Abstract
Introduction
In this cross-sectional study, we aimed to detect differences in cerebral blood flow (CBF) between subjects with Alzheimer's disease (AD), mild cognitive impairment (MCI), and subjective cognitive decline (SCD), using two-dimensional phase-contrast magnetic resonance imaging.
Methods
We included 74 AD patients (67 years, 51% female), 36 MCI patients (66 years, 33% female), and 62 patients with SCD (60 years, 32% female) from the Amsterdam Dementia Cohort. Patients with SCD are those who visited the memory clinic with subjective cognitive complaints without objective cognitive impairment. Whole-brain CBF (mL/100 g/min) was calculated using total volume flow measured with two-dimensional phase-contrast magnetic resonance imaging and normalized for brain volume.
Results
Mean CBF values (SD) were lower in AD compared to SCD (age and sex adjusted 70 ± 26 vs. 82 ± 24 mL/100 g/min, P < .05). Mean CBF values of MCI were comparable to AD. Across clinical groups, lower CBF was associated with lower scores on the Mini–Mental State Examination (age and sex adjusted stβ = 0.19 per mL/100 g/min; P = .02).
Discussion
Lower whole-brain CBF is seen in AD patients compared to SCD patients and is associated with worse cognitive function.
Keywords: Two-dimensional phase-contrast MRI, Cerebral blood flow, Alzheimer's disease, Neurodegeneration, Cognition
Highlights
-
•
The study consisted of a large sample of patients with AD, MCI, and controls.
-
•
CBF measured with 2D PC MRI differed between AD patients and controls.
-
•
Lower CBF was associated with worse cognitive function measured with MMSE.
-
•
2D PC MRI may be used as a marker for disease severity in a memory clinic.
1. Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disease caused by accumulation of amyloid plaques and neurofibrillary tangles, eventually leading to brain atrophy [1]. Accumulating evidence shows that patients with AD not only have brain atrophy but also have lower cerebral blood flow (CBF) compared to those with subjective cognitive decline (SCD). It is possible that neurodegeneration and small vessel disease (SVD) independently contribute to lower CBF in subjects with AD and that CBF may reflect total disease burden in AD [2], [3].
Several techniques can be used to measure CBF in subjects: single-photon emission computed tomography, positron emission tomography (PET), and arterial spin-labeling (ASL) magnetic resonance imaging (MRI) [2], [4], [5], [6], [7], [8], [9]. However, these techniques have several disadvantages. Single-photon emission computed tomography and PET are invasive, and ASL is very sensitive to measurement variability [8].
Two-dimensional phase-contrast (2D PC) imaging may be a promising alternative. This is a noninvasive, relatively fast, and cheap technique that measures the average flow in the carotid and basilar arteries. Furthermore, 2D PC imaging is an accurate and reproducible method to assess whole-brain CBF [10]. Two small studies and one larger study showed that 2D PC imaging is capable of measuring whole-brain CBF, which can be used to differentiate between subjects with SCD and with AD [6], [11], [12]. However, those studies did not adjust for brain volume. This is an important limitation because brain volume may be a confounder in the relationship between cerebral perfusion and cognition [13]. Two studies that did adjust for brain volume were performed in the general population and in subjects with diabetes mellitus, showing contradictory results for the association of CBF with cognition [13], [14].
We used 2D PC MRI to investigate differences in CBF, normalized for brain volume, in a large sample of subjects with AD, mild cognitive impairment (MCI), and SCD. In addition, we studied the association between CBF and cognitive function.
2. Methods
2.1. Subjects
In this cross-sectional study, 231 subjects were selected from the memory clinic–based Amsterdam Dementia Cohort. All subjects visited the memory clinic between October 2010 and May 2011 and underwent an extensive dementia screening, including medical history, neurological and physical examination, cognitive assessment, and brain MRI including 2D PC MRI [15]. The 2D PC image could not be used for the calculation of CBF when the lumen of (one of) the vessel(s) was not distinguishable from the static tissue (e.g., due to movement or artifacts). This led to the exclusion of 59 subjects.
In total, 172 subjects were included in the present study: 74 patients with AD, 36 patients with MCI, and 62 patients with SCD [16], [17]. We found no difference in demographics between the study subjects and the subjects who were excluded because of their 2D PC MRI. The excluded patients were evenly distributed over the diagnostic groups.
AD diagnosis according to the National Institute of Neurological and Communicative Disorders and Stroke - Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria [18], [19] and MCI diagnosis according to the Petersen criteria [20], [21] were made based on the consensus of a multidisciplinary team [15]. Subjects with SCD were those who visited our memory clinic with subjective cognitive complaints, but for them, clinical examination and neuropsychological tests were normal (i.e., criteria for MCI, dementia, or any other neurological or psychiatric disease that might cause cognitive decline were not met).
We collected information on presence of hypertension, hypercholesterolemia, and diabetes mellitus based on self-reported medical history and medication use. We dichotomized smoking into current and never/former smoking. The other cardiovascular risk factors were also dichotomized into present or absent. Mini–Mental State Examination (MMSE) scores were used as a measure of cognitive function.
The ethical review board of the VU University Medical Center approved the study. All subjects gave written informed consent to use their clinical information for research purpose.
2.2. MRI acquisition
All subjects underwent brain MRI imaging on a 3.0 T whole-body MRI scanner (Signa HDxt, General Electric Medical Systems, Milwaukee, WI, USA) using an eight-coil-channel head coil. The scan protocol has been described elsewhere more extensively [4].
The MRI protocol included a three-dimensional T1-weighted sequence (repetition time [TR] = 7.8 ms, echo time [TE] = 3 ms, inversion time = 450 ms, flip angle = 12°, and voxel sixe = 1.0 × 0.9 × 0.9 mm); a sagittal three-dimensional fluid-attenuated inversion recovery (TR = 8000 ms, TE = 123.6 ms, inversion time = 2350 ms, and voxel size = 1.0 × 1.0 × 1.0 mm); an axial 2D T2* gradient echo with an echo-planar read-out (TR = 5300 ms, TE ;= 25 ms, and voxel size = 1.0 × 0.5 × 0.5 mm); and an axial 2D proton density/T2-weighted (PD-T2) fast spin echo (TE = 20/112 ms, TR = 8680 ms, and voxel size = 1.0 × 0.5 × 0.5 mm). All scans were checked by neuroradiologists for gross abnormalities.
Medial temporal lobe atrophy (MTA) was rated on the coronal reconstructions of the T1-weighted images with scores ranging from 0 to 4 [22]. For analyses, we used the mean of left and right MTA scores and dichotomized this into low (<1.5) or high (>1.5). White matter hyperintensities (WMHs) were rated using the Fazekas scale, with scores ranging from 0 to 3, on the fluid-attenuated inversion recovery images [23]. The Fazekas score was dichotomized into low (0–1) and high (2–3). Microbleeds were defined as small round hypointense foci on T2*-weighted images, with a maximum diameter of 10 mm located in brain parenchyma. Lacunes were defined as deep lesions (3–15 mm) with cerebral spinal fluid (CSF)–like signal on all sequences. Microbleeds and lacunes were counted and for the analyses dichotomized into present or absent. The rater was blinded to the subjects' clinical data [2].
For the estimation of brain volume, not normalized by head size, T1 images were used. Preprocessing using FSL (version 5.0, www.fsl.fmrib.ox.ac.uk/fsl) included non-brain tissue removal [24], linear registration to standard space [25], and tissue segmentation [26], as previously described [4].
2.3. 2D PC MRI
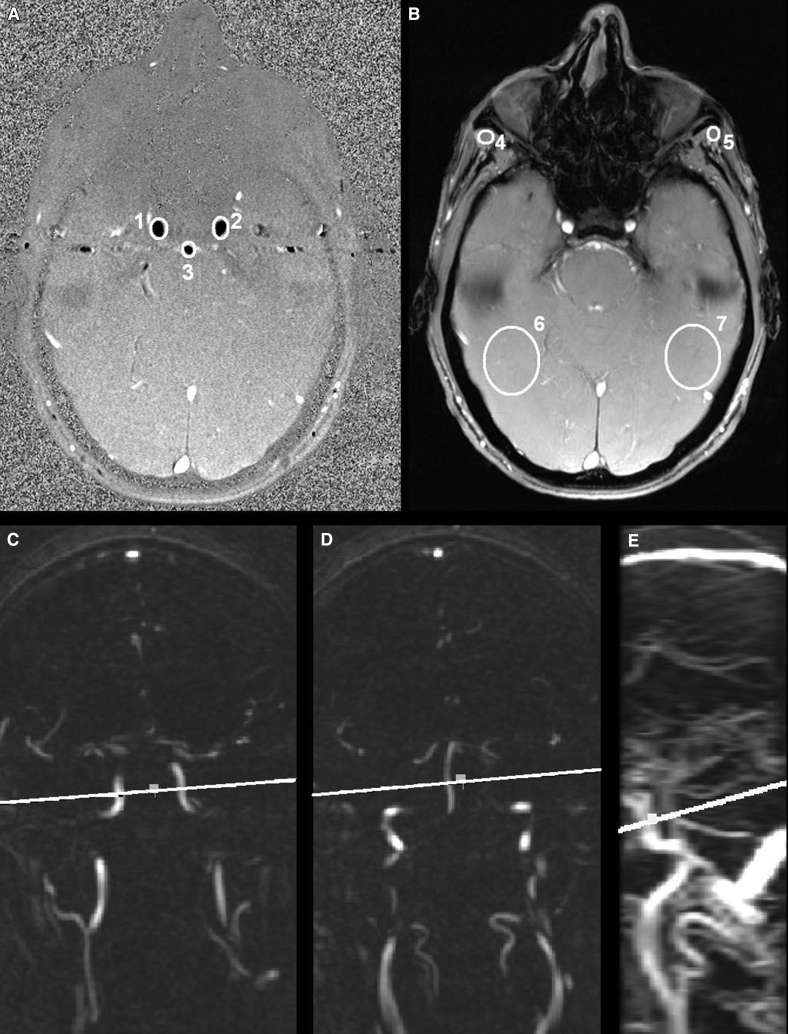
For flow measurement, nongated 2D PC imaging was performed. A transverse 2D PC image was made in a plane perpendicular to the internal carotids and basilar artery at the base of the skull (TR = 23.0 ms, TE = 7.2 ms, field of view = 26.0 cm2, matrix 512 × 512, flip angle = 15°, number of excitations = 4.0, bandwidth = 14.71 kHz, velocity encoding 100.00 cm/s, and slice thickness = 5.0 mm). For planning purposes, the 2D PC scan was preceded by a coronal 2D PC angiogram (slice thickness = 3 mm). Both the coronal source images and a sagittal maximum intensity projection (MIP) were used to plan the 2D PC image (Fig. 1).
Fig. 1.
Two-dimensional (2D) PC MRI. The images on the top row show the 2D PC phase image (A) and the 2D PC magnitude image (B). ROIs are drawn in white in the right carotid artery (1), the left carotid artery (2), and the basilar artery (3). The four background ROIs in static tissue are drawn in the sternocleidomastoid muscle on both sides (4 and 5) and two lower ROIs placed symmetrically relative to the three vessel ROIs (6 and 7). The images on the bottom row show the 2D PC magnetic resonance angiogram of carotid arteries (C), the basilar artery (D), and the sagittal 2D PC MIP image (E). The white line indicates the plane of the 2D PC phase and magnitude image. Abbreviations: MRI, magnetic resonance imaging; PC, phase-contrast; ROI, region of interest.
2.4. Flow calculations
Circular regions of interest (ROIs) were manually drawn around the basilar, the right, and the left carotid artery in the 2D PC image (Fig. 1). The ROIs were drawn on an enlarged PC image (magnitude 5x), making the placement of each ROI very exact. It was made sure that the ROI encompassed the entire lumen of the vessel, while including as few stationary tissue voxels as possible. The given mean value, comprising the velocity, and the area for each ROI was recorded. Volume flow of each vessel was calculated by multiplying the mean ROI velocity with the ROI area. Total volume flow was calculated by summing the volume flow of the three arteries and corrected for static flow offsets [27], [28]. Total volume flow was normalized to whole-brain volume to create whole-brain CBF in mL/100 g/min. Formulas are found in Supplementary Materials. Repeat analyses were performed at random in 16 scans (J.F.L. and I.S.v.M.), in which excellent agreement was achieved (intraclass correlation coefficient >0.99).
To correct for the stationary tissue voxels, we performed a first-order correction of background phase errors [27]. We drew four background ROIs in static tissue: two upper ROIs placed in the sternocleidomastoid muscle on both sides and two lower ROIs placed symmetrically relative to the three vessel ROIs (Fig. 1). We chose the sternocleidomastoid muscle for the upper background ROIs. This region was chosen to be sure that no large vessels were in the background ROI, affecting the measured offset values. The flow offset is known to vary spatially [28], and the symmetry of the background ROIs yields a first-order correction.
2.5. Statistical analysis
Differences between diagnostic groups were analyzed using one-way analyses of variance or χ2 tests. For ordinal measures, the Mann-Whitney U test was used. We studied the association of mean whole-brain CBF and diagnostic groups using the analysis of variance test. The association of CBF (independent variable) with MMSE scores (dependent variable) across and within diagnostic groups was studied with linear regression analysis. Furthermore, we studied the associations of CBF with the separate demographics using linear regression analyses.
All analyses were adjusted for age and sex and additionally for cardiovascular risk factors (hypertension, hypercholesterolemia, diabetes mellitus, and smoking) and separately for MRI markers of SVD (lacunes, WMHs, and microbleeds). All analyses were performed using software package SPSS (Chicago, IL, USA), version 22.0, for Windows.
3. Results
3.1. Demographics
Table 1 gives the patient characteristics for the total study sample by diagnosis groups. SCD patients were on average 6 years younger than those with MCI, and 7 years younger than AD patients. Compared to subjects with SCD, the AD group consisted of more women. As expected, MMSE scores were lower in AD than in MCI and SCD. No differences in cardiovascular risk factors were found between diagnostic groups.
Table 1.
Demographical and clinical characteristics for the total study group and according to clinical diagnosis
| Characteristics | Total (172) | SCD (62) | MCI (36) | AD (74) |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean (SD) | 64 (8.1) | 60 (8) | 66 (7)† | 67 (7)‡ |
| Females, n (%) | 70 (40.7) | 20 (32.3) | 12 (33.3) | 38 (51.4)∗ |
| MMSE score, mean (SD) | 25 (5.2) | 28 (1.9) | 27 (1.7) | 21 (5.4)§ |
| Vascular risk factors | ||||
| Hypertension, n (%) | 70 (40.7) | 23 (37.1) | 17 (47.2) | 30 (40.5) |
| Hypercholesterolemia, n (%) | 45 (26.2) | 14 (22.6) | 14 (38.9) | 17 (23.0) |
| Diabetes mellitus, n (%) | 21 (12.2) | 10 (16.1) | 6 (16.7) | 5 (6.8) |
| Smoking, n (%) | 21 (12.4) | 7 (11.5) | 4 (11.4) | 10 (13.7) |
| Missing, n = 3 | ||||
| Heart failure, n (%) | 5 (2.9%) | 1 (1.6%) | 2 (5.6%) | 2 (2.7%) |
| MRI characteristics | ||||
| WMH (Fazekas), median, (IQR) | 1 (0–1) | 1 (0–1) | 1 (0–2)∗ | 1 (0–2)∗ |
| Microbleed presence, n (%) | 43 (25.1) | 8 (12.9) | 14 (38.9)† | 21 (28.8)∗ |
| Lacune presence, n (%) | 13 (7.6) | 1 (1.6) | 8 (22.2)‡ | 4 (5.4)∗∗ |
| MTA, median (IQR) | 0.5 (0–1) | 0 (0–0.5) | 0.5 (0–1)∗ | 1 (0.5–2)§ |
| Brain volume (mL), mean (SD) | 1095.8 (113.7) | 1141.1 (119.1) | 1103.8 (99.5) | 1053.9 (100.5)∗∗ |
Abbreviations: AD, Alzheimer's disease; IQR, interquartile range; MCI, mild cognitive impairment; MMSE, Mini–Mental State Examination; MRI, magnetic resonance imaging; MTA = medial temporal lobe atrophy; SCD, subjective cognitive decline; SD, standard deviation; WMH = white matter hyperintensity.
NOTE. Available smoking status = SCD 61/62, MCI 35/36, and AD 73/74.
∗P < .05, †P ≤ .005, ‡P ≤ .001, compared to SCD; §P < .001 compared to MCI and SCD; ∗∗P < .05 compared to MCI and P < .001 compared to SCD.
Patients with MCI and AD had more WMHs, microbleeds, and MTA compared to subjects with SCD. AD patients also had higher MTA scores compared to MCI and lower brain volumes compared to patients with MCI and subjects with SCD. Patients with MCI had more lacunes than those with SCD and those with AD.
3.2. Cerebral blood flow
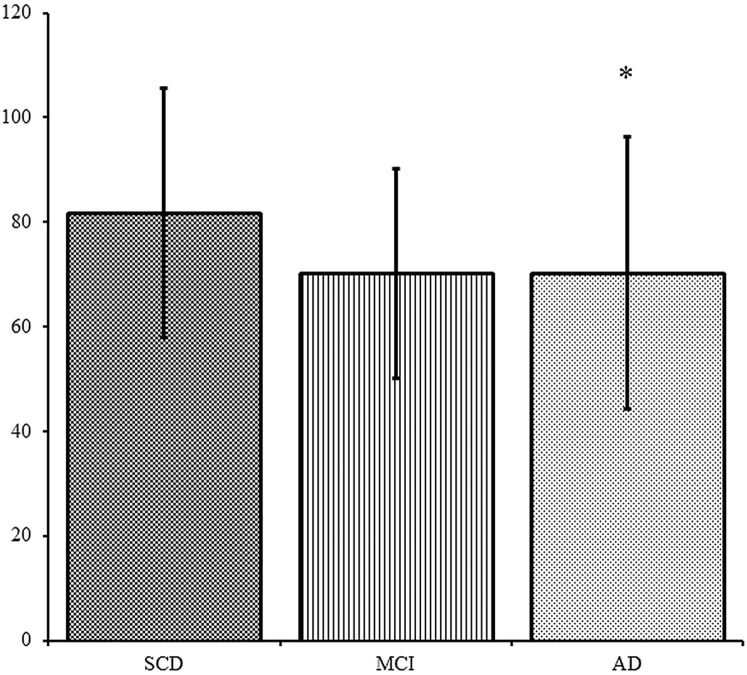
After adjusting for age and sex, CBF was lower in patients with AD compared to those with SCD (Fig. 2). After additionally adjusting for cardiovascular risk factors, CBF was lower in both patients with AD and MCI compared to subjects with SCD (mean difference [MD] =−10.2 ± standard error [SE] 4.5 and −10.4 ± SE 5.1; P = .02 and P < .05, respectively). When adjusting for age, sex, and SVD, CBF only differed between AD and SCD (MD = −10.0 ± SE 4.4; P = .03).
Fig. 2.
Mean CBF according to diagnostic groups. Mean CBF in mL/100 g/min, adjusted for age and sex. Error bars represent ±SD. ∗P < .05 compared to SCD. Abbreviations: AD, Alzheimer's disease; CBF, cerebral blood flow; MCI, mild cognitive impairment; SCD, subjective cognitive decline.
Across clinical groups, we found an association between higher CBF and higher scores on the MMSE (standardized beta [stβ] 0.19 per mL/100 g/min; P = .02), after correction for age and sex. After additional adjustment for cardiovascular risk factors, or for MRI markers of SVD, the associations remained almost identical (stβ 0.19 per mL/100 g/min; P = .01, and stβ 0.18 per mL/100 g/min; P = .02, respectively). No association of CBF with MMSE was found within each of the diagnostic groups.
Women had a higher CBF than men. Older age was associated with lower CBF. No differences in CBF were found between subjects with cardiovascular risk factors or with signs of SVD on MRI and those without. MTA score was also not associated with CBF in the whole group. Table 2 shows the results of the associations of these demographics with CBF.
Table 2.
Associations of demographical variables and cardiovascular risk factors with cerebral blood flow
| Characteristics | Total (n = 172) | P value |
|---|---|---|
| Age (per 10 years)∗ | −2.1 | .004 |
| Sex† (female) | 0.23 | .003 |
| Hypertension‡ | 0.03 | .71 |
| Hypercholesterolemia‡ | −0.01 | .93 |
| Diabetes mellitus‡ | 0.09 | .23 |
| Smoking‡ | −0.06 | .41 |
| WMH‡,§ | −0.10 | .18 |
| Microbleeds‡ | −0.21 | .10 |
| Lacunes‡ | 0.00 | 1.0 |
| MTA‡,‖ | −0.05 | .52 |
Abbreviations: MTA, medial temporal lobe atrophy; WMH = white matter hyperintensity.
NOTE. Numbers are standardized β's (mL/100 g/min).
Adjusted for sex.
Adjusted for age.
Adjusted for age and sex.
Dichotomized into low (0–1) and high (2–3).
Dichotomized into low (≤1.5) or high (≥1.5).
4. Discussion
In this memory clinic–based population, we found that whole-brain CBF was lower in AD patients compared with SCD patients and that lower CBF was associated with worse cognition across diagnostic groups after adjustment for age and sex. The mean CBF values of MCI patients were in comparable with AD patients.
Our results are in line with previous studies using several other techniques, showing lower CBF in subjects with AD compared to those with SCD [2], [4], [5], [6], [7], [8], [9]. No difference in CBF between MCI and AD subjects was found in the present study. A study investigating CBF changes in relation to predementia stages of AD also showed no difference in whole-brain CBF measured with ASL between patients with AD and stage-2 predementia patients with abnormal CSF amyloid β 1–42 (Aβ) and tau [5]. A different study showed that patients with AD had lower uncorrected CBF and corrected white matter CBF than those with MCI; however, no difference between these groups was found for corrected cortical CBF [4].
The findings from the present study strengthen the notion that CBF measured by PC MRI may be a potential biomarker for AD. The exact moment in the pathophysiological process where CBF becomes impaired relative to other biomarker changes needs yet to be established [8], [9], [29]. A previous study showed that compared to patients with SCD, CBF was lower in predementia patients when both Aβ and tau were abnormal in the CSF (stage 2) but was not lower when only Aβ in the CSF was abnormal (stage 1). This suggests that CBF changes occur further along the disease process than the accumulation of Aβ [5]. Another possible pathway leading to reduced CBF is through the presence of SVD [3]. However, in the present study, no associations between CBF and MTA were found, nor between CBF and SVD or cardiovascular risk factors. This suggests that reduced CBF in AD may be a reflection of other processes, for example, hypometabolism [30].
CBF values in our study were relatively high compared with those previously reported: our mean CBF for patients with SCD was 81.8 mL/100 g/min, whereas the mean CBF measured with ASL reported in the literature for controls is 50 mL/100 g/min [2], [3], [4], [5]. However, it may be possible that this difference is the result of the different approaches used by these techniques for the measuring CBF. ASL has some disadvantages related to its labeling sequences: low spatial resolution, sensitivity to transit time effects, and the low sensitivity to white matter CBF [9]. Most studies using ASL acquire only one postlabel delay time and could therefore confound the measurement of elderly with a decreased blood velocity [31]. PC MRI measures all the blood flowing toward the brain. This may lead to overestimation of whole brain perfusion as a fraction of the blood in the carotid and basilar arteries might go directly to the venous system without exchange with brain tissue [32]. Partial volume effects at the vessel boundary and intravoxel phase dispersion can also lead to a systematic overestimation of CBF measured with 2D PC MRI [33], [34], [35]. However, volume flow estimation is reported to be accurate when the number of voxels across the vessel diameter is three or more [36]. Our results can be considered accurate, as the smallest diameter of a vessel measured was 6 voxels.
No differences in presence of cardiovascular risk factors were found between AD, MCI, and SCD patients. Nonetheless, we decided to do additional adjustments for these factors because from a theoretical standpoint cardiovascular risk factors can be seen as confounders in the association between CBF and diagnostic groups, as it is known that they can have an additive effect in AD development [37].
This study has some limitations that need to be addressed. A limitation of the study was that 26% of the 2D PC images could not be used for the calculation of CBF because the lumen of (one of) the vessels was not distinguishable, leading to the exclusion of these subjects. For several reasons, the placement of a plane perpendicular to the vessels can be difficult for an MRI technician. The shape of the individual vessels can make parallel placement of the slice to all arteries impossible, and sometimes distinguishing each of the vessels can be challenging. In the future, automated slice positioning of the 2D PC flow image may help to improve precision by reducing measurement variability [35], [38]. Although we had to exclude patients because of difficulties with the placement of a plane perpendicular to all three vessels, we were able to find a difference between the patient groups with this technique. A second potential limitation of the present study is that SCD patients consisted of subjects with subjective cognitive complaints rather than healthy subjects from the community. This may have attenuated the difference between AD and MCI patients and patients with SCD. Third, it must be noted that in the present study subjects with MCI and AD were older than subjects with SCD and that older age was associated with lower total CBF in this study. This negative association of CBF with age has also been found in other studies [3], [11], [39], [40], [41]. We adjusted for age, and although some residual confounding by age cannot be excluded, we deem it unlikely that this would have had a large effect on our results. Finally, as a consequence of the cross-sectional study design, we were not able to study the prognostic value of 2D PC–measured CBF with regard to clinical progression, or in the case of the MCI subjects' progression toward AD. A prospective longitudinal study in cognitive intact elderly found a lower CBF using PC MRI at baseline as a predictor of subtle cognitive decline at 18 months, suggesting PC MRI can be used to predict cognitive decline [31]. A strength of our study is the relatively large sample size in each diagnostic group. An additional strength of our study is that we normalized whole-brain CBF for brain volume, a significant potential confounder in some previous studies [6], [11], [12].
Biomarker confirmation of Aβ using amyloid PET or CSF plays an important role in diagnosing AD, although cerebral Aβ deposition does not correlate well with cognitive status or disease stage [42]. Recent studies investigating the association of cognition and CBF measured with ASL have shown that CBF levels do correlate with cognitive impairment. Furthermore, a recent study showed that lower CBF values were associated with faster cognitive decline and might be able to predict future decline in a patient [16]. CBF may therefore be an imaging biomarker of disease severity [4], [5], [16], [17], [43], [44]. Biomarkers for disease severity are important for prognosis and may act as surrogate end points in clinical trials with disease-modifying drugs. In recap, CBF measured with ASL has potential to be important both in clinical as in research setting. In the present study, we have shown that CBF measured with 2D PC MRI also has potential as a marker for disease.
In conclusion, the present study shows that 2D PC MRI can detect differences in CBF between subjects with AD and SCD. 2D PC MRI is relatively cheap, fast, and noninvasive and may therefore be a promising technique for detecting CBF changes in both clinical and research setting.
Research in Context.
-
1.
Systematic review: We searched PubMed for publications regarding cerebral blood flow (CBF) and Alzheimer's disease (AD). We also searched for relevant publications in the reference lists of articles. Evidence suggests that CBF may be used as a determinant of cognitive decline in AD. In the past, several techniques have been used to measure CBF; however, each technique has specific disadvantages prohibiting widespread clinical use. Two-dimensional phase-contrast (2D PC) magnetic resonance imaging (MRI) may be a fast and cheap technique that can be used in clinical settings to measure whole-brain CBF.
-
2.
Interpretation: Our results suggest that 2D PC MRI may be a promising technique for detecting whole-brain CBF changes and that it may be used as an additive diagnostic marker for AD in a clinical setting.
-
3.
Future directions: Longitudinal studies using 2D PC MRI should investigate whether 2D PC MRI can be used to predict future decline in a patient.
Acknowledgments
Research of the VUmc Alzheimer Center is part of the neurodegeneration research program of the Amsterdam Neuroscience. The VUmc Alzheimer Center is supported by Alzheimer Nederland and Stichting VUmc fonds. The clinical database structure was developed with funding from Stichting Dioraphte.
J.F.L., I.S.v.M., and J.P.A.K. have nothing to disclose.
W.M.v.d.F. performs contract research for Boehringer Ingelheim and has been an invited speaker at Boehringer Ingelheim. Research programs of W.M.v.d.F. have been funded by ZonMW, NWO, EU-FP7, Alzheimer Nederland, Cardiovasculair Onderzoek Nederland, Stichting Dioraphte, Gieskes-Strijbis Fonds, Boehringer Ingelheim, Piramal Neuroimaging, Roche BV, Janssen Stellar, and Combinostics. All funding is paid to her institution.
P.S. has acquired grant support (for the institution) from GE Healthcare, Nutricia Research, Piramal, and MERCK. In the past 2 years, he has received consultancy/speaker fees (paid to the institution) from Lilly, Biogen, Novartis, Probiodrug, Roche, and EIP Pharma.
F.B. serves as a consultant for Biogen-Idec, Janssen Alzheimer Immunotherapy, Bayer-Schering, Merck-Serono, Roche, Novartis, Genzyme, and Sanofi-Aventis. F.B. has received sponsoring from EU-H2020, IMDI, SMSR, TEVA, Novartis, Toshiba, and IMI. He serves on the editorial boards of Radiology, Brain, Neuroradiology, MSJ, and Neurology.
N.D.P. serves on the advisory board of Boehringer Ingelheim, Forum, and Probiodrug and has provided consultancy services for Sanofi and Takeda. He has been a speaker at symposia organized by Janssen and Novartis. He has received research support from Alzheimer Nederland (STREAM-VCI project number WE.03-2012-02). N.D.P. is the CEO and co-owner of the Brain Research Center, Amsterdam, the Netherlands.
Footnotes
The authors have declared that no conflict of interest exists.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.dadm.2017.10.001.
Supplementary data
References
- 1.Jack C.R., Jr., Albert M.S., Knopman D.S., McKhann G.M., Sperling R.A., Carrillo M.C. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benedictus M.R., Binnewijzend M.A., Kuijer J.P., Steenwijk M.D., Versteeg A., Vrenken H. Brain volume and white matter hyperintensities as determinants of cerebral blood flow in Alzheimer's disease. Neurobiol Aging. 2014;35:2665–2670. doi: 10.1016/j.neurobiolaging.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Vernooij M.W., van der Lugt A., Ikram M.A., Wielopolski P.A., Vrooman H.A., Hofman A. Total cerebral blood flow and total brain perfusion in the general population: the Rotterdam Scan Study. J Cereb Blood Flow Metab. 2008;28:412–419. doi: 10.1038/sj.jcbfm.9600526. [DOI] [PubMed] [Google Scholar]
- 4.Binnewijzend M.A., Kuijer J.P., Benedictus M.R., van der Flier W.M., Wink A.M., Wattjes M.P. Cerebral blood flow measured with 3D pseudocontinuous arterial spin-labeling MR imaging in Alzheimer disease and mild cognitive impairment: a marker for disease severity. Radiology. 2013;267:221–230. doi: 10.1148/radiol.12120928. [DOI] [PubMed] [Google Scholar]
- 5.Binnewijzend M.A., Benedictus M.R., Kuijer J.P., van der Flier W.M., Teunissen C.E., Prins N.D. Cerebral perfusion in the predementia stages of Alzheimer's disease. Eur Radiol. 2016;26:506–514. doi: 10.1007/s00330-015-3834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roher A.E., Debbins J.P., Malek-Ahmadi M., Chen K., Pipe J.G., Maze S. Cerebral blood flow in Alzheimer's disease. Vasc Health Risk Manag. 2012;8:599–611. doi: 10.2147/VHRM.S34874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alsop D.C., Dai W., Grossman M., Detre J.A. Arterial spin labeling blood flow MRI: its role in the early characterization of Alzheimer's disease. J Alzheimers Dis. 2010;20:871–880. doi: 10.3233/JAD-2010-091699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hays C.C., Zlatar Z.Z., Wierenga C.E. The utility of cerebral blood flow as a biomarker of preclinical Alzheimer's disease. Cell Mol Neurobiol. 2016;36:167–179. doi: 10.1007/s10571-015-0261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wierenga C.E., Hays C.C., Zlatar Z.Z. Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer's disease. J Alzheimers Dis. 2014;42:S411–S419. doi: 10.3233/JAD-141467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spilt A., Box F.M., van der Geest R.J., Reiber J.H., Kunz P., Kamper A.M. Reproducibility of total cerebral blood flow measurements using phase contrast magnetic resonance imaging. J Magn Reson Imaging. 2002;16:1–5. doi: 10.1002/jmri.10133. [DOI] [PubMed] [Google Scholar]
- 11.Spilt A., Weverling-Rijnsburger A.W., Middelkoop H.A., van Der Flier W.M., Gussekloo J., de Craen A.J. Late-onset dementia: structural brain damage and total cerebral blood flow. Radiology. 2005;236:990–995. doi: 10.1148/radiol.2363041454. [DOI] [PubMed] [Google Scholar]
- 12.de Eulate R.G., Goni I., Galiano A., Vidorreta M., Recio M., Riverol M. Reduced cerebral blood flow in mild cognitive impairment assessed using phase-contrast MRI. J Alzheimers Dis. 2017;58:585–595. doi: 10.3233/JAD-161222. [DOI] [PubMed] [Google Scholar]
- 13.Poels M.M., Ikram M.A., Vernooij M.W., Krestin G.P., Hofman A., Niessen W.J. Total cerebral blood flow in relation to cognitive function: the Rotterdam Scan Study. J Cereb Blood Flow Metab. 2008;28:1652–1655. doi: 10.1038/jcbfm.2008.62. [DOI] [PubMed] [Google Scholar]
- 14.Tiehuis A.M., Vincken K.L., van den Berg E., Hendrikse J., Manschot S.M., Mali W.P. Cerebral perfusion in relation to cognitive function and type 2 diabetes. Diabetologia. 2008;51:1321–1326. doi: 10.1007/s00125-008-1041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Flier W.M., Pijnenburg Y.A., Prins N., Lemstra A.W., Bouwman F.H., Teunissen C.E. Optimizing patient care and research: the Amsterdam Dementia Cohort. J Alzheimers Dis. 2014;41:313–327. doi: 10.3233/JAD-132306. [DOI] [PubMed] [Google Scholar]
- 16.Benedictus M.R., Leeuwis A.E., Binnewijzend M.A., Kuijer J.P., Scheltens P., Barkhof F. Lower cerebral blood flow is associated with faster cognitive decline in Alzheimer's disease. Eur Radiol. 2017;27:1169–1175. doi: 10.1007/s00330-016-4450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leeuwis A.E., Benedictus M.R., Kuijer J.P.A., Binnewijzend M.A.A., Hooghiemstra A.M., Verfaillie S.C.J. Lower cerebral blood flow is associated with impairment in multiple cognitive domains in Alzheimer's disease. Alzheimers Dement. 2017;13:531–540. doi: 10.1016/j.jalz.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 18.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 19.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen R.C. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 21.Petersen R.C., Stevens J.C., Ganguli M., Tangalos E.G., Cummings J.L., DeKosky S.T. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 22.Scheltens P., Launer L.J., Barkhof F., Weinstein H.C., van Gool W.A. Visual assessment of medial temporal lobe atrophy on magnetic resonance imaging: interobserver reliability. J Neurol. 1995;242:557–560. doi: 10.1007/BF00868807. [DOI] [PubMed] [Google Scholar]
- 23.Fazekas F., Chawluk J.B., Alavi A., Hurtig H.I., Zimmerman R.A. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 24.Smith S.M. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y., Brady M., Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 27.Bernstein M.A., Zhou X.J., Polzin J.A., King K.F., Ganin A., Pelc N.J. Concomitant gradient terms in phase contrast MR: analysis and correction. Magn Reson Med. 1998;39:300–308. doi: 10.1002/mrm.1910390218. [DOI] [PubMed] [Google Scholar]
- 28.Lankhaar J.W., Hofman M.B., Marcus J.T., Zwanenburg J.J., Faes T.J., Vonk-Noordegraaf A. Correction of phase offset errors in main pulmonary artery flow quantification. J Magn Reson Imaging. 2005;22:73–79. doi: 10.1002/jmri.20361. [DOI] [PubMed] [Google Scholar]
- 29.Poels M.M., Ikram M.A., van der Lugt A., Hofman A., Niessen W.J., Krestin G.P. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology. 2012;78:326–333. doi: 10.1212/WNL.0b013e3182452928. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y., Wolk D.A., Reddin J.S., Korczykowski M., Martinez P.M., Musiek E.S. Voxel-level comparison of arterial spin-labeled perfusion MRI and FDG-PET in Alzheimer disease. Neurology. 2011;77:1977–1985. doi: 10.1212/WNL.0b013e31823a0ef7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xekardaki A., Rodriguez C., Montandon M.L., Toma S., Tombeur E., Herrmann F.R. Arterial spin labeling may contribute to the prediction of cognitive deterioration in healthy elderly individuals. Radiology. 2015;274:490–499. doi: 10.1148/radiol.14140680. [DOI] [PubMed] [Google Scholar]
- 32.Vestergaard M.B., Lindberg U., Aachmann-Andersen N.J., Lisbjerg K., Christensen S.J., Rasmussen P. Comparison of global cerebral blood flow measured by phase-contrast mapping MRI with 15 O-H2 O positron emission tomography. J Magn Reson Imaging. 2017;45:692–699. doi: 10.1002/jmri.25442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lagerstrand K.M., Lehmann H., Starck G., Vikhoff-Baaz B., Ekholm S., Forssell-Aronsson E. Method to correct for the effects of limited spatial resolution in phase-contrast flow MRI measurements. Magn Reson Med. 2002;48:883–889. doi: 10.1002/mrm.10288. [DOI] [PubMed] [Google Scholar]
- 34.Dolui S., Wang Z., Wang D.J., Mattay R., Finkel M., Elliott M. Comparison of non-invasive MRI measurements of cerebral blood flow in a large multisite cohort. J Cereb Blood Flow Metab. 2016;36:1244–1256. doi: 10.1177/0271678X16646124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng S.L., Su P., Wang F.N., Cao Y., Zhang R., Lu H. Optimization of phase-contrast MRI for the quantification of whole-brain cerebral blood flow. J Magn Reson Imaging. 2015;42:1126–1133. doi: 10.1002/jmri.24866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hofman M.B., Visser F.C., van Rossum A.C., Vink Q.M., Sprenger M., Westerhof N. In vivo validation of magnetic resonance blood volume flow measurements with limited spatial resolution in small vessels. Magn Reson Med. 1995;33:778–784. doi: 10.1002/mrm.1910330606. [DOI] [PubMed] [Google Scholar]
- 37.Bangen K.J., Nation D.A., Clark L.R., Harmell A.L., Wierenga C.E., Dev S.I. Interactive effects of vascular risk burden and advanced age on cerebral blood flow. Front Aging Neurosci. 2014;6:159. doi: 10.3389/fnagi.2014.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu P., Lu H., Filbey F.M., Pinkham A.E., McAdams C.J., Adinoff B. Automatic and reproducible positioning of phase-contrast MRI for the quantification of global cerebral blood flow. PLoS One. 2014;9:e95721. doi: 10.1371/journal.pone.0095721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bangen K.J., Restom K., Liu T.T., Jak A.J., Wierenga C.E., Salmon D.P. Differential age effects on cerebral blood flow and BOLD response to encoding: associations with cognition and stroke risk. Neurobiol Aging. 2009;30:1276–1287. doi: 10.1016/j.neurobiolaging.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leenders K.L., Perani D., Lammertsma A.A., Heather J.D., Buckingham P., Healy M.J.R. Cerebral blood-flow, blood-volume and oxygen utilization–normal values and effect of age. Brain. 1990;113:27–47. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]
- 41.Buijs P.C., Krabbe-Hartkamp M.J., Bakker C.J., de Lange E.E., Ramos L.M., Breteler M.M. Effect of age on cerebral blood flow: measurement with ungated two-dimensional phase-contrast MR angiography in 250 adults. Radiology. 1998;209:667–674. doi: 10.1148/radiology.209.3.9844657. [DOI] [PubMed] [Google Scholar]
- 42.Jack C.R., Jr., Lowe V.J., Weigand S.D., Wiste H.J., Senjem M.L., Knopman D.S. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wierenga C.E., Dev S.I., Shin D.D., Clark L.R., Bangen K.J., Jak A.J. Effect of mild cognitive impairment and APOE genotype on resting cerebral blood flow and its association with cognition. J Cereb Blood Flow Metab. 2012;32:1589–1599. doi: 10.1038/jcbfm.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kitagawa K., Oku N., Kimura Y., Yagita Y., Sakaguchi M., Hatazawa J. Relationship between cerebral blood flow and later cognitive decline in hypertensive patients with cerebral small vessel disease. Hypertens Res. 2009;32:816–820. doi: 10.1038/hr.2009.100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.