Abstract
BACKGROUND:
No retrievable and repositionable second generation transcatheter aortic valve is available in China. Here, we report the first-in-man implantation of the retrievable and repositionable VenusA-Plus valve.
METHODS:
A 76-year-old patient with symptomatic severe aortic stenosis and high surgical risk (STS 13.8%) was recommended for transcatheter aortic valve replacement (TAVR) by heart valve team. Type 0 bicuspid aortic valve with asymmetric calcification was identified by dual source computed tomography, and the unfavorable anatomies increased the possibility of malposition and paravalvular leakage during TAVR. Therefore, we used the retrievable and repositionable VenusA-Plus valve for the patient.
RESULTS:
Transfemoral TAVR was performed under local anesthesia with sedation, and a 26mm VenusA-Plus valve was successfully implanted. No transvalvular pressure gradient and trace paravalvular leakage were found.
CONCLUSION:
The successful first-in-man implantation indicates the retrievable and repositionable VenusA-Plus valve is feasible in complicated TAVR cases such as bicuspid aortic valve.
Keywords: Transcatheter aortic valve replacement, VenusA-Plus valve, Retrievable, Repositionable, Bicuspid aortic valve
INTRODUCTION
Transcatheter aortic valve replacement (TAVR) is a safe and effective alternative treatment to surgical aortic valve replacement (SAVR) for patients with symptomatic severe aortic stenosis (AS) who are at increased surgical risk (STS or EuroSCORE II≥4% or logistic EuroSCORE I≥10%).[1] In patients who received first generation devices, bicuspid AS had more frequent moderate-to-severe PVL especially with the self-expanding device.[2] However, in China, patients presenting for TAVR have a significantly higher frequency of bicuspid valve morphology and more severe leaflets calcification.[3,4] Although VenusA-valve (Venus Medtech Inc., Hangzhou, China) was approved by the China Food and Drug Administration (CFDA) as the first domestic self-expanding transcatheter heart valve (THV), Chinese patients needed the second generation THV with the ability to retrieve and reposition especially in cases of unfavorable anatomies such as bicuspid AS and heavily calcification.
VenusA-Plus valve (Venus Medtech Inc., Hangzhou, China) is built upon the VenusA-Valve and is the second generation THV with an improved and reinforced shaft and capsule allowing for retrievability and repositionability. Herein, we described the first-in-man implantation with this new device in a bicuspid AS patient.
CASE
A 76-year-old lady referred to our hospital due to symptomatic severe aortic stenosis with aortic valve area of 0.55 cm2, mean pressure gradient of 46 mmHg and maximum velocity of 4.42 m/s. With the comorbidities of coronary artery disease, hypertension, type 2 diabetes mellitus, history of right lower extremity amputation due to bone tumor and NYHA functional class IV, the STS score was calculated as 13.8%. The heart valve team recommended TAVR. Pre-TAVR dual source computed tomography evaluation showed type 0 bicuspid aortic valve with asymmetric calcification. The unfavorable anatomies increase the possibility of malposition and paravalvular leakage during TAVR using the first generation of self-expanding valve, such as CoreValve (Medtronic, Minneapolis, MN, USA) and Venus A valve. The second generation retrievable and repositionable VenusA-Plus valve was preferred in this unfavorable situation and ethical permission was obtained from the hospital. With the 21.2 mm in diameter annulus (Figure 1A) and asymmetric calcified leaflets (Figure 1B), a 26 mm VenusA-Plus (Figure 1C) valve was selected. The TAVR procedure was performed through transfemoral access under local anesthesia with sedation on November 23, 2017. Pre-dilation with a 20-mm Z-MED balloon (NuMED, Inc., Hopkinton, New York) was performed before the VenusA-Plus valve was implanted. The valve was then successfully crossed through the aortic arch and implanted in an ideal position without second attempt. The pressure gradient dropped from 44 mmHg to 0 mmHg with trivial paravalvular leak (Figure 1D).
Figure 1.
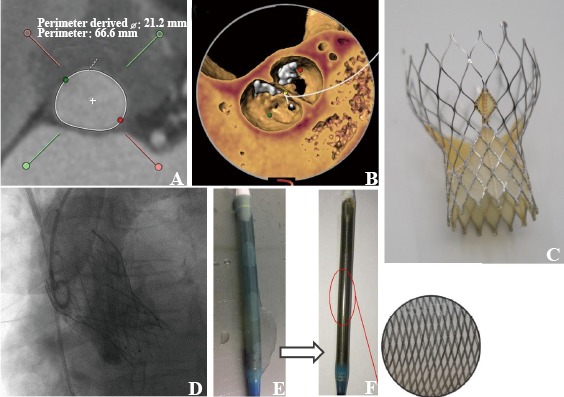
VenusA-Plus valve: first-in-man experience. A: aortic annulus; B: type 0 bicuspid aortic valve with asymmetric calcifi cation; C: The Venus A-Plus valve; D: The fi nal position of valve; E: capsule of VenusA-Valve; F: reinforced capsule of VenusA-Plus valve.
Pre-discharge TTE revealed an aortic valve area of 1.35 mm2, mean pressure gradient of 5 mmHg and maximum velocity of 1.6 m/s. The patient’s NYHA functional class was improved to class III without stroke and new pacemaker implantation.
DISCUSSION
The TAVR situation in China is in its infancy with a very limited number of cases. The aortic root anatomies of TAVR candidates are much more complicated than those in western countries.[3,4] Therefore, the need of new generation retrievable and repositionable THV is urgent in China.
….VenusA-valve is the first approved THV by CFDA with a similar design to the CoreValve but a stronger radial force designed for bicuspid aortic valve and severe calcification. However, the first generation VenusA valve system is not retrievable and repositionable. It is very challenging to have a retrievable delivery system for the higher expansion force VenusA-Valve. The VenusA-Plus retrievable delivery system has the same profile and working length as the current Venus-A delivery catheter. However, its shaft and capsule are reinforced with wire mesh to have enough pushability while maintain its flexibility (Figure 1E and F).
The successful first-in-man implantation indicates that the retrievable and repositionable VenusA-Plus valve is feasible in complicated TAVR cases such as bicuspid aortic valve. Future multi-center clinical trials are necessary before the new device will be widely used.
ACKNOWLEDGMENTS
The authors thank the Advanced Technique Research of Valvular Heart Disease Treatment Project (2015C03028) for their support of this study.
Footnotes
Funding: This study was supported by Advanced Technique Research of Valvular Heart Disease Treatment Project (2015C03028).
Ethical approval: This study protocol was approved by ethical comittee of the Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China.
Conflicts of interests: The authors declare that they have no competing interests. The funders had no role in the design, conduct, analysis, or interpretation of data or in writing the manuscript.
Contributors: JW, XL, FG, AD, MK, JC, YX and MY performed the TAVR procedure. YH, LY, QZ, QZ, CL and LW evaluated this patient for TAVR. XL, YH and CL wrote the manuscript. JW revised and edited the manuscript. All authors read and approved the final manuscript.
REFERENCES
- 1.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38(36):2739–91. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 2.Yoon SH, Bleiziffer S, De Backer O, Delgado V, Arai T, Ziegelmueller J, et al. Outcomes in Transcatheter Aortic Valve Replacement for Bicuspid Versus Tricuspid Aortic Valve Stenosis. J Am Coll Cardiol. 2017;69(21):2579–89. doi: 10.1016/j.jacc.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Liu XB, Jiang JB, Zhou QJ, Pu ZX, He W, Dong AQ, et al. Evaluation of the safety and efficacy of transcatheter aortic valve implantation in patients with a severe stenotic bicuspid aortic valve in a Chinese population. J Zhejiang Univ Sci B. 2015;16(3):208–14. doi: 10.1631/jzus.B1500017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jilaihawi H, Wu Y, Yang Y, Xu L, Chen M, Wang J, et al. Morphological characteristics of severe aortic stenosis in China:imaging corelab observations from the first Chinese transcatheter aortic valve trial. Catheter Cardiovasc Interv. 2015;85(Suppl 1):752–61. doi: 10.1002/ccd.25863. [DOI] [PubMed] [Google Scholar]


