It is becoming increasingly understood that the current paradigms of in vitro antimicrobial susceptibility testing may have significant shortcomings in predicting activity in vivo. This study evaluated the activity of several antibiotics alone and in combination against clinical isolates of Salmonella enterica serotype Newport (meningitis case) utilizing both conventional and physiological media. In addition, the interactions of these antibiotics with components of the innate immune system were evaluated. Azithromycin, which has performed quite well clinically despite high MICs in conventional media, was shown to be more active in physiological media and to enhance innate immune system killing. Alternatively, chloramphenicol did not show enhanced immune system killing, paralleling its inferior clinical performance to other antibiotics that have been used to treat Salmonella meningitis. These findings are important additions to the building understanding of current in vitro antimicrobial assay limitations that hopefully will amount to future improvements in these assays to better predict clinical efficacy and activity in vivo.
KEYWORDS: cathelicidin, innate immunity, meningitis, Salmonella
ABSTRACT
This study examines the pharmacodynamics of antimicrobials that are used to treat Salmonella with each other and with key components of the innate immune system. Antimicrobial synergy was assessed using time-kill and checkerboard assays. Antimicrobial interactions with innate immunity were studied by employing cathelicidin LL-37, whole-blood, and neutrophil killing assays. Ceftriaxone and ciprofloxacin were found to be synergistic in vitro against Salmonella enterica serotype Newport. Ceftriaxone, ciprofloxacin, and azithromycin each demonstrated synergy with the human cathelicidin defense peptide LL-37 in killing Salmonella. Exposure of Salmonella to sub-MICs of ceftriaxone resulted in enhanced susceptibility to LL-37, whole blood, and neutrophil killing. The activity of antibiotics in vivo against Salmonella may be underestimated in bacteriologic media lacking components of innate immunity. The pharmacodynamic interactions of antibiotics used to treat Salmonella with each other and with components of innate immunity warrant further study in light of recent findings showing in vivo selection of antimicrobial resistance by single agents in this pathogen.
IMPORTANCE It is becoming increasingly understood that the current paradigms of in vitro antimicrobial susceptibility testing may have significant shortcomings in predicting activity in vivo. This study evaluated the activity of several antibiotics alone and in combination against clinical isolates of Salmonella enterica serotype Newport (meningitis case) utilizing both conventional and physiological media. In addition, the interactions of these antibiotics with components of the innate immune system were evaluated. Azithromycin, which has performed quite well clinically despite high MICs in conventional media, was shown to be more active in physiological media and to enhance innate immune system killing. Alternatively, chloramphenicol did not show enhanced immune system killing, paralleling its inferior clinical performance to other antibiotics that have been used to treat Salmonella meningitis. These findings are important additions to the building understanding of current in vitro antimicrobial assay limitations that hopefully will amount to future improvements in these assays to better predict clinical efficacy and activity in vivo.
INTRODUCTION
Salmonellosis is one of the most common causes of diarrhea worldwide. Global estimates are 90,000 deaths from nontyphoidal and 180,000 deaths from typhoidal salmonellosis, while annual estimates in the United States are that about 1.2 million cases and 450 deaths occur from nontyphoidal salmonellosis (1, 2).
Salmonella meningitis is an unusual complication of salmonellosis in developed countries, although it is an emerging threat in resource-poor parts of the world. Most cases described in the literature occur in very young children or otherwise immunocompromised adults and are most commonly caused by typhoidal strains (3–5). However, rare cases have been described in immunocompetent adults, including in one neurosurgical patient (6, 7). Outcomes are generally poor, and treatment is not standardized due to the small number of reported cases (8). Nontyphoidal Salmonella meningitis in immunocompetent adults is exceedingly rare, and as a result, therapy is even less defined. Given the very complex pharmacodynamic interactions between coadministered antibiotics and endogenous immune factors, including the potential selection of antimicrobial resistance (9), we examined how different antibiotics influence Salmonella susceptibility to killing by cathelicidin LL-37, a critical immune component in meningeal infection (10, 11), as well as whole blood and neutrophils.
RESULTS
Antimicrobial susceptibility and synergy assays.
Susceptibility testing was performed for cerebrospinal fluid (CSF) and blood Salmonella strains using cation-adjusted Mueller-Hinton broth (CA-MHB) and RPMI 1640 medium supplemented with 10% Luria-Bertani broth (RPMI+10% LB). MIC results for ceftriaxone (CRO), ciprofloxacin (CIP), azithromycin (AZM), chloramphenicol (CM), and cathelicidin LL-37 are shown in Table 1. The respective MICs in RPMI+10% LB and CA-MHB of CRO (0.063 versus 0.125 mg/liter), CIP (0.0125 versus 0.05 mg/liter), and AZM (4 versus >32 mg/liter) were consistently lower in the physiologic medium. No difference in MIC was observed for CM (0.5 mg/liter) between the two media.
TABLE 1 .
MIC of antimicrobials against Salmonella strains from CSF and blood
|
Salmonella
sample |
MIC in mediuma |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| CRO (mg/liter) |
CIP (mg/liter) |
AZM (mg/liter) |
CM (mg/liter) |
LL-37 in R (μM)b |
|||||
| M | R | M | R | M | R | M | R | ||
| CSF | 0.125 | 0.063 | 0.05 | 0.0125 | >32 | 4 | 0.5 | 0.5 | 32 |
| Blood | 0.125 | 0.063 | 0.05 | 0.0125 | >32 | 4 | 0.5 | 0.5 | 32 |
CRO, ceftriaxone; CIP, ciprofloxacin; AZM, azithromycin; CM, chloramphenicol; M, cation-adjusted Mueller-Hinton broth; R, RPMI+10% LB.
LL-37 testing was performed only in RPMI-LB, as it is not active in MHB.
Kill curves at 2×, 4×, and 8× MIC were performed for CRO, CIP, and CM in both CA-MHB and RPMI+10% LB and for AZM in RPMI+10% LB for the CSF Salmonella isolate. The results in Fig. S1 in the supplemental material demonstrate bactericidal activity for all drugs except CM, which was bacteriostatic.
Kill curves against CSF Salmonella enterica strain in MHB (top row) and RPMI+10% LB (bottom row) using various antibiotics at 2×, 4×, and 8× MIC. Azithromycin was assayed only in RPMI+10% LB, as it was inactive in MHB. Download FIG S1, PDF file, 0.1 MB (108.7KB, pdf) .
Copyright © 2017 Sakoulas et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
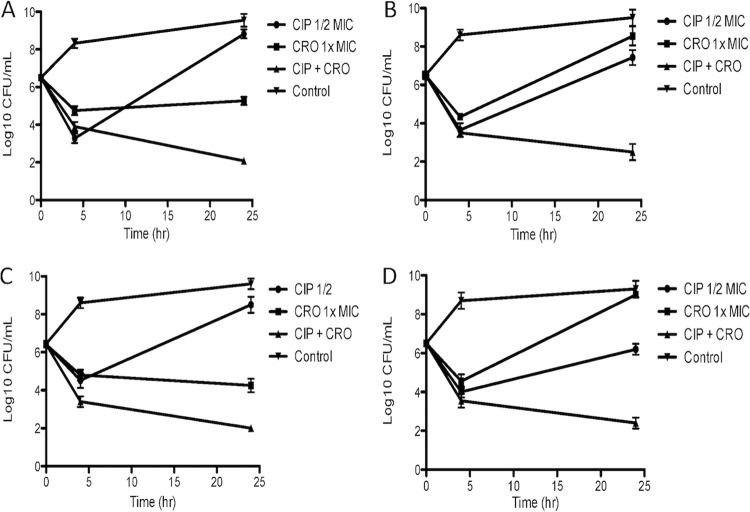
Checkerboard studies between CRO, CIP, and AZM are shown in Table 2, with synergy exhibited between (i) CRO and CIP in RPMI+10% LB, (ii) CRO and CIP in CA-MHB, and (iii) CRO and AZM in RPMI+10% LB based on fractional inhibitory concentration index (FICI). Additionally, time-kill assays using both standard bacteriologic CA-MHB and supplemented mammalian tissue culture medium RPMI+10% LB were performed to assess synergy and bactericidal activity for CIP+CRO, and the results are shown in Fig. 1. For both CSF and blood Salmonella isolates, the combination CRO+CIP in both CA-MHB and RPMI+10% LB demonstrated bactericidal activity (>3-log10-CFU/ml killing), whereas each drug alone resulted in either growth or stasis at the concentrations used (1× MIC of CRO, 1/2 MIC of CIP [Fig. 1]).
TABLE 2 .
FICIs of checkerboard assays between antibiotics using a Salmonella CSF isolate
| Combinationa | FICI in: |
|
|---|---|---|
| RPMI | MHB | |
| CRO+CIP | 0.3125 | 0.50 |
| CRO+AZM | 0.50 | NAb |
| CIP+AZM | 1 | NA |
| CRO+LL-37 | 0.5 | NA |
| CIP+LL-37 | 0.5 | NA |
| AZM+LL-37 | 0.75 | NA |
| CM+LL-37 | 1 | NA |
CRO, ceftriaxone; CIP, ciprofloxacin; AZM, azithromycin; CM, chloramphenicol.
NA, not applicable, as AZM and LL-37 are not active in MHB.
FIG 1 .
Representative killing assays combining 1× MIC of ceftriaxone (CRO) with 1/2 MIC of ciprofloxacin (CIP) for a Salmonella CSF isolate (MHB in panel A and RPMI+10% LB in panel B) and bloodstream isolate (MHB in panel C and RPMI+10% LB in panel D).
Pharmacodynamic interaction between human cathelicidin LL-37 and administered antibiotics.
Both the CSF and bloodstream Salmonella enterica isolates showed an LL-37 MIC of 32 μM (Table 1).
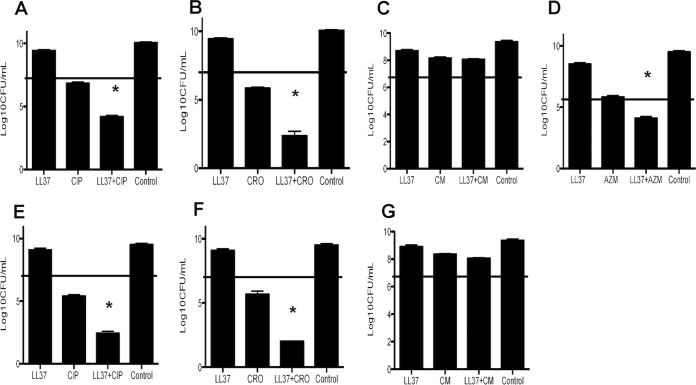
The pharmacodynamic relationships between various antibiotics (CRO, CIP, AZM, and CM) and LL-37 were first determined by checkerboard assays. Results in Table 2 demonstrate synergy between CRO and LL-37 (FICI = 0.5) and CIP and LL-37 (FICI = 0.5) and additivity for AZM+LL-37 (FICI = 0.75) and CM+LL-37 (FICI = 1). Killing assays using 1/2 MIC of LL-37 (16 μM), with or without 1/2 MIC of CIP, CRO, AZM, and CM, were performed. The killing assay results in Fig. 2 demonstrate a 2- to 3-log10-CFU/ml reduction of viable bacteria at 6 h for LL-37+CIP, LL-37+CRO, and LL-37+AZM. However, there was no killing observed when combining LL-37 and CM.
FIG 2 .
Assessment of activity of LL-37 (16 mM) in combination with various antibiotics against Salmonella. Shown are results of CFU counts of a Salmonella enterica CSF isolate (A, B, C, and D) or blood isolate (E, F, and G) in the presence of 1/2 MIC of LL-37 and 1/2 MIC of antibiotic after 6 h. The starting inoculum is shown by the horizontal line. CIP, ciprofloxacin; CRO, ceftriaxone; CM, chloramphenicol; AZM, azithromycin; Control, medium alone. *, P < 0.05 versus either agent alone.
We next assessed the influence of overnight pretreatment of Salmonella enterica with sub-MICs of CRO, CIP, or CM on subsequent bacterial killing by LL-37. Figure 3 demonstrates that growth in sub-MIC CRO greatly sensitizes the pathogen to killing by LL-37 in both strains (P = 0.002 for the CSF isolate and P = 0.006 for the blood isolate versus the paired antibiotic-untreated control). However, this phenomenon was not observed for CIP or CM, which attenuated the killing activity of LL-37. This achieve significance only with chloramphenicol against the CSF strain (P = 0.04 versus control).
FIG 3 .
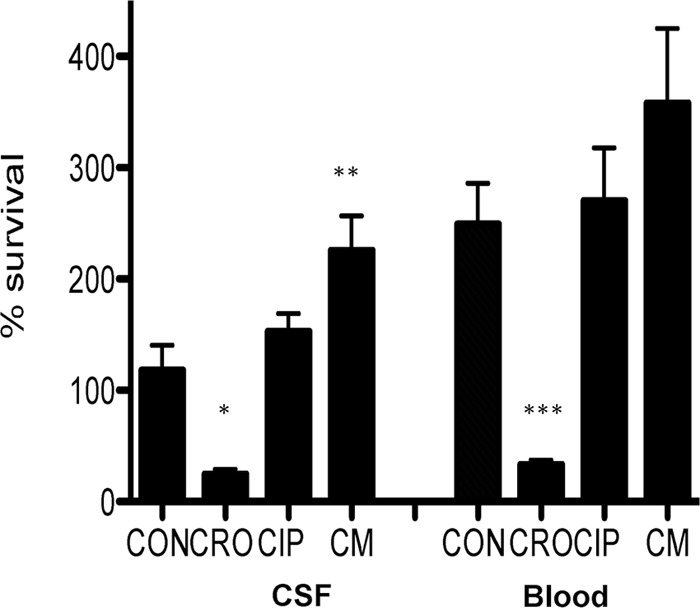
Assessment of activity of LL-37 (16 mM) against CSF and blood isolates from a patient with Salmonella enterica meningitis after growth in LB broth containing 0.05 mg/liter ceftriaxone (CRO), 0.0125 mg/liter ciprofloxacin (CIP), or 0.5 mg/liter chloramphenicol (CM) compared to antibiotic-free LB (control [CON]). *, P = 0.002 versus control; **, P = 0.04 versus control; ***, P = 0.006 versus control.
Antibiotic exposure and sensitization to innate immune-mediated killing.
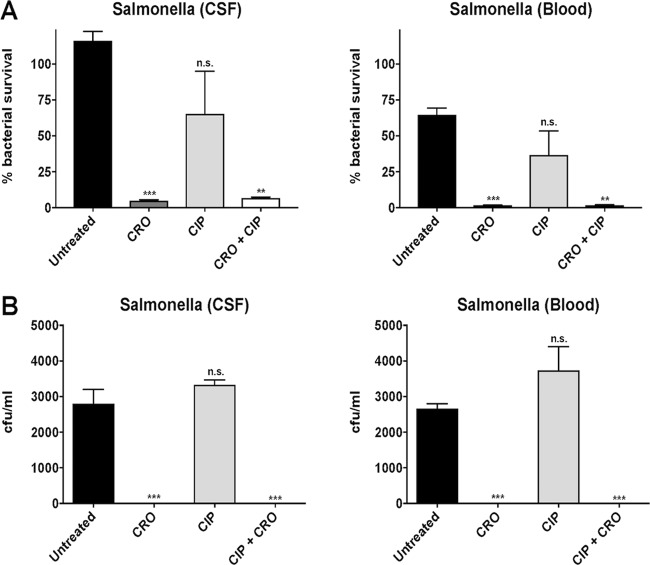
Neutrophil killing assays of CSF and blood Salmonella isolates were performed following exposure to antibiotic-free medium (untreated) or pretreatment with 1/4 MIC of CRO, 1/4 MIC of CIP, or 1/4 MIC of CM. CRO sensitizes Salmonella enterica serotype Newport to neutrophil killing, whereas CM and CIP do not (Fig. 4). Sub-MIC concentrations of CRO and CRO+CIP sensitize Salmonella to neutrophil killing (Fig. 5A) and whole-blood killing (Fig. 5B), whereas CIP alone does not. Additionally, neutrophil killing assays of the Salmonella isolates pretreated with 1/4 MIC LL-37 were performed following exposure to antibiotic-free medium (untreated) or 1× MIC of CRO, 1× MIC of CIP, or 1× MIC of CM (conditions predetermined by pilot studies). Pretreatment with LL-37 notably sensitized the patient’s Salmonella enterica serotype Newport isolates to neutrophil killing in the presence of 1× MIC of CRO, 1× MIC of CIP, and 1× MIC of CM (Fig. 6).
FIG 4 .
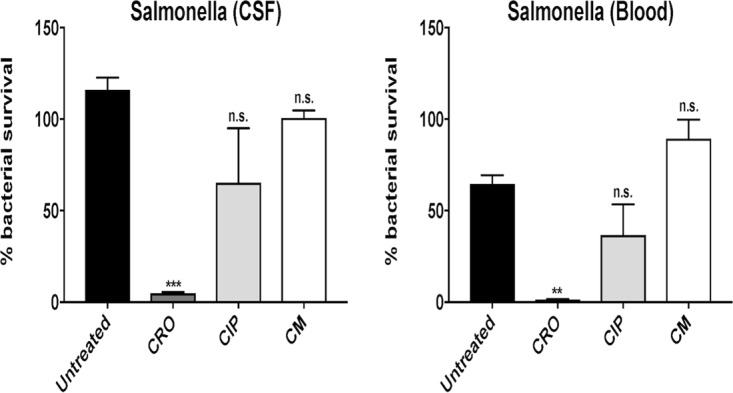
Neutrophil killing assays of CSF and blood Salmonella isolates were performed following exposure to antibiotic-free medium (untreated) or pretreatment with 1/4 MIC of ceftriaxone (CRO), 1/4 MIC of ciprofloxacin (CIP), or 1/4 MIC of chloramphenicol (CM). CRO sensitizes Salmonella enterica serotype Newport to neutrophil killing. Data are plotted as mean ± SEM and represent the percentage of bacterial survival remaining following 15 min of neutrophil exposure compared to the initial inoculum. **, P < 0.005, and n.s., no statistical significance, by two-way analysis of variance (ANOVA) and comparing pretreated bacteria to untreated bacteria.
FIG 5 .
The percentage of bacterial survival and number of colony-forming units per milliliter of bacteria recovered from (A) neutrophil killing and (B) whole-blood killing assays, respectively, were significantly reduced for Salmonella enterica serotype Newport CSF and blood isolates pretreated with 1/4 MIC of ceftriaxone (CRO) and 1/4 MIC of CRO + 1/4 MIC of ciprofloxacin (CIP) but not 1/4 MIC of CIP alone. Data are plotted as mean ± SEM and represent the percentage of bacterial survival remaining following 15 min of neutrophil exposure compared to the initial inoculum or CFU per milliliter recovered following 60 min of exposure to whole blood. **, P < 0.005, ***, P < 0.0005, and n.s., no statistical significance, by two-way ANOVA and comparing pretreated bacteria to untreated bacteria.
FIG 6 .
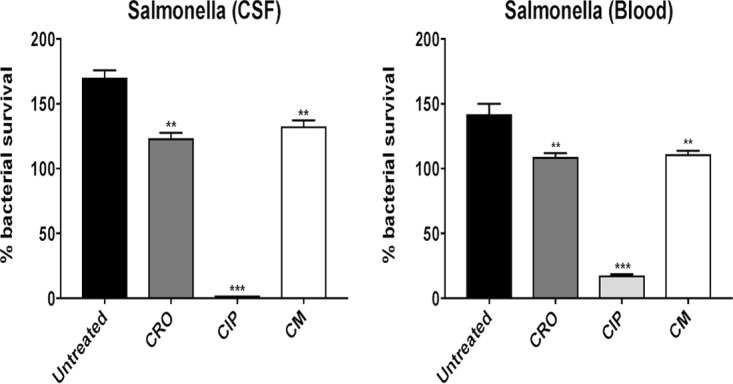
Neutrophil killing assays of CSF and blood Salmonella isolates pretreated with 1/4 MIC of LL-37 were performed following exposure to antibiotic-free medium (untreated) or 1× MIC of ceftriaxone (CRO), 1× MIC of ciprofloxacin (CIP), or 1× MIC of chloramphenicol (CM). Pretreatment with LL-37 sensitizes Salmonella enterica serotype Newport to neutrophil killing in the presence of 1× MIC of CRO, 1× MIC of CIP, and 1× MIC of CM. The percentage of bacterial survival was most prominently reduced in the presence of CIP. Data are plotted as mean ± SEM and represent the percentage of bacterial survival remaining following 15 min of neutrophil exposure compared to the initial inoculum. **, P < 0.005, ***, P < 0.0005, and n.s., no statistical significance, by two-way ANOVA and comparing bacteria with antibiotic to bacteria without antibiotic (untreated).
DISCUSSION
Limitations of in vitro antimicrobial susceptibility testing methods as predictors of in vivo treatment efficacy are increasingly being recognized (12, 13). Such limitations in assays traditionally performed in bacterial growth media stem from two basic interrelated concepts: (i) the inability of bacterial media to replicate the in vivo environment (e.g., pH, availability of nutrients, and drug permeability) and (ii) the absence of host innate immunity factors that likely have important pharmacodynamic interactions with administered antibiotics. For example, we have previously shown the clinical importance of β-lactams as adjunctive therapy in treating β-lactam-resistant Gram-positive pathogens, such as methicillin-resistant Staphylococcus aureus (14, 15) and vancomycin-resistant Enterococcus (16), and that the macrolide antibiotic AZM may demonstrate excellent in vivo activity against some resistant Gram-negative bacteria (17, 18). A recent study of Salmonella that demonstrated tissue-specific selection of antimicrobial-resistant subpopulations that may serve as a reservoir of treatment failure that escapes detection by standard bacteriological media (9) provides further evidence that the paradigms of antimicrobial susceptibility testing established decades ago require a reevaluation for improvement.
While antimicrobials used to treat invasive S. enterica serovar Typhimurium infections have been examined extensively in vitro and in animal models of infection (19), the role of host immunity factors in enhancing their activities has not been examined. In this study, we found (i) consistent synergy between CRO and CIP (the agents used to successfully treat the patient) against both the CSF and blood isolates in physiologic RPMI+10% LB by checkerboard and time-kill assays, (ii) synergistic killing of Salmonella by combinations of sub-MICs of LL-37 and CRO, CIP, and AZM, with a most pronounced effect between CRO and LL-37, and (iii) sensitization to killing of Salmonella by human neutrophils and whole blood after growth in 1/4 MICs of CRO and CRO+CIP, but not CIP or CM. CM, a bacteriostatic agent against the Salmonella strains tested, showed no synergy with LL-37.
Due to its rarity, the treatment of Salmonella meningitis is far from standardized. In the largest assessment to date, Owusu-Ofori and Scheld describe 126 cases: most infections were due to Salmonella Typhimurium, and all but 2 occurred in children less than 2 years of age (4). In this retrospective analysis, clinical outcomes were significantly better in patients receiving 3rd-generation cephalosporins, quinolones, or imipenem compared to conventional therapies such as CM, clotrimoxazole, or ampicillin. Among the latter group, CM (the only bacteriostatic agent examined in this study) fared the most poorly in that 35 out of 54 patients (65%) relapsed or died (4). This compared to successful treatment in 83% and 89% of patients receiving 3rd-generation cephalosporins and quinolones, respectively (4). Paralleling this inferior clinical performance, the results from this study with CM showed (i) bacteriostatic activity at 2×, 4×, and 8× MIC in both RPMI+10% LB and CA-MHB media and (ii) additive activity with LL-37 (FICI = 1). This contrasted with CRO and CIP, which were each noted to have bactericidal activity at 2×, 4×, and 8× MIC in both RPMI+10% LB and CA-MHB media and synergy in combination with LL-37 (FICI = 0.5). While prior work has shown antagonism of LL-37 activity by bacteriostatic antibiotics against Escherichia coli and S. aureus (20), the lack of synergy of CM with LL-37 demonstrates another collateral benefit of bactericidal antibiotics in that they enhance bacterial killing by innate immune mechanisms. These data highlight the importance of antimicrobial activity beyond the “susceptible versus resistant” designation reported by clinical microbiology laboratories when treating serious infections such as meningitis.
The effects of various antibiotics on the sensitivity of Salmonella to killing by components of human innate immunity were of interest. Cathelicidin LL-37 is a critical host defense antimicrobial peptide in the pathogenesis of bacterial meningitis, and therefore cooperative activity between LL-37 and the antibiotics used to treat meningitis is anticipated to be an important parameter to ensure successful treatment (10). While this has not been extensively studied, we previously found that ceftaroline demonstrated this synergy with LL-37 to a much greater degree than vancomycin, paralleling its superior performance in a difficult case of Streptococcus pneumoniae meningitis (11). In the present study, we found that pretreatment of the Salmonella isolates with 1/2 MIC of CRO, CIP, or AZM enhanced killing by LL-37 but resulted in either stasis or growth alone (Fig. 2).
It is worth mentioning that AZM has been used successfully to treat enteric fever due to Salmonella (21). While the relationship of MICs measured in standard microbiological media such as CA-MHB to clinical outcome is not entirely clear, the demonstration of a severalfold reduction in AZM MIC in RPMI+10% LB medium suggests that further clinical-microbiological correlation studies with AZM are warranted. A recent study demonstrated that the addition of bicarbonate to standard bacteriological media approximates susceptibility testing results obtained in physiologic media (13). The same investigators demonstrated that the susceptibility results obtained using bicarbonate-buffered bacteriological media or physiologic media were more reflective of antimicrobial performance in vivo using animal models of infection (13). It appears the in vivo activity of AZM against Salmonella may be vastly underappreciated using conventional antimicrobial susceptibility methods, a theme that appears to be recurring with Gram-negative bacteria given previously published data (17, 18, 22). A more accurate assessment of antibiotic activity in vivo may be appreciated by considering antibiotic interactions with various components of the innate immune system, understanding variability in antibiotic activity based on bacterial inoculum, and utilizing susceptibility testing conditions that better recapitulate the host environment, including medium type and pH.
MATERIALS AND METHODS
Bacterial strains.
The Salmonella enterica serotype Newport strains examined in this study were the bloodstream and cerebrospinal fluid (CSF) isolates from a patient who was successfully treated and whose lumboperitoneal shunt was salvaged without removal using CRO and CIP.
Antimicrobial susceptibility testing.
CIP, CRO, and CM were purchased from Sigma-Aldrich (St. Louis, MO). AZM in 500-mg lyophilized vials was purchased from Baxter Healthcare (Deerfield, IL). Human cathelicidin LL-37 was purchased from AnaSpec, Inc. (Fremont, CA), diluted to a stock concentration of 640 μM, and stored as frozen single-use 50-μl aliquots. Antimicrobial susceptibility testing and checkerboard assays were performed in cation-adjusted Mueller-Hinton broth (CA-MHB) and in RPMI 1640 medium supplemented with 10% Luria-Bertani broth (RPMI+10% LB) according to CLSI methods using freshly thawed bacteria from the −80°C freezer plated on Todd-Hewitt agar (THA) (23). Checkerboard assays (8 by 8) were run twice, and the results were interpreted by calculating the fractional inhibitory concentration index (FICI). The FICI is calculated using the formula FICabx1 + FICabx2 = FICI, where FICabx1 = the MIC of antibiotic 1 in combination divided by the MIC of antibiotic 1 alone and FICabx2 = the MIC of antibiotic 2 in combination divided by the MIC of antibiotic 2 alone. An FICI of <0.5 defines synergy, >0.5 to <1.0 additivity, >1 to <4.0 indifference, and >4.0 antagonism (24). In vitro killing assays were performed with single drugs and in various combinations using CA-MHB or RPMI+10% LB with starting inocula of approximately 5 × 106 CFU/ml, and sampling was performed at 4 and 24 h. LL-37 killing assays were performed in RPMI+10% LB using starting inocula of 5 × 105 CFU/ml as previously described. Bacterial survival was assessed at 2 h in the LL-37 killing assays on antibiotic-exposed bacteria and at 6 h in the studies involving LL-37 combined with antibiotics. For the experiments examining the effects of antibiotics on sensitization to LL-37 killing, bacteria were grown overnight in CA-MHB, pelleted, washed once in phosphate-buffered saline (PBS), and then resuspended in fresh RPMI+10% LB for addition to LL-37 killing assays. Bacterial counts were enumerated on THA plates.
Neutrophil killing assays.
Human neutrophils were isolated from healthy donors using the PolymorphPrep system (Axis-Shield) under protocols approved by the University of California San Diego Human Subjects Institutional Review Board for use in established bacterial killing assays with minor modifications (14). Neutrophils were used to seed a 96-well plate (2 × 105 cells/well). In experiment A, cells were infected at a multiplicity of infection (MOI) of 10 with Salmonella CSF or blood isolates that were grown overnight without antibiotic or with antibiotics (1/4 MIC of CRO, 1/4 MIC of CIP, 1/4 MIC of CM, or 1/4 MIC CRO + 1/4 MIC of CIP). In experiment B, cells were infected at a multiplicity of infection (MOI) of 10 with Salmonella CSF or blood isolates grown to stationary phase overnight with 1/4 MIC of LL-37 and then exposed to neutrophils without antibiotics or with antibiotics (1× MIC of CRO, 1× MIC of CIP, or 1× MIC of CM). After incubation for 15 min at 37°C and 5% CO2, the cells were lysed with 0.025% Triton X-100 and serially diluted in phosphate-buffered saline (PBS), and the total number of remaining bacteria was enumerated on Luria agar (LA) plates. The percentage of bacterial survival at 15 min was calculated as the mean ± standard error of the mean (SEM) of the initial inoculum.
Whole-blood killing assay.
Blood from healthy donors was collected using heparinized syringes. Salmonella CSF or blood isolates were grown to stationary phase overnight without antibiotic or with antibiotic (1/4 MIC of CRO, 1/4 MIC of CIP, 1/4 MIC of CM, or 1/4 MIC of CRO + 1/4 MIC of CIP). A total of 2 × 103 CFU of the stationary-phase bacteria were mixed with 200 μl of whole human blood in siliconized tubes and incubated at 37°C with orbital rotation for 60 min, 25-µl aliquots were removed, and cells were lysed with 0.025% Triton X-100, serially diluted in PBS, and plated onto LA for enumeration of surviving CFU (25, 26). These studies were approved by the University of California San Diego Human Research Protections Program.
ACKNOWLEDGMENTS
This research was supported by 1U01AI124316-01 National Institutes of Health grant HD071600 (G.S. and V.N.).
G.S. has received speaking honoraria from Allergan, Sunovion, and the Medicines Company and consulting fees from Allergan. V.N. has received research grant support from Roche Pharma and is on the Scientific Advisory Board of Cidara Therapeutics.
REFERENCES
- 1.Centers for Disease Control and Prevention Salmonella. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/salmonella/general/technical.html. Accessed 25 August 2017. [Google Scholar]
- 2.GBD 2015 Mortality and Causes of Death Collaborators 2016. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu HM, Huang WY, Lee ML, Yang AD, Chaou KP, Hsieh LY. 2011. Clinical features, acute complications, and outcome of Salmonella meningitis in children under one year of age in Taiwan. BMC Infect Dis 11:30. doi: 10.1186/1471-2334-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owusu-Ofori A, Scheld WM. 2003. Treatment of Salmonella meningitis: two case reports and a review of the literature. Int J Infect Dis 7:53–60. doi: 10.1016/S1201-9712(03)90043-9. [DOI] [PubMed] [Google Scholar]
- 5.Keddy KH, Sooka A, Musekiwa A, Smith AM, Ismail H, Tau NP, Crowther-Gibson P, Angulo FJ, Klugman KP. 2015. Clinical and microbiological features of Salmonella meningitis in a South African population, 2003–2013. Clin Infect Dis 61:S272–S282. doi: 10.1093/cid/civ685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aissaoui Y, Azendour H, Balkhi H, Haimeur C, Atmani M. 2006. Postoperative meningitis caused by an unusual etiological agent: Salmonella enteritidis. Neurochirurgie 52:547–550. doi: 10.1016/S0028-3770(06)71365-0. [DOI] [PubMed] [Google Scholar]
- 7.Metan G, Alp E, Esel D, Aygen B, Sumerkan B. 2005. Salmonella enteritidis: an unusual meningitis agent in an adult patient. Mikrobiyol Bul 2005:509–512. [PubMed] [Google Scholar]
- 8.Price EH, de Louvois J, Workman MR. 2000. Antibiotics for Salmonella meningitis in children. J Antimicrob Chemother 46:653–655. doi: 10.1093/jac/46.5.653. [DOI] [PubMed] [Google Scholar]
- 9.Kubicek-Sutherland JZ, Heithoff DM, Ersoy SC, Shimp WR, House JK, Marth JD, Smith JW, Mahan MJ. 2015. Host-dependent induction of transient antibiotic resistance: a prelude to treatment failure. EBioMedicine 2:1169–1178. doi: 10.1016/j.ebiom.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merres J, Höss J, Albrecht LJ, Kress E, Soehnlein O, Jansen S, Pufe T, Tauber SC, Brandenburg LO. 2014. Role of the cathelicidin-related antimicrobial peptide in inflammation and mortality in a mouse model of bacterial meningitis. J Innate Immun 6:205–218. doi: 10.1159/000353645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakoulas G, Nonejuie P, Kullar R, Pogliano J, Rybak MJ, Nizet V. 2015. Examining the use of ceftaroline in the treatment of Streptococcus pneumoniae meningitis with reference to human cathelicidin LL-37. Antimicrob Agents Chemother 59:2428–2431. doi: 10.1128/AAC.04965-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doern GV, Brecher SM. 2011. The clinical predictive value (or lack thereof) of the results of in vitro antimicrobial susceptibility tests. J Clin Microbiol 49:S11–S14. doi: 10.1128/JCM.00580-11. [DOI] [Google Scholar]
- 13.Ersoy SC, Heithoff DM, Barnes L, Tripp GK, House JK, Marth JD, Smith JW, Mahan MJ. 2017. Correcting a fundamental flaw in the paradigm for antimicrobial susceptibility testing. EBioMedicine 20:173–181. doi: 10.1016/j.ebiom.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakoulas G, Okumura CY, Thienphrapa W, Olson J, Nonejuie P, Dam Q, Pogliano J, Yeaman MR, Hensler ME, Bayer AS, Nizet V. 2014. Nafcillin enhances innate immune-mediated killing of methicillin-resistant Staphylococcus aureus. J Mol Microbiol 92:139–149. doi: 10.1007/s00109-013-1100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhand A, Bayer AS, Pogliano J, Yang SJ, Bolaris M, Nizet WG, Sakoulas G. 2011. Use of antistaphylococcal beta-lactams to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: role of daptomycin binding. Clin Infect Dis 53:158–163. doi: 10.1093/cid/cir340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakoulas G, Bayer AS, Pogliano J, Tsuji BT, Yang SJ, Mishra NN, Nizet V, Yeaman MR, Moise PA. 2012. Ampicillin enhances daptomycin- and cationic host defense peptide-mediated killing of ampicillin- and vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother 56:838–844. doi: 10.1128/AAC.05551-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin L, Nonejuie P, Munguia J, Hollands A, Olson J, Dam Q, Kumaraswamy M, Rivera H, Corriden R, Rohde M, Hensler ME, Burkart MD, Pogliano J, Sakoulas G, Nizet V. 2015. Azithromycin synergizes with cationic antimicrobial peptides to exert bactericidal and therapeutic activity against highly multi-drug resistant Gram-negative bacterial pathogens. EBioMedicine 2:690–698. doi: 10.1016/j.ebiom.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumaraswamy M, Lin L, Olson J, Sun CF, Nonejuie P, Corriden R, Döhrmann S, Ali SR, Amaro D, Rohde M, Pogliano J, Sakoulas G, Nizet V. 2016. Standard susceptibility testing overlooks potent azithromycin activity and cationic peptide synergy against multidrug-resistant Stenotrophomonas maltophilia. J Antimicrob Chemother 71:1264–1269. doi: 10.1093/jac/dkv487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bryan JP, Scheld WM. 1992. Therapy of experimental meningitis due to Salmonella enteridis. Antimicrob Agents Chemother 36:949–954. doi: 10.1128/AAC.36.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kristian SA, Timmer AM, Liu GY, Lauth X, Sal-Man N, Rosenfeld Y, Shai Y, Gallo RL, Nizet V. 2007. Impairment of innate immune killing mechanisms by bacteriostatic antibiotics. FASEB J 21:1107–1116. doi: 10.1096/fj.06-6802com. [DOI] [PubMed] [Google Scholar]
- 21.Hassing RJ, Goessens WHF, van Pelt W, Mevius DJ, Stricker BH, Molhoek N, Verbon A, van Genderen PJJ. 2014. Salmonella subtypes with increased MICs for azithromycin in travelers returned to the Netherlands. Emerg Infect Dis 20:705–708. doi: 10.3201/eid2004.131536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buyck JM, Plésiat P, Traore H, Vanderbist F, Tulkens PM, Van Bambeke F. 2012. Increased susceptibility of Pseudomonas aeruginosa to macrolides and ketolides in eukaryotic cell culture media and biological fluids due to decreased expression of oprM and increased outer-membrane permeability. Clin Infect Dis 55:534–542. doi: 10.1093/cid/cis473. [DOI] [PubMed] [Google Scholar]
- 23.CLSI 2016. Performance standards for antimicrobial susceptibility testing, 26th ed. CLSI supplement M100S. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 24.Moody J, Knapp C. Tests to assess bactericidal activity, p 5.10.11.11–15.10.13.16. In Garcia LS (ed), Clinical microbiology procedures handbook. ASM Press, Washington, DC. [Google Scholar]
- 25.Kristian SA, Datta V, Weidenmaier C, Kansal R, Fedtke I, Peschel A, Gallo RL, Nizet V. 2005. d-Alanylation of teichoic acids promotes group A streptococcus antimicrobial peptide resistance, neutrophil survival, and epithelial cell invasion. J Bacteriol 187:6719–6725. doi: 10.1128/JB.187.19.6719-6725.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Köckritz-Blickwede M, Chow OA, Nizet V. 2009. Fetal calf serum contains heat-stable nucleases that degrade neutrophil extracellular traps. Blood 114:5245–5246. doi: 10.1182/blood-2009-08-240713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kill curves against CSF Salmonella enterica strain in MHB (top row) and RPMI+10% LB (bottom row) using various antibiotics at 2×, 4×, and 8× MIC. Azithromycin was assayed only in RPMI+10% LB, as it was inactive in MHB. Download FIG S1, PDF file, 0.1 MB (108.7KB, pdf) .
Copyright © 2017 Sakoulas et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.