age of 80, patients with a BMI > 30 kg/m2, patients with CKD, patients with concomitant CAD and those in class III–IV heart failure.
This study is the most current volume-outcome analysis of TAVR data in the US. Data from the National Inpatient Sample from 2012 has been previously published. They concluded that in-hospital mortality and bleeding was higher at low-volume centers when compared to high-volume centers. Moreover, the volume-outcome relationship was even more pronounced in patients undergoing TAVR via alternative access. That dataset was limited because patient level information was not available and outcomes were compared using International Classification of Diseases (ICD) codes from the discharge diagnoses. This difference in mortality and bleeding could therefore have been attributed to differences in baseline characteristics and unrelated to actual differences in volume.11
Institutional learning curves for TAVR have been systematically reviewed within the PARTNER-I trial. It took an average of 28–30 cases to consistently achieve a low risk of 30-day major adverse events in the PARTNER-I study. Institutions entering the trial later had an abbreviated learning curve, suggesting that group learning can attenuate the learning curve and early lessons can be rapidly integrated into the group experience.12 It is unknown whether these learning curve numbers can be replicated outside the rigorous oversight of a clinical trial.
A limitation of this analysis is that the low and intermediate-volume centers enrolled patients at different times in their learning curve for TAVR. This may have given the high volume site an unfair advantage. In spite of this seeming advantage, major outcomes were not different between sites. This was a testament to abbreviated learning curves, group learning and the advantage of oversight and sharing of best practices from the large-volume center.
Data from the Columbia HeartSource Experience has shown that high-volume centers can transmit expertise to an outlying smaller center outside the confines of a clinical trial.13 Transmission of knowledge across sites can potentially enable low-volume centers performing TAVR to achieve risk-adjusted outcomes that are comparable with high-volume affiliates.
However, in bivariate analysis, patients with the highest heart failure class, highest STS-PROM scores, highest age, and worst renal function fared substantially better at the high-volume center. These findings are in line with previously published data. Risk-based stratification for patients undergoing TAVR has been described with patients deemed to be clinically high-risk or undergoing non-conventional TAVR (e.g. valve-in-valve) being referred to a high-volume center.14 Although this type of hub-and-spoke model has been successfully adopted in British Columbia, the findings have yet to be replicated in the US.15
There are several important limitations to this study, including the nonrandomized, retrospective design. Unlike the NIS dataset, which has potential for coding errors and misrepresentations of procedure volume, used in previous volume-outcome analyses, the dataset used in this study was dedicated, robust, with no patients lost to follow up. The 181 patients represent real world data in 2015 before the approval of the lower profile Sapien 3 valve. All patients in this study were treated with second-generation transcatheter heart valves and hence the proportion of cases done via alternative access was high. This may not be representative of current practice where the majority of procedures are done via the transfemoral approach. The small sample size especially at the low volume center may have also introduced selection bias into the primary outcome of the study. However, inclusion of the large volume center in the bivariate analysis, and its emergence as a predictor for improved outcomes helps resolve some of the bias.
5. Conclusions
An association between TAVR volume and patient outcome exists, with better outcomes at the large-volume center compared to intermediate and low-volume centers. Furthermore, certain patients, like those with a STS-PROM >12, may benefit from undergoing TAVR at a large-volume center. As TAVR becomes mainstream for intermediate and low risk patients, the procedure might become a single operator procedure similar to PCI or CABG, so the next focus of research should be operator and/or specialty specific. The findings of this study warrant a randomized analysis to validate or refute these findings. A longer term follow up is continuing and those findings will add more information.
Disclosure statement
All authors mentioned have no disclosures to report.
Authorship decleration
All authors meet the authorship criteria according to the latest guidelines of the International Committee of Medical Journal Editors, and all authors are in agreement with the manuscript.
References
- 1.Hannan E.L., Samadashvili Z., Stamato N. Utilization and 1-year mortality for transcatheter aortic valve replacement and surgical aortic valve replacement in new york patients with aortic stenosis: 2011 to 2012. JACC Cardiovasc Interv. 2016;9(6):578–585. doi: 10.1016/j.jcin.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 2.Holmes D.R., Jr., Brennan J.M., Rumsfeld J.S. Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA. 2015;313(10):1019–1028. doi: 10.1001/jama.2015.1474. [DOI] [PubMed] [Google Scholar]
- 3.Carroll J. Insights from the U.S. TVT Registry. Paper presented at: Transcatheter Valve Therapy; Chicago IL; June 5, 2015.
- 4.de Biasi A.R., Paul S., Nasar A. National analysis of short-term outcomes and volume-outcome relationships for transcatheter aortic valve replacement in the era of commercialization. Cardiology. 2016;133(1):58–68. doi: 10.1159/000440694. [DOI] [PubMed] [Google Scholar]
- 5.Murugiah K., Wang Y., Desai N. Hospital variation in outcomes for transcatheter aortic valve replacement among medicare beneficiaries, 2011 to 2013. J Am Coll Cardiol. 2015;66(23):2678–2679. doi: 10.1016/j.jacc.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walters D.L., Webster M., Pasupati S. Position statement for the operator and institutional requirements for a transcatheter aortic valve implantation (TAVI) program. Heart Lung Circ. 2015;24(March (3)):219–223. doi: 10.1016/j.hlc.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 7.AbuRahma A.F., Stone P.A., Srivastava M. The effect of surgeon’s specialty and volume on the perioperative outcome after carotid endarterectomy. J Vasc Surg. 2013;58:666–672. doi: 10.1016/j.jvs.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Chikwe J., Cavallaro P., Itagaki S. National Outcomes in acute aortic dissection: influence of surgeon and institutional volume on operative mortality. Ann Thoracic Surg. 2013;95:1563–1569. doi: 10.1016/j.athoracsur.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 9.Lin X., Tao H., Cai M. A systematic review and meta-analysis of the relationship between hospital volume and the outcomes of percutaneous coronary intervention. Medicine (Baltimore) 2016;95(5):e2687. doi: 10.1097/MD.0000000000002687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Attilio D., Clancy S. Episode of care costs for transcatheter aortic valve replacement in the United States. J Am Coll Cardiol Interv. 2016;9(4_S):S47–S48. [Google Scholar]
- 11.Badheka A.O., Patel N.J., Panaich S.S. Effect of hospital volume on outcomes of transcatheter aortic valve implantation. Am J Cardiol. 2015;116(August (4)):587–594. doi: 10.1016/j.amjcard.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Minha S., Waksman R., Satler L. Learning curves for transfemoral transcatheter aortic valve replacement in the partner-1 trial: success and safety. Catheter Cardiovasc Interv. 2016;87(January):165–175. doi: 10.1002/ccd.26121. [DOI] [PubMed] [Google Scholar]
- 13.Green P., Rosner G., Leon M. Counterpoint: access to transcatheter aortic valve replacement should not be limited to high-volume surgical centers. J Thorac Cardiovasc Surg. 2013;145(6):1444–1445. doi: 10.1016/j.jtcvs.2013.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stub D., Lauck S., Lee M. Regional systems of care to optimize outcomes in patients undergoing transcatheter aortic valve replacement. J Am Coll Cardiol Interv. 2015;15:1944–1951. doi: 10.1016/j.jcin.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Mack M. Balancing optimal outcomes with access to care: it can Be done! JACC Cardiovasc Interv. 2015;8(December (15)):1952–1953. doi: 10.1016/j.jcin.2015.10.023. [DOI] [PubMed] [Google Scholar]