Abstract
Background
Tetralogy of Fallot and absent pulmonary valve (TOF/APV) is associated with significant pulmonary artery dilatation and airway compression.
Methods
We performed a retrospective review of 73 consecutive patients who underwent repair for TOF/APV between January 2005–August 2015. Mean age was 6.4 ± 5.6 years (28 days–22 years). The right ventricular outflow tract (RVOT) was reconstructed using varied techniques. Freedom from RVOT gradients and re-operation was studied.
Results
There were four (5.5%) early deaths, two each in infants and older children. Median ICU stay was 2 days (range, 1–12 days). Mean ICU stay for, infants, children and adults, was 6.5 ± 6.04, 2.75 ± 2.45, and 2.33 ± 1.03 days, respectively (p = 0.0762). Median hospital stay was 6 days (range, 4 to 15 days). Mean hospital stay for, infants and children and adults was 7 ± 2, 6.75 ± 2.39, and 6.33 ± 1.63 days, respectively (p = 0.325). Mean follow up was 65 ± 36.6 months (median 56 months, range 7–126 months). On follow up echocardiography, 14 (21.21%) had no pulmonary regurgitation. 21 (31.81%) had mild PR patients, 8 (12.12%) moderate PR and 19 (28.78%) had severe PR. There were five (7.5%) reoperations.
Five and ten-year survival was 95% ± 2.12 and 92.3% ± 3.45 respectively. Freedom from RVOT reoperation was 93 ± 2.62% and 89 ± 3.87% at 5 and 10 years.
Conclusions
In contrast to children and adults with TOF/APV, infants carry significant early mortality. But the mid-term outcome for patients who survive the initial repair of TOF/APV is acceptable. However, these patients require constant surveillance and irrespective of the methods of RVOT management, the reoperation rates are expected to be high as more of these patients survive into adulthood.
Keywords: Tetralogy of Fallot, Absent pulmonary valve, Airway compression
1. Introduction
In 1847, Chevers was the first to describe the syndrome of Tetralogy of Fallot with absent pulmonary valve,1 which included two unique features, pulmonary regurgitation secondary to an absent or rudimentary pulmonary valve and aneurysmal dilatation of the pulmonary trunk and pulmonary arteries apart from features of Tetralogy of Fallot.
The distinctive feature of Tetralogy of Fallot with absent pulmonary valve (TOF-APV) is airway obstruction due to tracheobronchial compression that results from massive dilatation of the main pulmonary artery (PA) and its first- and second-order branches and from the abnormal branching of segmental arteries.2 Consequently, tracheomalacia and bronchomalacia determine the timing and severity of respiratory compromise as well as morbidity and mortality of these patients.
Several modifications of surgical techniques have emphasized reduction of dilated pulmonary arteries, establishment of pulmonary valve competence using valved conduits, and relief of obstruction of the tracheobronchial tree by means of suspension of the compressing pulmonary arteries, translocation of the pulmonary artery, or even segmental resection of the lung.
Although most concur regarding the need for early intervention in patients with TOF-APV to reduce the extent and severity of tracheal stenosis and bronchomalacia, considerable controversies exist regarding the best method of right ventricle (RV)–pulmonary artery (PA) connection, as well as the most efficacious surgical management of the dilated PAs. Reported techniques have included complete excision of the dilated PAs and reconstruction with homograft tissue, performance of a LeCompte maneuver, subtotal resection with flap augmentation and arteriopexy, and anterior or posterior resection and reduction pulmonary arterioplasty.3, 4, 5, 6, 7, 8, 9, 10
The RV enlargement that ensues reduces exercise capacity and is a risk factor for late arrhythmias, and sudden death.
In this study, we analyzed the mid-term results of patients who underwent surgery for Tetralogy of Fallot with absent pulmonary valve at our center in the last 10 years.
2. Patients and methods
2.1. Patients
Institute ethical clearance was obtained prior to commencing this study. Review of the Department of Cardiac Surgery database of the All India Institute of Medical Sciences, India identified 73 patients with the diagnosis of Tetralogy of Fallot with absent pulmonary valve between January 2005 and August 2015. A review of each patient’s complete medical record was performed. Data was collected for demographic profiling which included, age and weight at surgery, symptoms at presentation and preoperative saturation. Patients’ records were obtained detailing their need for pre-operative ventilatory support, features suggestive of congestive heart failure and presence of other cardiac anomalies. Preoperative echocardiography findings were recorded for right ventricle dimensions and function, RVOT gradient, pulmonary valve annulus size and the size of the left and right pulmonary arteries. The type of surgery was dependent on surgeon preference and patient anatomy. The surgical procedure was documented and included either the use of a transannular patch, monocuspid or bicuspid valved homograft, a hetrograft or a homograft conduit. Information was extracted from the hospital records about the intraoperative transesophageal echocardiography (TEE) findings which was performed in all the patients to assess adequacy of surgery using the Philips Ultrasound transducer Model iE33 (Philips, Bothell, WI, USA) and appropriate size TEE probes, so that patients across all ages and weights and even below 5 kg (smallest patient in our study weighed 3.1 kg) underwent this imaging.
Immediate post operative mortality and morbidity were recorded which included length of ICU and hospital stay, use of extracorporeal membrane oxygenator support and ICU- associated complications (Duration of mechanical ventilation/inotropes).
In all surviving patients, a pre-discharge echocardiography was obtained, documenting, ventricular function and RVOT gradient (if any), presence of any residual VSD and presence or absence of pulmonary or tricuspid regurgitation.
Follow-up data were obtained from the medical records and patients were requested to report for a follow-up evaluation and investigations. Follow-up was complete in 66 of 72 hospital survivors, and ranged from 7 to 126 months (mean 64 ± 36.6 months, median 56 months). Follow up data included any mortality in the intervening years, presence of persistent respiratory symptoms requiring ventilatory support or reoperation. All reoperations and their results were documented. When the patients came for follow up, repeat echocardiography was performed to look for right ventricular dimensions and function, presence and degree of pulmonary and tricuspid regurgitation, any RVOT gradient or residual VSD. All patients were also evaluated for neurological symptoms.
Patients also underwent routine electrocardiography (ECG) and were assessed for QRS duration and presence of arrhythmias. If arrhythmia was detected, then patients underwent Holter evaluation.
2.2. Operative techniques
All procedures were performed through a midline sternotomy. Cardiopulmonary bypass was established via standard aortic and bicaval venous cannulation. The left ventricle was decompressed by venting through the inter-atrial septum or the right superior pulmonary vein. Mild hypothermia (28–32 °C) and antegrade cold blood cardioplegia were used for myocardial protection.
All patients underwent complete repair. Enlargement of the right ventricular outflow tract (RVOT) was performed by resecting the hypertrophied muscle bundles through an incision in the RVOT to serve as the site of the RV-PA conduit or the infundibular patch.
The approach to the pulmonary artery dilatation differed with the age of the patient and the degree of compression. Sixty-three patients (86.3%) did not require any intervention while ten patients (13.7%) underwent either pulmonary artery plication (8/10, 80%) or reduction the aneurysmal pulmonary artery by excising elliptical strips of arterial wall (2/10, 20%).
The VSD was closed using a Dacron patch, usually by a trans-atrial approach (Table 1).
Table 1.
Operative Details of the patients.
| RVOT reconstruction technique | |
| CONTEGRA Valved conduit | 22 (30.1%) |
| Monocusp homograft | 21 (28.76%) |
| Transannular patch | 15 (0.21%) |
| Nunn’s bicuspid handmade valve | 6 (0.082%) |
| Transannular patch with monocusp | 6 (0.082%) |
| PTFE tube graft with monocusp valve | 3 (0.05%) |
| Pulmonary artery management | |
| No intervention | 63 (86.3%) |
| Pulmonary artery plication | 8 |
| Reduction | 2 |
The right ventricle to pulmonary artery continuity was reconstructed by CONTEGRA (Medtronic Inc, Minnesota, USA) valved bovine jugular vein conduit (22/73, 30.1%), transannular patch along with Nunn’s bicuspid handmade PTFE valve (6/73, 8.22%), monocusp pulmonary/aortic homograft (21/73, 28.76%), PTFE tube graft with monocusp (3/73, 4.11%), transannular patch with monocusp valve (6/73, 8.22%) and RVOT reconstruction with transannular patch (15/73, 20.55%). Currently at our institution, all patients with TOF/APV undergo RVOT reconstruction with either a homograft or monocusp valve with plication or reduction in size of pulmonary artery if required.
Pre-and post-operative TEE data were obtained from the medical records as described above. Complete operative data are summarized in Table 2.
Table 2.
Clinical profile of all the patients.
| Number of patients | 73 | |
| Male | 56 | |
| Female | 17 | |
| Mean ± S.D. (range) | Median | |
| Age of surgery | 6.4 ± 5.7 years (28 days–22 years) |
4 years |
| Age range | ||
| Neonate (<1 month) | 1 | |
| Infants (1 month–1 year) | 6 | |
| Children (1–18 years) | 60 | |
| Adults (>18 years) | 6 | |
| Weight at surgery | 10.6 kg ± 2.1 (3.1–65 kg) | 10 kg |
| Preoperative Sao2 (%) | 80.13% ± 14.1 (62%–99%) | 80% |
| Preoperative intubation | 18 | |
| Previous surgery | 8 | |
2.3. Data analysis
Data analysis was performed using STATA version 5 (College Station, TX, USA). Variables were compared using the Wilcoxon rank sum test. Data are presented as median and range. For survival analysis, the date of surgery was taken as zero time. For survival analysis, the patients were censored at the time of death or withdrawn alive at the time of last follow-up. To assess freedom from death or reoperation, patients were censored at the time of death or reoperation, or withdrawn alive at the time of last follow-up. The Kaplan-Meier survival estimates with 95% confidence limits (CLs) are provided. Survival distributions between groups were compared using the log rank test.
3. Results
3.1. Demographics
Seventy-three patients underwent surgery for TOF-APV during the stipulated study period. There were 56 males and 17 female patients. The mean age of repair was 6.4 ± 5.6 years (median 4 years, range 28 days–22 years). Median weight at repair was 10 kg (range 3.1–65 kg). Age of initial repair included one neonate, six infants, sixty children and six adults.
The clinical profile of the entire cohort is summarized in Table 2.
The patients presented with symptoms of breathlessness on exertion (40/73, 54.8%), cyanosis (20/73, 27.4%) and palpitations (25/73, 34.2%). The average preoperative saturation was 80.13% (median 80%, range of 62%–99%). 48% of the patients (35/73) presented with failure to thrive and ten patients (13.7%) presented with abdominal distension and pedal edema suggestive of congestive heart failure (Table 3).
Table 3.
Patients’ characteristics.
| Total patients | 73 | |
|---|---|---|
| Patients’ characteristics | Number of patients | Percent |
| Shortness of Breath | 40 | 54.8% |
| Cyanosis | 20 | 27.4% |
| Palpitation | 25 | 34.2% |
| Failure to thrive | 35 | 47.94% |
| Congestive heart failure | 10 | 13.7% |
| Pre- operative saturation | Mean 80.13% ± 14.05 | Median 80% |
| Range | 62%–99% | |
Seven patients had undergone modified Blalock-Taussig shunt outside and one patient had undergone a failed attempted pulmonary valve balloon dilatation. Eighteen patients had respiratory distress requiring preoperative mechanical ventilatory support.
Patients’ functional class was a median of II, with 19 patients who presented with a functional class >II. Sixteen patients were in functional class I, while 38 patients were in functional class II. Fifteen patients were in functional class III and four patients were in class IV.
Inotropic support was calculated in all the patients to quantify inotrope use.11 It was calculated as inotropic score = dopamine [mg/kg/min] + dobutamine [mg/kg/min] + 100 × epinephrine [mg/kg/min].
3.2. Operative and immediate post-operative results
Mean cardiopulmonary bypass (CPB) time was 125 ± 32 min (median 75 min, range 62 min to 220 min) and mean aortic cross clamp time was 47 ± 25 min (median 45 min, range 32–65 min). One patient required operative revision after reinstitution of cardiopulmonary bypass, for residual RVOT obstruction following trans-annular patch that was revised with a homograft (Table 4).
Table 4.
Analysis of previous studies.
| Study | Total Patients | Infant Mortality | Mean Follow-up (years) | Late Deaths | Late reoperations |
|---|---|---|---|---|---|
| Snir et al. (1991) | 22 | 25 | 3.6 | 1 | 1 |
| Watterson et al. (1992) | 19 | 17 | Not reported | 1 | 1 |
| Godart et al. (1996) | 37 | 20 | 5 | 1 | 1 |
| McDonnell et al. (1998) | 8 | 33 | 5.5 | 1 | 1 |
Taken from McDonnell et al., Absent Pulmonary Valve Syndrome, Ann Thorac Surg 1999;67:1391–1396.16
More than 5 days of ventilator support was considered prolonged. In the post operative period, prolonged ventilator support was required in five (6.85%) patients, all less than two years of age. All the five patients had required hospitalization and medical stabilization prior to surgery and had been on intermittent ventilator support preoperatively. Four patients out of five had presented with features of respiratory distress due to repeated broncho-pulmonary infection and episodes of respiratory obstruction and were being electively ventilated. One patient had history of hemoptysis requiring positive pressure ventilation preoperatively and required prolonged ventilation in the post-operative period.
In the immediate postoperative period, echocardiography was performed for all surviving patients prior to discharge.
Elective inotropic support consisted of 5–10 mcg/kg/ml of inj. Dobutamine. wa
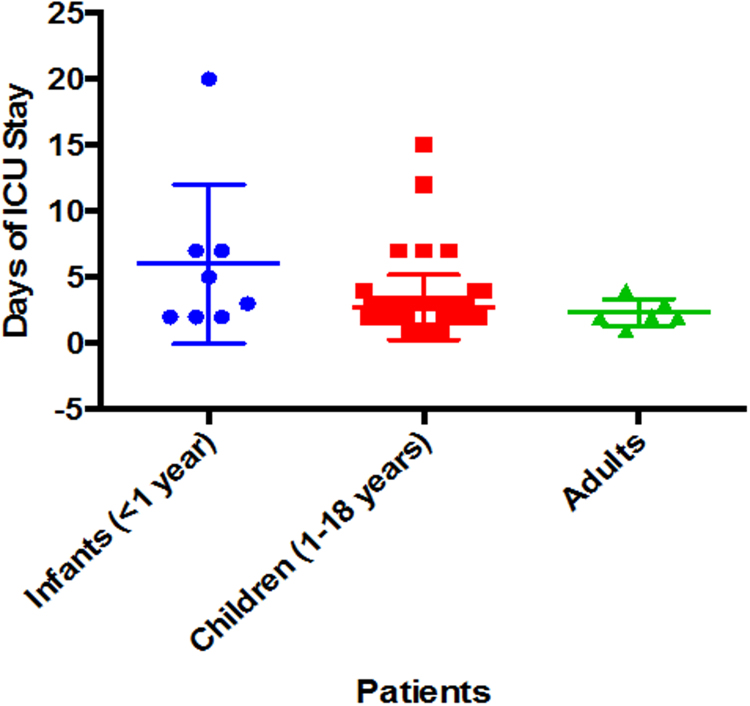
Median ICU stay following surgical repair was 2 days (range, 1–12 days). Mean ICU stay for, infants, children and adults was 6.5 ± 6.04, 2.75 ± 2.45, and 2.33 ± 1.03 days, respectively (p = 0.0762, not significant) (Fig. 1).
Fig. 1.
Days of ICU stay.
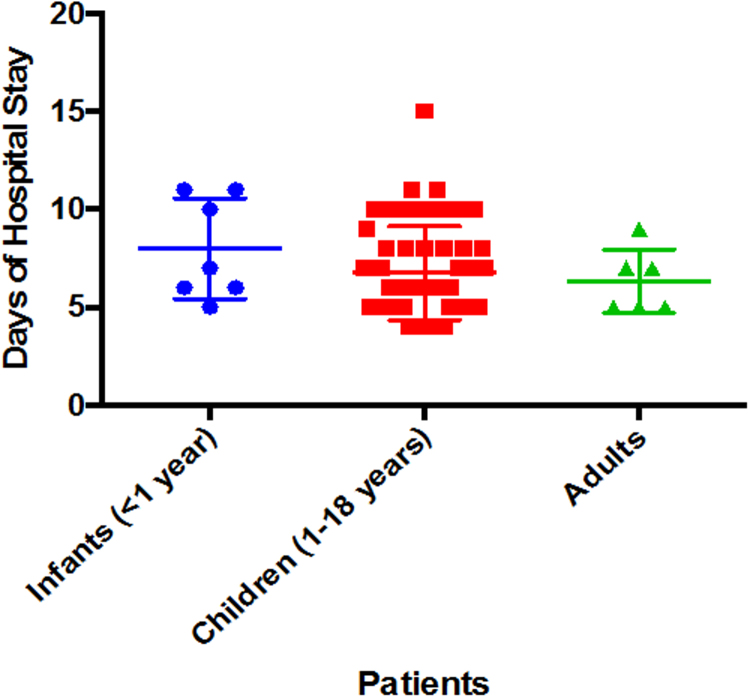
Median hospital stay, following surgery was 6 days (range, 4–15 days). Mean hospital stay for, infants, children and adults was 7 ± 2, 6.75 ± 2.39, and 6.33 ± 1.63 days, respectively (p = 0.325, not significant) (Fig. 2). In the immediate post-operative period, improvement of functional class was seen and postoperative saturation increased to median 99% (range 97–100%).
Fig. 2.
Days of Hospital Stay.
Operative mortality, defined as death within the initial postoperative hospitalization or within 30 days from surgery, was 5.5% (4/73). There were four deaths in total where two were infants and two were children. One patient was in shock preoperatively and was taken emergently to the operating room, two patients, age 3 months and 6 years expired on the 5th and 7th postoperative day from severe myocardial dysfunction, one death was in a neonate who died because of ventilator associated pneumonia on the 15th post operative day.
Several variables were analyzed to assess if they were risk factors for operative mortality. These variables included age, sex, and weight of patients, the duration of cardio- pulmonary bypass and aortic cross clamp and preoperative ventilator support. None of these variables were identified as independent risk factors on multivariable analysis.
Thirty patients (41.1%) had no pulmonary regurgitation (PR), 22 (30.1%) had mild PR, 15 (20.5%) and moderate PR and 2 (2.73%) had severe PR. Forty-three patients had no tricuspid regurgitation, while twelve, six, five and one had trivial, mild, moderate and severe tricuspid regurgitation. For the three patients with significant pulmonary and tricuspid regurgitation, none were symptomatic. Patients were managed conservatively on medical therapy and followed up closely in the out-patient clinic.
3.3. Late results
During the mean follow up period of 65 ± 36.6 months (median 56 months, range 7–126 months), three patients were lost to follow up and could not be contacted. Out of the rest 66 patients, one death was reported. The patient was a case of fronto-parietal infarct in the post-operative period and succumbed due to neurological causes ten months post operatively.
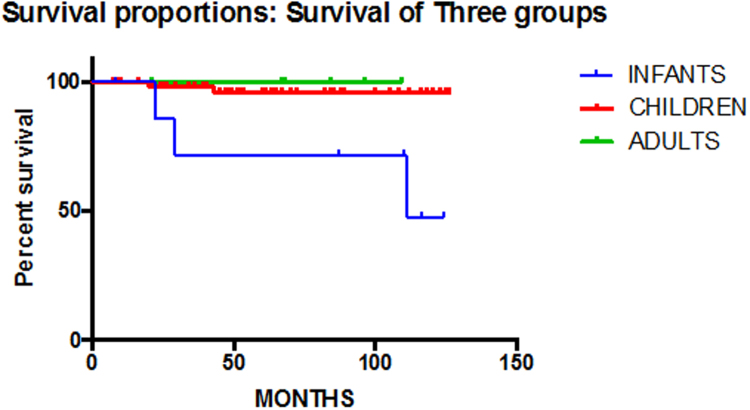
Overall survival at 5 and 10 years was 95% ± 2.12 and 92.3% ± 3.45, respectively (Fig. 3), Four out of five deaths were in patients undergoing initial surgery, with the remaining death was due to neurological cause ten moths post operatively.
Fig. 3.
Survival proportions.
The follow up period for the cohort of 66 patients were 64 ± 36.6 months (median 56 months, range 7–126 months). The patients were asked to come in for clinical assessment and investigations.
Two patients (3.03%) developed reactive airway disease in the late postoperative period and required follow up in the department of pulmonary medicine.
All the patients underwent a detailed physical examination and routine investigations. On electrocardiography, 1/66 (1.51%) patient was found to have first-degree heart block that had persisted from the time of surgery. In three other patients who had wide P-R interval in the immediate post-operative period, the heart block had resolved. Four (4.84%) patients had normal P-R interval with RBBB pattern. Neither condition required any further investigations.
On follow up echocardiography, patients 14 (21.21%) had no pulmonary regurgitation 21 (31.81%) had mild PR patients, 8 (12.12%) moderate PR and 19 (28.78%) had severe PR. All patients were asymptomatic and were treated conservatively. Patients were educated about adverse symptoms like exercise intolerance and were advised to come for frequent follow up.
Thirty-five patients (53.03%) had no tricuspid regurgitation, while 23 (34.84%), one (1.51%), and four (6.06%) had mild, moderate and severe tricuspid regurgitation respectively. All four patients with severe tricuspid regurgitation were asymptomatic. Routine liver function tests were not found to be elevated. Patients were started on diuretics and were advised close follow up. Significant RVOT gradient was seen in 3/6 patients (>35 mm Hg).
There were total of five (7.5%) reoperations. Three patients (4.54%) required reoperation for RV-PA conduit change due to significant RVOT gradient. One patient underwent conduit replacement with a larger size conduit for significant conduit obstruction. The patient presented with complaints of breathlessness. On echocardiography, he was found to have severe RV dysfunction (RSVP = 106 mm Hg + RAP) and severe tricuspid regurgitation. He underwent conduit revision. One patient underwent conduit revision for dilated RVOT with focal out-pouching of the conduit.
Freedom from re-intervention at 5 year and 10 years was 93 ± 2.62% and 89 ± 3.87% respectively.
Using the Cox multivariable regression model, we were unable to identify any significant demographic or operative factors for long-term survival or freedom from reoperation.
4. Discussion
There has been a reduction in mortality for patients undergoing repair of congenital heart defects in recent years due to improved techniques and the use of homografts/hetrografts. However, despite improvements in surgical techniques, cardiopulmonary bypass, and critical care, the mortality for infants with TOF/APV remains significantly greater than for infants with simple TOF.12, 13, 14
Substantial controversy exists regarding the management of symptomatic patients especially regarding arterioplasty of the branch PAs and the establishment of the RV-PA connection.
Most centers incorporate pulmonary arterioplasty or plication in the repair to diminish the size of the proximal pulmonary arteries and relieve airway obstruction.13, 14, 15 In the present day surgical practice, CT and MRI can help in decision making about the need for pulmonary artery reduction to alleviate respiratory compression. There is less agreement, however, concerning the method of RVOT reconstruction. Some centers have advocated utilization of either a valved homograft conduit or use of a transannular patch incorporating a monocusp valve.13, 14 In his article on surgical management of TOF-APV, Jonas described his technique of placement of a valved homograft conduit in the RVOT.13 In 2014, Yong et al. from Royal Children’s Hospital, Melbourne, Australia, described long-term follow-up of 52 patients who underwent surgery between 1975 and 2013. They had a median follow-up of 13 years with overall survival at 5, 10, and 20 years was 81%. Preoperative ventilation (P < 0.009) was the only risk factor for overall mortality. Freedom from late reoperation at 5, 10, and 20 years was 80%, 70%, and 52%, respectively. No difference in reoperation rates was found between valved conduit, monocusp, or valveless techniques. Risk factors for late reoperation on multivariate analysis were prematurity (P < 0.001) and neonatal primary repair (P < 0.007). Longer postoperative ventilation periods were predicted by preoperative ventilation (P < 0.001) and surgery during infancy (P ¼ 0.01).15
In our study, we found the total infant mortality to be 7.7% and no mortality in the adult group with a ten- year follow up. We were able to follow up 66/73 patients (90.4%). We found that preoperative ventilatory support does not alter the outcome of the surgery. Our study population was diverse, ranging from 1 month old infant to a 22-year-old adult patient. In spite of dealing with such a diverse population, our median ICU stay and hospital stay was two days and six days respectively. Minimal inotropic support was used during this period and post operatively patients demonstrated improved functional status.
Our mean follow up was 5.33 ± 3.05 years. During this period, we lost six patients to follow up. Five patients came back for reoperation and rest remain in annual follow up.
4.1. Study limitations
This study is a retrospective analysis and data are limited by the information available in the medical record. Because of the rarity of TOF/APV, the study population is small and thus statistical power is limited. The patient population has changed in recent years and surgical techniques have evolved. In spite of our best efforts, complete patient follow up could not be achieved.
5. Conclusion
While early mortality in patients with TOF APV is significant, the mid-term outcome for patients who survive the initial repair is acceptable. However, these patients require constant surveillance and irrespective of the methods used for RVOT management, the reoperation rates are expected to be high as more of these patients survive into adulthood.
Conflict of interest
None.
References
- 1.Chevers N. Recherches sur les maladies del’ artère pulmonaire. Arch Gen Med. 1847;15:488–508. [Google Scholar]
- 2.Rabinovitch M., Grady S., David I. Compression of intrapulmonary bronchi by abnormally branching pulmonary arteries associated with absent pulmonary valves. Am J Cardiol. 1982;50:804–813. doi: 10.1016/0002-9149(82)91238-3. [DOI] [PubMed] [Google Scholar]
- 3.Ilbawi M.N., Idriss F.S., Muster A.J., Wessel H.U., Paul M.H., DeLeon S.Y. Tetralogy of Fallot with absent pulmonary valve. Should valve insertion be part of the intracardiac repair? J Thorac Cardiovasc Surg. 1981;81:906–915. [PubMed] [Google Scholar]
- 4.Hew C.C., Daebritz S.H., Zurakowski D., del Nido P.I., Mayer J.E., Jr., Jonas R.A. Valved homograft replacement of aneurysmal pulmonary arteries for severely symptomatic absent pulmonary valve syndrome. Ann Thorac Surg. 2002;73:1778–1785. doi: 10.1016/s0003-4975(02)03511-7. [DOI] [PubMed] [Google Scholar]
- 5.Nolke L., Azakie A., Anagnostopoulos P.V., Alphonso N., Karl T.R. The Lecompte maneuver for relief of airway compression in absent pulmonary valve syndrome. Ann Thorac Surg. 2006;81:1802–1807. doi: 10.1016/j.athoracsur.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Hraska V. Repair of tetralogy of Fallot with absent pulmonary valve using a new approach. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2005;1:132–134. doi: 10.1053/j.pcsu.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Kirshbom P.M., Jaggers J.J., Ungerleider R.M. Tetralogy of Fallot with absent pulmonary valve: simplified technique for homograft repair. J Thorac Cardiovasc Surg. 1999;118:1125–1127. doi: 10.1016/S0022-5223(99)70116-9. [DOI] [PubMed] [Google Scholar]
- 8.Kreutzer C., Schlichter A., Kreutzer G. Tetralogy of Fallot with absent pulmonary valve: a surgical technique for complete repair. J Thorac Cardiovasc Surg. 1999;117:192–194. doi: 10.1016/s0022-5223(99)70488-5. [DOI] [PubMed] [Google Scholar]
- 9.Conte S., Serraf A., Godart F. Technique to repair tetralogy of Fallot with absent pulmonary valve. Ann Thorac Surg. 1997;63:1489–1491. doi: 10.1016/s0003-4975(97)00327-5. [DOI] [PubMed] [Google Scholar]
- 10.Yang J.H., Jun T.G., Park P.W. Factors related to the durability of a homograft monocusp valve inserted during repair of tetralogy of Fallot as based on the mid- to long-term outcomes. Cardiol Young. 2008;18:141–146. doi: 10.1017/S1047951108001923. [DOI] [PubMed] [Google Scholar]
- 11.Wernovsky G., Wypij D., Jonas R.A. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation. 1995;92:2226–2235. doi: 10.1161/01.cir.92.8.2226. [DOI] [PubMed] [Google Scholar]
- 12.Lakier J.B., Stanger P., Heymann M.A., Hoffman J.I.E., Rudolph A.M. Tetralogy of Fallot with absent pulmonary valve: natural history and hemodynamic considerations. Circulation. 1974;50:167–175. doi: 10.1161/01.cir.50.1.167. [DOI] [PubMed] [Google Scholar]
- 13.Jonas R.A. Surgical management of absent pulmonary valve syndrome. World J Pediatr Congenit Heart Surg. 2016;7:600–604. doi: 10.1177/2150135116651838. [DOI] [PubMed] [Google Scholar]
- 14.Saygi M., Haydin S., Guzeltas A., Odemis E., Yeniterzi M. A rare congenital cardiac anomaly in adulthood: tetralogy of Fallot with absent pulmonary valve syndrome. World J Pediatr Congenit Heart Surg. 2014;5:330–333. doi: 10.1177/2150135113513477. [DOI] [PubMed] [Google Scholar]
- 15.Yong M.S., Yim D., Brizard C.P. Long-term outcomes of patients with absent pulmonary valve syndrome: 38 years of experience. Ann Thorac Surg. 2014;97:1671–1677. doi: 10.1016/j.athoracsur.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 16.McDonnell B.E., Ragg G.W., Gaynor J.W. Absent pulmonary valve syndrome. Ann Thorac Surg. 1999;67:1391–1396. doi: 10.1016/s0003-4975(99)00250-7. [DOI] [PubMed] [Google Scholar]